Abstract
Phthalate exposure has been associated with adverse reproductive outcomes and oxidative stress is a potential mechanism by which they act. However, few human studies have explored co-exposure confounding or joint effects. Furthermore, most studies examine associations between biomarkers of exposure and oxidative stress from the same urine sample. We investigated single-exposure, co-exposure-adjusted, and joint associations between phthalate metabolites and oxidative stress in the Environment and Reproductive Health (EARTH) study among couples undergoing fertility treatment. We examined cross-sectional associations in both women and men, and longitudinal associations in women. Urine was collected in the follicular phase (women only) and at the time of fertility procedure (women and men), and analyzed for 11 phthalate metabolites. Urine from the time of fertility procedure was analyzed for oxidative stress biomarkers, including free 8-iso-prostaglandin F2α (8-iso-PGF2α), its primary metabolite (2,3-dinor-5,6-dihydro-15-F2t-isoprostane [F2-ISoP-M]), and prostaglandin F2α (PGF2α). Linear mixed effects models were used to estimate single-exposure associations. Bayesian Kernel Machine Regression (BKMR) was used to adjust for co-exposures and to estimate joint effects. Among women, we observed positive associations between all phthalate metabolites and oxidative stress biomarkers in single-exposure models, but there was clear co-exposure confounding. For instance, in a single-exposure model, we estimated a 63% (95% confidence interval: 51, 77) increase in the 8-iso-PGF2α metabolite per interquartile range (IQR) difference in mono-n-butyl phthalate (MBP) versus a 34% (95% credible interval: 12, 60) increase in co-adjusted models. However, several phthalate metabolites remained associated with oxidative stress in co-exposure models, and the joint effects of all exposures were high (e.g., an 114% increase in the 8-iso-PGF2α metabolite per IQR difference in all exposures). Longitudinal results were also attenuated compared to cross-sectional results in women; however, the joint effect of all exposures and the 8-iso-PGF2α metabolite remained positive and statistically significant (11% increase per IQR difference in all exposures, 95% credible interval: 0.2, 23). In men, associations were generally less pronounced, although the joint effect of the mixture on 8-iso-PGF2α was above the null. Because oxidative stress is related to reproductive success among couples seeking fertility treatment, mitigating phthalate exposure should be considered as a potentially beneficial measure.
Keywords: phthalates, oxidative stress, mixtures, pre-conception, fertility, Bayesian kernel machine regression
1. Introduction
Phthalates are a class of chemicals heavily used in everyday products predominantly for the purpose of softening plastic. Phthalates as plasticizers can be found in many household items including flooring, carpeting, food containers and packaging, and children’s toys. Additionally, these are used as preservatives and fillers in medications and dietary supplements, and as solvents in products including cosmetics, fragrances, and pesticides (ATSDR, 1995; ATSDR, 1997; ATSDR, 2001; ATSDR, 2019). Phthalates are quickly metabolized in the body but due to their widespread production and use, much of the US population is exposed daily (ATSDR, 1995; ATSDR, 1997; ATSDR, 2001; ATSDR, 2019; Hannon and Flaws, 2015).
Exposure to phthalates is thought to induce oxidative stress in humans, and increasing epidemiological evidence has shown phthalate metabolites are associated with oxidative stress biomarkers in many US populations (Ferguson et al., 2014; Ferguson et al., 2011; Ferguson et al., 2012; Holland et al., 2016; Wu et al., 2017) and worldwide (Dong et al., 2018; Duan et al., 2017; Liu et al., 2019; Yuan et al., 2020). Oxidative stress, an imbalance between oxygenated molecules and antioxidants, may interfere with reproduction. In our previous investigation of couples seeking fertility treatment, circulating measures of oxidative stress have been shown to have non-linear associations with treatment success in women (Rosen et al., 2019).
One limitation to the previous studies that have assessed the relationship between oxidative stress and phthalate exposure is the focus on single exposure associations (Guo et al., 2014; Wu et al., 2017). In a previous analysis in pregnant women, we used adaptive elastic net to identify the most important compounds within a mixture of phthalate metabolites and phenols for the association with oxidative stress biomarkers (Ferguson et al., 2019b). However, the cumulative effect of exposure is also of interest from a public health perspective. The association between preconception phthalate mixtures and oxidative stress has rarely been examined in women, though, oxidative stress levels may be important for pregnancy success as noted above. Additionally, these relationships have been examined seldom in men. Thus, this study aims to assess the association between urinary phthalate metabolites, individually and as a mixture, with circulating oxidative stress biomarkers, including 8-iso-prostaglandin F2α (8-iso-PGF2α), its primary metabolite (2,3-dinor-5,6-dihydro-15-F2t-isoprostane [F2-ISoP-M]), and prostaglandin F2α (PGF2α), in people seeking fertility treatment. Our mixture analyses examine single phthalate metabolite effects after accounting for co-exposure confounding and estimate joint effects.
A secondary aim of the analysis is to investigate potential bias of measuring both the exposure and outcome in the same urine sample. Because phthalates are rapidly metabolized in the human body (Frederiksen et al., 2007), and 8-iso-PGF2α is generated quickly and also rapidly metabolized (Helmersson and Basu, 1999), examining associations between biomarkers from the same point in time may be ideal. However, when biomarkers are analyzed in the same sample, residual confounding from urine dilution or other aspects of metabolism may occur. In this unique study, phthalate metabolite concentrations in women were measured in urine samples collected at the same time as oxidative stress biomarkers and additionally in samples collected approximately one week prior. Thus, we had the opportunity to examine longitudinal associations between exposure measurements and oxidative stress biomarkers in this study as well.
2. Materials and Methods
2.1. Study population
The Environment and Reproductive Health (EARTH) study is a prospective preconception cohort study of couples undergoing fertility treatment for examining determinants of fertility (Messerlian et al., 2018). Women between the ages of 18 and 45 undergoing fertility treatment at the Massachusetts General Hospital (MGH) Fertility Center from November 2007 through April 2015 were recruited and eligible for inclusion in the study if contributing their own oocytes. Their partners, if male and between the ages of 18 and 51, were also eligible for inclusion. The Human Studies Institutional Review Boards at Harvard T.H. Chan School of Public Health and MGH approved EARTH study protocols and all participants submitted informed consent prior to study initiation. Additionally, the current analysis was deemed exempt by the NIEHS IRB.
We collected 1 urine sample per male participant and up to 2 urine samples per female partner in each fertility treatment cycle. Women provided samples in the follicular phase and approximately one week later at the time of treatment (day of oocyte retrieval for IVF, day of insemination for IUI), and men provided samples at the time of treatment. In the current analysis, study participants who provided a urine sample at the time of fertility treatment with measured phthalate metabolite concentrations and oxidative stress biomarkers were included. Multiple cycles, if available, were included for each study participant. The current analysis included urine samples from 903 women-cycles (mean 2.03 cycles per woman, range 1-9) and 437 men-cycles (mean 1.87 cycles per man, range 1-8). Men and women were examined separately for all analyses.
