Abstract
Background:
Early life phthalate exposures may disrupt metabolism but results from human studies are inconsistent and few have examined body composition during adolescence. We investigated associations of gestational and childhood urinary phthalate biomarker concentrations with body composition at age 12 years.
Methods:
We used data from 206 mother-child pairs in a prospective pregnancy and birth cohort enrolled in Cincinnati, OH from 2003–2006. We measured nine phthalate metabolites in spot urine samples collected twice from mothers during pregnancy and up to seven times from children at 1, 2, 3, 4, 5, 8, and 12 years. At age 12 years, we assessed fat and lean mass of the whole body and android and gynoid subregions, and visceral fat area with dual x-ray absorptiometry, and calculated android to gynoid %fat ratio and age- and sex-standardized fat and lean mass index z-scores. Using a multiple informant model, we estimated covariate-adjusted associations between urinary phthalate biomarker concentrations at each time period and outcomes at age 12 years. We assessed effect measure modification by child sex using stratified models.
Results:
Generally, urinary mono-benzyl phthalate (MBzP) concentrations were modestly associated with lower fat and lean mass. Each 10-fold increase in urinary MBzP concentrations during gestation and at ages 5 and 8 years was associated with a −0.34 (95%CI: −0.72, 0.05), −0.44 (95% CI: −0.83, −0.05), and −0.35 (95% CI: −0.71, 0.00) z-score difference in lean body mass index, respectively. Urinary monoethyl phthalate, mono-(3-carboxypropyl) phthalate, and summed di(2-ethylhexyl) phthalate metabolites were associated with greater lean mass at some exposure periods. Slightly weaker but similar patterns of association were found with other body composition measures; associations did not differ by child sex.
Conclusion:
While most associations were weak, exposure to certain phthalates during gestation and childhood may be associated with adolescent body composition, particularly lean mass.
Keywords: phthalates, prenatal exposures, childhood exposures, environmental epidemiology, fat mass, adolescent health
1. Introduction
Phthalates, high production volume synthetic chemicals, are the most commonly used plasticizers in the world and are also scent retainers and emollients (Schettler 2006). Although some phthalates, including di(2-ethylhexyl) phthalate (DEHP), are prohibited for use in children’s toys and child care items (Consumer Product Safety Improvement Act 2008), phthalates are ubiquitous and several phthalate metabolites are detected in over >95% of urine samples in the United States (Centers for Disease Control and Prevention 2021). There is concern that phthalate exposures during gestation and early life may cause alterations in metabolism and growth given that these are periods of heightened developmental susceptibility (Braun 2017; Lawler et al. 2015).
The prevalence of childhood overweight and obesity is increasing: 1 in 3 United States children are overweight or obese (Kumar and Kelly 2017). Prevention of obesity is critically important, particularly during the adolescent period, given that obese adolescents are more likely to become overweight or obese as adults (Wright et al. 2001). Compared with obesity status during childhood, adolescent obesity is more strongly related with obesity in adulthood because risk factors change with age and pubertal development (Al-Hamad and Raman 2017; Morrison et al. 2007; Morrison et al. 2008; Zimmet et al. 2007).
Dual energy X-ray absorptiometry (DXA) is a standard clinical tool that provides accurate and precise estimates of adipose mass (Laskey 1996). DXA is a more accurate way to measure body composition than bioelectrical impedance and measures of anthropometry such as body mass index (BMI) (Wang et al. 2010; Weber et al. 2013). DXA also quantifies the distribution of body fat, which is more predictive of disease than overall body fat mass (Després et al. 1989; Goran and Gower 1999; Kaul et al. 2012; Krotkiewski et al. 1983; Lapidus et al. 1984; Larsson et al. 1984).
Prior studies have assessed prenatal or early-life phthalate exposures in relation to obesity-related measures in childhood (Philips et al. 2017), but only one prior epidemiological study has used DXA measures of body composition (Carlsson et al. 2018). Carlsson et al. 2018 reported no associations between adolescent urinary phthalate biomarkers and adolescent fat mass index among children aged 8 to 16 years in the Copenhagen Puberty Study; however, the study was cross-sectional with a relatively small sample size (n=107) (Carlsson et al. 2018).
Thus, we investigated associations of urinary phthalate metabolite concentrations during gestation and childhood with body composition at age 12 years to identify periods of heightened susceptibility.
2. Materials and Methods
2.1. Study Participants
We used data from women and children in the Health Outcomes and Measures of the Environment (HOME) Study, an ongoing prospective cohort study that enrolled pregnant woman from the Cincinnati, Ohio area during 2003 to 2006 (Braun et al. 2017; Braun et al. 2020). The HOME Study was designed to investigate relations between environmental chemical exposures and child health and development. Cohort details have been described previously (Braun et al. 2017; Braun et al. 2020). Briefly, cohort eligibility requirements included pregnant women who were 18 years or older, 16 ± 3 weeks of gestation, spoke English, lived in a home built before 1978, and had no history of HIV infection or other medical conditions. Of the 1,263 eligible women we approached, 468 (37%) agreed to participate in our study. Of these 67 pregnant women dropped out before delivery leaving 401 women who delivered 389 singletons, 3 stillbirths, and 9 sets of twins (Braun et al. 2017). Since delivery, the HOME Study has completed assessments at follow-up visits occurring at 4-weeks, 1, 2, 3, 4, 5, and 8 years, and follow-up rates ranged from 48% to 94% (Braun et al. 2020). Of the 441 children invited to participate in the 12-year follow-up visit, including women and children who re-enrolled in the HOME study after dropping out of the study before delivery, 256 (242 singletons and 7 twin sets) (58%) completed the visit (Braun et al. 2020) (Supplemental Figure 1).
After trained research assistants explained study protocols, all women provided written consent for themselves and their children. At the 12-year follow-up visit, research assistants also explained study protocols to the adolescents, and they provided written assent. The Cincinnati Children’s Hospital Medical Center (CCHMC) and participating delivery hospitals’ Institutional Review Boards (IRB) approved the HOME Study. The Centers for Disease Control and Prevention (CDC) also relied on CCHMC IRB.
2.2. Prenatal and Early Childhood Phthalate Exposure Assessment
Women provided two spot urine samples at an average of 16.0 (range: 10.4–22.6) and 26.5 (range: 19.1–34.6) weeks of gestation. Children provided up to seven spot urine samples at the follow-up visits. Urine samples were collected into either surgical inserts placed into a diaper for children who were not toilet-trained, into a training toilet lined with inserts for children who were being toilet-trained, or into polypropylene specimen cups for children who were toilet-trained and for pregnant women. After collection, all urine samples were stored at −20° C. Maternal and child urine samples were analyzed at the CDC for the following urinary phthalate metabolites: monoethyl phthalate (MEP), mono-n-butyl phthalate (MnBP), mono-isobutyl phthalate (MiBP), mono(3-carboxypropyl) phthalate (MCPP), monobenzyl phthalate (MBzP), and four metabolites of DEHP: mono(2-ethylhexyl) phthalate (MEHP), mono(2-ethyl-5hydroxyhexyl) phthalate (MEHHP), mono(2-ethyl-5-oxohexyl) phthalate (MEOHP), and mono(2ethyl-5-carboxypentyl) phthalate (MECPP) with isotope dilution high-performance liquid chromatography coupled with tandem mass spectrometry as previously described (Silva et al. 2004). Limits of detection (LOD) ranged from 0.2 to 1.2 ng/mL depending on the metabolite (Silva et al. 2004). Urinary creatinine was measured in each sample to account for urinary dilution.
