Abstract
INTRODUCTION:
To understand associations between a new measure of illness burden and care experiences in a large, national sample of Medicare beneficiaries surveyed before or after a cancer diagnosis.
MATERIALS AND METHODS:
The SEER-CAHPS Illness Burden Index (SCIBI) was previously developed using Surveillance, Epidemiology, and End Results (SEER)–Consumer Assessment of Healthcare Providers and Systems (CAHPS) linked data. The SCIBI provides a standardized morbidity score based on self- and other-reported information from 8 domains and proxies relative risk of 12-month, all-cause mortality among people surveyed before or after a cancer diagnosis. We analyzed a population of Medicare beneficiaries (n = 116,735; 49% fee-for-service and 51% Medicare Advantage [MA]; 73% post-cancer diagnosis) surveyed 2007 – 2013 to understand how their SCIBI scores were associated with 12 different care experience measures. Frequentist and Bayesian multivariable regression models adjusted for standard case-mix adjustors, enrollment type, timing of cancer diagnoses relative to survey, and survey year.
RESULTS AND DISCUSSION:
SCIBl scores were associated (P < .001) in frequentist models with better ratings of Health Plan (coefficient ± standard error: 0.33 ± 0.08) and better Getting Care Quickly scores (0.51 ± 0.09). In Bayesian models, individuals with higher illness burden had similar results on the same two measures and also reported reliably worse Overall Care experiences (coefficient ± posterior SD: −0.17 ± 0.06). Illness burden may influence how people experience care or report those experiences. Individuals with greater illness burdens may need intensive care coordination and multilevel interventions before and after a cancer diagnosis.
Keywords: comorbidity, care experiences, survey data, claims data, cancer
INTRODUCTION
As people continue to live longer after a cancer diagnosis, the relative impact of other chronic health conditions on health outcomes continues to grow.1 Older individuals who suffer from chronic conditions in addition to cancer experience poorer health status and quality of life,2 higher mortality,3 and shorter survival.4–6 Many patients, particularly those with early stage cancer, are more likely to die from chronic conditions other than their primary cancer,7 yet guidelines for treating patients with multiple chronic conditions are limited.8
Researchers have increasingly recognized important associations between health status, multiple chronic conditions, and patient experiences with care, particularly among individuals with cancer.9–11 Care experiences among cancer patients are influenced by a range of factors, including demographic characteristics and health status.12 National care experience measures, as publicly reported by the Consumer Assessment of Healthcare Providers and Systems (CAHPS) surveys, are thus adjusted for factors that have been shown to affect responses.13–16
Prior research has found that Medicare CAHPS respondents with cancer who rated their general or mental health status as excellent or very good were more likely than those with worse self-reported health to indicate excellent experiences.17,18 Although the single-item health status measures used in those studies have been widely used,19–21 a more comprehensive measure of morbidity may shed additional light on the association between illness burden and care experiences among people with cancer.
In a cross-agency collaboration, the Surveillance, Epidemiology, and End Results (SEER) cancer registry data from the National Cancer Institute have been linked with the Center for Medicare and Medicaid Services (CMS) data from Medicare CAHPS surveys, as well as Medicare claims and enrollment files. The data resource is known as SEER-CAHPS and was launched publicly in 2016.22 Detailed descriptions of the SEER-CAHPS linkage have been previously reported.23
The linked data were previously used to develop and validate a machine learning-derived measure of comorbidity called the SEER-CAHPS Illness Burden Index, or SCIBI. 24,25 The SCIBI score is an omnibus measure that proxies the relative risk of all-cause mortality in the 12 months after CAHPS survey response.24,25 The version we used for this analysis is the SCIBI-Concurrent Basic score, which incorporates factors including functional status, other conditions, and health services use that is associated with high medical need, serious illness, frailty, and the end of life. SCIBI-CB does not include standard case-mix adjustment variables, including age, education, and proxy response. The score is scaled such that higher values indicate higher relative risks of mortality within 12 months of survey response. SCIBI is based on a combination of self-reported (survey) measures, claims-based measures, administrative data, and cancer registry data from the SEER program.
In the current analysis, our goals were to examine differences in illness burden (SCIBI) scores in a large Medicare population with a documented incidence of cancer, surveyed either before or after their diagnosis, and analyze associations between illness burdens and care experiences, controlling for standard case-mix adjustors used in public reporting. The SCIBI was developed as a tool specifically for this data resource and to enable researchers to better control for illness burden. This study provides an application of the tool and baseline comparative information for a large sample, so that future researchers have a benchmark for their own analyses.
