Abstract
Objective:
Enlarged perivascular spaces have emerged as markers of cerebral small vessel disease and are linked to perivascular drainage dysfunction. The Apolipoprotein E ɛ4 allele is the strongest genetic risk factor for cerebral amyloid angiopathy and Alzheimer’s neuropathology, but the underlying mechanisms remain unclear. We studied the relationship between APOE-ɛ4 and the topography and burden of enlarged perivascular spaces to elucidate underlying mechanisms between APOE-ɛ4 and adverse clinical outcomes.
Methods:
We included 3,564 Framingham Heart Study participants with available genotypes and magnetic resonance imaging. Enlarged perivascular spaces in the basal ganglia and centrum semiovale were rated using a validated scale. We related APOE-ɛ4 allele presence to high burden of enlarged perivascular spaces in each region and a mixed score reflecting high burden in both regions using multivariable logistic regression. Exploratory analyses incorporated presence of cerebral microbleeds and assessed effect modification by hypertension.
Results:
Mean age was 60.7 years (SD: 14.6), 1644 (46.1%) were male, 1486 (41.8%) were hypertensive, and 836 (23.5%) participants were APOE-ɛ4 carriers. APOE-ɛ4 was associated with high burden of enlarged perivascular spaces in the centrum semiovale (OR 1.45, 95% CI: 1.16, 1.81) and mixed regions (OR: 1.37; 95% CI: 1.11, 1.68). Associations were slightly stronger in hypertensive subjects.
Interpretation:
The APOE-ɛ4 allele plays a modest role in the burden of enlarged perivascular spaces in the centrum semiovale. Further studies are needed to clarify the underlying small vessel disease type in community-dwelling individuals with predominant centrum semiovale enlarged perivascular spaces, which may be hypertensive angiopathy in our sample.
Introduction
Enlarged perivascular spaces (ePVS) visible on magnetic resonance imaging (MRI) represent an emerging marker of cerebral small vessel disease (CSVD), which is associated with greater risk of stroke and dementia.1 Perivascular spaces are in the interface between cerebral vessels and brain parenchyma and are considered to represent drainage routes for cerebral metabolites, including beta amyloid (Aβ).2 When visible on MRI, perivascular spaces are considered to be enlarged, reflecting CSVD and glymphatic dysfunction, and may help further understand the preclinical pathophysiology leading to stroke and adverse cognitive outcomes. Prior literature suggests a role for glymphatic dysfunction in the pathogenesis of CSVD2 but it is likely that once advanced CSVD is established, a vicious cycle ensues where CSVD triggers perivascular drainage dysfunction, in turn promoting further CSVD development.
The brain topography of enlarged perivascular space (ePVS) burden has been attributed to different types of CSVD; lobar (centrum semiovale, CSO) distribution has been attributed to cerebral amyloid angiopathy (CAA), deep (basal ganglia, BG) distribution to cerebral hypertensive angiopathy, and mixed locations in the BG and CSO attributed to an interplay of both.3–5 CSO ePVS have been associated with amyloid PET positivity in patients with spontaneous ICH6, thus suggesting a role for ePVS in the CSO as markers of CAA. Additionally, juxtacortical ePVS have been associated with cerebral microbleeds (CMBs),7 specifically lobar CMBs, which are also considered to be markers of CAA, while BG ePVS have been related to deep location CMBs.4 Thus, it has been suggested that CMBs in the CSO may reflect CAA, while those in deep regions or mixed lobar and deep regions may reflect hypertensive angiopathy.8,9
The apolipoprotein E (APOE) ɛ4 allele has been related to higher risk of lobar intracerebral hemorrhage (ICH)10 and Alzheimer’s disease (AD).11 APOE-ɛ4 is strongly associated with CAA, which is the main cause of primary lobar ICH in the elderly12 and is frequently encountered in patients with vascular dementia13 and AD.14 APOE-ɛ4 allele presence may lead to impaired perivascular clearance of Aβ15, which could be reflected in higher burden of ePVS in lobar regions. On the other hand, APOE-ɛ4 may also interact synergistically with hypertension, both in its effects on stroke and dementia risk and subclinical manifestations of CSVD.16 In a prior meta-analysis, APOE-ɛ4 was related to higher burden of MRI markers of small vessel disease, including white matter hyperintensities and cerebral microbleeds.17 Thus, a potential mechanism linking presence of the ɛ4 allele with stroke and dementia is through the main forms of CSVD: CAA and hypertensive angiopathy.
However, the relation of APOE genotype and ePVS is unclear, and study of this relationship may provide insight into the early pathophysiology linking APOE genotypes with risk of stroke and dementia, which may help improve preventive strategies. We hypothesized that APOE-ɛ4 is associated with higher ePVS burden and the association will vary according to brain topography, in turn reflecting different underlying predominant CSVD subtypes. We hypothesized stronger associations with high ePVS burden in the CSO and in mixed brain regions and associations modified by hypertension status.
Methods
Study Sample
We included participants from the following cohorts from the Framingham Heart Study (FHS): Original, Offspring, Third Generation, OMNI 1, and New Offspring Spouse. The FHS began in 1948 and enrolled 5,209 residents of Framingham, MA into the Original cohort to study the epidemiology of cardiovascular disease. Starting in 1971, 5,124 children of participants of the Original cohort were enrolled into the Offspring cohort. Recruitment for the Third Generation started in 2002 and comprised of 4,095 participants who had at least one parent in the Offspring cohort. The New Offspring Spouse cohort, which began at the same time as the Third Generation, enrolled 103 parents of Third Generation participants not previously enrolled in the Offspring cohort. Lastly, the OMNI 1 cohort, starting in 1994, enrolled 506 participants to increase racial diversity of the FHS.18 The Institutional Review Board of Boston University Medical Center approved the study protocol and informed consent was obtained from all subjects.
