Abstract
Background:
Air pollution has been linked to obesity while higher ambient temperatures typically reduce metabolic demand in a compensatory manner. Both relationships may impact glucose metabolism, thus we examined the association between intermediate- and long-term exposure to fine particulate matter (PM2.5) and ambient temperature and glycated hemoglobin (HbA1c), a longer-term marker of glucose control.
Methods:
We assessed 3-month, 6-month, and 12-month average air pollution and ambient temperature at 1-km2 spatial resolution via satellite remote sensing models (2013–2019), and assessed HbA1c at four, six, and eight years postpartum in women enrolled in the Programming Research in Obesity, Growth, Environment and Social Stressors (PROGRESS) cohort based in Mexico City. PM2.5 and ambient temperature were matched to participants’ addresses and confirmed by GPS tracker. Using linear mixed-effects models, we examined the association between 3-month, 6-month, and 12-month average PM2.5 and ambient temperature with repeated log-transformed HbA1c values. All models included a random intercept for each woman and were adjusted for calendar year, season, and individual-level confounders (age, marital status, smoking, alcohol consumption level, and education level).
Results:
We analyzed 1,265 HbA1c measurements of 484 women. Per 1 μg/m3 increase in 3-month and 6-month PM2.5, HbA1c levels increased by 0.28% (95% confidence interval (95 %CI): 0.14, 0.42%) and 0.28% (95 %CI: 0.04, 0.52%) respectively. No association was seen for 12-month average PM2.5. Per 1 °C increase in ambient temperature, HbA1c levels decreased by 0.63% (95 %CI: −1.06, −0.21%) and 0.61% (95 %CI: −1.08, −0.13%), while the 12-month average again is not associated with HbA1c.
Conclusions:
Intermediate-term exposure to PM2.5 and ambient temperature are associated with opposing changes in HbA1c levels, in this region of high PM2.5 and moderate temperature fluctuation. These effects, measurable in mid-adult life, may portend future risk of type 2 diabetes and possible heart disease.
Keywords: Air pollution, Particulate matter, Temperature, Diabetes, Glycated hemoglobin, HbA1c
1. Introduction
Particulate matter (PM) pollution is one of the world’s leading environmental problems and is commonly assessed by inhalable particles of different aerodynamic diameter reflecting size in microns (PM10 or PM2.5). PM has been linked to multiple health problems including heart disease, with smaller particles posing a greater threat than larger ones (U.S. Environmental Protection Agency (EPA), 2016). PM2.5 has been linked to higher risk of ischemic heart disease, atherosclerosis, and altered cardiac autonomic function (Brook et al., 2010; Du et al., 2016; Pope and Dockery, 2006). Similarly, higher ambient temperature is a metabolic stressor that has been studied as a risk factor for cardiovascular health, and moderately high ambient temperatures in conjunction with high humidity may result in increased illness and death due to their effects on the autonomic and cardiovascular systems (U.S. Centers for Disease Control and Prevention (CDC), 2020). These links may also be partially mediated through impaired glucose metabolism, which often precedes the development of cardiovascular disease (Blauw et al., 2017; Chen et al., 2016; Liu et al., 2016; Lucht et al., 2018; Riant et al., 2018; Sasso et al., 2004; Tran and Wang, 2019; Wolf et al., 2016; Yitshak-Sade et al., 2017).
Glycated hemoglobin (HbA1c) reflects non-enzymatic binding of glucose to hemoglobin in the red blood cell and is a longer-term marker of blood glucose levels than serum glucose as hemoglobin is found exclusively in red blood cells, and has a half-life of approximately 120 days. Relative to fasting blood glucose which provides only a measure of glucose concentration present in an individual’s blood at a given time point, HbA1c is a much more stable measurement of blood glucose that does not capture postprandial fluctuations but reflects integrated measures of glucose over time, and can provide insight on the cumulative glycemic history over a two to three month period (Sherwani et al., 2016). Existing studies have found that long-term variability of HbA1c increases the risk of cardiovascular morbidity, including coronary artery disease, heart failure, and atrial fibrillation (Gu et al., 2018, 2017; Lee et al., 2013; Luk et al., 2013).
With regards to PM2.5 and ambient temperature effects on glucose metabolism, numerous mechanistic studies have provided plausibility for different biological pathways, including oxidative stress and inflammation (Panni et al., 2016; Sørensen et al., 2003a, 2003b; Ward-Caviness et al., 2016; Wilker et al., 2012; Wu et al., 2017; Xu et al., 2019). Because the impact of environmental exposures may vary based on the mechanisms by which they induce metabolic changes, it is critical to explore the effects of PM2.5 and ambient temperature using different time metrics, including that of the short- (i.e., days to weeks), intermediate- (i.e., months), and long-term (i.e., years). Furthermore, women of child bearing age may be more vulnerable to these environmental health effects due to metabolic changes around pregnancy. Multiparity is a cause of weight gain for women of reproductive age and is known to increase the risk of developing diabetes. As such, women of child bearing age and would be an optimal population for investigating such effects (Boyles et al., 2021; Rebholz et al., 2012).
Although some existing studies have investigated the relationship between PM2.5 and glucose metabolism (Moody et al., 2019; Yitshak-Sade et al., 2016), few studies have explored the association between ambient temperature and glucose metabolism (Tien et al., 2016; Tseng et al., 2005), and even fewer have studied these two exposures simultaneously with formally defined time-metrics of exposure. We were able to address this gap with satellite-based models our group has recently developed over the study area (Gutíerrez-Avila et al., 2021; Just et al., 2015), which provides detailed spatiotemporal resolved PM2.5 and ambient temperature levels that can used to calculate discrete exposure windows of interest.
In this study, we investigate the association between intermediate-and long-term exposure to PM2.5 and ambient temperature and HbA1c. We make use of the aforementioned exposure models and comprehensive longitudinal health data of mothers from the Programming Research in Obesity, Growth, Environment and Social Stressors (PROGRESS) cohort, from which the availability of detailed health data allows us to best identify the potential associations of interest in our study. We hypothesize that intermediate- and long-term increases in PM2.5 and decreases in ambient temperature exposures are associated with higher HbA1c levels.
2. Methods
2.1. Study population
Women in PROGRESS were initially recruited through clinics in the Mexican Social Security Institute (Instituto Mexicano del Seguro Social, Spanish acronym IMSS) from July 2007 to February 2011 during their 2nd trimester of pregnancy and were longitudinally followed thereafter. Extensive information was collected from participants during initial and follow up visits through questionnaires and laboratory sampling, including biomarkers for metal exposures, accelerometry data, and demographic information. All women participating in the study provided written informed consent before taking part in the study. More details about the cohort and enrollment can be found in an existing publication (Braun et al., 2014).
This initial study protocol for PROGRESS was approved by the institutional review boards at the Mexico National Institute of Public Health, the Mexico National Institute of Perinatology, the IMSS, and the Icahn School of Medicine at Mount Sinai.
