Cardiorespiratory fitness (CRF) and habitual physical activity (PA) are hallmarks of cardiometabolic health1. Greater PA leads to higher CRF2, reduced cardiovascular risk, and improved longevity, but the molecular mechanisms linking PA and CRF are incompletely elucidated. Given a growing literature around proteomic, metabolomic, and genomic methods for studying exercise, large population-based studies with careful phenotyping may offer unique insights into determinants and mechanisms underlying CRF. To better understand the influence of PA on metabolism, we related circulating metabolites to two components of PA (moderate-vigorous PA [MVPA] and steps/day) and to sedentary time (SED) in >1000 community-dwelling individuals. By aligning relations of PA measures and circulating metabolites with previously published data on metabolite responses to acute exercise3, we sought to prioritize metabolites that are associated with PA/SED and that may be modifiable with acute exercise and implicated in CRF (Panel A).
Figure 1.
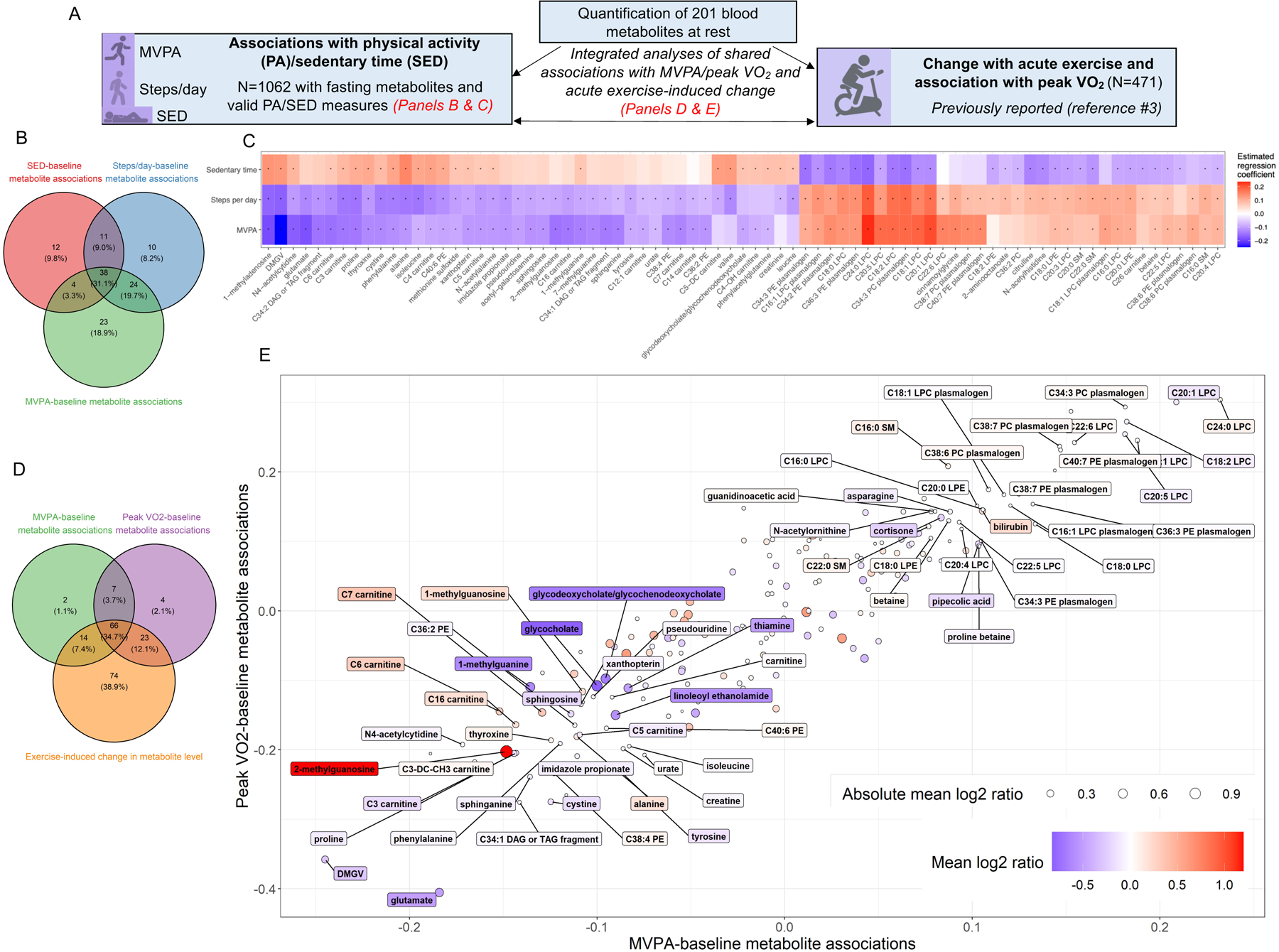
Panel (A) includes a schematic of the study design. Panel (B) includes a Venn diagram illustrating the overlap of statistically significant (at FDR 5%) associations of resting metabolites with MVPA, steps/day, and SED (N=1062; linear models relating metabolite [dependent variable] to PA measure with adjustment for age, sex, location, season, and device wear time). Metabolite levels were log-transformed and rank-normalized (resulting in mean=0 and SD=1) for analysis. In Panel (C), the regression coefficients from individual associations of metabolites and MVPA, steps/day, and SED are shown for all metabolites associated with ≥2 PA measures, in a Heatmap format. The “*” indicates associations that are statistically significant (at FDR 5%). Panel (D) shows a Venn diagram displaying statistically significant associations (at FDR 5%) of metabolites with MVPA, associations of resting metabolites with peak VO2 (N=471, from prior report3; linear models relating peak VO2 [dependent variable] to metabolite with adjustment for age and sex), and change in metabolite level from rest to peak exercise (N=411, from prior report3; the log2 fold change in metabolite levels were assessed using 2-tailed t-tests). Panel (E), metabolites were plotted according to their association with MVPA (X-axis), association with peak VO2 (Y-axis) and their exercise-induced change (circle). The circle size reflects the magnitude of change from rest to peak exercise (expressed as the absolute value of the mean log2[peak/rest] ratio), and the coloring of the dot and the label reflect the magnitude and direction of change in the log2[peak/rest] ratio. The 66 individual metabolites with statistically significant (at FDR 5%) associations with MVPA and peak VO2 and with significant changes with exercise are labeled. Abbreviations: SED, sedentary time; MVPA, moderate-vigorous physical activity.
