Abstract
Preterm birth, the leading cause of neonatal morbidity and mortality worldwide, results from preterm labor, a syndrome that includes multiple etiologies. In this review, we have summarized the immune mechanisms implicated in intra-amniotic inflammation, the best-characterized cause of preterm labor and birth. While the intra-amniotic inflammatory responses driven by microbes (infection) or alarmins (sterile) have some overlap in the participating cellular and molecular processes, the distinct natures of these two conditions necessitate the implementation of specific approaches to prevent adverse pregnancy and neonatal outcomes. Intra-amniotic infection can be treated using the correct antibiotics, whereas sterile intra-amniotic inflammation could potentially be treated using a combination of anti-inflammatory drugs (e.g., betamethasone, inflammasome inhibitors, etc.). Recent evidence also supports a role for fetal T-cell activation as a newly described trigger for preterm labor and birth in a subset of cases. Moreover, here we also provide evidence of two potential immune mechanisms responsible for a subset of preterm births formerly considered to be idiopathic. First, the impairment of maternal Tregs can lead to preterm birth, likely due to the loss of immunosuppressive activity resulting in unleashed effector T-cell responses. Second, homeostatic macrophages were shown to be essential for maintaining pregnancy and promoting fetal development, and the adoptive transfer of homeostatic M2-polarized macrophages shows great promise for preventing inflammation-induced preterm birth. Collectively, in this review, we discuss established and novel immune mechanisms responsible for preterm birth and highlight potential targets for novel strategies aimed at preventing the multi-etiological syndrome of preterm labor.
In brief:
The syndrome of preterm labor comprises multiple established and novel etiologies. This review summarizes the distinct immune mechanisms implicated in preterm labor and birth and highlights potential strategies for its prevention.
Introduction
Preterm birth affects over 15 million pregnancies annually and remains a leading cause of neonatal morbidity and mortality (Liu et al., 2015, Chawanpaiboon et al., 2019). In addition to its drastic short-term consequences, preterm birth can have lasting effects on development that can persist into adulthood (Abitbol and Rodriguez, 2012, Carmody and Charlton, 2013, O’Reilly et al., 2013, Blencowe et al., 2013, Ream and Lehwald, 2018). Preterm deliveries are largely spontaneous, with the remainder being iatrogenic (i.e., medically indicated) (Goldenberg et al., 2008, Romero et al., 2014a). Spontaneous preterm birth results from preterm labor, a syndrome that includes multiple causal and associated etiologies (Romero et al., 2014a). Among these, inflammation of the amniotic cavity (i.e., intra-amniotic inflammation) is the most well-established cause (Romero et al., 2006b, Romero et al., 2007, Goldenberg et al., 2008, Romero et al., 2014a). Intra-amniotic inflammation can occur in two different contexts: the first results from the invasion of the amniotic cavity by microbes, termed intra-amniotic infection, whereas the second takes place in the absence of microbes and is associated with an increase in endogenous danger signals or alarmins, termed sterile intra-amniotic inflammation (Romero et al., 2006b, Romero et al., 2007, Goldenberg et al., 2008, Romero et al., 2014a, Romero et al., 2014b, Romero et al., 2014c, Romero et al., 2015b, Romero et al., 2015c). Importantly, a growing body of evidence has indicated that, although these two inflammatory states have similar clinical outcomes, they are intrinsically distinct from one another (Romero et al., 2014c, Romero et al., 2015a, Bhatti et al., 2020, Motomura et al., 2021a). Therefore, understanding the differences between the pathogenesis of intra-amniotic infection and sterile intra-amniotic inflammation is essential for determining the correct patient management.
A large proportion of spontaneous preterm births, however, are not associated with inflammation of the amniotic cavity and fetus (whether microbial or sterile), and have thus been grouped into the broad category of idiopathic preterm birth (Goldenberg et al., 2008, Barros et al., 2015). Among the proposed causes of idiopathic preterm birth, a breakdown of maternal-fetal tolerance has been put forward as a potential trigger for maternal inflammatory responses leading to the onset of preterm labor (Romero et al., 2014a). Pregnancy represents a tightly controlled maternal immune response that requires a delicate balance between effective host defense against potential infection (Bizargity et al., 2009, Arenas-Hernandez et al., 2016, van Egmond et al., 2016, van der Zwan et al., 2018) and maintaining tolerance of the foreign conceptus (Aluvihare et al., 2004, Taglauer et al., 2010, Munoz-Suano et al., 2011, Mold and McCune, 2012, Arck and Hecher, 2013, Erlebacher, 2013). Moreover, recent evidence has suggested that the fetus itself can exhibit immune responses and must therefore also tolerate the mother (Mold et al., 2008, Ivarsson et al., 2013, McGovern et al., 2017, Frascoli et al., 2018), given that fetal T-cell activation is associated with preterm labor and birth (Frascoli et al., 2018, Gomez-Lopez et al., 2019g). Such bidirectional tolerance is therefore the result of complex immunological adaptations that occur both systemically and locally (i.e., at the maternal-fetal interface), of which the latter involves both innate and adaptive cellular immune components (Croy et al., 1985, Aluvihare et al., 2004, Sasaki et al., 2004, Houser et al., 2011, Svensson et al., 2011, Bartmann et al., 2014, Vacca et al., 2015, Doisne et al., 2015, St Louis et al., 2016, Xu et al., 2016, Gomez-Lopez et al., 2017a, Xu et al., 2018b, Miller et al., 2018, Jiang et al., 2018, Vazquez et al., 2019, Arenas-Hernandez et al., 2019, Leng et al., 2019, Salvany-Celades et al., 2019, Gomez-Lopez et al., 2020, Gomez-Lopez et al., 2021a). In particular, regulatory T cells (Tregs) are considered important antigen-specific mediators of maternal-fetal tolerance through their suppression of potentially harmful effector T-cell responses (Zenclussen et al., 2005, Darrasse-Jèze et al., 2006, Kahn and Baltimore, 2010, Shima et al., 2010, Rowe et al., 2011, Samstein et al., 2012, Rowe et al., 2012, Chen et al., 2013, Diao et al., 2021), and thus the dysfunction of these cells has been implicated in preterm birth (Schober et al., 2012, Gomez-Lopez et al., 2020). Indeed, we recently provided mechanistic evidence supporting a critical role for Tregs in late pregnancy by demonstrating that the loss of these cells leads to preterm birth in mice (Gomez-Lopez et al., 2020). However, Treg dysfunction/deficiency only seems to be responsible for a small subset of preterm births (Gomez-Lopez et al., 2020); thus, we reasoned that other immune cells are contributing to maternal-fetal tolerance and may therefore be implicated in a breakdown of this process leading to preterm birth. Macrophages are considered to be important for preserving immune homeostasis at the maternal-fetal interface (Hunt et al., 1984, Gustafsson et al., 2008, Svensson et al., 2011, Svensson-Arvelund et al., 2015, Svensson-Arvelund and Ernerudh, 2015, Chambers et al., 2020, Abassi et al., 2020, Gomez-Lopez et al., 2021a); however, the importance of these cells in late pregnancy had not yet been demonstrated. Using an animal model of macrophage depletion, we showed that the loss of these cells in late gestation resulted in preterm birth as well as neonatal mortality (Gomez-Lopez et al., 2021a). Importantly, we also showed that the restoration of homeostatic macrophages could prevent inflammation-associated preterm birth and adverse neonatal outcomes in mice, further demonstrating the importance of these cells for pregnancy maintenance (Gomez-Lopez et al., 2021a). Therefore, deciphering the contributions of these immune cell subsets to successful pregnancy may allow for the identification of novel approaches that can be used to prevent preterm labor and birth.
In this review, we first discuss the discovery, clinical definitions, and immune mechanisms implicated in intra-amniotic infection and sterile intra-amniotic inflammation. Moreover, we discuss the current and potential approaches that can be used to treat these two distinct clinical conditions. Next, we discuss the activation of the fetal immune system as a novel mechanism leading to preterm birth. We then focus on the mechanisms whereby maternal effector T cells, Tregs, and macrophages participate in successful pregnancy. In addition, we review the evidence implicating each subset in the pathophysiology of preterm labor and birth, and potential therapeutic approaches that can be used to target these cells. We aim to provide an overview of key immunological processes implicated in preterm labor and birth, which can provide deeper understanding, highlight gaps in knowledge, and provide potential targets for future therapies that can be used to treat this devastating obstetrical syndrome.
Intra-amniotic infection: the most well-known etiology of preterm labor and birth
The amniotic cavity has been classically thought to be a sterile compartment (Perez-Munoz et al., 2017), and therefore the detection of viable microorganisms in the amniotic fluid is considered to be abnormal. Microbial invasion of the amniotic cavity (MIAC) can elicit a local inflammatory response (i.e., microbial-associated intra-amniotic inflammation) (Naeye and Ross, 1982, Romero et al., 1991b, Romero et al., 1993c, Martinez-Varea et al., 2017, Gomez-Lopez et al., 2018c, Gomez-Lopez et al., 2019b, Galaz et al., 2020c, Galaz et al., 2020a). Microbial-associated intra-amniotic inflammation, referred to hereafter as intra-amniotic infection, is defined as the presence of microbes together with intra-amniotic inflammation [i.e., increased concentrations of IL-6 or MMP-8 (Park et al., 2001, Yoon et al., 2001)] (Goldenberg et al., 2008, Romero et al., 2014a, Romero et al., 2014b, Romero et al., 2014c, Romero et al., 2015b, Romero et al., 2015c), and is strongly associated with preterm labor and delivery (Romero et al., 2001, Goncalves et al., 2002, Goldenberg et al., 2008, Bastek et al., 2011, Romero et al., 2014a). Although a small subset of patients with intra-amniotic infection may progress to a systemic maternal infection (i.e., clinical chorioamnionitis (Gibbs et al., 1982, Gibbs and Duff, 1991)), the majority of women with intra-amniotic infection/inflammation are asymptomatic, supporting the subclinical nature of this condition (Gravett et al., 1986, Gibbs et al., 1992, Romero et al., 2006a, Romero et al., 2007, Goldenberg et al., 2008). Notably, microbiological analyses of the amniotic fluid suggest that approximately 25% of all spontaneous preterm births are related to infection (Gibbs et al., 1992, Romero et al., 2001, Goncalves et al., 2002, Goldenberg et al., 2008, Romero et al., 2014a). The proportion of patients with intra-amniotic infection is variable among the different clinical obstetric scenarios. On average, the rate of positive amniotic fluid culture among numerous studies analyzing women with preterm labor and intact membranes is approximately 10% (Goncalves et al., 2002), and this rate is increased to over 20% among those who ultimately deliver preterm (Romero et al., 1989c). Among the studies of women with preterm prelabor rupture of membranes (PPROM), the mean rate of a positive amniotic fluid culture is about 30% at time of admission (Romero et al., 1988b, Goncalves et al., 2002, Romero et al., 2015b) and can reach up to 75% if the sample is collected at labor onset (Romero et al., 1988b). Furthermore, it has been shown using molecular microbiological techniques that a significant proportion of patients with PPROM who presented a negative amniotic fluid culture with intra-amniotic inflammation yielded a positive bacterial signal (DiGiulio et al., 2010, Romero et al., 2015b, Theis et al., 2020), suggesting the presence of non-cultivable microorganisms. Intra-amniotic infection is also present in up to 50% of women with cervical insufficiency (Romero et al., 1992a, Lee et al., 2008, Bujold et al., 2008, Oh et al., 2010, Lisonkova et al., 2014), as well as in one out of ten women with a sonographic short cervix (Hassan et al., 2006, Romero et al., 2015c). Although it is well known that a short cervix is considered a powerful predictor of preterm birth (Andersen et al., 1990, Iams et al., 1996, Heath et al., 1998, Berghella et al., 1999, Hassan et al., 2000, Romero, 2007, Rosenbloom et al., 2020, Gudicha et al., 2021), the presence of intra-amniotic infection among these patients confers an increased risk of early preterm delivery (i.e., before 34 weeks) compared to those without infection (Hassan et al., 2006, Romero et al., 2015c). Importantly, intra-amniotic infection is also associated with adverse neonatal outcomes, including increased morbidity and mortality (Yoon et al., 1996a, Yoon et al., 1999, Yoon et al., 2000b, Berger et al., 2004, Kirchner et al., 2007). Taken together, a large body of clinical evidence has implicated intra-amniotic infection as being strongly linked to spontaneous preterm labor and birth, thereby increasing the already high basal risk in a subset of women, including those with a short cervix.
To establish a causal link between intra-amniotic infection and preterm birth, multiple experimental approaches using animal models have been widely utilized (Dombroski et al., 1990, Gravett et al., 1994, Fidel et al., 2003, Novy et al., 2009, Boldenow et al., 2016, Gomez-Lopez et al., 2018a, Garcia-Flores et al., 2018, Faro et al., 2019, Motomura et al., 2020b). The intra-amniotic administration of microorganisms or their products (e.g., lipopolysaccharide or LPS) has been shown to induce preterm labor and birth in different animal models (Dombroski et al., 1990, Gravett et al., 1994, Fidel et al., 2003, Elovitz and Mrinalini, 2004, Novy et al., 2009, Boldenow et al., 2016, Gomez-Lopez et al., 2018a, Garcia-Flores et al., 2018, Faro et al., 2019, Motomura et al., 2020b, Stranik et al., 2020, Cappelletti et al., 2021). By comparing different routes of LPS administration (intra-amniotic, intra-uterine, and intra-peritoneal), we showed that only the intra-amniotic injection of this bacterial product resembles the subclinical nature of intra-amniotic inflammation/infection (Gomez-Lopez et al., 2018a), in which the activation of the common pathway of labor typically occurs in the absence of systemic symptoms such as fever (Gravett et al., 1986, Romero et al., 1988b, Romero et al., 1989c, Gibbs et al., 1992, Romero et al., 2006a, Romero et al., 2007, Goldenberg et al., 2008). Therefore, a causal link between the presence of microorganisms or their products in the amniotic cavity and the onset of preterm labor was established.
How do microorganisms invade the amniotic cavity? Multiple routes have been proposed whereby microorganisms can reach the intra-amniotic space: 1) ascension from the lower genital tract; 2) hematogenous dissemination through the placenta (transplacental infection); 3) retrograde seeding from the peritoneal cavity through the fallopian tubes; and 4) accidental inoculation (i.e., iatrogenic) at the time of invasive procedures such as amniocentesis, cordocentesis, or chorionic villous sampling. A large number of investigations support the ascending route as the most common pathway of intrauterine infection (Romero et al., 1989c, Romero et al., 1990b, Romero et al., 2019, Oh et al., 2019a). Indeed, we recently investigated the bacterial profiles of amniotic fluids and vaginal swabs taken at the time of amniocentesis in women with intra-amniotic infection using conventional culture, matrix-assisted laser desorption ionization-time of flight mass spectrometry (MALDI-TOF), and 16S ribosomal RNA (rRNA) gene sequencing (Romero et al., 2019). We found that the bacterial profiles of amniotic fluid are largely consistent with those of the vagina, thus generating solid evidence supporting the ascending route of microbial invasion of the amniotic cavity (Romero et al., 2019). Moreover, animal models have demonstrated the biological plausibility of ascending intra-amniotic infection (Vornhagen et al., 2016, Suff et al., 2018, Pavlidis et al., 2020, Gilbert et al., 2021, Spencer et al., 2021). The most common microorganisms cultured from the amniotic fluid of women with intra-amniotic infection include Ureaplasma spp., Mycoplasma hominis, Gardnerella vaginalis, and Streptococcus agalactiae, among others (Romero et al., 1989c, Yoon et al., 1998, DiGiulio et al., 2010, Mendz et al., 2013, Romero et al., 2014c, Romero et al., 2015d), all of which can be found in the vagina (Romero et al., 1989c, Romero et al., 2019). Additional evidence in favor of an ascending model of intra-amniotic infection comes from the demonstration of greater signs of inflammation/infection in tissues near the cervix. For example, histological inflammation is more common and severe in the cervical zone of the chorioamniotic membranes (Malak and Bell, 1994, McLaren et al., 1999, El Khwad et al., 2005, Nhan-Chang et al., 2010, Gomez-Lopez et al., 2011, Elfayomy and Almasry, 2014, Marcellin et al., 2017). Similarly, intra-amniotic inflammation is more prevalent in the first (i.e., closer to the cervix) rather than the second fetus in twin pregnancies affected by preterm labor with intact membranes (Romero et al., 1990b, Oh et al., 2019a). Thus, a four-stage ascending process of intrauterine infection from the lower genital tract has been classically proposed (Romero et al., 1988a, Goncalves et al., 2002). This process includes an initial alteration of the vaginal microbiome, characterized by a reduction in the proportion of commensal bacteria such as Lactobacillus spp. and the abnormal growth of pathological organisms (e.g., Neisseria gonorrhoeae, Chlamydia trachomatis, Ureaplasma parvum, Mycoplasma, and Gardnerella vaginalis, among others) (stage I). Then, these pathological microorganisms gain access to the intrauterine cavity (stage II), causing a localized inflammatory reaction in the decidua and chorion. Here, the microorganisms invade the amniotic cavity through the chorionic vessels or by directly crossing the intact membranes (stage III). Lastly, microorganisms in the amniotic cavity gain access to the fetus through different routes of entry, including the fetal mucosal tissues or the invasion of the fetal villous circulation (stage IV). A systemic dissemination of microbes from these fetal sites can occur, leading to fetal inflammatory response syndrome (FIRS) (Gomez et al., 1998, Romero et al., 1998, Pacora et al., 2002, Madsen-Bouterse et al., 2010, Jung et al., 2020, Para et al., 2021). Such fetal compromise may explain the increased risk of short- and long-term complications as well as death in neonates born to women with intra-amniotic infection (Yoon et al., 1998, Hitti et al., 2001, Yoon et al., 2003, Berger et al., 2004, Kirchner et al., 2007, Arayici et al., 2014, Kostlin-Gille et al., 2021).
In addition to the classical ascending route of intra-amniotic infection, the invasion of the amniotic cavity can also be caused by trans-placental infection in a small fraction of patients (Romero et al., 1989c, Goldenberg et al., 2008). Furthermore, maternal infections such as urinary tract infections (Kass, 1962, Romero et al., 1989b, Wing et al., 2014) and malaria (Menendez et al., 2000, Desai et al., 2007), among others (Kourtis et al., 2014, Fouks et al., 2018), have been associated with preterm labor and birth. Interestingly, molecular tools for bacterial detection in amniotic fluid have been useful to show the presence of microorganisms normally found in the oral cavity (e.g. Fusobacterium nucleatum and Streptococcus spp.) in pregnant patients at term (Bearfield et al., 2002), further supporting the proposed relationship between periodontal disease and adverse pregnancy outcomes through hematogenous dissemination (Goepfert et al., 2004, Le et al., 2021, Uwambaye et al., 2021). Collectively, these findings suggest that maternal bacteremia and trans-placental passage could account for some cases of intra-amniotic infection. However, additional research is required to establish a direct link between extra-uterine infectious conditions and spontaneous preterm labor and birth.
Host immune defense mechanisms in intra-amniotic infection
Once microbes have entered the amniotic cavity, different local and systemic mechanisms of host immune defense are elicited in both the maternal and the fetal compartments (Romero et al., 1989c, Gibbs and Duff, 1991, Romero et al., 2006a, Lee et al., 2006, Romero et al., 2007, Lee et al., 2007, Gotsch et al., 2007, Romero et al., 2014a). The local inflammatory response towards microbes invading the amniotic cavity is characterized by an infiltration of leukocytes, including both innate and adaptive immune cells (Romero et al., 1991b, Romero et al., 1993c, Gomez et al., 1994, Yoon et al., 1996b, Martinez-Varea et al., 2017, Gomez-Lopez et al., 2018c, Gomez-Lopez et al., 2019b, Galaz et al., 2020c, Galaz et al., 2020a, Galaz et al., 2020b, Gomez-Lopez et al., 2021c), as well as increased amniotic fluid concentrations of cytokines, chemokines and prostaglandins (Romero et al., 1986, Saito et al., 1993, Romero et al., 1993b, Hsu et al., 1998a, Hsu et al., 1998b, Yoon et al., 2001, Figueroa et al., 2005, Cobo et al., 2014, Park et al., 2016, Tarca et al., 2017, Peiris et al., 2020, Bhatti et al., 2020, McCartney et al., 2021, Peiris et al., 2021). Immunophenotyping of the cellular component of this immune response in patients with intra-amniotic infection has revealed that the most common cells involved in the local inflammatory response are neutrophils and monocytes/macrophages, as well as to a lesser extent T cells, B cells, and NK cells (Martinez-Varea et al., 2017, Gomez-Lopez et al., 2018c, Gomez-Lopez et al., 2019g, Galaz et al., 2020a, Galaz et al., 2020c). Using DNA fingerprinting and fluorescence in situ hybridization, we have shown that both fetal and maternal neutrophils can access the amniotic cavity and participate in host defense against intra-amniotic infection/inflammation (Gomez-Lopez et al., 2017f). Similarly, monocytes/macrophages present in the amniotic fluid of patients with demonstrated infection/inflammation can originate from both the mother and the fetus (Gomez-Lopez et al., 2019d). However, in cases of intra-amniotic infection leading to preterm labor and birth, the majority of neutrophils and monocytes/macrophages in the amniotic fluid are derived from the fetus (Sampson et al., 1997, Gomez-Lopez et al., 2017f, Gomez-Lopez et al., 2019d). By contrast, a predominant maternal origin has been shown for neutrophils (Gomez-Lopez et al., 2017f) and monocytes/macrophages (Gomez-Lopez et al., 2019d) detected in the amniotic fluid of women with intra-amniotic infection/inflammation who delivered at term. Therefore, both the mother and fetus can display a local immune response to microbes in the amniotic cavity.
The immune response towards microbes invading the amniotic cavity requires the orchestration of multiple leukocyte functions. As first responders to infection, neutrophils are characterized by a variety of host defense mechanisms, including phagocytic capacity, the release of antimicrobial products and immune mediators, and the formation of neutrophil extracellular traps (NETs) (Mantovani et al., 2011, Burn et al., 2021). NETs are web-like structures composed of DNA, histones, and antimicrobial products such as neutrophil elastase that can trap microbes (Brinkmann et al., 2004, Fuchs et al., 2007, Brinkmann and Zychlinsky, 2012). Notably, we and others have demonstrated that neutrophils in amniotic fluid are capable of performing the abovementioned host defense mechanisms including phagocytosis (Gomez-Lopez et al., 2017b), release of antimicrobial products and immune mediators such as lactoferrin, defensins, tumor necrosis factor (TNF)-α and macrophage inflammatory protein-1β (Heller et al., 1995, Otsuki et al., 1999, Pacora et al., 2000a, Maymon et al., 2001, Espinoza et al., 2003, Gravett et al., 2004, Soto et al., 2007, Martinez-Varea et al., 2017, Varrey et al., 2018, Para et al., 2020), and formation of NETs (Gomez-Lopez et al., 2017g, Galaz et al., 2020c). Similarly, neutrophils infiltrating the chorioamniotic membranes in response to intra-amniotic infection also have the capacity to form NETs (Boldenow et al., 2016, Gomez-Lopez et al., 2017c, Tong et al., 2019, Tong et al., 2021). On the other hand, one of the primary functions of monocytes/macrophages is the production and secretion of pro-inflammatory cytokines (Serbina et al., 2008), which is consistent with reports of such cells expressing interleukin (IL)-1β and IL-1α in the amniotic cavity of patients with intra-amniotic infection (Martinez-Varea et al., 2017, Galaz et al., 2020c). Consistent with these distinct roles of neutrophils and monocytes/macrophages in intra-amniotic infection, high-throughput RNA sequencing analysis revealed differing transcriptomic profiles in these cell types (Gomez-Lopez et al., 2021c), thus highlighting the complexity of the local cellular innate immune responses in women with intra-amniotic infection. In addition, the inflammatory mediators detected in the amniotic fluid are mainly related to innate immune cells (i.e., neutrophils and monocyte/macrophages) (Gomez-Lopez et al., 2019b, Galaz et al., 2020a). Although the number of amniotic fluid T and B cells is also increased in patients with intra-amniotic infection/inflammation (Gomez-Lopez et al., 2018c), their contribution to the integrated immune response remains less clear, given the overwhelming presence of innate immune cells. Yet, we recently proposed a role for the T-cell cytokine IL-22 in the host response against microbes invading the amniotic cavity by demonstrating the participation of this cytokine in the intra-amniotic inflammatory milieu that occurs prior to Ureaplasma parvum-induced preterm birth in mice, which was prevented by IL-22 deficiency (Gershater et al., 2022, Accepted). Thus, the cellular immune response of women at risk for spontaneous preterm birth with demonstrated intra-amniotic infection is greater than in those without infection, and is primarily driven by neutrophils, monocytes/macrophages, and, to a lesser extent, T cells (Fig. 1A).
Fig. 1. Distinct immune responses in the amniotic cavity of women with preterm labor.
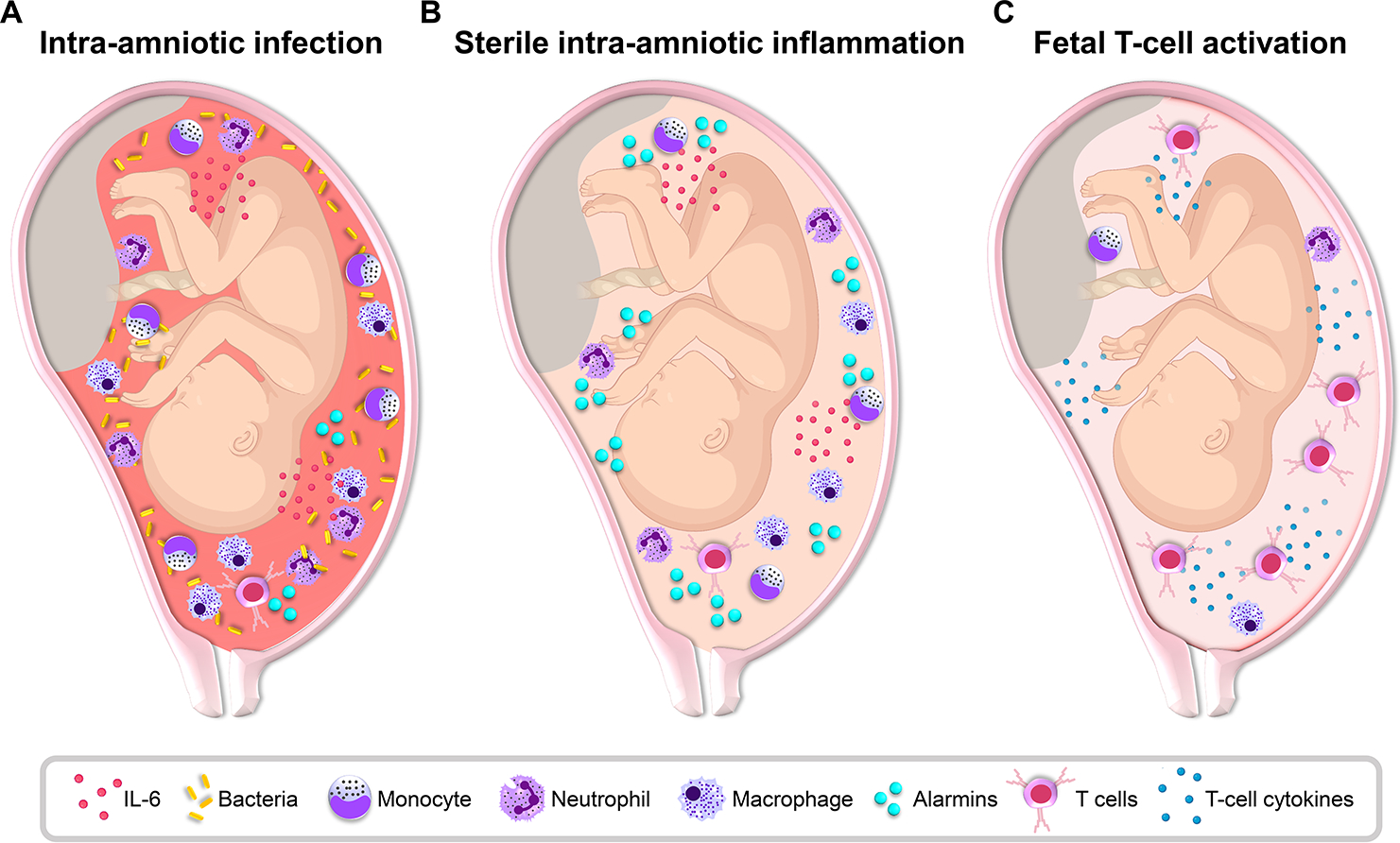
Representative diagrams of the fetus and amniotic cavity showing the causative agents and responding immune cells associated with distinct immune responses leading to preterm labor. (A) Intra-amniotic infection is typically triggered by the ascending invasion of bacteria from lower genital tract and is characterized by a massive local immune response including elevated concentrations of inflammatory mediators such as interleukin (IL)-6 and abundant neutrophils, monocytes/macrophages, and T cells. (B) Sterile intra-amniotic inflammation can be triggered by endogenous alarmins and involves elevated concentrations of inflammatory mediators such as IL-6 and a mild infiltration of immune cells such as neutrophils, monocytes/macrophages, and T cells. (C) A subset of cases of preterm labor and birth, formerly considered to be idiopathic, can be driven by the fetal immune system, as indicated by the activation and increased presence of fetal T cells and their mediators in the amniotic cavity. This immune response may also include the activation of amniotic fluid resident innate immune cells such as neutrophils and monocytes/macrophages.