2.2. Urinary Phthalate Metabolite Measurements
Urine samples were collected in a sterile polypropylene specimen cup. Specific gravity (SG) was measured at room temperature using a handheld refractometer (National Instrument Company, Inc., Baltimore, MD, USA) calibrated with deionized water before each measurement. Each urine was divided into aliquots, frozen, and stored at −80 °C. Urine samples were shipped on dry ice overnight to the Center for Disease Control (CDC) where they were stored at or below −40 °C until analysis. The CDC laboratory is certified by the Health Care Financing Administration to comply with the requirements set forth in the Clinical Laboratory Improvement Amendments of 1988 (CLIA ’88). All analytical measurements follow strict quality control/quality assurance according to CLIA guidelines. For example, along with study samples, each analytical run includes a set of calibrators, reagent blanks, and high- and low-concentration quality control (QC) materials. Concentrations of the QCs were evaluated using standard statistical probability rules (Caudill et al., 2008).
Online solid-phase extraction coupled with isotope dilution-high-performance liquid chromatography-tandem mass spectrometry was used to quantify urinary concentrations of phthalate metabolites by the Centers for Disease Control and Prevention (CDC) (Mínguez-Alarcón et al., 2019). All urine samples examined in the present analysis were analyzed for a panel of 11 phthalate metabolites, including: mono-ethyl phthalate (MEP), mono-n-butyl phthalate (MBP), monobenzyl phthalate (MBzP), mono-isobutyl phthalate (MiBP), mono (2-ethylhexyl) phthalate (MEHP), mono (2-ethyl-5-carboxypentyl) phthalate (MECPP), mono (2-ethyl-5-hydroxyhexyl) phthalate (MEHHP), mono (2-ethyl-5-oxohexyl) phthalate (MEOHP), mono(3-carboxypropyl) phthalate (MCPP), monocarboxyoctyl phthalate (MCOP), and monocarboxy-isononyl phthalate (MCNP). Urinary phthalate metabolite concentrations below the limit of detection (LOD) were replaced with the LOD divided by the square root of 2. The molar sum of di-2-ethylhexyl phthalate metabolites (ΣDEHP) was calculated as (MEHP/278.34 + MEHHP/294.34 + MEOHP/292.33 + MECPP/308.33) and used in all analyses in lieu of individual DEHP metabolites.
2.3. Oxidative Stress Measurements
Free 8-iso-PGF2α, F2-ISoP-M, and PGF2α were also measured in urine samples from the same time points and used as biomarkers of oxidative stress. F2-ISoP-M is a main metabolite of 8-iso-PGF2α and may be a better indicator of whole-body oxidative stress compared to the parent compound (Milne et al., 2007). This is because kidney or bladder oxidative stress could confound levels of 8-iso-PGF2α in the urine sample, whereas the metabolite is formed in the liver so urinary concentrations would not have disproportionate representation from what is formed in the kidney or bladder. Concentrations were measured via gas chromatography-negative ion chemical ionization-mass spectrometry with stable isotope dilution (Milne et al., 2007). Briefly, 0.5mL urine was diluted to a volume of 10mL with 0.01N HCl containing 1.0ng of [2H4]-15-F2t-IsoP ([2H4]-8-iso-PGF2a; Cayman Chemical, Ann Arbor, MI). The solution was adjusted to pH 3 with 1N HCl prior to extraction on first a C-18 Sep-Pak extraction cartridge and subsequently a silica Sep-Pak extraction cartridge (Waters Corporation, Milford, MA USA). Analytes were then converted to the pentafluorobenzyl (PFB) ester, trimethylsilyl (TMS) ether derivatives for GC-MS analysis. GC/NICI-MS was carried out on an Agilent 5973 Inert Mass Selective Detector that is coupled with an Agilent 6890n Network GC system (Agilent Labs, Torrance, CA) that is interfaced with an Agilent computer.
8-iso-PGF2α can be derived through multiple pathways that reflect both oxidative stress and inflammation (van ’t Erve et al., 2019). Using a novel formula based on the relative proportions of 8-iso-PGF2α and PGF2α in the sample, we calculate the fraction of 8-iso-PGF2α that originates from oxidative lipid damage or upregulation of specific inflammation pathways (van ’t Erve et al., 2015). Briefly, the formulae were developed based on observations from in vitro, animal, and human data on the generation of 8-iso-PGF2α and PGF2α in response to activation of either pathway. The ratio of free 8-iso-PGF2α to PGF2α is based on the following formula (Van’t Erve et al., 2016), where CLP is the chemical lipid peroxidation and PGHS is the lipid peroxidation by prostaglandin-endoperoxide synthases:
The fraction of 8-iso-PGF2α produced by chemical lipid peroxidation can then be calculated as follows. (Note, the chemical fraction of 8-iso-PGF2α refers to the quantity of 8-iso-PGF2α from the chemical pathway and is not a proportion.)
An R program and tutorial for performing the calculations are available from: https://www.niehs.nih.gov/research/resources/software/tox-pharm/tools/pgf/index.cfm.
2.4. Statistical Analysis
Demographic characteristics were summarized based on information from the first study cycle with median (interquartile range [IQR]) or n (%). Distributions of phthalate metabolites and oxidative stress biomarker concentrations were summarized using median (IQR), also for the first study cycle. Spearman correlations were used to summarize pairwise associations among phthalate metabolite concentrations, also from the first study visit. For these descriptive analyses, concentrations of all biomarkers were corrected for urine dilution using the following formula: Bc = B[(SGmed-1)/(SG-1)], where SGmed is the median sg concentration in the EARTH population (1.015), B is the raw biomarker concentration, Bc is the sg-corrected biomarker concentration, and SG is the specific gravity of the individual sample. For subsequent analyses, we modeled raw concentrations of exposure and oxidative stress biomarkers and included specific gravity as a covariate, as this approach incurs less bias (Barr et al., 2005).
For all analyses, urinary biomarkers and phthalate metabolites were natural log transformed and then mean-centered and divided by their standard deviation so that all beta estimates would reflect the difference in outcome per 1 standard deviation increase in log-phthalate metabolite. Subsequently, all effect estimates were converted to reflect the change in outcome per interquartile range (IQR) difference in exposure. Additionally, all models included subject-specific random intercepts to accommodate multiple measurements of exposure and outcome. Single exposure associations were assessed via linear mixed-effects models. Mixture effects were estimated using Bayesian Kernel Machine Regression (BKMR) and assessed in two ways (Bobb et al., 2015). First, the co-exposure estimates were calculated to examine the association for each individual phthalate metabolite while all other phthalate metabolite concentrations are fixed at their median. Second, we estimated the joint effect of simultaneously increasing all phthalate metabolites within the mixture from the 25th to 75th percentile. Using single exposure and mixture models described above, we also estimated the phthalate metabolite associations with the chemical fraction of 8-iso-PGF2α.