Participants who underwent brain MRI and had ePVS assessments were included in the present study. Brain MRI data were collected at exam cycles 25 through 31 for the Original cohort (1998 – 2010), exam 7 through 9 for the Offspring cohort (1998–2014), exams 1 and 2 for the Third Generation (2008–2011), exam 2 for New Offspring Spouses (2008–2010), and 2 through 4 for OMNI 1 (1999–2013).
In the FHS, there are 10,589 available MRI scans from 5,594 subjects, of which 4,658 MRI scans from 3,998 participants had ePVS assessment. Exclusion criteria for the present study included history of dementia, stroke, and other neurological conditions (e.g. head trauma, multiple sclerosis, brain tumor) that could affect brain MRI measurements. After these exclusions, 4,199 MRI scans from 3,710 participants remained. 22% of participants underwent multiple MRI examinations, with some participants undergoing multiple MRIs in an exam cycle. Among these participants with multiple measurements, we first selected measurements corresponding to a unique primary clinic exam. Then we selected the latest MRI examination for each participant to use in the analysis. After removing 146 participants with missing genotype data, the total sample size was 3,564 (177 Original, 1482 Offspring, 17 New Offspring Spouse, 1758 Third Generation, and 130 OMNI 1 participants). The sample selection flow chart is shown in Figure 1.
Figure 1:
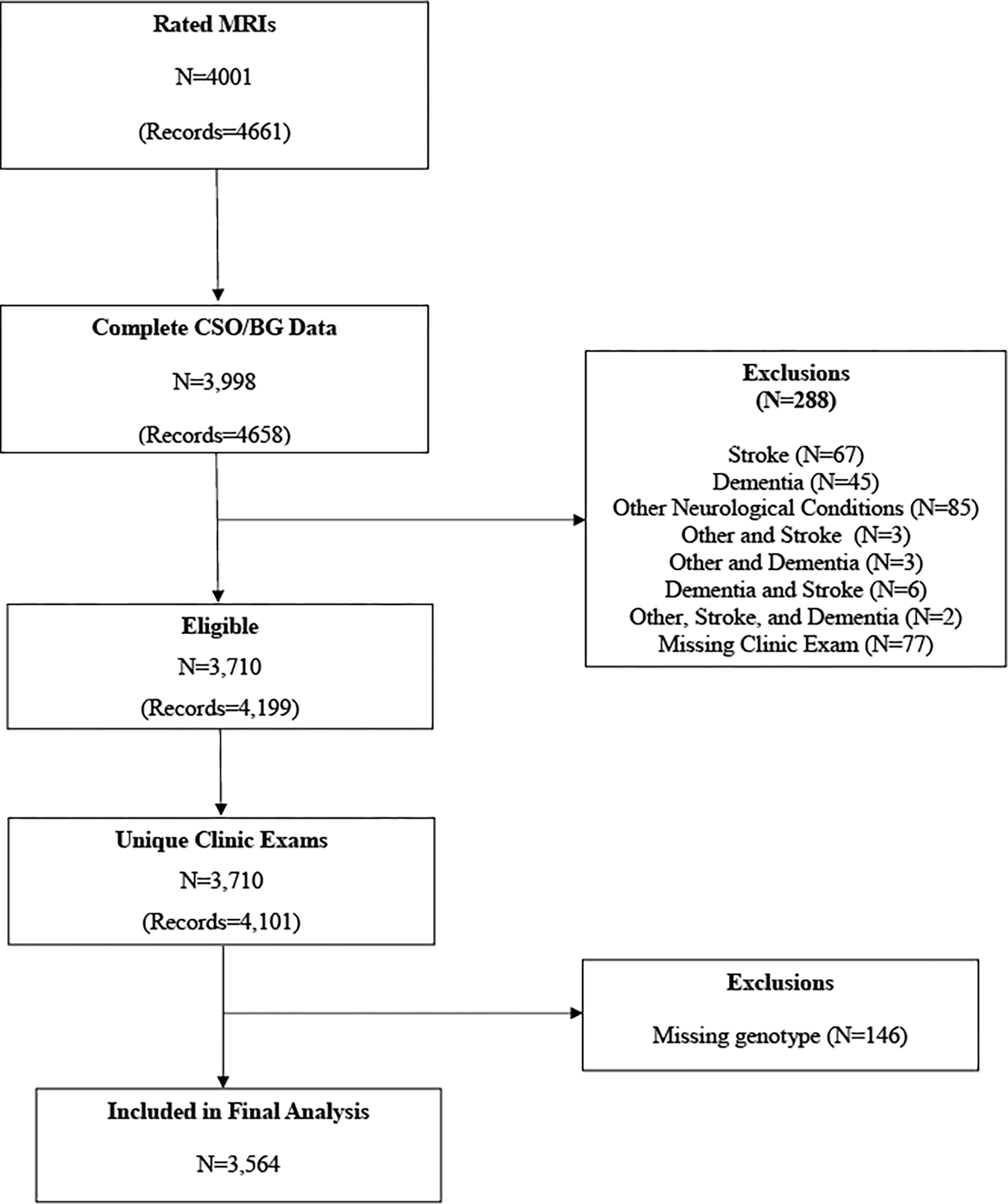
Sample Selection
Brain MRI: Acquisition and Analysis
Brain MRI acquisition measures and image processing methods have been described in detail.19,20 Participants were imaged on a 1T (1999–2005) or 1.5T (after 2005) Magnetom scanner (Siemens Medical, Erlangen, Germany).
ePVS Ratings
MRI characteristics of enlarged perivascular spaces were based on consensus criteria by the Standards for Reporting Vascular Changes on Neuroimaging Criteria (STRIVE consortium).21 ePVS met the following criteria: diameter less than 3mm, signal intensity similar to cerebrospinal fluid on all sequences, adherence to the course of penetrating vessels, and linear (parallel to the penetrating vessel) or round/ovoid (perpendicular to the penetrating vessel).