Urinary phthalate metabolite concentrations below the LOD were given a value of LOD/√2 for data analysis. To adjust for urinary dilution, we used covariate-adjusted standardization (O’Brien et al. 2016). Covariates in our creatinine prediction model for samples collected during pregnancy included gestational age at time of collection, maternal race/ethnicity, age, mid-gestation BMI, pregnancy complications, family income, and height. Covariates in our creatinine prediction model for samples collected from children included child race/ethnicity, age, BMI, family income, height, and sex. Then, due to their non-normal distributions, we log10 transformed the covariate-adjusted creatinine standardized urinary phthalate metabolite concentrations to reduce the influence of outlying values. If a woman provided more than one sample during pregnancy, we averaged the two log10-transformed covariate-adjusted creatinine-standardized urinary phthalate concentrations for analysis.
Urinary concentrations of MEHP, MnBP, and MiBP could not be quantified at 1–3 years of age because these metabolites were detected in the diaper inserts (Watkins et al. 2014a). We only used the three oxidative DEHP metabolites (MEOHP, MEHHP, MECPP) to have a consistent DEHP measure across the maternal and child samples. Therefore, we calculated the molar sum of DEHP metabolites (∑DEHP) by dividing each of the three metabolites by its molar mass and summing the concentrations.
2.3. Adolescent Body Composition
We measured height, weight, and BMI at the 12-year study visit. Weight was obtained to the nearest 0.01 kg using a ScaleTronix scale (White Plains, NY) while children were dressed in undergarments. Height was measured to the nearest 0.1 cm with an Ayrton Stadiometer Model S100 (Prior Lake, Minnesota). Trained research assistants obtained measurements in triplicate, and the average of the three measurements was used. We calculated age- and sex-standardized height, weight, and BMI z-scores using CDC reference data from U.S. children (Kuczmarski et al. 2000).
Body composition was assessed at the 12-year study visit with DXA using a Hologic Horizon densitometer (Hologic Inc, Marlborough, MA) by trained and experienced technicians. Scans were analyzed using the United States National Health and Nutrition Examination Survey (NHANES) body composition analysis calibration. We calculated whole body fat mass index (FMI) as whole-body fat mass (kg) divided by height (m)2. Whole-body lean body mass index (LBMI), a measure of skeletal muscle mass and lean soft tissues that does not include bone mineral content, was calculated as lean body mass (kg) divided by height (m)2. We calculated age- and sex-standardized FMI and LBMI z-scores using pediatric reference curves generated from NHANES data (Weber et al. 2013). Android obesity, which consists of excess human adipose tissue mainly around the trunk and upper body, has been associated with an increased risk for metabolic and cardiovascular disease while gynoid fat distribution, which consists of human adipose tissue storage in the lower body, may be protective (Samsell et al. 2014). We calculated android to gynoid percent fat ratio as percent fat measured in the android region divided by percent fat measured in the gynoid region. We assessed visceral fat as the cross-sectional area (cm2) of fat inside the abdominal area. To account for differences in total body fat among children, models with visceral fat as an outcome were also adjusted for FMI z-scores.
2.4. Covariates
Mothers completed a standardized interview that was administered by trained research assistants during the second and third trimesters of pregnancy and each child follow-up visit to ascertain demographic and other maternal and child characteristics. Maternal nutritional factors during pregnancy, such as vitamin use and fruit and vegetable consumption, were assessed using standardized interviews during the second or third trimester. Tobacco smoke exposure during gestation was measured using the average of serum cotinine concentrations measured at 16 and 26 weeks of pregnancy as previously described (Bernert et al. 1997; Braun et al. 2010). Additional pregnancy and delivery information was ascertained from medical chart reviews. Child physical activity at age 12 years was measured using the Physical Activity Questionnaire for Older Children (PAQ-C), a detailed self-administered 7-day recall instrument that measures physical activity among children between 8 and 14-years of age (Kowalski et al. 2004).
We used a directed acyclic graph (DAG) to identify potential confounders that may be associated with both phthalate exposures during pregnancy and early childhood and body composition during adolescence (Supplemental Figure 2). Our final adjusted models included child sex and the following maternal baseline covariates: race/ethnicity, average cotinine concentration during pregnancy (ng/mL), pregnancy weight gain, mid-pregnancy BMI (<25, 25–30, or >30 kg/m2), prenatal vitamin use (“Never”, “Weekly/Monthly”, or “Daily”), prenatal fruit and vegetable consumption (“Monthly”, “Weekly”, “Daily”). We adjusted for household income level at each prenatal and follow-up visit. In addition to potential confounders, we adjusted for PAQ-C composite scores at age 12 years to improve precision of our estimates.
2.5. Statistical Analyses
Of the 256 mother-child pairs in the cohort who returned for the age 12-year follow-up visit we excluded twins (n=14), those who were missing urinary metabolite measures during pregnancy and childhood or DXA measures (n=19), and those with missing covariates (maternal anthropometry during pregnancy n=15; serum cotinine concentrations n=2), leaving 206 children in our analytic sample.
We examined pregnancy and early childhood urinary phthalate metabolite distributions at each time point using descriptive statistics and generating boxplots. We also calculated Pearson correlation coefficients among the pregnancy and six postnatal measurements. Next, we described the central tendency and distribution of DXA and anthropometry measurements at age 12 years. All 206 children in our sample had maternal phthalates measured at least once during gestation. Regarding postnatal phthalate biomarker measures, 34% (n=69) had all 7 postnatal phthalate biomarker measures, 25% (n=52) had 6 postnatal phthalate biomarker measures, 16% (n=33) had 5 postnatal phthalate biomarker measures, 11% (n=23) had 4 postnatal phthalate biomarker measures, 6% (n=13) had 3 postnatal phthalate biomarker measures, 5% (n=10) had 2 phthalate biomarker measure, and only 3% (n=6) had prenatal and the 12-year visit phthalate biomarker. We used multiple informant models to examine potential associations between repeated urinary phthalate biomarker concentrations and adolescent body composition. This method has been previously described (Sanchez et al. 2011) and used in other HOME Study analyses (Jackson-Browne et al. 2018; Shoaff et al. 2017; Stacy et al. 2017). Briefly, a multiple informant model utilizes generalized estimating equations (GEEs) for parameter estimation with embedded separate linear regression models for each exposure period. In separate models for each phthalate biomarker, we used the multiple informant model to jointly-estimate a difference in adolescent body composition per 10-fold increase in urinary phthalate biomarker concentrations in each exposure period. We included the same covariates in models for all exposure periods. Although associations of phthalate biomarker concentrations with outcomes are not co-adjusted, the multiple informant model allows for a statistical test of whether associations differ by exposure period. We considered associations to be significantly different by exposure period when the phthalate biomarker X exposure period product term p-value was ≤0.20.
We used SAS software, version 9.4. (SAS Institute, Inc., Cary, North Carolina) and RStudio, version 1.2.509 (RStudio: Integrated Development Environment for R) for data analyses.
2.6. Exploratory Analysis
We examined whether child sex modified associations between phthalate biomarkers and adolescent body composition using sex-stratified multiple informant models.
Results
Women in our study were predominantly Non-Hispanic White (59%), aged 25–30 years at delivery (61%), had a household income during pregnancy between $40,000-$80,000 (34%), and had a mid-pregnancy BMI less than 25 kg/m2 (41.7%) (Table 1). Most women reported daily prenatal vitamin use (72.8%) and weekly (52%) or daily (43%) fruit and vegetable consumption during pregnancy (Table 1). Children were predominantly female (54%) and had a mean PAQ-C score of 1.5 (SD: 0.6) at age 12 years (Table 1). Characteristics were similar among women included in our study sample and those with complete prenatal covariates at enrollment (Supplemental Table 1). Generally, covariate-adjusted creatinine-standardized urinary concentrations of MEP and ∑DEHP were higher than other phthalate biomarkers (Supplemental Table 2). Concentrations of MEP and MnBP were highest during pregnancy whereas concentrations of MCPP, MBzP, and ∑DEHP were highest at the 3-year visit and MiBP concentrations were highest at the 4-year visit (Supplemental Table 2). Phthalate biomarker concentrations were lowest at the 12-year visit (Supplemental Table 2).