Covariates which resulted in a > 10% change in effect estimates for any metabolite-biomarker pair in single exposure linear mixed-effects models were included in all adjusted models. These included: education (<college graduate/college graduate), race (white/other), smoking status (ever/never), infertility diagnosis (male/female factor/unexplained), age (years), and body mass index (BMI; kg/m2). Fertility treatment procedure (IVF/IUI) and urinary specific gravity were also included as time-varying covariates. For single-exposure analyses, missing covariates were imputed using multivariate imputation via chained equations and all model estimates were pooled across multiple imputations using Rubin’s rules (Buuren and Groothuis-Oudshoom, 2010; Rubin, 2004). For BKMR models, five chains were run for 200,000 iterations, each on a multiply imputed data set with a 50,000 burn-in period and 150 iterate thinning interval. Default priors were used, and the parallel chains were used to assess diagnostics including convergence via the multivariate potential scale reduction factor and trace plots (Brooks and Gelman, 1998).
In our secondary analysis, we estimated longitudinal associations in women between phthalate metabolite concentrations analyzed in urine from the follicular phase and oxidative stress biomarker concentrations in urine from the time of treatment, roughly one week later (n=382 women, 735 women-cycles). These models took the same form as our primary models, which accounted for repeated measures across participant with subject-specific random intercepts, and accounted for the same covariates.
3. Results
3.1. Descriptive statistics
The analysis sample consisted of 445 women contributing 903 cycles and 234 men contributing 437 cycles. At the first cycle, women had a median age of 35 years, were typically of healthy BMI (median = 23 kg/m2) and were primarily white (83%), college educated (91%) and never smokers (72%) (Table 1). Furthermore, 39% of infertility diagnoses were of unexplained origin. Men were slightly older (median = 36 years old) and slightly heavier (BMI median = 27 kg/m2), and mostly college educated (84%) and white (87%).
Table 1.
Demographic characteristics at first study visit for women and men enrolled in the EARTH study—median (IQR) or n (%).
| Women (n = 445) | Men (n = 234) | |
|---|---|---|
| Age (years) | 35.0 (32.0, 38.0) | 36.0 (32.9, 39.6) |
| missing | 0 | 1 |
| BMI (kg/m2) | 23.4 (21.2, 26.4) | 26.9 (24.4, 29.4) |
| missing | 2 | 1 |
| Race | ||
| White | 368 (82.7) | 202 (86.7) |
| Other | 77 (17.3) | 31 (13.3) |
| missing | 0 | 1 |
| Education | ||
| < College graduate | 36 (9.1) | 30 (16.0) |
| College graduate | 361 (90.9) | 157 (84.0) |
| missing | 48 | 47 |
| Smoking Status | ||
| Never | 320 (71.9) | 152 (65.2) |
| Ever | 125 (28.1) | 81 (34.8) |
| missing | 0 | 1 |
| Primary infertility diagnosis | ||
| Female Factor | 144 (32.4) | 76 (32.5) |
| Male Factor | 126 (28.3) | 70 (29.9) |
| Unexplained | 175 (39.3) | 88 (37.6) |
| Fertility treatment | ||
| IUI | 233 (52.4) | 106 (45.3) |
| IVF | 212 (47.6) | 128 (54.7) |
Abbreviations: IQR, interquartile range; BMI, body mass index; IUI, intrauterine insemination; IVF, in vitro fertilization. Note: If multiple infertility diagnoses were present, the primary or most important diagnosis was used.
Phthalate metabolite and oxidative stress biomarker concentrations from the time of fertility treatment at the first study cycle are presented in Table 2. Phthalate metabolite concentrations were similar in men and women, and Spearman correlations between phthalate metabolites were also largely similar within men and women (Figure 1). High positive correlations in women were observed between MCOP and MCPP (0.80). Similarly, high positive correlations in men were observed between MCOP and MCPP (0.76). Associations between first phthalate metabolite measurements and demographic characteristics of the study participants are shown in Supplemental Tables 2 and 3 for women and men, respectively. Phthalate metabolite levels tended to be higher among participants with less than a college education compared to those with a college degree among women, and among participants of other race/ethnicity compared to white participants for both men and women.
Table 2.
Specific gravity-corrected urinary phthalate metabolite and oxidative stress biomarker concentrations measured in the first cycle from women and men in the EARTH study: 50th percentile (25th, 75th percentiles) (ng/mL).a
| Women (n = 445) | Men (n = 234) | |
|---|---|---|
| Phthalate metabolites | ||
|
| ||
| MEP | 49.2 (18.6, 129.0) | 43.0 (17.4, 119.4) |
| MBP | 9.4 (3.7, 17.9) | 10.8 (6.7, 19.2) |
| MBzP | 2.7 (1.0, 5.7) | 3.3 (1.8, 6.1) |
| MiBP | 6.0 (2.7, 10.8) | 6.7 (4.2, 12.6) |
| ΣDEHP | 0.1 (0.06, 0.3) | 0.1 (0.09, 0.4) |
| MCPP | 3.1 (1.2, 7.9) | 3.4 (1.8, 8.5) |
| MCOP | 23.1 (7.5, 67.9) | 25.2 (9.6, 74.4) |
| MCNP | 4.0 (2.3, 8.7) | 3.5 (1.9, 7.4) |
|
| ||
| Oxidative stress biomarkers | ||
|
| ||
| 8-iso-PGF2α | 1.3 (0.9, 1.8) | 1.0 (0.8, 1.4) |
| F2-IsoP-M | 0.5 (0.4, 0.7) | 0.6 (0.4, 1.0) |
| PGF2α | 1.2 (0.8, 1.8) | 1.4 (0.8, 2.7) |
Units for ΣDEHP are in nmol/mL.
Abbreviations: MEP, Mono-ethyl phthalate; MBP, Mono-n-butyl phthalate; MBzP, Monobenzyl phthalate; MiBP, Mono-isobutyl phthalate; ΣDEHP. sum of di(2-ethylhexyl) phthalates; MCPP, Mono(3-carboxypropyl) phthalate; MCOP, Monocarboxyoctyl phthalate; MCNP, Monocarboxy-isononyl phthalate; 8-iso-PGF2α, 8-iso-prostaglandin F2α; F2-IsoP-M, 2,3-dinor-5,6-dihydro-15-F2t-isoprostane; PGF2α, prostaglandin F2α.
Figure 1.