2.2. Outcome assessment
We obtained HbA1c measurements from women enrolled in the PROGRESS cohort. HbA1c was measured in whole blood for all participants, and samples were run on an InnovaStar analyzer (DiaSys) using a turbidimetric immunoassay at the National Institute of Perinatology, Mexico. For the purposes of this study, HbA1c measurements were taken from participants during the 48-month, 72-month, and 96-month postpartum follow-up visits, available for the years 2013 to 2019. We excluded HbA1c measurements from our analytic dataset for which address or covariate information were missing.
2.3. Exposure assessment
We generated PM2.5 and mean ambient temperature estimates from our novel satellite-based models, which provided daily predictions at 1-km2 resolutions for the locations and exposure windows included in our study (2013–2019). In brief, in both models we used hybrid satellite-based modeling approaches, utilizing a combination of data from satellites, atmospheric simulations, land use datasets, and ground monitoring stations. Our models leveraged mixed effect modeling and machine-learning based approaches and was rigorously evaluated using leave-one-monitor-out cross-validation methods. The PM2.5 model showed excellent performance with a cross-validated R2 of 0.72. The ambient temperature model also performed very well, with annual cross-validated R2′s ranging from 0.78 to 0.95. More in depth information about the approach of the models can be found in existing publications (Gutíerrez-Avila et al., 2021; Just et al., 2015). Exposure estimates were assigned to study participants based on their geocoded home addresses and the corresponding PM2.5 and ambient temperature 1-km2 exposure grids from our satellite-based models. Information on latitude and longitude of each participant address was collected at each visit by field workers, and participants were assigned the corresponding exposure grids associated with the address provided until a new address is provided at a follow-up visit, during which we assign new exposure grids based on the address provided. Information on moving dates was retrieved using an address history questionnaire, which was also used to validate the GPS coordinates collected at each visit by field workers geocoding the participants’ addresses.
We a priori defined exposures as moving averages of PM2.5 and ambient temperature at different windows before blood for HbA1c measurement was drawn. We defined intermediate-term exposure as 3-month and 6-month averaged exposures, and long-term exposure as 12-month averaged exposures prior to the time of the visit, based on biological plausibility and findings from current research (Chuang et al., 2011; Moody et al., 2019; Yitshak-Sade et al., 2016).
2.4. Covariates
Information on age, marital status, smoking status, alcohol consumption, and education was collected through verbally administered questionnaires during monitoring visits. BMI was calculated based on measured weights and heights during each monitoring visit. Weight was measured using the InBody 270 or InBody 370 Body Composition Analyzers (Biospace Inc, 1996); height was measured using a Seca 206 roller measuring tape.
2.5. Statistical analysis
We employed linear mixed-effects models to investigate the relationship between 3-month, 6-month, and 12-month average PM2.5 and ambient temperature with HbA1c values. For each exposure window, we tested the association with ambient temperature and PM2.5 simultaneously. As the mean HbA1c distribution was highly skewed, we natural log-transformed HbA1c values. In the models, we included random intercepts for each participant to accommodate for multiple visits, and we adjusted for calendar year, season (nominal variable with three categories: May – October (rainy), and November – February (dry cold), and March – April (warm)), and individual-level characteristics at each visit, including age, body mass index (BMI) (both continuous variables), marital status (married/lifetime partner or single/separated), smoking status (yes or no), alcohol consumption level (once or twice a week/once a month, less than once a month but at least once a year, or never drank alcohol), and education level (middle school or less/don’t know, technical post-middle/high school and high school, or college/graduate school).
We selected confounders based on the availability of covariates in our cohort that best aligned with existing literature in this area of research. For the adjustment of seasonal and time trends, we selected the best fitting model based on the Bayesian Information Criterion (BIC).
Coefficients were antilog-transformed to the original units, and all results are presented as the percent change in the outcome and 95% confidence intervals per 1 μg/m3 increase in PM2.5 and per 1 °C increase in average ambient temperature. We assessed potential non-linearity in all exposure–response relationships using penalized splines for PM2.5 and average ambient temperature, and we found no evidence of deviation from linearity. We therefore treated the exposures as linear. To explore potential effect modification between PM2.5 and ambient temperature exposures, we repeated the models adding continuous or categorical (by tertiles of exposures) interaction terms between the exposures. We also tested effect modification between our exposures with other covariates of interest, including age, BMI, and education level.
2.6. Sensitivity analysis
We added several sensitivity analyses to make sure our findings are robust. First, we excluded subjects that were diagnosed with diabetes (N = 44 women; n = 121 HbA1c measurements). Diabetes diagnosis was established by 2 or more measurements of fasting glucose greater or equal to 126 mg/dL or HbA1c higher than 6.5% (American Diabetes Association, 2010).
Second, although the cohort visits were conducted 48-month, 72-month, and 96- months following the index pregnancy, some women had additional pregnancies during the follow up period. Because pregnancy may alter glucose/HbA1c levels, we also conducted a separate sensitivity analysis excluding HbA1c results obtained while the women were pregnant (n = 24 HbA1c measurements).
Finally, since a portion of HbA1c measurements were excluded due to missing data, we repeated the analysis using stabilized inverse probability weights to avoid selection bias. We modeled the probability of having complete information using a logistic regression with the following predictors: age, marital status, pregnancy status, alcohol consumption, education level, visit number, month, and year. All statistical analyses were performed using the R Statistical Software, version 4.0.3 (Foundation for Statistical Computing, Vienna, Austria). The lmerTest package was used to construct mixed effects models used in this analysis.
3. Results
We included 1,265 HbA1c measurements of 484 women. Most of the women had information on three HbA1c measurements (75.0%), 11.4% had two measurements, and 13.6% had one. The average age at measurement was 28 years, 40.7% did not have a high school diploma, and 62.6% were past or current smokers (Table 1). The average BMI of the population was 28.2 (overweight), the fasting glucose level of women across measurements was 91.9 mg/dL, and the average HbA1c level was 5.5%. 5.2% of the HbA1c measurements were greater than 6.5%. Of the 1,656 HbA1c measurements performed, 23.6% (n = 391) of the HbA1c measurements were excluded from our analytic dataset due to missing information, driven mostly by missing or unmatchable address information (20.8%), but also by missing covariates information (2.8%). A flowchart of our study exclusion process is shown in Figure S1.
Table 1.
