Abstract
Background:
Gaps remain in understanding longitudinal patterns and predictors of perinatal depressive symptoms in sub-Saharan Africa. This study aimed to explore trajectories of depressive symptoms and associated factors from pregnancy through 9 months postpartum among Kenyan women.
Methods:
In this prospective cohort study, we analyzed data from the PrEP Implementation for Mothers in Antenatal Care study in which HIV-negative women were enrolled in pregnancy and followed through 9 months postpartum in 20 public sector maternal child health clinics in Western Kenya. Study nurses serially assessed depressive symptoms using the Center for Epidemiologic Studies Depression Scale (CESD-10), intimate partner violence (IPV) with the Hurt, Insult, Threaten, Scream scale, and social support with the Medical Outcomes Study scale. Generalized estimating equations were used to identify correlates of moderate-to-severe depressive symptoms (MSD) (CESD-10 score ≥10) and group-based trajectory modelling (GBTM) identified patterns.
Findings:
Among 3555 women, median age was 24.0 years (IQR 21.0–28.7), 1330 (38%) had low social support, and 278 (8%) reported IPV in pregnancy. All participants (3555, 100%) were female sex and all (3555, 100%) were African Kenyan ethnicity. Prevalence of MSD was higher in pregnancy than postpartum (870/3555, 24.5% vs. 597/3555, 16.8%, p<0.001). Five patterns of depressive symptoms were identified; persistent MSD in pregnancy and postpartum (295, 8%), MSD in pregnancy which resolved postpartum (139, 4%), MSD that emerged postpartum (40, 1%), chronically mild symptoms (2,709, 76%), and no depressive symptoms (372, 10%). Emergent MSD was associated with older age. Emergent, persistent, and resolving MSD were associated with pregnancy IPV; persistent MSD and resolving MSD with low social support and high HIV risk (p<0.05). MSD risk was 1.5- to 2.1-times higher with IPV, low social support, and partner HIV-positive status (p<0.05); 23% of perinatal MSD cases were attributable to low social support.
Interpretation:
One third of women had perinatal MSD; 13% (474) had higher severity phenotypes of resolving, persistent and emerging MSD that may require tailored interventions. Perinatal women with comorbid psychosocial stressors such as IPV and prior pregnancy loss should be prioritized for mental health services that augment social support within routine MCH care.
Funding:
National Institutes of Health
Keywords: Perinatal depression, Center for Epidemiologic Studies Depression Scale-10, sub-Saharan Africa, mental health, pregnancy, postpartum
MUHTASARI
Usuli wa utafiti:
Bado kuna mapengo katika kuelewa aina na vibashiri vya muda mrefu vya dalili za sonona kabla na baada ya kujifungua barani Afrika kusini mwa Sahara. Utafiti huu umelenga kuchunguza mielekeo ya dalili za sonona na sababu ambatanishi kutoka kipindi cha ujauzito hadi miezi 9 baada ya kujifungua miongoni mwa wanawake wa Kenya.
Njia:
Katika utafiti huu wa makundi yamuda mrefu, tulichambua data kutoka kwenye Utekelezaji wa utoaji dawa za kujikinga na maambukizi ya virusi vya ukimwi (PrEP) kwa akina Mama katika utafiti wa Utunzaji wa Ujauzito ambapo wanawake wajawazito wasio na maambukizi ya virusi vya ukimwi walifuatiliwa kwa kipindi cha hadi miezi 9 baada ya kujifungua katika kliniki 20 za sekta ya umma ya afya ya watoto Magharibi mwa Kenya. Wauguzi watafiti walitathmini kimfululizo dalili za sonona kwa kutumia Kipimo cha Sonona cha Kituo cha Tafiti za Kiepidemiolojia (CESD-10), ukatili wa mpenzi wa karibu (IPV) chenye kipimo cha Kuumiza, Kutusi, Kutishia, Kulia, na msaada wa kijamii na kipimo cha Utafiti wa Matokeo ya Kimatibabu. Milinganyo ya ujumla ya ukadiriaji ilitumika kutambua mifano ya dalili za wastani hadi kali za sonona (MSD) (Alama ya CESD-10 ≥10) na aina zilizotambulika za uundaji mwelekeo kulingana na kundi (GBTM).
Matokeo:
Kati ya wanawake 3555, umri wa wastani ulikuwa miaka 24 (IQR 21–28.7), 1330 (38%) walikuwa na msaada mdogo wa kijamii, na 278 (8%) ukatili wa mpenzi wa karibu yaani IPV katika ujauzito. Washiriki wote (3555, 100%) walikuwa jinsia ya kike na wote (3555, 100%) walikuwa Waafrika wenye asili ya Kenya. Uwepo wa dalili za wastani hadi kali za sonona yaani MSD ulikuwa mkubwa zaidi katika ujauzito kuliko baada ya kujifungua (870/3555, 24.5% dhidi ya 597/3555, 16.8%, p<0.001). Aina tano za dalili za sonona ziligundulika; dalili za wastani hadi kali za sonona yaani MSD zilizo endelevu katika ujauzito na baada ya kujifungua (295, 8%), dalili za wastani hadi kali za sonona yaani MSD zilizoisha baada ya kujifungua (139, 4%), dalili za wastani hadi kali za sonona yaani MSD zilizotokea baada ya kujifungua (40, 1%), dalili nyepesi za muda mrefu (2,709, 76%), na wasio na dalili za sonona (372, 10%). Dalili za wastani hadi kali za sonona yaani MSD zilizojitokeza zilihusishwa na umri mkubwa zaidi. Dalili za wastani hadi kali za sonona yaani MSD za kujitokeza, endelevu, na zinazoisha zilihusishwa na ukatili wa mwenzi wa karibu yaani IPV katika ujauzito; dalili za wastani hadi kali za sonona yaani MSD zilizo endelevu na zinazoisha zilihusishwa na msaada mdogo wa kijamii na hatari kubwa ya kupata virusi ya ukimwi (p<0.05). Hatari ya dalili za wastani hadi kali za sonona yaani MSD ilikuwa kubwa zaidi kwa mara 1.5 hadi 2.8 kwenye ukatili wa mpenzi wa karibu yaani IPV, msaada mdogo wa kijamii, na hali ya mpenzi kuwa na virusi vya ukimwi. (p<0.05); 23% ya kesi za dalili za wastani hadi kali za sonona yaani MSD wakati wa ujauzito hadi baada ya kujifungua zilichangiwa na msaada mdogo wa kijamii.
Tafsiri:
Theluthi moja ya wanawake walikuwa na dalili za wastani hadi kali za sonona yaani MSD; 13% (474) walikuwa na aina za dalili za wastani hadi kali za sonona yaani MSD za kuisha, endelevu, na zinazojitokeza zenye ukali zaidi ambazo zinaweza kuhitaji hatua stahiki. Wanawake walio kipindi cha ujauzito hadi baada ya kujifungua wenye visababishi vya kisaikolojia na kijamii vya msongo wa mawazo kama vile ukatili wa mwenzi wa karibu yaani IPV na upotezaji ujauzito hapo kabla wanapaswa kupewa kipaumbele kwenye huduma za afya ya akili ambazo zitaongezea msaada wa kijamii ndani ya matunzo ya kawaida ya Afya ya Mama na Mtoto yaani MCH.
Ufadhili:
Taasisi za Taifa za Afya
Introduction
Perinatal depression is the most common morbidity during pregnancy and postpartum, affecting about 10% of pregnant and breastfeeding women worldwide, with higher frequency in low- and middle-income countries (LMIC).1 Mental health issues are among the top causes of pregnancy-related death;2 a wide spectrum of adverse outcomes may follow perinatal depression, including maternal suicide, adverse perinatal outcomes, poor maternal-infant bonding, suboptimal infant growth, and child mental health problems.3
Existing studies on perinatal depression in sub-Saharan Africa (SSA) highlight high prevalence of postpartum depression (17%)4 — even higher during pregnancy (26.3%).5 Known cofactors of perinatal depression globally, and in SSA, include social and comorbid factors like intimate partner violence (IPV), social support, socioeconomic status, physical illness, history of mental health issues, prior adverse perinatal outcomes, and relationship factors.1,4,6–8 Experiencing IPV during pregnancy is associated with 3-times higher frequency of depression.4,9 Lacking support from partners, family, and friends is similarly related to reporting higher depressive symptoms.1
However, gaps remain in understanding perinatal depression in sub-Saharan Africa (SSA).10 Most studies to date are cross-sectional, and do not characterize changes in depressive symptoms throughout the perinatal period to inform appropriate timing and groups for intervention.11 The lack of longitudinal data limits understanding of the causal directionality of relationships with modifiable cofactors and makes it unclear whether prior or current exposures have the strongest effect on maternal mental health. Understanding the combination and timing of factors influencing experience of higher-severity perinatal depression patterns could inform appropriate intervention.