Framingham Heart Study (FHS) Generation 3 participants wore omnidirectional accelerometers (Philips Respironics) during waking hours for eight days following their third study visit (2016–2019)2. We included 1062 participants with valid accelerometry data (≥10 hours; ≥3 days) and metabolite profiling performed on fasting peripheral blood samples. MVPA was defined as counts >1535/minute and was log-transformed for analysis. SED was defined as counts <100/minute (standardized to an 18-hour day). Metabolite profiling was performed as described3. Participants also underwent maximum effort cardiopulmonary exercise testing at their third study visit (exercise time 11.9±2.1 minutes) with blood sampling at rest and peak exercise; metabolic changes and associations with peak CRF (VO2) were reported (N=471)3. A Benjamini-Hochberg false-discovery rate (FDR) of 5% was used to account for multiplicity for all analyses. The study was approved by Institutional Review Boards at Massachusetts General Hospital and Boston University Medical Campus. All participants provided written informed consent. Analyses were performed in SAS 9.4 and R. Data supporting the study findings will be made available on reasonable request.
The study sample (N=1062) was 52% women and had the following characteristics: age, 54±8 years; body mass index, 27.7±5.2 kg/m2; MVPA, 22.8±20.0 minutes/day; steps/day, 7964±3739; SED, 13.4±1.4 hours/day; peak VO2, 23.8±7.4 ml/kg/min. (Additional clinical characteristics and a full list of assayed metabolites are available upon request.) In linear models adjusted for age, sex, device wear time, season, and participant location (New England vs. other, accounting for seasonal difference), 89, 83, and 65 of 201 assayed metabolites were associated with MVPA, steps/day, and SED, respectively (Panel B). The majority of statistically significant metabolites were common among ≥2 PA/SED measures and demonstrated directional concordance (Panel C). Lower PA/higher SED was directly associated with metabolites previously linked with increased cardiometabolic risk (e.g., dimethylguanidino valeric acid [DMGV], glutamate, isoleucine, valine, tyrosine, phenylalanine, sphingosine, glycodeoxycholate4). Similarly, several putatively “cardioprotective” metabolites (e.g., betaine, plasmalogen species) were associated with higher PA/lower SED. We also observed relations of PA measures with metabolites with less clearly defined roles in CRF and cardiometabolic disease (e.g., nucleoside derivatives 1-methyladenosine, 2-methylguanosine, and pteridine derivative xanothopterin).
Next, we identified metabolites that were not only associated with greater PA but that were also modified by acute exercise and related to CRF (peak VO2). Of 89 MVPA-associated metabolites, 66 (74%) demonstrated statistically significant peak VO2 associations and changes with acute exercise (Panel D). Individual metabolites were broadly concordant in the direction of association with MVPA and peak VO2 (Panel E). However, among metabolites that were positively and negatively associated with MVPA/peak VO2, we observed variability in the direction of change with acute exercise. For example, DMGV and glutamate were associated with lower MVPA/peak VO2 and decreased with acute exercise, whereas betaine and plasmologens were associated with higher MVPA/peak VO2 and increased with exercise. Directional concordance across MVPA/peak VO2 associations and changes with acute exercise may identify metabolites relevant to exercise-mediated benefits on cardiometabolic health. Indeed, DMGV has been shown to decrease with acute exercise3 and longer-term exercise training5 and is linked to cardiometabolic disease. By contrast, we observed discordance regarding exercise-induced changes and MVPA/peak VO2 associations for several metabolites including acylcarnitines, 1- and 2-methylguanosine, cortisone, and lysophasphatidylcholines. Metabolites with opposing directionality may be involved in substrate availability/handling during increased energy requirements. For example, increased circulating acylcarnitines may reflect augmented mitochondrial lipid utilization4.