Descriptive statistics for exposures, confounders, and outcome.
| Exposures | Analyzed Dataset (n = 1,265; N = 484) |
|---|---|
| PM2.5 (μg/m3), Mean ± Standard Deviation (SD) | |
| Daily average | 20.7 ± 9.2 |
| 3-month average | 20.6 ± 4.2 |
| 6-month average | 20.9 ± 3.0 |
| 12-month average | 20.7 ± 1.8 |
| Ambient temperature (°C), Mean ± SD | |
| Daily average | 15.6 ± 2.5 |
| 3-month average | 15.7 ± 1.9 |
| 6-month average | 15.6 ± 1.7 |
| 12-month average | 15.3 ± 1.4 |
| Population characteristics | |
| Age (years), Mean ± SD | 27.8 ± 5.7 |
| Fasting glucose level (mg/dL), Mean ± SD | 91.9 ± 21.3 |
| HbA1c (%), Mean ± SD | 5.5 ± 0.8 |
| BMI, Mean ± SD | 28.2 ± 5.2 |
| Ever Smoker, % (n) | |
| Yes | 62.6 (792) |
| No | 37.4 (473) |
| Marital Status, % (n) | |
| Married/Life-time partner | 81.3 (1,029) |
| Single/Separated | 18.7 (236) |
| Alcohol consumption, % (n) | |
| Once or twice a week/Once a month | 13.8 (175) |
| Less than once a month but at least once a year | 53.8 (680) |
| Has never drank alcohol | 32.4 (410) |
| Highest school level, % (n) | |
| Middle school or less/Don’t know | 40.7 (515) |
| Technical post-middle/high school and high school | 42.1 (533) |
| College or graduate | 17.2 (217) |
n refers to the number of HbA1c measurements, while N refers to the number of women.
The average daily PM2.5 concentration across the study period was 20.7 μg/m3, and the average ambient temperature across the study period was 15.6 °C. The Pearson correlation between daily PM2.5 and daily average ambient temperature was 0.10. We additionally compared the baseline characteristics of measurements included and excluded from the study and found them to be mostly similar. Among measurements excluded from the analysis, however, we found that BMI, alcohol consumption, and education distributions were significantly different (Table S1). Descriptive statistics by examination wave and by number of measurements per participant are also shown in the supplemental materials (Table S2 and S3).
Distributions of daily PM2.5 and daily mean ambient temperature levels are shown in Fig. 1, time series plots of daily PM2.5 and daily mean ambient temperature are shown in Figure S2, and boxplots of monthly ambient temperature are shown in Figure S3. Exposure-response relationships modeled with penalized splines used to assess non-linearity are shown in Figure S4. For 3-month and 6-month exposures, we did not detect any evidence of non-linearity. For 12-month exposures, although we detected potentially nonlinear relationships, the penalized splines were not significant. Furthermore, the observed nonlinear relationships appeared to be driven by outliers in 12-month PM2.5 and average ambient temperature (i.e., less than 18 μg/m3 for PM2.5 and less than 13 °C), which constitutes less than 2% and 5% of our dataset, respectively.
Fig. 1.
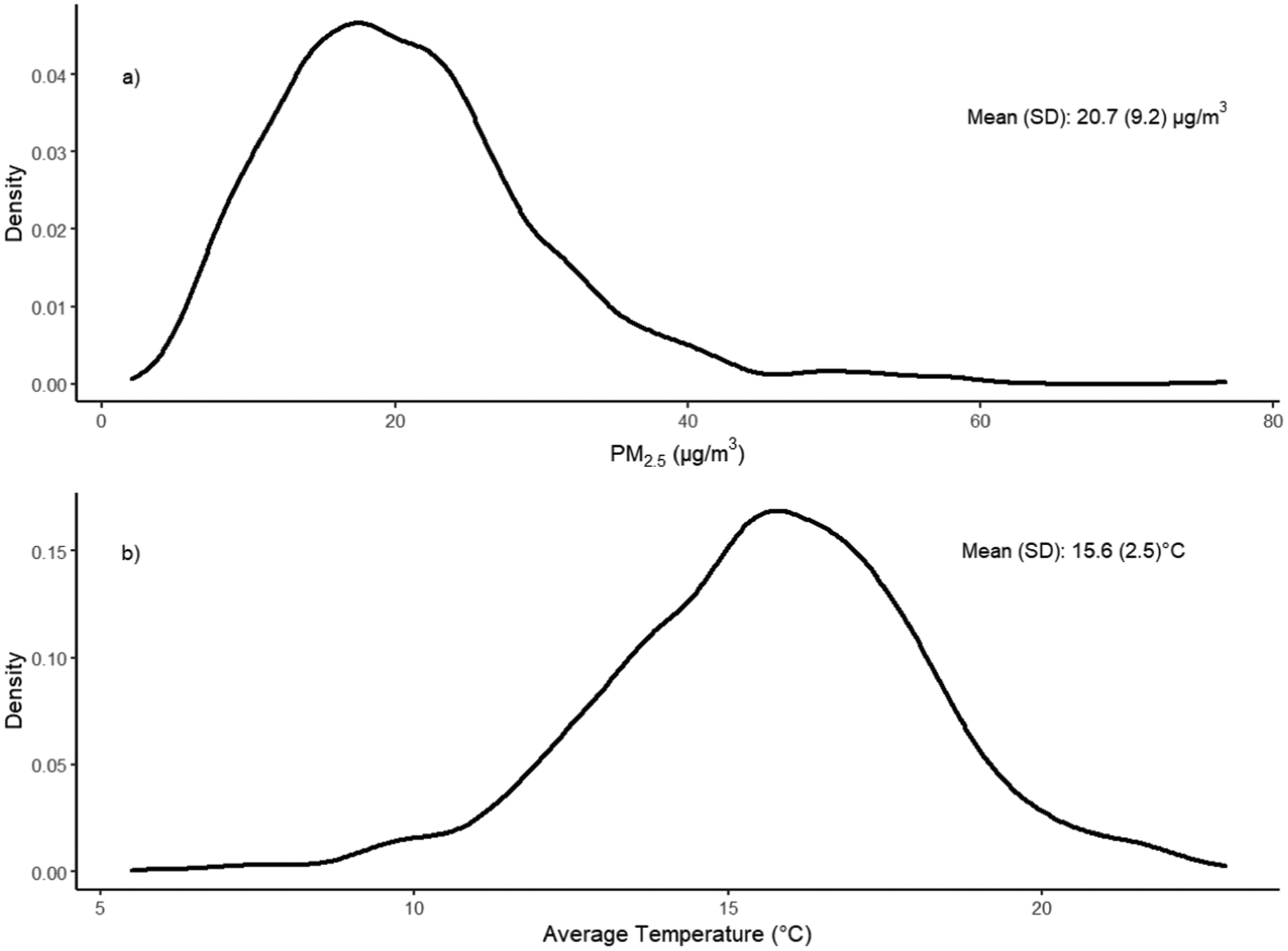
Distributions of a) daily PM2.5 and b) daily average temperature.