Treatment of intra-amniotic infection
Considering the above demonstrations showing a strong relationship between intra-amniotic infection and spontaneous preterm birth as well as its adverse consequences, numerous randomized clinical trials have attempted to manage such risks using antibiotic therapy. Multiple clinical trials in patients with PPROM indicated that antibiotic therapy is associated with a longer latency period (time between the onset of PPROM and delivery) as well as reduced rates of clinical chorioamnionitis and neonatal sepsis (Mercer and Arheart, 1995, Kenyon et al., 2001a, Kenyon et al., 2013). Thus, antibiotics are considered a standard of care for women with PPROM (Ehrenberg and Mercer, 2001, Yudin et al., 2009, Thomson et al., 2019, American College of Obstetricians and Gynecologists, 2020). However, most studies evaluating the potential usefulness of antibiotics to prolong gestational length and reduce neonatal morbidity in women with preterm labor and intact membranes have been unsuccessful (Newton et al., 1989, Romero et al., 1993a, Gordon et al., 1995, Kenyon et al., 2001b). Such disparity in the success of antibiotic treatment could be explained by the greater prevalence of intra-amniotic infection in women with PPROM compared to those with preterm labor and intact membranes (Goncalves et al., 2002), and thus the benefits of antibiotics lies in their inherent function of killing bacteria or inhibiting bacterial growth. Therefore, it is imperative to evaluate the microbial and inflammatory status of the amniotic fluid to select the subset of women with preterm labor and intact membranes who will benefit from antibiotic treatment. Indeed, recent investigations have demonstrated that the utilization of an appropriate antibiotic regimen can improve adverse perinatal outcomes in women with preterm labor and intact membranes (Yoon et al., 2019), PPROM (Lee et al., 2016a, Lee et al., 2016b), or cervical insufficiency (Oh et al., 2019b, Yeo et al., 2021) who were diagnosed with intra-amniotic infection/inflammation. This antibiotic regimen includes clarithromycin, ceftriaxone and metronidazole, based on their pharmacokinetics (Kafetzis et al., 1983, Visser and Hundt, 1984, Amon, 1985, Matsuda et al., 1988, Witt et al., 2003, Park et al., 2012) and broad coverage for bacteria that are typically found in the amniotic fluid (Romero et al., 1989c, Yoon et al., 1998, DiGiulio et al., 2010, Mendz et al., 2013, Romero et al., 2014b, Romero et al., 2014c, Romero et al., 2015b, Romero et al., 2015d, Romero et al., 2015c). Specifically, the use of clarithromycin is strongly supported by its coverage of genital mycoplasmas as well as more efficient trans-placental passage than other macrolides (Witt et al., 2003, Park et al., 2012). Such protective effects of clarithromycin were recently demonstrated by the reduced rates of preterm birth and neonatal mortality observed in mice treated with this antibiotic after the intra-amniotic injection of Ureaplasma parvum (Motomura et al., 2020b). Furthermore, ceftriaxone and metronidazole offer excellent antimicrobial coverage for aerobic and anaerobic bacteria, respectively (Klein and Cunha, 1995, Freeman et al., 1997, Lamb et al., 2002, Brook et al., 2013), and can also cross the placenta efficiently (Kafetzis et al., 1983, Visser and Hundt, 1984, Amon, 1985, Matsuda et al., 1988). Therefore, this recent evidence supports the use of amniocentesis to evaluate the infectious status of the amniotic fluid and the treatment of intra-amniotic infection with the optimal antibiotic therapy.
Sterile intra-amniotic inflammation: the new kid on the block among the etiologies of preterm labor and birth
Discovery of sterile intra-amniotic inflammation
As mentioned above, the presence of intra-amniotic inflammation has been traditionally attributed to the host defense processes triggered by microbes invading the amniotic cavity (Romero et al., 1987, Romero et al., 1988c, Romero et al., 1989c, Gibbs et al., 1992). However, several clinical investigations have reported that a subset of women diagnosed with intra-amniotic inflammation (as indicated by elevated amniotic fluid IL-6 concentrations (Yoon et al., 2001)) had negative amniotic fluid cultures (Hitti et al., 1997, Yoon et al., 2000a, Yoon et al., 2001, Gardella et al., 2004, DiGiulio et al., 2008, DiGiulio et al., 2010, Combs et al., 2014). Two potential explanations for this phenomenon can be proposed: 1) this subset of women had intra-amniotic inflammation that was initiated by non-cultivable or fastidious microorganisms, or 2) such inflammation was driven by non-infectious processes, as observed in gout, rheumatoid arthritis, or other sterile inflammatory diseases (Busso and So, 2010, Goh and Midwood, 2012, So and Martinon, 2017). Over the past two decades, the advancement of molecular microbiological techniques has allowed for the detection and identification of non-cultivable microbes in the amniotic fluid (Jalava et al., 1996, Oyarzun et al., 1998, Kim et al., 2003, Gardella et al., 2004, DiGiulio et al., 2008, Romero et al., 2014b, Combs et al., 2014, Burnham et al., 2020, Theis et al., 2020, Stinson et al., 2020). Accordingly, our group has utilized a combination of conventional culture and molecular microbiological techniques [PCR-electrospray ionization mass spectrometry (PCR-ESI/MS)](Romero et al., 2014b) as well as microbial cell-free DNA (cfDNA) (Burnham et al., 2020) to demonstrate the absence of bacterial or viral nucleic acids in a subset of women with intra-amniotic inflammation, a condition that has been consequently termed “sterile intra-amniotic inflammation” (Romero et al., 2014c, Romero et al., 2015b, Romero et al., 2015c, Romero et al., 2015a, Burnham et al., 2020) (Fig. 1B). Upon the establishment of this new clinical entity, a new line of investigation has been undertaken by our group and others to evaluate the prevalence and underlying mechanisms of sterile intra-amniotic inflammation. Importantly, sterile intra-amniotic inflammation is more common than intra-amniotic infection in women with preterm labor with intact membranes (Romero et al., 2014c) as well as in women with an asymptomatic sonographic short cervix (Romero et al., 2015c) or cervical insufficiency (Chalupska et al., 2021). Moreover, women with sterile intra-amniotic inflammation have pregnancy and neonatal outcomes similar to women with intra-amniotic infection (Romero et al., 2014c, Combs et al., 2014). The clinical outcomes of sterile intra-amniotic inflammation correlate with the presence of acute inflammatory lesions in the placenta (i.e., acute histologic chorioamnionitis and funisitis), again resembling the outcomes of microbial-associated intra-amniotic inflammation (Romero et al., 2014c). Thus, sterile intra-amniotic inflammation has emerged as a new entity with pregnancy and neonatal consequences as devastating as those associated with infection, and therefore warrants deeper investigation.
Progress in the understanding of sterile intra-amniotic inflammation
As an initial effort to distinguish the inflammatory processes taking place in sterile intra-amniotic inflammation from those observed in intra-amniotic infection, our group performed a network analysis of the cytokines and other known inflammatory mediators in the amniotic fluid of women who underwent preterm labor with intact membranes and were diagnosed with either sterile intra-amniotic inflammation or intra-amniotic infection (Romero et al., 2015a). This network analysis revealed the enrichment of IL-1α and high mobility group box 1 (HMGB1) in the amniotic fluid of women with sterile intra-amniotic inflammation (Romero et al., 2015a). Multiple studies have demonstrated the importance of IL-1α in the physiologic and pathologic processes of parturition (Romero et al., 1989a, Romero et al., 1990a, Nadeau-Vallee et al., 2016a, Nadeau-Vallee et al., 2016b, Nadeau-Vallee et al., 2017b, Equils et al., 2020), as evidenced by its increased concentrations in the amniotic fluid of women with intra-amniotic inflammation (Romero et al., 1992b) as well as mechanistic demonstrations that the systemic administration of IL-1 induced preterm parturition in mice (Romero et al., 1991a), which could be prevented by pre-treatment with the IL-1 receptor antagonist (IL-1RA) (Romero and Tartakovsky, 1992). Clinical investigations have also shown that women with sterile intra-amniotic inflammation who had higher amniotic fluid concentrations of HMGB1 delivered sooner than women with lower concentrations, implicating this mediator in the pathological process of preterm labor and birth (Romero et al., 2014c). Notably, both IL-1α and HMGB1 are known damage-associated molecular patterns (DAMPs) or alarmins (Oppenheim and Yang, 2005, Lotze et al., 2007, Bianchi, 2007, Rider et al., 2017), and thus our network analysis hinted at a key role for alarmins in the pathophysiology of sterile intra-amniotic inflammation.
Alarmins are considered part of a broad class of molecules, termed danger signals, that alert the innate and adaptive immune system and thus trigger host defense mechanisms (Oppenheim and Yang, 2005, Lotze et al., 2007, Bianchi, 2007, Rider et al., 2017). Danger signals that are derived from exogenous sources, such as microbes, are called pathogen-associated molecular patterns (PAMPs) (Oppenheim and Yang, 2005, Lotze et al., 2007, Bianchi, 2007, Rider et al., 2017). Yet, it is now well known that immune activation can also be induced by endogenous DAMPs or alarmins (Oppenheim and Yang, 2005, Lotze et al., 2007, Bianchi, 2007, Rider et al., 2017). Multiple defining characteristics have been described for alarmins: 1) they are rapidly released upon non-programmed cell death (i.e., necrosis) but not apoptosis; 2) viable cells can also release alarmins via specialized secretion systems or by the endoplasmic reticulum-Golgi secretion pathway; 3) as danger signals, alarmins can participate in the recruitment and activation of innate immune cells via pattern recognition receptors (PRR); and 4) alarmins contribute to the restoration of homeostasis by promoting healing of tissues damaged by inflammation (Oppenheim and Yang, 2005, Lotze et al., 2007, Bianchi, 2007, Rider et al., 2017). In addition, studies have shown that alarmins can be released upon cellular senescence (Huang et al., 2015), a state of cellular aging in which cell division has been halted (Campisi and d’Adda di Fagagna, 2007, Munoz-Espin and Serrano, 2014, Di Micco et al., 2021). Classical alarmins include HMGB1 (Wang et al., 1999, Harris and Raucci, 2006), S100 proteins (Foell et al., 2007), IL-1α (Werman et al., 2004, Tracy et al., 2012, Di Paolo and Shayakhmetov, 2016), and heat-shock protein 70 (HSP70) (Asea et al., 2000), among others (Bianchi, 2007). Importantly, each of the abovementioned alarmins has been demonstrated to be increased in amniotic fluid of women with intra-amniotic inflammation who underwent preterm labor and birth (Romero et al., 1992b, Friel et al., 2007, Romero et al., 2011, Romero et al., 2012, Romero et al., 2014c, Baumbusch et al., 2016, Son et al., 2019, Chaiworapongsa et al., 2008). Based on these observations, we have undertaken a series of translational investigations to establish a causal link between elevated amniotic fluid concentrations of alarmins and preterm birth. The ultrasound-guided intra-amniotic administration of HMGB1 or S100B, at pathological concentrations found in women with sterile intra-amniotic inflammation, induced preterm birth in mice (Gomez-Lopez et al., 2016c, Gomez-Lopez et al., 2019c, Galaz et al., 2021). Similarly, the intra-amniotic injection of the alarmins IL-1α, HSP70, or S100A12 also reduced gestational length, thereby increasing the rate of preterm birth (Motomura et al., 2020a, Schwenkel et al., 2021, Motomura et al., 2021b). Importantly, the intra-amniotic injection of alarmins also induced adverse fetal and neonatal outcomes, as evidenced by a fetal inflammatory response (Kallapur et al., 2011) and increased mortality at birth (Gomez-Lopez et al., 2019c, Motomura et al., 2020a, Motomura et al., 2021b, Schwenkel et al., 2021) as well as postnatal changes such as alterations in respiratory parameters (Emerson et al., 1997, Willet et al., 2002), systemic cortisol levels, and concentration of lung surfactant proteins (Emerson et al., 1997) in neonates. Hence, we have provided solid evidence demonstrating a causal link between the elevated amniotic fluid concentrations of alarmins and preterm birth and adverse neonatal outcomes.
In search of the putative mechanisms whereby alarmins in the amniotic cavity can induce preterm labor and birth, we first turned to the human chorioamniotic membranes. The chorioamniotic membranes are the tissues surrounding the amniotic cavity containing the fetus (Bourne, 1962), and their activation is a component of the common pathway of labor (Norwitz et al., 1999, Romero et al., 2006b, Smith, 2007, Romero et al., 2014c). Given that the chorioamniotic membranes are in direct contact with the amniotic fluid, in vitro studies were undertaken to evaluate the pathways that were affected upon exposure of these tissues to HMGB1 (Bredeson et al., 2014, Plazyo et al., 2016, Menon et al., 2016). We showed that HMGB1 induced a pro-inflammatory response in the chorioamniotic membranes by increasing the secretion of IL-6 and mature IL-1β as well as by upregulating the expression of pro-inflammatory transcripts such as NFKB1, ILIB, IL6, TNF, IL1A, and IFNG and the HMGB1 receptors RAGE and TLR2 (Plazyo et al., 2016). Notably, HMGB1 exposure also upregulated the mRNA and protein expression of the inflammasome components nucleotide-binding oligomerization domain (NOD)-like receptor (NLR) family pyrin domain-containing 3 protein (NLRP3), NOD 1 and 2, and absent in melanoma 2 (AIM2) in the chorioamniotic membranes (Plazyo et al., 2016). Similarly, we have demonstrated the release of IL-6 and IL-8, the upregulated mRNA expression of NFKB1, ILIB, IL6, RAGE, TLR2, NOD, and the increased protein expression of NLRP3 in the chorioamniotic membranes exposed to S100A12 (Motomura et al., 2021b). Taken together, our results demonstrate that alarmins act by inducing inflammatory responses in the human chorioamniotic membranes, a process that involves the NLRP3 inflammasome.
Inflammasomes are cytoplasmic high-molecular-weight protein complexes that coordinate inflammatory responses (Martinon et al., 2002, Mariathasan and Monack, 2007, van de Veerdonk et al., 2011, Gross et al., 2011, Henao-Mejia et al., 2012, Franchi and Nunez, 2012, Swanson et al., 2019). The basic structure of an NLR inflammasome consists of an inflammasome sensor molecule, the adaptor protein ASC (an apoptosis-associated speck-like protein), and pro-caspase-1 (Martinon et al., 2002, Mariathasan and Monack, 2007, van de Veerdonk et al., 2011, Gross et al., 2011, Henao-Mejia et al., 2012, Franchi and Nunez, 2012, Swanson et al., 2019). Upon activation, the inflammasome complex induces the autocatalytic cleavage of pro-caspase-1 into its active form, which can cleave pro-IL-1β and pro-IL-18 into their mature and released forms (Black et al., 1989, Kostura et al., 1989, Cerretti et al., 1992, Thornberry et al., 1992, Gu et al., 1997, Ghayur et al., 1997). These cytokines, which play a key role in term and preterm parturition (Romero et al., 1989a, Romero et al., 1990a, Romero et al., 1991a, Romero et al., 1992b, Romero and Tartakovsky, 1992, Pacora et al., 2000b, Girard et al., 2014), and other components of the NLRP3 inflammasome are increased in amniotic fluid of women who underwent spontaneous term or preterm labor, and such an increase is more pronounced in the presence of intra-amniotic inflammation (Romero et al., 1992b, Pacora et al., 2000b, Gotsch et al., 2008). Furthermore, NLRP3 inflammasome components are also expressed by the chorioamniotic membranes of women with spontaneous term or preterm parturition (Gotsch et al., 2008, Lappas, 2014, Gomez-Lopez et al., 2017e, Gomez-Lopez et al., 2017i, Gomez-Lopez et al., 2017h, Bryant et al., 2017, Romero et al., 2018, Gomez-Lopez et al., 2018b, Gomez-Lopez et al., 2019a, Motomura et al., 2021a), indicating a role for the NLRP3 inflammasome in the inflammatory physiologic or pathologic process of labor. In line with these findings, human and animal studies have shown the increased expression of caspase-1 and mature IL-1β in the chorioamniotic membranes exposed to HMGB1 (Plazyo et al., 2016), IL-1α (Motomura et al., 2020a), S100B (Gomez-Lopez et al., 2019c), and S100A12 (Motomura et al., 2021b). It is also worth mentioning that there is evidence of a causal relationship between the intra-amniotic (Baggia et al., 1996, Sadowsky et al., 2000, Sadowsky et al., 2006, Presicce et al., 2015) or intra-uterine (Yoshimura and Hirsch, 2005) administration of IL-1β and preterm labor in animals.
Once the inflammasome is activated, the ASC adaptor protein assembles into a large intracellular complex known as a “speck” (Fernandes-Alnemri et al., 2007, Vajjhala et al., 2012). Such ASC specks can function as alarmins upon their release into the extracellular space (Balci-Peynircioglu et al., 2008, Baroja-Mazo et al., 2014, Franklin et al., 2014), and thus their detection can serve as an indicator of in vivo inflammasome activation (Stutz et al., 2013). Notably, ASC concentrations were increased in amniotic fluid of women with labor at term (Panaitescu et al., 2019), and were higher in women with clinical chorioamnionitis at term and either sterile or microbial-associated intra-amniotic inflammation (Gomez-Lopez et al., 2019e). Importantly, amniotic fluid ASC concentrations were also increased in patients undergoing preterm labor with either sterile intra-amniotic inflammation or intra-amniotic infection (Gomez-Lopez et al., 2018b), providing additional confirmation that both alarmins and microbes can induce inflammasome activation in the amniotic cavity.
The final step of inflammasome activation is pyroptosis, a type of programmed cell death characterized by the release of cytosolic contents through pores formed in the cell membrane by gasdermin D (GSDMD) (Gaidt and Hornung, 2016, Sborgi et al., 2016, Aglietti and Dueber, 2017, Shi et al., 2017), a protein that is cleaved by active caspase-1 and caspase-11 (Kayagaki et al., 2015, Shi et al., 2015). Amniotic fluid concentrations of GSDMD have been utilized as a readout of in vivo pyroptosis in the amniotic cavity of women who underwent spontaneous term labor and those with spontaneous preterm labor with intact membranes (Gomez-Lopez et al., 2019f, Gomez-Lopez et al., 2021b). Specifically, GSDMD was detectable in the amniotic fluid and chorioamniotic membranes of women with preterm labor and sterile intra-amniotic inflammation or intra-amniotic infection; moreover, the presence of GSDMD was associated with elevated protein expression of caspase-1 and IL-1β in the chorioamniotic membranes (Gomez-Lopez et al., 2019f). These results provide evidence that women with sterile intra-amniotic inflammation undergo inflammasome-mediated pyroptosis in the intra-amniotic space.
A central question that arose from the abovementioned studies is: what is the origin of the alarmins in the amniotic cavity? Alarmins can be released during cellular senescence (Huang et al., 2015) and as a result of tissue injury or non-programmed cellular death (Oppenheim and Yang, 2005, Lotze et al., 2007, Bianchi, 2007, Rider et al., 2017). Notably, cellular senescence of the chorioamniotic membranes has been considered a physiological mechanism of parturition at term (Behnia et al., 2015, Polettini et al., 2015, Bonney et al., 2016, Velarde and Menon, 2016), and in particular, decidual senescence has been proposed as an independent mechanism involved in non-infection-related spontaneous preterm labor (Hirota et al., 2010, Hirota et al., 2011, Romero et al., 2014a, Deng et al., 2016, Cha and Aronoff, 2017). Hence, we evaluated whether the chorioamniotic membranes from women undergoing preterm labor without acute histologic chorioamnionitis exhibit cellular senescence (Gomez-Lopez et al., 2017d). Such tissues presented signs of cellular senescence (Gomez-Lopez et al., 2017d), and thus represent a potential source of alarmins in the amniotic cavity of women with sterile intra-amniotic inflammation who underwent preterm labor and birth.
The studies described herein provide evidence for a role of alarmins and the NLRP3 inflammasome in the chorioamniotic membranes in sterile intra-amniotic inflammation. However, whether the intra-amniotic inflammatory response driven by alarmins is distinct from that initiated by invading microbes is a subject of ongoing investigation. We sought to characterize the transcriptomic differences between the chorioamniotic membranes from women who underwent spontaneous preterm labor with intact membranes and sterile intra-amniotic inflammation and those with intra-amniotic infection by utilizing RNA sequencing (Motomura et al., 2021a). Significant transcriptomic differences were found in the chorioamniotic membranes from women with sterile intra-amniotic inflammation compared to the other study groups, and the immune response in this tissue was milder than that induced by microbes. Importantly, such a response included the upregulation of transcripts for the alarmin S100A8 as well as the inflammasome-related molecules PYCARD, AIM2, and NLRC4 (Motomura et al., 2021a). Furthermore, the chorioamniotic membrane transcriptomes from women with intra-amniotic infection clustered separately from those of women with sterile intra-amniotic inflammation or without inflammation, thus further confirming the distinct nature of the immune response taking place during sterile intra-amniotic inflammation (Motomura et al., 2021a).
Collectively, the abovementioned investigations implicate sterile intra-amniotic inflammation as a new clinical entity that can lead to adverse perinatal outcomes. Such a distinct inflammatory response is triggered by alarmins and involves the activation of the NLRP3 inflammasome in the amniotic cavity.
Can we treat sterile intra-amniotic inflammation to prevent preterm birth?
In general, the treatment of sterile inflammatory processes includes the use of anti-inflammatory medications such as non-steroidal anti-inflammatory (Fullerton, 2013) or corticosteroid (Dougherty and Schneebeli, 1955, Coutinho and Chapman, 2011, Busillo and Cidlowski, 2013) drugs. Other treatments that specifically decrease the concentration of alarmins driving sterile inflammation have also been utilized to treat gout (Terkeltaub, 2003, Pacher et al., 2006, Khanna et al., 2012). However, the majority of these drugs utilized to treat sterile inflammation-related pathologies are not approved for use during pregnancy. Therefore, to date there is no approved treatment for sterile intra-amniotic inflammation. Given the abovementioned role of the NLRP3 inflammasome in sterile intra-amniotic inflammation, we have proposed that the inhibition of NLRP3 inflammasome activation could be used to improve perinatal outcomes. MCC950 is a specific inhibitor of the NLRP3 inflammasome that has been utilized in multiple animal models of diseases such as colitis (Perera et al., 2018), traumatic brain injury (Ismael et al., 2018a, Xu et al., 2018a), and stroke (Ismael et al., 2018b), among others (van der Heijden et al., 2017, Zhai et al., 2018). Therefore, we induced sterile intra-amniotic inflammation in mice via the ultrasound-guided intra-amniotic injection of S100B and showed that treatment with MCC950 drastically reduced preterm birth and neonatal mortality (Gomez-Lopez et al., 2019c). As further proof of this mechanism, we induced sterile intra-amniotic inflammation in Nlrp3−/− mice using IL-1α or S100B and showed that preterm birth and neonatal mortality were similarly reduced ((Motomura et al., 2020a) and Gomez-Lopez et al., unpublished data). However, MCC950 is not approved for use during pregnancy and requires additional research to assess its safety in this regard. Given that IL-1β is a product of inflammasome activation, animal models of intra-amniotic or intra-uterine injection of IL-1β have been utilized to test treatments for intra-amniotic inflammation-associated preterm birth (Sadowsky et al., 2000, Sadowsky et al., 2003, Yoshimura and Hirsch, 2005). Pre-treatment with indomethacin, a tocolytic agent that can be used to delay preterm labor, reduced uterine contractions in catheterized macaques intra-amniotically injected with IL-1β (Sadowsky et al., 2000). Yet, in humans, indomethacin is only recommended for use until 32 weeks of gestation, given the increased risk of closure of the ductus arteriosus in fetuses exposed to it, which limits its potential utility for preventing preterm birth (Vermillion et al., 1997, Macones et al., 2001, American College of Obstetricians and Gynecologists, 2016). Similarly, pre-treatment with either dexamethasone or IL-10 reduced the uterine contractility and the intra-amniotic inflammation induced by the intra-amniotic administration of IL-1β in macaques (Sadowsky et al., 2003). Moreover, pre-treatment with a non-competitive IL-1 receptor ligand, Rytvela, prevented intra-uterine IL-1β-induced preterm birth in mice (Nadeau-Vallee et al., 2017a). Yet, further studies are required to address the safety and usefulness of this promising tool during human pregnancy. Furthermore, to date there are no predictive tools for determining women at risk of sterile intra-amniotic inflammation, and thus pre-treatments are difficult to translate into a clinical setting. Therefore, we explored approaches currently approved for use during pregnancy that could be used to treat sterile intra-amniotic inflammation.
The drug development process can take several years from the discovery to their approval (Kaitin, 2010, Hughes et al., 2011). This process is even more complex during pregnancy due to the physiological changes of pregnant women and the imminent risk of fetal damage (Sheffield et al., 2014, Chappell and David, 2016, Ren et al., 2021). Therefore, the repurposing of drugs that are already approved to be utilized during pregnancy is an optimal approach. Under this premise, and given the urgency to find a treatment for sterile intra-amniotic inflammation, we have investigated two medications that are widely utilized during pregnancy: betamethasone (Galaz et al., 2021) and clarithromycin (Galaz et al., submitted). Betamethasone is a corticosteroid that has become the standard of care for women at risk of delivering preterm, as it has been shown to accelerate fetal organ maturation (American College of Obstetricians and Gynecologists, 2017, McGoldrick et al., 2020). As a corticosteroid, betamethasone has been demonstrated to reduce inflammatory processes in multiple clinical settings (Corbett et al., 1993, Corbel et al., 1999, Matsuo et al., 2009, Ly and Amici, 2018, Zhao et al., 2021). Thus, we recently utilized our model of HMGB1-induced sterile intra-amniotic inflammation to demonstrate that treatment with betamethasone prevented preterm birth; yet, it did not reduce neonatal mortality (Galaz et al., 2021). Ongoing research is investigating the mechanisms whereby betamethasone can extend gestational length. On the other hand, clarithromycin is a macrolide that, together with other antibiotics, has emerged as an effective treatment to be used in the context of intra-amniotic infection/inflammation in women with preterm labor with intact membranes (Yoon et al., 2019), PPROM (Lee et al., 2016a, Lee et al., 2016b), and cervical insufficiency (Oh et al., 2019b, Yeo et al., 2021). Clarithromycin exhibits potent anti-inflammatory properties by acting through the NF-κB and AP-1 pathways (Kikuchi et al., 2002, Yamamoto et al., 2017). Moreover, clarithromycin is the macrolide that most efficiently crosses the placenta (Witt et al., 2003). Importantly, a recent study showed that clarithromycin reduced the amniotic fluid concentrations of IL-6 in women with PPROM and sterile intra-amniotic inflammation (Kacerovsky et al., 2020). Therefore, we undertook a series of animal experiments to investigate whether clarithromycin can be utilized to prevent preterm birth and adverse neonatal outcomes in a model of alarmin-induced sterile intra-amniotic inflammation as well as the underlying mechanisms of action (Galaz et al., submitted). We demonstrated that treatment with clarithromycin prevented HMGB1-induced preterm birth by interfering with the common pathway of parturition as evidenced by dysregulated expression of contractility-associated proteins and inflammatory mediators in the intra-uterine tissues. Notably, clarithromycin improved neonatal mortality by dampening inflammation in the placenta as well as in the fetal lung, intestine, liver, and spleen (Galaz et al., submitted). However, HMGB1-induced neonatal mortality was not fully rescued by clarithromycin treatment. It is worth mentioning that women at risk of preterm birth due to intra-amniotic inflammation/infection are treated with both corticosteroids and antibiotics simultaneously (Lee et al., 2016b, Oh et al., 2019b, Yoon et al., 2019, American College of Obstetricians and Gynecologists, 2020). Hence, further research is required to address whether the combination of betamethasone and clarithromycin, or different drugs that could have synergistic effects, can be used to treat the adverse pregnancy and neonatal outcomes caused by sterile intra-amniotic inflammation.
A unique type of intra-amniotic inflammation driven by fetal T-cell activation: a novel mechanism for preterm labor and birth
The clinical definition of intra-amniotic inflammation considers only the elevated amniotic concentrations of established biomarkers such as IL-6 or MMP-8 (Park et al., 2001, Yoon et al., 2001). Yet, inflammation as a general concept comprises systemic or tissue-wide reaction involving a diverse array of cellular and soluble immune mediators at sites of infection or injury (Abbas et al., 2016). In line with this concept, it has been demonstrated that the intra-amniotic inflammatory response involves the active participation of both maternal and fetal immune cells. Indeed, a pioneer study showed that fetal innate immune cells in the human umbilical cord blood are activated in cases of preterm labor leading to preterm birth compared to term deliveries (Berry et al., 1995). Notably, in this study the cord blood was obtained prior to birth (via cordocentesis), and only a fraction of the preterm neonates were exposed to microbes, thereby suggesting that the fetus itself is able to respond or cause the process of labor (Berry et al., 1995). More recently, we showed that the cord blood of preterm neonates has a population of central memory Th1 cells that is absent in term neonates (Frascoli et al., 2018). Such T cells specifically responded to maternal alloantigens and induced myometrial contractility in vitro (Frascoli et al., 2018). Last, the adoptive transfer of activated T cells into the fetal mice induced pregnancy loss, providing in vivo evidence of a role for activated T cells in adverse pregnancy outcomes (Frascoli et al., 2018). Hence, these studies provided insight into the functions of the fetal adaptive immune system and suggested that the fetus could trigger preterm labor. To confirm these findings, we utilized amniotic fluid samples, which allow the study of the in utero fetal immune response (Gomez-Lopez et al., 2018c). Specifically, we have previously demonstrated the presence of multiple immune cell populations in the amniotic fluid that vary throughout gestation in the absence of intra-amniotic inflammation (Gomez-Lopez et al., 2018c). A prior study also noted that fetal innate lymphoid cells (ILCs) are present in amniotic fluid of women in the absence of intra-amniotic inflammation/infection and that such cells expressed a phenotype indicative of intra-epithelial localization (Marquardt et al., 2016), suggesting that they are derived from fetal tissues and can respond to intra-amniotic inflammation. In light of the above evidence, we evaluated amniotic fluid samples and showed that T cells represent a major subset of leukocytes in preterm pregnancies and that such cells were of fetal origin, as indicated by DNA fingerprinting (Gomez-Lopez et al., 2019g). We also found that fetal CD4+ T cells, but none of the other evaluated leukocyte subsets, were increased in amniotic fluid of women with idiopathic preterm labor (i.e., without intra-amniotic inflammation/infection), which represents the largest subset of preterm labor cases (Gomez-Lopez et al., 2019g). Consistent with the abovementioned findings in amniotic fluid ILCs (Marquardt et al., 2016), fetal T cells expressed markers indicative of mucosal residence, confirming that these cells did not originate from the fetal circulation (Gomez-Lopez et al., 2019g). Furthermore, fetal CD4+ T cells from amniotic fluid samples express cytokines typical of T-cell activation (IL-2, IL-4, and IL-13), suggesting a mild and distinct immune response in idiopathic preterm labor. Moreover, in vitro experiments showed that umbilical cord blood T cells from neonates born to mothers who underwent idiopathic preterm labor and birth displayed enhanced responsiveness compared to those from neonates delivered at term (Gomez-Lopez et al., 2019g), providing novel evidence that fetal T-cell activation is associated with idiopathic preterm labor and birth (Gomez-Lopez et al., 2019g). Last, the ultrasound-guided intra-amniotic injection of activated neonatal CD4+ T cells in pregnant mice resulted in preterm delivery, demonstrating that activated fetal T cells are capable of triggering preterm parturition and, therefore, represent a new mechanism of disease for idiopathic preterm birth (Gomez-Lopez et al., 2019g) (Fig. 1C). Yet, further studies are warranted to understand the mechanisms responsible for the premature activation of fetal T cells in the amniotic cavity.