Table 1.
Characteristics of women and their children in the HOME Study, 2003–2006 (N=206).
| n (%) | |
|---|---|
| Maternal Age at Delivery | |
| 18–25 | 50 (24) |
| > 25–35 | 125 (61) |
| > 35 | 31 (15) |
| Maternal Race/Ethnicity | |
| Non-Hispanic White | 121 (59) |
| Non-Hispanic Black | 73 (35) |
| Other | 12 (6) |
| Household Income During Pregnancy | |
| > $80K | 54 (26) |
| $40–80K | 69 (34) |
| $20–40K | 32 (16) |
| < $20K | 51 (25) |
| Maternal Weight Gain during Pregnancy (lbs) (mean, SD) | 21.4 (10.9) |
| Maternal Mid-Pregnancy BMI (kg/m 2 ) | |
| <25 (under or normal weight) | 86 (42) |
| 25–<30 (overweight) | 68 (33) |
| ≥30 (obese) | 52 (25) |
| Tobacco Smoke Exposure during Pregnancy 1 | |
| Unexposed | 61 (30) |
| Secondhand Smoke | 123 (60) |
| Active Smoker | 22 (11) |
| Prenatal Vitamin Use | |
| Never | 23 (11) |
| Weekly/Monthly | 33 (16) |
| Daily | 150 (73) |
| Prenatal Fruit and Vegetable Consumption | |
| Monthly | 10 (5) |
| Weekly | 108 (52) |
| Daily | 88 (43) |
| Child Sex (Male) | 95 (46) |
| Physical Activity Score at Age 12 (mean, SD) | 2 (0.6) |
Smoking status is based on pregnancy serum cotinine concentrations;<0.015 ng/mL was classified as unexposed, 0.014 to ≤3.0 ng/mL was classified as secondhand smoke, and>3.0 ng/mL was considered active smoking exposure.
We collected DXA and anthropometric data at an average age of 12.4 (± 0.7) years. Compared to the reference population of U.S. adolescents of the same age and sex, our study population had a higher mean (±SD) FMI z-score (0.23 ± 0.82), BMI z-score (0.39 ±1.2), height z-score (0.45 ± 1.2), and weight z-score (0.50 ± 1.1) and lower LBMI z-score (−0.33 ± 1.2) (Table 2). FMI was similar among males and females, but males had lower z-scores for LBMI, BMI, height, and weight, lower android to gynoid % fat ratio, and higher visceral fat area compared to females (p-values <0.01) (Table 2). DXA outcomes were moderately to strongly correlated with anthropometry measures (Pearson’s ρ = 0.24–0.93) (Supplemental Table 3). While FMI and LBMI were moderately correlated with each other (ρ = 0.64), they were both highly correlated with BMI (ρ = 0.86 and 0.89, respectively) (Supplemental Table 3). Results from the multiple informant models suggest that most estimates were null, and most phthalate biomarker X exposure period interaction p-values were not statistically significant (Supplemental Tables 4–10). However, some associations between urinary phthalate biomarker concentrations and body composition or anthropometry outcomes at age 12 years differed based on the timing of exposure (Supplemental Tables 4–10). For FMI z-scores, we observed statistically significant associations with urinary MnBP, MiBP, and MBzP concentrations at some exposure periods, with associations generally stronger for concentrations measured in middle childhood (Figure 1; Supplemental Table 4). For example, while urinary MBzP concentrations at other time points were not associated with FMI, a 10-fold increase in urinary MBzP concentrations at age 5- and 8-years was associated with 0.27 (95% CI: −0.55, 0.01) and 0.20 (95% CI: −0.46, 0.05) lower FMI z-scores, respectively (Figure 1, Supplemental Table 4). Additionally, we observed positive associations with some urinary phthalate biomarker concentrations during middle childhood and 12-year fat mass index z-scores. For example, a 10-fold increase in 4-year MCPP and ∑DEHP concentrations was associated with a 0.40 (95% CI: −0.02, 0.83) and 0.30 (95% CI: −0.11, 0.34) higher 12-year FMI z-scores, respectively (Figure 1; Supplemental Table 4). Still, the phthalate biomarker exposure period interaction p-values were not statistically significant for FMI z-scores except for MiBP (Figure 1; Supplemental Table 4).
Table 2.
Dual energy x-ray absorptiometry (DXA) and anthropometry measurements among 206 12-year-old children in the HOME Study by child sex, mean (SD).
| Overall (N=206) | Males (N=95) | Females (N=111) | |
|---|---|---|---|
| DXA | |||
| Fat mass index (FMI) z-score | 0.23 (0.82) | 0.24 (0.71) | 0.22 (0.90) |
| Lean body mass index (LBMI) z-score | −0.33 (1.1) | −0.51 (1.1) | −0.18 (1.2) |
| Android to gynoid %fat ratio | 0.81 (0.12) | 0.79 (0.11) | 0.82 (0.13) |
| Cross-sectional area (cm2) of fat inside abdominal cavity | 45.7 (21.1) | 50.5 (13.8) | 41.5 (25.1) |
| Anthropometry | |||
| Body mass index (BMI) z-score | 0.39 (1.2) | 0.17 (1.2) | 0.57 (1.1) |
| Height z-score | 0.45 (1.2) | 0.37 (1.2) | 0.52 (1.1) |
| Weight z-score | 0.50 (1.2) | 0.26 (1.2) | 0.70 (1.2) |
Figure 1.