METHODS
Study Population
Our Institutional Review Board declared this research exempt [45CFR46.101(b)] from IRB review. For this analysis, we included individuals aged 18 or older who responded to a Medicare CAHPS survey between 2007 and 2013. SEER-CAHPS includes Medicare beneficiaries diagnosed with cancer (including benign tumors) while residing in a SEER region. We stratified by whether survey response was before or after a first cancer diagnosis and whether the beneficiary was enrolled in Medicare Advantage (MA) or fee-for-service (FFS). We excluded a few individuals missing their date of cancer diagnosis (either year or month) and a small number of individuals who had a death date before the survey response date or diagnosis date. The stage variable we used was the summ2k1 variable, Summary Staging, which uses all the information available in the medical record. According to the SEER documentation, all cases are blank for a number of sites, including many of the head and neck cancers.26 In addition, nearly 20% of the sample had benign tumors.
Measures
CAHPS measures included 5 global ratings (Overall Care, Personal Doctor, Specialist, Health Plan/Medicare, Prescription Drug Plan) and 7 composite measures (Doctor Communication, Getting Needed Care, Getting Needed Prescription Drugs, Getting Care Quickly, Care Coordination, Health Plan Customer Service, and PDP Customer Service). All measures were available for all years of the study except for the Care Coordination measure, which was limited to surveys returned in 2012 or 2013. The number of survey respondents analyzed for each measure ranged from 22,282 to 103,286 (see Technical Appendix, Table A-1).
The global ratings are scored from 0 to 10, with 0 being the worst possible and 10 the best possible. Composite measure scores range from 0 to 100 and are calculated based on prior psychometric research that grouped different survey items into logical clusters representing single concepts. The customer service measures encompass getting needed information, being treated with courtesy and respect by customer service staff, and getting information about which services or medicines are covered and how much they cost. Further details about how each measure is defined are available online.22 Following prior research, the 5 global ratings were linearly rescaled to a possible range of 0–100 for comparability and ease of interpretation.27
Statistical Analysis
Our analysis included both frequentist and Bayesian estimations due to concerns about the applicability of OLS estimation to the data. The inclusion and agreement of both methods reinforces the robustness of the results.
We estimated frequentist ordinary least-squares (OLS) multivariable regression models for each outcome measure, with the exposure being SCIBI z-score (standardized to have an overall population mean of 0 and SD of 1) and controlling for sex, year, survey timing (before or after diagnosis), and Medicare enrollment (MA or FFS). We also adjusted for standard case-mix adjustment variables used in public reporting of MA and PDP CAHPS Survey results: age, education, general health status, mental health status, received help responding or proxy answered questions for respondent, dual (Medicare-Medicaid) enrollment or low-income subsidy, and survey language.
After exploring alternative specifications, we used survey-weighted ordinary least squares (OLS) models to identify significant associations between SCIBI scores and CAHPS measures. Bonferroni corrections were applied to adjust for multiple comparisons; the significance level was set at P < .004.
We had concerns about whether the data met the usual assumptions of the frequentist OLS models for the outcome measures, which are ordinal (as is common in survey data) and asymmetrically distributed, rather than normally distributed. While the large sample size of the data alleviated those concerns to some degree, we followed up the OLS analysis with a Bayesian analysis that relaxed the distributional assumptions of the frequentist model. Our Bayesian models were implemented using the BAS package for R.28 The Bayesian models used uniform, noninformative priors for each variable. We used the survey weights from the frequentist analyses as probability weights for the response covariates in the Bayesian models. Analyses were conducted in R version 3.6.0, SAS v. 9.4 (SAS Institute, Cary, NC) and Stata/MP v. 15.1 (StataCorp LLC, College Station, TX).
RESULTS
Descriptive Results
The analytic sample included 116,735 survey respondents with cancer information in our data. About half (49%) were enrolled in FFS at the time of their survey, and 73% were surveyed after diagnosis. Among people surveyed before diagnosis, 6% died within 12 months of their survey response; among those surveyed after diagnosis, 7% died within 12 months. Among people with SCIBI scores in the top percentile (>99th percentile) of the distribution, 59% died; in the bottom quartile (≤26th percentile), about 1% died.
Sociodemographic (Table 1) and clinical (Table 2) characteristics show that the four tabulation groups were dissimilar, with different distributions on most of the characteristics examined. Comparing the tabulation groups on clinical characteristics (Table 2) reveals that the group reporting the highest prevalence of having multiple chronic conditions was FFS enrollees with cancer surveyed pre-diagnosis (nearly 19%). FFS enrollees were more likely than MA enrollees to report poor/fair health (vs. good/very good/excellent), depression, pain that interferes with activities, a physical condition that interferes with independence, and needing help with routine tasks.
Table 1.
