Abstract
Background & Aims:
Patients with colon cancer who prematurely discontinue postoperative chemotherapy may have an increased risk of disease recurrence and death. This study tested the hypothesis that the quantity and distribution of abdominal adipose tissue predict premature chemotherapy discontinuation.
Methods:
This cohort study included 533 patients with stage II-III colon cancer who initiated a planned regimen of 24-weeks of 5-fluorouracil, leucovorin, and oxaliplatin (FOLFOX) chemotherapy. The primary exposures were body mass index (BMI) and computed tomography-derived abdominal adiposity measures (e.g., visceral, subcutaneous, and intramuscular adipose tissue). The primary endpoint was premature chemotherapy discontinuation, defined as receiving <6 cycles of FOLFOX. Generalized linear models quantified the relative risk (RR) of premature chemotherapy discontinuation adjusted for age, sex, cancer stage, height, and muscle area, using two-sided statistical tests.
Results:
Forty-two patients [7.9% (95% CI: 5.7, 10.5)] prematurely discontinued chemotherapy. Visceral adipose tissue [RR: 3.27 (95% CI: 1.26, 8.49)] and intramuscular adipose tissue [RR: 2.79 (95% CI: 1.09, 7.12)] were statistically significantly associated with an increased risk of premature chemotherapy discontinuation. BMI [RR: 2.07 (95% CI: 0.75, 5.73)] and subcutaneous adipose tissue [RR: 2.32 (95% CI: 0.91, 5.94)] were not statistically significantly associated with premature chemotherapy discontinuation.
Conclusion:
Among patients with stage II-III colon cancer who initiate postoperative chemotherapy, excess visceral and intramuscular adiposity may be risk factors for the premature discontinuation of chemotherapy.
Keywords: adverse event, chemotherapy dosing, obesity, pharmacology
INTRODUCTION
In patients with resected high-risk stage II and stage III colon cancer, a postoperative chemotherapy regimen improves disease-free and overall survival versus surgery alone. For the past two decades, a postoperative regimen that combines a fluoropyrimidine and oxaliplatin has been the standard of care for patients with stage III disease and selected patients with high-risk stage II disease (1, 2). The duration of postoperative chemotherapy has evolved, initially being 12-months, then six months, and recently for low-risk patients, three months was non-inferior to six months (3, 4). No less than three months of chemotherapy is recommended for patients with stage III or high-risk stage II colon cancer (3, 4). One in ten patients with colon cancer who initiate postoperative 5-fluorouracil and oxaliplatin (FOLFOX) chemotherapy do not complete at least six cycles [e.g., three months; (5)], and those who prematurely discontinue chemotherapy have an increased risk of disease recurrence and death (6–8). Risk factors for premature chemotherapy discontinuation are limited and often not modifiable [e.g., age, female sex (9, 10)].
Obesity is a chronic, relapsing, and progressive disease characterized by excess adiposity (11). More than one-in-three patients with colon cancer have obesity (12), which may influence the tolerability and efficacy of chemotherapy (13). Obesity is commonly diagnosed using body mass index (BMI), which scales body weight to height, and does not distinguish muscle from distinct adipose tissue depots [e.g., visceral, subcutaneous, and intramuscular adiposity; (14)]. This is relevant because adiposity explains 14% of oxaliplatin pharmacokinetic variation in patients with gastrointestinal cancer, and patients with low muscle and excess adiposity are 45% more likely to experience severe dose-limiting toxicity (15). Low skeletal muscle is an established predictor of treatment-related toxicity in various solid tumors (16) and explains the pharmacokinetic variability of 5-fluorouracil in colorectal cancer (17). Adiposity is correlated with muscle, such that patients with a higher BMI have, on average, more adipose tissue and more skeletal muscle (18). However, it is not known if the quantity and distribution of adipose tissue predict premature chemotherapy discontinuation independently of muscle in patients with colon cancer (19).
This study tested the hypothesis that the quantity and distribution of adiposity, measured with abdominal computed tomography imaging, is associated with premature chemotherapy discontinuation in patients with stage II-III colon cancer who initiated postoperative FOLFOX chemotherapy.