Three investigators (JRR, AS, PP) rated the MRI scans and were blinded to the subjects’ demographic and clinical information and other imaging sequences. ePVS ratings were performed on T2-weighted axial MRI sequences using a validated method which groups ePVS by brain topography into centrum semiovale (CSO) and basal ganglia (BG).22 The CSO area includes MRI slices above the roof of the lateral ventricles up through the subcortical white matter in cerebral hemispheres. The BG region involves the deep structures including the caudate nucleus, internal capsule, thalamus, lent inform nucleus, external capsule and insular cortex. We also used a mixed grouping method to describe ePVS burden in both regions.
The burden of ePVS was categorized into grades based on ePVS counts: I (1–10), II (11–20), III (20–40) and IV (>40). A subset of scans (legacy scans from the older MRI dataset above) were rated using coronal acquisitions due to the higher resolution in this sequence than in the axial views, following the method we recently reported (F.R. Lara & A. Scruton, unpublished data). In scans with available axial and coronal slices with good resolution, independent ePVS ratings were done in each region, blinded to ratings of the other view (i.e. ratings done using axial views were blinded and independent of ratings using coronal views). We observed that ratings using coronal or axial views were highly correlated (ICC=0.90) in both brain regions.
ePVS rating reliability measures
Intra-rater reproducibility was assessed using 200 scans (JRR, PP) or 20 scans (AS) on two separate occasions two to four weeks apart. The order of scans was changed randomly between the two reading sessions. The intra-rater reliability was good to excellent (ICC: basal ganglia, JRR and PP=0.76, AS=0.81; centrum semiovale JRR = 0.81, PP=0.76, AS=0.83).
Inter-rater reproducibility measures were also compared between the primary rater (JRR) and secondary raters (PP, AS) using 200 scans (JRR vs PP), and 20 scans (JRR vs AS). Inter-rater reliability comparing two independent readers was excellent (ICC basal ganglia JRR vs PP = 0.86, JRR vs. AS =0.81; centrum semiovale JRR vs. PP= 0.8, JRR vs. AS= 0.81).
Cerebral microbleeds (CMB)
Ratings of CMBs were performed following published guidelines23 and methods have been described in detail.24 Reproducibility of CMB ratings in the FHS is good to excellent.25 Presence of CMBs were categorized according to their brain location: any CMB (CMB in any brain region), lobar only, deep only, and mixed location (lobar or deep).
APOE Genotype
APOE genotyping in the Framingham Heart Study has been described elsewhere.26 Participants carrying the ɛ2/ɛ4, ɛ3/ɛ4, or ɛ4/ɛ4 genotype were defined as APOE-ɛ4 carriers. Participants carrying the ɛ2/ɛ2, ɛ2/ɛ3, ɛ3/ɛ3 genotypes were defined as APOE-ɛ4 non-carriers.
Other clinical characteristics
Demographic and clinical characteristics of interest were measured at the closest exam cycle to MRI (up to 5 years before or 2 years after MRI). Systolic and diastolic blood pressures (mmHg) were each taken as the average of the Framingham clinic physician’s two measurements and treated as continuous variables. Hypertension was evaluated using the JNC-7 criteria as SBP ≥140 mm Hg or DBP≥90 mm Hg or use of antihypertensive medications. Current cigarette smoking (yes/no) was defined as self-reported use in the year prior to the examination. Diabetes was diagnosed either by fasting plasma glucose ≥126 mg/dL (Offspring, Third Generation, NOS, and OMNI I examinations), non-fasting plasma glucose ≥200 mg/dL (Original cohort examinations), or treatment with insulin or an oral hypoglycemic agent. Lastly, current use of antihypertensive medications (yes/no) was collected for our sample.
Statistical Analysis
Descriptive statistics of demographic variables and risk factors are described for the overall sample, including means and standard deviations of continuous variables and frequencies and relative frequencies of categorical variables.
To reflect the predominantly presumed underlying form of CSVD (CAA or hypertensive angiopathy) our main outcomes of interest were CSO ePVS, BG ePVS, and ePVS in the mixed CSO-BG regions. Ratings in the CSO and BG were assigned independently and irrespective of ratings in the other region. In each brain region, we defined high burden ePVS as counts greater than 20 (i.e. grade III or IV) to reflect high ePVS burden as previously suggested.4 This definition was used to create a mixed score to reflect the number of regions with high ePVS burden (0=none, 1=either CSO or BG region, 2=both CSO and BG regions), which likely represents the combined effect of CSVD subtypes or advanced hypertensive angiopathy.
Multivariable logistic regression was used to investigate the association between APOE-ɛ4 presence and ePVS burden. We used ordinal logistic regression for CSO-BG mixed score and binary logistic regression for high burden CSO and BG ePVS. In secondary analyses, we also evaluated CSO and BG ePVS (grades I-IV) using ordinal and multinomial logistic regression models. However, we do not report results from these models because of violation of the proportional odds assumption in the case of the ordinal logistic regression models and convergence failure in the case of the generalized logit models.
Models were adjusted for age at MRI, sex, FHS cohort, and time interval between exam cycle and brain MRI acquisition. Additional models further adjusted for the following vascular risk factors: hypertension status, history of diabetes, and current smoking status. Seventeen participants in the New Offspring Spouse cohort were combined into the Third Generation cohort for analysis since these cohorts had the same enrollment periods. Adjustment for MRI sequence used for ePVS ratings (axial vs. coronal) did not change results.