Sociodemographic characteristics of individuals with cancer in the SEER-CAHPS Illness Burden Index sample
| Group 1 | Group 2 | Group 3 | Group 4 | |
|---|---|---|---|---|
| Surveyed pre-diagnosis | Surveyed post-diagnosis | |||
|
| ||||
| MA, %* (Unweighted n=16,222) | FFS, %* (Unweighted n=15,647) | MA, %* (Unweighted n=42,834) | FFS, %* (Unweighted n=42,032) | |
|
| ||||
| Age in yrs on survey date | ||||
| 18–64 | 6.1 | 8.8 | 5.8 | 6.6 |
| 65–69 | 22.4 | 23.8 | 18.8 | 19.7 |
| 70–74 | 24.8 | 22.7 | 22.5 | 22.1 |
| 75–79 | 21.4 | 19.5 | 21.1 | 20.1 |
| 80–84 | 15.2 | 15.0 | 17.3 | 17.1 |
| 85 or older | 10.1 | 10.2 | 14.4 | 14.4 |
| Female | 50.8 | 51.5 | 51.0 | 51.1 |
| Race/ethnicity | ||||
| White, non-Hispanic | 79.0 | 84.3 | 78.8 | 86.2 |
| Black, non-Hispanic | 11.5 | 8.9 | 10.2 | 6.7 |
| Hispanic, any race | 2.3 | 1.8 | 2.2 | 1.3 |
| Asian, non-Hispanic | 3.8 | 2.5 | 4.5 | 2.5 |
| Other/unknown | 3.4 | 2.5 | 4.4 | 3.2 |
| Married | 52.5 | 52.3 | 66.9 | 69.1 |
| Lives alone | 31.1 | 30.8 | 30.3 | 29.3 |
| Continuously enrolled | 98.7 | 93.1 | 98.9 | 93.6 |
| Dual (Medicare + Medicaid) | 15.9 | 14.8 | 12.7 | 10.0 |
| Neighborhood poverty level | ||||
| <5% | 19.4 | 20.9 | 23.6 | 27.0 |
| 5 to <10% | 27.1 | 25.8 | 24.7 | 24.4 |
| 10 to <20% | 30.4 | 29.6 | 22.9 | 22.1 |
| 20%+ | 22.0 | 21.7 | 14.0 | 12.6 |
| Missing | 1.1 | 2.0 | 14.8 | 13.8 |
| Educational attainment | ||||
| Less than 12 years | 21.1 | 20.3 | 17.6 | 14.4 |
| 12 to 15 years | 51.9 | 50.9 | 52.4 | 51.5 |
| 16 or more years | 17.3 | 21.0 | 21.8 | 27.1 |
| Missing | 9.6 | 7.8 | 8.1 | 7.0 |
| Urban/rural residence | ||||
| Metropolitan | 95.6 | 86.7 | 95.9 | 88.9 |
| Non-metropolitan, non-rural | 3.5 | 10.6 | 3.2 | 8.8 |
| Rural | 0.7 | 2.6 | 0.7 | 2.1 |
| Census region | ||||
| Northeast | 12.8 | 18.6 | 11.9 | 18.1 |
| Midwest | 7.0 | 11.6 | 10.4 | 14.8 |
| South | 21.1 | 27.9 | 16.5 | 19.1 |
| West | 59.0 | 41.9 | 61.2 | 47.9 |
Source: Authors’ analysis of SEER-CAHPS, 2007–2013. FFS: fee-for-service; MA: Medicare Advantage.
Unweighted percentages shown.
Table 2.
Clinical characteristics of individuals with cancer in the SEER-CAHPS Illness Burden Index sample
| Group 1 | Group 2 | Group 3 | Group 4 | |
|---|---|---|---|---|
| Surveyed pre-diagnosis | Surveyed post-diagnosis | |||
|
| ||||
| MA (Unweighted n=16,222) |
FFS (Unweighted n=15,647) |
MA (Unweighted n=42,834) |
FFS (Unweighted n=42,032) |
|
|
| ||||
| Point estimates | ||||
| SCIBI z-score - mean | −0.19 | −0.23 | 0.07 | 0.13 |
| Median | −0.28 | −0.47 | −0.13 | −0.42 |
| Range | −0.50 to 4.00 | −0.50 to 6.00 | −0.50 to 8.12 | −0.50 to 13.32 |
| SF-12 Physical Component (v12) - mean | 41.3 | 38.3 | 39.2 | 37.1 |
| SF-12 Mental Component (v12) - mean | 54.4 | 52.5 | 53.5 | 52.6 |
| Comorbidity count - mean | 0.85 | 0.89 | 1.4 | 1.5 |
| Percentages | ||||
| Poor/fair general health status | 25.7 | 31.7 | 29.9 | 33.7 |
| Poor/fair mental health status | 9.3 | 11.9 | 11.1 | 11.4 |
| Depression (PHQ-2 ≥ 3) | 29.7 | 36.1 | 32.7 | 37.7 |
| Proxy respondent or assistance | 12.0 | 13.6 | 13.3 | 13.5 |
| Has had a condition/problem lasting 3+ months | 91.3 | 92.8 | 92.0 | 93.7 |
| Current smoker | 14.8 | 15.5 | 8.6 | 8.6 |