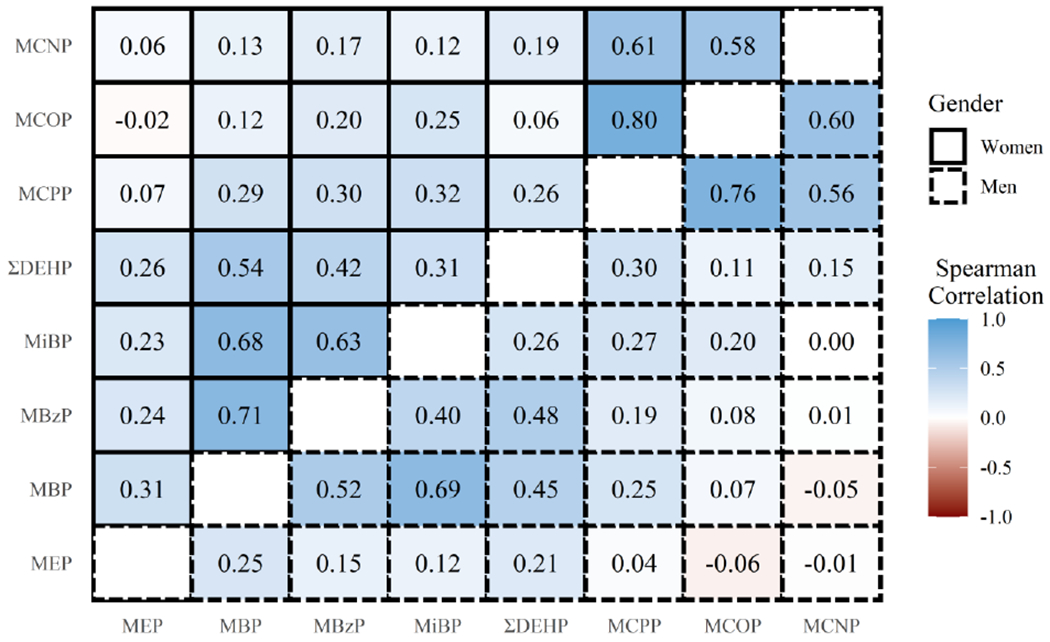
Spearman correlation matrix of specific gravity-corrected urinary phthalate metabolite concentrations for EARTH study participants by gender at first cycle. Upper and lower triangles of the matrix correspond to phthalate metabolite correlations for women and men, respectively.
3.2. Cross-sectional associations among women and men
Figure 2 displays cross-sectional associations between the oxidative stress biomarkers and urinary phthalate metabolites by gender, in single-exposure models and in co-exposure models (numeric values presented in Supplemental Tables 4 and 5 for women and men, respectively). In single-exposure models, positive associations were observed between all oxidative stress biomarker measures and phthalate metabolites in women. The magnitude of the associations for all oxidative stress biomarkers was greatest for MBP (43-63% increase in oxidative stress biomarker per IQR difference in exposure), MiBP (30-52% increase per IQR difference), and MBzP (33-49% increase per IQR difference). In men, no associations were observed between phthalates and PGF2α. However, some positive associations were observed between phthalate metabolites and 8-iso-PGF2α and its main metabolite. As in women, associations were greatest in magnitude for associations between these two oxidative stress biomarkers and MBP (16-19%), MBzP (17-18%), and MiBP (22-24%), but they were also high for ΣDEHP (14%).
Figure 2.
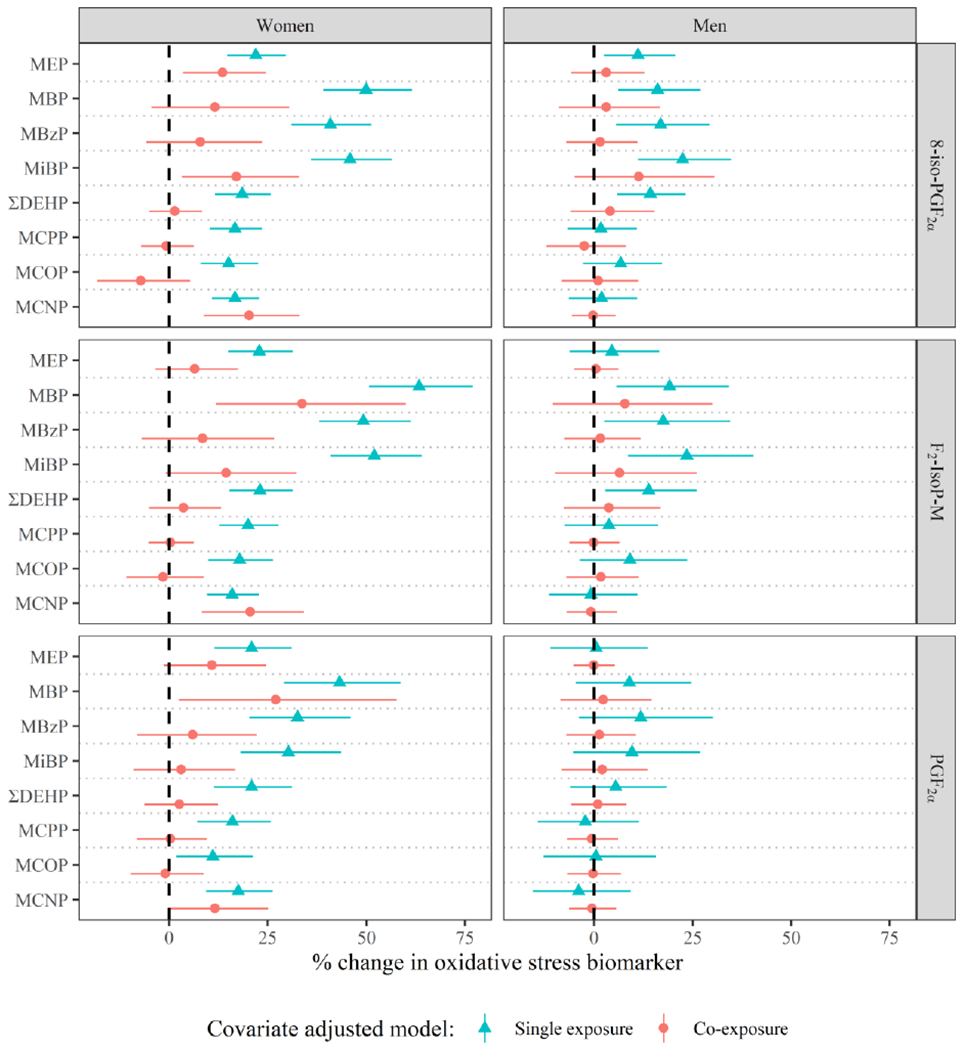
Adjusteda percent change (95% Confidence Interval for single exposure models, 95% Credible Interval for co-exposure models) in oxidative stress biomarker concentration per interquartile range difference in urinary phthalate metabolite concentration by gender, in models adjusted for demographic covariates (green triangles) and additionally jointly adjusted for all other phthalate metabolites (red circles).
aSingle exposure and co-exposure models include the following covariates: education (±college graduate), race (white/other), smoking status (ever/never), infertility diagnosis (male/female factor/unexplained), age (years), body mass index (kg/m2), current cycle fertility treatment procedure (IVF/IUI), and current cycle urine specific gravity. Co-exposure models were estimated using Bayesian Kernel Machine regression with all exposures included, and estimates display the percent change for a difference in a specific phthalate metabolite from the 25th percentile to the 75th percentile while holding all other phthalate metabolites in the mixture fixed at their respective 50th percentiles.
The associations between phthalate metabolites and measures of oxidative stress were attenuated in both women and men when jointly co-adjusting for other phthalate metabolites. In women, MEP, MBP, MiBP, and MCNP maintained positive associations, but not consistently across all oxidative stress biomarkers. For instance, the most stable associations from single exposure to co-exposure models in women were for MCNP, which remained positively associated with all three oxidative stress biomarkers in jointly adjusted models (14-22% increase per IQR difference) except for PGF2α. In men, effect estimates were more similar between single exposure and co-exposure models. However, no phthalate metabolites maintained statistical significance in co-exposure models.