In exploratory analyses, we stratified our analyses by hypertension status to investigate whether hypertension modifies the association between APOE ɛ4 allele presence and ePVS burden in each brain region and in the mixed regions. We also used logistic regression analysis to relate ePVS burden to the presence of CMBs (overall and by brain topography) to further elucidate the likely underlying predominant CSVD type. Two new ePVS categorizations were used as independent variables in this analysis: high ePVS burden strictly in the CSO (i.e. excluding any participants with concurrent high BG ePVS burden), and high burden ePVS strictly in the BG (i.e. excluding participants who had concurrent high CSO burden) to reflect exclusive topography of ePVS, presumably CAA or hypertensive angiopathy for the CSO and BG respectively.
All analyses were performed using Statistical Analysis System (SAS) software version 9.4 (SAS Institute, Cary, NC). A two-sided p-value <0.05 was considered statistically significant.
Results
Baseline characteristics by ePVS grade and region are described in Table 1. We observed older age and higher proportions of vascular risk factors among participants with high ePVS burden across all brain regions. After adjusting for age, sex, FHS cohort, and time interval between clinic exam and MRI, we observed that APOE-ɛ4 presence was associated with higher burden of ePVS in the CSO region (OR: 1.45, 95% CI: 1.16, 1.81; p=0.002) (Table 2). We also found that APOE-ɛ4 was associated with high ePVS burden in mixed regions (OR: 1.37, 95% CI: 1.11, 1.68; p=0.004), likely driven by CSO ePVS associations. There was no significant association between APOE-ɛ4 and ePVS burden in the BG region (OR: 1.15, 95% CI: 0.86, 1.53, p=0.23). Similar estimates were observed when additionally adjusting for smoking status, hypertension, and diabetes.
Table 1:
Sample characteristics overall and stratified by ePVS burden
| Centrum Semiovale (CSO) | Basal Ganglia (BG) | CSO-BG Mixed Scorea | ||||||
|---|---|---|---|---|---|---|---|---|
| Overall N=3,564 | Grade I-II N=2,933 | Grade III-IV N=630 | Grade I-II N=3,216 | Grade III-IV N=348 | None N=2,809 | One N=530 | Both N=224 | |
| Clinical Characteristics | ||||||||
| Male, n (%) | 1644 (43) | 1376 (47) | 267 (42) | 1497 (47) | 147 (42) | 1322 (47) | 228 (43) | 93 (42) |
| Age at exam closest to baseline MRI, yrs, mean [SD] | 59.1 (14.8) | 55.8 (14) | 72.2 (10.9) | 56.9 (14.2) | 75.4 (9.8) | 55.1 (13.6) | 70.7 (10.8) | 76.5 (9.5) |
| Age at MRI, yrs, mean [SD] | 61.1 (14.5) | 57.9 (13.8) | 73.9 (10.6) | 59.0 (14) | 77.1 (9.4) | 57.1 (13.4) | 72.4 (10.5) | 78.2 (9.1) |
| APOE ε4+, n (%) | 836 (23) | 663 (23) | 173 (27) | 752 (23) | 84 (24) | 639 (23) | 137 (26) | 60 (27) |
| APOE genotypes, n (%) | ||||||||
| ε2/ε2 | 19 (1) | 17 (1) | 2 (0) | 18 (1) | 1 (0) | 17 (1) | 1 (0) | 1 (0) |
| ε2/ε3 | 442 (12) | 383 (13) | 79 (12) | 415 (12) | 47 (13) | 363 (12) | 72 (13) | 27 (11) |
| ε2/ε4 | 75 (2) | 57 (2) | 20 (3) | 74 (2) | 3 (1) | 55 (2) | 21 (4) | 1 (0) |
| ε3/ε3 | 2267 (64) | 1968 (64) | 413 (61) | 2148 (64) | 233 (63) | 1880 (64) | 356 (62) | 145 (62) |
| ε3/ε4 | 699 (20) | 583 (19) | 146 (22) | 654 (19) | 75 (20) | 562 (19) | 113 (20) | 54 (23) |
| ε4/ε4 | 62 (2) | 48 (2) | 14 (2) | 54 (2) | 8 (2) | 47 (2) | 8 (1) | 7 (3) |
| FHS cohort, n (%) | ||||||||
| Original | 177 (5) | 72 (2) | 105 (17) | 108 (3) | 69 (20) | 59 (2) | 62 (12) | 56 (25) |
| Offspring | 1482 (42) | 1049 (36) | 432 (69) | 1243 (39) | 239 (69) | 958 (34) | 375 (71) | 148 (66) |
| NOS/Third Gen | 1775 (50) | 1700 (58) | 75 (12) | 1748 (54) | 27 (8) | 1690 (60) | 68 (13) | 17 (8) |
| OMNI 1 | 130 (4) | 112 (4) | 18 (3) | 117 (4) | 13 (4) | 102 (4) | 25 (5) | 3 (1) |
| Time between exam and MRI, yrs, mean (SD) | 2.0 (1.1) | 2.1 (1.0) | 1.7 (1.1) | 2.0 (1.1) | 1.6 (1.1) | 2.1 (1.0) | 1.7 (1.1) | 1.6 (1.2) |
| Vascular risk factors | ||||||||
| Systolic blood pressure, mm Hg, mean [SD] | 123.0 (17.3) | 120.9 (16.3) | 131.2 (18.8) | 121.4 (16.5) | 135.2 (18.9) | 120.4 (16.0) | 129.6 (18.8) | 136.2 (18.5) |
| Diastolic blood pressure, mm Hg, mean [SD] | 73.1 (9.8) | 73.5 (9.6) | 71.1 (10.3) | 73.2 (9.6) | 73 (9.9) | 73.6 (9.5) | 71.4 (10.3) | 70.9 (10.7) |
| Hypertensionb, n (%) | 1486 (42) | 1063 (36) | 423 (67) | 1223 (38) | 263 (76) | 975 (35) | 336 (64) | 175 (78) |
| Current smokers, n (%) | 272 (8) | 226 (8) | 46 (7) | 256 (8) | 16 (5) | 221 (8) | 40 (8) | 11 (5) |