Maternal immune contributions to the etiology of preterm labor and birth
Effector and regulatory T cells
The earliest hypotheses for the seemingly paradoxical nature of pregnancy were prompted by advances in the understanding of immunological tolerance, most notably those pioneered by Peter Medawar (Medawar, 1953, Billington, 2003), the father of reproductive immunology. In one of the most widely recognized works in the field of reproduction, Medawar postulated several reasons why the maternal immune system did not reject the fetus: 1) complete anatomic separation of the mother and fetus; 2) lack of antigenic potential by the fetus; or 3) inertness or unresponsiveness of the maternal immune system (Medawar, 1953). While later investigations have disqualified the first two hypotheses, the third has since been shown to have merit. Rather than complete inertness, it is now clear that pregnancy represents a state of immunological tolerance during which the mother must tolerate the semi-allograft fetus (Chaouat et al., 1979, Bonney and Onyekwuluje, 2003, Zenclussen et al., 2005, Robertson et al., 2009, Kahn and Baltimore, 2010, Shima et al., 2010, Zenclussen et al., 2010, Dimova et al., 2011, Rowe et al., 2012, Samstein et al., 2012, Ramhorst et al., 2012, Shima et al., 2015), with a growing body of evidence suggesting that the fetus also tolerates the mother (Mold et al., 2008, Ivarsson et al., 2013, McGovern et al., 2017, Frascoli et al., 2018). The maternal immune system is constantly exposed to foreign paternal/fetal antigens (Herzenberg et al., 1979, Bianchi et al., 1996, Knight et al., 1998, Germain et al., 2007, Holland et al., 2012, Stenqvist et al., 2013, Gohner et al., 2017, Tong et al., 2018, Arenas-Hernandez et al., 2021), resulting in a series of local (Erlebacher et al., 2007, Bizargity et al., 2009, Samstein et al., 2012, Shima et al., 2015) and systemic (Chaouat et al., 1979, Bonney and Onyekwuluje, 2003, Aluvihare et al., 2004, Zenclussen et al., 2005, Bizargity et al., 2009, Shima et al., 2010, Kahn and Baltimore, 2010, Rowe et al., 2012, Samstein et al., 2012) immunological adaptations that are collectively termed “maternal-fetal tolerance.” Among the mediators that foster and sustain maternal-fetal tolerance, adaptive immune cells such as effector T cells (Vargas et al., 1993, Tilburgs et al., 2006, Taglauer et al., 2008, Wang et al., 2015, Powell et al., 2017, Terzieva et al., 2019, Arenas-Hernandez et al., 2019) and regulatory T cells (Tregs) (Aluvihare et al., 2004, Sasaki et al., 2004, Heikkinen et al., 2004, Sindram-Trujillo et al., 2004, Wang et al., 2015, Tsuda et al., 2018, Salvany-Celades et al., 2019, Gomez-Lopez et al., 2020) perform critical functions. Both cell types participate in mediating the complex immunological scenario of pregnancy in which maternal-fetal tolerance must occur (Bonney and Onyekwuluje, 2003, Aluvihare et al., 2004, Zenclussen et al., 2005, Kahn and Baltimore, 2010, Shima et al., 2010, Samstein et al., 2012, Rowe et al., 2012, Svensson-Arvelund et al., 2015, Shima et al., 2015) but also remain vigilant against external threats (e.g., infection) (Bizargity et al., 2009, Arenas-Hernandez et al., 2016, van Egmond et al., 2016, van der Zwan et al., 2018). Therefore, disruptions or alterations in the activity of these different immune cell subsets is often associated with adverse pregnancy outcomes (Sasaki et al., 2004, Zenclussen et al., 2005, Shima et al., 2010, Jianjun et al., 2010, Yamada et al., 2012, Care et al., 2018, Tsuda et al., 2018, Arenas-Hernandez et al., 2019, Tsuda et al., 2021).
Effector T cells
After encountering their specific antigen, circulating naïve T cells proliferate and differentiate to perform effector functions and eliminate threats (Bachmann et al., 1999). Such T cells are subsequently termed “memory” T cells and can be subdivided into central, effector, and terminally differentiated effector memory T cells based on their functional state and localization to secondary lymphoid organs or the circulation (Sallusto et al., 1999, Geginat et al., 2003, D’Asaro et al., 2006). Moreover, CD4+ and CD8+ T cells can differentiate into one of several effector subsets including T helper type 1 (Th1)/T cytotoxic type 1 (Tc1), Th2/Tc2, Th9/Tc9, and Th17/Tc17 cells according to stimuli provided by the surrounding microenvironment (Brummelman et al., 2018, Saravia et al., 2019). Due to the tightly controlled immunological balance at the maternal-fetal interface, effector T cells were conventionally thought to be absent from this compartment. Indeed, the combination of tissue-specific anti-T-cell mechanisms (Daya et al., 1987, Nancy et al., 2012) together with the presence of Tregs (Aluvihare et al., 2004, Heikkinen et al., 2004, Sasaki et al., 2004, Svensson-Arvelund et al., 2015, Tsuda et al., 2018, Salvany-Celades et al., 2019, Gomez-Lopez et al., 2020) and other homeostatic immune cells such as macrophages (Hunt et al., 1984, Gustafsson et al., 2008, Houser et al., 2011, Svensson et al., 2011, Svensson-Arvelund et al., 2015, Xu et al., 2016, Gomez-Lopez et al., 2021a) makes the decidua a largely unwelcoming site for effector T cells, and as a consequence some of the T cells residing in this compartment display exhausted or senescent phenotypes (Wang et al., 2015, van der Zwan et al., 2018, Slutsky et al., 2019). However, as the end of pregnancy approaches, both human (Gomez-Lopez et al., 2009, Gomez-Lopez et al., 2011, Gomez-Lopez et al., 2013) and animal (Heyborne et al., 1992, Furcron et al., 2015, Arenas-Hernandez et al., 2016, St Louis et al., 2016, Gomez-Lopez et al., 2017a, Arenas-Hernandez et al., 2019, Gomez-Lopez et al., 2020, Stas et al., 2020) studies have demonstrated that T cells migrate to the maternal-fetal interface, where they acquire distinct activated phenotypes (Sindram-Trujillo et al., 2003, Tilburgs et al., 2009a, Tilburgs et al., 2009b, Tilburgs et al., 2010, Arenas-Hernandez et al., 2019). Indeed, decidual T cells show increased expression of activation markers such as CD25, CD38, or CD69 (Abadia-Molina et al., 1996, Sindram-Trujillo et al., 2004, Arenas-Hernandez et al., 2019) as well as labor-associated inflammatory mediators (Joachim et al., 2001, Gomez-Lopez et al., 2013).
Given their participation in normal parturition at term, effector T cells have also been implicated in the premature onset of labor. The invasion of cytotoxic T cells into the decidual tissues, termed chronic histological chorioamnionitis, is frequently observed in pregnancies with complications such as spontaneous preterm birth (Kim et al., 2015). More recently, we showed that effector and activated T cells expressing perforin and granzyme B are enriched at the maternal-fetal interface of women who underwent spontaneous preterm labor and birth (Arenas-Hernandez et al., 2019) (Fig. 2A). Consistent with such human findings, the administration of an anti-CD3 antibody to pregnant mice induced the systemic activation of T cells, resulting in preterm birth (Gomez-Lopez et al., 2016b, Arenas-Hernandez et al., 2019) through inflammatory mechanisms that are distinct from those observed in other well-known preterm birth models (Fidel et al., 1994, Dudley et al., 1996, Nadeem et al., 2016, Gomez-Lopez et al., 2018a, Arenas-Hernandez et al., 2019). Furthermore, we recently demonstrated that a subset of decidual T cells co-express IL-22 and RORγt, and that such cells are enriched in women who underwent preterm labor and birth (Gershater et al., 2022, Accepted). The expression of these two molecules, together with the absence of IL-17A, allowed us to propose that such decidual T cells belong to the Th22 subset (Duhen et al., 2009, Liu et al., 2009, Nograles et al., 2009, Trifari et al., 2009). Our finding that Th22-like cells are present at the maternal-fetal interface of women with preterm labor is in line with a previous study showing that decidual IL-22-expressing T cells are implicated in pregnancy loss (Logiodice et al., 2019). Hence, T cells expressing IL-22 are present at the maternal-fetal interface in early and late pregnancy, where they seem to participate in the mechanisms involved in obstetrical disease.
Fig. 2. Regulatory T cells and homeostatic macrophages: two potential mechanisms of ‘idiopathic’ preterm labor.
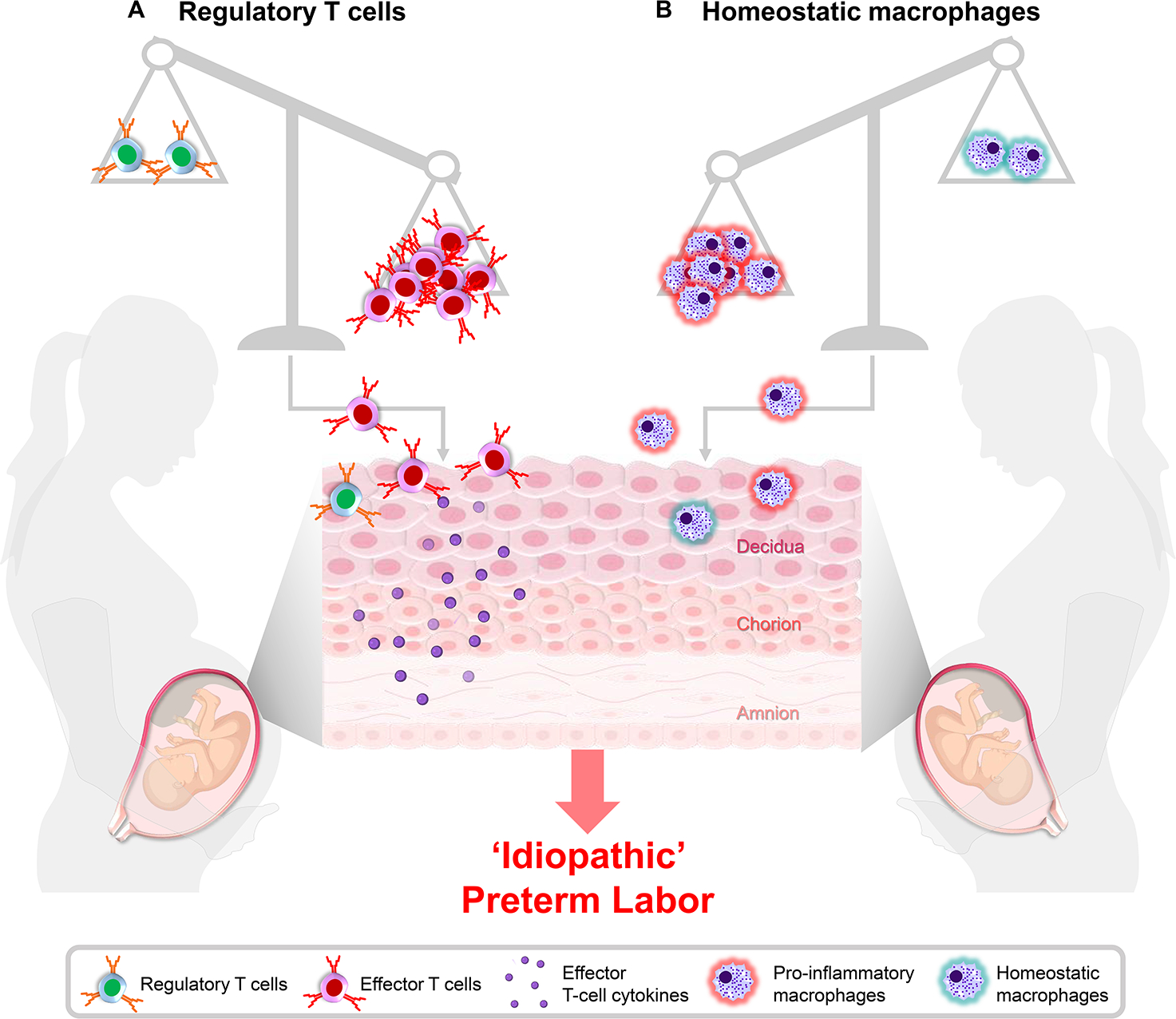
(A) Regulatory T cells serve to suppress effector T cells, thereby preventing a maternal anti-fetal immune response. When the balance between regulatory and effector T cells is disrupted, the activation and infiltration of effector T cells at the maternal-fetal interface can occur, leading to preterm labor and birth. (B) Homeostatic macrophages are important sentinels of the maternal-fetal interface that act as non-antigen specific mediators of maternal-fetal homeostasis and promote fetal development. The inadequate function of these cells can permit the acquisition of a pro-inflammatory phenotype by decidual macrophages as a consequence of preterm labor, emphasizing the importance of homeostatic macrophages in late pregnancy. Importantly, homeostatic macrophages may represent a therapeutic approach to prevent preterm labor and birth.
IL-17-producing T cells (Th17 cells) are a subset of conventionally pro-inflammatory effector T cells that participate in host defense at mucosal/barrier surfaces (Stockinger and Omenetti, 2017). Such cells have been reported as residing at the maternal-fetal interface in early pregnancy (Wu et al., 2014, Lombardelli et al., 2016), and a disruption of the balance between Th17 cells and Tregs is implicated in early pregnancy complications (i.e., spontaneous abortion) (Wang et al., 2010, Nakashima et al., 2010, Lee et al., 2011, Lee et al., 2012, Wu et al., 2016, Zhu et al., 2017). In late pregnancy, studies have suggested that Th17 cells also contribute to the pathophysiology of preeclampsia (Santner-Nanan et al., 2009, Saito, 2010, Fu et al., 2014, Zhang et al., 2018). Notably, such cells are more prevalent in the chorioamniotic membranes from cases of preterm birth with acute chorioamnionitis than in cases without, suggesting that Th17 cells are associated with inflammatory processes at the maternal-fetal interface of women with preterm labor and birth (Ito et al., 2010, Fedorka et al., 2021). In addition to Th17 cells, we have proposed that, under specific conditions, IL-22 is expressed by maternal T cells in the uterine decidua of women with preterm labor and birth (Gershater et al., 2022, Accepted). Under pathological circumstances associated with maternal T-cell activation, IL-22 can cross the maternal-fetal interface and reach the amniotic cavity where it is sensed by the fetal and gestational tissues, causing fetal injury that can lead to neonatal death (Gershater et al., 2022, Accepted).
Regulatory T cells
Regulatory T cells play an important role in mediating immune tolerance in a variety of clinical contexts such as autoimmune disease and transplantation. Consequently, it is unsurprising that Tregs are critical drivers of maternal-fetal tolerance (Chaouat et al., 1979, Bonney and Onyekwuluje, 2003, Aluvihare et al., 2004, Sasaki et al., 2004, Heikkinen et al., 2004, Zenclussen et al., 2005, Robertson et al., 2009, Kahn and Baltimore, 2010, Shima et al., 2010, Samstein et al., 2012, Rowe et al., 2012, Jiang et al., 2014, Svensson-Arvelund et al., 2015, Shima et al., 2015, Bonney, 2016, Tsuda et al., 2018, Salvany-Celades et al., 2019, Gomez-Lopez et al., 2020, Zhang et al., 2021). Conventional Tregs are described as CD4+CD25+Foxp3+ cells that display potent immunosuppressive functions (Sakaguchi et al., 1985, Fontenot et al., 2003). The Treg subset includes thymic/natural Tregs and peripheral/induced Tregs (Yuan and Malek, 2012, Abbas et al., 2013): whereas natural Tregs are considered important in the context of autoimmunity (Jordan et al., 2001, Apostolou et al., 2002, Kumar et al., 2019), peripheral Tregs contribute to mucosal immunity (Haribhai et al., 2011, Josefowicz et al., 2012), which includes maternal-fetal tolerance (Sasaki et al., 2004, Zenclussen et al., 2005, Robertson et al., 2009, Guerin et al., 2009, Zenclussen et al., 2010, Samstein et al., 2012, Rowe et al., 2012, Robertson et al., 2018, Schjenken et al., 2020). Tregs exhibit their immunosuppressive functions through several mechanisms, including secretion of TGF-β (Read et al., 2000, Nakamura et al., 2001) and IL-10 (Asseman et al., 1999, Annacker et al., 2001) that exert paracrine actions on surrounding cells. While a number of studies have established the importance of Tregs during pregnancy establishment and maintenance (Zenclussen et al., 2005, Darrasse-Jèze et al., 2006, Kahn and Baltimore, 2010, Shima et al., 2010, Rowe et al., 2011, Samstein et al., 2012, Rowe et al., 2012, Chen et al., 2013, Diao et al., 2021), recent investigations have also pointed to a role for these adaptive immune cells in late pregnancy (Gomez-Lopez et al., 2020), when most obstetrical diseases manifest (Goldenberg et al., 2008). Tregs are present at the maternal-fetal interface throughout the third trimester prior to labor at term (Tilburgs et al., 2006, Tilburgs et al., 2008, Galazka et al., 2009, Salvany-Celades et al., 2019, Gomez-Lopez et al., 2020), where their proportions were altered compared to women who delivered without labor (Sindram-Trujillo et al., 2004, Galazka et al., 2009). Such observations have been confirmed in mice, where a decidual Treg population was reported (Furcron et al., 2015, Furcron et al., 2016, Gomez-Lopez et al., 2016a). Thus, Tregs represent a component of the immune repertoire at the maternal-fetal interface in late pregnancy.
Alterations in systemic or local Tregs (Sasaki et al., 2007, Prins et al., 2009, Quinn et al., 2011, Nguyen et al., 2017, Tsuda et al., 2018) as well as the Th17/Treg balance (Santner-Nanan et al., 2009, Ding et al., 2019) have been implicated in the pathogenesis of preeclampsia, emphasizing the importance of this adaptive immune subset throughout pregnancy. Yet, little was known of a direct contribution of these cells to spontaneous preterm labor and birth. Accordingly, we recently reported that a subset of women with idiopathic preterm labor and birth displayed a reduction in functional Tregs at the maternal-fetal interface (Gomez-Lopez et al., 2020). This finding correlates with clinical reports showing that women who underwent preterm labor and birth have reduced numbers and function of Tregs in the maternal circulation (Xiong et al., 2010, Schober et al., 2012). Consequently, we utilized a murine model of maternal Treg depletion to demonstrate that the systemic deficiency of Tregs can lead to preterm birth in first and repeat pregnancies; moreover, the loss of such cells induces adverse neonatal outcomes (Gomez-Lopez et al., 2020). The mechanisms whereby the loss of Tregs induces adverse perinatal outcomes involved alterations in the cellular and soluble immune responses in the mother and at the maternal-fetal interface as well as dysregulation of developmental and cellular processes in the placenta (Gomez-Lopez et al., 2020). As a secondary effect, the loss of Tregs also increased maternal susceptibility to preterm birth induced by the administration of LPS (Gomez-Lopez et al., 2020). Importantly, the observed adverse perinatal outcomes were rescued by the adoptive transfer of polyclonal expanded Tregs (Gomez-Lopez et al., 2020). Therefore, we suggested that Tregs play a central role during late pregnancy by modulating systemic and local cellular responses, and that alterations in the proportions or functionality of such cells can promote a pro-inflammatory environment resulting in the development of obstetrical complications such as spontaneous preterm labor in addition to increased susceptibility to infection-induced preterm birth (Fig. 2A).
Potential interventions
The evidence presented above underscores the importance of the balance between Treg immunosuppressive functions throughout pregnancy and the controlled activity of effector T cells for successful maternal-fetal tolerance. Thus, pregnancy interventions that promote such a balance, whether directly or as a secondary effect, are of great interest. We have shown that two commonly utilized treatments, vaginal progesterone and human chorionic gonadotropin (hCG), both display immunomodulatory effects that include an increased proportion of Tregs in the decidual tissues in mice (Furcron et al., 2015, Furcron et al., 2016). Such an effect is likely mediated through the glucocorticoid receptor (GR) by which progesterone can induce conventional T-cell apoptosis, thereby resulting in an increased proportion of Tregs (Engler et al., 2017). We have also shown that progesterone exerts complementary reduction of inflammatory responses at the maternal-fetal interface in a model of systemic maternal T-cell activation-induced preterm birth (Arenas-Hernandez et al., 2019), and thus such a treatment can address both components of effector T cell/Treg imbalance associated with preterm birth.
A number of different cellular and molecular approaches directed at conventional T cells or Tregs have been utilized to treat conditions such as autoimmune disease or graft-versus-host disease (GVHD) (Esensten et al., 2018, Ferreira et al., 2019). While successful in some cases, the potential application of such treatments during pregnancy highlights some challenges. With regard to cellular approaches, multiple clinical trials have shown benefits of administering ex vivo expanded polyclonal Tregs to patients with various diseases (Esensten et al., 2018); yet, animal studies have indicated that infusion of antigen-specific Tregs may be more potent in conditions such as diabetes (Green et al., 2002, Tang et al., 2004, Esensten et al., 2018). In one of these studies, it was demonstrated that adoptively transferring Tregs derived from the pancreatic lymph nodes to recipient mice prevented diabetes development (Green et al., 2002); however, such a strategy would be largely non-applicable in humans, particularly during pregnancy, as obtaining Tregs from lymphoid organs adjacent to the target tissue is not feasible. Nonetheless, we have shown that the adoptive transfer of polyclonal Tregs in mice could successfully prevent the impending adverse pregnancy outcomes induced by a reduction of these cells (Gomez-Lopez et al., 2020). For the majority of diseases, the use of engineered T-cell receptors (TCRs) or chimeric antigen receptor (CAR) Tregs can be applied at least in theory, given that candidate antigens have typically been identified (Ferreira et al., 2019). On the other hand, while it is presumed that paternal/fetal antigens are those driving maternal anti-fetal rejection, the identification of epitopes that could be used to engineer specific Tregs is difficult and remains a major limitation of immunological investigations during pregnancy.
Aside from cellular therapies, strategies to take advantage of the mediators required for and produced by effector and regulatory T cells have also been explored for treating inflammatory diseases. Interleukin-2 is essential for the development and suppressive functions of Tregs, which constitutively express CD25 [a component of the high-affinity IL-2 receptor (Tang, 2015)]. Thus, it was reasoned that low-dose IL-2 treatment could preferentially boost Tregs without initiating systemic immune activation (Tang, 2015). Prior and ongoing clinical trials have shown some success in alleviating symptoms in patients with GVHD or systemic lupus erythematosus (SLE), among others (Ferreira et al., 2019), and it is possible that a combination of expanded Treg infusion together with low-dose IL-2 could ensure that transferred cells are maintained and even expanded. However, normal pregnancy is characterized by low-grade systemic inflammation and immune activation (Sacks et al., 1998, Naccasha et al., 2001, Kraus et al., 2012) that can be exacerbated in the context of disease, and thus substantial research is required to evaluate whether the administration of IL-2 during pregnancy would be detrimental. The corticosteroid prednisone is clinically used in a variety of immune-related diseases and can promote the function and expansion of Tregs (Fu et al., 2019). Indeed, in vitro prednisone treatment increased the proportion of Tregs among isolated first trimester decidual lymphocytes and inhibited Th17 cells (Fu et al., 2017). Moreover, a clinical trial provided evidence that prednisone increased peripheral Tregs and improved pregnancy success in repeated implantation failure (RIF) patients (Huang et al., 2021). Last, vitamin D exerts well-documented anti-inflammatory effects that include induction of IL-10 and inhibition of Th9 and Th17 cells (Palmer et al., 2011, Korf et al., 2012). Indeed, given its modulation of the Treg/Th17 ratio, insufficient vitamin D has been implicated in pregnancy complications such as preeclampsia (Muyayalo et al., 2019, Ribeiro et al., 2021), preterm labor (Zahran et al., 2018), and recurrent pregnancy loss (Ji et al., 2019), which could be improved by supplementation (Chen et al., 2020). Thus, a number of treatment options exist that could be utilized to boost Treg functions and numbers and potentially improve pregnancy outcomes.
An alternative approach to boosting Tregs could be the targeted inhibition of specific mediators released by effector T-cell subsets. An ongoing line of investigation in our laboratory has indicated that the cytokine IL-22, which is primarily produced by the Th22 subset (in the absence of IL-17A) at the maternal-fetal interface, can cross from the maternal side to the amniotic cavity and be sensed by the binding protein expressed by fetal and gestational tissues, causing tissue damage and leading to adverse short- and long-term neonatal outcomes (Gershater et al., 2022, Accepted). Thus, in the context of specific pregnancy complications, therapeutic approaches directed at diminishing the concentrations or activity of specific T cell-associated mediators could also represent a viable approach.
Homeostatic and pro-inflammatory macrophages
The evidence provided above demonstrates that Tregs are an essential component of maternal-fetal tolerance. Yet, we have shown using animal models that the impaired presence of Tregs in late pregnancy only accounts for a small proportion of preterm births (Gomez-Lopez et al., 2020). Therefore, we considered that other immune cells besides Tregs must participate in maintaining maternal-fetal homeostasis. Macrophages constitute the second-largest population of leukocytes (20–30%) at the maternal-fetal interface (Lessin et al., 1988, Williams et al., 2009), and contribute to multiple processes required for pregnancy establishment including embryo implantation (Jaiswal et al., 2012, Care et al., 2013, Schumacher et al., 2018), trophoblast invasion (Tan et al., 2014, Ding et al., 2021), and spiral artery remodeling (Hazan et al., 2010, Lash et al., 2016). In addition, a large number of investigations have explored the phenotypes of decidual macrophages and noted that the majority of this subset display unique functions and phenotypes that most closely resemble anti-inflammatory or M2-like macrophages (Hunt et al., 1984, Gustafsson et al., 2008, Nagamatsu and Schust, 2010, Svensson et al., 2011, Houser et al., 2011, Svensson-Arvelund et al., 2015, Svensson-Arvelund and Ernerudh, 2015, Xu et al., 2016, Chambers et al., 2020). Therefore, we recently proposed that macrophages in the uterine decidua exert anti-inflammatory functions during late pregnancy that promote maternal-fetal homeostasis and thereby sustain gestation prior to term labor (Gomez-Lopez et al., 2021a) (Fig. 2B). We applied a model of pregnant Cd11bDTR/DTR mice wherein CD11b+ macrophages could be depleted upon administration of diphtheria toxin (Duffield et al., 2005, Cailhier et al., 2005), which has been shown to impair fetal development (Yellon et al., 2019) and result in pregnancy loss in early gestation (Robertson et al., 2008, Care et al., 2013). Using this approach, we demonstrated that macrophage depletion in late pregnancy resulted in preterm birth in the majority of dams (75%) (Gomez-Lopez et al., 2021a). More importantly, the majority of pups born to macrophage-depleted dams did not survive past the first 24 hours of life, suggesting that the loss of maternal macrophages may have adverse effects beyond intra-uterine life (Gomez-Lopez et al., 2021a). A causal link between maternal macrophage depletion and adverse pregnancy outcomes was mechanistically demonstrated by adoptively transferring bone marrow-derived macrophages (BMDMs) into macrophage-depleted dams, which successfully reduced the preterm birth rate but did not improve neonatal survival (Gomez-Lopez et al., 2021a). We reasoned that, given the homeostatic phenotypes attributed to macrophages at the maternal-fetal interface (Hunt et al., 1984, Gustafsson et al., 2008, Nagamatsu and Schust, 2010, Svensson et al., 2011, Houser et al., 2011, Svensson-Arvelund et al., 2015, Svensson-Arvelund and Ernerudh, 2015, Xu et al., 2016, Chambers et al., 2020), the in vitro polarization of BMDMs prior to their adoptive transfer may improve their effectiveness. We utilized our translationally-relevant model of intra-amniotic inflammation induced by the ultrasound-guided intra-amniotic injection of LPS to demonstrate that the administration of M2-polarized macrophages not only drastically reduced preterm births but decreased neonatal mortality, thereby demonstrating the importance of homeostatic macrophages for pregnancy maintenance as well as neonatal survival (Gomez-Lopez et al., 2021a). Molecular investigation revealed that the adoptive transfer of M2-polarized macrophages reduced inflammation in the maternal circulation and amniotic fluid as well as downregulated inflammatory gene expression in fetal tissues such as the brain and lung (Gomez-Lopez et al., 2021a), providing mechanistic insight into the protective effects of these homeostatic cells for the mother and fetus.
Although the majority of macrophages at the maternal-fetal interface display homeostatic or anti-inflammatory phenotypes, the presence of pro-inflammatory macrophages has also been documented in the context of physiological (Repnik et al., 2008, Xu et al., 2016, Chambers et al., 2020) or pathological (Hamilton et al., 2012, Xu et al., 2016) labor. During normal labor at term, an influx of myeloid cells such as macrophages occurs at the maternal-fetal interface (Young et al., 2002, Osman et al., 2003, Shynlova et al., 2013, Xu et al., 2016, Sharps et al., 2020) as well as in the uterine (Thomson et al., 1999, Mackler et al., 1999, Young et al., 2002, Osman et al., 2003, Gomez-Lopez et al., 2010, Wahid et al., 2015) and cervical (Bokström et al., 1997, Sakamoto et al., 2005, Yellon et al., 2008, Timmons et al., 2009, Payne et al., 2012, Myers, 2012) tissues as part of the inflammatory response required for parturition to occur, although the requirement of macrophages for cervical ripening is still controversial (Word et al., 2005, Timmons and Mahendroo, 2006, Gonzalez et al., 2009, Gonzalez et al., 2011a). Indeed, we have documented that women who undergo labor at term have increased proportions of pro-inflammatory M1-like macrophages in the decidua compared to term deliveries without labor (Xu et al., 2016). Consistently, women who undergo preterm labor display greater numbers of decidual macrophages compared to women delivering at term without labor (Hamilton et al., 2012, Xu et al., 2016), driven by a rise in macrophages presenting an M1-like phenotype (Xu et al., 2016, Gomez-Lopez et al., 2021a). Moreover, macrophage numbers are increased in human uterine tissues during preterm labor (Xu et al., 2016, Gomez-Lopez et al., 2021a) as well as in the cervix of mice undergoing LPS-induced preterm birth (Timmons et al., 2009, Gonzalez et al., 2011b, Gomez-Lopez et al., 2020). Together, the above studies demonstrate that macrophages in the uterine decidua are polarized towards a pro-inflammatory phenotype as part of the processes of term and preterm labor (Fig. 2B).
Potential macrophage-related interventions for preterm labor
Currently, multiple anti-inflammatory therapies have been tested to prevent preterm labor caused by inflammation (Wakabayashi et al., 2013, Sykes et al., 2014, Xu et al., 2016, Chin et al., 2016, Kadam et al., 2017, Garcia-Flores et al., 2018, Gomez-Lopez et al., 2019c). Given the demonstrated importance of macrophage polarization for pregnancy outcomes, we showed that rosiglitazone can be used to treat LPS-induced preterm birth by activating the PPARγ pathway, thereby reducing decidual macrophage-mediated inflammation (Xu et al., 2016). However, we reasoned that the adoptive transfer of M2-polarized macrophages may represent a more direct approach. The in vitro generation of M2-polarized macrophages have been studied and applied as therapeutic treatments in cancer (Andreesen et al., 1998), diabetes (Parsa et al., 2012), neuropathic pain (Pannell et al., 2016), and other inflammatory diseases (Wang et al., 2007, Weber et al., 2007, Hunter et al., 2010), with minimal adverse effects being reported (Andreesen et al., 1998). Investigations in which the transferred M2 macrophages were tracked suggested that these cells will preferentially target inflamed tissues, such as the pancreas in diabetic mice (Parsa et al., 2012). Moreover, the intrathecal administration of M2 macrophages yielded a significant neurological improvement in human patients undergoing treatment for stroke (Chernykh et al., 2016). Such clinical and animal studies, together with current knowledge of the important role for macrophages throughout gestation, hinted that this strategy could be useful during pregnancy. Accordingly, we recently demonstrated that adoptively transferring macrophages polarized to an M2-like phenotype in vitro prevented preterm birth and, more importantly, improved neonatal outcomes in a model of LPS-induced intra-amniotic inflammation (Gomez-Lopez et al., 2021a). Such outcomes point to the potential viability of M2-polarized macrophages as a treatment option for women at risk for delivering preterm. It is worth mentioning that such a treatment would not be suitable for all cases of spontaneous preterm labor. Indeed, as described above, administering an antibiotic regimen is considered the optimal approach for treating spontaneous preterm labor associated with intra-amniotic infection (Yudin et al., 2009, Lee et al., 2016b, Yoon et al., 2019). Thus, the adoptive transfer of M2-polarized macrophages could represent a treatment option for sterile intra-amniotic inflammation; yet, further research is required to demonstrate the feasibility of such an approach.