Fig. 2 shows the percent change in HbA1c levels associated with 3-month, 6-month, and 12-month average PM2.5 and ambient temperature. Results are presented per 1 μg/m3 increase in PM2.5 and per 1 °C increase in average ambient temperature. We observed a significant increase in HbA1c levels associated with mean PM2.5 exposure 3-months (0.28% change, 95% confidence interval (95 %CI): 0.14, 0.42%), and 6-months (0.28% change, 95 %CI: 0.04, 0.52%) before the HbA1c measurements. We observed negative associations between ambient temperature exposure and HbA1c levels. Per 1 °C increase in 3-month and 6-month average ambient temperature exposure, HbA1c levels decreased by 0.63% (95 %CI: −1.06, −0.21%) and 0.61% (95 %CI: −1.08, −0.13%), respectively. 12-month average PM2.5 or ambient temperature exposure were not associated with HbA1c in our analysis. We did not find evidence for an interaction between ambient temperature and PM2.5 in any of the exposure windows for both continuous and categorical interaction terms (Table S4). We did find significant interaction terms between 3-month and 6-month PM2.5 with age, but did not find evidence for interaction with other covariates (Table S5). Results of sensitivity analyses were very similar to that of the main analyses and the inference did not change (Figure S5 and Table S6).
Fig. 2.
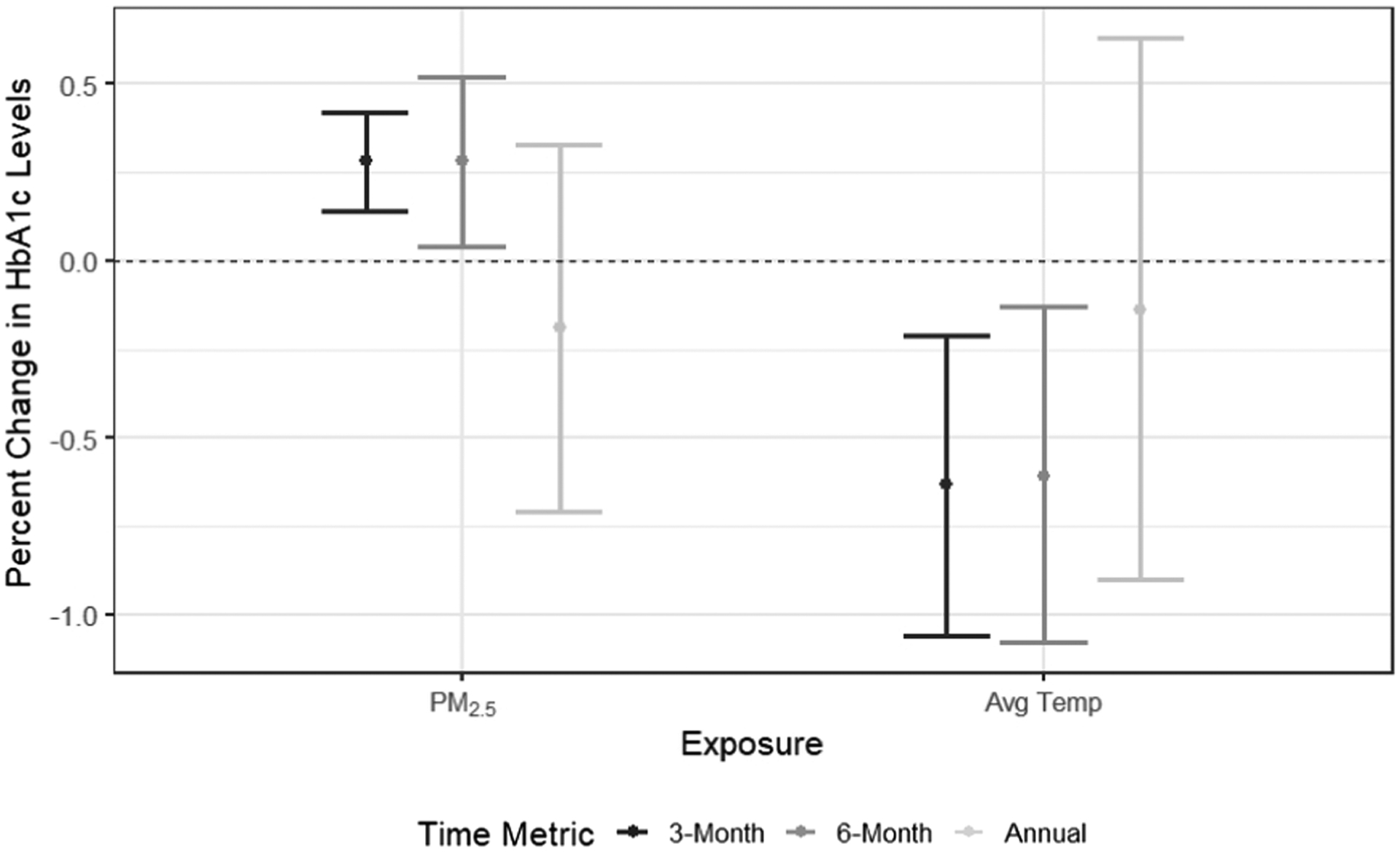
Percent increase in HbA1C levels associated with PM2.5 (per 1 μg/m3) and average temperature (per 1 °C) for different time metrics.
4. Discussion
Using data from satellite-based PM2.5 and ambient temperature models and seven years of the PROGRESS cohort longitudinal data on exposure and HbA1c, we investigated the relationship between 3-month, 6-month, and 12-month average PM2.5 and ambient temperature and repeated measures of HbA1c over time. We found positive associations for 3-month and 6-month PM2.5 with HbA1c, and negative associations for 3-month and 6-month ambient temperature with HbA1c. The results of our analyses were robust to additional sensitivity analyses.
Several studies are consistent with our findings for PM2.5, although none specifically studied women of child bearing age. Women of child bearing age were the target population for our research question of interest because they may be more vulnerable to environmental health effects due to pregnancy-related metabolic changes (Boyles et al., 2021), and we indeed found air pollution and ambient temperature exposures to be related to HbA1c in this population. Yitshak-Sade et al. investigated the association of PM10 and PM2.5 with blood glucose and HbA1c in a large retrospective cohort of adults with cardiovascular risk factors in Southern Israel (Yitshak-Sade et al., 2016). They found positive associations between 3-month PM10 with both outcomes, and 3-month PM2.5 with HbA1c. Moody et al. explored the association for prenatal and perinatal exposure of PM2.5 with HbA1c of children aged 4 to 7 years in the PROGRESS cohort (Moody et al., 2019). They found that prenatal PM2.5 was associated with higher HbA1c levels in children from age 4 to 5 years and 6 to 7 years, and also identified relevant sex-specific critical exposure windows. Numerous studies have also explored the association between long-term exposure to air pollution and diabetes-related outcomes including blood glucose, HbA1c, and biomarkers for insulin resistance (Liu et al., 2016; Lucht et al., 2018; Riant et al., 2018; Wolf et al., 2016). Both Riant et al. and Lucht et al. found positive albeit small associations between PM and HbA1c, which is consistent with the results of our study. There are a couple of mechanisms that may explain the association between PM2.5 and HbA1c. One is through the effects of PM2.5′s effects on oxidative stress, which can lead to pro-inflammatory processes that result in the development of metabolic syndromes, including diabetes (Lim and Thurston, 2019). Another is through the disruption of mitochondrial function, which can result in the decrease of brown adipose tissue, essential in the regulation of glucose homeostasis (Xu et al., 2011). As a long-term marker of glycemic control, HbA1c would reflect all of these potential changes to glucose levels in the body.