| Physical condition interferes with independence | 18.8 | 23.8 | 22.5 | 27.1 |
| Need help with personal care | 6.8 | 9.2 | 9.5 | 11.3 |
| Need assistance with routine tasks | 16.9 | 22.1 | 20.9 | 25.7 |
| Activity limitations | ||||
| Bathing | 11.5 | 14.2 | 13.4 | 14.9 |
| Dressing | 8.9 | 11.4 | 11.0 | 12.0 |
| Eating | 4.8 | 5.4 | 5.9 | 6.2 |
| Getting in/out of chairs | 18.0 | 21.0 | 19.0 | 21.4 |
| Walking | 27.8 | 32.6 | 29.5 | 31.7 |
| Using the toilet | 7.0 | 8.2 | 8.4 | 9.0 |
| Climbing stairs | 52.3 | 62.8 | 56.8 | 65.6 |
| Moderate activities | 47.8 | 59.1 | 52.6 | 61.8 |
| Social activities | 7.2 | 11.2 | 9.3 | 11.5 |
| Pain interferes | 18.2 | 25.2 | 19.2 | 25.8 |
| Has a lot of energy a little/none of the time | 18.8 | 25.4 | 24.5 | 29.0 |
| Specific conditions reported | ||||
| Heart attack or angina | 23.9 | 28.0 | 25.0 | 27.7 |
| Stroke | 9.6 | 9.6 | 10.6 | 10.3 |
| COPD | 20.0 | 21.3 | 19.6 | 20.0 |
| Diabetes | 32.4 | 31.4 | 34.0 | 30.4 |
| Cancer | 7.8 | 9.6 | 70.9 | 73.9 |
| 2+ self-reported chronic conditions | 17.6 | 18.9 | 15.7 | 15.5 |
| Cancer-specific clinical characteristics | Not used | |||
| Stage at diagnosis | ||||
| In situ | 8.9 | 9.8 | ||
| Local | 44.0 | 43.9 | ||
| Regional | 12.8 | 12.6 | ||
| Distant | 4.4 | 4.8 | ||
| N/A, unknown, or unstaged | 1.7 | 2.0 | ||
| Missing | 28.3 | 27.0 | ||
| Cancer site | ||||
| Bladder | 4.6 | 5.0 | ||
| Breast | 23.0 | 22.4 | ||
| Colorectal | 4.5 | 4.5 | ||
| Lung | 3.9 | 4.2 | ||
| Prostate | 24.5 | 23.4 | ||
| Renal | 2.3 | 2.4 | ||
| Skin (melanoma) | 6.9 | 7.8 | ||
| Other | 30.2 | 30.4 | ||
| Primary/first cancer is malignant* | 83.5 | 82.4 | ||
Source: Authors’ analysis of SEER-CAHPS, 2007–2013. Unweighted percentages of non-missing responses shown. FFS: fee-for-service; MA: Medicare Advantage; N/A: not applicable; PHQ-2: Patient Health Questionnaire-2 question version; SF-12: the Medical Outcomes Study Short Form - 12 question version.
People with benign tumors were included in sample.
Multivariable Regression Results
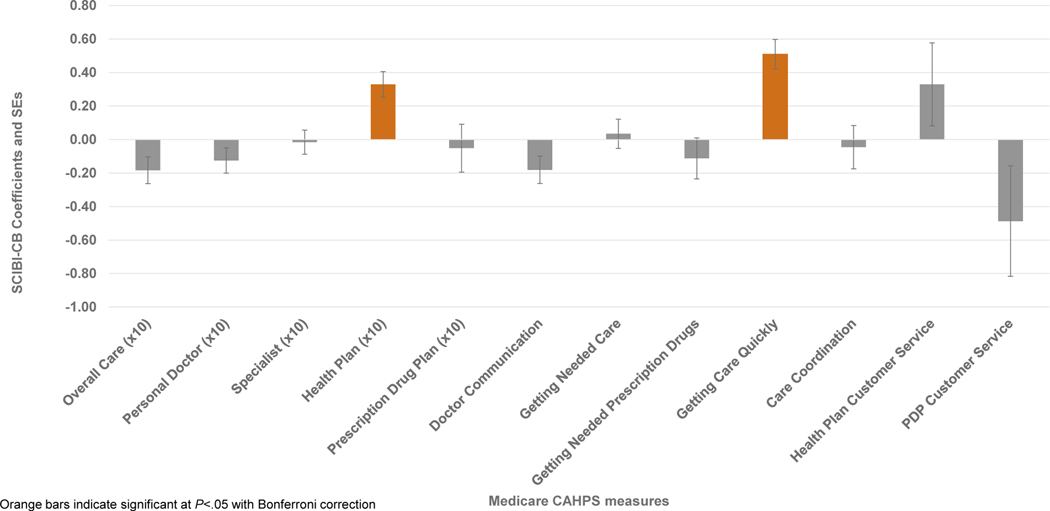
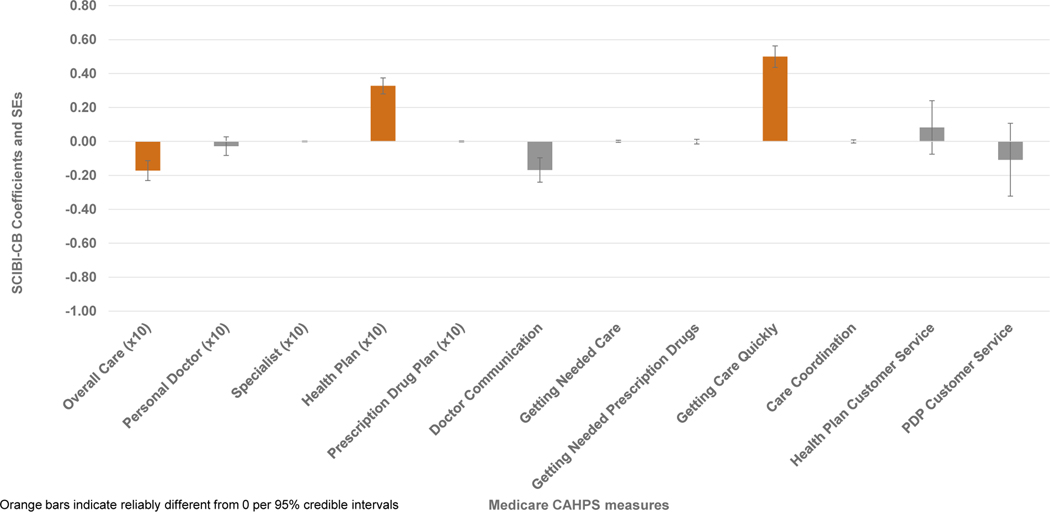
In frequentist linear regressions, SCIBI scores were significantly associated with better ratings of Health Plan (coefficient ± standard error: 0.33 ± 0.08) and better Getting Care Quickly scores (0.51 ± 0.09; Figure 1). Figure 2 provides the point estimates and 95% credible intervals for the Bayesian associations between each measure and z-normalized SCIBI score, which strongly supported the conclusions of the frequentist models. In Bayesian models, individuals with higher illness burden had similar results on the same two measures and also reported reliably worse Overall Care experiences (coefficient ± posterior SD: −0.17 ± 0.06). The coefficients that were significant in the frequentist analysis had the same sign and a posterior probability of greater than 99% of being non-zero in the Bayesian analysis. The 95% credible intervals around those coefficients were uniformly more compact than the frequentist 95% confidence intervals. As indicated, the Bayesian analyses supports the same conclusions as the frequentist analyses with stronger evidence in the form of more precise interval estimates and without the assumptions of frequentist linear regression.