MATERIALS & METHODS
Study Population and Design
The Colorectal, Sarcopenia, Cancer And Near-term Survival (C-SCANS) study was derived from the Kaiser Permanente Northern California (KPNC) cancer registry. The C-SCANS cohort included all patients aged 18 to 80 years diagnosed with stage I to III invasive colorectal cancer from 2006 to 2011 who underwent surgical resection with curative intent (20). The current study was restricted to patients with colon cancer who initiated FOLFOX chemotherapy within six months after surgical resection (21). When this cohort study was initiated, the oral fluoropyrimidine, capecitabine, was infrequently used in clinical practice at KPNC. The study investigators obtained a waiver of written informed consent. The KPNC institutional review board approved this study (CN-12BCaan-04-H).
Measures of Body Composition
Medical assistants measured height (m) and weight (kg) with standardized clinical procedures at diagnosis. Body mass index (BMI) was calculated as weight divided by the square of height (kg/m2). The quantity and distribution of adipose tissue were measured using preoperative computed tomography images initially collected for clinical purposes (e.g., diagnostic cancer staging) using sliceOmatic software (V5.0, TomoVision, Montreal, Canada). A single-slice transverse image at the third lumbar vertebra was used because cross-sectional tissue areas in this region correlate with whole-body skeletal muscle and adipose tissue volumes (22, 23). Tissues were defined with a semi-automated procedure using Hounsfield unit (HU) thresholds of −29 to 150 for skeletal muscle, −150 to −50 for visceral adipose tissue, and −190 to −30 for subcutaneous adipose tissue. Intramuscular adiposity was operationalized as the average radiation attenuation of skeletal muscle; in statistical regression models, this variable was scaled inversely to represent greater intramuscular adiposity [e.g., lower muscle radiation attenuation indicates greater accumulation of intramyocellular triglyceride (24, 25)]. Two blinded research staff members analyzed a randomly selected sub-sample of 50 computed tomography images. The coefficients of variation for visceral, subcutaneous, and intramuscular adiposity were 2.7%, 1.2%, and 0.7%, respectively (26). One blinded research staff member analyzed the remaining images.
Covariates
The KPNC electronic medical record was used to obtain information on age and sex at diagnosis. The KPNC cancer registry was used to obtain information on the cancer stage (American Joint Committee on Cancer [AJCC 7th edition; (27)], and body surface area (28, 29). Covariate data were 100% complete for all variables.
Study Outcomes
The primary endpoint of premature chemotherapy discontinuation was defined as receiving less than six cycles of FOLFOX, as documented in the electronic chemotherapy pharmacy infusion records (19). The threshold of six cycles of FOLFOX was selected since no less than three months (e.g., six cycles) of chemotherapy is recommended for patients with stage III or high-risk stage II colon cancer (3, 4). The endpoints databases were constructed by research staff blinded to the quantity and distribution of adipose tissue.
Statistical Analysis
Baseline characteristics are presented as means ± SDs or counts and percentages. The Pearson correlation coefficient (r) and coefficient of determination (R2) were used to quantify the strength of the association between BMI and visceral, subcutaneous, and intramuscular adipose tissue. The quantity and distribution of adipose tissue were modeled with restricted cubic splines. Cubic splines accommodate nonlinearity, provide statistically efficient and visually intuitive descriptions of associations, and eliminate the need to arbitrarily categorize continuously distributed variables (30). Generalized linear models were used to quantify the relative risk (RR) and absolute risk difference (ARD) of the association between adipose tissue and premature chemotherapy discontinuation (31). The RR and ARD were calculated from the regression spline model for two comparisons: 1) the 5th versus 95th percentile values of the adipose tissue distribution; and 2) each standard deviation increase in adipose tissue. Models were multivariable-adjusted for age, sex, cancer stage, patient height (14), and muscle area. Linearity and nonlinearity were examined visually using spline plots and statistically using likelihood ratio tests. All statistical tests were two-sided.