| Diabetes mellitus, n (%) | 372 (11) | 272 (9) | 100 (17) | 324 (10) | 48 (15) | 254 (9) | 88 (18) | 30 (15) |
| Antihypertensive use, n (%) | 1219 (34) | 867 (30) | 352 (57) | 994 (31) | 225 (65) | 789 (28) | 283 (54) | 147 (66) |
| Cerebral Microbleeds | ||||||||
| Any CMB N=2,046 | 100 (5) | 78 (4) | 22 (10) | 85 (4) | 15 (17) | 76 (4) | 11 (7) | 13 (20) |
| Any Lobar N=2,032 | 86 (4) | 68 (4) | 18 (9) | 73 (4) | 13 (15) | 66 (4) | 9 (6) | 11 (17) |
| Any Deep N=1971 | 25 (1) | 15 (1) | 10 (5) | 20 (1) | 5 (6) | 15 (1) | 5 (3) | 5 (9) |
| Deep and Lobar N=1957 | 11 (1) | 5 (0) | 6 (3) | 8 (0) | 3 (4) | 5 (0) | 3 (2) | 3 (5) |
| Deep or Mixed N=1971 | 25 (1) | 15 (1) | 10 (5) | 20 (1) | 5 (6) | 15 (1) | 5 (3) | 5 (9) |
Number of CSO-BG regions with high ePVS burden (0=none, 1=one region, or 2=both regions); high burden is defined as grade III or IV ePVS
Hypertension is defined as SBP ≥140 mmHg or DBP ≥90 mmHg and/or use of antihypertensive medication
Table 2:
Association Between APOE-ɛ4 and High Burden of ePVS by Brain Region
| Odds Ratios (95% CI) | |||
|---|---|---|---|
| Centrum Semiovale (CSO) | Basal Ganglia (BG) | Mixed CSO-BG Scorea | |
| Model 1 | |||
| APOE ε4+ | 1.45 (1.16, 1.81)* | 1.15 (0.86, 1.53) | 1.37 (1.11, 1.68)* |
| Model 2 | |||
| APOE4 ε4+ | 1.46 (1.16, 1.83)* | 1.19 (0.89, 1.60) | 1.38 (1.12, 1.71)* |
ePVS = enlarged perivascular spaces; CSO = centrum semiovale; BG = basal ganglia; CI = confidence interval; FHS = Framingham Heart Study
High burden of ePVS is defined as grades III or IV in the respective region (i.e. counts > 20). Model 1 adjusts for age, sex, time interval between examination and brain MRI, and FHS cohort. Model 2 additionally adjusts for smoking status, diabetes, and hypertension status. Binary logistic regression was used for high burden of ePVS in the CSO and BG regions. Ordinal logistic regression was used for CSO-BG mixed score where probabilities were cumulated over higher outcome values.
p < 0.05
Number of CSO-BG regions with high ePVS burden (0=none, 1=one region, or 2=both regions)
Tests for interactions between APOE-ɛ4 and hypertension status were not significant in either region nor the mixed regions. In our pre-specified exploratory analysis stratified by hypertension status, the relation of APOE-ɛ4 and ePVS burden was slightly stronger and statistically significant only in participants with hypertension in the CSO and mixed regions (Table 3). There was no significant association between APOE-ɛ4 and ePVS burden in the BG region, regardless of hypertension status. Additional adjustment for vascular risk factors did not change the estimates of the stratified analysis.
Table 3.
Exploratory Analyses of the Association Between APOE-ɛ4 and High Burden of ePVS Stratified by Hypertension Status
| Odds Ratio (95% Confidence Interval) | |||
|---|---|---|---|
| Centrum Semiovale (CSO) | Basal Ganglia (BG) | Mixed CSO-BG Scorea | |
| Model 1 | |||
| APOE ε4+ (Hypertensive) | 1.47 (1.11, 1.95)* | 1.29 (0.92, 1.81) | 1.45 (1.11, 1.88)* |
| APOE ε4+ (Non-hypertensive) | 1.37 (0.95, 1.97) | 0.86 (0.49, 1.51) | 1.19 (0.85, 1.68) |
| Model 2 | |||
| APOE ε4+ (Hypertensive) | 1.49 (1.12, 1.99)* | 1.32 (0.93, 1.89) | 1.47 (1.13, 1.93)* |
| APOE ε4+ (Non-hypertensive) | 1.35 (0.93, 1.96) | 0.93 (0.52, 1.64) | 1.20 (0.84, 1.70) |
ePVS = enlarged perivascular spaces; CSO = centrum semiovale; BG = basal ganglia; CI = confidence interval; FHS = Framingham Heart Study
High burden of ePVS is defined as grades III or IV in the respective region (i.e. counts > 20). Model 1 adjusts for age, sex, time interval between examination and brain MRI, and FHS cohort. Model 2 additionally adjusts for smoking status, diabetes, and hypertension status. Binary logistic regression was used for high burden of ePVS in the CSO and BG regions. Ordinal logistic regression was used for CSO-BG mixed score where probabilities were cumulated over higher outcome values.
p < 0.05
Number of CSO-BG regions with high ePVS burden (0=none, 1=one region, or 2=both regions)
CMB data were available in 2032 out of 3564 (57%) participants with ePVS ratings. The number of participants in subgroups of high ePVS grades were much smaller than in the entire study sample, and the samples differed in several characteristics. Thus, results relating ePVS and CMB are exploratory and should be interpreted with caution. Of note, the sample with CMB data was on average nine years younger and had a lower prevalence of hypertension (29% vs 42%).