Identifying trajectories of depressive symptoms over time is increasingly used to understand patterns of perinatal depression and to define characteristics of individuals within distinct trajectories.12,13,14 Group-based trajectory modelling is a rigorous, data-driven approach that uses maximum likelihood estimation to identify distinct groups with similar prospective patterns of a serially measured outcome.15 To date, no longitudinal evaluations have assessed patterns of MSD among perinatal populations in Eastern African settings. Understanding patterns and determinants of perinatal depression, could contribute insights to understanding peripartum depression and inform efficient mental health service integration and delivery within maternal child health (MCH) settings in SSA.
We aimed to serially evaluate timing, patterns, and predictors of perinatal depressive symptoms, and assess social factors over time among perinatal women attending MCH services in Kenya.
Methods
Study design and participants
This longitudinal analysis was nested in the PrEP implementation for Mothers in Antenatal Care study (PrIMA)--a cluster randomized trial conducted in Western Kenya to compare two models for pre-exposure prophylaxis implementation among pregnant women (NCT03070600).16 Women attending antenatal care (ANC) within the 20 MCH clinics in Siaya and Homa Bay counties were screened and enrolled between January 2018 – June 2019. Pregnant women were eligible for enrollment if they were HIV-uninfected, ≥15 years old (the age of emancipation in pregnancy in Kenya), and were able to provide consent.
Procedures
Study nurses administered questionnaires in Kiswahili, Dholuo, or English languages using tablet-based REDCap surveys. We collected information about demographics, pregnancy history, partner characteristics, and psychosocial factors. To determine history of intimate partner violence (IPV), we used the 4-item Hurt, Insult, Threaten, Scream Scale with a cut-point of 10 or greater to define IPV (absolute score range: 4–20).17 We used the 18-item Medical Outcomes Study Social Support Survey (MOS-SSS; higher scores, higher social support, range: 18–90).18 We dichotomized this variable with a cut-point of 72 or greater for low social support to enhance interpretability. We defined household crowding – a marker of socioeconomic status19 – as the ratio of people per room in a residence greater than the median (>2 people/room). We evaluated HIV risk using a validated risk score developed to predict HIV acquisition among perinatal women in SSA, which includes predictors of having a male partner with unknown HIV status (risk score of 6), number of lifetime sexual partners (score of 1 per partner), and recent syphilis diagnosis (within past 6 months; score of 5).20 A HIV risk score of >6 is considered “high”, corresponding to 8.9 HIV infections per 100 person-years.20
Outcomes
Experience of depressive symptoms was collected serially during pregnancy (enrollment visit), 6 weeks postpartum, and 9 months postpartum using the 10-item Center for Epidemiologic Studies Depression Scale (CESD-10) where each item depicts a discrete depressive symptom. Participants rate items from 0–3 based on past-week frequency. Higher total scores denote higher severity of depressive symptoms (absolute score range: 0–30). A validated cut-point of 10 or greater denotes moderate-to-severe depressive symptoms (CESD-10 score ≥ 10; MSD).21
Choice of primary measure
We selected the CESD-10 to measure depressive symptoms among perinatal Kenyan women in the PrIMA study for multiple reasons. Its common application in large-scale epidemiologic studies globally improves the comparability of our findings across settings. As a 10-item scale with Likert scale response options, the burden of administration is low thus facilitating efficient screening in busy public sector MCH clinics. The CESD-10 was validated among a general population in South Africa, with acceptable-to-excellent internal consistency across three sub-populations (Cronbach’s alpha: 0.69–0.89), and excellent ability to detect major depressive disorder (MDD) defined by the Mini-International Neuropsychiatric Interview (MINI) in area under the Receiver Operating Characteristic curves (AUROC) in three sub-populations (AUROC: 0.81 [95% CI: 0.71–0.90], 0.93 [95% CI: 0.90–0.96], 0.94 [95% CI: 0.89–0.99]):22 The 20-item CESD was evaluated for validity and reliability in a Ugandan perinatal population, with high internal consistency (Cronbach’s alpha: 0.92) and good detection of MDD with the MINI (AUROC: 0.82).23 The Kenyan Ministry of Health does not currently maintain policy guidelines specifying a perinatal depression screening scale for use in MCH settings; we will align future studies with tools specified in Kenyan policies.
Statistical analysis
Participants were included in the present analysis if they had complete depressive symptom information at the enrollment visit during pregnancy and at least one postpartum visit. The prevalence of MSD was calculated at each study visit (pregnancy, 6 weeks, 9 months postpartum). Demographic characteristics were evaluated using descriptive statistics.
We identified correlates of MSD during the perinatal period (pregnancy – 9 months postpartum) using univariable and multivariable generalized estimating equation models (GEE) with Poisson family, log link, independent correlation structure, robust standard errors, clustered by participant. In the multivariable model, we included variables hypothesized à priori to be associated with perinatal depression according to our conceptual model (Appendix) or if they were associated with perinatal MSD (alpha ≤0.1) in univariable analysis. The confounding variables included in multivariable GEE models were: age (years), not married/living with a partner (yes/no), completed education (years), household crowding (yes/no), multiparous (yes/no), prior pregnancy loss (yes/no), lifetime sexual partners >2 (yes/no), partner living with HIV (yes/no), partner HIV status unknown (yes/no), transactional sex (yes/no), forced sex (yes/no), sexually transmitted infection (yes/no), ever drinks alcohol (time-varying [current visit]; yes/no), low social support (time-varying [current visit]; yes/no), IPV (time-varying [current visit]; yes/no), pregnancy vs. postpartum (yes/no), and facility.. We estimated population attributable risk percentages (PAR%) for each correlate of perinatal MSD.
We identified discrete trajectories of perinatal depressive symptoms using GBTM – a method that groups individuals with similar patterns of an outcome measured over time using maximum likelihood estimation,12 which has been used to identify trajectories of depression.13,14 As a data-driven approach, researchers do not form the distinct groups themselves, but instead use model fitting to identify latent clusters. Depressive symptoms were modelled using the continuous CESD-10 score (censored normal distribution) and time as days since enrollment. We fit the GBTM using the two-step process outlined by Nagin et al.12 We hypothesized depressive symptoms would follow two to five distinct and clinically meaningful trajectories.12 Thus, we fit a set of four models, each with a different number of trajectories (2–5) and used the Bayesian Information Criterion (BIC) values to identify the best-fitting model with the optimal number of trajectory groups (Appendix).12 During this step, we modeled all trajectory groups with cubic polynomial terms, such that differences in BIC values only described fit differences due to varying the number of trajectories. Next, we fixed the number of trajectories (identified in the prior step) and ran models that differed in polynomial form of each trajectory group (e.g., linear, quadratic, cubic). We ran a separate model for each potential combination of polynomial forms, identifying the model with the optimal combination of trajectory group functional forms using BIC values.12
We assessed adequacy of the final GBTM through methods described by Nagin et al.15 Group-based trajectory models calculate every individual’s likelihood of belonging to each trajectory group, summarized as the “posterior probabilities of group membership”. If the proportion of women belonging to each trajectory group was not meaningfully different (<5%) from the sum of individual posterior probabilities of group membership, we deemed the model was adequate. Further, we determined if the average posterior probability of membership in each group among the individuals assigned to that group was at least 70%.12
Nonrandom attrition in the study population over time is accommodated by a GBTM-extension method, which allows attrition to differ by trajectory group, thus relaxing the assumption of missingness at random.12,25 Among participants with a CESD-10 score in pregnancy and at least 1 postpartum score, 78% (2783/3555) had a CESD-10 score at the 6 week visit, 90% had a CESD-10 score at the 9 month postpartum visit (3205/3555), and 69% (2438/3555) had scores for all three visits.
To illuminate each group’s member profile, we assessed descriptive statistics for characteristics we identified as correlates of perinatal MSD. We evaluated these factors as baseline predictors of depressive symptom group membership by fitting a multivariable multinomial logistic regression model. Analyses were conducted using Stata 15.
Ethical considerations
The study protocol, informed consent forms, and data collection tools were approved by the Kenyatta National Hospital-University of Nairobi Ethics Research Committee and University of Washington Human Subjects Review Committee. All participants provided written informed consent.
Role of funding sources
Funding agencies had no role in writing this manuscript or submitting for publication.