Finally, we examined the overall impact of phthalate metabolites on each oxidative stress biomarker. Figure 3 displays the adjusted percent change in each oxidative stress biomarkers per IQR increase in all urinary phthalate metabolite concentrations within the mixture. In women, the percent increases in oxidative stress biomarkers were all estimated to be greater than 72% (72% - 114% per IQR increase). As in models of individual phthalate metabolites, associations with oxidative stress, rather than inflammation, appeared to be driving these associations. Among men, associations were lesser in magnitude, but still elevated for 8-iso-PGF2α (24% increase per IQR difference in all phthalate metabolites). The co-exposure associations between urinary phthalate metabolites and the chemical fraction of 8-iso-PGF2α were similar to the overall associations with 8-iso-PGF2α for women and null in men (Supplemental Figure 1). Focusing on joint mixture results in men, the positive associations observed in 8-iso-PGF2α per simultaneous increase in metabolite concentrations were not apparent with the chemical fraction of 8-iso-PGF2α (Supplemental Figure 2). This suggests that the associations with overall 8-iso-PGF2α could have been driven by inflammation rather than oxidative stress.
Figure 3.
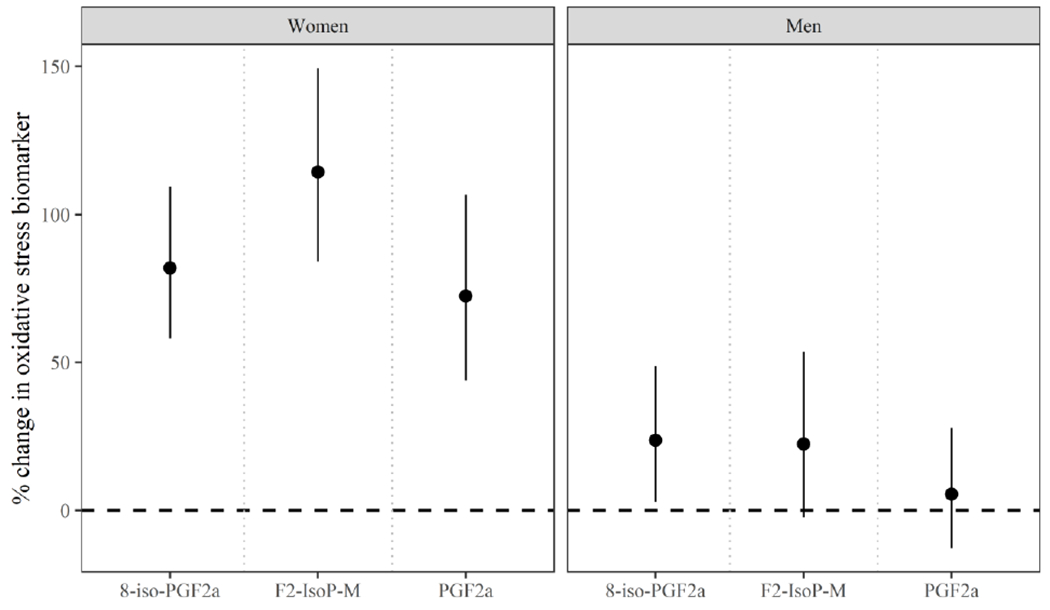
Adjusteda percent change (95% credible interval) in oxidative stress biomarker concentration per joint increase in all urinary phthalate metabolite concentrations from the 25th to 75th percentile by gender.
aModels include the following covariates: education (±college graduate), race (White/Other), smoking status (ever/never), infertility diagnosis (male/female factor/unexplained), age (years), body mass index (kg/m2), current cycle fertility treatment procedure (IVF/IUI), and current cycle urine specific gravity.
3.3. Longitudinal associations among women
In our secondary longitudinal analysis in women examining follicular phase phthalate metabolite concentrations with oxidative stress biomarkers measured at the time of fertility treatment, we observed associations that were attenuated compared to those in our primary models (Figure 4). Comparing results from single-exposure models, all effect estimates from longitudinal models were greatly attenuated in magnitude compared to those from cross-sectional models. For example, % increase per IQR difference in individual phthalate metabolites and the 8-iso-PGF2α metabolite ranged from 16 - 63 for cross-sectional models but only 2.9 – 9.8 for longitudinal models. However, associations between almost all phthalate metabolites and the 8-iso-PGF2α metabolite remained statistically significant. Regarding results from co-exposure models, all associations from cross-sectional analyses were attenuated to the null in longitudinal analyses.
Figure 4.
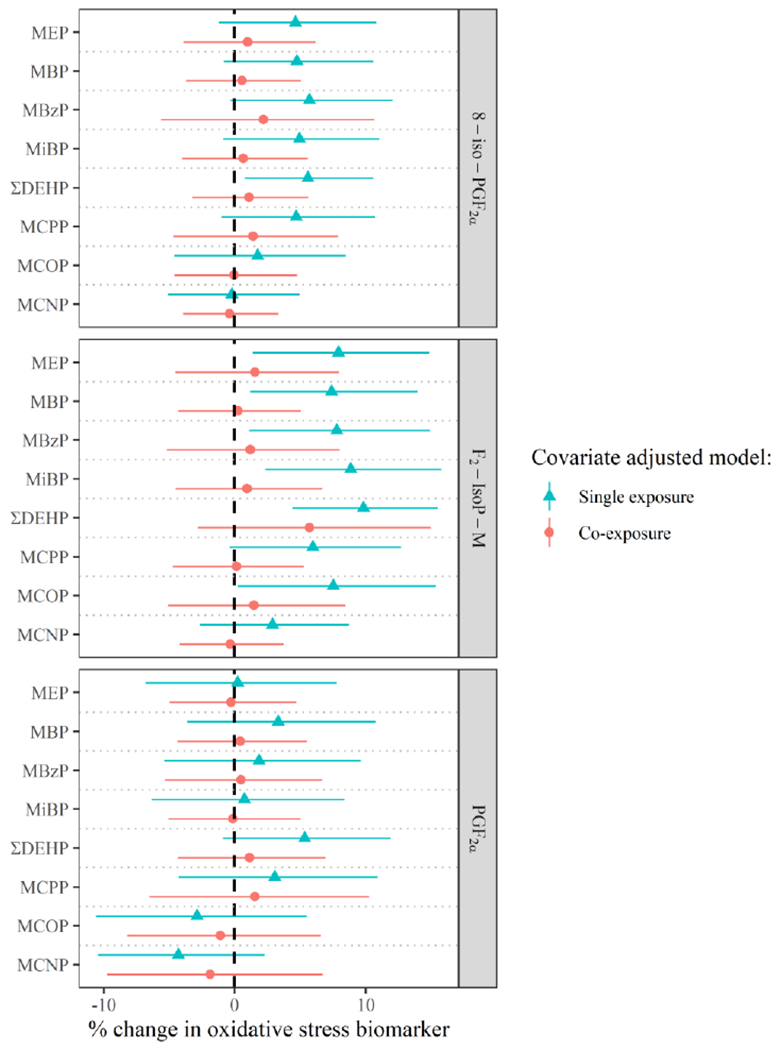
Adjusteda percent change (95% Confidence Interval for single exposure models, 95% Credible Interval for co-exposure models) in oxidative stress biomarker concentration per interquartile range difference in urinary phthalate metabolite concentration, in longitudinal models adjusted for demographic covariates (green triangles) and additionally jointly adjusted for all other phthalate metabolites (red circles).
aSingle exposure and co-exposure models include the following covariates: education (±college graduate), race (white/other), smoking status (ever/never), infertility diagnosis (male/female factor/unexplained), age (years), body mass index (kg/m2), current cycle fertility treatment procedure (IVF/IUI), urine specific gravity at treatment, and urine specific gravity during the follicular phase. Co-exposure models were estimated using Bayesian Kernel Machine regression with all exposures included, and estimates display the percent change for an increase in a specific phthalate metabolite from the 25th percentile to the 75th percentile while holding all other phthalate metabolites in the mixture fixed at their respective 50th percentiles.