Descriptive statistics show that the prevalence of any CMB increased with ePVS burden irrespective of brain region. However, we no longer observed significant associations between APOE-ɛ4 with CSO ePVS (OR: 1.23, 95% CI: 0.83, 1.82) or the mixed score (OR: 1.17, 95% CI: 0.81, 1.70) in the reduced sample. Additional adjustment for any CMB presence did not change the results.
In multivariable analyses adjusting for age, sex, cohort, and time interval, we observed there was only a significant association between high burden CSO ePVS and deep location CMBs (OR: 2.64, 95% CI: 1.02, 6.88) (Table 4). However, this association was no longer present when we only considered participants with strictly high CSO burden (OR 1.37, 95% CI: 0.51, 3.72). Due to a lack of data, we were unable to observe the effects strictly high BG burden on CMBs.
Table 4.
Exploratory Analyses of the Association Between High Burden of ePVS and Cerebral Microbleed Presence by Brain Region
| Odds Ratio (95% CI) | ||||
|---|---|---|---|---|
| Location | Any CMB | Strictly Lobar | Strictly Deep | Deep or Lobar |
| Centrum Semiovale (CSO)a | 1.08 (0.61, 1.93) | 0.94 (0.50, 1.78) | 2.64 (1.02, 6.88)* | 3.22 (0.77, 13.43) |
| Strictly CSOb | 0.71 (0.35, 1.43) | 0.70 (0.32, 1.49) | 1.37 (0.51, 3.72) | 2.25 (0.61, 8.35) |
| Basal Ganglia (BG)c | 1.39 (0.72, 2.69) | 1.23 (0.53, 2.54) | 1.76 (0.61, 5.02) | 1.07 (0.24, 4.81) |
| Mixed CSO-BG scored (score 1 vs 0) | 0.75 (0.37, 1.52) | 0.72 (0.34, 1.53) | 1.64 (0.53, 5.11) | 2.46 (0.51, 11.97) |
| Mixed CSO-BG scored (score 2 vs 0) | 1.50 (0.71, 3.16) | 1.23 (0.54, 2.82) | 3.26 (0.98, 10.81) | 2.63 (0.43, 16.13) |
ePVS = enlarged perivascular spaces; CSO = centrum semiovale; BG = basal ganglia; CMB = cerebral microbleeds; CI = confidence interval; FHS = Framingham Heart Study;
High burden of enlarged perivascular spaces (ePVS) is defined as grade III or IV (i.e. counts greater than 20). Models adjust for age, sex, FHS cohort, time interval between examination and MRI.
p < 0.05
Includes all participants with high CSO burden regardless of BG ratings
Includes participants with exclusively high CSO burden, excluding those who also have high BG ePVS burden
Includes all participants with high BG burden regardless of CSO ratings
Number of CSO-BG regions with high ePVS burden (0=none, 1=one region, or 2=both regions)
Discussion
The main findings of our study are an association of the APOE-ɛ4 allele with higher burden of ePVS in the CSO brain region, and with higher burden of ePVS in mixed CSO and BG regions. No significant associations were observed between APOE-ɛ4 presence and ePVS burden in the BG region.
We expand prior studies by reporting the relation of APOE genotype with ePVS in a large sample of asymptomatic community-dwelling individuals. Prior studies have related the APOE-ɛ4 allele to CAA and MRI markers considered to represent CAA, such as intracerebral hemorrhage10, posterior distribution of WMH17, and cortical superficial siderosis.27 Our findings suggest that APOE-ɛ4 allele presence may relate differently to ePVS according to brain topography. Our results contrast with a prior report from the Three-City (3C)-Dijon MRI Study reporting no significant associations between the APOE genotype and ePVS.28 However, there are some noteworthy differences that may account for these findings, such as differences in the ePVS rating method, MRI sequence used, and inclusion of a much younger sample in our study.
We did not observe any significant associations of APOE-ɛ4 with high ePVS burden in the basal ganglia. This result also contrasts with a prior report based on a small sample where APOE-ɛ4 was only associated with ePVS in the BG regions.29 This study used different ePVS rating scales, MRI sequence, and had a much smaller sample (N=41 APOE-ɛ4 carriers) with unclear proportion of participants with high CSO burden, limiting their ability to conduct multivariable analyses. However, our subgroup analyses were also limited by the smaller number of participants with high burden ePVS in the BG, which may have decreased statistical power.
Our exploratory analyses sought to understand the relation of ePVS topography with the likely underlying type of CSVD. In the pre-specified stratified analyses by hypertension status, we observed that the relation of APOE-ɛ4 presence and high CSO and mixed ePVS burden was slightly stronger in participants with hypertension. While we recognize that statistical interaction was not significant in our analyses, there may be an interplay of hypertension and APOE-ɛ4 which may increase the risk of perivascular drainage dysfunction and cerebral small vessel disease represented by high ePVS burden. The relation of ePVS with deep CMB discussed below seem to also support this observation. Furthermore, a prior animal study using confocal optic microscopy demonstrated that higher blood pressure and hypertension resulted in impaired cerebral perivascular flow.30
Although APOE-ɛ4 has been associated with beta amyloid deposition, leading to development of CAA and AD pathology,31 APOE-ɛ4 also interacts with hypertension to promote hypertensive angiopathy and its downstream effects. In a prior study of patients with ICH, APOE-ɛ4 presence and dose (one or two alleles) showed a significant interaction with systolic blood pressure where individuals with elevated blood pressure and presence of an ɛ4 allele had increased risk of recurrent ICH and small vessel ischemic stroke.16 APOE-ɛ4 also interacts with elevated blood pressure to promote cognitive impairment.32 Thus, a synergistic interaction of APOE-ɛ4 with hypertension may be implicated in impaired perivascular drainage and CSVD represented by ePVS.