Figure 1. Coefficients and standard errors from frequentist linear regressions estimating associations between SCIBI z-scores and care experience measures.
Source: Authors’ analysis of SEER-CAHPS, 2007–2013. Orange bars represent significant associations at P<.004. Regressions adjusted for sex, year, survey timing (before or after diagnosis), Medicare enrollment (MA or FFS), age, education, general health status, mental health status, received help responding or proxy answered questions for respondent, dual (Medicare-Medicaid) enrollment or low-income subsidy, and survey language.
Figure 2. Associations between SCIBI z-scores and care experience measures from survey-weighted Bayesian Model Averaging.
Source: Authors’ analysis of SEER-CAHPS, 2007–2013. Orange bars represent estimates that were reliably different from 0 according to Bayesian Model Averaging-based regressions adjusted for sex, year, survey timing (before or after diagnosis), Medicare enrollment (MA or FFS), age, education, general health status, mental health status, received help responding or proxy answered questions for respondent, dual (Medicare-Medicaid) enrollment or lowincome subsidy, and survey language.
DISCUSSION
Summary of Main Findings
Our omnibus measure of illness burden, the SCIBI score, was associated with 3 out of 12 care experience measures (Health Plan, Getting Care Quickly, and Overall Care). Individuals who had greater morbidity per their SCIBI score—whether before or after a cancer diagnosis— reported better care experiences on Health Plan and Getting Care Quickly measures than healthier individuals. This was confirmed by both frequentist and Bayesian analyses. The Bayesian model averaging approach also identified a reliable association between greater morbidity and worse Overall Care ratings that was not significant in the frequentist model.
Comparisons with Prior Studies
This study is novel in its examination of associations between illness burden and outcomes among people with cancer using a comprehensive measure of illness burden that incorporates self-report and medical claims data. It is also consistent with prior studies showing that outcome measures are affected by illness burden. Several prior studies using SEER-CAHPS have found that patients with better general or mental health status were significantly more likely to indicate excellent experience with nearly all measures examined.10,17,29
As in the current study, a prior SEER-CAHPS study of dual (Medicare/Medicaid) enrollees found that they gave higher ratings of their Health Plan than non-duals, but lower ratings on Overall Care.28 Lower cost-sharing responsibilities for duals could be one explanation for that finding. Similarly, it is possible that sicker individuals rate their health plans higher than healthier individuals because they appreciate the benefits they receive once their deductibles are met. Supporting this hypothesis, among those with higher health plan ratings in this study, DME, inpatient care, and SNF utilization rates were higher, and a greater proportion were dual enrollees. Future research is needed to understand how total and out-of-pocket expenditures affect care experiences.