Conclusion
In summary, here we have provided an overview of the immune mechanisms implicated in intra-amniotic inflammation, the best-characterized cause of preterm labor and birth. While the inflammatory processes driven by microbes (infection) or alarmins (sterile) have some overlap in their participating cellular and molecular processes, the distinct natures of these two conditions necessitate the implementation of specific approaches to prevent adverse pregnancy and neonatal outcomes (Fig. 1A&B). Intra-amniotic infection can be treated using the right antibiotics, whereas sterile intra-amniotic inflammation could be potentially treated using a combination of anti-inflammatory drugs (e.g., betamethasone, inflammasome inhibitors, etc.). Importantly, we have also described the current evidence supporting fetal T-cell activation as a newly described trigger for preterm labor and birth in a subset of cases (Fig. 1C). These findings represent an exciting area of future research focused on further elucidating the fetal immune mechanisms implicated in such a response. Recently, we also provided evidence of two potential immune mechanisms responsible for a subset of preterm births formerly considered to be idiopathic. In the first, we have shown that the impairment of maternal Tregs leads to preterm birth, likely due to the loss of immunosuppressive activity resulting in unleashed effector T-cell responses (Fig. 2A). Second, we have demonstrated the importance of homeostatic macrophages for maintaining pregnancy and promoting fetal development as well as the effectiveness of the adoptive transfer of M2-polarized macrophages for preventing inflammation-induced preterm birth (Fig. 2B). Collectively, in this review, we have discussed established and novel immune mechanisms responsible for preterm birth and highlighted potential targets for novel strategies aimed at preventing the multi-etiological syndrome of preterm labor.
KEY POINTS.
Intra-amniotic inflammation, driven by microbes ascending from the lower genital tract (intra-amniotic infection) or by alarmins (i.e. danger signals) released upon cellular stress or damage (sterile intra-amniotic inflammation), is the best-established causal link to preterm labor and birth. While intra-amniotic infection can be treated using the correct antibiotic regimen, sterile intra-amniotic inflammation currently lacks approved treatment. Yet, ongoing investigations have identified several promising approaches that could be used to treat women with this clinical condition.
Pregnancy involves unique mechanisms of maternal-fetal tolerance. Recent investigations have expanded this concept by showing that fetal T cells respond to maternal antigens and are implicated in premature onset of labor leading to preterm birth.
Maternal-fetal tolerance includes several mechanisms that regulate maternal T cells, including the induction of an exhausted or senescent state, silencing of T-cell chemoattractant expression at the maternal-fetal interface, and the expansion of regulatory T cells (Tregs). A breakdown of such maternal-fetal tolerance, either through the aberrant activation of effector T cells or the impaired functionality of Tregs, can result in preterm labor and birth.
Macrophages represent a critical cellular component of the maternal-fetal interface. Such cells exert anti-inflammatory functions to promote maternal-fetal homeostasis until term, when they acquire a pro-inflammatory phenotype to promote labor. The importance of macrophages was further established by showing that the impairment of these cells during pregnancy results in preterm labor and birth. Importantly, homeostatic macrophages may represent a viable cellular approach to treat sterile intra-amniotic inflammation.
Funding
This research was supported by the Perinatology Research Branch, Division of Obstetrics and Maternal-Fetal Medicine, Division of Intramural Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, U.S. Department of Health and Human Services (NICHD/NIH/DHHS) under Contract No. HHSN275201300006C. This research was also supported by the Wayne State University Perinatal Initiative in Maternal, Perinatal and Child Health. R.R. has contributed to this work as part of his official duties as an employee of the United States Federal Government. The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Footnotes
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of this review.
REFERENCES
- Abadia-Molina AC, Ruiz C, Montes MJ, King A, Loke YW Olivares EG 1996. Immune phenotype and cytotoxic activity of lymphocytes from human term decidua against trophoblast. J Reprod Immunol, 31, 109–23. [DOI] [PubMed] [Google Scholar]
- Abassi Z, Knaney Y, Karram T Heyman SN 2020. The Lung Macrophage in SARS-CoV-2 Infection: A Friend or a Foe? Front Immunol, 11, 1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbas AK, Benoist C, Bluestone JA, Campbell DJ, Ghosh S, Hori S, Jiang S, Kuchroo VK, Mathis D, Roncarolo MG, et al. 2013. Regulatory T cells: recommendations to simplify the nomenclature. Nat Immunol, 14, 307–8. [DOI] [PubMed] [Google Scholar]
- Abbas AK, Lichtman AH, Pillai S, Baker DL Baker A 2016. Basic immunology : functions and disorders of the immune system.
- Abitbol CL Rodriguez MM 2012. The long-term renal and cardiovascular consequences of prematurity. Nat Rev Nephrol, 8, 265–74. [DOI] [PubMed] [Google Scholar]
- Aglietti RA Dueber EC 2017. Recent Insights into the Molecular Mechanisms Underlying Pyroptosis and Gasdermin Family Functions. Trends Immunol, 38, 261–271. [DOI] [PubMed] [Google Scholar]
- Aluvihare VR, Kallikourdis M Betz AG 2004. Regulatory T cells mediate maternal tolerance to the fetus. Nat Immunol, 5, 266–71. [DOI] [PubMed] [Google Scholar]
- American College of Obstetricians and Gynecologists 2016. Practice Bulletin No. 171: Management of Preterm Labor. Obstet Gynecol, 128, e155–64. [DOI] [PubMed] [Google Scholar]
- American College of Obstetricians and Gynecologists 2017. Committee Opinion No. 713: Antenatal Corticosteroid Therapy for Fetal Maturation. Obstet Gynecol, 130, e102–e109. [DOI] [PubMed] [Google Scholar]
- American College of Obstetricians and Gynecologists 2020. Prelabor Rupture of Membranes: ACOG Practice Bulletin, Number 217. Obstet Gynecol, 135, e80–e97. [DOI] [PubMed] [Google Scholar]
- Amon I 1985. Placental transfer of metronidazole. J Perinat Med, 13, 97–8. [DOI] [PubMed] [Google Scholar]
- Andersen HF, Nugent CE, Wanty SD Hayashi RH 1990. Prediction of risk for preterm delivery by ultrasonographic measurement of cervical length. Am J Obstet Gynecol, 163, 859–67. [DOI] [PubMed] [Google Scholar]
- Andreesen R, Hennemann B Krause SW 1998. Adoptive immunotherapy of cancer using monocyte-derived macrophages: rationale, current status, and perspectives. J Leukoc Biol, 64, 419–26. [DOI] [PubMed] [Google Scholar]
- Annacker O, Pimenta-Araujo R, Burlen-Defranoux O, Barbosa TC, Cumano A Bandeira A 2001. CD25+ CD4+ T cells regulate the expansion of peripheral CD4 T cells through the production of IL-10. J Immunol, 166, 3008–18. [DOI] [PubMed] [Google Scholar]
- Apostolou I, Sarukhan A, Klein L Von Boehmer H 2002. Origin of regulatory T cells with known specificity for antigen. Nat Immunol, 3, 756–63. [DOI] [PubMed] [Google Scholar]
- Arayici S, Kadioglu Simsek G, Oncel MY, Eras Z, Canpolat FE, Oguz SS, Uras N, Zergeroglu S Dilmen U 2014. The effect of histological chorioamnionitis on the short-term outcome of preterm infants </=32 weeks: a single-center study. J Matern Fetal Neonatal Med, 27, 1129–33. [DOI] [PubMed] [Google Scholar]
- Arck PC Hecher K 2013. Fetomaternal immune cross-talk and its consequences for maternal and offspring’s health. Nat Med, 19, 548–56. [DOI] [PubMed] [Google Scholar]
- Arenas-Hernandez M, Romero R, Gershater M, Tao L, Xu Y, Garcia-Flores V, Pusod E, Miller D, Galaz J, Motomura K, et al. 2021. Specific innate immune cells uptake fetal antigen and display homeostatic phenotypes in the maternal circulation. J Leukoc Biol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arenas-Hernandez M, Romero R, St Louis D, Hassan SS, Kaye EB Gomez-Lopez, N. 2016. An imbalance between innate and adaptive immune cells at the maternal-fetal interface occurs prior to endotoxin-induced preterm birth. Cell Mol Immunol, 13, 462–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arenas-Hernandez M, Romero R, Xu Y, Panaitescu B, Garcia-Flores V, Miller D, Ahn H, Done B, Hassan SS, Hsu CD, et al. 2019. Effector and Activated T Cells Induce Preterm Labor and Birth That Is Prevented by Treatment with Progesterone. J Immunol, 202, 2585–2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asea A, Kraeft SK, Kurt-Jones EA, Stevenson MA, Chen LB, Finberg RW, Koo GC Calderwood SK 2000. HSP70 stimulates cytokine production through a CD14-dependant pathway, demonstrating its dual role as a chaperone and cytokine. Nat Med, 6, 435–42. [DOI] [PubMed] [Google Scholar]
- Asseman C, Mauze S, Leach MW, Coffman RL Powrie F 1999. An essential role for interleukin 10 in the function of regulatory T cells that inhibit intestinal inflammation. J Exp Med, 190, 995–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann MF, Barner M, Viola A Kopf M 1999. Distinct kinetics of cytokine production and cytolysis in effector and memory T cells after viral infection. Eur J Immunol, 29, 291–9. [DOI] [PubMed] [Google Scholar]
- Baggia S, Gravett MG, Witkin SS, Haluska GJ Novy MJ 1996. Interleukin-1 beta intra-amniotic infusion induces tumor necrosis factor-alpha, prostaglandin production, and preterm contractions in pregnant rhesus monkeys. J Soc Gynecol Investig, 3, 121–6. [DOI] [PubMed] [Google Scholar]
- Balci-Peynircioglu B, Waite AL, Schaner P, Taskiran ZE, Richards N, Orhan D, Gucer S, Ozen S, Gumucio D Yilmaz E 2008. Expression of ASC in renal tissues of familial mediterranean fever patients with amyloidosis: postulating a role for ASC in AA type amyloid deposition. Exp Biol Med (Maywood), 233, 1324–33. [DOI] [PubMed] [Google Scholar]
- Baroja-Mazo A, Martin-Sanchez F, Gomez AI, Martinez CM, Amores-Iniesta J, Compan V, Barbera-Cremades M, Yague J, Ruiz-Ortiz E, Anton J, et al. 2014. The NLRP3 inflammasome is released as a particulate danger signal that amplifies the inflammatory response. Nat Immunol, 15, 738–48. [DOI] [PubMed] [Google Scholar]
- Barros FC, Papageorghiou AT, Victora CG, Noble JA, Pang R, Iams J, Cheikh Ismail L, Goldenberg RL, Lambert A, Kramer MS, et al. 2015. The distribution of clinical phenotypes of preterm birth syndrome: implications for prevention. JAMA Pediatr, 169, 220–9. [DOI] [PubMed] [Google Scholar]
- Bartmann C, Segerer SE, Rieger L, Kapp M, Sütterlin M Kämmerer U 2014. Quantification of the predominant immune cell populations in decidua throughout human pregnancy. Am J Reprod Immunol, 71, 109–19. [DOI] [PubMed] [Google Scholar]
- Bastek JA, Gomez LM Elovitz MA 2011. The role of inflammation and infection in preterm birth. Clin Perinatol, 38, 385–406. [DOI] [PubMed] [Google Scholar]
- Baumbusch MA, Buhimschi CS, Oliver EA, Zhao G, Thung S, Rood K Buhimschi IA 2016. High Mobility Group-Box 1 (HMGB1) levels are increased in amniotic fluid of women with intra-amniotic inflammation-determined preterm birth, and the source may be the damaged fetal membranes. Cytokine, 81, 82–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bearfield C, Davenport ES, Sivapathasundaram V Allaker RP 2002. Possible association between amniotic fluid micro-organism infection and microflora in the mouth. BJOG, 109, 527–33. [DOI] [PubMed] [Google Scholar]
- Behnia F, Taylor BD, Woodson M, Kacerovsky M, Hawkins H, Fortunato SJ, Saade GR Menon R 2015. Chorioamniotic membrane senescence: a signal for parturition? Am J Obstet Gynecol, 213, 359 e1–16. [DOI] [PubMed] [Google Scholar]
- Berger A, Witt A, Haiden N, Kretzer V, Heinze G Pollak, A. 2004. Amniotic cavity cultures, blood cultures, and surface swabs in preterm infants--useful tools for the management of early-onset sepsis? J Perinat Med, 32, 446–52. [DOI] [PubMed] [Google Scholar]
- Berghella V, Daly SF, Tolosa JE, Divito MM, Chalmers R, Garg N, Bhullar A Wapner RJ 1999. Prediction of preterm delivery with transvaginal ultrasonography of the cervix in patients with high-risk pregnancies: does cerclage prevent prematurity? Am J Obstet Gynecol, 181, 809–15. [DOI] [PubMed] [Google Scholar]
- Berry SM, Romero R, Gomez R, Puder KS, Ghezzi F, Cotton DB Bianchi DW 1995. Premature parturition is characterized by in utero activation of the fetal immune system. Am J Obstet Gynecol, 173, 1315–20. [DOI] [PubMed] [Google Scholar]
- Bhatti G, Romero R, Rice GE, Fitzgerald W, Pacora P, Gomez-Lopez N, Kavdia M, Tarca AL Margolis L 2020. Compartmentalized profiling of amniotic fluid cytokines in women with preterm labor. PLoS One, 15, e0227881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi DW, Zickwolf GK, Weil GJ, Sylvester S Demaria MA 1996. Male fetal progenitor cells persist in maternal blood for as long as 27 years postpartum. Proc Natl Acad Sci U S A, 93, 705–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi ME 2007. DAMPs, PAMPs and alarmins: all we need to know about danger. J Leukoc Biol, 81, 1–5. [DOI] [PubMed] [Google Scholar]
- Billington WD 2003. The immunological problem of pregnancy: 50 years with the hope of progress. A tribute to Peter Medawar. J Reprod Immunol, 60, 1–11. [DOI] [PubMed] [Google Scholar]
- Bizargity P, Del Rio R, Phillippe M, Teuscher C Bonney EA 2009. Resistance to lipopolysaccharide-induced preterm delivery mediated by regulatory T cell function in mice. Biol Reprod, 80, 874–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black RA, Kronheim SR, Merriam JE, March CJ Hopp TP 1989. A pre-aspartate-specific protease from human leukocytes that cleaves pro-interleukin-1 beta. J Biol Chem, 264, 5323–6. [PubMed] [Google Scholar]
- Blencowe H, Lee AC, Cousens S, Bahalim A, Narwal R, Zhong N, Chou D, Say L, Modi N, Katz J, et al. 2013. Preterm birth-associated neurodevelopmental impairment estimates at regional and global levels for 2010. Pediatr Res, 74 Suppl 1, 17–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bokström H, Brännström M, Alexandersson M Norström A 1997. Leukocyte subpopulations in the human uterine cervical stroma at early and term pregnancy. Hum Reprod, 12, 586–90. [DOI] [PubMed] [Google Scholar]
- Boldenow E, Gendrin C, Ngo L, Bierle C, Vornhagen J, Coleman M, Merillat S, Armistead B, Whidbey C, Alishetti V, et al. 2016. Group B Streptococcus circumvents neutrophils and neutrophil extracellular traps during amniotic cavity invasion and preterm labor. Sci Immunol, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonney EA 2016. Immune Regulation in Pregnancy: A Matter of Perspective? Obstet Gynecol Clin North Am, 43, 679–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonney EA, Krebs K, Saade G, Kechichian T, Trivedi J, Huaizhi Y Menon R 2016. Differential senescence in feto-maternal tissues during mouse pregnancy. Placenta, 43, 26–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonney EA Onyekwuluje J 2003. The H-Y response in mid-gestation and long after delivery in mice primed before pregnancy. Immunol Invest, 32, 71–81. [DOI] [PubMed] [Google Scholar]
- Bourne G 1962. The foetal membranes. A review of the anatomy of normal amnion and chorion and some aspects of their function. Postgrad Med J, 38, 193–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bredeson S, Papaconstantinou J, Deford JH, Kechichian T, Syed TA, Saade GR Menon R 2014. HMGB1 promotes a p38MAPK associated non-infectious inflammatory response pathway in human fetal membranes. PLoS One, 9, e113799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, Weinrauch Y Zychlinsky A 2004. Neutrophil extracellular traps kill bacteria. Science, 303, 1532–5. [DOI] [PubMed] [Google Scholar]
- Brinkmann V Zychlinsky A 2012. Neutrophil extracellular traps: is immunity the second function of chromatin? J Cell Biol, 198, 773–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brook I, Wexler HM Goldstein EJ 2013. Antianaerobic antimicrobials: spectrum and susceptibility testing. Clin Microbiol Rev, 26, 526–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brummelman J, Pilipow K Lugli E 2018. The Single-Cell Phenotypic Identity of Human CD8(+) and CD4(+) T Cells. Int Rev Cell Mol Biol, 341, 63–124. [DOI] [PubMed] [Google Scholar]
- Bryant AH, Bevan RJ, Spencer-Harty S, Scott LM, Jones RH Thornton CA 2017. Expression and function of NOD-like receptors by human term gestation-associated tissues. Placenta, 58, 25–32. [DOI] [PubMed] [Google Scholar]
- Bujold E, Morency AM, Rallu F, Ferland S, Tetu A, Duperron L, Audibert F Laferriere C 2008. Bacteriology of amniotic fluid in women with suspected cervical insufficiency. J Obstet Gynaecol Can, 30, 882–887. [DOI] [PubMed] [Google Scholar]
- Burn GL, Foti A, Marsman G, Patel DF Zychlinsky A 2021. The Neutrophil. Immunity, 54, 1377–1391. [DOI] [PubMed] [Google Scholar]
- Burnham P, Gomez-Lopez N, Heyang M, Cheng AP, Lenz JS, Dadhania DM, Lee JR, Suthanthiran M, Romero R De Vlaminck I 2020. Separating the signal from the noise in metagenomic cell-free DNA sequencing. Microbiome, 8, 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busillo JM Cidlowski JA 2013. The five Rs of glucocorticoid action during inflammation: ready, reinforce, repress, resolve, and restore. Trends Endocrinol Metab, 24, 109–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busso N So A 2010. Mechanisms of inflammation in gout. Arthritis Res Ther, 12, 206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cailhier JF, Partolina M, Vuthoori S, Wu S, Ko K, Watson S, Savill J, Hughes J Lang RA 2005. Conditional macrophage ablation demonstrates that resident macrophages initiate acute peritoneal inflammation. J Immunol, 174, 2336–42. [DOI] [PubMed] [Google Scholar]
- Campisi J D’adda Di Fagagna F 2007. Cellular senescence: when bad things happen to good cells. Nat Rev Mol Cell Biol, 8, 729–40. [DOI] [PubMed] [Google Scholar]
- Cappelletti M, Presicce P, Feiyang M, Senthamaraikannan P, Miller LA, Pellegrini M, Sim MS, Jobe AH, Divanovic S, Way SS, et al. 2021. The induction of preterm labor in rhesus macaques is determined by the strength of immune response to intrauterine infection. PLoS Biol, 19, e3001385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Care AS, Bourque SL, Morton JS, Hjartarson EP, Robertson SA Davidge ST 2018. Reduction in Regulatory T Cells in Early Pregnancy Causes Uterine Artery Dysfunction in Mice. Hypertension, 72, 177–187. [DOI] [PubMed] [Google Scholar]
- Care AS, Diener KR, Jasper MJ, Brown HM, Ingman WV Robertson SA 2013. Macrophages regulate corpus luteum development during embryo implantation in mice. J Clin Invest, 123, 3472–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmody JB Charlton JR 2013. Short-term gestation, long-term risk: prematurity and chronic kidney disease. Pediatrics, 131, 1168–79. [DOI] [PubMed] [Google Scholar]
- Cerretti DP, Kozlosky CJ, Mosley B, Nelson N, Van Ness K, Greenstreet TA, March CJ, Kronheim SR, Druck T, Cannizzaro LA, et al. 1992. Molecular cloning of the interleukin-1 beta converting enzyme. Science, 256, 97–100. [DOI] [PubMed] [Google Scholar]
- Cha JM Aronoff DM 2017. A role for cellular senescence in birth timing. Cell Cycle, 16, 2023–2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaiworapongsa T, Erez O, Kusanovic JP, Vaisbuch E, Mazaki-Tovi S, Gotsch F, Than NG, Mittal P, Kim YM, Camacho N, et al. 2008. Amniotic fluid heat shock protein 70 concentration in histologic chorioamnionitis, term and preterm parturition. J Matern Fetal Neonatal Med, 21, 449–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalupska M, Kacerovsky M, Stranik J, Gregor M, Maly J, Jacobsson B Musilova I 2021. Intra-Amniotic Infection and Sterile Intra-Amniotic Inflammation in Cervical Insufficiency with Prolapsed Fetal Membranes: Clinical Implications. Fetal Diagn Ther, 48, 58–69. [DOI] [PubMed] [Google Scholar]
- Chambers M, Rees A, Cronin JG, Nair M, Jones N Thornton CA 2020. Macrophage Plasticity in Reproduction and Environmental Influences on Their Function. Front Immunol, 11, 607328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaouat G, Voisin GA, Escalier D Robert P 1979. Facilitation reaction (enhancing antibodies and suppressor cells) and rejection reaction (sensitized cells) from the mother to the paternal antigens of the conceptus. Clin Exp Immunol, 35, 13–24. [PMC free article] [PubMed] [Google Scholar]
- Chappell LC David AL 2016. Improving the Pipeline for Developing and Testing Pharmacological Treatments in Pregnancy. PLoS Med, 13, e1002161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chawanpaiboon S, Vogel JP, Moller AB, Lumbiganon P, Petzold M, Hogan D, Landoulsi S, Jampathong N, Kongwattanakul K, Laopaiboon M, et al. 2019. Global, regional, and national estimates of levels of preterm birth in 2014: a systematic review and modelling analysis. Lancet Glob Health, 7, e37–e46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T, Darrasse-Jèze G, Bergot AS, Courau T, Churlaud G, Valdivia K, Strominger JL, Ruocco MG, Chaouat G Klatzmann D 2013. Self-specific memory regulatory T cells protect embryos at implantation in mice. J Immunol, 191, 2273–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Diao L, Lian R, Qi L, Yu S, Liu S, Lin S, Xue Z Zeng Y 2020. Potential impact of maternal vitamin D status on peripheral blood and endometrium cellular immunity in women with recurrent implantation failure. Am J Reprod Immunol, 84, e13243. [DOI] [PubMed] [Google Scholar]
- Chernykh ER, Shevela EY, Starostina NM, Morozov SA, Davydova MN, Menyaeva EV Ostanin AA 2016. Safety and Therapeutic Potential of M2 Macrophages in Stroke Treatment. Cell Transplant, 25, 1461–71. [DOI] [PubMed] [Google Scholar]
- Chin PY, Dorian CL, Hutchinson MR, Olson DM, Rice KC, Moldenhauer LM Robertson SA 2016. Novel Toll-like receptor-4 antagonist (+)-naloxone protects mice from inflammation-induced preterm birth. Sci Rep, 6, 36112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobo T, Kacerovsky M Jacobsson B 2014. Amniotic fluid infection, inflammation, and colonization in preterm labor with intact membranes. Am J Obstet Gynecol, 211, 708. [DOI] [PubMed] [Google Scholar]
- Combs CA, Gravett M, Garite TJ, Hickok DE, Lapidus J, Porreco R, Rael J, Grove T, Morgan TK, Clewell W, et al. 2014. Amniotic fluid infection, inflammation, and colonization in preterm labor with intact membranes. Am J Obstet Gynecol, 210, 125 e1–125 e15. [DOI] [PubMed] [Google Scholar]
- Corbel M, Lagente V, Theret N, Germain N, Clement B Boichot E 1999. Comparative effects of betamethasone, cyclosporin and nedocromil sodium in acute pulmonary inflammation and metalloproteinase activities in bronchoalveolar lavage fluid from mice exposed to lipopolysaccharide. Pulm Pharmacol Ther, 12, 165–71. [DOI] [PubMed] [Google Scholar]
- Corbett MC, Hingorani M, Boulton JE Shilling JS 1993. Subconjunctival betamethasone is of benefit after cataract surgery. Eye (Lond), 7 (Pt 6), 744–8. [DOI] [PubMed] [Google Scholar]
- Coutinho AE Chapman KE 2011. The anti-inflammatory and immunosuppressive effects of glucocorticoids, recent developments and mechanistic insights. Mol Cell Endocrinol, 335, 2–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croy BA, Gambel P, Rossant J Wegmann TG 1985. Characterization of murine decidual natural killer (NK) cells and their relevance to the success of pregnancy. Cell Immunol, 93, 315–26. [DOI] [PubMed] [Google Scholar]
- D’asaro M, Dieli F, Caccamo N, Musso M, Porretto F Salerno A 2006. Increase of CCR7- CD45RA+ CD8 T cells (T(EMRA)) in chronic graft-versus-host disease. Leukemia, 20, 545–7. [DOI] [PubMed] [Google Scholar]
- Darrasse-Jèze G, Klatzmann D, Charlotte F, Salomon BL Cohen JL 2006. CD4+CD25+ regulatory/suppressor T cells prevent allogeneic fetus rejection in mice. Immunol Lett, 102, 106–9. [DOI] [PubMed] [Google Scholar]
- Daya S, Rosenthal KL Clark DA 1987. Immunosuppressor factor(s) produced by decidua-associated suppressor cells: a proposed mechanism for fetal allograft survival. Am J Obstet Gynecol, 156, 344–50. [DOI] [PubMed] [Google Scholar]
- Deng W, Cha J, Yuan J, Haraguchi H, Bartos A, Leishman E, Viollet B, Bradshaw HB, Hirota Y Dey SK 2016. p53 coordinates decidual sestrin 2/AMPK/mTORC1 signaling to govern parturition timing. J Clin Invest, 126, 2941–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai M, Ter Kuile FO, Nosten F, Mcgready R, Asamoa K, Brabin B Newman RD 2007. Epidemiology and burden of malaria in pregnancy. Lancet Infect Dis, 7, 93–104. [DOI] [PubMed] [Google Scholar]
- Di Micco R, Krizhanovsky V, Baker D D’adda Di Fagagna F 2021. Cellular senescence in ageing: from mechanisms to therapeutic opportunities. Nat Rev Mol Cell Biol, 22, 75–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Paolo NC Shayakhmetov DM 2016. Interleukin 1alpha and the inflammatory process. Nat Immunol, 17, 906–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diao L, Hierweger AM, Wieczorek A, Arck PC Thiele K 2021. Disruption of Glucocorticoid Action on CD11c(+) Dendritic Cells Favors the Generation of CD4(+) Regulatory T Cells and Improves Fetal Development in Mice. Front Immunol, 12, 729742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Digiulio DB, Romero R, Amogan HP, Kusanovic JP, Bik EM, Gotsch F, Kim CJ, Erez O, Edwin S Relman DA 2008. Microbial prevalence, diversity and abundance in amniotic fluid during preterm labor: a molecular and culture-based investigation. PLoS One, 3, e3056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Digiulio DB, Romero R, Kusanovic JP, Gomez R, Kim CJ, Seok KS, Gotsch F, Mazaki-Tovi S, Vaisbuch E, Sanders K, et al. 2010. Prevalence and diversity of microbes in the amniotic fluid, the fetal inflammatory response, and pregnancy outcome in women with preterm pre-labor rupture of membranes. Am J Reprod Immunol, 64, 38–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimova T, Nagaeva O, Stenqvist AC, Hedlund M, Kjellberg L, Strand M, Dehlin E Mincheva-Nilsson L 2011. Maternal Foxp3 expressing CD4+ CD25+ and CD4+ CD25- regulatory T-cell populations are enriched in human early normal pregnancy decidua: a phenotypic study of paired decidual and peripheral blood samples. Am J Reprod Immunol, 66 Suppl 1, 44–56. [DOI] [PubMed] [Google Scholar]
- Ding H, Dai Y, Lei Y, Wang Z, Liu D, Li R, Shen L, Gu N, Zheng M, Zhu X, et al. 2019. Upregulation of CD81 in trophoblasts induces an imbalance of Treg/Th17 cells by promoting IL-6 expression in preeclampsia. Cell Mol Immunol, 16, 302–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding J, Yang C, Zhang Y, Wang J, Zhang S, Guo D, Yin T Yang J 2021. M2 macrophage-derived G-CSF promotes trophoblasts EMT, invasion and migration via activating PI3K/Akt/Erk1/2 pathway to mediate normal pregnancy. J Cell Mol Med, 25, 2136–2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doisne JM, Balmas E, Boulenouar S, Gaynor LM, Kieckbusch J, Gardner L, Hawkes DA, Barbara CF, Sharkey AM, Brady HJ, et al. 2015. Composition, Development, and Function of Uterine Innate Lymphoid Cells. J Immunol, 195, 3937–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dombroski RA, Woodard DS, Harper MJ Gibbs RS 1990. A rabbit model for bacteria-induced preterm pregnancy loss. Am J Obstet Gynecol, 163, 1938–43. [DOI] [PubMed] [Google Scholar]
- Dougherty TF Schneebeli GL 1955. The use of steroids as anti-inflammatory agents. Ann N Y Acad Sci, 61, 328–48. [DOI] [PubMed] [Google Scholar]
- Dudley DJ, Branch DW, Edwin SS Mitchell MD 1996. Induction of preterm birth in mice by RU486. Biol Reprod, 55, 992–5. [DOI] [PubMed] [Google Scholar]
- Duffield JS, Forbes SJ, Constandinou CM, Clay S, Partolina M, Vuthoori S, Wu S, Lang R Iredale JP 2005. Selective depletion of macrophages reveals distinct, opposing roles during liver injury and repair. J Clin Invest, 115, 56–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duhen T, Geiger R, Jarrossay D, Lanzavecchia A Sallusto F 2009. Production of interleukin 22 but not interleukin 17 by a subset of human skin-homing memory T cells. Nat Immunol, 10, 857–63. [DOI] [PubMed] [Google Scholar]