RESULTS
Characteristics of the Study Cohort
The average (SD) age of the 533 patients was 58.7 (11.3) years, 55.9% were women, and 85.6% had stage III colon cancer (Table 1). The mean (SD) body surface area was 1.89 (0.26) m2, BMI was 28.7 (6.3) kg/m2, visceral adipose tissue was 147.2 (101.4) cm2, subcutaneous adipose tissue was 229.3 (123.9) cm2, and muscle attenuation was 40.2 (9.4) HU. BMI was correlated with visceral adipose tissue [r =0.61 (95% CI: 0.56, 0.67); Figure S1A], subcutaneous adipose tissue [r = 0.86 (95% CI: 0.83, 0.88); Figure S1B], and muscle attenuation [r = –0.42 (95% CI: –0.49, –0.35); Figure S1C].
Table 1.
Baseline characteristics of the study population overall and stratified by premature chemotherapy discontinuation status
| Characteristic | Overall Cohort (n = 533) | Premature Chemotherapy Discontinuation | P | |
|---|---|---|---|---|
| Yes (n = 42) | No (n = 491) | |||
| Age, mean (SD), y | 58.7 (11.3) | 65.0 (11.6) | 58.2 (11.1) | <0.001 |
| Sex | 0.33 | |||
| Male | 235 (44.1) | 15 (35.7) | 220 (44.8) | |
| Female | 298 (55.9) | 27 (64.3) | 271 (55.2) | |
| Stage | 0.18 | |||
| II | 77 (14.4) | 9 (21.4) | 68 (13.8) | |
| III | 456 (85.6) | 33 (78.6) | 423 (86.2) | |
| Mean Body Surface Area (SD), m2 | 1.89 (0.26) | 1.84 (0.24) | 1.89 (0.26) | 0.18 |
| Mean Body Weight (SD), kg | 82.0 (20.7) | 78.6 (19.6) | 82.3 (20.8) | 0.25 |
| Mean Height (SD), m | 1.68 (0.10) | 1.67 (0.10) | 1.68 (0.10) | 0.41 |
| Mean BMI (SD), kg/m2 | 28.7 (6.3) | 28.0 (6.4) | 28.8 (6.3) | 0.44 |
| Mean Muscle Area (SD), cm2 | 140.9 (37.1) | 125.1 (29.8) | 142.3 (37.4) | 0.004 |
| Mean Visceral Adipose Tissue Area (SD), cm2 | 147.2 (101.4) | 153.8 (98.1) | 146.7 (101.8) | 0.66 |
| Mean Subcutaneous Adipose Tissue Area (SD), cm2 | 229.3 (123.9) | 231.7 (129.0) | 229.1 (123.6) | 0.90 |
| Mean Muscle Attenuation (SD), HU | 40.2 (9.4) | 36.6 (9.7) | 40.5 (9.3) | 0.009 |
Abbreviations: BMI, body mass index (calculated as weight [kg] divided by the square of height [m]; kg/m2); HU, Hounsfield units.
Forty-two patients [7.9% (95% CI: 5.7, 10.5)] prematurely discontinued chemotherapy. Those who prematurely discontinued chemotherapy had a similar body surface area [between group difference (Δ): 0.06 m2 (95% CI: –0.03, 0.14)], and received a similar first cycle relative dose intensity of 5-fluorouracil [Δ: –0.001% (95% CI: –0.04, 0.04)] and oxaliplatin [Δ: –0.01% (95% CI:–0.03, 0.01)], as compared with patients who did not prematurely discontinue chemotherapy.
Effects of Adipose Tissue Quantity and Distribution on Premature Chemotherapy Discontinuation
BMI was not statistically significantly associated with premature chemotherapy discontinuation [RR: 2.07 (95% CI: 0.75, 5.73); Figure 1A]; the RR of premature chemotherapy discontinuation for each SD (6.3 kg/m2) increase in BMI was 1.26 (95% CI: 0.93, 1.72). Visceral adipose tissue was statistically significantly associated with an increased risk of premature chemotherapy discontinuation [RR: 3.27 (95% CI: 1.26, 8.49); Figure 1B]; the RR of premature chemotherapy discontinuation for each SD (101.4 cm2) increase in visceral adipose tissue was 1.45 (95% CI: 1.06, 1.96). Subcutaneous adipose tissue was not statistically significantly associated with premature chemotherapy discontinuation [RR: 2.32 (95% CI: 0.91, 5.94); Figure 1C]; the RR of premature chemotherapy discontinuation for each SD (123.9 cm2) increase in subcutaneous adipose tissue was 1.32 (95% CI: 0.99, 1.76). Intramuscular adipose tissue was statistically significantly associated with an increased risk of premature chemotherapy discontinuation [RR: 2.79 (95% CI: 1.09, 7.12)]; Figure 1D]; the RR of premature chemotherapy discontinuation for each SD (9.4 HU) increase in intramuscular adipose tissue was 1.37 (95% CI: 1.02, 1.86). The conclusions of the above-described results were comparable on the ARD scale [Figure S2].