An analysis of Medicare CAHPS responses among people with end-stage renal disease (ESRD) found that those with ESRD reported better experiences than those without on 7 out of 10 measures. Recent research found that Medicare Current Beneficiary Survey respondents with more ADL limitations reported lower ratings of care, although the relationship was not linear.29 Another study found that measures of care quality (effective acute and chronic treatment) were often worse among cancer survivors with comorbid conditions when compared to noncancer controls with the same conditions, although results varied among types of cancer.30 Prior research using Home Health CAHPS data also supports the idea that there may be a need to adjust for morbidity when examining patient experiences of care.31 The current publicly reported Medicare CAHPS measures are adjusted for both GHS and MHS.
We did not examine the associations between strictly claims-based comorbidity indices and care experience measures in this paper, since about half of our sample was enrolled in MA and thus had no claims data. However, previous analyses using a FFS subgroup of older Medicare beneficiaries (n = 9,305) surveyed up to 5 years after a cancer diagnosis found associations between care experiences and the NCI Combined Index, comorbidity count, and a study-specific measure of burden.32 Specifically, the Kent et al. analysis reported that individuals with higher morbidity burden rated their Personal Doctor, Specialist Physician, and Doctor’s Communication higher, while rating Getting Care Quickly lower; however, results varied depending on how morbidity was defined.
Caveats and Limitations
Limitations of this analysis are important to take into consideration when interpreting our findings. Our sample included FFS and MA Medicare beneficiaries of all ages, but only in areas covered by the SEER cancer registry program (which includes roughly 30% of the US population). Of note, between 42–61% of the sample (depending on the survey year and Medicare program enrollment) resided in the West Census region. In addition, the Medicare CAHPS surveys have relatively low (and declining) response rates.33 Nonetheless, response rates are a poor proxy for nonresponse bias.34,35 As a further protection against nonresponse bias, all analyses used person-level poststratification weights that account for sample design and nonresponse.
While much prior research supports the validity of CAHPS items, the Medicare CAHPS survey is a general population survey that asks patients to think about their most frequently seen physicians. Results using SEER data linked with CAHPS data targeted at the population examined here, such as the CAHPS Cancer Care Survey, might find different results. Finally, while the SCIBI incorporates information on multiple measures of health (including mental health), many unmeasured factors may also influence care experiences, such as a particular patient’s expectations around care quality.28
Implications for Policy and Practice
The results of our study point to the need to improve care experiences overall, particularly for cancer survivors with compromised health status. Patients with worse health status and other chronic conditions may not only suffer from their diseases but may also experience the burden of having to manage treatment and follow-up related care in concert with managing their health conditions. It is encouraging that patients report better scores on Getting Care Quickly when they have higher illness burdens. Many patients see several medical specialists and often must coordinate their own care, which may consist of multiple treatment regimens. Cancer patients with poorer health status also may have higher utilization rates than those without other chronic conditions.36 Given the complexity of cancer care, it is critical to understand and address an individual’s total illness burden and its effect on care.
Over the past decade, many new health policies have been enacted in the US, such as Medicaid expansion and the many other provisions of the Affordable Care Act. In addition, the Oncology Care Model (OCM), launched in 2016, has aimed to reform oncology care by bundling reimbursement based on chemotherapy episodes. Our study sample extends through 2013; however, we have no reason to believe that the study’s findings would be any different if we used more contemporary data. Most of the policy changes affect those who were previously uninsured, as opposed to those covered by Medicare. The OCM only directly affects those Medicare beneficiaries diagnosed with cancers who are treated with chemotherapy at a participating practice (as of July 2021, there were 126 participating practices),30 although it is possible that there have been (or will be) spill-over effects in the larger cancer population. Future research is needed to understand how the OCM may influence care experiences in future, especially around care coordination and access to care.
Existing measures of morbidity adjustment are inadequate or unavailable for over half the SEER-CAHPS population. Single-item measures of health status are separately accounted for in case-mix analysis, but may not fully capture an individual’s illness burden. Data on self-reported comorbidities and physical function are complex and cumbersome to include in multivariable modeling, especially because they are often colinear. This paper demonstrates the utility of the SCIBI scores as a method for controlling for illness burden and provides benchmark data on their associations with care experiences in a large sample.
Conclusions
In conclusion, our findings suggest associations between illness burden and a few care experience measures. Our findings highlight the need for additional investigation, since cancer patients with greater morbidity often need more tailored and coordinated care. Future research should examine the use of the SCIBI in longitudinal and interventional studies to support symptom management and improved care quality for cancer patients.
Acknowledgments:
The authors gratefully acknowledge Diana Zabala and Amy Huebeler of RTI International for assistance and programming.
Funding for this research was provided to LML, JC, JK, and MTH under National Cancer Institute contract #HHSN-261–2015-00132U.
TECHNICAL APPENDIX
Table A-1.