- Ehrenberg HM Mercer BM 2001. Antibiotics and the management of preterm premature rupture of the fetal membranes. Clin Perinatol, 28, 807–18. [DOI] [PubMed] [Google Scholar]
- El Khwad M, Stetzer B, Moore RM, Kumar D, Mercer B, Arikat S, Redline RW, Mansour JM Moore JJ 2005. Term human fetal membranes have a weak zone overlying the lower uterine pole and cervix before onset of labor. Biol Reprod, 72, 720–6. [DOI] [PubMed] [Google Scholar]
- Elfayomy AK Almasry SM 2014. Expression of tumor necrosis factor-alpha and vascular endothelial growth factor in different zones of fetal membranes: a possible relation to onset of labor. J Mol Histol, 45, 243–57. [DOI] [PubMed] [Google Scholar]
- Elovitz MA Mrinalini C 2004. Animal models of preterm birth. Trends Endocrinol Metab, 15, 479–87. [DOI] [PubMed] [Google Scholar]
- Emerson GA, Bry K, Hallman M, Jobe AH, Wada N, Ervin MG Ikegami M 1997. Intra-amniotic interleukin-1 alpha treatment alters postnatal adaptation in premature lambs. Biol Neonate, 72, 370–9. [DOI] [PubMed] [Google Scholar]
- Engler JB, Kursawe N, Solano ME, Patas K, Wehrmann S, Heckmann N, Luhder F, Reichardt HM, Arck PC, Gold SM, et al. 2017. Glucocorticoid receptor in T cells mediates protection from autoimmunity in pregnancy. Proc Natl Acad Sci U S A, 114, E181–E190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Equils O, Kellogg C, Mcgregor J, Gravett M, Neal-Perry G Gabay C 2020. The role of the IL-1 system in pregnancy and the use of IL-1 system markers to identify women at risk for pregnancy complicationsdagger. Biol Reprod, 103, 684–694. [DOI] [PubMed] [Google Scholar]
- Erlebacher A 2013. Mechanisms of T cell tolerance towards the allogeneic fetus. Nat Rev Immunol, 13, 23–33. [DOI] [PubMed] [Google Scholar]
- Erlebacher A, Vencato D, Price KA, Zhang D Glimcher LH 2007. Constraints in antigen presentation severely restrict T cell recognition of the allogeneic fetus. J Clin Invest, 117, 1399–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esensten JH, Muller YD, Bluestone JA Tang Q 2018. Regulatory T-cell therapy for autoimmune and autoinflammatory diseases: The next frontier. J Allergy Clin Immunol, 142, 1710–1718. [DOI] [PubMed] [Google Scholar]
- Espinoza J, Chaiworapongsa T, Romero R, Edwin S, Rathnasabapathy C, Gomez R, Bujold E, Camacho N, Kim YM, Hassan S, et al. 2003. Antimicrobial peptides in amniotic fluid: defensins, calprotectin and bacterial/permeability-increasing protein in patients with microbial invasion of the amniotic cavity, intra-amniotic inflammation, preterm labor and premature rupture of membranes. J Matern Fetal Neonatal Med, 13, 2–21. [DOI] [PubMed] [Google Scholar]
- Faro J, Romero R, Schwenkel G, Garcia-Flores V, Arenas-Hernandez M, Leng Y, Xu Y, Miller D, Hassan SS Gomez-Lopez N 2019. Intra-amniotic inflammation induces preterm birth by activating the NLRP3 inflammasome. Biol Reprod, 100, 1290–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedorka CE, El-Sheikh Ali H, Walker OF, Scoggin KE, Dini P, Loux SC, Troedsson MHT Ball BA 2021. The imbalance of the Th17/Treg axis following equine ascending placental infection. J Reprod Immunol, 144, 103268. [DOI] [PubMed] [Google Scholar]
- Fernandes-Alnemri T, Wu J, Yu JW, Datta P, Miller B, Jankowski W, Rosenberg S, Zhang J Alnemri ES 2007. The pyroptosome: a supramolecular assembly of ASC dimers mediating inflammatory cell death via caspase-1 activation. Cell Death Differ, 14, 1590–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira LMR, Muller YD, Bluestone JA Tang Q 2019. Next-generation regulatory T cell therapy. Nat Rev Drug Discov, 18, 749–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fidel P, Ghezzi F, Romero R, Chaiworapongsa T, Espinoza J, Cutright J, Wolf N Gomez R 2003. The effect of antibiotic therapy on intrauterine infection-induced preterm parturition in rabbits. J Matern Fetal Neonatal Med, 14, 57–64. [DOI] [PubMed] [Google Scholar]
- Fidel PL Jr., Romero R, Wolf N, Cutright J, Ramirez M, Araneda H Cotton DB 1994. Systemic and local cytokine profiles in endotoxin-induced preterm parturition in mice. Am J Obstet Gynecol, 170, 1467–75. [DOI] [PubMed] [Google Scholar]
- Figueroa R, Garry D, Elimian A, Patel K, Sehgal PB Tejani N 2005. Evaluation of amniotic fluid cytokines in preterm labor and intact membranes. J Matern Fetal Neonatal Med, 18, 241–7. [DOI] [PubMed] [Google Scholar]
- Foell D, Wittkowski H, Vogl T Roth J 2007. S100 proteins expressed in phagocytes: a novel group of damage-associated molecular pattern molecules. J Leukoc Biol, 81, 28–37. [DOI] [PubMed] [Google Scholar]
- Fontenot JD, Gavin MA Rudensky AY 2003. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol, 4, 330–6. [DOI] [PubMed] [Google Scholar]
- Fouks Y, Amit S, Many A, Haham A, Mandel D Shinar S 2018. Listeriosis in pregnancy: under-diagnosis despite over-treatment. J Perinatol, 38, 26–30. [DOI] [PubMed] [Google Scholar]
- Franchi L Nunez G 2012. Immunology. Orchestrating inflammasomes. Science, 337, 1299–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin BS, Bossaller L, De Nardo D, Ratter JM, Stutz A, Engels G, Brenker C, Nordhoff M, Mirandola SR, Al-Amoudi A, et al. 2014. The adaptor ASC has extracellular and ‘prionoid’ activities that propagate inflammation. Nat Immunol, 15, 727–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frascoli M, Coniglio L, Witt R, Jeanty C, Fleck-Derderian S, Myers DE, Lee TH, Keating S, Busch MP, Norris PJ, et al. 2018. Alloreactive fetal T cells promote uterine contractility in preterm labor via IFN-gamma and TNF-alpha. Sci Transl Med, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman CD, Klutman NE Lamp KC 1997. Metronidazole. A therapeutic review and update. Drugs, 54, 679–708. [DOI] [PubMed] [Google Scholar]
- Friel LA, Romero R, Edwin S, Nien JK, Gomez R, Chaiworapongsa T, Kusanovic JP, Tolosa JE, Hassan SS Espinoza J 2007. The calcium binding protein, S100B, is increased in the amniotic fluid of women with intra-amniotic infection/inflammation and preterm labor with intact or ruptured membranes. J Perinat Med, 35, 385–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu B, Tian Z Wei H 2014. TH17 cells in human recurrent pregnancy loss and pre-eclampsia. Cell Mol Immunol, 11, 564–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu XQ, Cai JY, Huang QY, Li DJ, Li N Li MJ 2017. Prednisone may induce immunologic tolerance by activating the functions of decidual immune cells in early pregnancy. Oncotarget, 8, 102191–102198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu XQ, Cai JY Li MJ 2019. Prednisone may rebuild the immunologic homeostasis: Alteration of Th17 and Treg cells in the lymphocytes from rats’ spleens after treated with prednisone-containing serum. Mol Genet Genomic Med, 7, e00800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs TA, Abed U, Goosmann C, Hurwitz R, Schulze I, Wahn V, Weinrauch Y, Brinkmann V Zychlinsky A 2007. Novel cell death program leads to neutrophil extracellular traps. J Cell Biol, 176, 231–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fullerton JN 2013. Use of non-steroidal anti-inflammatory drugs (NSAIDs) as immunomodulatory agents. BMJ, 347, f4984. [DOI] [PubMed] [Google Scholar]
- Furcron AE, Romero R, Mial TN, Balancio A, Panaitescu B, Hassan SS, Sahi A, Nord C Gomez-Lopez N 2016. Human Chorionic Gonadotropin Has Anti-Inflammatory Effects at the Maternal-Fetal Interface and Prevents Endotoxin-Induced Preterm Birth, but Causes Dystocia and Fetal Compromise in Mice. Biol Reprod, 94, 136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furcron AE, Romero R, Plazyo O, Unkel R, Xu Y, Hassan SS, Chaemsaithong P, Mahajan A Gomez-Lopez N 2015. Vaginal progesterone, but not 17α-hydroxyprogesterone caproate, has antiinflammatory effects at the murine maternal-fetal interface. Am J Obstet Gynecol, 213, 846.e1–846.e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaidt MM Hornung V 2016. Pore formation by GSDMD is the effector mechanism of pyroptosis. EMBO J, 35, 2167–2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galaz J, Romero R, Arenas-Hernandez M, Panaitescu B, Para R Gomez-Lopez N 2021. Betamethasone as a potential treatment for preterm birth associated with sterile intra-amniotic inflammation: a murine study. J Perinat Med, 49, 897–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galaz J, Romero R, Slutsky R, Xu Y, Motomura K, Para R, Pacora P, Panaitescu B, Hsu CD, Kacerovsky M, et al. 2020a. Cellular immune responses in amniotic fluid of women with preterm prelabor rupture of membranes. J Perinat Med, 48, 222–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galaz J, Romero R, Xu Y, Miller D, Levenson D, Para R, Varrey A, Hsu R, Tong A, Hassan SS, et al. 2020b. Cellular immune responses in amniotic fluid of women with a sonographic short cervix. J Perinat Med, 48, 665–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galaz J, Romero R, Xu Y, Miller D, Slutsky R, Levenson D, Hsu CD Gomez-Lopez N 2020c. Cellular immune responses in amniotic fluid of women with preterm clinical chorioamnionitis. Inflamm Res, 69, 203–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galazka K, Wicherek L, Pitynski K, Kijowski J, Zajac K, Bednarek W, Dutsch-Wicherek M, Rytlewski K, Kalinka J, Basta A, et al. 2009. Changes in the subpopulation of CD25+ CD4+ and FOXP3+ regulatory T cells in decidua with respect to the progression of labor at term and the lack of analogical changes in the subpopulation of suppressive B7-H4 macrophages--a preliminary report. Am J Reprod Immunol, 61, 136–46. [DOI] [PubMed] [Google Scholar]
- Garcia-Flores V, Romero R, Miller D, Xu Y, Done B, Veerapaneni C, Leng Y, Arenas-Hernandez M, Khan N, Panaitescu B, et al. 2018. Inflammation-Induced Adverse Pregnancy and Neonatal Outcomes Can Be Improved by the Immunomodulatory Peptide Exendin-4. Front Immunol, 9, 1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardella C, Riley DE, Hitti J, Agnew K, Krieger JN Eschenbach D 2004. Identification and sequencing of bacterial rDNAs in culture-negative amniotic fluid from women in premature labor. Am J Perinatol, 21, 319–23. [DOI] [PubMed] [Google Scholar]
- Geginat J, Lanzavecchia A Sallusto F 2003. Proliferation and differentiation potential of human CD8+ memory T-cell subsets in response to antigen or homeostatic cytokines. Blood, 101, 4260–6. [DOI] [PubMed] [Google Scholar]
- Germain SJ, Sacks GP, Sooranna SR, Sargent IL Redman CW 2007. Systemic inflammatory priming in normal pregnancy and preeclampsia: the role of circulating syncytiotrophoblast microparticles. J Immunol, 178, 5949–56. [DOI] [PubMed] [Google Scholar]
- Gershater M, Romero R, Arenas-Hernandez M, Galaz J, Motomura K, Tao L, Xu Y, Miller D, Pique-Regi R, Martinez G, et al. 2022, Accepted. Interleukin-22 Plays a Dual Role in the Amniotic Cavity: Tissue Injury and Host Defense against Microbes in Preterm Labor. J Immunol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghayur T, Banerjee S, Hugunin M, Butler D, Herzog L, Carter A, Quintal L, Sekut L, Talanian R, Paskind M, et al. 1997. Caspase-1 processes IFN-gamma-inducing factor and regulates LPS-induced IFN-gamma production. Nature, 386, 619–23. [DOI] [PubMed] [Google Scholar]
- Gibbs RS, Blanco JD, St Clair PJ Castaneda YS 1982. Quantitative bacteriology of amniotic fluid from women with clinical intraamniotic infection at term. J Infect Dis, 145, 1–8. [DOI] [PubMed] [Google Scholar]
- Gibbs RS Duff P 1991. Progress in pathogenesis and management of clinical intraamniotic infection. Am J Obstet Gynecol, 164, 1317–26. [DOI] [PubMed] [Google Scholar]
- Gibbs RS, Romero R, Hillier SL, Eschenbach DA Sweet RL 1992. A review of premature birth and subclinical infection. Am J Obstet Gynecol, 166, 1515–28. [DOI] [PubMed] [Google Scholar]
- Gilbert NM, Foster LR, Cao B, Yin Y, Mysorekar IU Lewis AL 2021. Gardnerella vaginalis promotes group B Streptococcus vaginal colonization, enabling ascending uteroplacental infection in pregnant mice. Am J Obstet Gynecol, 224, 530 e1–530 e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girard S, Heazell AE, Derricott H, Allan SM, Sibley CP, Abrahams VM Jones RL 2014. Circulating cytokines and alarmins associated with placental inflammation in high-risk pregnancies. Am J Reprod Immunol, 72, 422–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goepfert AR, Jeffcoat MK, Andrews WW, Faye-Petersen O, Cliver SP, Goldenberg RL Hauth JC 2004. Periodontal disease and upper genital tract inflammation in early spontaneous preterm birth. Obstet Gynecol, 104, 777–83. [DOI] [PubMed] [Google Scholar]
- Goh FG Midwood KS 2012. Intrinsic danger: activation of Toll-like receptors in rheumatoid arthritis. Rheumatology (Oxford), 51, 7–23. [DOI] [PubMed] [Google Scholar]
- Gohner C, Plosch T Faas MM 2017. Immune-modulatory effects of syncytiotrophoblast extracellular vesicles in pregnancy and preeclampsia. Placenta, 60 Suppl 1, S41–S51. [DOI] [PubMed] [Google Scholar]
- Goldenberg RL, Culhane JF, Iams JD Romero R 2008. Epidemiology and causes of preterm birth. Lancet, 371, 75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Lopez N, Arenas-Hernandez M, Romero R, Miller D, Garcia-Flores V, Leng Y, Xu Y, Galaz J, Hassan SS, Hsu CD, et al. 2020. Regulatory T Cells Play a Role in a Subset of Idiopathic Preterm Labor/Birth and Adverse Neonatal Outcomes. Cell Rep, 32, 107874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Lopez N, Estrada-Gutierrez G, Jimenez-Zamudio L, Vega-Sanchez R Vadillo-Ortega F 2009. Fetal membranes exhibit selective leukocyte chemotaxic activity during human labor. J Reprod Immunol, 80, 122–31. [DOI] [PubMed] [Google Scholar]
- Gomez-Lopez N, Garcia-Flores V, Chin PY, Groome HM, Bijland MT, Diener KR, Romero R Robertson SA 2021a. Macrophages exert homeostatic actions in pregnancy to protect against preterm birth and fetal inflammatory injury. JCI Insight, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Lopez N, Guilbert LJ Olson DM 2010. Invasion of the leukocytes into the fetal-maternal interface during pregnancy. J Leukoc Biol, 88, 625–33. [DOI] [PubMed] [Google Scholar]
- Gomez-Lopez N, Motomura K, Miller D, Garcia-Flores V, Galaz J Romero R 2019a. Inflammasomes: Their Role in Normal and Complicated Pregnancies. J Immunol, 203, 2757–2769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Lopez N, Olson DM Robertson SA 2016a. Interleukin-6 controls uterine Th9 cells and CD8(+) T regulatory cells to accelerate parturition in mice. Immunol Cell Biol, 94, 79–89. [DOI] [PubMed] [Google Scholar]
- Gomez-Lopez N, Romero R, Arenas-Hernandez M, Ahn H, Panaitescu B, Vadillo-Ortega F, Sanchez-Torres C, Salisbury KS Hassan SS 2016b. In vivo T-cell activation by a monoclonal αCD3ε antibody induces preterm labor and birth. Am J Reprod Immunol, 76, 386–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Lopez N, Romero R, Arenas-Hernandez M, Panaitescu B, Garcia-Flores V, Mial TN, Sahi A Hassan SS 2018a. Intra-amniotic administration of lipopolysaccharide induces spontaneous preterm labor and birth in the absence of a body temperature change. J Matern Fetal Neonatal Med, 31, 439–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Lopez N, Romero R, Arenas-Hernandez M, Schwenkel G, St Louis D, Hassan SS Mial TN 2017a. In vivo activation of invariant natural killer T cells induces systemic and local alterations in T-cell subsets prior to preterm birth. Clin Exp Immunol, 189, 211–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Lopez N, Romero R, Galaz J, Xu Y, Panaitescu B, Slutsky R, Motomura K, Gill N, Para R, Pacora P, et al. 2019b. Cellular immune responses in amniotic fluid of women with preterm labor and intra-amniotic infection or intra-amniotic inflammation. Am J Reprod Immunol, 82, e13171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Lopez N, Romero R, Garcia-Flores V, Leng Y, Miller D, Hassan SS, Hsu CD Panaitescu B 2019c. Inhibition of the NLRP3 inflammasome can prevent sterile intra-amniotic inflammation, preterm labor/birth, and adverse neonatal outcomesdagger. Biol Reprod, 100, 1306–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Lopez N, Romero R, Garcia-Flores V, Xu Y, Leng Y, Alhousseini A, Hassan SS Panaitescu B 2017b. Amniotic fluid neutrophils can phagocytize bacteria: A mechanism for microbial killing in the amniotic cavity. Am J Reprod Immunol, 78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Lopez N, Romero R, Leng Y, Garcia-Flores V, Xu Y, Miller D Hassan SS 2017c. Neutrophil extracellular traps in acute chorioamnionitis: A mechanism of host defense. Am J Reprod Immunol, 77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Lopez N, Romero R, Leng Y, Xu Y, Slutsky R, Levenson D, Pacora P, Jung E, Panaitescu B Hsu CD 2019d. The origin of amniotic fluid monocytes/macrophages in women with intra-amniotic inflammation or infection. J Perinat Med, 47, 822–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Lopez N, Romero R, Maymon E, Kusanovic JP, Panaitescu B, Miller D, Pacora P, Tarca AL, Motomura K, Erez O, et al. 2019e. Clinical chorioamnionitis at term IX: in vivo evidence of intra-amniotic inflammasome activation. J Perinat Med, 47, 276–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Lopez N, Romero R, Panaitescu B, Leng Y, Xu Y, Tarca AL, Faro J, Pacora P, Hassan SS Hsu CD 2018b. Inflammasome activation during spontaneous preterm labor with intra-amniotic infection or sterile intra-amniotic inflammation. Am J Reprod Immunol, 80, e13049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Lopez N, Romero R, Panaitescu B, Miller D, Zou C, Gudicha DW, Tarca AL, Para R, Pacora P, Hassan SS, et al. 2021b. Gasdermin D: in vivo evidence of pyroptosis in spontaneous labor at term. J Matern Fetal Neonatal Med, 34, 569–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Lopez N, Romero R, Plazyo O, Panaitescu B, Furcron AE, Miller D, Roumayah T, Flom E Hassan SS 2016c. Intra-Amniotic Administration of HMGB1 Induces Spontaneous Preterm Labor and Birth. Am J Reprod Immunol, 75, 3–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Lopez N, Romero R, Plazyo O, Schwenkel G, Garcia-Flores V, Unkel R, Xu Y, Leng Y, Hassan SS, Panaitescu B, et al. 2017d. Preterm labor in the absence of acute histologic chorioamnionitis is characterized by cellular senescence of the chorioamniotic membranes. Am J Obstet Gynecol, 217, 592 e1–592 e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Lopez N, Romero R, Tarca AL, Miller D, Panaitescu B, Schwenkel G, Gudicha DW, Hassan SS, Pacora P, Jung E, et al. 2019f. Gasdermin D: Evidence of pyroptosis in spontaneous preterm labor with sterile intra-amniotic inflammation or intra-amniotic infection. Am J Reprod Immunol, 82, e13184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Lopez N, Romero R, Varrey A, Leng Y, Miller D, Done B, Xu Y, Bhatti G, Motomura K, Gershater M, et al. 2021c. RNA Sequencing Reveals Diverse Functions of Amniotic Fluid Neutrophils and Monocytes/Macrophages in Intra-Amniotic Infection. J Innate Immun, 13, 63–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Lopez N, Romero R, Xu Y, Garcia-Flores V, Leng Y, Panaitescu B, Miller D, Abrahams VM Hassan SS 2017e. Inflammasome assembly in the chorioamniotic membranes during spontaneous labor at term. Am J Reprod Immunol, 77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Lopez N, Romero R, Xu Y, Leng Y, Garcia-Flores V, Miller D, Jacques SM, Hassan SS, Faro J, Alsamsam A, et al. 2017f. Are amniotic fluid neutrophils in women with intraamniotic infection and/or inflammation of fetal or maternal origin? Am J Obstet Gynecol, 217, 693 e1–693 e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Lopez N, Romero R, Xu Y, Miller D, Arenas-Hernandez M, Garcia-Flores V, Panaitescu B, Galaz J, Hsu CD, Para R, et al. 2019g. Fetal T Cell Activation in the Amniotic Cavity during Preterm Labor: A Potential Mechanism for a Subset of Idiopathic Preterm Birth. J Immunol, 203, 1793–1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Lopez N, Romero R, Xu Y, Miller D, Leng Y, Panaitescu B, Silva P, Faro J, Alhousseini A, Gill N, et al. 2018c. The immunophenotype of amniotic fluid leukocytes in normal and complicated pregnancies. Am J Reprod Immunol, 79, e12827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Lopez N, Romero R, Xu Y, Miller D, Unkel R, Shaman M, Jacques SM, Panaitescu B, Garcia-Flores V Hassan SS 2017g. Neutrophil Extracellular Traps in the Amniotic Cavity of Women with Intra-Amniotic Infection: A New Mechanism of Host Defense. Reprod Sci, 24, 1139–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Lopez N, Romero R, Xu Y, Plazyo O, Unkel R, Leng Y, Than NG, Chaiworapongsa T, Panaitescu B, Dong Z, et al. 2017h. A Role for the Inflammasome in Spontaneous Preterm Labor With Acute Histologic Chorioamnionitis. Reprod Sci, 24, 1382–1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Lopez N, Romero R, Xu Y, Plazyo O, Unkel R, Than NG, Chaemsaithong P, Chaiworapongsa T, Dong Z, Tarca AL, et al. 2017i. A Role for the Inflammasome in Spontaneous Labor at Term with Acute Histologic Chorioamnionitis. Reprod Sci, 24, 934–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Lopez N, Vadillo-Perez L, Hernandez-Carbajal A, Godines-Enriquez M, Olson DM Vadillo-Ortega F 2011. Specific inflammatory microenvironments in the zones of the fetal membranes at term delivery. Am J Obstet Gynecol, 205, 235.e15–24. [DOI] [PubMed] [Google Scholar]
- Gomez-Lopez N, Vega-Sanchez R, Castillo-Castrejon M, Romero R, Cubeiro-Arreola K Vadillo-Ortega F 2013. Evidence for a role for the adaptive immune response in human term parturition. Am J Reprod Immunol, 69, 212–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez R, Romero R, Galasso M, Behnke E, Insunza A Cotton DB 1994. The value of amniotic fluid interleukin-6, white blood cell count, and gram stain in the diagnosis of microbial invasion of the amniotic cavity in patients at term. Am J Reprod Immunol, 32, 200–10. [DOI] [PubMed] [Google Scholar]
- Gomez R, Romero R, Ghezzi F, Yoon BH, Mazor M Berry SM 1998. The fetal inflammatory response syndrome. Am J Obstet Gynecol, 179, 194–202. [DOI] [PubMed] [Google Scholar]
- Goncalves LF, Chaiworapongsa T Romero R 2002. Intrauterine infection and prematurity. Ment Retard Dev Disabil Res Rev, 8, 3–13. [DOI] [PubMed] [Google Scholar]
- Gonzalez JM, Dong Z, Romero R Girardi G 2011a. Cervical remodeling/ripening at term and preterm delivery: the same mechanism initiated by different mediators and different effector cells. PLoS One, 6, e26877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez JM, Franzke CW, Yang F, Romero R Girardi G 2011b. Complement activation triggers metalloproteinases release inducing cervical remodeling and preterm birth in mice. Am J Pathol, 179, 838–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez JM, Xu H, Chai J, Ofori E Elovitz MA 2009. Preterm and term cervical ripening in CD1 Mice (Mus musculus): similar or divergent molecular mechanisms? Biol Reprod, 81, 1226–32. [DOI] [PubMed] [Google Scholar]
- Gordon M, Samuels P, Shubert P, Johnson F, Gebauer C Iams J 1995. A randomized, prospective study of adjunctive ceftizoxime in preterm labor. Am J Obstet Gynecol, 172, 1546–52. [DOI] [PubMed] [Google Scholar]
- Gotsch F, Romero R, Chaiworapongsa T, Erez O, Vaisbuch E, Espinoza J, Kusanovic JP, Mittal P, Mazaki-Tovi S, Kim CJ, et al. 2008. Evidence of the involvement of caspase-1 under physiologic and pathologic cellular stress during human pregnancy: a link between the inflammasome and parturition. J Matern Fetal Neonatal Med, 21, 605–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotsch F, Romero R, Kusanovic JP, Mazaki-Tovi S, Pineles BL, Erez O, Espinoza J Hassan SS 2007. The fetal inflammatory response syndrome. Clin Obstet Gynecol, 50, 652–83. [DOI] [PubMed] [Google Scholar]
- Gravett MG, Hummel D, Eschenbach DA Holmes KK 1986. Preterm labor associated with subclinical amniotic fluid infection and with bacterial vaginosis. Obstet Gynecol, 67, 229–37. [DOI] [PubMed] [Google Scholar]
- Gravett MG, Novy MJ, Rosenfeld RG, Reddy AP, Jacob T, Turner M, Mccormack A, Lapidus JA, Hitti J, Eschenbach DA, et al. 2004. Diagnosis of intra-amniotic infection by proteomic profiling and identification of novel biomarkers. JAMA, 292, 462–9. [DOI] [PubMed] [Google Scholar]
- Gravett MG, Witkin SS, Haluska GJ, Edwards JL, Cook MJ Novy MJ 1994. An experimental model for intraamniotic infection and preterm labor in rhesus monkeys. Am J Obstet Gynecol, 171, 1660–7. [DOI] [PubMed] [Google Scholar]
- Green EA, Choi Y Flavell RA 2002. Pancreatic lymph node-derived CD4(+)CD25(+) Treg cells: highly potent regulators of diabetes that require TRANCE-RANK signals. Immunity, 16, 183–91. [DOI] [PubMed] [Google Scholar]
- Gross O, Thomas CJ, Guarda G Tschopp J 2011. The inflammasome: an integrated view. Immunol Rev, 243, 136–51. [DOI] [PubMed] [Google Scholar]
- Gu Y, Kuida K, Tsutsui H, Ku G, Hsiao K, Fleming MA, Hayashi N, Higashino K, Okamura H, Nakanishi K, et al. 1997. Activation of interferon-gamma inducing factor mediated by interleukin-1beta converting enzyme. Science, 275, 206–9. [DOI] [PubMed] [Google Scholar]
- Gudicha DW, Romero R, Kabiri D, Hernandez-Andrade E, Pacora P, Erez O, Kusanovic JP, Jung E, Paredes C, Berry SM, et al. 2021. Personalized assessment of cervical length improves prediction of spontaneous preterm birth: a standard and a percentile calculator. Am J Obstet Gynecol, 224, 288 e1–288 e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerin LR, Prins JR Robertson SA 2009. Regulatory T-cells and immune tolerance in pregnancy: a new target for infertility treatment? Hum Reprod Update, 15, 517–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafsson C, Mjosberg J, Matussek A, Geffers R, Matthiesen L, Berg G, Sharma S, Buer J Ernerudh J 2008. Gene expression profiling of human decidual macrophages: evidence for immunosuppressive phenotype. PLoS One, 3, e2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton S, Oomomian Y, Stephen G, Shynlova O, Tower CL, Garrod A, Lye SJ Jones RL 2012. Macrophages infiltrate the human and rat decidua during term and preterm labor: evidence that decidual inflammation precedes labor. Biol Reprod, 86, 39. [DOI] [PubMed] [Google Scholar]
- Haribhai D, Williams JB, Jia S, Nickerson D, Schmitt EG, Edwards B, Ziegelbauer J, Yassai M, Li SH, Relland LM, et al. 2011. A requisite role for induced regulatory T cells in tolerance based on expanding antigen receptor diversity. Immunity, 35, 109–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris HE Raucci A 2006. Alarmin(g) news about danger: workshop on innate danger signals and HMGB1. EMBO Rep, 7, 774–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan S, Romero R, Hendler I, Gomez R, Khalek N, Espinoza J, Nien JK, Berry SM, Bujold E, Camacho N, et al. 2006. A sonographic short cervix as the only clinical manifestation of intra-amniotic infection. J Perinat Med, 34, 13–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan SS, Romero R, Berry SM, Dang K, Blackwell SC, Treadwell MC Wolfe HM 2000. Patients with an ultrasonographic cervical length < or =15 mm have nearly a 50% risk of early spontaneous preterm delivery. Am J Obstet Gynecol, 182, 1458–67. [DOI] [PubMed] [Google Scholar]
- Hazan AD, Smith SD, Jones RL, Whittle W, Lye SJ Dunk CE 2010. Vascular-leukocyte interactions: mechanisms of human decidual spiral artery remodeling in vitro. Am J Pathol, 177, 1017–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath VC, Southall TR, Souka AP, Elisseou A Nicolaides KH 1998. Cervical length at 23 weeks of gestation: prediction of spontaneous preterm delivery. Ultrasound Obstet Gynecol, 12, 312–7. [DOI] [PubMed] [Google Scholar]
- Heikkinen J, Möttönen M, Alanen A Lassila O 2004. Phenotypic characterization of regulatory T cells in the human decidua. Clin Exp Immunol, 136, 373–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller KA, Greig PC Heine RP 1995. Amniotic-fluid Lactoferrin: A Marker for Subclinical Intraamniotic Infection Prior to 32 Weeks Gestation. Infect Dis Obstet Gynecol, 3, 179–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henao-Mejia J, Elinav E, Strowig T Flavell RA 2012. Inflammasomes: far beyond inflammation. Nat Immunol, 13, 321–4. [DOI] [PubMed] [Google Scholar]