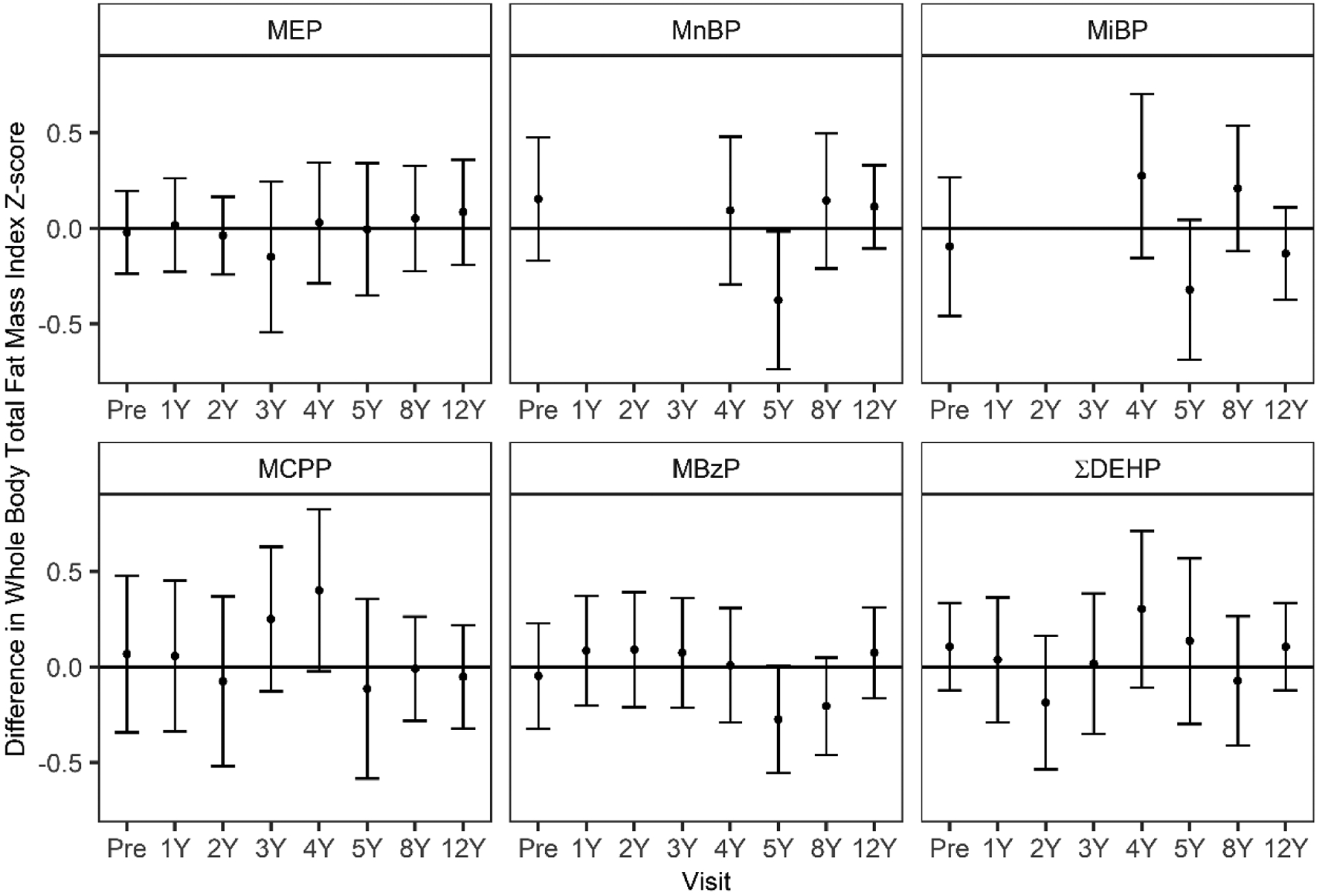
Adjusted differences in 12-year fat mass index (FMI) z-score per 10-fold increase in pregnancy and childhood urinary phthalate biomarker concentrations.
Differences and 95% confidence intervals (CI) estimated in multiple informants models adjusted for maternal race/ethnicity, smoking during pregnancy, pregnancy weight gain, maternal mid-pregnancy BMI, prenatal vitamin use, prenatal fruit and vegetable consumption, physical activity at age 12, income level at each visit, and for overall models, child sex. Urinary concentrations of MEHP, MnBP, and MiBP were unable to be quantified at 1–3 years of age because these metabolites were detected in the diaper inserts. *Phthalate metabolite by exposure period p-value.
For LBMI, associations with urinary MBzP concentrations depended on the timing of exposure (MBzP X exposure period interaction p-value = 0.10) (Figure 2, Supplemental Table 5). Urinary MBzP concentrations during gestation, at 5- and 8-years of age, but not in early childhood, were associated with lower LBMI z-scores (Figure 2, Supplemental Table 5). A 10-fold increase in urinary MBzP concentrations at 5- and 8-years of age was associated with a 0.44 (95% CI: −0.83, −0.05) and a 0.35 (95% CI: −0.71, 0.00) lower LBMI z-score (Figure 2, Supplemental Table 5). We also observed positive associations between some urinary phthalate biomarker concentrations and 12-year LBMI z-scores. In particular, a 10-fold increase in 12-year ∑DEHP was associated with 0.56 (95% CI: 0.05, 1.07) higher LBMI z-scores at 12 years of age (Figure 2; Supplemental Table 5).
Figure 2.
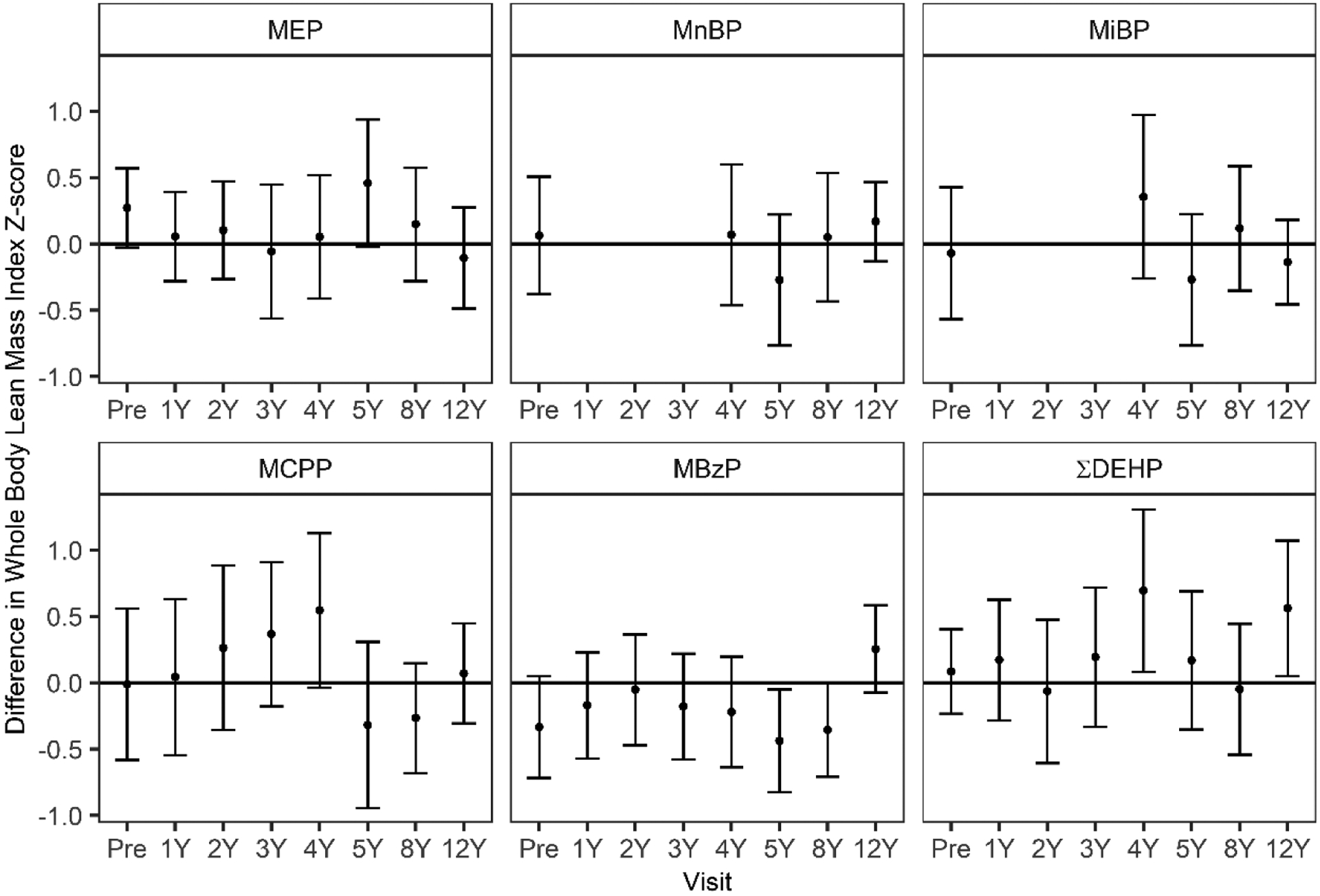
Adjusted differences in 12-year lean body mass index (LBMI) z-score per 10-fold increase in pregnancy and childhood urinary phthalate metabolite biomarker concentrations.