Availability of outcome measures by year and tabulation group and number of beneficiaries included in each regression model
| Measure | Model N | Years available |
|---|---|---|
|
| ||
| Global ratings | ||
| Overall Rating of Care | 95,470 | All |
| Personal Doctor | 86,765 | All |
| Specialist | 65,584 | All |
| Health Plan | 103,286 | All |
| Prescription Drug Plan | 74,846 | All |
| Composite scores | ||
| Getting Needed Care | 83,339 | All |
| Getting Care Quickly | 98,281 | All |
| Getting Needed Prescription Drug | 73,229 | All |
| Care Coordination | 22,282 | 2012–2013 |
| Doctor Communication | 87,474 | All |
| Health Plan Customer Service | 29,920 | All |
| PDP Customer Service | 22,425 | All |
Footnotes
Disclaimer: This article was prepared as part of the authors’ (MTH, MAM, AWS), official duties as employees of the US Federal Government. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the National Cancer Institute.
Conflict of interest statement: The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Bluethmann SM, Mariotto AB, Rowland JH. Anticipating the “Silver Tsunami”: Prevalence Trajectories and Comorbidity Burden among Older Cancer Survivors in the United States. Cancer Epidemiol Biomarkers Prev. Jul 2016;25(7):1029–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smith AW, Reeve BB, Bellizzi KM, et al. Cancer, comorbidities, and health-related quality of life of older adults. Health Care Financ Rev. Summer 2008;29(4):41–56. [PMC free article] [PubMed] [Google Scholar]
- 3.Chen RC, Royce TJ, Extermann M, Reeve BB. Impact of age and comorbidity on treatment and outcomes in elderly cancer patients. Seminars in radiation oncology. Oct 2012;22(4):265–271. [DOI] [PubMed] [Google Scholar]
- 4.Bradley CJ, Dahman B, Anscher M. Prostate cancer treatment and survival: evidence for men with prevalent comorbid conditions. Medical care. Jun 2014;52(6):482–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mariotto AB, Wang Z, Klabunde CN, Cho H, Das B, Feuer EJ. Life tables adjusted for comorbidity more accurately estimate noncancer survival for recently diagnosed cancer patients. Journal of clinical epidemiology. Dec 2013;66(12):1376–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Patnaik JL, Byers T, Diguiseppi C, Denberg TD, Dabelea D. The influence of comorbidities on overall survival among older women diagnosed with breast cancer. Journal of the National Cancer Institute. Jul 20 2011;103(14):1101–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Albertsen PC, Moore DF, Shih W, Lin Y, Li H, Lu-Yao GL. Impact of comorbidity on survival among men with localized prostate cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. Apr 1 2011;29(10):1335–1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lugtenberg M, Burgers JS, Clancy C, Westert GP, Schneider EC. Current guidelines have limited applicability to patients with comorbid conditions: a systematic analysis of evidence-based guidelines. PloS one. 2011;6(10):e25987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Foglino S, Bravi F, Carretta E, Fantini MP, Dobrow MJ, Brown AD. The relationship between integrated care and cancer patient experience: A scoping review of the evidence. Health Policy. 2016;120(1):55–63. [DOI] [PubMed] [Google Scholar]
- 10.Mollica M, Lines LM, Halpern MT, et al. Patient experiences of cancer care: scoping review, future directions, and introduction of a new data resource: Surveillance Epidemiology and End Results-Consumer Assessment of Healthcare Providers and Systems (SEER-CAHPS). Patient Experience Journal. 2017;4(1):103–121. [Google Scholar]
- 11.Lines LM, Mollica M, Halpern MT, Buckenmaier S, Kirschner J, Wilder-Smith A. Care experiences among Medicare beneficiaries with cancer: A cross-study overview of published results to date from SEER-CAHPS. [Blog post]. 2019; https://www.themedicalcareblog.com/seer-cahps-cross-study-overview/. Accessed Mar. 30, 2020.
- 12.Corbett T, Bridges J. Multimorbidity in older adults living with and beyond cancer. Current opinion in supportive and palliative care. 2019;13(3):220–224. [DOI] [PubMed] [Google Scholar]
- 13.Centers for Medicare and Medicaid Services. Case-mix coefficients for MA & PDP CAHPS. 2016; http://www.ma-pdpcahps.org/globalassets/ma-pdp/case-mix-adjustment/case-mix-coefficients-for-2016-mcahps-data_final-w-o-7_0-508-v2.pdf. Accessed Sept. 19, 2017.