Results
Among PrIMA study participants, 97% (4299/4447) attended at least 1 postpartum visit when depressive symptom information was collected, of which 91% (3882/4257) had complete depressive symptom data in pregnancy; 92% of those (3555/3882) also had depressive symptom data in postpartum and were included in this analysis. All participants (n=3555, 100%) were female sex and all (n=3555, 100%) were African Kenyan ethnicity. Participants contributed a median of 379 person-days of follow-up time (Interquartile range [IQR]: 334–430, total 1,330,257 person-days). The median age was 24.0 (interquartile range [IQR]: 21.0–28.7), the majority (3021, 86%) were married, and most had previously experienced pregnancy (2693, 76%) (Table 1). Women had a median education of 10 years (IQR: 8–12); only 14% (491) of women were formally employed. About half (1725, 49%) of women experienced household crowding (>2 people/room). Over 1 in 3 women (1330, 38%) experienced low social support during pregnancy (median social support score:75 [IQR: 62–88]). About 35% (1257) of women were deemed high risk for HIV acquisition. Intimate partner violence was reported by 8% (278) of women during pregnancy. We did not detect differences in social support, IPV, HIV risk, or frequency of MSD during pregnancy between those included in the analysis because they had depressive symptom data in pregnancy and postpartum versus those not included (pregnancy scores only) (Appendix).
Table 1.
Baseline characteristics of PrIMA study participants included in depression analyses (n=3555)
| Demographic characteristics | n | n or median | % or IQR |
|---|---|---|---|
| Age (years) | 3553 | 24.0 | 21.0–28.7 |
| Older adults (>24 years) | 3553 | 1552 | 43.7 |
| Gestational age (enrollment) | 3555 | 24.0 | 20.0–29.4 |
| Not married or not living with a partner | 3522 | 501 | 14.2 |
| Completed education (years) | 3488 | 10.0 | 8.0–12.0 |
| Regularly employed | 3515 | 491 | 14.0 |
| Household crowding (≥2 people/room) | 3529 | 1725 | 48.9 |
| Pregnancy history | |||
| Multigravida | 3551 | 2693 | 75.8 |
| Prior pregnancy loss | 3544 | 447 | 12.6 |
| Prior preterm birth | 3555 | 29 | 0.8% |
| Partnership and sexual behavior characteristics | |||
| Lifetime sexual partners | 3549 | 2.0 | 2.0–3.0 |
| Lifetime sexual partners (>2) | 3549 | 2957 | 83.3 |
| Partner age difference >10 years (among those with a partner) | 2734 | 428 | 15.7 |
| Partner living with HIV | 3507 | 144 | 4.1 |
| Partner HIV status unknown | 3507 | 1054 | 30.1 |
| High HIV risk1 | 3555 | 1257 | 35.4 |
| Transactional sex ever (last 6 mo.) | 3549 | 57 | 1.6 |
| Forced to have sex against her will (last 6 mo.) | 3550 | 199 | 5.6 |
| Sexually transmitted infection (enrollment) | 3550 | 91 | 2.6 |
| Psychosocial characteristics | |||
| Ever drink alcohol | 3539 | 141 | 4–0 |
| Social support score2 | 3489 | 75.0 | 62.0–88.0 |
| Low social support2 (MOS-SSS score <72) | 3489 | 1330 | 38.1 |
| Intimate partner violence3 (HITS score ≥10) | 3550 | 278 | 7–8 |
We evaluated HIV risk using the Pintye et al.17 risk score (high HIV risk: score >6 = “Yes”, score ≤6 = “No”).
We evaluated social support using the 18-item Medical Outcomes Study social support score (MOS-SSS), defining low social support as scores below 72 (Low social support: MOS-SSS score score <72 = “Yes”, MOS-SSS score ≥ 72 = “No”).
We evaluated intimate partner violence using the 4-item Hurt, Insult, Threaten, and Scream scale (HITS), defining intimate partner violence as scores of 10 and above (IPV: HITS score ≥10 = “Yes”, HITS score <10 = “No”).
One in four women (24.5%, 870/3555, 95% CI:23.1–25.9) had MSD at enrollment during pregnancy (median gestational age: 25 weeks, IQR: 20–30) (Appendix). Prevalence of MSD was lower during postpartum (16.8%, 597/3555; 95% CI:15.6–18.1; p-value<0.001 from McNemar’s test of pregnancy vs. postpartum), and declined from 6 weeks (12.2%, 340/2783, 95% CI: 11.0–13.5) to 9 months postpartum (10.5%; 336/3210, 95% CI: 9.4–11.6; p-value: 0.033 from McNemar’s test for 6 weeks vs. 9 months postpartum). Overall, 8.7% (308/3555, 95% CI: 7.8–9.6) of women had MSD only in postpartum and 33.1% (1178/3555, 95% CI: 31.6–34.7) of women experienced MSD at some point during the perinatal period. Frequency of MSD during pregnancy varied significantly by facility (range: 6/150, 4.0% - 119/179, 66.0%, p<0.001) (Appendix).
Women who were not married or living with a partner had over 50% higher frequency of MSD compared to married women (adjusted Relative Risk [aRR]: 1.6, 95% CI: 1.3–1.8) (Table 2, Figure 1). Frequency of MSD was twice as high among those reporting IPV (aRR: 2.1, 95% CI: 1.8–2.4). Having low social support was associated with nearly twice the frequency of perinatal MSD (aRR: 1.7, 95% CI: 1.6–2.0). Associations for both IPV and social support with MSD were stronger with current than prior experiences. Women with a partner living with HIV (aRR: 1.5, 95% CI: 1.2–1.8), a partner with unknown HIV status (aRR: 1.2, 95% CI: 1.1–1.4), and those with over 2 lifetime sexual partners (aRR: 1.2, 95% CI: 1.1–1.4) had about 20% higher frequency of MSD compared to those without these factors. Women who were forced to have sex against their will had 50% higher frequency of MSD compared to those without this experience (aRR: 1.5, 95% CI: 1.2–1.7). Recent sexually transmitted infection (STI) within 6 months of enrollment was associated with 30% higher MSD prevalence than those not reporting recent STI (aRR: 1.3, 95% CI: 1.0–1.7). Frequency of MSD during pregnancy was higher than postpartum MSD (aRR: 2.1, 95% CI: 1.9–2.3).
Table 2.
Longitudinal correlates of perinatal moderate-to-severe depressive symptoms from pregnancy through 9 months postpartum (n=3555)
| Univariable Analysis Moderate-to-severe depressive symptoms (MSD)d vs. No MSD | Multivariable Analysis MSD vs. No MSD1 | Population attributable risk % (PAR%)2 | ||||||
|---|---|---|---|---|---|---|---|---|
| RR | 95% CI | p-value | aRR | 95% CI | p-value | PAR% | 95% CI | |
| Baseline factors | ||||||||
| Demographic characteristics | ||||||||
| Age (years) | 1.01 | 1.00–1.02 | 0.046 | 1.00 | 0.99–1.01 | 0.950 | ||
| Older age (>24 years) | 1.07 | 0.97–1.19 | 0.179 | |||||
| Not married or not living with a partner | 1.14 | 0.99–1.31 | 0.068 | 1.55 | 1.31–1.82 | <0.001 | 5.58 | 3.34–7.78 |
| Completed education (years) | 1.00 | 0.98–1.01 | 0.612 | 1.00 | 0.98–1.01 | 0.808 | ||
| Regularly employed | 0.92 | 0.78–1.07 | 0.270 | |||||
| Household crowding (>2 people/room) | 1.17 | 1.05–1.30 | 0.003 | 1.15 | 1.04–1.27 | 0.008 | 6.79 | 1.65–11.67 |
| Pregnancy characteristics | ||||||||
| Multigravida | 1.20 | 1.05–1.36 | 0.006 | 1.08 | 0.92–1.26 | 0.346 | ||
| Prior pregnancy loss | 1.25 | 1.08–1.44 | 0.003 | 1.06 | 0.93–1.22 | 0.356 | ||
| Prior preterm birth | 0.99 | 0.52–1.95 | 0.979 | |||||
| Partnership and sexual behavior characteristics | ||||||||
| Lifetime sexual partners > 2 | 1.36 | 1.15–1.60 | <0.001 | 1.19 | 1.01–1.40 | 0.034 | 14.12 | 1.03–25.48 |
| Partner age difference >10 years | 1.04 | 0.89–1.23 | 0.605 | |||||
| Partner living with HIV | 1.20 | 0.97–1.50 | 0.096 | 1.48 | 1.22–1.78 | <0.001 | 1.68 | 0.75–2.60 |
| Partner HIV status unknown | 1.47 | 1.33–1.64 | <0.001 | 1.23 | 1.09–1.38 | 0.001 | 6.80 | 2.86–10.58 |
| High HIV riska | 1.48 | 1.34–1.64 | <0.001 | |||||
| Transactional sex (last 6 mo.) | 1.53 | 1.11–2.13 | 0.010 | 1.01 | 0.70–1.45 | 0.953 | ||
| Forced to have sex (last 6 mo) | 1.53 | 1.28–1.84 | <0.001 | 1.47 | 1.24–1.74 | <0.001 | 2.65 | 1.32–3.96 |
| Sexually transmitted infection (last 6 mo) | 1.72 | 1.37–2.15 | <0.001 | 1.32 | 1.03–1.70 | 0.028 | 1.18 | 0.01–2.13 |
| Psychosocial factors (baseline) | ||||||||
| Ever drinks alcohol | 1.46 | 1.17–1.82 | 0.001 | |||||
| Low social supportb | 1.82 | 1.64–2.01 | <0.001 | |||||
| Intimate partner violencec | 2.00 | 1.76–2.28 | <0.001 | |||||
|
Psychosocial factors (time-varying; currently) |
||||||||
| Ever drinks alcohol | 1.63 | 1.29–2.04 | <0.001 | 1.06 | 0.84–1.35 | 0.621 | ||
| Low social supportb | 2.40 | 2.18–2.64 | <0.001 | 1.74 | 1.56–1.95 | <0.001 | 23.34 | 18.77–27.65 |
| Intimate partner violencec | 2.83 | 2.52–3.18 | <0.001 | 2.07 | 1.81–2.36 | <0.001 | 7.57 | 5.83–9.27 |
| Pregnancy vs. postpartum | 2.17 | 2.00–2.36 | <0.001 | 2.07 | 1.85–2.31 | <0.001 | ||
Adjusted relative risks are additionally adjusted for the variables depicted here, as well as facility, since moderate-to-severe depressive symptoms varied significantly by facility (Appendix).