There are relatively few existing studies that have explored the association between ambient temperature and HbA1c, although with climate change the interest in the relationship between ambient temperature and health is growing. Tseng et al. investigated seasonal variations in population HbA1c levels over a 2-year period and identified that HbA1c levels were higher in the winter and lower in the summer (Tseng et al., 2005). We are only aware of a single epidemiologic study that explored the association between ambient temperature and HbA1c, which, consistent with our findings, also found a negative association (Tien et al., 2016). It is possible that HbA1c in our study may be serving as a marker of metabolic changes that are driven by temperature. Metabolism in general scales inversely with temperature going up to generate heat in cold spells and down to reduce body temperature during heat waves (Clarke and Fraser, 2004). Numerous studies have indicated that heat exposure appears to improve glucose metabolism (Hanssen et al., 2015; Moellering and Smith, 2012; Pallubinsky et al., 2020). From this perspective, the inverse relationship between ambient temperature and HbA1c observed in our study is consistent with known effects of temperature on metabolism. In times of high ambient temperature, the body would want to slow its metabolism as a compensatory mechanism, since metabolism produces heat (Çatak et al., 2018). Likewise, when ambient temperature is low, the body will want to generate heat and thus increase the rate of metabolism. Studies in this area of research have yielded conflicting results, with many studies finding that HbA1c levels are higher in the winter months and lower in summer months (Gikas et al., 2009; Higgins et al., 2009; Hou et al., 2017; Pereira et al., 2015; Sakura et al., 2010; Shen et al., 2019), but also many that found positive correlations between ambient temperature and HbA1c and higher HbA1c levels in the spring (Alghamdi et al., 2020; Kim et al., 2014; Raphael et al., 2021). Regardless of the direction of association, glucose levels would be affected by these changes. It is interesting that our results were strongest for 3- and 6-month intervals of ambient temperature and weakest for 12 months. We note that a 12-month ambient temperature average would include both the highest and lowest average ambient temperatures and the effects would tend to cancel each other with regards to HbA1c. It is not surprising then that 3-and 6-month intervals are better able to capture effects of ambient temperature on HbA1c.
Similar to our findings, Yitshak-Sade et al. found a significant increase in HbA1c levels, associated with intermediate-term exposure to PM2.5 (Yitshak-Sade et al., 2016). Unlike our findings, Chuang et al. found that long-term exposure to PM2.5 was associated with increases of serum glucose and HbA1c in a population in Taiwan, where ambient temperatures are relatively high year-round (Chuang et al., 2011). It is possible that we were unable to detect any long-term associations in our analysis due to the relatively small variability of the exposures in our study area. Furthermore, unlike many existing studies of ambient temperature and health which found nonlinear associations (Ye et al., 2012), we found a linear association with ambient temperature. This can be attributed to the hot climate and low ambient temperature variability in the studied region. It is also interesting that we detected significant negative interactions only for 3- and 6-month PM2.5 with age. There is ample evidence of positive interactive effects between PM2.5 and age, as air pollution have larger effects on susceptible populations such as the elderly (Gouveia and Fletcher, 2000; Zhang et al., 2021). However, because our study population focuses only on women of child bearing age, our population is relatively young with a narrow age range, and these interactions may only be indicative of an effect in this specific population. Therefore, we believe our detected interactions should be interpreted with caution.
The health effects associated with environmental exposure from ambient sources such as air pollution and ambient temperature tend to be relatively small, and our study is no exception. However, given the ubiquity of the exposure, impaired glucose levels as a result of ambient exposures can translate into potentially large effects on larger populations (Rajagopalan and Brook, 2012). As a well-known risk factor of cardiovascular disease, even very small differences in glucose and glycemic control may translate to clinically meaningful variations in disease risk (Gerstein, 1997).
Our study has a few limitations. First and foremost, a sizeable portion of our HbA1c measurements (23.6%) were excluded from this analysis due to missing information, which may result in potentially biased effect estimates and introduce potential missing data bias into our study if missingness is associated with either our exposures or outcome of interest. However, we compared baseline characteristics of our analyzed and excluded datasets and did not find the excluded data to be substantially different in most categories from that of our analyzed data aside from maternal education, which is the only socioeconomic indicator we adjust for in our statistical models. We acknowledge that these differences in material education may reflect underlying differences between the analyzed and excluded populations and result in the lack of generalizability to participants with different educational statuses. (Table S1). We did though conduct a sensitivity analysis using inverse probability weighting to eliminate potential selection bias, and our inference did not change (Table S6). Furthermore, the limited existing sample size may be the reason we are unable to detect statistical significance in some of our results, such as the PM2.5 and ambient temperature interaction terms. Second, this study utilizes modeled ambient PM2.5 and ambient temperature data, which are a proxy for personal-level exposures and may be prone to exposure measurement error. We are, however, confident in our modeled exposure results, which were obtained through a rigorous multi-stage model, cross-validated, and then matched to the geocoded home addresses of the study participants. We also acknowledge that there may be a number of potential covariates missing from our model for which data is not available, including detailed diabetes history of all participants and lifestyle factors (e.g., exercise, diet, fluid intake, etc.), and that exercise in particular may be a potential confounder. Additionally, diabetes history was not collected in all study visits. However, to overcome this weakness, we used fasting glucose and HbA1c levels from blood test results to create an indicator for diabetes diagnosis based on guidelines from the American Diabetes Association, which we utilized in our sensitivity analysis. We also included a large set of covariates (calendar year, season, age, marital status, smoking, alcohol consumption level, and education level) that are consistent with the literature and believe would adequately control for most sources of potential confounding, although we recognize that certain covariates (such as smoking) may benefit from more detailed classification. Lastly, PROGRESS participants were recruited in IMSS, which is the largest health provider in Mexico and covers more than 55% of the Mexican population. PROGRESS participants are thus likely sourced from a representative population of Mexico City. However, findings of this study might not be generalizable to other women of reproductive age due to the small sample size, and we are unable to verify the representativeness of our study population.
Our study has several strengths. Our study is longitudinal by design, which allows us to more confidently establish temporality. Furthermore, PROGRESS collected detailed baseline information that allow us to adequately adjust for numerous potential confounders of interest, including age, BMI, marital status, smoking status, alcohol consumption level, and education level. Lastly, the findings of our study were highly robust to sensitivity analyses, and both reaffirm existing studies (for PM2.5) and provides a novel finding (for ambient temperature) in this area of research.