Differences and 95% confidence intervals (CI) estimated in multiple informants models adjusted for maternal race/ethnicity, smoking during pregnancy, pregnancy weight gain, maternal mid-pregnancy BMI, prenatal vitamin use, prenatal fruit and vegetable consumption, physical activity at age 12, income level at each visit, and for overall models, child sex. Urinary concentrations of MEHP, MnBP, and MiBP were unable to be quantified at 1–3 years of age because these metabolites were detected in the diaper inserts. *Phthalate metabolite by exposure period p-value.
Similar patterns were found among the remaining DXA outcomes (Supplemental Tables 6–7), and the three anthropometry outcomes (Supplemental Tables 8–10). Generally, after stratifying by child sex, associations among females and males were similar to overall observations albeit slightly stronger among females (Supplemental Tables 8–10). For example, a 10-fold increase in urinary MBzP concentrations during gestation and 8-years of age was associated with a 0.20 (95% CI: −0.75, 0.35) and 0.09 (95% CI: −0.63, 0.46) lower LBMI z-score among boys and a 0.42 (95% CI: −1.03, 0.20) and 0.67 (95% CI: −1.17, 0.17) lower LBMI z-score among girls, respectively (Supplemental Table 4).
Discussion
In this prospective pregnancy and birth cohort in Cincinnati, OH, urinary phthalate biomarkers were generally not associated with DXA or anthropometry measures at age 12 years, and most associations did not depend on the timing of exposure. However, urinary MBzP concentrations were modestly associated with lower fat and lean mass, while MEP, MCPP, and ∑DEHP concentrations were associated with greater lean mass at some exposure periods.
The use of BMI as a measure of adiposity has limitations, especially in adolescents (Weber et al. 2013). DXA measures of body composition among children and adolescents have been shown to be highly correlated with the criterion 4-compartment model (Boeke et al. 2013; Sopher et al. 2004). We are aware of only one previous study that examined associations between phthalate exposure biomarkers and adolescent adiposity measured by DXA. Using a cross-sectional study design, Carlsson et al. 2018 reported no associations between urinary phthalate biomarkers and FMI among 107 Caucasian adolescents aged 8 to 16 in the Copenhagen Puberty Study (Carlsson et al. 2018). We also found few associations between urinary phthalate biomarker concentrations and body composition at age 12 years, except for positive cross-sectional associations of urinary MBzP and ∑DEHP concentrations with LBMI. However, cross-sectional associations may reflect reverse causation given that phthalate metabolites have short half-lives in urine and thus are unlikely to affect concurrent body composition.
We observed associations of certain phthalate biomarkers with LBMI, which was not assessed in the Carlsson et al 2018 study. Our findings of somewhat stronger associations of phthalate biomarker concentrations with LBMI than with FMI may suggest that muscle is more affected by phthalate exposures than body fat. Previous studies relying on BMI have not been able to make this distinction given that BMI is strongly correlated with both FMI and LBMI. However, a previous study of 481 South Korean mother-child pairs reported that maternal MnBP and ∑DEHP concentrations were associated with lower skeletal muscle index, a measure of lean mass using bio-electrical impedance analysis, among girls (but not boys) at age 6 years (Lee et al. 2020). The study found associations with BMI, but not with FMI, providing additional evidence that associations of phthalate metabolites with BMI in the literature may be more reflective of differences in lean mass than fat mass. This finding may reflect anti-androgenic activity of phthalates given the important role of androgens in muscle growth. Previous animal and epidemiological studies suggest that androgen is positively associated with muscle growth and strength and that both pregnancy and childhood exposure to phthalates may decrease offspring’s testosterone production both in the fetal period and during childhood (Bornehag et al. 2015; Meeker and Ferguson 2014; Parks et al. 2000; Sinha et al. 2014; Swan 2008; Wang et al. 2015).
Previous research in the HOME Study cohort found no associations between pregnancy urinary phthalate biomarker concentrations and BMI, waist circumference, or percent body fat measured with bio-electrical impedance analysis at 8 years of age. However, ∑DEHP and MEP concentrations at age 5 and 8 years were associated with increased body fat at age 8 years while earlier childhood concentrations were not (Shoaff et al. 2017). MBzP concentrations at most periods were associated with reduced adiposity with the strongest associations observed with prenatal exposure (Shoaff et al. 2017). These findings were replicated in the current analysis which extended follow-up to age 12 years. The persistence of these associations in adolescence is important because obesity in adolescence is more strongly associated with cardiometabolic outcomes in adulthood than obesity in childhood (Al-Hamad and Raman 2017; Morrison et al. 2007; Morrison et al. 2008; Wright et al. 2001; Zimmet et al. 2007).
In addition to Shoaff et al. 2017, we are aware of six prospective studies that have examined pregnancy or early-life phthalate exposures and adolescent adiposity using anthropometry or bioelectrical impedance (Bowman et al. 2019; Deierlein et al. 2016; Harley et al. 2017; Heggeseth et al. 2019; Yang et al. 2017, 2018). Five of these prospective studies focused on gestational phthalate exposures (Bowman et al. 2019; Harley et al. 2017; Heggeseth et al. 2019; Yang et al. 2017, 2018), and one study focused on childhood phthalate exposures (Deierlein et al. 2016).
Two studies examined prospective associations of gestational phthalate exposures with anthropometry measures among 345 participants in the CHAMACOS cohort (Harley et al. 2017; Heggeseth et al. 2019). Both studies found that increases in prenatal MEP, MBzP, and ∑DEHP metabolite concentrations were positively associated with BMI z-scores, waist circumference, percent body fat, and increased odds of being overweight or obese. Heggeseth et al. 2019 also reported associations between gestational phthalate metabolite concentrations and growth trajectories, with sex-specific analyses (Heggeseth et al. 2019). Yang et al. 2017 and Yang et al. 2018 examined prospective associations of prenatal phthalate exposures with anthropometry measures among 249 children in the Early Life Exposure to Environmental Toxicants Study (Yang et al. 2017, 2018). Yang et al. 2017 found an inverse relationship between prenatal urinary MBzP concentrations and BMI z-scores among children age 8–14 years. Yang et al. 2018 reported sex-specific effects between gestational phthalate exposures and growth trajectories from birth until 14 years of age. Among females there was a positive association between MECPP and BMI growth trajectories and among males there was a negative association between MiBP, MBzP, MEHP, and MEHHP and BMI growth trajectories (Yang et al. 2018). Although we were underpowered to detect differences in associations by sex, we also found stronger associations among females for several phthalate biomarker – outcome relations.
In a prospective pregnancy and birth cohort in Mexico City with 223 mother-child pairs, Bowman et al. 2019 found that prenatal MiBP, MBP, and MBzP concentrations were positively associated with BMI z-score, waist circumference, and skinfold thickness among females aged 9–17 years of age. Among males, Bowman et al. 2019 observed positive associations between maternal MBzP concentrations in pregnancy and adolescent BMI z score and waist circumference. In contrast, we found generally null or inverse associations of maternal urinary concentrations of these phthalate metabolites with FMI and BMI z-scores at age 12 years. Differences in findings may relate to ages of body composition assessment or differences in confounder adjustment. Bowman et al. adjusted only for specific gravity, maternal education, and maternal age whereas we accounted for a variety of maternal characteristics that we identified as potential confounders based on prior literature.
In the only other prospective study of childhood exposures, Deierlein et al. 2016 observed positive associations between low molecular weight phthalate metabolite concentrations measured at age 6–8 years and change in BMI and waist circumference between ages 7–13 years among 1,017 females in the Breast Cancer and Environment Research Program (Deierlein et al. 2016). While we found null or negative associations between low molecular weight phthalate biomarker concentrations during childhood and FMI and BMI among females, we assessed body composition at a single time point rather than change over time.