Population attributable risk % were calculated for all factors significant at p-value<0.05 in multivariable analysis, except for pregnancy status since the number of study visits taking place during pregnancy versus postpartum influenced the PAR%.
We evaluated HIV risk using the Pintye et al.17 risk score (high HIV risk: score >6 = “Yes”, score ≤6 = “No”).
We evaluated social support using the 18-item Medical Outcomes Study social support score (MOS-SSS), defining low social support as scores below 72 (Low social support: MOS-SSS score <72 = “Yes”, MOS-SSS score ≥ 72 = “No”).
We evaluated intimate partner violence using the 4-item Hurt, Insult, Threaten, and Scream scale (HITS), defining intimate partner violence as scores of 10 and above (IPV: HITS score ≥10 = “Yes”, HITS score <10 = “No”).
We evaluated moderate-to-severe depressive symptoms using the 10-item Center for Epidemiologic Studies Depression Scale (CESD-10), defining MSD as scores of 10 and above (MSD: CESD-10 score ≥10 = “Yes”, CESD-10 score <10 = “No”).
Figure 1.
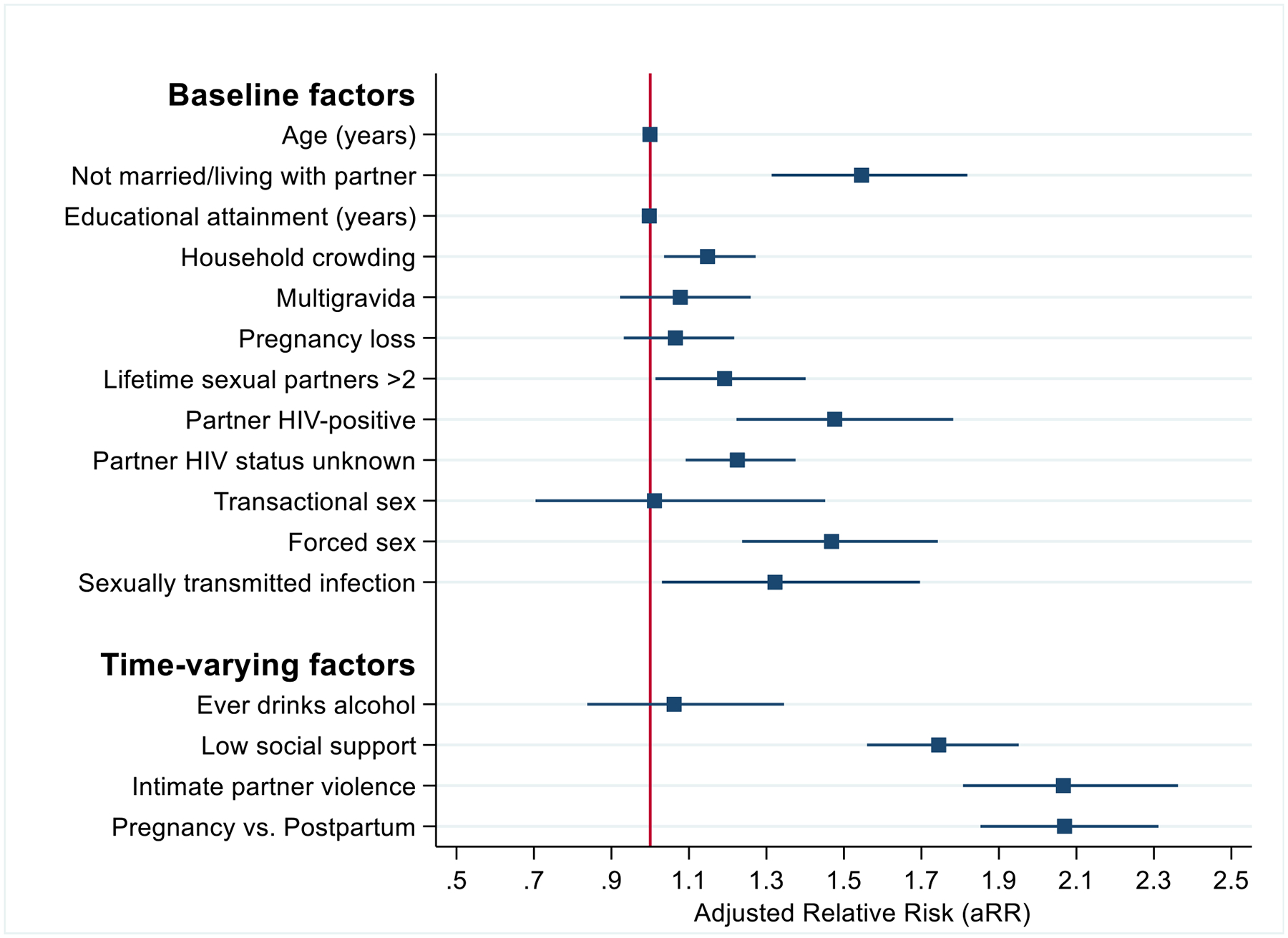
Correlates of ever perinatal MSD and population attributable risk percentages
Age, household crowding, prior pregnancy loss, alcohol use, and transactional sex were not associated with MSD after adjusting for confounders in multivariable analyses (Table 2, Figure 1). In sensitivity analyses, we evaluated correlates of MSD during pregnancy separately from postpartum. Low social support, IPV, and lifetime number of sexual partners were associated with MSD in both periods with other cofactors associated with only pregnancy or only postpartum MSD (Appendix).
Based on population attributable risk proportions, over one in five (23.3%, 95% CI: 18.8–27.7) (Table 2) perinatal MSD cases were attributable to low social support. The next highest proportion of perinatal MSD cases was attributable to having more than 2 lifetime sexual partners (14.1%, 95% CI: 1.0–25.5). IPV accounted for 8% of perinatal MSD cases (7.6%, 95% CI: 5.8–9.3), while experiencing household crowding accounted for 7% of perinatal MSD cases (6.8%, 95% CI: 1.7–11.7), and being unmarried accounted for about 6% of MSD cases (5.6%, 95% CI: 3.3–7.8). Nearly 7% of the perinatal MSD cases were attributable to having a partner with unknown HIV status (6.8%, 95% CI: 2.9–10.6). About 2% of perinatal MSD cases were attributable to having a partner living with HIV (1.7%, 95% CI: 0.8–2.6), and 1% were attributable to having a recent STI (1.1%, 95% CI: 0.01–2.1).