5. Conclusion
Intermediate-term, but not long-term, exposure to PM2.5 and ambient temperature are associated with opposing alterations in HbA1c in women of child bearing age, although these patterns may differ under extreme weather conditions. Taken together, these effects may be important indicators of risk for type 2 diabetes and cardiovascular disease later in life and should be monitored seriously.
Supplementary Material
Acknowledgements
Research reported in this publication was supported by the National Institute of Environmental Health Sciences (NIEHS) of the National Institutes of Health under award numbers R01ES013744, R01ES032242, R24ES028522, and P30ES023515.
Footnotes
Competing Interests
The author(s) declare that they have no competing interests.
CRediT authorship contribution statement
Mike Z. He: Conceptualization, Methodology, Software, Formal analysis, Writing – original draft, Writing – review & editing, Visualization. Itai Kloog: Software, Resources, Writing – review & editing, Supervision. Allan C. Just: Methodology, Writing – review & editing. Iván Gutiérrez-Avila: Data curation, Writing – review & editing. Elena Colicino: Methodology, Writing – review & editing. Martha M. Téllez-Rojo: Investigation, Resources, Data curation, Writing – review & editing. María Luisa Pizano-Zárate: Investigation, Writing – review & editing. Marcela Tamayo-Ortiz: Investigation, Writing – review & editing. Alejandra Cantoral: Writing – review & editing, Project administration. Diana C. Soria-Contreras: Writing – review & editing, Project administration. Andrea A. Baccarelli: Resources, Writing – review & editing. Robert O. Wright: Investigation, Resources, Writing – review & editing, Funding acquisition. Maayan Yitshak-Sade: Conceptualization, Methodology, Writing – review & editing, Supervision.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.envint.2022.107298.
References
- Alghamdi AS, Alqadi A, Jenkins RO, Haris PI, 2020. Higher Ambient Temperature Is Associated with Worsening of HbA1c Levels in a Saudi Population. SSRN Electron. J 14, 881–891. 10.2139/ssrn.3487754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Diabetes Association, 2010. Diagnosis and classification of diabetes mellitus. Diabetes Care 33. 10.2337/dc10-S062. [DOI] [Google Scholar]
- Biospace Inc, 1996. InBody370 User’s Manual. Cerritos, CA. [Google Scholar]
- Blauw LL, Aziz NA, Tannemaat MR, Blauw CA, de Craen AJ, Pijl H, Rensen PCN, 2017. Diabetes incidence and glucose intolerance prevalence increase with higher outdoor temperature. BMJ Open Diabetes Res. Care 5 (1), e000317. 10.1136/bmjdrc-2016-000317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyles AL, Beverly BE, Fenton SE, Jackson CL, Jukic AMZ, Sutherland VL, Baird DD, Collman GW, Dixon D, Ferguson KK, Hall JE, Martin EM, Schug TT, White AJ, Chandler KJ, 2021. Environmental Factors Involved in Maternal Morbidity and Mortality. J. Women’s Heal 30 (2), 245–252. 10.1089/jwh.2020.8855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun JM, Wright RJ, Just AC, Power MC, Tamayo M, Schnaas L, Hu H, Wright RO, Tellez-rojo MM, 2014. Relationships between lead biomarkers and diurnal salivary cortisol indices in pregnant women from Mexico City : a cross-sectional study. Environ. Heal 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brook RD, Rajagopalan S, Pope CA, Brook JR, Bhatnagar A, Diez-Roux AV, Holguin F, Hong Y, Luepker RV, Mittleman MA, Peters A, Siscovick D, Smith SC, Whitsel L, Kaufman JD, 2010. Particulate Matter Air Pollution and Cardiovascular Disease. Circulation 121 (21), 2331–2378. 10.1161/CIR.0b013e3181dbece1. [DOI] [PubMed] [Google Scholar]
- Çatak J, Semerciöz S, Yalçinkaya BH, Yilmaz B, Özilgen M, 2018. Bioenergy Conversion. Compr. Energy Syst 4–5, 1131–1158. 10.1016/B978-0-12-809597-3.00447-8. [DOI] [Google Scholar]
- Chen Z, Salam MT, Toledo-Corral C, Watanabe RM, Xiang AH, Buchanan TA, Habre R, Bastain TM, Lurmann F, Wilson JP, Trigo E, Gilliland FD, 2016. Ambient air pollutants have adverse effects on insulin and glucose homeostasis in mexican americans. Diabetes Care 39, 547–554. 10.2337/dc15-1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang K-J, Yan Y-H, Chiu S-Y, Cheng T-J, 2011. Long-term air pollution exposure and risk factors for cardiovascular diseases among the elderly in Taiwan. Occup. Environ. Med 68 (1), 64–68. 10.1136/oem.2009.052704. [DOI] [PubMed] [Google Scholar]
- Clarke A, Fraser KPP, 2004. Why does metabolism scale with temperature? Funct. Ecol 18 (2), 243–251. 10.1111/j.0269-8463.2004.00841.x. [DOI] [Google Scholar]
- Du Y, Xu X, Chu M, Guo Y, Wang J, 2016. Air particulate matter and cardiovascular disease: The epidemiological, biomedical and clinical evidence. J. Thorac. Dis 8, E8–E19. 10.3978/j.issn.2072-1439.2015.11.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerstein HC, 1997. Glucose: a Continuous Risk Factor for Cardiovascular Disease. Diabet. Med 14 (S3), S25–S31. [DOI] [PubMed] [Google Scholar]
- Gikas A, Sotiropoulos A, Pastromas V, Papazafiropoulou A, Apostolou O, Pappas S, 2009. Seasonal variation in fasting glucose and HbA1c in patients with type 2 diabetes. Prim. Care Diabetes 3 (2), 111–114. 10.1016/j.pcd.2009.05.004. [DOI] [PubMed] [Google Scholar]
- Gouveia N, Fletcher T, 2000. Time series analysis of air pollution and mortality: Effects by cause, age and socioeconomic status. J. Epidemiol. Community Health 54, 750–755. 10.1136/jech.54.10.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu J, Fan Y-Q, Zhang J-F, Wang C-Q, 2018. Association of hemoglobin A1c variability and the incidence of heart failure with preserved ejection fraction in patients with type 2 diabetes mellitus and arterial hypertension. Hell. J. Cardiol 59 (2), 91–97. 10.1016/j.hjc.2017.08.001. [DOI] [PubMed] [Google Scholar]