- Herzenberg LA, Bianchi DW, Schroder J, Cann HM Iverson GM 1979. Fetal cells in the blood of pregnant women: detection and enrichment by fluorescence-activated cell sorting. Proc Natl Acad Sci U S A, 76, 1453–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyborne KD, Cranfill RL, Carding SR, Born WK O’brien RL 1992. Characterization of gamma delta T lymphocytes at the maternal-fetal interface. J Immunol, 149, 2872–8. [PubMed] [Google Scholar]
- Hirota Y, Cha J, Yoshie M, Daikoku T Dey SK 2011. Heightened uterine mammalian target of rapamycin complex 1 (mTORC1) signaling provokes preterm birth in mice. Proc Natl Acad Sci U S A, 108, 18073–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirota Y, Daikoku T, Tranguch S, Xie H, Bradshaw HB Dey SK 2010. Uterine-specific p53 deficiency confers premature uterine senescence and promotes preterm birth in mice. J Clin Invest, 120, 803–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitti J, Riley DE, Krohn MA, Hillier SL, Agnew KJ, Krieger JN Eschenbach DA 1997. Broad-spectrum bacterial rDNA polymerase chain reaction assay for detecting amniotic fluid infection among women in premature labor. Clin Infect Dis, 24, 1228–32. [DOI] [PubMed] [Google Scholar]
- Hitti J, Tarczy-Hornoch P, Murphy J, Hillier SL, Aura J Eschenbach DA 2001. Amniotic fluid infection, cytokines, and adverse outcome among infants at 34 weeks’ gestation or less. Obstet Gynecol, 98, 1080–8. [DOI] [PubMed] [Google Scholar]
- Holland OJ, Linscheid C, Hodes HC, Nauser TL, Gilliam M, Stone P, Chamley LW Petroff MG 2012. Minor histocompatibility antigens are expressed in syncytiotrophoblast and trophoblast debris: implications for maternal alloreactivity to the fetus. Am J Pathol, 180, 256–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houser BL, Tilburgs T, Hill J, Nicotra ML Strominger JL 2011. Two unique human decidual macrophage populations. J Immunol, 186, 2633–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu CD, Meaddough E, Aversa K, Hong SF, Lee IS, Bahodo-Singh RO, Lu LC Copel JA 1998a. Dual roles of amniotic fluid nitric oxide and prostaglandin E2 in preterm labor with intra-amniotic infection. Am J Perinatol, 15, 683–7. [DOI] [PubMed] [Google Scholar]
- Hsu CD, Meaddough E, Aversa K, Hong SF, Lu LC, Jones DC Copel JA 1998b. Elevated amniotic fluid levels of leukemia inhibitory factor, interleukin 6, and interleukin 8 in intra-amniotic infection. Am J Obstet Gynecol, 179, 1267–70. [DOI] [PubMed] [Google Scholar]
- Huang J, Xie Y, Sun X, Zeh HJ 3rd, Kang R, Lotze MT Tang D 2015. DAMPs, ageing, and cancer: The ‘DAMP Hypothesis’. Ageing Res Rev, 24, 3–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Q, Wu H, Li M, Yang Y Fu X 2021. Prednisone improves pregnancy outcome in repeated implantation failure by enhance regulatory T cells bias. J Reprod Immunol, 143, 103245. [DOI] [PubMed] [Google Scholar]
- Hughes JP, Rees S, Kalindjian SB Philpott KL 2011. Principles of early drug discovery. Br J Pharmacol, 162, 1239–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt JS, Manning LS Wood GW 1984. Macrophages in murine uterus are immunosuppressive. Cell Immunol, 85, 499–510. [DOI] [PubMed] [Google Scholar]
- Hunter MM, Wang A, Parhar KS, Johnston MJ, Van Rooijen N, Beck PL Mckay DM 2010. In vitro-derived alternatively activated macrophages reduce colonic inflammation in mice. Gastroenterology, 138, 1395–405. [DOI] [PubMed] [Google Scholar]
- Iams JD, Goldenberg RL, Meis PJ, Mercer BM, Moawad A, Das A, Thom E, Mcnellis D, Copper RL, Johnson F, et al. 1996. The length of the cervix and the risk of spontaneous premature delivery. National Institute of Child Health and Human Development Maternal Fetal Medicine Unit Network. N Engl J Med, 334, 567–72. [DOI] [PubMed] [Google Scholar]
- Ismael S, Nasoohi S Ishrat T 2018a. MCC950, the Selective Inhibitor of Nucleotide Oligomerization Domain-Like Receptor Protein-3 Inflammasome, Protects Mice against Traumatic Brain Injury. J Neurotrauma, 35, 1294–1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ismael S, Zhao L, Nasoohi S Ishrat T 2018b. Inhibition of the NLRP3-inflammasome as a potential approach for neuroprotection after stroke. Sci Rep, 8, 5971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito M, Nakashima A, Hidaka T, Okabe M, Bac ND, Ina S, Yoneda S, Shiozaki A, Sumi S, Tsuneyama K, et al. 2010. A role for IL-17 in induction of an inflammation at the fetomaternal interface in preterm labour. J Reprod Immunol, 84, 75–85. [DOI] [PubMed] [Google Scholar]
- Ivarsson MA, Loh L, Marquardt N, Kekalainen E, Berglin L, Bjorkstrom NK, Westgren M, Nixon DF Michaelsson J 2013. Differentiation and functional regulation of human fetal NK cells. J Clin Invest, 123, 3889–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaiswal MK, Mallers TM, Larsen B, Kwak-Kim J, Chaouat G, Gilman-Sachs A Beaman KD 2012. V-ATPase upregulation during early pregnancy: a possible link to establishment of an inflammatory response during preimplantation period of pregnancy. Reproduction, 143, 713–25. [DOI] [PubMed] [Google Scholar]
- Jalava J, Mantymaa ML, Ekblad U, Toivanen P, Skurnik M, Lassila O Alanen A 1996. Bacterial 16S rDNA polymerase chain reaction in the detection of intra-amniotic infection. Br J Obstet Gynaecol, 103, 664–9. [DOI] [PubMed] [Google Scholar]
- Ji J, Zhai H, Zhou H, Song S, Mor G Liao A 2019. The role and mechanism of vitamin D-mediated regulation of Treg/Th17 balance in recurrent pregnancy loss. Am J Reprod Immunol, 81, e13112. [DOI] [PubMed] [Google Scholar]
- Jiang TT, Chaturvedi V, Ertelt JM, Kinder JM, Clark DR, Valent AM, Xin L Way SS 2014. Regulatory T cells: new keys for further unlocking the enigma of fetal tolerance and pregnancy complications. J Immunol, 192, 4949–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X, Du MR, Li M Wang H 2018. Three macrophage subsets are identified in the uterus during early human pregnancy. Cell Mol Immunol, 15, 1027–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jianjun Z, Yali H, Zhiqun W, Mingming Z Xia Z 2010. Imbalance of T-cell transcription factors contributes to the Th1 type immunity predominant in pre-eclampsia. Am J Reprod Immunol, 63, 38–45. [DOI] [PubMed] [Google Scholar]
- Joachim RA, Hildebrandt M, Oder J, Klapp BF Arck PC 2001. Murine stress-triggered abortion is mediated by increase of CD8+ TNF-alpha+ decidual cells via substance P. Am J Reprod Immunol, 45, 303–9. [DOI] [PubMed] [Google Scholar]
- Jordan MS, Boesteanu A, Reed AJ, Petrone AL, Holenbeck AE, Lerman MA, Naji A Caton AJ 2001. Thymic selection of CD4+CD25+ regulatory T cells induced by an agonist self-peptide. Nat Immunol, 2, 301–6. [DOI] [PubMed] [Google Scholar]
- Josefowicz SZ, Niec RE, Kim HY, Treuting P, Chinen T, Zheng Y, Umetsu DT Rudensky AY 2012. Extrathymically generated regulatory T cells control mucosal TH2 inflammation. Nature, 482, 395–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung E, Romero R, Yeo L, Diaz-Primera R, Marin-Concha J, Para R, Lopez AM, Pacora P, Gomez-Lopez N, Yoon BH, et al. 2020. The fetal inflammatory response syndrome: the origins of a concept, pathophysiology, diagnosis, and obstetrical implications. Semin Fetal Neonatal Med, 25, 101146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kacerovsky M, Romero R, Stepan M, Stranik J, Maly J, Pliskova L, Bolehovska R, Palicka V, Zemlickova H, Hornychova H, et al. 2020. Antibiotic administration reduces the rate of intraamniotic inflammation in preterm prelabor rupture of the membranes. Am J Obstet Gynecol, 223, 114 e1–114 e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadam L, Gomez-Lopez N, Mial TN, Kohan-Ghadr HR Drewlo S 2017. Rosiglitazone Regulates TLR4 and Rescues HO-1 and NRF2 Expression in Myometrial and Decidual Macrophages in Inflammation-Induced Preterm Birth. Reprod Sci, 24, 1590–1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kafetzis DA, Brater DC, Fanourgakis JE, Voyatzis J Georgakopoulos P 1983. Ceftriaxone distribution between maternal blood and fetal blood and tissues at parturition and between blood and milk postpartum. Antimicrob Agents Chemother, 23, 870–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn DA Baltimore D 2010. Pregnancy induces a fetal antigen-specific maternal T regulatory cell response that contributes to tolerance. Proc Natl Acad Sci U S A, 107, 9299–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaitin KI 2010. Deconstructing the drug development process: the new face of innovation. Clin Pharmacol Ther, 87, 356–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallapur SG, Kramer BW, Nitsos I, Pillow JJ, Collins JJ, Polglase GR, Newnham JP Jobe AH 2011. Pulmonary and systemic inflammatory responses to intra-amniotic IL-1alpha in fetal sheep. Am J Physiol Lung Cell Mol Physiol, 301, L285–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kass EH 1962. Pyelonephritis and bacteriuria. A major problem in preventive medicine. Ann Intern Med, 56, 46–53. [DOI] [PubMed] [Google Scholar]
- Kayagaki N, Stowe IB, Lee BL, O’rourke K, Anderson K, Warming S, Cuellar T, Haley B, Roose-Girma M, Phung QT, et al. 2015. Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling. Nature, 526, 666–71. [DOI] [PubMed] [Google Scholar]
- Kenyon S, Boulvain M Neilson JP 2013. Antibiotics for preterm rupture of membranes. Cochrane Database Syst Rev, CD001058. [DOI] [PubMed] [Google Scholar]
- Kenyon SL, Taylor DJ, Tarnow-Mordi W Group OC 2001a. Broad-spectrum antibiotics for preterm, prelabour rupture of fetal membranes: the ORACLE I randomised trial. ORACLE Collaborative Group. Lancet, 357, 979–88. [DOI] [PubMed] [Google Scholar]
- Kenyon SL, Taylor DJ, Tarnow-Mordi W Group OC 2001b. Broad-spectrum antibiotics for spontaneous preterm labour: the ORACLE II randomised trial. ORACLE Collaborative Group. Lancet, 357, 989–94. [DOI] [PubMed] [Google Scholar]
- Khanna D, Fitzgerald JD, Khanna PP, Bae S, Singh MK, Neogi T, Pillinger MH, Merill J, Lee S, Prakash S, et al. 2012. 2012 American College of Rheumatology guidelines for management of gout. Part 1: systematic nonpharmacologic and pharmacologic therapeutic approaches to hyperuricemia. Arthritis Care Res (Hoboken), 64, 1431–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi T, Hagiwara K, Honda Y, Gomi K, Kobayashi T, Takahashi H, Tokue Y, Watanabe A Nukiwa T 2002. Clarithromycin suppresses lipopolysaccharide-induced interleukin-8 production by human monocytes through AP-1 and NF-kappa B transcription factors. J Antimicrob Chemother, 49, 745–55. [DOI] [PubMed] [Google Scholar]
- Kim CJ, Romero R, Chaemsaithong P Kim JS 2015. Chronic inflammation of the placenta: definition, classification, pathogenesis, and clinical significance. Am J Obstet Gynecol, 213, S53–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M, Kim G, Romero R, Shim SS, Kim EC Yoon BH 2003. Biovar diversity of Ureaplasma urealyticum in amniotic fluid: distribution, intrauterine inflammatory response and pregnancy outcomes. J Perinat Med, 31, 146–52. [DOI] [PubMed] [Google Scholar]
- Kirchner L, Helmer H, Heinze G, Wald M, Brunbauer M, Weninger M Zaknun D 2007. Amnionitis with Ureaplasma urealyticum or other microbes leads to increased morbidity and prolonged hospitalization in very low birth weight infants. Eur J Obstet Gynecol Reprod Biol, 134, 44–50. [DOI] [PubMed] [Google Scholar]
- Klein NC Cunha BA 1995. Third-generation cephalosporins. Med Clin North Am, 79, 705–19. [DOI] [PubMed] [Google Scholar]
- Knight M, Redman CW, Linton EA Sargent IL 1998. Shedding of syncytiotrophoblast microvilli into the maternal circulation in pre-eclamptic pregnancies. Br J Obstet Gynaecol, 105, 632–40. [DOI] [PubMed] [Google Scholar]
- Korf H, Wenes M, Stijlemans B, Takiishi T, Robert S, Miani M, Eizirik DL, Gysemans C Mathieu C 2012. 1,25-Dihydroxyvitamin D3 curtails the inflammatory and T cell stimulatory capacity of macrophages through an IL-10-dependent mechanism. Immunobiology, 217, 1292–300. [DOI] [PubMed] [Google Scholar]
- Kostlin-Gille N, Hartel C, Haug C, Gopel W, Zemlin M, Muller A, Poets CF, Herting E Gille C 2021. Epidemiology of Early and Late Onset Neonatal Sepsis in Very Low Birthweight Infants: Data From the German Neonatal Network. Pediatr Infect Dis J, 40, 255–259. [DOI] [PubMed] [Google Scholar]
- Kostura MJ, Tocci MJ, Limjuco G, Chin J, Cameron P, Hillman AG, Chartrain NA Schmidt JA 1989. Identification of a monocyte specific pre-interleukin 1 beta convertase activity. Proc Natl Acad Sci U S A, 86, 5227–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kourtis AP, Read JS Jamieson DJ 2014. Pregnancy and infection. N Engl J Med, 370, 2211–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus TA, Engel SM, Sperling RS, Kellerman L, Lo Y, Wallenstein S, Escribese MM, Garrido JL, Singh T, Loubeau M, et al. 2012. Characterizing the pregnancy immune phenotype: results of the viral immunity and pregnancy (VIP) study. J Clin Immunol, 32, 300–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar P, Marinelarena A, Raghunathan D, Ragothaman VK, Saini S, Bhattacharya P, Fan J, Epstein AL, Maker AV Prabhakar BS 2019. Critical role of OX40 signaling in the TCR-independent phase of human and murine thymic Treg generation. Cell Mol Immunol, 16, 138–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb HM, Ormrod D, Scott LJ Figgitt DP 2002. Ceftriaxone: an update of its use in the management of community-acquired and nosocomial infections. Drugs, 62, 1041–89. [DOI] [PubMed] [Google Scholar]
- Lappas M 2014. Caspase-1 activation is increased with human labour in foetal membranes and myometrium and mediates infection-induced interleukin-1beta secretion. Am J Reprod Immunol, 71, 189–201. [DOI] [PubMed] [Google Scholar]
- Lash GE, Pitman H, Morgan HL, Innes BA, Agwu CN Bulmer JN 2016. Decidual macrophages: key regulators of vascular remodeling in human pregnancy. J Leukoc Biol, 100, 315–25. [DOI] [PubMed] [Google Scholar]
- Le QA, Eslick GD, Coulton KM, Akhter R, Condous G, Eberhard J Nanan R 2021. Does Treatment of Gingivitis During Pregnancy Improve Pregnancy Outcomes? A Systematic Review and Meta-Analysis. Oral Health Prev Dent, 19, 565–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Romero R, Kim SM, Chaemsaithong P, Park CW, Park JS, Jun JK Yoon BH 2016a. A new anti-microbial combination prolongs the latency period, reduces acute histologic chorioamnionitis as well as funisitis, and improves neonatal outcomes in preterm PROM. J Matern Fetal Neonatal Med, 29, 707–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Romero R, Kim SM, Chaemsaithong P Yoon BH 2016b. A new antibiotic regimen treats and prevents intra-amniotic inflammation/infection in patients with preterm PROM. J Matern Fetal Neonatal Med, 29, 2727–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SE, Romero R, Jung H, Park CW, Park JS Yoon BH 2007. The intensity of the fetal inflammatory response in intraamniotic inflammation with and without microbial invasion of the amniotic cavity. Am J Obstet Gynecol, 197, 294 e1–6. [DOI] [PubMed] [Google Scholar]
- Lee SE, Romero R, Kim CJ, Shim SS Yoon BH 2006. Funisitis in term pregnancy is associated with microbial invasion of the amniotic cavity and intra-amniotic inflammation. J Matern Fetal Neonatal Med, 19, 693–7. [DOI] [PubMed] [Google Scholar]
- Lee SE, Romero R, Park CW, Jun JK Yoon BH 2008. The frequency and significance of intraamniotic inflammation in patients with cervical insufficiency. Am J Obstet Gynecol, 198, 633 e1–8. [DOI] [PubMed] [Google Scholar]
- Lee SK, Kim JY, Hur SE, Kim CJ, Na BJ, Lee M, Gilman-Sachs A Kwak-Kim J 2011. An imbalance in interleukin-17-producing T and Foxp3+ regulatory T cells in women with idiopathic recurrent pregnancy loss. Hum Reprod, 26, 2964–71. [DOI] [PubMed] [Google Scholar]
- Lee SK, Kim JY, Lee M, Gilman-Sachs A Kwak-Kim J 2012. Th17 and regulatory T cells in women with recurrent pregnancy loss. Am J Reprod Immunol, 67, 311–8. [DOI] [PubMed] [Google Scholar]
- Leng Y, Romero R, Xu Y, Galaz J, Slutsky R, Arenas-Hernandez M, Garcia-Flores V, Motomura K, Hassan SS, Reboldi A, et al. 2019. Are B cells altered in the decidua of women with preterm or term labor? Am J Reprod Immunol, 81, e13102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lessin DL, Hunt JS, King CR Wood GW 1988. Antigen expression by cells near the maternal-fetal interface. Am J Reprod Immunol Microbiol, 16, 1–7. [DOI] [PubMed] [Google Scholar]
- Lisonkova S, Sabr Y Joseph KS 2014. Diagnosis of subclinical amniotic fluid infection prior to rescue cerclage using gram stain and glucose tests: an individual patient meta-analysis. J Obstet Gynaecol Can, 36, 116–122. [DOI] [PubMed] [Google Scholar]
- Liu L, Oza S, Hogan D, Perin J, Rudan I, Lawn JE, Cousens S, Mathers C Black RE 2015. Global, regional, and national causes of child mortality in 2000–13, with projections to inform post-2015 priorities: an updated systematic analysis. Lancet, 385, 430–40. [DOI] [PubMed] [Google Scholar]
- Liu Y, Yang B, Zhou M, Li L, Zhou H, Zhang J, Chen H Wu C 2009. Memory IL-22-producing CD4+ T cells specific for Candida albicans are present in humans. Eur J Immunol, 39, 1472–9. [DOI] [PubMed] [Google Scholar]
- Logiodice F, Lombardelli L, Kullolli O, Haller H, Maggi E, Rukavina D Piccinni MP 2019. Decidual Interleukin-22-Producing CD4+ T Cells (Th17/Th0/IL-22+ and Th17/Th2/IL-22+, Th2/IL-22+, Th0/IL-22+), Which Also Produce IL-4, Are Involved in the Success of Pregnancy. Int J Mol Sci, 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombardelli L, Logiodice F, Aguerre-Girr M, Kullolli O, Haller H, Casart Y, Berrebi A, L’faqihi-Olive FE, Duplan V, Romagnani S, et al. 2016. Interleukin-17-producing decidual CD4+ T cells are not deleterious for human pregnancy when they also produce interleukin-4. Clin Mol Allergy, 14, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotze MT, Deisseroth A Rubartelli A 2007. Damage associated molecular pattern molecules. Clin Immunol, 124, 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ly S Amici JM 2018. Role of betamethasone valerate 2.250 mg medicated plaster in the treatment of psoriasis and other dermatological pathologies: a review. Drugs Context, 7, 212539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackler AM, Iezza G, Akin MR, Mcmillan P Yellon SM 1999. Macrophage trafficking in the uterus and cervix precedes parturition in the mouse. Biol Reprod, 61, 879–83. [DOI] [PubMed] [Google Scholar]
- Macones GA, Marder SJ, Clothier B Stamilio DM 2001. The controversy surrounding indomethacin for tocolysis. Am J Obstet Gynecol, 184, 264–72. [DOI] [PubMed] [Google Scholar]
- Madsen-Bouterse SA, Romero R, Tarca AL, Kusanovic JP, Espinoza J, Kim CJ, Kim JS, Edwin SS, Gomez R Draghici S 2010. The transcriptome of the fetal inflammatory response syndrome. Am J Reprod Immunol, 63, 73–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malak TM Bell SC 1994. Structural characteristics of term human fetal membranes: a novel zone of extreme morphological alteration within the rupture site. Br J Obstet Gynaecol, 101, 375–86. [DOI] [PubMed] [Google Scholar]
- Mantovani A, Cassatella MA, Costantini C Jaillon S 2011. Neutrophils in the activation and regulation of innate and adaptive immunity. Nat Rev Immunol, 11, 519–31. [DOI] [PubMed] [Google Scholar]
- Marcellin L, Schmitz T, Messaoudene M, Chader D, Parizot C, Jacques S, Delaire J, Gogusev J, Schmitt A, Lesaffre C, et al. 2017. Immune Modifications in Fetal Membranes Overlying the Cervix Precede Parturition in Humans. J Immunol, 198, 1345–1356. [DOI] [PubMed] [Google Scholar]
- Mariathasan S Monack DM 2007. Inflammasome adaptors and sensors: intracellular regulators of infection and inflammation. Nat Rev Immunol, 7, 31–40. [DOI] [PubMed] [Google Scholar]
- Marquardt N, Ivarsson MA, Sundstrom E, Akesson E, Martini E, Eidsmo L, Mjosberg J, Friberg D, Kublickas M, Ek S, et al. 2016. Fetal CD103+ IL-17-Producing Group 3 Innate Lymphoid Cells Represent the Dominant Lymphocyte Subset in Human Amniotic Fluid. J Immunol, 197, 3069–3075. [DOI] [PubMed] [Google Scholar]
- Martinez-Varea A, Romero R, Xu Y, Miller D, Ahmed AI, Chaemsaithong P, Chaiyasit N, Yeo L, Shaman M, Lannaman K, et al. 2017. Clinical chorioamnionitis at term VII: the amniotic fluid cellular immune response. J Perinat Med, 45, 523–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinon F, Burns K Tschopp J 2002. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell, 10, 417–26. [DOI] [PubMed] [Google Scholar]
- Matsuda S, Suzuki M, Shimizu T, Ishikawa M, Souma A, Fujimoto S, Makinoda S, Chimura T, Watanabe T, Oda T, et al. 1988. [Pharmacokinetic and clinical evaluations of ceftriaxone in perinatal infections in obstetrics and gynecology]. Jpn J Antibiot, 41, 1251–60. [PubMed] [Google Scholar]
- Matsuo Y, Ishihara T, Ishizaki J, Miyamoto K, Higaki M Yamashita N 2009. Effect of betamethasone phosphate loaded polymeric nanoparticles on a murine asthma model. Cell Immunol, 260, 33–8. [DOI] [PubMed] [Google Scholar]
- Maymon E, Romero R, Chaiworapongsa T, Kim JC, Berman S, Gomez R Edwin S 2001. Value of amniotic fluid neutrophil collagenase concentrations in preterm premature rupture of membranes. Am J Obstet Gynecol, 185, 1143–8. [DOI] [PubMed] [Google Scholar]
- Mccartney SA, Kapur R, Liggitt HD, Baldessari A, Coleman M, Orvis A, Ogle J, Katz R, Rajagopal L Adams Waldorf KM 2021. Amniotic fluid interleukin 6 and interleukin 8 are superior predictors of fetal lung injury compared with maternal or fetal plasma cytokines or placental histopathology in a nonhuman primate model. Am J Obstet Gynecol, 225, 89 e1–89 e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mcgoldrick E, Stewart F, Parker R Dalziel SR 2020. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst Rev, 12, CD004454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mcgovern N, Shin A, Low G, Low D, Duan K, Yao LJ, Msallam R, Low I, Shadan NB, Sumatoh HR, et al. 2017. Human fetal dendritic cells promote prenatal T-cell immune suppression through arginase-2. Nature, 546, 662–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mclaren J, Taylor DJ Bell SC 1999. Increased incidence of apoptosis in non-labour-affected cytotrophoblast cells in term fetal membranes overlying the cervix. Hum Reprod, 14, 2895–900. [DOI] [PubMed] [Google Scholar]
- Medawar PB 1953. Some Immunological and Endocrinological Problems Raised by the Evolution of Viviparity in Vetebrates. Symp Soc Exp Biol, 7, 320–328. [Google Scholar]
- Mendz GL, Kaakoush NO Quinlivan JA 2013. Bacterial aetiological agents of intra-amniotic infections and preterm birth in pregnant women. Front Cell Infect Microbiol, 3, 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menendez C, Ordi J, Ismail MR, Ventura PJ, Aponte JJ, Kahigwa E, Font F Alonso PL 2000. The impact of placental malaria on gestational age and birth weight. J Infect Dis, 181, 1740–5. [DOI] [PubMed] [Google Scholar]
- Menon R, Behnia F, Polettini J, Saade GR, Campisi J Velarde M 2016. Placental membrane aging and HMGB1 signaling associated with human parturition. Aging (Albany NY), 8, 216–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer BM Arheart KL 1995. Antimicrobial therapy in expectant management of preterm premature rupture of the membranes. Lancet, 346, 1271–9. [DOI] [PubMed] [Google Scholar]
- Miller D, Motomura K, Garcia-Flores V, Romero R Gomez-Lopez N 2018. Innate Lymphoid Cells in the Maternal and Fetal Compartments. Front Immunol, 9, 2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mold JE Mccune JM 2012. Immunological tolerance during fetal development: from mouse to man. Adv Immunol, 115, 73–111. [DOI] [PubMed] [Google Scholar]
- Mold JE, Michaëlsson J, Burt TD, Muench MO, Beckerman KP, Busch MP, Lee TH, Nixon DF Mccune JM 2008. Maternal alloantigens promote the development of tolerogenic fetal regulatory T cells in utero. Science, 322, 1562–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motomura K, Romero R, Galaz J, Tarca AL, Done B, Xu Y, Leng Y, Garcia-Flores V, Arenas-Hernandez M, Theis KR, et al. 2021a. RNA Sequencing Reveals Distinct Immune Responses in the Chorioamniotic Membranes of Women with Preterm Labor and Microbial or Sterile Intra-amniotic Inflammation. Infect Immun, 89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motomura K, Romero R, Garcia-Flores V, Leng Y, Xu Y, Galaz J, Slutsky R, Levenson D Gomez-Lopez N 2020a. The alarmin interleukin-1alpha causes preterm birth through the NLRP3 inflammasome. Mol Hum Reprod, 26, 712–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motomura K, Romero R, Plazyo O, Garcia-Flores V, Gershater M, Galaz J, Miller D Gomez-Lopez N 2021b. The alarmin S100A12 causes sterile inflammation of the human chorioamniotic membranes as well as preterm birth and neonatal mortality in micedagger. Biol Reprod, 105, 1494–1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motomura K, Romero R, Xu Y, Theis KR, Galaz J, Winters AD, Slutsky R, Garcia-Flores V, Zou C, Levenson D, et al. 2020b. Intra-Amniotic Infection with Ureaplasma parvum Causes Preterm Birth and Neonatal Mortality That Are Prevented by Treatment with Clarithromycin. mBio, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz-Espin D Serrano M 2014. Cellular senescence: from physiology to pathology. Nat Rev Mol Cell Biol, 15, 482–96. [DOI] [PubMed] [Google Scholar]
- Munoz-Suano A, Hamilton AB Betz AG 2011. Gimme shelter: the immune system during pregnancy. Immunol Rev, 241, 20–38. [DOI] [PubMed] [Google Scholar]
- Muyayalo KP, Huang XB, Qian Z, Li ZH, Mor G Liao AH 2019. Low circulating levels of vitamin D may contribute to the occurrence of preeclampsia through deregulation of Treg/Th17 cell ratio. Am J Reprod Immunol, 82, e13168. [DOI] [PubMed] [Google Scholar]
- Myers DA 2012. The recruitment and activation of leukocytes into the immune cervix: further support that cervical remodeling involves an immune and inflammatory mechanism. Biol Reprod, 87, 107. [DOI] [PubMed] [Google Scholar]
- Naccasha N, Gervasi MT, Chaiworapongsa T, Berman S, Yoon BH, Maymon E Romero R 2001. Phenotypic and metabolic characteristics of monocytes and granulocytes in normal pregnancy and maternal infection. Am J Obstet Gynecol, 185, 1118–23. [DOI] [PubMed] [Google Scholar]
- Nadeau-Vallee M, Chin PY, Belarbi L, Brien ME, Pundir S, Berryer MH, Beaudry-Richard A, Madaan A, Sharkey DJ, Lupien-Meilleur A, et al. 2017a. Antenatal Suppression of IL-1 Protects against Inflammation-Induced Fetal Injury and Improves Neonatal and Developmental Outcomes in Mice. J Immunol, 198, 2047–2062. [DOI] [PubMed] [Google Scholar]
- Nadeau-Vallee M, Obari D, Beaudry-Richard A, Sierra EM, Beaulac A, Maurice N, Olson DM Chemtob S 2017b. Preterm Birth and Neonatal Injuries: Importance of Interleukin-1 and Potential of Interleukin-1 Receptor Antagonists. Curr Pharm Des, 23, 6132–6141. [DOI] [PubMed] [Google Scholar]
- Nadeau-Vallee M, Obari D, Palacios J, Brien ME, Duval C, Chemtob S Girard S 2016a. Sterile inflammation and pregnancy complications: a review. Reproduction, 152, R277–R292. [DOI] [PubMed] [Google Scholar]
- Nadeau-Vallee M, Obari D, Quiniou C, Lubell WD, Olson DM, Girard S Chemtob S 2016b. A critical role of interleukin-1 in preterm labor. Cytokine Growth Factor Rev, 28, 37–51. [DOI] [PubMed] [Google Scholar]
- Nadeem L, Shynlova O, Matysiak-Zablocki E, Mesiano S, Dong X Lye S 2016. Molecular evidence of functional progesterone withdrawal in human myometrium. Nat Commun, 7, 11565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naeye RL Ross SM 1982. Amniotic fluid infection syndrome. Clin Obstet Gynaecol, 9, 593–607. [PubMed] [Google Scholar]
- Nagamatsu T Schust DJ 2010. The immunomodulatory roles of macrophages at the maternal-fetal interface. Reprod Sci, 17, 209–18. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Kitani A Strober W 2001. Cell contact-dependent immunosuppression by CD4(+)CD25(+) regulatory T cells is mediated by cell surface-bound transforming growth factor beta. J Exp Med, 194, 629–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashima A, Ito M, Shima T, Bac ND, Hidaka T Saito S 2010. Accumulation of IL-17-positive cells in decidua of inevitable abortion cases. Am J Reprod Immunol, 64, 4–11. [DOI] [PubMed] [Google Scholar]