Five discrete depressive symptom groups were illuminated by group-based trajectory modelling (Figure 2). Women with MSD persisting from pregnancy into postpartum clustered within one depressive symptom group (“Persistent MSD”, 295/3555, 8.3%) with a cubic polynomial form. In another group, women had MSD that emerged in postpartum following mild depressive symptoms during pregnancy (“Emergent MSD”, 40/3555, 1.1%, cubic). About 4% of women had MSD in pregnancy which resolved to mild depressive symptoms postpartum (“Resolving MSD”, 139/3555, 3.9%, quadratic). The majority of women had chronically mild depressive symptoms throughout the perinatal period (“Chronic mild”, 2709/3555, 76.2%, quartic). The model fitting process using maximum likelihood estimation grouped women into this category for their similarity in average “mild” depressive symptom scores over time. Some of the women included in this group experienced one episode of MSD with a CESD-10 score just above 10 during the perinatal period (Appendix). About one in ten women never had depressive symptoms from pregnancy through 9 months postpartum (“No depressive symptoms”, 372/3555, 10.5%, linear).
Figure 2.
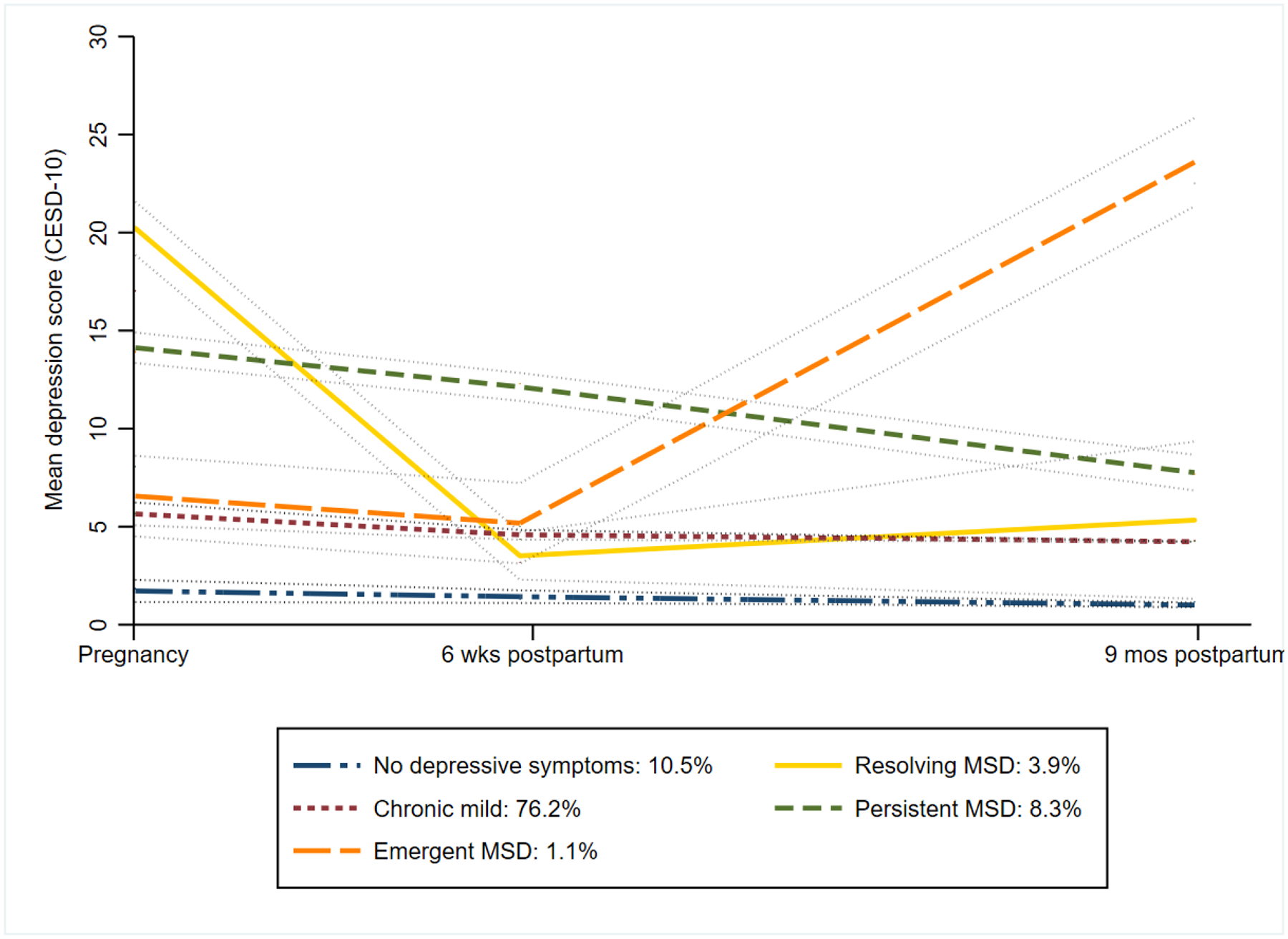
Trajectories of depressive symptoms from group-based trajectory model (n=3555)
Group-based trajectory model was fit using Center for Epidemiologic Studies Depression Scale (CESD-10) as a continuous score (range 0–30), modelled as censored normal distribution, over time since enrollment (days).
This was the best fitting model (BIC = −26648.8, AIC = −26525.5, Log Likelihood =−26485.5) and model adequacy was supported (Appendix). The average posterior probability of being allocated to a depressive symptom group among women assigned to that group was >70% for all groups (absolute range: 71.9%–90.8%). The proportion of women in each depressive symptom group was well-aligned (≤5%) with the proportion expected in each group based on the sum of posterior probabilities.
We further evaluated the time-varying influence of IPV and low social support on depressive symptom patterns by including them as predictors in the GBTM model. The adjusted patterns were uniform in shape over time, stratified by severity level, implying the contributions of these factors to the dynamic shapes differentiating depressive symptom groups (Figure 3).
Figure 3.
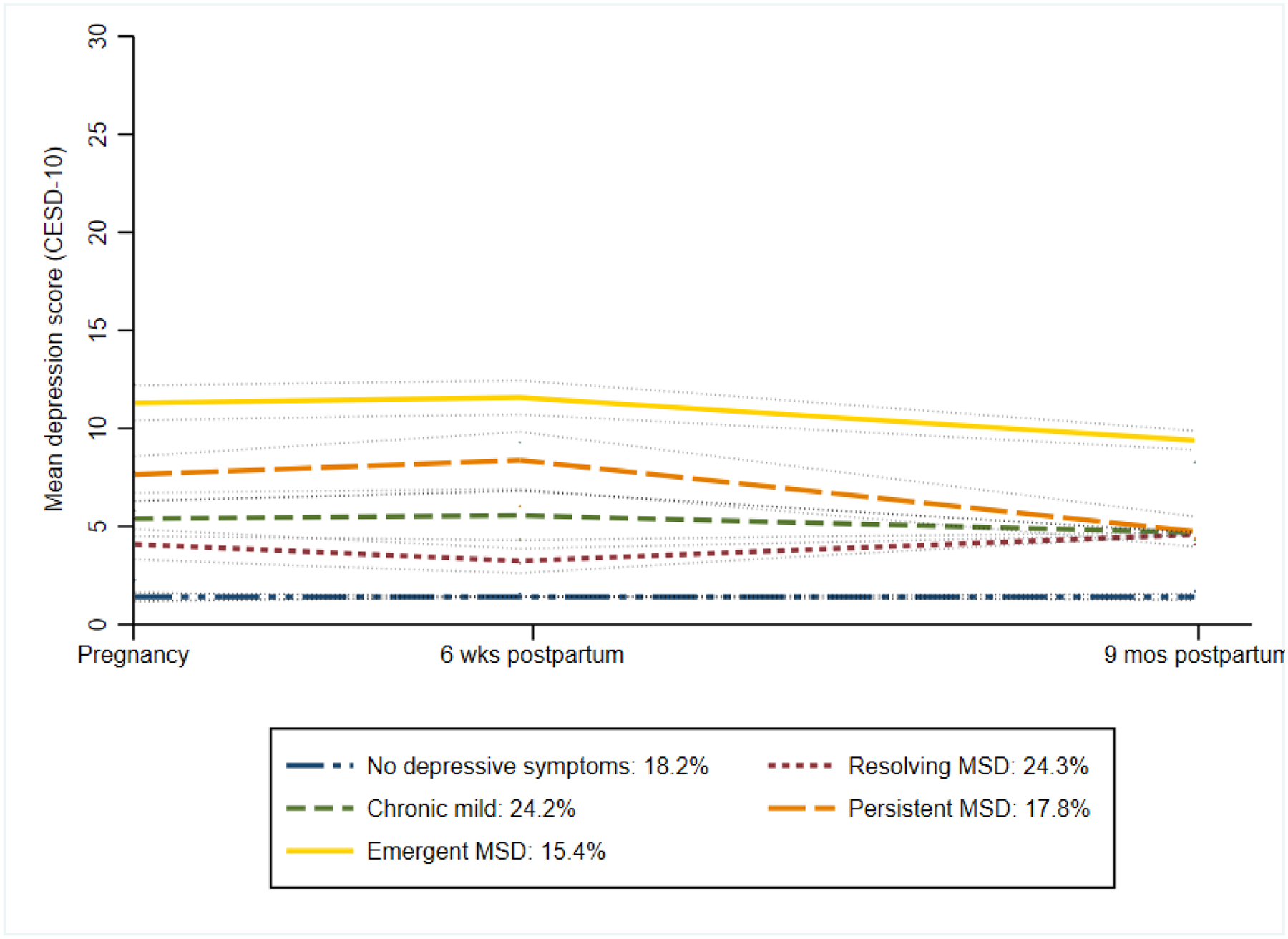
Trajectories of moderate-to-severe depressive symptoms from group-based trajectory model, adjusted for time-varying intimate partner violence and low social support (n=3555)
Group-based trajectory model was fit using Center for Epidemiologic Studies Depression Scale (CESD-10) as a continuous score (range 0–30), modelled as censored normal distribution, over time since enrollment (days), and time-varying covariates (Intimate Partner Violence, Low social support)
Generally, members of the “Persistent MSD” or “Resolving MSD” group had the highest effect sizes for predictors followed by the “Emergent MSD” group, when compared to the “no depressive symptoms” group (Figure 4, Appendix). Membership in the “Persistent MSD” and “Resolving MSD” groups was associated with IPV, low social support, and high HIV risk (effect sizes range: 1.7–6.2). Prior pregnancy loss was also associated with being in the “Persistent MSD” group (adjusted Odds Ratio [aOR]: 1.9, 95% CI: 1.2–3.1). The strongest predictor of “Resolving MSD” was IPV experienced during pregnancy (aOR: 6.2, 95% CI: 31–12.3), whereas the strongest predictor of “Persistent MSD” was low social support (aOR: 5.3, 95% CI: 3.8–7.5). Older age (>24 years old) was associated with membership in the Emergent MSD group (aOR: 2.2, 95% CI: 1.1–4.4), as was IPV (aOR: 4.0, 95% CI: 1.4–11.4).