- 14.Mayer LA, Elliott MN, Haas A, Hays RD, Weinick RM. Less Use of Extreme Response Options by Asians to Standardized Care Scenarios May Explain Some Racial/Ethnic Differences in CAHPS Scores. Medical care. Jan 2016;54(1):38–44. [DOI] [PubMed] [Google Scholar]
- 15.O’Malley AJ, Zaslavsky AM, Elliott MN, Zaborski L, Cleary PD. Case-Mix Adjustment of the CAHPS® Hospital Survey. Health services research. 2005;40(6p2):2162–2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martino SC, Elliott MN, Cleary PD, et al. Psychometric Properties of an Instrument to Assess Medicare Beneficiaries’ Prescription Drug Plan Experiences. Health care financing review. 2009;30(3):41–53. [PMC free article] [PubMed] [Google Scholar]
- 17.Halpern MT, Urato MP, Kent EE. The health care experience of patients with cancer during the last year of life: Analysis of the SEER-CAHPS data set. Cancer. 2017;123(2):336–344. [DOI] [PubMed] [Google Scholar]
- 18.Halpern MT, Urato MP, Lines LM, Cohen JB, Arora NK, Kent EE. Healthcare experience among older cancer survivors: analysis of the SEER-CAHPS dataset. Journal of geriatric oncology. 2018;9(3):194–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.DeSalvo KB, Bloser N, Reynolds K, He J, Muntner P. Mortality Prediction with a Single General Self-Rated Health Question: A Meta-Analysis. Journal of General Internal Medicine. 2006;21(3):267–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jylhä M. What is self-rated health and why does it predict mortality? Towards a unified conceptual model. Social science & medicine. 2009;69(3):307–316. [DOI] [PubMed] [Google Scholar]
- 21.Macias C, Gold PB, Öngür D, Cohen BM, Panch T. Are Single-Item Global Ratings Useful for Assessing Health Status? Journal of Clinical Psychology in Medical Settings. 2015;22(4):251–264. [DOI] [PubMed] [Google Scholar]
- 22.National Cancer Institute. SEER-CAHPS Data Documentation. 2017; https://healthcaredelivery.cancer.gov/seer-cahps/aboutdata/documentation.html. Accessed Jan. 20, 2017. [Google Scholar]
- 23.Chawla N, Urato M, Ambs A, et al. Unveiling SEER-CAHPS: a new data resource for quality of care research. Journal of general internal medicine. 2015;30(5):641–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lines LM, Cohen J, Halpern MT, Kent EE, Mollica M. Random Survival Forests Using Linked Data to Measure Illness Burden Among People With Cancer: Development and Internal Validation of the SEER-CAHPS Illness Burden Index. Oral presentation presented at AcademyHealth Annual Research Meeting; June 4, 2019, 2019; Washington, DC. [Google Scholar]
- 25.Lines LM, Cohen J, Kirschner J, et al. Random Survival Forests Using Linked Data to Measure Illness Burden Among Individuals Before or After a Cancer Diagnosis: Development and Internal Validation of the SEER-CAHPS Illness Burden Index. Int J Medical Informatics. 2020; 10.1016/j.ijmedinf.2020.104305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.National Cancer Institute. Localized/Regional/Distant Stage Adjustments. 2021; https://seer.cancer.gov/seerstat/variables/seer/yr1975_2018/lrd_stage/index.html. Accessed Oct. 18, 2021.
- 27.Paddison CA, Elliott MN, Haviland AM, et al. Experiences of care among Medicare beneficiaries with ESRD: Medicare Consumer Assessment of Healthcare Providers and Systems (CAHPS) survey results. American journal of kidney diseases. 2013;61(3):440–449. [DOI] [PubMed] [Google Scholar]
- 28.BAS: Bayesian variable selection and model averaging using Bayesian adaptive sampling [computer program]. CRAN Comprehensive R Archive Network; 2018. [Google Scholar]
- 29.Lines LM, Cohen J, Halpern MT, Smith AW, Kent EE. Care experiences among dually enrolled older adults with cancer: SEER-CAHPS, 2005–2013. Cancer Causes & Control. 2019;30(10):1137–1144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Centers for Medicare and Medicaid Innovation. Oncology Care Model Overview. 2021; https://innovation.cms.gov/innovation-models/oncology-care. Accessed Dec. 9, 2021.
- 31.Chen H, Tilford J, Wan F, Schuldt R. CMS HCC risk scores and home health patient experience measures. Am J Manage Care. 2018;24(10):e319–24. [PubMed] [Google Scholar]
- 32.Kent EE, Mollica M, Klabunde CN, et al. Examining the relative influence of multimorbidity on variations in older cancer patients’ experiences with care. ASCO Quality. 2018. Chicago, IL. [Google Scholar]
- 33.Vogeli C, Shields AE, Lee TA, et al. Multiple chronic conditions: prevalence, health consequences, and implications for quality, care management, and costs. J Gen In-tern Med. Dec 2007;22(Suppl 3):391–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.. Centers for Medicare and Medicaid Services. Medicare CAHPS Response Rates. https://ma-pdpcahps.org/en/historic-data/; 2019. (Accessed Oct. 31, 2019).
- 35.Davern M. Nonresponse rates are a problematic indicator of nonresponse bias in sur-vey research. Health Serv Res. 2013;48(3):905–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Groves RM, Peytcheva E. The impact of nonresponse rates on nonresponse bias: a metaanalysis. Public Opin Q. 2008;72(2):167–89. [Google Scholar]