Models of the joint effects of the phthalate metabolite mixture and oxidative stress biomarkers showed effect estimates that were again attenuated compared to those from cross-sectional models (Figure 5). However, the association between the phthalate mixture and the 8-iso-PGF2α metabolite remained significantly elevated where an IQR difference in the phthalate metabolite mixture was associated with a 11% (95% credible interval=0.2, 23) higher 8-iso-PGF2α metabolite concentration.
Figure 5.
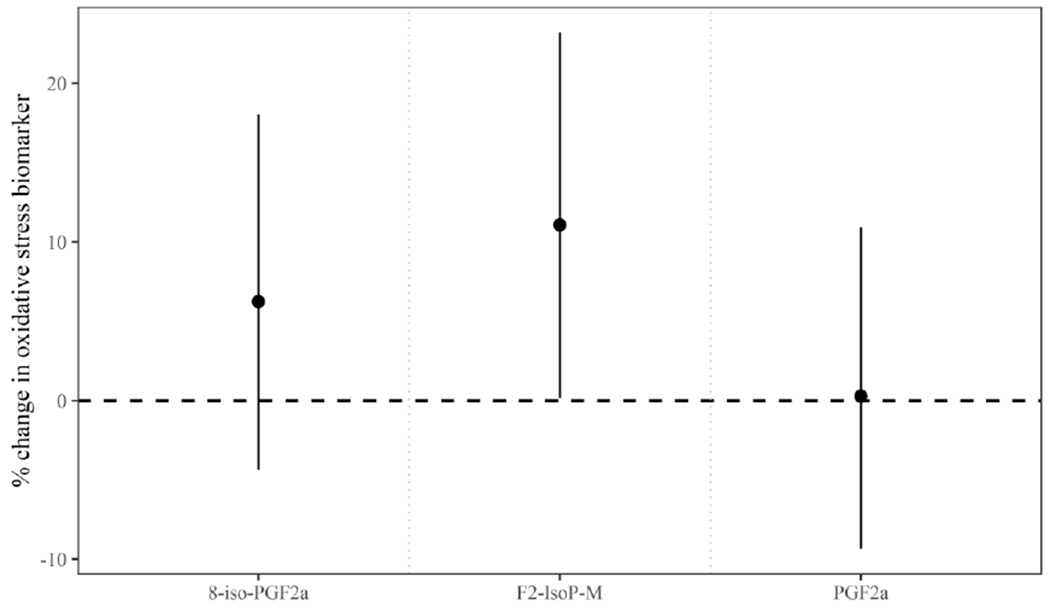
Adjusteda percent change (95% credible interval) in oxidative stress biomarker concentration per joint increase in all urinary phthalate metabolite concentrations from the 25th to 75th percentile among women in longitudinal models.
aModels include the following covariates: education (±college graduate), race (White/Other), smoking status (ever/never), infertility diagnosis (male/female factor/unexplained), age (years), body mass index (kg/m2), current cycle fertility treatment procedure (IVF/IUI), specific gravity measured in urine from the treatment cycle, and specific gravity measured in urine from the follicular phase.
4. Discussion
Among men and women undergoing fertility treatment, we observed positive associations between urinary phthalate metabolites and oxidative stress markers in single-exposure models; however, the magnitude of most associations was attenuated in multi-exposure models. The overall impact of the phthalate metabolite mixture on oxidative stress biomarkers was quite high, and greater in magnitude in women than in men. These findings suggest that exposure to phthalates among these couples is associated with elevated systemic oxidative stress and that co-exposure confounding as well as joint effects are important to consider in these relationships.
Our findings from single exposure models are consistent with findings from other studies on the association between urinary phthalate metabolites and oxidative stress biomarkers in adults (Chang et al., 2019; Dong et al., 2018; Duan et al., 2017; Ferguson et al., 2014; Ferguson et al., 2011; Ferguson et al., 2012; Ferguson et al., 2015; Guo et al., 2014; Holland et al., 2016; Liu et al., 2019; van ’t Erve et al., 2019; Wu et al., 2017; Yuan et al., 2020). Among these studies, few have accounted for co-exposure confounding or estimated cumulative effects of exposure, as we did in the present analysis. In a study of pregnant women, the magnitude of the association between phthalate metabolites and oxidative stress biomarkers was attenuated in models that were mutually adjusted, as was observed in our findings (Ferguson et al., 2015). Our analysis further illustrates that co-exposure confounding is potentially highly impactful, as demonstrated by attenuations in jointly adjusted models versus single exposure models. Additionally, our study highlights the strong cumulative effects of exposure to phthalates on oxidative stress biomarkers, particularly among women.
In studies including men and women, gender differences in the association between phthalate exposure and oxidative stress have been observed. Most of these studies indicate that associations are stronger in women compared to men, which is consistent with what we observed in the present analysis. In a study using data form the National Health and Nutrition Examination Survey (NHANES) between 1999 – 2006, MEP was found to be positively associated with bilirubin in women but null in men (Ferguson et al., 2012). Duan et al. (2017) explored associations between phthalate metabolites and malondialdehyde (MDA) in a cohort of Chinese adults with type 2 diabetes, and associations were generally positive but smaller in magnitude for men compared to women. Kim et al. observed stronger associations between urinary phthalate metabolites and MDA in women compared to men from an elderly population (Kim et al., 2013). Finally, in a pre-conception cohort of US couples, positive associations between MEP and 8-hydroxydeoxyguanosine (8-OHdG) were observed among women but were null in their male partners, though the opposite was true for MiBP (Guo et al., 2014).
The reasons for differences between the exposure-response associations in men and women are not clear. One plausible explanation could be differential expression of peroxisome proliferator-activated receptors in men and women. Phthalates are known to bind to these receptors and it is potentially through this mechanism that they could induce oxidative stress (Rusyn et al., 2006). PPARs may have sexually dimorphic expression or activity (Vidal-Puig et al., 1997; Zhang et al., 2012), which could thus explain the differences in associations observed here. An alternative explanation, if associations are not causal but induced because of unmeasured confounding by urinary excretion, is that sex differences are attributable to differences in metabolism and excretion of phthalates or oxidative stress markers in men and women. This idea may be supported by evidence showing differences in urinary specific gravity by sex in a recent study (Kuiper et al., 2021). Furthermore, in this study population, correlations between specific gravity and biomarkers of exposure and outcome were higher among women compared to men (Supplemental Figure 3). For example, the correlation between uncorrected urinary 8-iso-PGF2a and specific gravity was 0.82 for women and 0.76 for men. A final explanation for differences by sex in our study is that sample size was almost double in women compared to men, although this would more likely influence precision rather than effect estimates.