Evidence from experimental studies suggests that gestational and childhood phthalate exposures may influence body composition through multiple mechanisms (Grun and Blumberg 2009; Heindel et al. 2015). First, several phthalates have been shown to have weak estrogenic activity and strong anti-androgen activity (Borch et al. 2006; Harris et al. 1997; Jobling et al. 1995; Sohoni and Sumpter 1998), which could influence sex-specific patterns of growth (Heindel et al. 2017). As noted above, androgens play an important role in skeletal muscle development which may underly associations with LBMI in our study. Second, phthalates modify peroxisome proliferator-activated receptors (PPARs) (Desvergne et al. 2009; Pereira-Fernandes et al. 2013; Taxvig et al. 2012), which are predominately located in adipose tissues and regulate adipocyte differentiation, reduced inflammation, and lipid and carbohydrate metabolism (Millar 2013; Philips et al. 2017). Animal studies also suggest that perinatal exposures to DEHP and MEHP increase visceral fat and adipose tissue in adult mice through PPAR modulation (Desvergne et al. 2009; Pereira-Fernandes et al. 2013; Taxvig et al. 2012). Insulin has been shown to promote protein synthesis and prohibit proteolysis in muscle tissues, therefore modification of PPARs by chemical metabolic disruptors may contribute to insulin resistance and subsequent muscle protein loss (Guillet and Boirie 2005). Additionally, phthalates have been associated with increased oxidative stress markers in pregnant women, which is associated with increased inflammation (Ferguson et al. 2014). Both chronic inflammation and oxidative damage has also been associated with muscle atrophy and may be another mechanism for our observed decreases in LBMI in our study (Meng and Yu 2010; Ryall et al. 2008). Finally, phthalates may alter the production of adipokines, which are important cell signaling proteins involved in metabolic regulation and function including appetite, thermogenesis stimulation, fatty acid oxidation, decreased glucose, and reduced body weight and fat (Philips et al. 2017). Taken together, these potential mechanisms suggest that phthalates may cause inflammation and endothelial dysfunction that can lead to changes in body composition during adolescence and continue into adulthood (Philips et al. 2017).
Our study has many strengths. First, in addition to measures of anthropometry we had DXA measures of body composition. DXA is an accurate way to measure body composition and provides measures of body fat, lean, and bone mass (Wang et al. 2010; Weber et al. 2013). A second strength of our study is the repeated phthalate biomarker measurements during pregnancy and childhood, which allowed us to formally assess potential periods of heightened susceptibility to phthalate exposures using multiple informants modeling (Buckley et al. 2019a; Sanchez et al. 2011). Evaluating potential periods of heightened susceptibility is important to determine whether there are particular sensitive periods of growth and development to target for potential interventions such as education programs or chemical policy regulation. Overall, we did not find consistent evidence of a single period of susceptibility in our study. Next, we collected a rich set of covariates during pregnancy and adolescence and adjusted for a variety of potential baseline and time-varying confounders.
Our study also has several limitations. First, due to our modest sample-size we had relatively low power to detect exposure-time period interactions or differences by child sex. There is potential for selection bias due to loss to follow-up because a greater proportion of women who returned for follow-up visits from 1 to 5 years following delivery were Non-Hispanic White, well educated, and had higher household income compared to those who did not return for follow-up visits (Braun et al. 2017). However, baseline covariate distributions in our sample were similar to the full study and we previously reported no evidence of selection bias in associations of prenatal phthalate exposures and child BMI as loss to follow-up was not associated with maternal urinary phthalate biomarker concentrations (Buckley et al. 2016). Next, there may be residual confounding. Diet is a major source of phthalate exposures (Buckley et al. 2019b) and is also associated with body composition. While we lacked a detailed or standardized measure of gestational or childhood diet, we adjusted for fruit and vegetable consumption during pregnancy as well as other measured confounders that may help to block backdoor paths related to dietary intake (Supplemental Figure 2). Finally, children in our study were in various stages of puberty. DXA measures were age- and sex- standardized to account for variability in measures by age and sex. However, we did not adjust for puberty as it may be a mediator of associations. Pregnancy and early childhood phthalate concentrations have been associated with pubertal timing (Berger et al. 2018; Sears and Braun 2020; Watkins et al. 2014b; Watkins et al. 2017) and puberty is associated with body composition (Siervogel et al. 2003).
In this study, urinary phthalate biomarker concentrations during pregnancy and ages 1–12 years of age were generally not associated with body composition or anthropometry measures during adolescence. Although most associations were not modified by timing of exposure, phthalate biomarker concentrations at some exposure periods were associated with DXA outcomes. . Larger studies are warranted to further evaluate potential sex-specific effects and interrogate potential associations of phthalate exposures with measures of lean body mass.
Supplementary Material
Acknowledgements
This work was supported by grants from the National Institute of Environmental Health Sciences of the National Institutes of Health (R01ES030078, R01ES025214, R01ES014575, R01ES020349, R01ES027224, P01ES011261, T32ES00714). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. We are grateful to the HOME Study participants for the time they have given to the study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Publisher's Disclaimer: Disclaimer
Publisher's Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the CDC. Use of trade names is for identification only and does not imply endorsement by the CDC, the Public Health Service, or the US Department of Health and Human Services.
The Cincinnati Children’s Hospital Medical Center (CCHMC) and participating delivery hospitals’ Institutional Review Boards (IRB) approved the HOME Study. The Centers for Disease Control and Prevention (CDC) also relied on CCHMC IRB.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Al-Hamad D, Raman V. 2017. Metabolic syndrome in children and adolescents. Transl Pediatr 6:397–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkins JL, Whincup PH, Morris RW, Lennon LT, Papacosta O, Wannamethee SG. 2014. Sarcopenic obesity and risk of cardiovascular disease and mortality: A population-based cohort study of older men. J Am Geriatr Soc 62:253–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger K, Eskenazi B, Kogut K, Parra K, Lustig RH, Greenspan LC, et al. 2018. Association of prenatal urinary concentrations of phthalates and bisphenol a and pubertal timing in boys and girls. Environmental Health Perspectives 126:097004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernert JT Jr., Turner WE, Pirkle JL, Sosnoff CS, Akins JR, Waldrep MK, et al. 1997. Development and validation of sensitive method for determination of serum cotinine in smokers and nonsmokers by liquid chromatography/atmospheric pressure ionization tandem mass spectrometry. Clin Chem 43:2281–2291. [PubMed] [Google Scholar]
- Boeke CE, Oken E, Kleinman KP, Rifas-Shiman SL, Taveras EM, Gillman MW. 2013. Correlations among adiposity measures in school-aged children. BMC pediatrics 13:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borch J, Metzdorff SB, Vinggaard AM, Brokken L, Dalgaard M. 2006. Mechanisms underlying the anti-androgenic effects of diethylhexyl phthalate in fetal rat testis. Toxicology 223:144–155. [DOI] [PubMed] [Google Scholar]
- Bornehag CG, Carlstedt F, Jönsson BA, Lindh CH, Jensen TK, Bodin A, et al. 2015. Prenatal phthalate exposures and anogenital distance in swedish boys. Environ Health Perspect 123:101–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman A, Peterson KE, Dolinoy DC, Meeker JD, Sánchez BN, Mercado-Garcia A, et al. 2019. Phthalate exposures, DNA methylation and adiposity in mexican children through adolescence. Frontiers in Public Health 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun JM, Daniels JL, Poole C, Olshan AF, Hornung R, Bernert JT, et al. 2010. A prospective cohort study of biomarkers of prenatal tobacco smoke exposure: The correlation between serum and meconium and their association with infant birth weight. Environmental Health 9:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun JM. 2017. Early-life exposure to edcs: Role in childhood obesity and neurodevelopment. Nat Rev Endocrinol 13:161–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun JM, Kalloo G, Chen A, Dietrich KN, Liddy-Hicks S, Morgan S, et al. 2017. Cohort profile: The health outcomes and measures of the environment (home) study. Int J Epidemiol 46:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun JM, Buckley JP, Cecil KM, Chen A, Kalkwarf HJ, Lanphear BP, et al. 2020. Adolescent follow-up in the health outcomes and measures of the environment (home) study: Cohort profile. BMJ open 10:e034838–e034838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley JP, Engel SM, Braun JM, Whyatt RM, Daniels JL, Mendez MA, et al. 2016. Prenatal phthalate exposures and body mass index among 4 to 7 year old children: A pooled analysis. Epidemiology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley JP, Hamra GB, Braun JM. 2019a. Statistical approaches for investigating periods of susceptibility in children’s environmental health research. Current environmental health reports. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley JP, Kim H, Wong E, Rebholz CM. 2019b. Ultra-processed food consumption and exposure to phthalates and bisphenols in the us national health and nutrition examination survey, 2013–2014. Environ Int 131:105057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson A, Sorensen K, Andersson AM, Frederiksen H, Juul A. 2018. Bisphenol a, phthalate metabolites and glucose homeostasis in healthy normal-weight children. Endocr Connect 7:232–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. 2021. Fourth national report on human exposure to environmental chemicals, updated tables, march 2021.