Figure 4.
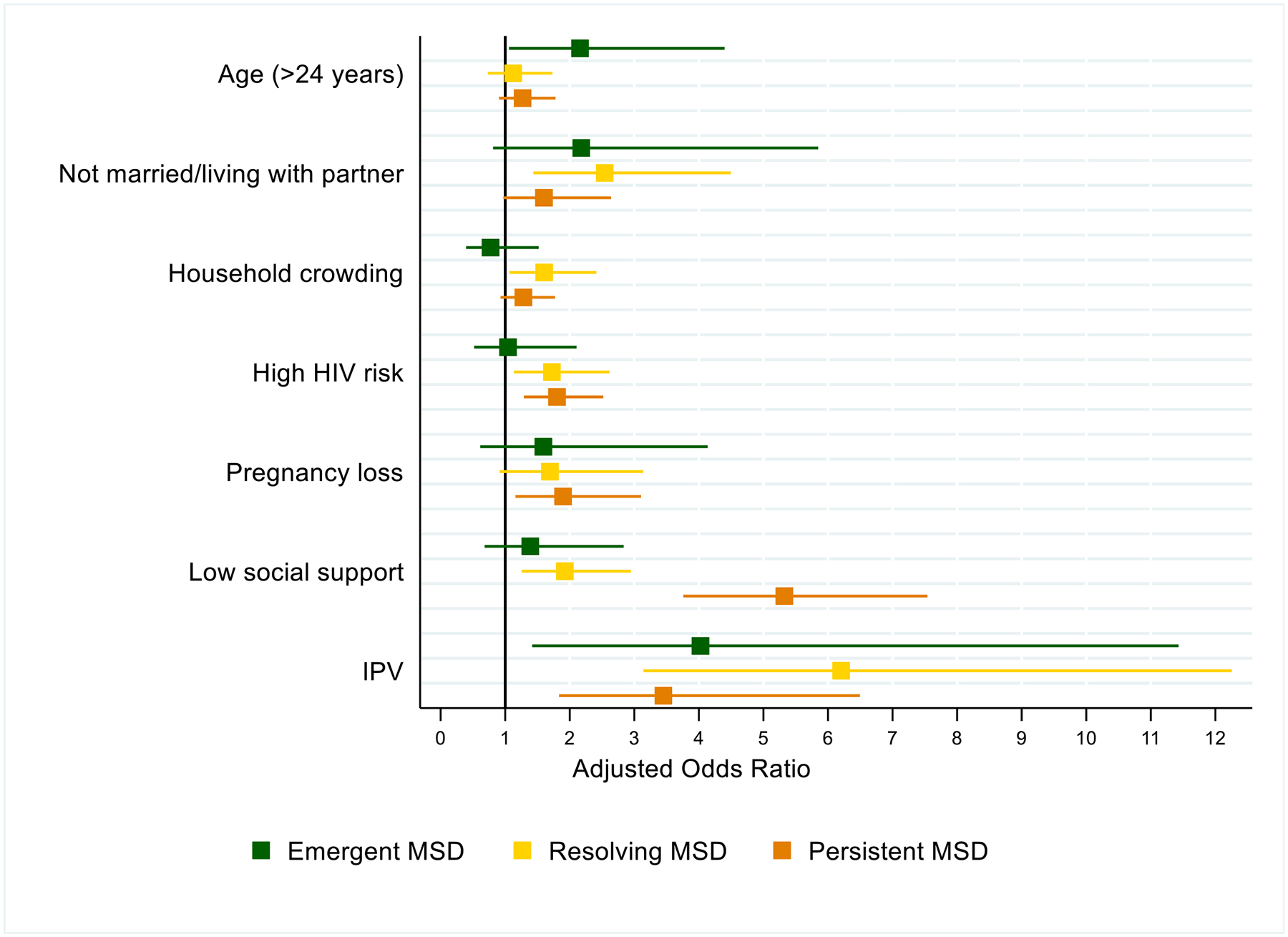
Predictors of trajectory membership: results from multivariable multinomial logistic regression with GBTM groups
Within the “Persistent MSD” group, the majority (65%, 190/295) of women reported low social support during pregnancy which remained through 9 months postpartum—the highest across all groups (Appendix). Over half experienced high HIV risk (48%, 141/295) and household crowding (53%, 156/295). One in six of “Persistent” group members reported IPV during pregnancy (17%, 51/295)—the second highest proportion across groups, and IPV prevalence decreased over time. Median CESD-10 scores were relatively consistent across visits.
Members of the “Emergent MSD” depressive symptom group had a high frequency of reported IPV (15%, 6/40) during pregnancy, which increased slightly by the 9-month postpartum visit (18%, 7/40). About 15% (6/40) of “Emergent MSD” members had a prior pregnancy loss, which was the second highest frequency across all groups. Median CESD-10 scores were severe at the 9-month visit (22, IQR: 20–27).
The “Resolving MSD” group had the largest proportion of women with high HIV risk (49%, 68/139) and the largest proportion of unmarried women (27, 20%). Over 1 in 5 women reported IPV during pregnancy (25%, 34/139), which was also the highest frequency across groups. Prevalence of IPV was lower at 9 months postpartum (4%, 5/139). This group reported low social support consistently across visits. Median CESD-10 scores were severe during pregnancy (19, IQR: 17–22), mild thereafter. The “Chronic mild” and “No depressive symptoms” groups had similar characteristics.
Discussion
In this large prospective analysis among perinatal Kenyan women followed from pregnancy through 9 months postpartum in public sector MCH care, we found high prevalence (33%) of MSD at least once during the perinatal period, with higher frequency in pregnancy than postpartum. To our knowledge, this is the largest study to evaluate perinatal depressive symptom trajectories among women in a LMIC setting-- the first in Eastern Africa, to our knowledge.
Five distinct trajectories described phenotypes of depressive symptom experiences over time. About 13% of women had a higher severity depressive symptom pattern with MSD that either persisted throughout the perinatal period, resolved after pregnancy, or emerged postpartum. Most women had mild depressive symptoms throughout the perinatal period; some with episodic symptom scores just above the threshold for MSD. IPV, low social support, or being at high risk for HIV were cofactors of perinatal MSD and predicted membership in higher severity depressive symptom groups.