While our analysis is limited by the lack of repeated measures of CRF after subjecting participants to exercise training, the results provide a snapshot at the intersection of PA and CRF, allowing future targeted studies to evaluate modifiability of selected metabolites with exercise training and how results may differ by sex and underlying health status. Traditional cross-sectional approaches to identifying molecular biomarkers of PA or CRF rely on single measures. By evaluating the full landscape of exercise (e.g., habitual PA, peak CRF, acute exercise perturbation), our approach more directly assessed the potential implications of molecular signatures of PA, thereby highlighting specific molecular signatures of PA that may be more amenable to genetic and mechanistic interrogation.
Sources of Funding:
The Framingham Heart Study is supported by the National Heart, Lung and Blood Institute NHLBI Contracts N01-HC-25195, HHSN268201500001I, and 75N92019D00031. This work was supported by NIH grants K23-HL138260 and R01-HL156975 (MN), R01-HL131029 (Dr. Vasan and GDL), and AHA grant 15GPSGC24800006 (GDL). Dr. Nayor is supported by a Career Investment Award from the Department of Medicine, Boston University School of Medicine. Dr. Malhotra was supported by the National Heart, Lung, and Blood Institute (R01HL142809), the American Heart Association (18TPA34230025), and the Wild Family Foundation. Dr. Vasan is supported in part by the Evans Medical Foundation and the Jay and Louis Coffman Endowment from the Department of Medicine, Boston University School of Medicine.
Disclosures:
Dr. Spartano acknowledges research support from the Alzheimer’s Association and has also received funding from Novo Nordisk for a MD-initiated research grant unrelated to the current paper. Dr. Murthy has stock holdings in General Electric and receives research funding from Siemens. Dr. Malhotra receives research funding from Bayer and Amgen and serves as a consultant for Myokardia/BMS and Third Pole. Dr. Malhotra is a co-founder of Patch, Inc. Dr. Malhotra is a co-inventor for a patent on pharmacologic BMP inhibitors (along with Mass General Brigham) for which he receives royalties. Dr. Malhotra receives royalties from UpToDate for scientific content authorship. Dr. Murabito has served as a guest lecturer/consultant at Merck. Dr. Shah is supported in part by grants from the National Institutes of Health and the American Heart Association. In the past 12 months, Dr. Shah has served as a consultant for Myokardia (ongoing) and Best Doctors (ongoing), receives research funding from Amgen (concluded), had minor stock holdings in Gilead, and his spouse has current stock holdings in Pfizer. Dr. Shah is a co-inventor on a patent for ex-RNAs signatures of cardiac remodeling. Dr. Lewis acknowledges research funding from the National Institutes of Health and the American Heart Association as well as Amgen, Cytokinetics, Applied Therapeutics, AstraZeneca, Sonivie in relation to projects and clinical trials investigating exercise capacity that are distinct from this work. He has served as a scientific advisor for Pfizer, Merck, Boehringer-Ingelheim, Novartis, American Regent, Relypsa, Cyclerion, Cytokinetics, and Amgen and receives royalties from UpToDate for scientific content authorship related to exercise physiology.
References
- 1.Ross R, Blair SN, Arena R, Church TS, Despres JP, Franklin BA, Haskell WL, Kaminsky LA, Levine BD, Lavie CJ, et al. Importance of Assessing Cardiorespiratory Fitness in Clinical Practice: A Case for Fitness as a Clinical Vital Sign: A Scientific Statement From the American Heart Association. Circulation. 2016;134:e653–e699. [DOI] [PubMed] [Google Scholar]
- 2.Nayor M, Chernofsky A, Spartano NL, Tanguay M, Blodgett JB, Murthy VL, Malhotra R, Houstis NE, Velagaleti RS, Murabito JM, et al. Physical activity and fitness in the community: the Framingham Heart Study. Eur Heart J. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nayor M, Shah RV, Miller PE, Blodgett JB, Tanguay M, Pico AR, Murthy VL, Malhotra R, Houstis NE, Deik A, et al. Metabolic Architecture of Acute Exercise Response in Middle-Aged Adults in the Community. Circulation. 2020;142:1905–1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheng S, Shah SH, Corwin EJ, Fiehn O, Fitzgerald RL, Gerszten RE, Illig T, Rhee EP, Srinivas PR, Wang TJ, et al. Potential Impact and Study Considerations of Metabolomics in Cardiovascular Health and Disease: A Scientific Statement From the American Heart Association. Circ Cardiovasc Genet. 2017;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Robbins JM, Herzig M, Morningstar J, Sarzynski MA, Cruz DE, Wang TJ, Gao Y, Wilson JG, Bouchard C, Rankinen T, et al. Association of Dimethylguanidino Valeric Acid With Partial Resistance to Metabolic Health Benefits of Regular Exercise. JAMA Cardiol. 2019;4:636–643. [DOI] [PMC free article] [PubMed] [Google Scholar]