- Gu J, Fan YQ, Zhang JF, Wang CQ, 2017. Impact of long-term glycemic variability on development of atrial fibrillation in type 2 diabetic patients. Anatol. J. Cardiol 18, 410–416. 10.14744/AnatolJCardiol.2017.7938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutíerrez-Avila I, Arfer KB, Wong S, Rush J, Kloog I, Just AC, 2021. A spatiotemporal reconstruction of daily ambient temperature using satellite data in the Megalopolis of Central Mexico from 2003 to 2019. Int. J. Climatol 41 (8), 4095–4111. 10.1002/joc.7060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanssen MJW, Hoeks J, Brans B, van der Lans AAJJ, Schaart G, van den Driessche JJ, Jörgensen JA, Boekschoten MV, Hesselink MKC, Havekes B, Kersten S, Mottaghy FM, van Marken Lichtenbelt WD, Schrauwen P, 2015. Short-term cold acclimation improves insulin sensitivity in patients with type 2 diabetes mellitus. Nat. Med 21 (8), 863–865. 10.1038/nm.3891. [DOI] [PubMed] [Google Scholar]
- Higgins T, Saw S, Sikaris K, Wiley CL, Cembrowski GC, Lyon AW, Khajuria A, Tran D, 2009. Seasonal variation in hemoglobin A1c: Is it the same in both hemispheres? J. Diabetes Sci. Technol 3 (4), 668–671. 10.1177/193229680900300408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou L, Li M, Huang X, Wang L, Sun P, Shi R, Ding L, Pang S, 2017. Seasonal variation of hemoglobin A1c levels in patients with type 2 diabetes. Int. J. Diabetes Dev. Ctries 37 (4), 432–436. 10.1007/s13410-016-0500-y. [DOI] [Google Scholar]
- Just AC, Wright RO, Schwartz J, Coull BA, Baccarelli AA, Tellez-Rojo MM, Moody E, Wang Y, Lyapustin A, Kloog I, 2015. Using High-Resolution Satellite Aerosol Optical Depth to Estimate Daily PM2.5 Geographical Distribution in Mexico City. Environ. Sci. Technol 49 (14), 8576–8584. 10.1021/acs.est.5b00859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YJ, Park S, Yi W, Yu KS, Kim TH, Oh TJ, Choi J, Cho YM, 2014. Seasonal variation in hemoglobin a1c in Korean patients with type 2 diabetes mellitus. J. Korean Med. Sci 29, 550–555. 10.3346/jkms.2014.29.4.550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee EJ, Kim YJ, Kim TN, Kim TI, Lee WK, Kim M-K, Park JH, Rhee BD, 2013. A1c Variability Can Predict Coronary Artery Disease in Patients with Type 2 Diabetes with Mean A1c Levels Greater than 7. Endocrinol. Metab 28 (2), 125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim CC, Thurston GD, 2019. Air Pollution, Oxidative Stress, and Diabetes: a Life Course Epidemiologic Perspective. Curr. Diab. Rep 19 (8) 10.1007/s11892-019-1181-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Yang C, Zhao Y, Ma Z, Bi J, Liu Y, Meng X, Wang Y, Cai J, Kan H, Chen R, 2016. Associations between long-term exposure to ambient particulate air pollution and type 2 diabetes prevalence, blood glucose and glycosylated hemoglobin levels in China. Environ. Int 92–93, 416–421. 10.1016/j.envint.2016.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucht SA, Hennig F, Matthiessen C, Ohlwein S, Icks A, Moebus S, Jöckel KH, Jakobs H, Hoffmann B, 2018. Air pollution and glucose metabolism: An analysis in non-diabetic participants of the Heinz Nixdorf recall study. Environ. Health Perspect 126, 047001-1-047001-10. 10.1289/EHP2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luk AOY, Ma RCW, Lau ESH, Yang X, Lau WWY, Yu LWL, Chow FCC, Chan JCN, So W-Y, 2013. Risk association of HbA1c variability with chronic kidney disease and cardiovascular disease in type 2 diabetes: prospective analysis of the Hong Kong Diabetes Registry. Diabetes. Metab. Res. Rev 29 (5), 384–390. 10.1002/dmrr.2404. [DOI] [PubMed] [Google Scholar]
- Moellering DR, Smith DL, 2012. Ambient Temperature and Obesity. Curr. Obes. Rep 1 (1), 26–34. 10.1007/s13679-011-0002-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moody EC, Cantoral A, Tamayo-Ortiz M, Pizáno-Zarate ML, Schnaas L, Kloog I, Oken E, Coull B, Baccarelli A, Téllez-Rojo MM, Wright RO, Just AC, 2019. Association of Prenatal and Perinatal Exposures to Particulate Matter With Changes in Hemoglobin A 1c Levels in Children Aged 4 to 6 Years. JAMA Netw. Open 2 (12), e1917643. 10.1001/jamanetworkopen.2019.17643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallubinsky H, Phielix E, Dautzenberg B, Schaart G, Connell NJ, de Wit-Verheggen V, Havekes B, van Baak MA, Schrauwen P, van Marken Lichtenbelt WD, 2020. Passive exposure to heat improves glucose metabolism in overweight humans. Acta Physiol. 229, 1–13. 10.1111/apha.13488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panni T, Mehta AJ, Schwartz JD, Baccarelli AA, Just AC, Wolf K, 2016. Pollution in Three Study Populations : KORA F3, KORA F4, and the Normative Aging Study 124, 983–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira M.T.R.e.P., Lira D, Bacelar C, Oliveira JC, Carvalho A.C.d., 2015. Seasonal variation of haemoglobin A1c in a Portuguese adult population. Arch. Endocrinol. Metab 59 (3), 231–235. 10.1590/2359-3997000000043. [DOI] [PubMed] [Google Scholar]
- Pope CA, Dockery DW, 2006. 2006 CRITICAL REVIEW Health Effects of Fine Particulate Air Pollution : Lines that Connect. J. Air Waste Manag. Assoc 709–742. [DOI] [PubMed] [Google Scholar]
- Rajagopalan S, Brook RD, 2012. Air pollution and type 2 diabetes: Mechanistic insights. Diabetes 61, 3037–3045. 10.2337/db12-0190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raphael A, Friger M, Biderman A, 2021. Seasonal variations in HbA1c among type 2 diabetes patients on a semi-arid climate between the years 2005–2015. Prim. Care Diabetes 15 (3), 502–506. 10.1016/j.pcd.2020.11.013. [DOI] [PubMed] [Google Scholar]
- Rebholz SL, Jones T, Burke KT, Jaeschke A, Tso P, D’Alessio DA, Woollett LA, 2012. Multiparity leads to obesity and inflammation in mothers and obesity in male offspring. Am. J. Physiol. – Endocrinol. Metab 302 (4), 449–457. 10.1152/ajpendo.00487.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riant M, Meirhaeghe A, Giovannelli J, Occelli F, Havet A, Cuny D, Amouyel P, Dauchet L, 2018. Associations between long-term exposure to air pollution, glycosylated hemoglobin, fasting blood glucose and diabetes mellitus in northern France. Environ. Int 120, 121–129. 10.1016/j.envint.2018.07.034. [DOI] [PubMed] [Google Scholar]