Whether ePVS could represent a surrogate treatment target in the pathway to dementia and stroke risk needs further investigation. Of note, other MRI markers have been studied as surrogate markers. For instance, the Systolic Blood Pressure Intervention Trial (SPRINT) found that intensive treatment of hypertension resulted in smaller increases of white matter lesion volumes compared to standard treatment.33
Exploratory analyses relating ePVS to CMBs were based on the premise that CMBs in strictly lobar regions are considered to reflect CAA, while CMBs in strictly deep or mixed lobar and deep regions are attributed to hypertensive angiopathy.8,9 However, these analyses relating ePVS to CMB presence were limited by noteworthy differences with the primary study sample since only 57% participants in our study had both CMB and ePVS data. This may explain why we no longer observed associations between APOE-ɛ4 with ePVS in the CSO nor the mixed score regions in the reduced sample, even after adjusting for CMB presence.
In the reduced sample, high burden of CSO ePVS was associated with strictly deep CMBs but not strictly lobar CMBs. We no longer observed this association in analyses restricted to strictly CSO ePVS excluding any participants with concurrent high BG ePVS ratings. These findings suggest that hypertensive angiopathy may be the predominant underlying type of small vessel disease in our sample with high CSO ePVS burden. Our exploratory analyses by hypertension status also seem to support this hypothesis as noted above. Nevertheless, we highlight the presence of large differences in the subsamples with available CMB data, and our study cannot exclude that advanced CSO ePVS represent CAA in other cohorts, or in patients with clinical intracerebral hemorrhage. Complete CMB data for our sample is required to make more definitive conclusions regarding predominant underlying forms of CSVD.
The present study has several strengths, which include a large community-based sample. Interpretation of brain MRI was done by readers blinded to all clinical data. Among its limitations, FHS participants are of predominantly white, European descent, which limits generalizability of our findings to similar populations.
In our statistical analyses, important underlying assumptions were not met for ordinal logistic regression. Furthermore, few participants had grade IV ePVS in the CSO and BG regions in our sample (3.7% and 1.2% respectively), which precluded examining the full range of ePVS ratings because of convergence failure in generalized logits models. Thus, ePVS grades in the CSO and BG regions were dichotomized. A larger sample is needed to utilize generalized logit models for multinomial responses in future analysis.
Further analysis will investigate the relationship between ePVS and neuropathology, specifically amyloid deposition using brain tissue and PET scan data, and characterization of arteriolosclerosis by ePVS topography. We also aim to explore various underlying mechanisms of ePVS and to confirm its relation with incident stroke and dementia as suggested by previous studies.
Summary for Social Media If Published.
If you and/or a co-author has a Twitter handle that you would like to be tagged, please enter it here.
-
What is the current knowledge on the topic? (one to two sentences)
APOE4 and enlarged perivascular spaces are independently associated with increased risk of dementia and stroke, but their relation has not been clearly established.
-
What question did this study address? (one to two sentences)
Is APOE4 associated with enlarged perivascular spaces (representing perivascular drainage dysfunction and cerebral small vessel disease) and is the relation different depending on ePVS topography?
-
What does this study add to our knowledge? (one to two sentences)
Presence of APOE-ɛ4 alleles is one of the strongest genetic risk factors for Alzheimer Disease. This study supports APOE-ɛ4 relation with ePVS in the centrum semiovale and mixed brain regions, and generates the hypothesis that these relations may reflect underlying hypertensive angiopathy.
-
How might this potentially impact on the practice of neurology? (one to two sentences)
Our study adds much needed insight into the relation of genetic factors and ePVS, which are subclinical markers of cerebral small vessel disease and glymphatic dysfunction and are considered to precede clinical stroke and dementia.
Acknowledgements
This work (design and conduct of the study, collection and management of the data) was supported by the Framingham Heart Study’s National Heart, Lung, and Blood Institute contract (N01-HC-25195; HHSN268201500001I) and by grants from the National Institute on Aging (R01 AG059725, AG008122; K23AG038444; R03 AG048180-01A1; AG033193); NIH grant (1RO1 HL64753; R01 HL076784; 1 R01 AG028321, P30 AG010129, NS017950), and NHLBI grants (HL67288 and 2K24HL04334).
Footnotes
Potential Conflicts of Interest: The authors do not report any conflicts of interest.