Given this study’s unique design, we were able to examine phthalate metabolite concentrations in samples collected ~1 week prior to samples in which oxidative stress biomarkers were measured as a sensitivity analysis among women. In general, the longitudinal effect estimates were greatly attenuated compared to cross-sectional effect estimates in single-exposure, co-exposure, and joint models. Notably, however, the effect estimate for the joint effect of phthalates on the 8-iso-PGF2a metabolite remained positive and statistically significant in the longitudinal model, although the effect estimate was markedly lower (11%) compared to what we observed in cross-sectional models (114%). The attenuation of effects could be explained in two ways. It is possible that there are unmeasured factors relating to metabolism and excretion of phthalates and oxidative stress biomarkers that are confounding cross-sectional associations. Alternatively, because phthalate metabolites and oxidative stress biomarkers both have short half-lives (Frederiksen et al., 2007; Helmersson and Basu, 1999), associations between compounds measured in samples collected one week apart might be poorer estimates of the true exposure-response relationship. We cannot shed light on what the “ideal” study might be, but our results highlight the need to carefully consider potential connotations of measuring exposures and outcomes in the same sample in future work.
There is limited literature on the association between phthalate metabolites and oxidative stress biomarkers among couples undergoing fertility treatment. However, this association may be particularly important in this setting as we previously observed that the oxidative stress biomarkers measured in this study were associated with reduced IVF or IUI success in a non-linear manner (Rosen et al., 2019). Oxidative stress in other studies has also been associated with reproductive parameters in men and women, as well as success of fertility treatment (Du Plessis et al., 2008; Ruder et al., 2008; Tremellen, 2008). Thus, understanding environmental factors that impact oxidative stress in these populations is crucial. Of the existing studies, the biological matrix in which oxidative stress biomarkers are analyzed (e.g., urine, seminal fluid, follicular fluid) as well as the oxidative stress biomarkers themselves (8-iso-PGF2α, 8-OHdG, others) are quite varied; however, most studies have observed associations between one or more phthalate metabolites and oxidative stress (Al-Saleh et al., 2019; Liu et al., 2019; Wu et al., 2017; Yuan et al., 2020).
These and our results suggest a potentially important role of oxidative stress in the relationship between phthalate exposure and reproductive success among couples undergoing fertility treatment. A number of studies have utilized mediation analyses to further demonstrate a role of oxidative stress in the association between phthalate exposure and outcome of interest (Al-Saleh et al., 2019; Ferguson et al., 2017; Liu et al., 2019). However, such methods have not accounted for possible co-exposure confounding, the potential for interaction, or cumulative effects. Given our findings which illustrate co-exposure confounding but also strong joint effects, such methodologic advancements should be a focus of future work.
There were limitations to this analysis. The results of this study may not be generalizable to the preconception population, since couples undergoing fertility treatment tend to be an older, more educated, sub-fertile, and of higher socioeconomic status compared to other individuals who are trying to get pregnant. Additionally, we did not adjust our analyses for other chemical exposures which may be correlated with phthalate exposure and similarly create oxidative stress. However, in a previous study, we observed that when phthalate biomarker associations were adjusted for the correlated phenol biomarkers, the associations for phthalate metabolites remained significant (Ferguson et al., 2019a).
Despite the limitations delineated, this study has numerous strengths. First, it provides a large sample of men and women with repeated measures of exposure and outcome that is sufficiently powered to examine associations between phthalate exposures and oxidative stress. Second, we analyzed several biomarkers of oxidative stress using highly specific mass spectrometry. 8-iso-PGF2α is recognized as a highly accurate marker of oxidative stress that is responsive to environmental exposures (Kadiiska et al., 2005). Third, this study adds to the current body of literature by explicitly characterizing the degree of co-exposure confounding as well as joint associations. Finally, we were able to assess these associations in longitudinal models among women, demonstrating marked attenuation of effect estimates compared to cross-sectional models, but that even when exposure and outcome were measured one week apart there was a joint effect of the phthalate metabolite mixture on the 8-iso-PGF2α metabolite.
5. Conclusion:
Among sub-fertile couples, we found evidence of joint associations between phthalate metabolites and circulating oxidative stress biomarkers among women, and, to a lesser extent, men, as well as clear evidence of co-exposure confounding in the phthalate-oxidative stress relationship. These findings highlight the importance of examining joint effects as well as utilizing methods that account for co-exposure confounding in the study of phthalates. Furthermore, because oxidative stress is related to reproductive success among couples seeking fertility treatment, mitigating phthalate exposure should be considered as a potentially beneficial measure.
Supplementary Material
Funding:
This research was supported in part by the Intramural Research Program of the National Institute of Environmental Health Sciences (NIEHS ZIA ES103314) and NIEHS grants (R01ES022955, R01ES009718, R01ES00002).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Human Subjects Research: The Human Studies Institutional Review Boards at Harvard T.H. Chan School of Public Health and MGH approved EARTH study protocols and all participants submitted informed consent prior to study initiation. Additionally, the current analysis was deemed exempt by the NIEHS IRB.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Al-Saleh I, et al. , 2019. Exposure to phthalates in couples undergoing in vitro fertilization treatment and its association with oxidative stress and DNA damage. Environmental research. 169, 396–408. [DOI] [PubMed] [Google Scholar]
- ATSDR, Toxicological profile for diethyl phthalate. In: A. f. T. S. a. D. Registry, (Ed.). U.S. Department of Health and Human Services, Public Health Service, Atlanta, GA, 1995. [Google Scholar]
- ATSDR, Toxicological profile for Di-n-octylphthalate (DNOP). In: A. f. T. S. a. D. Registry, (Ed.). U.S. Department of Health and Human Services, Public Health Service, Atlanta, GA, 1997. [Google Scholar]
- ATSDR, Toxicological profile for Di-n-butyl Phthalate. In: A. f. T. S. a. D. Registry, (Ed.). U.S. Department of Health and Human Services, Public Health Service, Atlanta, GA, 2001. [Google Scholar]
- ATSDR, Toxicological profile for Di(2-ethylhexyl)phthalate (DEHP). In: A. f. T. S. a. D. Registry, (Ed.). U.S. Department of Health and Human Services, Public Health Service, Atlanta, GA, 2019. [Google Scholar]
- Barr DB, et al. , 2005. Urinary creatinine concentrations in the US population: implications for urinary biologic monitoring measurements. Environmental health perspectives. 113, 192–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobb JF, et al. , 2015. Bayesian kernel machine regression for estimating the health effects of multi-pollutant mixtures. Biostatistics. 16, 493–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks SP, Gelman A, 1998. General Methods for Monitoring Convergence of Iterative Simulations. Journal of Computational and Graphical Statistics. 7, 434–455. [Google Scholar]