- Sakura H, Tanaka Y, Iwamoto Y, 2010. Seasonal fluctuations of glycated hemoglobin levels in Japanese diabetic patients. Diabetes Res. Clin. Pract 88 (1), 65–70. 10.1016/j.diabres.2009.12.011. [DOI] [PubMed] [Google Scholar]
- Sasso FC, Carbonara O, Nasti R, Campana B, Marfella R, Torella M, Nappi G, Torella R, Cozzolino D, 2004. Glucose Metabolism and Coronary Heart Disease in Patients with Normal Glucose Tolerance. J. Am. Med. Assoc 291, 1857–1863. 10.1001/jama.291.15.1857. [DOI] [PubMed] [Google Scholar]
- Shen EX, Moses RG, Oats JJN, Lowe J, McIntyre HD, 2019. Seasonality, temperature and pregnancy oral glucose tolerance test results in Australia. BMC Pregnancy Childbirth 19, 263. 10.1186/s12884-019-2413-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwani SI, Khan HA, Ekhzaimy A, Masood A, Sakharkar MK, 2016. Significance of HbA1c test in diagnosis and prognosis of diabetic patients. Biomark. Insights 11, 95–104. 10.4137/Bmi.s38440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sørensen M, Autrup H, Hertel O, Wallin H, Knudsen LE, Loft S, 2003. Personal exposure to PM2. 5 and biomarkers of DNA damage. Cancer Epidemiol. Biomarkers Prev 12, 191–196. [PubMed] [Google Scholar]
- Sørensen M, Daneshvar B, Hansen M, Dragsted LO, Hertel O, Knudsen L, Loft S, 2002. Personal PM2.5 exposure and markers of oxidative stress in blood. Environ. Health Perspect 111 (2), 161–165. 10.1289/ehp.5646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tien K-J, Yang C-Y, Weng S-F, Liu S-Y, Hsieh M-C, Chou C-W, 2016. The impact of ambient temperature on HbA1c in Taiwanese type 2 diabetic patients: The most vulnerable subgroup. J. Formos. Med. Assoc 115 (5), 343–349. 10.1016/j.jfma.2015.03.010. [DOI] [PubMed] [Google Scholar]
- Tran DH, Wang ZV, 2019. Glucose Metabolism in Cardiac Hypertrophy and Heart Failure. J. Am. Heart Assoc 8, 1–18. 10.1161/JAHA.119.012673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng C-L, Brimacombe M, Xie M, Rajan M, Wang H, Kolassa J, Crystal S, Chen T-C, Pogach L, Safford M, 2005. Seasonal patterns in monthly hemoglobin A1c values. Am. J. Epidemiol 161 (6), 565–574. 10.1093/aje/kwi071. [DOI] [PubMed] [Google Scholar]
- U.S. Centers for Disease Control and Prevention (CDC), 2020. Heat Exposure and Cardiovascular Health: A Summary for Health Departments.
- U.S. Environmental Protection Agency (EPA), 2016. Particulate Matter (PM) Pollution [WWW Document] URL https://www.epa.gov/pm-pollution/particulate-matter-pm-basics#PM%0Ahttps://www.epa.gov/pm-pollution/particulate-matter-pm-basics#PM (accessed 6.1.20).
- Ward-Caviness CK, Nwanaji-Enwerem JC, Wolf K, Wahl S, Colicino E, Trevisi L, Kloog I, Just AC, Vokonas P, Cyrys J, Gieger C, Schwartz J, Baccarelli AA, Schneider A, Peters A, 2016. Long-term exposure to air pollution is associated with biological aging. Oncotarget 7, 74510–74525. 10.18632/oncotarget.12903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilker EH, Yeh G, Wellenius GA, Davis RB, Phillips RS, Mittleman MA, 2012. Ambient Temperature and Biomarkers of Heart Failure: A Repeated Measures Analysis. Environ. Health Perspect 120 (8), 1083–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf K, Popp A, Schneider A, Breitner S, Hampel R, Rathmann W, Herder C, Roden M, Koenig W, Meisinger C, Peters A, 2016. Association between long-term exposure to air pollution and biomarkers related to insulin resistance, subclinical inflammation, and adipokines. Diabetes 65, 3314–3326. 10.2337/db15-1567. [DOI] [PubMed] [Google Scholar]
- Wu S, Yang D, Pan L, Shan J, Li H, Wei H, Wang B, Huang J, Baccarelli AA, Shima M, Deng F, Guo X, 2017. Ambient temperature and cardiovascular biomarkers in a repeated-measure study in healthy adults: A novel biomarker index approach. Environ. Res 156, 231–238. 10.1016/j.envres.2017.02.036. [DOI] [PubMed] [Google Scholar]
- Xu H, Brook RD, Wang T, Song X, Feng B, Yi T, Liu S, Wu R, Chen J, Zhang Y, Liu S, Zhao Q, Wang Y, Zheng L, Huo Y, Rajagopalan S, Li J, Huang W, 2019. Short-term effects of ambient air pollution and outdoor temperature on biomarkers of myocardial damage, inflammation and oxidative stress in healthy adults. Environ. Epidemiol 3, e078 10.1097/ee9.0000000000000078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z, Xu X, Zhong M, Hotchkiss IP, Lewandowski RP, Wagner JG, Bramble LA, Yang Y, Wang A, Harkema JR, Lippmann M, Rajagopalan S, Chen LC, Sun Q, 2011. Ambient particulate air pollution induces oxidative stress and alterations of mitochondria and gene expression in brown and white adipose tissues. Part. Fibre Toxicol 8, 1–14. 10.1186/1743-8977-8-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye X, Wolff R, Yu W, Vaneckova P, Pan X, Tong S, 2012. Ambient temperature and morbidity: A review of epidemiological evidence. Environ. Health Perspect 120 (1), 19–28. 10.1289/ehp.1003198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yitshak-Sade M, Kloog I, Liberty IF, Schwartz J, Novack V, 2016. The Association Between Air Pollution Exposure and Glucose and Lipid Levels. J. Clin. Endocrinol. Metab 101, 2460–2467. 10.1210/jc.2016-1378. [DOI] [PubMed] [Google Scholar]
- Yitshak-Sade M, Kloog I, Novack V, 2017. Do air pollution and neighborhood greenness exposures improve the predicted cardiovascular risk? Environ. Int 107, 147–153. 10.1016/j.envint.2017.07.011. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Wang J, Kwong JC, Burnett RT, van Donkelaar A, Hystad P, Martin RV, Bai L.i., McLaughlin J, Chen H, 2021. Long-term exposure to air pollution and mortality in a prospective cohort: The Ontario Health Study. Environ. Int 154, 106570. 10.1016/j.envint.2021.106570. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