References
- 1.Rensma SP, van Sloten TT, Launer LJ, Stehouwer CDA. Cerebral small vessel disease and risk of incident stroke, dementia and depression, and all-cause mortality: a systematic review and meta-analysis. Neurosci Biobehav Rev 2018;90:164–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mestre H, Kostrikov S, Mehta RI, Nedergaard M. Perivascular spaces, glymphatic dysfunction, and small vessel disease. Clin Sci (Lond) 2017;131(17):2257–2274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Charidimou A, Meegahage R, Fox Z, et al. Enlarged perivascular spaces as a marker of underlying arteriopathy in intracerebral haemorrhage: a multicentre MRI cohort study. J Neurol Neurosurg Psychiatry 2013;84(6):624–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Charidimou A, Boulouis G, Pasi M, et al. MRI-visible perivascular spaces in cerebral amyloid angiopathy and hypertensive arteriopathy. Neurology 2017;88(12):1157–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shams S, Martola J, Charidimou A, et al. Topography and determinants of magnetic resonance imaging (MRI)-visible perivascular spaces in a large memory clinic cohort. J Am Heart Assoc 2017;6(9):e006279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Raposo N, Planton M, Payoux P, et al. Enlarged perivascular spaces and florbetapir uptake in patients with intracerebral hemorrhage. Eur J Nucl Med Mol Imaging 2019;46(11):2339–2347. [DOI] [PubMed] [Google Scholar]
- 7.Bouvy WH, van Veluw SJ, Kuijf HJ, et al. Microbleeds colocalize with enlarged juxtacortical perivascular spaces in amnestic mild cognitive impairment and early Alzheimer’s disease: a 7 Tesla MRI study. J Cereb Blood Flow Metab 2020;40(4):739–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vernooij MW, van der Lugt A, Ikram MA, et al. Prevalence and risk factors of cerebral microbleeds: the Rotterdam Scan Study. Neurology 2008;70(14):1208–1214. [DOI] [PubMed] [Google Scholar]
- 9.Jung YH, Jang H, Park SB, et al. Strictly lobar microbleeds reflect amyloid angiopathy regardless of cerebral and cerebellar compartments. Stroke 2020;51(12):3600–3607. [DOI] [PubMed] [Google Scholar]
- 10.Biffi A, Sonni A, Anderson CD, et al. Variants at APOE influence risk of deep and lobar intracerebral hemorrhage. Ann Neurol 2010;68(6):934–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Corder E, Saunders A, Strittmatter W, et al. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science 1993;261(5123):921–923. [DOI] [PubMed] [Google Scholar]
- 12.Verghese PB, Castellano JM, Holtzman DM. Apolipoprotein E in Alzheimer’s disease and other neurological disorders. Lancet Neurol 2011;10(3):241–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haglund M, Sjöbeck M, Englund E. Severe cerebral amyloid angiopathy characterizes an underestimated variant of vascular dementia. Dement Geriatr Cogn Disord 2004;18(2):132–137. [DOI] [PubMed] [Google Scholar]
- 14.Kalaria RN, Kenny RA, Ballard CG, et al. Towards defining the neuropathological substrates of vascular dementia. J Neurol Sci 2004;226(1–2):75–80. [DOI] [PubMed] [Google Scholar]
- 15.Hawkes CA, Sullivan PM, Hands S, et al. Disruption of arterial perivascular drainage of amyloid-β from the brains of mice expressing the human APOE ε4 allele. PLOS One 2012;7(7):e41636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Biffi A, Murphy MP, Kubiszewski P, et al. APOE genotype, hypertension severity and outcomes after intracerebral haemorrhage. Brain Commun 2019;1(1):fcz018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schilling S, DeStefano AL, Sachdev PS, et al. APOE genotype and MRI markers of cerebrovascular disease: systematic review and meta-analysis. Neurology 2013;81(3):292–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tsao CW, Vasan RS. Cohort profile: the Framingham Heart Study (FHS): overview of milestones in cardiovascular epidemiology. Int J Epidemiol 2015;44(6):1800–1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.DeCarli C, Massaro J, Harvey D, et al. Measures of brain morphology and infarction in the Framingham Heart Study: establishing what is normal. Neurobiol Aging 2005;26(4):491–510. [DOI] [PubMed] [Google Scholar]
- 20.Sarnowski C, Satizabal CL, DeCarli C, et al. Whole genome sequence analyses of brain imaging measures in the Framingham Study. Neurology 2018;90(3):e188–e196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wardlaw JM, Smith EE, Biessels GJ, et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol 2013;12(8):822–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Potter GM, Chappell FM, Morris Z, Wardlaw JM. Cerebral perivascular spaces visible on magnetic resonance imaging: development of a qualitative rating scale and its observer reliability. Cerebrovasc Dis 2015;39(3–4):224–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Greenberg SM, Vernooij MW, Cordonnier C, et al. Cerebral microbleeds: a guide to detection and interpretation. Lancet Neurol 2009;8(2):165–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Romero JR, Preis SR, Beiser A, et al. Risk factors, stroke prevention treatments, and prevalence of cerebral microbleeds in the Framingham Heart Study. Stroke 2014;45(5):1492–1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Romero JR, Demissie S, Beiser A, et al. Relation of plasma β-amyloid, clusterin, and tau with cerebral microbleeds: Framingham Heart Study. Ann. Clin. Transl. Neurol. 2020;7(7):1083–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Elosua R, Ordovas JM, Cupples LA, et al. Association of APOE genotype with carotid atherosclerosis in men and women: the Framingham Heart Study. J Lipid Res 2004;45(10):1868–1875. [DOI] [PubMed] [Google Scholar]
- 27.Shams S, Martola J, Charidimou A, et al. Cortical superficial siderosis: prevalence and biomarker profile in a memory clinic population. Neurology 2016;87(11):1110–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhu YC, Tzourio C, Soumaré A, et al. Severity of dilated Virchow-Robin spaces is associated with age, blood pressure, and MRI markers of small vessel disease: a population-based study. Stroke 2010;41(11):2483–2490. [DOI] [PubMed] [Google Scholar]
- 29.Luo X, Jiaerken Y, Yu X, et al. Associations between APOE genotype and cerebral small-vessel disease: a longitudinal study. Oncotarget 2017;8(27):44477–44489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mestre H, Tithof J, Du T, et al. Flow of cerebrospinal fluid is driven by arterial pulsations and is reduced in hypertension. Nat Commun 2018;9(1):4878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jeon SY, Byun MS, Yi D, et al. Influence of hypertension on brain amyloid deposition and Alzheimer’s disease signature neurodegeneration. Neurobiol Aging 2019;75:62–70. [DOI] [PubMed] [Google Scholar]
- 32.Oberlin LE, Manuck SB, Gianaros PJ, et al. Blood pressure interacts with APOE ε4 to predict memory performance in a midlife sample. Neuropsychology 2015;29(5):693–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.SPRINT MIND Investigators for the SPRINT Research Group, Nasrallah IM, Pajewski NM, et al. Association of intensive vs standard blood pressure control with cerebral white matter lesions. JAMA 2019;322(6):524–534. [DOI] [PMC free article] [PubMed] [Google Scholar]