- Consumer Product Safety Improvement Act. 2008. 11 u.S.C. Fed Reg 110–314. [Google Scholar]
- Deierlein AL, Wolff MS, Pajak A, Pinney SM, Windham GC, Galvez MP, et al. 2016. Longitudinal associations of phthalate exposures during childhood and body size measurements in young girls. Epidemiology 27:492–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Després JP, Moorjani S, Ferland M, Tremblay A, Lupien PJ, Nadeau A, et al. 1989. Adipose tissue distribution and plasma lipoprotein levels in obese women. Importance of intra-abdominal fat. Arteriosclerosis 9:203–210. [DOI] [PubMed] [Google Scholar]
- Desvergne B, Feige JN, Casals-Casas C. 2009. Ppar-mediated activity of phthalates: A link to the obesity epidemic? Mol Cell Endocrinol 304:43–48. [DOI] [PubMed] [Google Scholar]
- Ferguson KK, Cantonwine DE, Rivera-Gonzalez LO, Loch-Caruso R, Mukherjee B, Anzalota Del Toro LV, et al. 2014. Urinary phthalate metabolite associations with biomarkers of inflammation and oxidative stress across pregnancy in puerto rico. Environ Sci Technol 48:7018–7025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fielding RA, Vellas B, Evans WJ, Bhasin S, Morley JE, Newman AB, et al. 2011. Sarcopenia: An undiagnosed condition in older adults. Current consensus definition: Prevalence, etiology, and consequences. International working group on sarcopenia. J Am Med Dir Assoc 12:249–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goran MI, Gower BA. 1999. Relation between visceral fat and disease risk in children and adolescents. Am J Clin Nutr 70:149S–156S. [DOI] [PubMed] [Google Scholar]
- Grun F, Blumberg B. 2009. Endocrine disrupters as obesogens. Mol Cell Endocrinol 304:19–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillet C, Boirie Y. 2005. Insulin resistance: A contributing factor to age-related muscle mass loss? Diabetes Metab 31 Spec No 2:5s20–25s26. [DOI] [PubMed] [Google Scholar]
- Harley KG, Berger K, Rauch S, Kogut K, Claus Henn B, Calafat AM, et al. 2017. Association of prenatal urinary phthalate metabolite concentrations and childhood bmi and obesity. Pediatr Res 82:405–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris CA, Henttu P, Parker MG, Sumpter JP. 1997. The estrogenic activity of phthalate esters in vitro. Environmental health perspectives 105:802–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heggeseth BC, Holland N, Eskenazi B, Kogut K, Harley KG. 2019. Heterogeneity in childhood body mass trajectories in relation to prenatal phthalate exposure. Environmental research 175:22–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heindel JJ, vom Saal FS, Blumberg B, Bovolin P, Calamandrei G, Ceresini G, et al. 2015. Parma consensus statement on metabolic disruptors. Environmental health : a global access science source 14:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heindel JJ, Blumberg B, Cave M, Machtinger R, Mantovani A, Mendez MA, et al. 2017. Metabolism disrupting chemicals and metabolic disorders. Reprod Toxicol 68:3–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imboden MT, Swartz AM, Finch HW, Harber MP, Kaminsky LA. 2017. Reference standards for lean mass measures using ge dual energy x-ray absorptiometry in caucasian adults. PloS one 12:e0176161–e0176161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson-Browne MS, Papandonatos GD, Chen A, Calafat AM, Yolton K, Lanphear BP, et al. 2018. Identifying vulnerable periods of neurotoxicity to triclosan exposure in children. Environmental health perspectives 126:057001–057001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jobling S, Reynolds T, White R, Parker MG, Sumpter JP. 1995. A variety of environmentally persistent chemicals, including some phthalate plasticizers, are weakly estrogenic. Environmental health perspectives 103:582–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaul S, Rothney MP, Peters DM, Wacker WK, Davis CE, Shapiro MD, et al. 2012. Dual-energy x-ray absorptiometry for quantification of visceral fat. Obesity (Silver Spring) 20:1313–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowalski KC, Crocker PR, Donen RM. 2004. The physical activity questionnaire for older children (paq-c) and adolescents (paq-a) manual. College of Kinesiology, University of Saskatchewan; 87:1–38. [Google Scholar]
- Krotkiewski M, Björntorp P, Sjöström L, Smith U. 1983. Impact of obesity on metabolism in men and women. Importance of regional adipose tissue distribution. J Clin Invest 72:1150–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuczmarski RJ, Odgen CL, Grummer-Strawn LM, Flegal KM, Guo SS, Wei R, et al. 2000. Cdc growth charts: United states. 2001/02/24 ed. Hyattsville, Maryland: National Center for Health Statistics. [Google Scholar]
- Kumar S, Kelly AS. 2017. Review of childhood obesity: From epidemiology, etiology, and comorbidities to clinical assessment and treatment. Mayo Clin Proc 92:251–265. [DOI] [PubMed] [Google Scholar]
- Lapidus L, Bengtsson C, Larsson B, Pennert K, Rybo E, Sjöström L. 1984. Distribution of adipose tissue and risk of cardiovascular disease and death: A 12 year follow up of participants in the population study of women in gothenburg, sweden. Br Med J (Clin Res Ed) 289:1257–1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson B, Svärdsudd K, Welin L, Wilhelmsen L, Björntorp P, Tibblin G. 1984. Abdominal adipose tissue distribution, obesity, and risk of cardiovascular disease and death: 13 year follow up of participants in the study of men born in 1913. Br Med J (Clin Res Ed) 288:1401–1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laskey MA. 1996. Dual-energy x-ray absorptiometry and body composition. Nutrition 12:45–51. [DOI] [PubMed] [Google Scholar]
- Lawler CP, Heindel JJ, Gray K, Schug TT, Blawas AM. 2015. Elucidating the links between endocrine disruptors and neurodevelopment. Endocrinology 156:1941–1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DW, Lim YH, Shin CH, Lee YA, Kim BN, Kim JI, et al. 2020. Prenatal exposure to di-(2-ethylhexyl) phthalate and decreased skeletal muscle mass in 6-year-old children: A prospective birth cohort study. Environmental research 182:109020. [DOI] [PubMed] [Google Scholar]
- Meeker JD, Ferguson KK. 2014. Urinary phthalate metabolites are associated with decreased serum testosterone in men, women, and children from nhanes 2011–2012. The Journal of clinical endocrinology and metabolism 99:4346–4352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng SJ, Yu LJ. 2010. Oxidative stress, molecular inflammation and sarcopenia. Int J Mol Sci 11:1509–1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millar JS. 2013. Novel benefits of peroxisome proliferator-activated receptors on cardiovascular risk. Current opinion in lipidology 24:233–238. [DOI] [PubMed] [Google Scholar]
- Morrison JA, Friedman LA, Gray-McGuire C. 2007. Metabolic syndrome in childhood predicts adult cardiovascular disease 25 years later: The princeton lipid research clinics follow-up study. Pediatrics 120:340–345. [DOI] [PubMed] [Google Scholar]
- Morrison JA, Friedman LA, Wang P, Glueck CJ. 2008. Metabolic syndrome in childhood predicts adult metabolic syndrome and type 2 diabetes mellitus 25 to 30 years later. The Journal of pediatrics 152:201–206. [DOI] [PubMed] [Google Scholar]