Our estimate of MSD during pregnancy (25%) aligned with the pooled prevalence from a recent meta-analysis among African women (26%),5 as did our estimate of postpartum depression prevalence (17%).4 Associations of perinatal MSD with IPV, low social support, and being unmarried are consistent with results from SSA4 and other LMICs.1 Few studies have assessed associations between HIV risk factors and depression among pregnant and breastfeeding women. Our findings echo associations between HIV risk behaviors and mental health outcomes among non-pregnant women at high HIV risk.26 Women with HIV risk factors in our study had higher frequency of perinatal depression, highlighting potentially synergistic benefits of integrating HIV prevention and mental health services within MCH settings.
To understand the impact of each cofactor on perinatal depression, we estimated population attributable risk proportions, which considers effect size and prevalence of the factor. Low social support accounted for >20% of perinatal depression cases, because low social support was common (50%) and doubled one’s risk for depression. About 7% of perinatal depression cases could be potentially avoided if all women were aware of their partners’ HIV status; another 7% if IPV was prevented. Our findings suggest that integrated efforts to enhance social support, increase partner HIV testing and eliminate IPV could decrease peripartum depression by 34%.27 Population attributable risk proportion should be considered in conjunction with feasibility. modifiability, and cost-effectiveness when selecting interventions to impact perinatal depression in resource-limited settings.
In GBTM analysis, 8% of women had persistent depression, similar to estimates from prior perinatal depressive GBTM analyses in South Africa (3.1%–8.6%).28–31 Women in this group had comorbid stressors of high HIV risk, prior pregnancy loss, and IPV, as well as the highest proportion of low social support through 9 months postpartum. Low social support in the context of multiple coexisting life stressors may perpetuate depressive symptoms in this group. About 4% of perinatal women had severe depressive scores during pregnancy with symptoms decreasing to mild or low levels after delivery, similar to a prior study (1.3%).31 This group had the highest frequency of IPV, high HIV risk, household crowding and the greatest proportion of unmarried women. Depressive symptoms for some women in this group may have resolved because HIV risk did not result in HIV acquisition or when IPV resolved postpartum. A small group (1.1%) had mild or low depressive symptoms during pregnancy that increased to MSD postpartum, similar to prior studies (2.2%–10.1%).28,30,31 Defining characteristics of this group included older age and high frequency of prior pregnancy loss. Unlike other groups, women in this group reported IPV more frequently 9 months postpartum, perhaps precipitating postpartum depressive symptoms. Median depressive symptom scores in this group reached the highest severity of any group. Our GBTM findings suggest that perinatal women with comorbid stressors should be prioritized for mental health services, particularly those reporting IPV, since IPV had the strongest influence on dynamic changes in depressive symptoms. Interventions that increase social support may interrupt persistent perinatal depression. Older women, especially those with prior pregnancy loss, should be monitored for emergent postpartum depression.
We identified a large group (76%) with chronic mild depressive symptoms throughout the perinatal period, as did similar studies in SSA (71.1%−91.5%).28,30,31 While median CESD-10 scores in this group were mild over time, some members had episodes of MSD with scores just above the referral threshold (referral score ≥10). They represented the remaining 20% of women ever reporting MSD besides the 13% with higher severity patterns. Based on these results, over half of perinatal MSD cases identified by CESD-10 screening may occur among women with a less concerning pattern of depressive symptoms. The higher severity groups identified in our study may offer a more accurate estimate of perinatal depression.32 The chronic mild group may be sufficiently supported by lower-intensity psychosocial services, whereas women with higher severity symptoms could be prioritized for mental health services with higher provider time and resource allocation. Cognitive behavioral therapy, interpersonal therapy, and problem-solving counseling have been shown to be effectively delivered by lay counselors in low-resource settings33 and could be integrated into MCH care in SSA, where high attendance to antenatal care and postnatal care is high (>90).
Our data were collected serially in a large, multisite randomized cluster trial. This enabled a trajectory analysis to better understand changes in maternal mental health over time using GBTM--a rigorous, data-driven method. We estimated PAR% to expand existing evidence about cofactors of perinatal depression on population-level impact. The PrIMA study did not enroll women living with HIV, which could limit generalizability of our findings in settings with high HIV prevalence. Our study did not include clinical diagnosis of depression by a clinician, thus we may have misclassified this outcome. We used a validated screening scale to classify MSD, which has been used in multiple studies in sub-Saharan Africa.22,23 We used GBTM extension methods to account for potential non-random attrition in this pragmatic trial.25 We acknowledge that we were unable to account for the contributions of unmeasured factors on changes in depressive symptoms over time, such as changing perceptions or attitudes about pregnancy outcomes, comorbid physical health conditions, and other mental health conditions (e.g., stress, anxiety).
A third of perinatal Kenyan women attending public sector MCH services had moderate-to-severe depressive symptoms at some point during the perinatal period, with higher prevalence in pregnancy compared to postpartum. A subset of women had severe MSD that persisted from pregnancy to postpartum, resolved during the postpartum period, or emerged postpartum. These groups could particularly benefit from mental health intervention. Women with mild depressive symptoms close to the screening threshold could be offered lower-intensity psychosocial support. Experience of IPV, lower social support, being unmarried, and having a partner known to be living with HIV or of unknown HIV status predicted perinatal MSD. Low social support accounted for the highest proportion of depression cases and was associated with higher severity patterns. Interventions addressing depressive symptoms which enhance social support and increase partner HIV testing should be integrated into MCH services and prioritized for those reporting IPV and prior pregnancy loss.
Supplementary Material
Research in context.
Evidence before this study
Trajectories of perinatal depressive symptoms are not well-understood globally, particularly in low- and middle-income countries (LMICs) of sub-Saharan Africa (SSA). Statistical methods to evaluate developmental trajectories over time, including latent growth curve models, growth curve mixture models, and group-based trajectory models (GBTM), have been increasingly applied to perinatal depressive symptoms over the last two decades, yet few such analyses have been conducted in LMIC populations. We searched PubMed for articles published in English published any time before February 13, 2022 using the following keywords: “depressive symptoms” OR “depression” AND “trajectories” OR “trajectory” OR “patterns” AND “pregnancy” OR “postpartum” OR “perinatal” AND all sub-Saharan African countries as defined by the World Health Organization. Our search identified only 6 analyses characterizing pregnancy and postpartum depressive symptom trajectories in LMICs in Africa (Garman et al. 2019, Garman et al. 2019, Pellowski et al. 2019, Rotheram-Fuller et al. 2018, Barthel et al. 2017, and Barthel et al. 2016), 4 in South Africa and 2 in West Africa (Ghana, Cote d’Ivoire). These studies each identified at least two distinct trajectories of depressive symptoms among African women. Studies to date have not evaluated time-varying correlates of trajectory group membership. Perinatal depressive symptom trajectories have not been evaluated in East Africa, where prevalence of perinatal depression is high, maternal child health (MCH) care is well-attended, and mental health screening and treatment could be integrated in MCH for high impact.
Added value of this study
To our knowledge, we performed the first trajectory analysis of perinatal depressive symptoms that included evaluation of time-varying correlates in a LMIC setting. It is our understanding that this is the first evaluation of perinatal depressive symptom trajectories in East Africa – a setting with increasing momentum for integrating mental health services in MCH. We found five distinct trajectories of perinatal depressive symptoms among Kenyan women; a third of perinatal women had moderate-to-severe depressive symptoms at least once during pregnancy and postpartum. Three groups had higher severity phenotypes of resolving, persisting, and emerging depression that may have distinct etiologies. Our results highlight that perinatal mental health services should augment social support, address intimate partner violence, and tailor care for women with prior pregnancy loss, as these factors influenced the timing, severity, and impact of perinatal depression.
Implications of all the available evidence
Perinatal depression is a leading cause of maternal morbidity and mortality and can influence long-term health of mothers and their children. The World Health Organization encourages expanded case identification and treatment for mental disorders through the Mental Health Gap Action Programme (mhGAP), especially in widely attended care settings like maternal and child health. However, global guidelines do not prioritize specific mental health interventions for perinatal depression or indicate who should receive these services. Our results provide new insights into phenotypes that should be prioritized for mental health care in MCH settings to optimize impact. Simultaneously screening for depressive symptoms, intimate partner violence, social support, HIV risk, and prior pregnancy loss could identify women with comorbid psychosocial stressors for higher-intensity mental health intervention. Services that improve social support, promote partner HIV testing, and address IPV may reduce or prevent perinatal depression in LMICs, especially if received during pregnancy. Our results inform next steps for implementing perinatal mental health interventions in MCH settings globally.