- Nancy P, Tagliani E, Tay CS, Asp P, Levy DE Erlebacher A 2012. Chemokine gene silencing in decidual stromal cells limits T cell access to the maternal-fetal interface. Science, 336, 1317–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton ER, Dinsmoor MJ Gibbs RS 1989. A randomized, blinded, placebo-controlled trial of antibiotics in idiopathic preterm labor. Obstet Gynecol, 74, 562–6. [PubMed] [Google Scholar]
- Nguyen TA, Kahn DA Loewendorf AI 2017. Maternal-Fetal rejection reactions are unconstrained in preeclamptic women. PLoS One, 12, e0188250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nhan-Chang CL, Romero R, Tarca AL, Mittal P, Kusanovic JP, Erez O, Mazaki-Tovi S, Chaiworapongsa T, Hotra J, Than NG, et al. 2010. Characterization of the transcriptome of chorioamniotic membranes at the site of rupture in spontaneous labor at term. Am J Obstet Gynecol, 202, 462 e1–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nograles KE, Zaba LC, Shemer A, Fuentes-Duculan J, Cardinale I, Kikuchi T, Ramon M, Bergman R, Krueger JG Guttman-Yassky E 2009. IL-22-producing “T22” T cells account for upregulated IL-22 in atopic dermatitis despite reduced IL-17-producing TH17 T cells. J Allergy Clin Immunol, 123, 1244–52.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norwitz ER, Robinson JN Challis JR 1999. The control of labor. N Engl J Med, 341, 660–6. [DOI] [PubMed] [Google Scholar]
- Novy MJ, Duffy L, Axthelm MK, Sadowsky DW, Witkin SS, Gravett MG, Cassell GH Waites KB 2009. Ureaplasma parvum or Mycoplasma hominis as sole pathogens cause chorioamnionitis, preterm delivery, and fetal pneumonia in rhesus macaques. Reprod Sci, 16, 56–70. [DOI] [PubMed] [Google Scholar]
- O’reilly M, Sozo F Harding R 2013. Impact of preterm birth and bronchopulmonary dysplasia on the developing lung: long-term consequences for respiratory health. Clin Exp Pharmacol Physiol, 40, 765–73. [DOI] [PubMed] [Google Scholar]
- Oh KJ, Hong JS, Romero R Yoon BH 2019a. The frequency and clinical significance of intra-amniotic inflammation in twin pregnancies with preterm labor and intact membranes. J Matern Fetal Neonatal Med, 32, 527–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh KJ, Lee SE, Jung H, Kim G, Romero R Yoon BH 2010. Detection of ureaplasmas by the polymerase chain reaction in the amniotic fluid of patients with cervical insufficiency. J Perinat Med, 38, 261–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh KJ, Romero R, Park JY, Lee J, Conde-Agudelo A, Hong JS Yoon BH 2019b. Evidence that antibiotic administration is effective in the treatment of a subset of patients with intra-amniotic infection/inflammation presenting with cervical insufficiency. Am J Obstet Gynecol, 221, 140 e1–140 e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppenheim JJ Yang D 2005. Alarmins: chemotactic activators of immune responses. Curr Opin Immunol, 17, 359–65. [DOI] [PubMed] [Google Scholar]
- Osman I, Young A, Ledingham MA, Thomson AJ, Jordan F, Greer IA Norman JE 2003. Leukocyte density and pro-inflammatory cytokine expression in human fetal membranes, decidua, cervix and myometrium before and during labour at term. Mol Hum Reprod, 9, 41–5. [DOI] [PubMed] [Google Scholar]
- Otsuki K, Yoda A, Saito H, Mitsuhashi Y, Toma Y, Shimizu Y Yanaihara T 1999. Amniotic fluid lactoferrin in intrauterine infection. Placenta, 20, 175–9. [DOI] [PubMed] [Google Scholar]
- Oyarzun E, Yamamoto M, Kato S, Gomez R, Lizama L Moenne A 1998. Specific detection of 16 micro-organisms in amniotic fluid by polymerase chain reaction and its correlation with preterm delivery occurrence. Am J Obstet Gynecol, 179, 1115–9. [DOI] [PubMed] [Google Scholar]
- Pacher P, Nivorozhkin A Szabo C 2006. Therapeutic effects of xanthine oxidase inhibitors: renaissance half a century after the discovery of allopurinol. Pharmacol Rev, 58, 87–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacora P, Chaiworapongsa T, Maymon E, Kim YM, Gomez R, Yoon BH, Ghezzi F, Berry SM, Qureshi F, Jacques SM, et al. 2002. Funisitis and chorionic vasculitis: the histological counterpart of the fetal inflammatory response syndrome. J Matern Fetal Neonatal Med, 11, 18–25. [DOI] [PubMed] [Google Scholar]
- Pacora P, Maymon E, Gervasi MT, Gomez R, Edwin SS, Yoon BH Romero R 2000a. Lactoferrin in intrauterine infection, human parturition, and rupture of fetal membranes. Am J Obstet Gynecol, 183, 904–10. [DOI] [PubMed] [Google Scholar]
- Pacora P, Romero R, Maymon E, Gervasi MT, Gomez R, Edwin SS Yoon BH 2000b. Participation of the novel cytokine interleukin 18 in the host response to intra-amniotic infection. Am J Obstet Gynecol, 183, 1138–43. [DOI] [PubMed] [Google Scholar]
- Palmer MT, Lee YK, Maynard CL, Oliver JR, Bikle DD, Jetten AM Weaver CT 2011. Lineage-specific effects of 1,25-dihydroxyvitamin D(3) on the development of effector CD4 T cells. J Biol Chem, 286, 997–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panaitescu B, Romero R, Gomez-Lopez N, Xu Y, Leng Y, Maymon E, Pacora P, Erez O, Yeo L, Hassan SS, et al. 2019. In vivo evidence of inflammasome activation during spontaneous labor at term. J Matern Fetal Neonatal Med, 32, 1978–1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pannell M, Labuz D, Celik MO, Keye J, Batra A, Siegmund B Machelska H 2016. Adoptive transfer of M2 macrophages reduces neuropathic pain via opioid peptides. J Neuroinflammation, 13, 262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Para R, Romero R, Miller D, Galaz J, Done B, Peyvandipour A, Gershater M, Tao L, Motomura K, Ruden DM, et al. 2021. The Distinct Immune Nature of the Fetal Inflammatory Response Syndrome Type I and Type II. Immunohorizons, 5, 735–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Para R, Romero R, Miller D, Panaitescu B, Varrey A, Chaiworapongsa T, Hassan SS, Hsu CD Gomez-Lopez N 2020. Human beta-defensin-3 participates in intra-amniotic host defense in women with labor at term, spontaneous preterm labor and intact membranes, and preterm prelabor rupture of membranes. J Matern Fetal Neonatal Med, 33, 4117–4132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park HS, Ahn BJ Jun JK 2012. Placental transfer of clarithromycin in human pregnancies with preterm premature rupture of membranes. J Perinat Med, 40, 641–6. [DOI] [PubMed] [Google Scholar]
- Park JS, Romero R, Yoon BH, Moon JB, Oh SY, Han SY Ko EM 2001. The relationship between amniotic fluid matrix metalloproteinase-8 and funisitis. Am J Obstet Gynecol, 185, 1156–61. [DOI] [PubMed] [Google Scholar]
- Park JY, Romero R, Lee J, Chaemsaithong P, Chaiyasit N Yoon BH 2016. An elevated amniotic fluid prostaglandin F2alpha concentration is associated with intra-amniotic inflammation/infection, and clinical and histologic chorioamnionitis, as well as impending preterm delivery in patients with preterm labor and intact membranes. J Matern Fetal Neonatal Med, 29, 2563–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsa R, Andresen P, Gillett A, Mia S, Zhang XM, Mayans S, Holmberg D Harris RA 2012. Adoptive transfer of immunomodulatory M2 macrophages prevents type 1 diabetes in NOD mice. Diabetes, 61, 2881–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlidis I, Spiller OB, Sammut Demarco G, Macpherson H, Howie SEM, Norman JE Stock SJ 2020. Cervical epithelial damage promotes Ureaplasma parvum ascending infection, intrauterine inflammation and preterm birth induction in mice. Nat Commun, 11, 199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne KJ, Clyde LA, Weldon AJ, Milford TA Yellon SM 2012. Residency and activation of myeloid cells during remodeling of the prepartum murine cervix. Biol Reprod, 87, 106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peiris HN, Romero R, Vaswani K, Gomez-Lopez N, Tarca AL, Gudicha DW, Erez O, Maymon E, Reed S Mitchell MD 2020. Prostaglandin and prostamide concentrations in amniotic fluid of women with spontaneous labor at term with and without clinical chorioamnionitis. Prostaglandins Leukot Essent Fatty Acids, 163, 102195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peiris HN, Romero R, Vaswani K, Reed S, Gomez-Lopez N, Tarca AL, Gudicha DW, Erez O, Maymon E Mitchell MD 2021. Preterm labor is characterized by a high abundance of amniotic fluid prostaglandins in patients with intra-amniotic infection or sterile intra-amniotic inflammation. J Matern Fetal Neonatal Med, 34, 4009–4024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera AP, Fernando R, Shinde T, Gundamaraju R, Southam B, Sohal SS, Robertson A. a. B., Schroder K, Kunde D Eri R 2018. MCC950, a specific small molecule inhibitor of NLRP3 inflammasome attenuates colonic inflammation in spontaneous colitis mice. Sci Rep, 8, 8618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Munoz ME, Arrieta MC, Ramer-Tait AE Walter J 2017. A critical assessment of the “sterile womb” and “in utero colonization” hypotheses: implications for research on the pioneer infant microbiome. Microbiome, 5, 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plazyo O, Romero R, Unkel R, Balancio A, Mial TN, Xu Y, Dong Z, Hassan SS Gomez-Lopez N 2016. HMGB1 Induces an Inflammatory Response in the Chorioamniotic Membranes That Is Partially Mediated by the Inflammasome. Biol Reprod, 95, 130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polettini J, Behnia F, Taylor BD, Saade GR, Taylor RN Menon R 2015. Telomere Fragment Induced Amnion Cell Senescence: A Contributor to Parturition? PLoS One, 10, e0137188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell RM, Lissauer D, Tamblyn J, Beggs A, Cox P, Moss P Kilby MD 2017. Decidual T Cells Exhibit a Highly Differentiated Phenotype and Demonstrate Potential Fetal Specificity and a Strong Transcriptional Response to IFN. J Immunol, 199, 3406–3417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Presicce P, Senthamaraikannan P, Alvarez M, Rueda CM, Cappelletti M, Miller LA, Jobe AH, Chougnet CA Kallapur SG 2015. Neutrophil recruitment and activation in decidua with intra-amniotic IL-1beta in the preterm rhesus macaque. Biol Reprod, 92, 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prins JR, Boelens HM, Heimweg J, Van Der Heide S, Dubois AE, Van Oosterhout AJ Erwich JJ 2009. Preeclampsia is associated with lower percentages of regulatory T cells in maternal blood. Hypertens Pregnancy, 28, 300–11. [DOI] [PubMed] [Google Scholar]
- Quinn KH, Lacoursiere DY, Cui L, Bui J Parast MM 2011. The unique pathophysiology of early-onset severe preeclampsia: role of decidual T regulatory cells. J Reprod Immunol, 91, 76–82. [DOI] [PubMed] [Google Scholar]
- Ramhorst R, Fraccaroli L, Aldo P, Alvero AB, Cardenas I, Leiros CP Mor G 2012. Modulation and recruitment of inducible regulatory T cells by first trimester trophoblast cells. Am J Reprod Immunol, 67, 17–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Read S, Malmström V Powrie F 2000. Cytotoxic T lymphocyte-associated antigen 4 plays an essential role in the function of CD25(+)CD4(+) regulatory cells that control intestinal inflammation. J Exp Med, 192, 295–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ream MA Lehwald L 2018. Neurologic Consequences of Preterm Birth. Curr Neurol Neurosci Rep, 18, 48. [DOI] [PubMed] [Google Scholar]
- Ren Z, Bremer AA Pawlyk AC 2021. Drug development research in pregnant and lactating women. Am J Obstet Gynecol, 225, 33–42. [DOI] [PubMed] [Google Scholar]
- Repnik U, Tilburgs T, Roelen DL, Van Der Mast BJ, Kanhai HH, Scherjon S Claas FH 2008. Comparison of macrophage phenotype between decidua basalis and decidua parietalis by flow cytometry. Placenta, 29, 405–12. [DOI] [PubMed] [Google Scholar]
- Ribeiro VR, Romao-Veiga M, Nunes PR, Matias ML, Peracoli JC Peracoli MTS 2021. Vitamin D modulates the transcription factors of T cell subsets to anti-inflammatory and regulatory profiles in preeclampsia. Int Immunopharmacol, 101, 108366. [DOI] [PubMed] [Google Scholar]
- Rider P, Voronov E, Dinarello CA, Apte RN Cohen I 2017. Alarmins: Feel the Stress. J Immunol, 198, 1395–1402. [DOI] [PubMed] [Google Scholar]
- Robertson SA, Care AS Moldenhauer LM 2018. Regulatory T cells in embryo implantation and the immune response to pregnancy. J Clin Invest, 128, 4224–4235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson SA, Guerin LR, Moldenhauer LM Hayball JD 2009. Activating T regulatory cells for tolerance in early pregnancy - the contribution of seminal fluid. J Reprod Immunol, 83, 109–16. [DOI] [PubMed] [Google Scholar]
- Robertson SA, Jasper MJ, Bromfield JJ, Care AS, Nakamura H Ingman WV 2008. The Role of Macrophages in Implantation and Early Pregnancy Success. Biology of Reproduction, 78, 274–275. [Google Scholar]
- Romero R 2007. Prevention of spontaneous preterm birth: the role of sonographic cervical length in identifying patients who may benefit from progesterone treatment. Ultrasound Obstet Gynecol, 30, 675–86. [DOI] [PubMed] [Google Scholar]
- Romero R, Brody DT, Oyarzun E, Mazor M, Wu YK, Hobbins JC Durum SK 1989a. Infection and labor. III. Interleukin-1: a signal for the onset of parturition. Am J Obstet Gynecol, 160, 1117–23. [DOI] [PubMed] [Google Scholar]
- Romero R, Chaiworapongsa T, Alpay Savasan Z, Xu Y, Hussein Y, Dong Z, Kusanovic JP, Kim CJ Hassan SS 2011. Damage-associated molecular patterns (DAMPs) in preterm labor with intact membranes and preterm PROM: a study of the alarmin HMGB1. J Matern Fetal Neonatal Med, 24, 1444–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero R, Chaiworapongsa T, Savasan ZA, Hussein Y, Dong Z, Kusanovic JP, Kim CJ Hassan SS 2012. Clinical chorioamnionitis is characterized by changes in the expression of the alarmin HMGB1 and one of its receptors, sRAGE. J Matern Fetal Neonatal Med, 25, 558–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero R, Dey SK Fisher SJ 2014a. Preterm labor: one syndrome, many causes. Science, 345, 760–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero R, Emamian M, Quintero R, Wan M, Hobbins JC Mitchell MD 1986. Amniotic fluid prostaglandin levels and intra-amniotic infections. Lancet, 1, 1380. [DOI] [PubMed] [Google Scholar]
- Romero R, Espinoza J, Goncalves LF, Kusanovic JP, Friel L Hassan S 2007. The role of inflammation and infection in preterm birth. Semin Reprod Med, 25, 21–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero R, Espinoza J, Goncalves LF, Kusanovic JP, Friel LA Nien JK 2006a. Inflammation in preterm and term labour and delivery. Semin Fetal Neonatal Med, 11, 317–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero R, Espinoza J, Kusanovic JP, Gotsch F, Hassan S, Erez O, Chaiworapongsa T Mazor M 2006b. The preterm parturition syndrome. BJOG, 113 Suppl 3, 17–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero R, Gomez-Lopez N, Winters AD, Jung E, Shaman M, Bieda J, Panaitescu B, Pacora P, Erez O, Greenberg JM, et al. 2019. Evidence that intra-amniotic infections are often the result of an ascending invasion - a molecular microbiological study. J Perinat Med, 47, 915–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero R, Gomez R, Chaiworapongsa T, Conoscenti G, Kim JC Kim YM 2001. The role of infection in preterm labour and delivery. Paediatr Perinat Epidemiol, 15 Suppl 2, 41–56. [DOI] [PubMed] [Google Scholar]
- Romero R, Gomez R, Ghezzi F, Yoon BH, Mazor M, Edwin SS Berry SM 1998. A fetal systemic inflammatory response is followed by the spontaneous onset of preterm parturition. Am J Obstet Gynecol, 179, 186–93. [DOI] [PubMed] [Google Scholar]
- Romero R, Gonzalez R, Sepulveda W, Brandt F, Ramirez M, Sorokin Y, Mazor M, Treadwell MC Cotton DB 1992a. Infection and labor. VIII. Microbial invasion of the amniotic cavity in patients with suspected cervical incompetence: prevalence and clinical significance. Am J Obstet Gynecol, 167, 1086–91. [DOI] [PubMed] [Google Scholar]
- Romero R, Grivel JC, Tarca AL, Chaemsaithong P, Xu Z, Fitzgerald W, Hassan SS, Chaiworapongsa T Margolis L 2015a. Evidence of perturbations of the cytokine network in preterm labor. Am J Obstet Gynecol, 213, 836 e1–836 e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero R, Kadar N, Hobbins JC Duff GW 1987. Infection and labor: the detection of endotoxin in amniotic fluid. Am J Obstet Gynecol, 157, 815–9. [DOI] [PubMed] [Google Scholar]
- Romero R, Mazor M, Brandt F, Sepulveda W, Avila C, Cotton DB Dinarello CA 1992b. Interleukin-1 alpha and interleukin-1 beta in preterm and term human parturition. Am J Reprod Immunol, 27, 117–23. [DOI] [PubMed] [Google Scholar]
- Romero R, Mazor M Tartakovsky B 1991a. Systemic administration of interleukin-1 induces preterm parturition in mice. Am J Obstet Gynecol, 165, 969–71. [DOI] [PubMed] [Google Scholar]
- Romero R, Mazor M, Wu YK, Sirtori M, Oyarzun E, Mitchell MD Hobbins JC 1988a. Infection in the pathogenesis of preterm labor. Semin Perinatol, 12, 262–79. [PubMed] [Google Scholar]
- Romero R, Miranda J, Chaemsaithong P, Chaiworapongsa T, Kusanovic JP, Dong Z, Ahmed AI, Shaman M, Lannaman K, Yoon BH, et al. 2015b. Sterile and microbial-associated intra-amniotic inflammation in preterm prelabor rupture of membranes. J Matern Fetal Neonatal Med, 28, 1394–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero R, Miranda J, Chaiworapongsa T, Chaemsaithong P, Gotsch F, Dong Z, Ahmed AI, Yoon BH, Hassan SS, Kim CJ, et al. 2014b. A novel molecular microbiologic technique for the rapid diagnosis of microbial invasion of the amniotic cavity and intra-amniotic infection in preterm labor with intact membranes. Am J Reprod Immunol, 71, 330–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero R, Miranda J, Chaiworapongsa T, Chaemsaithong P, Gotsch F, Dong Z, Ahmed AI, Yoon BH, Hassan SS, Kim CJ, et al. 2015c. Sterile intra-amniotic inflammation in asymptomatic patients with a sonographic short cervix: prevalence and clinical significance. J Matern Fetal Neonatal Med, 28, 1343–1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero R, Miranda J, Chaiworapongsa T, Korzeniewski SJ, Chaemsaithong P, Gotsch F, Dong Z, Ahmed AI, Yoon BH, Hassan SS, et al. 2014c. Prevalence and clinical significance of sterile intra-amniotic inflammation in patients with preterm labor and intact membranes. Am J Reprod Immunol, 72, 458–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero R, Miranda J, Kusanovic JP, Chaiworapongsa T, Chaemsaithong P, Martinez A, Gotsch F, Dong Z, Ahmed AI, Shaman M, et al. 2015d. Clinical chorioamnionitis at term I: microbiology of the amniotic cavity using cultivation and molecular techniques. J Perinat Med, 43, 19–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero R, Oyarzun E, Mazor M, Sirtori M, Hobbins JC Bracken M 1989b. Meta-analysis of the relationship between asymptomatic bacteriuria and preterm delivery/low birth weight. Obstet Gynecol, 73, 576–82. [PubMed] [Google Scholar]
- Romero R, Parvizi ST, Oyarzun E, Mazor M, Wu YK, Avila C, Athanassiadis AP Mitchell MD 1990a. Amniotic fluid interleukin-1 in spontaneous labor at term. J Reprod Med, 35, 235–8. [PubMed] [Google Scholar]
- Romero R, Quintero R, Nores J, Avila C, Mazor M, Hanaoka S, Hagay Z, Merchant L Hobbins JC 1991b. Amniotic fluid white blood cell count: a rapid and simple test to diagnose microbial invasion of the amniotic cavity and predict preterm delivery. Am J Obstet Gynecol, 165, 821–30. [DOI] [PubMed] [Google Scholar]
- Romero R, Quintero R, Oyarzun E, Wu YK, Sabo V, Mazor M Hobbins JC 1988b. Intraamniotic infection and the onset of labor in preterm premature rupture of the membranes. Am J Obstet Gynecol, 159, 661–6. [DOI] [PubMed] [Google Scholar]
- Romero R, Roslansky P, Oyarzun E, Wan M, Emamian M, Novitsky TJ, Gould MJ Hobbins JC 1988c. Labor and infection. II. Bacterial endotoxin in amniotic fluid and its relationship to the onset of preterm labor. Am J Obstet Gynecol, 158, 1044–9. [DOI] [PubMed] [Google Scholar]
- Romero R, Shamma F, Avila C, Jimenez C, Callahan R, Nores J, Mazor M, Brekus CA Hobbins JC 1990b. Infection and labor. VI. Prevalence, microbiology, and clinical significance of intraamniotic infection in twin gestations with preterm labor. Am J Obstet Gynecol, 163, 757–61. [DOI] [PubMed] [Google Scholar]
- Romero R, Sibai B, Caritis S, Paul R, Depp R, Rosen M, Klebanoff M, Sabo V, Evans J, Thom E, et al. 1993a. Antibiotic treatment of preterm labor with intact membranes: a multicenter, randomized, double-blinded, placebo-controlled trial. Am J Obstet Gynecol, 169, 764–74. [DOI] [PubMed] [Google Scholar]
- Romero R, Sirtori M, Oyarzun E, Avila C, Mazor M, Callahan R, Sabo V, Athanassiadis AP Hobbins JC 1989c. Infection and labor. V. Prevalence, microbiology, and clinical significance of intraamniotic infection in women with preterm labor and intact membranes. Am J Obstet Gynecol, 161, 817–24. [DOI] [PubMed] [Google Scholar]
- Romero R Tartakovsky B 1992. The natural interleukin-1 receptor antagonist prevents interleukin-1-induced preterm delivery in mice. Am J Obstet Gynecol, 167, 1041–5. [DOI] [PubMed] [Google Scholar]
- Romero R, Xu Y, Plazyo O, Chaemsaithong P, Chaiworapongsa T, Unkel R, Than NG, Chiang PJ, Dong Z, Xu Z, et al. 2018. A Role for the Inflammasome in Spontaneous Labor at Term. Am J Reprod Immunol, 79, e12440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero R, Yoon BH, Kenney JS, Gomez R, Allison AC Sehgal PB 1993b. Amniotic fluid interleukin-6 determinations are of diagnostic and prognostic value in preterm labor. Am J Reprod Immunol, 30, 167–83. [DOI] [PubMed] [Google Scholar]
- Romero R, Yoon BH, Mazor M, Gomez R, Diamond MP, Kenney JS, Ramirez M, Fidel PL, Sorokin Y, Cotton D, et al. 1993c. The diagnostic and prognostic value of amniotic fluid white blood cell count, glucose, interleukin-6, and gram stain in patients with preterm labor and intact membranes. Am J Obstet Gynecol, 169, 805–16. [DOI] [PubMed] [Google Scholar]
- Rosenbloom JI, Raghuraman N, Temming LA, Stout MJ, Tuuli MG, Dicke JM, Macones GA Cahill AG 2020. Predictive Value of Midtrimester Universal Cervical Length Screening Based on Parity. J Ultrasound Med, 39, 147–154. [DOI] [PubMed] [Google Scholar]
- Rowe JH, Ertelt JM, Aguilera MN, Farrar MA Way SS 2011. Foxp3(+) regulatory T cell expansion required for sustaining pregnancy compromises host defense against prenatal bacterial pathogens. Cell Host Microbe, 10, 54–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe JH, Ertelt JM, Xin L Way SS 2012. Pregnancy imprints regulatory memory that sustains anergy to fetal antigen. Nature, 490, 102–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacks GP, Studena K, Sargent K Redman CW 1998. Normal pregnancy and preeclampsia both produce inflammatory changes in peripheral blood leukocytes akin to those of sepsis. Am J Obstet Gynecol, 179, 80–6. [DOI] [PubMed] [Google Scholar]
- Sadowsky DW, Adams KM, Gravett MG, Witkin SS Novy MJ 2006. Preterm labor is induced by intraamniotic infusions of interleukin-1beta and tumor necrosis factor-alpha but not by interleukin-6 or interleukin-8 in a nonhuman primate model. Am J Obstet Gynecol, 195, 1578–89. [DOI] [PubMed] [Google Scholar]
- Sadowsky DW, Haluska GJ, Gravett MG, Witkin SS Novy MJ 2000. Indomethacin blocks interleukin 1beta-induced myometrial contractions in pregnant rhesus monkeys. Am J Obstet Gynecol, 183, 173–80. [DOI] [PubMed] [Google Scholar]
- Sadowsky DW, Novy MJ, Witkin SS Gravett MG 2003. Dexamethasone or interleukin-10 blocks interleukin-1beta-induced uterine contractions in pregnant rhesus monkeys. Am J Obstet Gynecol, 188, 252–63. [DOI] [PubMed] [Google Scholar]
- Saito S 2010. Th17 cells and regulatory T cells: new light on pathophysiology of preeclampsia. Immunol Cell Biol, 88, 615–7. [DOI] [PubMed] [Google Scholar]
- Saito S, Kasahara T, Kato Y, Ishihara Y Ichijo M 1993. Elevation of amniotic fluid interleukin 6 (IL-6), IL-8 and granulocyte colony stimulating factor (G-CSF) in term and preterm parturition. Cytokine, 5, 81–8. [DOI] [PubMed] [Google Scholar]
- Sakaguchi S, Fukuma K, Kuribayashi K Masuda T 1985. Organ-specific autoimmune diseases induced in mice by elimination of T cell subset. I. Evidence for the active participation of T cells in natural self-tolerance; deficit of a T cell subset as a possible cause of autoimmune disease. J Exp Med, 161, 72–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto Y, Moran P, Bulmer JN, Searle RF Robson SC 2005. Macrophages and not granulocytes are involved in cervical ripening. J Reprod Immunol, 66, 161–73. [DOI] [PubMed] [Google Scholar]
- Sallusto F, Lenig D, Förster R, Lipp M Lanzavecchia A 1999. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature, 401, 708–12. [DOI] [PubMed] [Google Scholar]
- Salvany-Celades M, Van Der Zwan A, Benner M, Setrajcic-Dragos V, Bougleux Gomes HA, Iyer V, Norwitz ER, Strominger JL Tilburgs T 2019. Three Types of Functional Regulatory T Cells Control T Cell Responses at the Human Maternal-Fetal Interface. Cell Rep, 27, 2537–2547.e5. [DOI] [PubMed] [Google Scholar]
- Sampson JE, Theve RP, Blatman RN, Shipp TD, Bianchi DW, Ward BE Jack RM 1997. Fetal origin of amniotic fluid polymorphonuclear leukocytes. Am J Obstet Gynecol, 176, 77–81. [DOI] [PubMed] [Google Scholar]
- Samstein RM, Josefowicz SZ, Arvey A, Treuting PM Rudensky AY 2012. Extrathymic generation of regulatory T cells in placental mammals mitigates maternal-fetal conflict. Cell, 150, 29–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santner-Nanan B, Peek MJ, Khanam R, Richarts L, Zhu E, Fazekas De St Groth B Nanan R 2009. Systemic increase in the ratio between Foxp3+ and IL-17-producing CD4+ T cells in healthy pregnancy but not in preeclampsia. J Immunol, 183, 7023–30. [DOI] [PubMed] [Google Scholar]
- Saravia J, Chapman NM Chi H 2019. Helper T cell differentiation. Cell Mol Immunol, 16, 634–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki Y, Darmochwal-Kolarz D, Suzuki D, Sakai M, Ito M, Shima T, Shiozaki A, Rolinski J Saito S 2007. Proportion of peripheral blood and decidual CD4(+) CD25(bright) regulatory T cells in pre-eclampsia. Clin Exp Immunol, 149, 139–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki Y, Sakai M, Miyazaki S, Higuma S, Shiozaki A Saito S 2004. Decidual and peripheral blood CD4+CD25+ regulatory T cells in early pregnancy subjects and spontaneous abortion cases. Mol Hum Reprod, 10, 347–53. [DOI] [PubMed] [Google Scholar]
- Sborgi L, Ruhl S, Mulvihill E, Pipercevic J, Heilig R, Stahlberg H, Farady CJ, Muller DJ, Broz P Hiller S 2016. GSDMD membrane pore formation constitutes the mechanism of pyroptotic cell death. EMBO J, 35, 1766–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schjenken JE, Moldenhauer LM, Zhang B, Care AS, Groome HM, Chan HY, Hope CM, Barry SC Robertson SA 2020. MicroRNA miR-155 is required for expansion of regulatory T cells to mediate robust pregnancy tolerance in mice. Mucosal Immunol, 13, 609–625. [DOI] [PubMed] [Google Scholar]
- Schober L, Radnai D, Schmitt E, Mahnke K, Sohn C Steinborn A 2012. Term and preterm labor: decreased suppressive activity and changes in composition of the regulatory T-cell pool. Immunol Cell Biol, 90, 935–44. [DOI] [PubMed] [Google Scholar]
- Schumacher A, Sharkey DJ, Robertson SA Zenclussen AC 2018. Immune Cells at the Fetomaternal Interface: How the Microenvironment Modulates Immune Cells To Foster Fetal Development. J Immunol, 201, 325–334. [DOI] [PubMed] [Google Scholar]
- Schwenkel G, Romero R, Slutsky R, Motomura K, Hsu CD Gomez-Lopez N 2021. HSP70: an alarmin that does not induce high rates of preterm birth but does cause adverse neonatal outcomes. J Matern Fetal Neonatal Med, 34, 4110–4118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serbina NV, Jia T, Hohl TM Pamer EG 2008. Monocyte-mediated defense against microbial pathogens. Annu Rev Immunol, 26, 421–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharps MC, Baker BC, Guevara T, Bischof H, Jones RL, Greenwood SL Heazell AEP 2020. Increased placental macrophages and a pro-inflammatory profile in placentas and maternal serum in infants with a decreased growth rate in the third trimester of pregnancy. Am J Reprod Immunol, 84, e13267. [DOI] [PubMed] [Google Scholar]