- Buuren S. v., Groothuis-Oudshoorn K, 2010. mice: Multivariate imputation by chained equations in R. Journal of statistical software. 1–68. [Google Scholar]
- Caudill SP, et al. , 2008. Multi-rule quality control for the age-related eye disease study. Stat Med. 27, 4094–106. [DOI] [PubMed] [Google Scholar]
- Chang WH, et al. , 2019. Sex hormones and oxidative stress mediated phthalate-induced effects in prostatic enlargement. Environ Int. 126, 184–192. [DOI] [PubMed] [Google Scholar]
- Dong R, et al. , 2018. The role of oxidative stress in cardiometabolic risk related to phthalate exposure in elderly diabetic patients from Shanghai. Environ Int. 121, 340–348. [DOI] [PubMed] [Google Scholar]
- Du Plessis SS, et al. , 2008. Impact of oxidative stress on IVF. Expert review of obstetrics & gynecology. 3, 539–554. [Google Scholar]
- Duan Y, et al. , 2017. Exposure to phthalates in patients with diabetes and its association with oxidative stress, adiponectin, and inflammatory cytokines. Environ Int. 109, 53–63. [DOI] [PubMed] [Google Scholar]
- Ferguson KK, et al. , 2014. Urinary phthalate metabolite associations with biomarkers of inflammation and oxidative stress across pregnancy in Puerto Rico. Environ Sci Technol. 48, 7018–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson KK, et al. , 2017. Mediation of the relationship between maternal phthalate exposure and preterm birth by oxidative stress with repeated measurements across pregnancy. Environmental health perspectives. 125, 488–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson KK, et al. , 2019a. Urinary concentrations of phenols in association with biomarkers of oxidative stress in pregnancy: assessment of effects independent of phthalates. Environment international. 131, 104903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson KK, et al. , 2019b. Urinary concentrations of phenols in association with biomarkers of oxidative stress in pregnancy: Assessment of effects independent of phthalates. Environ Int. 131, 104903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson KK, et al. , 2011. Urinary phthalate metabolites in relation to biomarkers of inflammation and oxidative stress: NHANES 1999-2006. Environ Res. 111, 718–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson KK, et al. , 2012. Exploration of oxidative stress and inflammatory markers in relation to urinary phthalate metabolites: NHANES 1999-2006. Environ Sci Technol. 46, 477–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson KK, et al. , 2015. Urinary phthalate metabolites and biomarkers of oxidative stress in pregnant women: a repeated measures analysis. Environ Health Perspect. 123, 210–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frederiksen H, et al. , 2007. Metabolism of phthalates in humans. Mol Nutr Food Res. 51, 899–911. [DOI] [PubMed] [Google Scholar]
- Guo Y, et al. , 2014. Urinary concentrations of phthalates in couples planning pregnancy and its association with 8-hydroxy-2’-deoxyguanosine, a biomarker of oxidative stress: longitudinal investigation of fertility and the environment study. Environ Sci Technol. 48, 9804–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannon PR, Flaws JA, 2015. The effects of phthalates on the ovary. Front Endocrinol (Lausanne). 6, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmersson J, Basu S, 1999. F2-isoprostane excretion rate and diurnal variation in human urine. Prostaglandins Leukot Essent Fatty Acids. 61, 203–5. [DOI] [PubMed] [Google Scholar]
- Holland N, et al. , 2016. Urinary Phthalate Metabolites and Biomarkers of Oxidative Stress in a Mexican-American Cohort: Variability in Early and Late Pregnancy. Toxics. 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadiiska ΜB, et al. , 2005. Biomarkers of oxidative stress study II: are oxidation products of lipids, proteins, and DNA markers of CC14 poisoning? Free Radio Biol Med. 38, 698–710. [DOI] [PubMed] [Google Scholar]
- Kim JH, et al. , 2013. Diethylhexyl phthalates is associated with insulin resistance via oxidative stress in the elderly: a panel study. PloS one. 8, e71392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuiper JR, et al. , 2021. Urinary specific gravity measures in the US population: Implications for the adjustment of non-persistent chemical urinary biomarker data. Environment international. 156, 106656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, et al. , 2019. Mediation of the relationship between phthalate exposure and semen quality by oxidative stress among 1034 reproductive-aged Chinese men. Environ Res. 179, 108778. [DOI] [PubMed] [Google Scholar]
- Messerlian C, et al. , 2018. The Environment and Reproductive Health (EARTH) Study: A Prospective Preconception Cohort. Hum Reprod Open. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milne GL, et al. , 2007. Quantification of F 2-isoprostanes as a biomarker of oxidative stress. Nature protocols. 2, 221–226. [DOI] [PubMed] [Google Scholar]
- Mínguez-Alarcón L, et al. , 2019. Urinary concentrations of bisphenol A, parabens and phthalate metabolite mixtures in relation to reproductive success among women undergoing in vitro fertilization. Environ Int. 126, 355–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen EM, et al. , 2019. Urinary oxidative stress biomarker levels and reproductive outcomes among couples undergoing fertility treatments. Hum Reprod. 34, 2399–2409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin DB, 2004. Multiple imputation for nonresponse in surveys. John Wiley & Sons. [Google Scholar]
- Ruder EH, et al. , 2008. Oxidative stress and antioxidants: exposure and impact on female fertility. Human reproduction update. 14, 345–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusyn I, et al. , 2006. Modes of action and species-specific effects of di-(2-ethylhexyl) phthalate in the liver. Critical reviews in toxicology. 36, 459–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremellen K, 2008. Oxidative stress and male infertility—a clinical perspective. Human reproduction update. 14, 243–258. [DOI] [PubMed] [Google Scholar]
- van ’t Erve TJ, et al. , 2015. Reinterpreting the best biomarker of oxidative stress: The 8-iso-PGF(2ó)/PGF(2ó) ratio distinguishes chemical from enzymatic lipid peroxidation. Free Radio Biol Med. 83, 245–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van ’t Erve TJ, et al. , 2019. Phthalates and Phthalate Alternatives Have Diverse Associations with Oxidative Stress and Inflammation in Pregnant Women. Environ Sci Technol. 53, 3258–3267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van’t Erve TJ, et al. , 2016. Reinterpreting the best biomarker of oxidative stress: The 8-iso-prostaglandin F2α/prostaglandin F2α ratio shows complex origins of lipid peroxidation biomarkers in animal models. Free Radical Biology and Medicine. 95, 65–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal-Puig AJ, et al. , 1997. Peroxisome proliferator-activated receptor gene expression in human tissues. Effects of obesity, weight loss, and regulation by insulin and glucocorticoids. The Journal of clinical investigation. 99, 2416–2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H, et al. , 2017. Urinary phthalate and phthalate alternative metabolites and isoprostane among couples undergoing fertility treatment. Environ Res. 153, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan XQ, et al. , 2020. Phthalate metabolites and biomarkers of oxidative stress in the follicular fluid of women undergoing in vitro fertilization. Sci Total Environ. 738, 139834. [DOI] [PubMed] [Google Scholar]
- Zhang MA, et al. , 2012. Peroxisome proliferator-activated receptor (PPAR) α and-γ regulate IFNγ and IL-17A production by human T cells in a sex-specific way. Proceedings of the National Academy of Sciences. 109, 9505–9510. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