Funding sources:
This work was supported by the National Institute of Allergy and Infectious Disease (R01 AI125498 to GJS), the Eunice Kennedy Shriver National Institute of Child Health and Human Development (F31HD101149 to AL, R01HD100201 to JP and R01 HD094630 to GJS). The funding agencies had no role in the writing of the manuscript or the decision to submit it for publication.
Declaration of interests:
This paper represents the opinions of the authors and is not meant to represent the position or opinions of organizations, nor the official position of any staff members. Ms. Larsen reports grants from NIH during the conduct of the study. Dr. John-Stewart reports grants from NIH, grants from CDC, grants from Thrasher, personal fees from UpToDate, personal fees from UW, grants from IMPAACT, outside the submitted work. Dr. Kinuthia reports grants from NIH, during the conduct of the study. Dr. Pintye reports grants from NIH during the conduct of the study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fisher J, Cabral de Mello M, Patel V, et al. Prevalence and determinants of common perinatal mental disorders in women in low- and lower-middle-income countries: a systematic review. Bull World Health Organ 2012; 90: 139–149H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oates M Perinatal psychiatric disorders: a leading cause of maternal morbidity and mortality. Br Med Bull 2003; 67: 219–29. [DOI] [PubMed] [Google Scholar]
- 3.Stein A, Pearson RM, Goodman SH, et al. Effects of perinatal mental disorders on the fetus and child. Lancet (London, England) 2014; 384: 1800–19. [DOI] [PubMed] [Google Scholar]
- 4.Dadi AF, Akalu TY, Baraki AG, Wolde HF. Epidemiology of postnatal depression and its associated factors in Africa: A systematic review and meta-analysis. PLoS One. 2020; 15. DOI: 10.1371/journal.pone.0231940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dadi AF, Wolde HF, Baraki AG, Akalu TY. Epidemiology of antenatal depression in Africa: A systematic review and meta-analysis. BMC Pregnancy Childbirth 2020; 20: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pilkington PD, Milne LC, Cairns KE, Lewis J, Whelan TA. Modifiable partner factors associated with perinatal depression and anxiety: A systematic review and meta-analysis. J Affect Disord 2015; 178: 165–80. [DOI] [PubMed] [Google Scholar]
- 7.Biaggi A, Conroy S, Pawlby S, Pariante CM. Identifying the women at risk of antenatal anxiety and depression: A systematic review. J Affect Disord 2016; 191: 62–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Howard LM, Oram S, Galley H, Trevillion K, Feder G. Domestic Violence and Perinatal Mental Disorders: A Systematic Review and Meta-Analysis. PLoS Med 2013; 10: e1001452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Howard LM, Molyneaux E, Dennis C-L, Rochat T, Stein A, Milgrom J. Non-psychotic mental disorders in the perinatal period. Lancet (London, England) 2014; 384: 1775–88. [DOI] [PubMed] [Google Scholar]
- 10.Atif N, Lovell K, Rahman A. Maternal mental health: The missing ‘m’ in the global maternal and child health agenda. Semin Perinatol 2015; 39: 345–52. [DOI] [PubMed] [Google Scholar]
- 11.Parsons CE, Young KS, Rochat TJ, Kringelbach ML, Stein A. Postnatal depression and its effects on child development: a review of evidence from low- and middle-income countries. Br Med Bull 2012; 101: 57–79. [DOI] [PubMed] [Google Scholar]
- 12.Nagin D. Group-based modeling of development. Harvard University Press, 2005. https://www-jstor-org.offcampus.lib.washington.edu/stable/j.ctvjf9z1f (accessed June 26, 2020). [Google Scholar]
- 13.Santos H, Tan X, Salomon R. Heterogeneity in perinatal depression: how far have we come? A systematic review. Arch Womens Ment Health 2017; 20: 11–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baron E, Bass J, Murray SM, Schneider M, Lund C. A systematic review of growth curve mixture modelling literature investigating trajectories of perinatal depressive symptoms and associated risk factors. J Affect Disord 2017; 223: 194–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nagin D. Group-based modeling of development. Harvard University Press, 2005. https://books.google.com/books/about/Group_Based_Modeling_of_Development.html?id=cE0mwX_ByVMC (accessed June 3, 2020). [Google Scholar]
- 16.JC D, J K, J P, et al. PrEP Implementation for Mothers in Antenatal Care (PrIMA): Study Protocol of a Cluster Randomised Trial. BMJ Open 2019; 9. DOI: 10.1136/BMJOPEN-2018-025122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rabin RF, Jennings JM, Campbell JC, Bair-Merritt MH. Intimate partner violence screening tools: a systematic review. Am J Prev Med 2009; 36: 439–445.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sherbourne CD, Stewart AL. The MOS social support survey. Soc Sci Med 1991; 32: 705–14. [DOI] [PubMed] [Google Scholar]
- 19.Melki IS, Beydoun HA, Khogali M, Tamim H, Yunis KA, National Collaborative Perinatal Neonatal Network (NCPNN). Household crowding index: a correlate of socioeconomic status and inter-pregnancy spacing in an urban setting. J Epidemiol Community Heal 2004; 58: 476–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pintye J, Drake AL, Kinuthia J, et al. A Risk Assessment Tool for Identifying Pregnant and Postpartum Women Who May Benefit From Preexposure Prophylaxis. Clin Infect Dis 2017; 64: 751–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Andresen EM, Malmgren JA, Carter WB, Patrick DL. Screening for depression in well older adults: evaluation of a short form of the CES-D (Center for Epidemiologic Studies Depression Scale). Am J Prev Med; 10: 77–84. [PubMed] [Google Scholar]
- 22.Baron EC, Davies T, Lund C. Validation of the 10-item Centre for Epidemiological Studies Depression Scale (CES-D-10) in Zulu, Xhosa and Afrikaans populations in South Africa. BMC Psychiatry 2017; 17: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.BK N, J A, A A, et al. Reliability and validity of the center for epidemiologic studies-depression scale in screening for depression among HIV-infected and -uninfected pregnant women attending antenatal services in northern Uganda: a cross-sectional study. BMC Psychiatry 2014; 14. DOI: 10.1186/S12888-014-0303-Y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen W, Qian L, Shi J, Franklin M. Comparing performance between log-binomial and robust Poisson regression models for estimating risk ratios under model misspecification. BMC Med Res Methodol 2018; 18: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haviland AM, Jones BL, Nagin DS. Group-based Trajectory Modeling Extended to Account for Nonrandom Participant Attrition. Sociol Methods Res 2011; 40: 367–90. [Google Scholar]
- 26.Larsen A, Kinuthia J, Lagat H, et al. Depression and HIV risk behaviors among adolescent girls and young women seeking family planning services in Western Kenya: https://doi.org/101177/0956462420920423 2020; 31: 652–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rothman KJ, Greenland S. Causation and Causal Inference in Epidemiology. https://doi.org/102105/AJPH2004059204 2011; 95. DOI: 10.2105/AJPH.2004.059204. [DOI] [PubMed] [Google Scholar]
- 28.Barthel D, Kriston L, Fordjour D, et al. Trajectories of maternal ante- and postpartum depressive symptoms and their association with child- and mother-related characteristics in a West African birth cohort study. PLoS One 2017; 12: e0187267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Garman EC, Schneider M, Lund C. Perinatal depressive symptoms among low-income South African women at risk of depression: trajectories and predictors. BMC Pregnancy Childbirth 2019; 19: 202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Garman EC, Cois A, Tomlinson M, Rotheram-Borus MJ, Lund C. Course of perinatal depressive symptoms among South African women: associations with child outcomes at 18 and 36 months. Soc Psychiatry Psychiatr Epidemiol 2019; published online Feb 25. DOI: 10.1007/s00127-019-01665-2. [DOI] [PubMed] [Google Scholar]
- 31.Pellowski JA, Bengtson AM, Barnett W, et al. Perinatal depression among mothers in a South African birth cohort study: Trajectories from pregnancy to 18 months postpartum. J Affect Disord 2019; 259: 279–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tsai AC. Reliability and validity of depression assessment among persons with HIV in sub-Saharan Africa: systematic review and meta-analysis. J Acquir Immune Defic Syndr 2014; 66: 503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chowdhary N, Sikander S, Atif N, et al. The content and delivery of psychological interventions for perinatal depression by non-specialist health workers in low and middle income countries: A systematic review. Best Pract Res Clin Obstet Gynaecol 2014; 28: 113–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