- O’Brien KM, Upson K, Cook NR, Weinberg CR. 2016. Environmental chemicals in urine and blood: Improving methods for creatinine and lipid adjustment. Environ Health Perspect 124:220–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks LG, Ostby JS, Lambright CR, Abbott BD, Klinefelter GR, Barlow NJ, et al. 2000. The plasticizer diethylhexyl phthalate induces malformations by decreasing fetal testosterone synthesis during sexual differentiation in the male rat. Toxicol Sci 58:339–349. [DOI] [PubMed] [Google Scholar]
- Pereira-Fernandes A, Demaegdt H, Vandermeiren K, Hectors TL, Jorens PG, Blust R, et al. 2013. Evaluation of a screening system for obesogenic compounds: Screening of endocrine disrupting compounds and evaluation of the ppar dependency of the effect. PloS one 8:e77481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philips EM, Jaddoe VWV, Trasande L. 2017. Effects of early exposure to phthalates and bisphenols on cardiometabolic outcomes in pregnancy and childhood. Reprod Toxicol 68:105–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryall JG, Schertzer JD, Lynch GS. 2008. Cellular and molecular mechanisms underlying age-related skeletal muscle wasting and weakness. Biogerontology 9:213–228. [DOI] [PubMed] [Google Scholar]
- Samsell L, Regier M, Walton C, Cottrell L. 2014. Importance of android/gynoid fat ratio in predicting metabolic and cardiovascular disease risk in normal weight as well as overweight and obese children. Journal of obesity 2014:846578–846578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez BN, Hu H, Litman HJ, Tellez-Rojo MM. 2011. Statistical methods to study timing of vulnerability with sparsely sampled data on environmental toxicants. Environ Health Perspect 119:409–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schettler T 2006. Human exposure to phthalates via consumer products. Int J Androl 29:134–139; discussion 181–135. [DOI] [PubMed] [Google Scholar]
- Sears CG, Braun JM. 2020. Phthalate exposure, adolescent health, and the need for primary prevention. Endocrinol Metab Clin North Am 49:759–770. [DOI] [PubMed] [Google Scholar]
- Shoaff J, Papandonatos GD, Calafat AM, Ye X, Chen A, Lanphear BP, et al. 2017. Early-life phthalate exposure and adiposity at 8 years of age. Environ Health Perspect 125:097008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siervogel RM, Demerath EW, Schubert C, Remsberg KE, Chumlea WC, Sun S, et al. 2003. Puberty and body composition. Horm Res 60:36–45. [DOI] [PubMed] [Google Scholar]
- Silva MJ, Barr DB, Reidy JA, Malek NA, Hodge CC, Caudill SP, et al. 2004. Urinary levels of seven phthalate metabolites in the u.S. Population from the national health and nutrition examination survey (nhanes) 1999–2000. Environmental health perspectives 112:331–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha I, Sinha-Hikim AP, Wagers AJ, Sinha-Hikim I. 2014. Testosterone is essential for skeletal muscle growth in aged mice in a heterochronic parabiosis model. Cell Tissue Res 357:815–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohoni P, Sumpter JP. 1998. Several environmental oestrogens are also anti-androgens. The Journal of endocrinology 158:327–339. [DOI] [PubMed] [Google Scholar]
- Sopher AB, Thornton JC, Wang J, Pierson RN Jr., Heymsfield SB, Horlick M. 2004. Measurement of percentage of body fat in 411 children and adolescents: A comparison of dual-energy x-ray absorptiometry with a four-compartment model. Pediatrics 113:1285–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stacy SL, Papandonatos GD, Calafat AM, Chen A, Yolton K, Lanphear BP, et al. 2017. Early life bisphenol a exposure and neurobehavior at 8years of age: Identifying windows of heightened vulnerability. Environment international 107:258–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenholm S, Harris TB, Rantanen T, Visser M, Kritchevsky SB, Ferrucci L. 2008. Sarcopenic obesity: Definition, cause and consequences. Curr Opin Clin Nutr Metab Care 11:693–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swan SH. 2008. Environmental phthalate exposure in relation to reproductive outcomes and other health endpoints in humans. Environmental research 108:177–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taxvig C, Dreisig K, Boberg J, Nellemann C, Schelde AB, Pedersen D, et al. 2012. Differential effects of environmental chemicals and food contaminants on adipogenesis, biomarker release and ppargamma activation. Mol Cell Endocrinol 361:106–115. [DOI] [PubMed] [Google Scholar]
- Wang Y, Cai L, Wu Y, Wilson RF, Weston C, Fawole O, et al. 2015. What childhood obesity prevention programmes work? A systematic review and meta-analysis. Obesity reviews : an official journal of the International Association for the Study of Obesity 16:547–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Heymsfield SB, Chen Z, Zhu S, Pierson RN. 2010. Estimation of percentage body fat by dual-energy x-ray absorptiometry: Evaluation by in vivo human elemental composition. Physics in medicine and biology 55:2619–2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins DJ, Eliot M, Sathyanarayana S, Calafat AM, Yolton K, Lanphear BP, et al. 2014a. Variability and predictors of urinary concentrations of phthalate metabolites during early childhood. Environ Sci Technol 48:8881–8890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins DJ, Téllez-Rojo MM, Ferguson KK, Lee JM, Solano-Gonzalez M, Blank-Goldenberg C, et al. 2014b. In utero and peripubertal exposure to phthalates and bpa in relation to female sexual maturation. Environmental research 134:233–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins DJ, Sánchez BN, Téllez-Rojo MM, Lee JM, Mercado-García A, Blank-Goldenberg C, et al. 2017. Phthalate and bisphenol a exposure during in utero windows of susceptibility in relation to reproductive hormones and pubertal development in girls. Environmental research 159:143–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber DR, Moore RH, Leonard MB, Zemel BS. 2013. Fat and lean bmi reference curves in children and adolescents and their utility in identifying excess adiposity compared with bmi and percentage body fat. The American journal of clinical nutrition 98:49–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright CM, Parker L, Lamont D, Craft AW. 2001. Implications of childhood obesity for adult health: Findings from thousand families cohort study. BMJ (Clinical research ed) 323:1280–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang TC, Peterson KE, Meeker JD, Sanchez BN, Zhang Z, Cantoral A, et al. 2017. Bisphenol a and phthalates in utero and in childhood: Association with child bmi z-score and adiposity. Environmental research 156:326–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang TC, Peterson KE, Meeker JD, Sanchez BN, Zhang Z, Cantoral A, et al. 2018. Exposure to bisphenol a and phthalates metabolites in the third trimester of pregnancy and bmi trajectories. Pediatr Obes 13:550–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmet P, Alberti G, Kaufman F, Tajima N, Silink M, Arslanian S, et al. 2007. The metabolic syndrome in children and adolescents. Lancet (London, England) 369:2059–2061. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