- Sheffield JS, Siegel D, Mirochnick M, Heine RP, Nguyen C, Bergman KL, Savic RM, Long J, Dooley KE Nesin M 2014. Designing drug trials: considerations for pregnant women. Clin Infect Dis, 59 Suppl 7, S437–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J, Gao W Shao F 2017. Pyroptosis: Gasdermin-Mediated Programmed Necrotic Cell Death. Trends Biochem Sci, 42, 245–254. [DOI] [PubMed] [Google Scholar]
- Shi J, Zhao Y, Wang K, Shi X, Wang Y, Huang H, Zhuang Y, Cai T, Wang F Shao F 2015. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature, 526, 660–5. [DOI] [PubMed] [Google Scholar]
- Shima T, Inada K, Nakashima A, Ushijima A, Ito M, Yoshino O Saito S 2015. Paternal antigen-specific proliferating regulatory T cells are increased in uterine-draining lymph nodes just before implantation and in pregnant uterus just after implantation by seminal plasma-priming in allogeneic mouse pregnancy. J Reprod Immunol, 108, 72–82. [DOI] [PubMed] [Google Scholar]
- Shima T, Sasaki Y, Itoh M, Nakashima A, Ishii N, Sugamura K Saito S 2010. Regulatory T cells are necessary for implantation and maintenance of early pregnancy but not late pregnancy in allogeneic mice. J Reprod Immunol, 85, 121–9. [DOI] [PubMed] [Google Scholar]
- Shynlova O, Nedd-Roderique T, Li Y, Dorogin A, Nguyen T Lye SJ 2013. Infiltration of myeloid cells into decidua is a critical early event in the labour cascade and post-partum uterine remodelling. J Cell Mol Med, 17, 311–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sindram-Trujillo A, Scherjon S, Kanhai H, Roelen D Claas F 2003. Increased T-cell activation in decidua parietalis compared to decidua basalis in uncomplicated human term pregnancy. Am J Reprod Immunol, 49, 261–8. [DOI] [PubMed] [Google Scholar]
- Sindram-Trujillo AP, Scherjon SA, Van Hulst-Van Miert PP, Kanhai HH, Roelen DL Claas FH 2004. Comparison of decidual leukocytes following spontaneous vaginal delivery and elective cesarean section in uncomplicated human term pregnancy. J Reprod Immunol, 62, 125–37. [DOI] [PubMed] [Google Scholar]
- Slutsky R, Romero R, Xu Y, Galaz J, Miller D, Done B, Tarca AL, Gregor S, Hassan SS, Leng Y, et al. 2019. Exhausted and Senescent T Cells at the Maternal-Fetal Interface in Preterm and Term Labor. J Immunol Res, 2019, 3128010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R 2007. Parturition. N Engl J Med, 356, 271–83. [DOI] [PubMed] [Google Scholar]
- So AK Martinon F 2017. Inflammation in gout: mechanisms and therapeutic targets. Nat Rev Rheumatol, 13, 639–647. [DOI] [PubMed] [Google Scholar]
- Son GH, Kim Y, Lee JJ, Lee KY, Ham H, Song JE, Park ST Kim YH 2019. MicroRNA-548 regulates high mobility group box 1 expression in patients with preterm birth and chorioamnionitis. Sci Rep, 9, 19746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto E, Espinoza J, Nien JK, Kusanovic JP, Erez O, Richani K, Santolaya-Forgas J Romero R 2007. Human beta-defensin-2: a natural antimicrobial peptide present in amniotic fluid participates in the host response to microbial invasion of the amniotic cavity. J Matern Fetal Neonatal Med, 20, 15–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer NR, Radnaa E, Baljinnyam T, Kechichian T, Tantengco O. a. G., Bonney E, Kammala AK, Sheller-Miller S Menon R 2021. Development of a mouse model of ascending infection and preterm birth. PLoS One, 16, e0260370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Louis D, Romero R, Plazyo O, Arenas-Hernandez M, Panaitescu B, Xu Y, Milovic T, Xu Z, Bhatti G, Mi QS, et al. 2016. Invariant NKT Cell Activation Induces Late Preterm Birth That Is Attenuated by Rosiglitazone. J Immunol, 196, 1044–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stas MR, Koch M, Stadler M, Sawyer S, Sassu EL, Mair KH, Saalmuller A, Gerner W Ladinig A 2020. NK and T Cell Differentiation at the Maternal-Fetal Interface in Sows During Late Gestation. Front Immunol, 11, 582065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenqvist AC, Nagaeva O, Baranov V Mincheva-Nilsson L 2013. Exosomes secreted by human placenta carry functional Fas ligand and TRAIL molecules and convey apoptosis in activated immune cells, suggesting exosome-mediated immune privilege of the fetus. J Immunol, 191, 5515–23. [DOI] [PubMed] [Google Scholar]
- Stinson L, Hallingstrom M, Barman M, Viklund F, Keelan J, Kacerovsky M, Payne M Jacobsson B 2020. Comparison of Bacterial DNA Profiles in Mid-Trimester Amniotic Fluid Samples From Preterm and Term Deliveries. Front Microbiol, 11, 415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockinger B Omenetti S 2017. The dichotomous nature of T helper 17 cells. Nat Rev Immunol, 17, 535–544. [DOI] [PubMed] [Google Scholar]
- Stranik J, Kacerovsky M, Vescicik P, Faist T, Jacobsson B Musilova I 2020. A rodent model of intra-amniotic inflammation/infection, induced by the administration of inflammatory agent in a gestational sac, associated with preterm delivery: a systematic review. J Matern Fetal Neonatal Med, 1–9. [DOI] [PubMed] [Google Scholar]
- Stutz A, Horvath GL, Monks BG Latz E 2013. ASC speck formation as a readout for inflammasome activation. Methods Mol Biol, 1040, 91–101. [DOI] [PubMed] [Google Scholar]
- Suff N, Karda R, Diaz JA, Ng J, Baruteau J, Perocheau D, Tangney M, Taylor PW, Peebles D, Buckley SMK, et al. 2018. Ascending Vaginal Infection Using Bioluminescent Bacteria Evokes Intrauterine Inflammation, Preterm Birth, and Neonatal Brain Injury in Pregnant Mice. Am J Pathol, 188, 2164–2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svensson-Arvelund J Ernerudh J 2015. The Role of Macrophages in Promoting and Maintaining Homeostasis at the Fetal-Maternal Interface. Am J Reprod Immunol, 74, 100–9. [DOI] [PubMed] [Google Scholar]
- Svensson-Arvelund J, Mehta RB, Lindau R, Mirrasekhian E, Rodriguez-Martinez H, Berg G, Lash GE, Jenmalm MC Ernerudh J 2015. The human fetal placenta promotes tolerance against the semiallogeneic fetus by inducing regulatory T cells and homeostatic M2 macrophages. J Immunol, 194, 1534–44. [DOI] [PubMed] [Google Scholar]
- Svensson J, Jenmalm MC, Matussek A, Geffers R, Berg G Ernerudh J 2011. Macrophages at the fetal-maternal interface express markers of alternative activation and are induced by M-CSF and IL-10. J Immunol, 187, 3671–82. [DOI] [PubMed] [Google Scholar]
- Swanson KV, Deng M Ting JP 2019. The NLRP3 inflammasome: molecular activation and regulation to therapeutics. Nat Rev Immunol, 19, 477–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sykes L, Macintyre DA, Teoh TG Bennett PR 2014. Anti-inflammatory prostaglandins for the prevention of preterm labour. Reproduction, 148, R29–40. [DOI] [PubMed] [Google Scholar]
- Taglauer ES, Adams Waldorf KM Petroff MG 2010. The hidden maternal-fetal interface: events involving the lymphoid organs in maternal-fetal tolerance. Int J Dev Biol, 54, 421–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taglauer ES, Trikhacheva AS, Slusser JG Petroff MG 2008. Expression and function of PDCD1 at the human maternal-fetal interface. Biol Reprod, 79, 562–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan W, Chen L, Guo L, Ou X, Xie D Quan S 2014. Relationship between macrophages in mouse uteri and angiogenesis in endometrium during the peri-implantation period. Theriogenology, 82, 1021–7. [DOI] [PubMed] [Google Scholar]
- Tang Q 2015. Therapeutic window of interleukin-2 for autoimmune diseases. Diabetes, 64, 1912–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Q, Henriksen KJ, Bi M, Finger EB, Szot G, Ye J, Masteller EL, Mcdevitt H, Bonyhadi M Bluestone JA 2004. In vitro-expanded antigen-specific regulatory T cells suppress autoimmune diabetes. J Exp Med, 199, 1455–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarca AL, Fitzgerald W, Chaemsaithong P, Xu Z, Hassan SS, Grivel JC, Gomez-Lopez N, Panaitescu B, Pacora P, Maymon E, et al. 2017. The cytokine network in women with an asymptomatic short cervix and the risk of preterm delivery. Am J Reprod Immunol, 78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terkeltaub RA 2003. Clinical practice. Gout. N Engl J Med, 349, 1647–55. [DOI] [PubMed] [Google Scholar]
- Terzieva A, Dimitrova V, Djerov L, Dimitrova P, Zapryanova S, Hristova I, Vangelov I Dimova T 2019. Early Pregnancy Human Decidua is Enriched with Activated, Fully Differentiated and Pro-Inflammatory Gamma/Delta T Cells with Diverse TCR Repertoires. Int J Mol Sci, 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theis KR, Romero R, Motomura K, Galaz J, Winters AD, Pacora P, Miller D, Slutsky R, Florova V, Levenson D, et al. 2020. Microbial burden and inflammasome activation in amniotic fluid of patients with preterm prelabor rupture of membranes. J Perinat Med, 48, 115–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson AJ, Royal College Of O. Gynaecologists 2019. Care of Women Presenting with Suspected Preterm Prelabour Rupture of Membranes from 24(+0) Weeks of Gestation: Green-top Guideline No. 73. BJOG, 126, e152–e166. [DOI] [PubMed] [Google Scholar]
- Thomson AJ, Telfer JF, Young A, Campbell S, Stewart CJ, Cameron IT, Greer IA Norman JE 1999. Leukocytes infiltrate the myometrium during human parturition: further evidence that labour is an inflammatory process. Hum Reprod, 14, 229–36. [PubMed] [Google Scholar]
- Thornberry NA, Bull HG, Calaycay JR, Chapman KT, Howard AD, Kostura MJ, Miller DK, Molineaux SM, Weidner JR, Aunins J, et al. 1992. A novel heterodimeric cysteine protease is required for interleukin-1 beta processing in monocytes. Nature, 356, 768–74. [DOI] [PubMed] [Google Scholar]
- Tilburgs T, Roelen DL, Van Der Mast BJ, De Groot-Swings GM, Kleijburg C, Scherjon SA Claas FH 2008. Evidence for a selective migration of fetus-specific CD4+CD25bright regulatory T cells from the peripheral blood to the decidua in human pregnancy. J Immunol, 180, 5737–45. [DOI] [PubMed] [Google Scholar]
- Tilburgs T, Roelen DL, Van Der Mast BJ, Van Schip JJ, Kleijburg C, De Groot-Swings GM, Kanhai HH, Claas FH Scherjon SA 2006. Differential distribution of CD4(+)CD25(bright) and CD8(+)CD28(−) T-cells in decidua and maternal blood during human pregnancy. Placenta, 27 Suppl A, S47–53. [DOI] [PubMed] [Google Scholar]
- Tilburgs T, Scherjon SA, Roelen DL Claas FH 2009a. Decidual CD8+CD28- T cells express CD103 but not perforin. Hum Immunol, 70, 96–100. [DOI] [PubMed] [Google Scholar]
- Tilburgs T, Scherjon SA, Van Der Mast BJ, Haasnoot GW, Versteeg VDV-MM, Roelen DL, Van Rood JJ Claas FH 2009b. Fetal-maternal HLA-C mismatch is associated with decidual T cell activation and induction of functional T regulatory cells. J Reprod Immunol, 82, 148–57. [DOI] [PubMed] [Google Scholar]
- Tilburgs T, Schonkeren D, Eikmans M, Nagtzaam NM, Datema G, Swings GM, Prins F, Van Lith JM, Van Der Mast BJ, Roelen DL, et al. 2010. Human decidual tissue contains differentiated CD8+ effector-memory T cells with unique properties. J Immunol, 185, 4470–7. [DOI] [PubMed] [Google Scholar]
- Timmons BC, Fairhurst AM Mahendroo MS 2009. Temporal changes in myeloid cells in the cervix during pregnancy and parturition. J Immunol, 182, 2700–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmons BC Mahendroo MS 2006. Timing of neutrophil activation and expression of proinflammatory markers do not support a role for neutrophils in cervical ripening in the mouse. Biol Reprod, 74, 236–45. [DOI] [PubMed] [Google Scholar]
- Tong M, Abrahams VM Chamley LW 2018. Immunological effects of placental extracellular vesicles. Immunol Cell Biol. [DOI] [PubMed] [Google Scholar]
- Tong M, Potter JA, Mor G Abrahams VM 2019. Lipopolysaccharide-Stimulated Human Fetal Membranes Induce Neutrophil Activation and Release of Vital Neutrophil Extracellular Traps. J Immunol, 203, 500–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong M, Smith AH Abrahams VM 2021. Activated Neutrophils Propagate Fetal Membrane Inflammation and Weakening through ERK and Neutrophil Extracellular Trap-Induced TLR-9 Signaling. J Immunol, 206, 1039–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracy EC, Bowman MJ, Henderson BW Baumann H 2012. Interleukin-1alpha is the major alarmin of lung epithelial cells released during photodynamic therapy to induce inflammatory mediators in fibroblasts. Br J Cancer, 107, 1534–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trifari S, Kaplan CD, Tran EH, Crellin NK Spits H 2009. Identification of a human helper T cell population that has abundant production of interleukin 22 and is distinct from T(H)-17, T(H)1 and T(H)2 cells. Nat Immunol, 10, 864–71. [DOI] [PubMed] [Google Scholar]
- Tsuda S, Nakashima A, Morita K, Shima T, Yoneda S, Kishi H Saito S 2021. The role of decidual regulatory T cells in the induction and maintenance of fetal antigen-specific tolerance: Imbalance between regulatory and cytotoxic T cells in pregnancy complications. Hum Immunol, 82, 346–352. [DOI] [PubMed] [Google Scholar]
- Tsuda S, Zhang X, Hamana H, Shima T, Ushijima A, Tsuda K, Muraguchi A, Kishi H Saito S 2018. Clonally Expanded Decidual Effector Regulatory T Cells Increase in Late Gestation of Normal Pregnancy, but Not in Preeclampsia, in Humans. Front Immunol, 9, 1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uwambaye P, Munyanshongore C, Rulisa S, Shiau H, Nuhu A Kerr MS 2021. Assessing the association between periodontitis and premature birth: a case-control study. BMC Pregnancy Childbirth, 21, 204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vacca P, Montaldo E, Croxatto D, Loiacono F, Canegallo F, Venturini PL, Moretta L Mingari MC 2015. Identification of diverse innate lymphoid cells in human decidua. Mucosal Immunol, 8, 254–64. [DOI] [PubMed] [Google Scholar]
- Vajjhala PR, Mirams RE Hill JM 2012. Multiple binding sites on the pyrin domain of ASC protein allow self-association and interaction with NLRP3 protein. J Biol Chem, 287, 41732–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van De Veerdonk FL, Netea MG, Dinarello CA Joosten LA 2011. Inflammasome activation and IL-1beta and IL-18 processing during infection. Trends Immunol, 32, 110–6. [DOI] [PubMed] [Google Scholar]
- Van Der Heijden T, Kritikou E, Venema W, Van Duijn J, Van Santbrink PJ, Slutter B, Foks AC, Bot I Kuiper J 2017. NLRP3 Inflammasome Inhibition by MCC950 Reduces Atherosclerotic Lesion Development in Apolipoprotein E-Deficient Mice-Brief Report. Arterioscler Thromb Vasc Biol, 37, 1457–1461. [DOI] [PubMed] [Google Scholar]
- Van Der Zwan A, Bi K, Norwitz ER, Crespo  C, Claas FHJ, Strominger JL Tilburgs T 2018. Mixed signature of activation and dysfunction allows human decidual CD8(+) T cells to provide both tolerance and immunity. Proc Natl Acad Sci U S A, 115, 385–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Egmond A, Van Der Keur C, Swings GM, Scherjon SA Claas FH 2016. The possible role of virus-specific CD8(+) memory T cells in decidual tissue. J Reprod Immunol, 113, 1–8. [DOI] [PubMed] [Google Scholar]
- Vargas ML, Sántos JL, Ruiz C, Montes MJ, Alemán P, García-Tortosa C García-Olivares E 1993. Comparison of the proportions of leukocytes in early and term human decidua. Am J Reprod Immunol, 29, 135–40. [DOI] [PubMed] [Google Scholar]
- Varrey A, Romero R, Panaitescu B, Miller D, Chaiworapongsa T, Patwardhan M, Faro J, Pacora P, Hassan SS, Hsu CD, et al. 2018. Human beta-defensin-1: A natural antimicrobial peptide present in amniotic fluid that is increased in spontaneous preterm labor with intra-amniotic infection. Am J Reprod Immunol, 80, e13031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazquez J, Chasman DA, Lopez GE, Tyler CT, Ong IM Stanic AK 2019. Transcriptional and Functional Programming of Decidual Innate Lymphoid Cells. Front Immunol, 10, 3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velarde MC Menon R 2016. Positive and negative effects of cellular senescence during female reproductive aging and pregnancy. J Endocrinol, 230, R59–76. [DOI] [PubMed] [Google Scholar]
- Vermillion ST, Scardo JA, Lashus AG Wiles HB 1997. The effect of indomethacin tocolysis on fetal ductus arteriosus constriction with advancing gestational age. Am J Obstet Gynecol, 177, 256–9; discussion 259–61. [DOI] [PubMed] [Google Scholar]
- Visser AA Hundt HK 1984. The pharmacokinetics of a single intravenous dose of metronidazole in pregnant patients. J Antimicrob Chemother, 13, 279–83. [DOI] [PubMed] [Google Scholar]
- Vornhagen J, Quach P, Boldenow E, Merillat S, Whidbey C, Ngo LY, Adams Waldorf KM Rajagopal L 2016. Bacterial Hyaluronidase Promotes Ascending GBS Infection and Preterm Birth. mBio, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahid HH, Dorian CL, Chin PY, Hutchinson MR, Rice KC, Olson DM, Moldenhauer LM Robertson SA 2015. Toll-Like Receptor 4 Is an Essential Upstream Regulator of On-Time Parturition and Perinatal Viability in Mice. Endocrinology, 156, 3828–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakabayashi A, Sawada K, Nakayama M, Toda A, Kimoto A, Mabuchi S, Kinose Y, Nakamura K, Takahashi K, Kurachi H, et al. 2013. Targeting interleukin-6 receptor inhibits preterm delivery induced by inflammation. Mol Hum Reprod, 19, 718–26. [DOI] [PubMed] [Google Scholar]
- Wang H, Bloom O, Zhang M, Vishnubhakat JM, Ombrellino M, Che J, Frazier A, Yang H, Ivanova S, Borovikova L, et al. 1999. HMG-1 as a late mediator of endotoxin lethality in mice. Science, 285, 248–51. [DOI] [PubMed] [Google Scholar]
- Wang SC, Li YH, Piao HL, Hong XW, Zhang D, Xu YY, Tao Y, Wang Y, Yuan MM, Li DJ, et al. 2015. PD-1 and Tim-3 pathways are associated with regulatory CD8+ T-cell function in decidua and maintenance of normal pregnancy. Cell Death Dis, 6, e1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang WJ, Hao CF, Yi L, Yin GJ, Bao SH, Qiu LH Lin QD 2010. Increased prevalence of T helper 17 (Th17) cells in peripheral blood and decidua in unexplained recurrent spontaneous abortion patients. J Reprod Immunol, 84, 164–70. [DOI] [PubMed] [Google Scholar]
- Wang Y, Wang YP, Zheng G, Lee VW, Ouyang L, Chang DH, Mahajan D, Coombs J, Wang YM, Alexander SI, et al. 2007. Ex vivo programmed macrophages ameliorate experimental chronic inflammatory renal disease. Kidney Int, 72, 290–9. [DOI] [PubMed] [Google Scholar]
- Weber MS, Prod’homme T, Youssef S, Dunn SE, Rundle CD, Lee L, Patarroyo JC, Stuve O, Sobel RA, Steinman L, et al. 2007. Type II monocytes modulate T cell-mediated central nervous system autoimmune disease. Nat Med, 13, 935–43. [DOI] [PubMed] [Google Scholar]
- Werman A, Werman-Venkert R, White R, Lee JK, Werman B, Krelin Y, Voronov E, Dinarello CA Apte RN 2004. The precursor form of IL-1alpha is an intracrine proinflammatory activator of transcription. Proc Natl Acad Sci U S A, 101, 2434–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willet KE, Kramer BW, Kallapur SG, Ikegami M, Newnham JP, Moss TJ, Sly PD Jobe AH 2002. Intra-amniotic injection of IL-1 induces inflammation and maturation in fetal sheep lung. Am J Physiol Lung Cell Mol Physiol, 282, L411–20. [DOI] [PubMed] [Google Scholar]
- Williams PJ, Searle RF, Robson SC, Innes BA Bulmer JN 2009. Decidual leucocyte populations in early to late gestation normal human pregnancy. J Reprod Immunol, 82, 24–31. [DOI] [PubMed] [Google Scholar]
- Wing DA, Fassett MJ Getahun D 2014. Acute pyelonephritis in pregnancy: an 18-year retrospective analysis. Am J Obstet Gynecol, 210, 219 e1–6. [DOI] [PubMed] [Google Scholar]
- Witt A, Sommer EM, Cichna M, Postlbauer K, Widhalm A, Gregor H Reisenberger K 2003. Placental passage of clarithromycin surpasses other macrolide antibiotics. Am J Obstet Gynecol, 188, 816–9. [DOI] [PubMed] [Google Scholar]
- Word RA, Landrum CP, Timmons BC, Young SG Mahendroo MS 2005. Transgene insertion on mouse chromosome 6 impairs function of the uterine cervix and causes failure of parturition. Biol Reprod, 73, 1046–56. [DOI] [PubMed] [Google Scholar]
- Wu HX, Jin LP, Xu B, Liang SS Li DJ 2014. Decidual stromal cells recruit Th17 cells into decidua to promote proliferation and invasion of human trophoblast cells by secreting IL-17. Cell Mol Immunol, 11, 253–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L, Li J, Xu HL, Xu B, Tong XH, Kwak-Kim J Liu YS 2016. IL-7/IL-7R signaling pathway might play a role in recurrent pregnancy losses by increasing inflammatory Th17 cells and decreasing Treg cells. Am J Reprod Immunol, 76, 454–464. [DOI] [PubMed] [Google Scholar]
- Xiong H, Zhou C Qi G 2010. Proportional changes of CD4+CD25+Foxp3+ regulatory T cells in maternal peripheral blood during pregnancy and labor at term and preterm. Clin Invest Med, 33, E422. [DOI] [PubMed] [Google Scholar]
- Xu X, Yin D, Ren H, Gao W, Li F, Sun D, Wu Y, Zhou S, Lyu L, Yang M, et al. 2018a. Selective NLRP3 inflammasome inhibitor reduces neuroinflammation and improves long-term neurological outcomes in a murine model of traumatic brain injury. Neurobiol Dis, 117, 15–27. [DOI] [PubMed] [Google Scholar]
- Xu Y, Romero R, Miller D, Kadam L, Mial TN, Plazyo O, Garcia-Flores V, Hassan SS, Xu Z, Tarca AL, et al. 2016. An M1-like Macrophage Polarization in Decidual Tissue during Spontaneous Preterm Labor That Is Attenuated by Rosiglitazone Treatment. J Immunol, 196, 2476–2491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Romero R, Miller D, Silva P, Panaitescu B, Theis KR, Arif A, Hassan SS Gomez-Lopez N 2018b. Innate lymphoid cells at the human maternal-fetal interface in spontaneous preterm labor. Am J Reprod Immunol, 79, e12820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada H, Takeda M, Maezawa Y, Ebina Y, Hazama R, Tanimura K, Wakui Y Shimada S 2012. A high dose intravenous immunoglobulin therapy for women with four or more recurrent spontaneous abortions. ISRN Obstet Gynecol, 2012, 512732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto S, Ogasawara N, Yamamoto K, Uemura C, Takaya Y, Shiraishi T, Sato T, Hashimoto S, Tsutsumi H, Takano K, et al. 2017. Mitochondrial proteins NIP-SNAP-1 and −2 are a target for the immunomodulatory activity of clarithromycin, which involves NF-kappaB-mediated cytokine production. Biochem Biophys Res Commun, 483, 911–916. [DOI] [PubMed] [Google Scholar]
- Yellon SM, Ebner CA Sugimoto Y 2008. Parturition and recruitment of macrophages in cervix of mice lacking the prostaglandin F receptor. Biol Reprod, 78, 438–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yellon SM, Greaves E, Heuerman AC, Dobyns AE Norman JE 2019. Effects of macrophage depletion on characteristics of cervix remodeling and pregnancy in CD11b-dtr mice. Biol Reprod, 100, 1386–1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo L, Romero R, Chaiworapongsa T, Para R, Johnson J, Kmak D, Jung E, Yoon BH Hsu CD 2021. Resolution of acute cervical insufficiency after antibiotics in a case with amniotic fluid sludge. J Matern Fetal Neonatal Med, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon BH, Chang JW Romero R 1998. Isolation of Ureaplasma urealyticum from the amniotic cavity and adverse outcome in preterm labor. Obstet Gynecol, 92, 77–82. [DOI] [PubMed] [Google Scholar]
- Yoon BH, Romero R, Kim KS, Park JS, Ki SH, Kim BI Jun JK 1999. A systemic fetal inflammatory response and the development of bronchopulmonary dysplasia. Am J Obstet Gynecol, 181, 773–9. [DOI] [PubMed] [Google Scholar]
- Yoon BH, Romero R, Kim M, Kim EC, Kim T, Park JS Jun JK 2000a. Clinical implications of detection of Ureaplasma urealyticum in the amniotic cavity with the polymerase chain reaction. Am J Obstet Gynecol, 183, 1130–7. [DOI] [PubMed] [Google Scholar]
- Yoon BH, Romero R, Lim JH, Shim SS, Hong JS, Shim JY Jun JK 2003. The clinical significance of detecting Ureaplasma urealyticum by the polymerase chain reaction in the amniotic fluid of patients with preterm labor. Am J Obstet Gynecol, 189, 919–24. [DOI] [PubMed] [Google Scholar]
- Yoon BH, Romero R, Moon JB, Shim SS, Kim M, Kim G Jun JK 2001. Clinical significance of intra-amniotic inflammation in patients with preterm labor and intact membranes. Am J Obstet Gynecol, 185, 1130–6. [DOI] [PubMed] [Google Scholar]
- Yoon BH, Romero R, Park JS, Kim CJ, Kim SH, Choi JH Han TR 2000b. Fetal exposure to an intra-amniotic inflammation and the development of cerebral palsy at the age of three years. Am J Obstet Gynecol, 182, 675–81. [DOI] [PubMed] [Google Scholar]
- Yoon BH, Romero R, Park JY, Oh KJ, Lee J, Conde-Agudelo A Hong JS 2019. Antibiotic administration can eradicate intra-amniotic infection or intra-amniotic inflammation in a subset of patients with preterm labor and intact membranes. Am J Obstet Gynecol, 221, 142 e1–142 e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon BH, Romero R, Yang SH, Jun JK, Kim IO, Choi JH Syn HC 1996a. Interleukin-6 concentrations in umbilical cord plasma are elevated in neonates with white matter lesions associated with periventricular leukomalacia. Am J Obstet Gynecol, 174, 1433–40. [DOI] [PubMed] [Google Scholar]
- Yoon BH, Yang SH, Jun JK, Park KH, Kim CJ Romero R 1996b. Maternal blood C-reactive protein, white blood cell count, and temperature in preterm labor: a comparison with amniotic fluid white blood cell count. Obstet Gynecol, 87, 231–7. [DOI] [PubMed] [Google Scholar]
- Yoshimura K Hirsch E 2005. Effect of stimulation and antagonism of interleukin-1 signaling on preterm delivery in mice. J Soc Gynecol Investig, 12, 533–8. [DOI] [PubMed] [Google Scholar]
- Young A, Thomson AJ, Ledingham M, Jordan F, Greer IA Norman JE 2002. Immunolocalization of proinflammatory cytokines in myometrium, cervix, and fetal membranes during human parturition at term. Biol Reprod, 66, 445–9. [DOI] [PubMed] [Google Scholar]
- Yuan X Malek TR 2012. Cellular and molecular determinants for the development of natural and induced regulatory T cells. Hum Immunol, 73, 773–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yudin MH, Van Schalkwyk J, Eyk NV, Infectious Diseases C Maternal Fetal Medicine C 2009. Antibiotic therapy in preterm premature rupture of the membranes. J Obstet Gynaecol Can, 31, 863–867. [DOI] [PubMed] [Google Scholar]
- Zahran AM, Zharan KM Hetta HF 2018. Significant correlation between regulatory T cells and vitamin D status in term and preterm labor. J Reprod Immunol, 129, 15–22. [DOI] [PubMed] [Google Scholar]
- Zenclussen AC, Gerlof K, Zenclussen ML, Sollwedel A, Bertoja AZ, Ritter T, Kotsch K, Leber J Volk HD 2005. Abnormal T-cell reactivity against paternal antigens in spontaneous abortion: adoptive transfer of pregnancy-induced CD4+CD25+ T regulatory cells prevents fetal rejection in a murine abortion model. Am J Pathol, 166, 811–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zenclussen ML, Thuere C, Ahmad N, Wafula PO, Fest S, Teles A, Leber A, Casalis PA, Bechmann I, Priller J, et al. 2010. The persistence of paternal antigens in the maternal body is involved in regulatory T-cell expansion and fetal-maternal tolerance in murine pregnancy. Am J Reprod Immunol, 63, 200–8. [DOI] [PubMed] [Google Scholar]
- Zhai Y, Meng X, Ye T, Xie W, Sun G Sun X 2018. Inhibiting the NLRP3 Inflammasome Activation with MCC950 Ameliorates Diabetic Encephalopathy in db/db Mice. Molecules, 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Lin Y, Li Y, Zhao D Du M 2021. Mesenchymal stem cells enhance Treg immunosuppressive function at the fetal-maternal interface. J Reprod Immunol, 148, 103366. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Liu Z, Tian M, Hu X, Wang L, Ji J Liao A 2018. The altered PD-1/PD-L1 pathway delivers the ‘one-two punch’ effects to promote the Treg/Th17 imbalance in pre-eclampsia. Cell Mol Immunol, 15, 710–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Asahina A, Asawanonda P, Frez ML, Imafuku S, Hyun Kim D, Theng C, Wang L, Zhang JA Zimmo, S. 2021. Systematic review and practical guidance on the use of topical calcipotriol and topical calcipotriol with betamethasone dipropionate as long-term therapy for mild-to-moderate plaque psoriasis. J Dermatol, 48, 940–960. [DOI] [PubMed] [Google Scholar]
- Zhu L, Chen H, Liu M, Yuan Y, Wang Z, Chen Y, Wei J, Su F Zhang J 2017. Treg/Th17 Cell Imbalance and IL-6 Profile in Patients With Unexplained Recurrent Spontaneous Abortion. Reprod Sci, 24, 882–890. [DOI] [PubMed] [Google Scholar]