Figure 1.
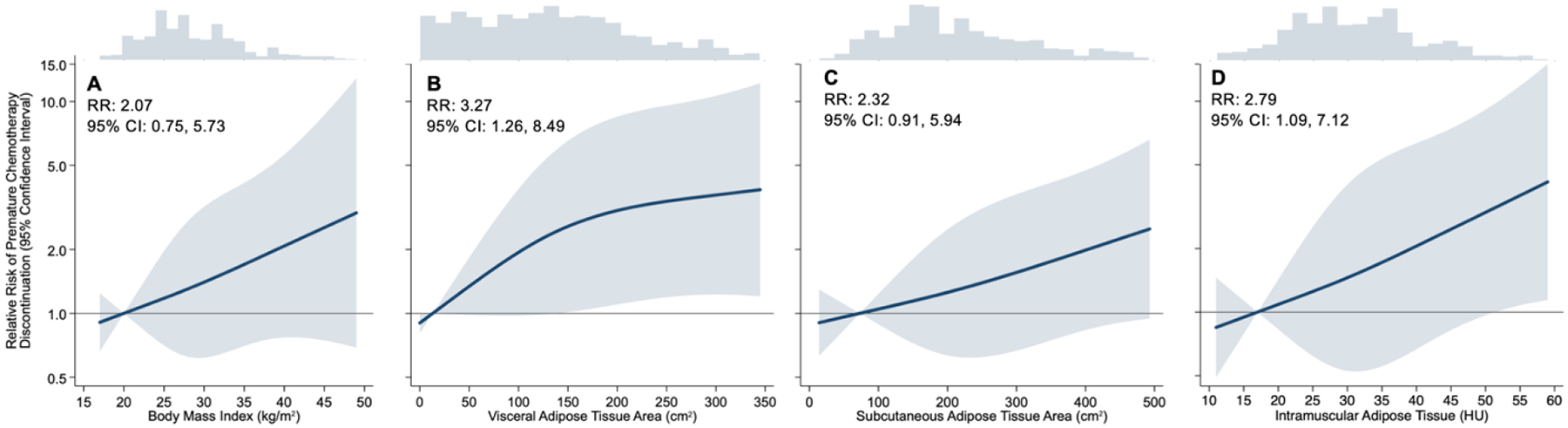
Restricted cubic spline estimates of premature chemotherapy discontinuation on the relative risk scale as a function of body mass index (Panel A); visceral adipose tissue area (Panel B); subcutaneous adipose tissue area (Panel C); and intramuscular adipose tissue (Panel D).
Relative risk (RR) estimates in the text are the contrast of the 5th and 95th percentiles. Shaded regions indicate 95% confidence bands for risk of premature chemotherapy discontinuation. Estimates are adjusted for age, sex, cancer stage, height, and muscle area. Note distinct x-axis scales; y-axis plotted on the logarithmic scale.
DISCUSSION
In this population-based cohort study, 1-in-12 patients with stage II-III colon cancer received less than six cycles (e.g., three months) of postoperative chemotherapy, when at the time this study was conducted, the standard of care was 12 cycles [e.g., six months; (2, 32)]. Visceral and intramuscular adiposity were statistically significantly associated with an increased risk of premature chemotherapy discontinuation. The associations between adiposity and chemotherapy discontinuation were independent of skeletal muscle. BMI and subcutaneous adipose tissue were not statistically significantly associated with premature chemotherapy discontinuation.
The association between excess adiposity and premature chemotherapy discontinuation is pharmacologically plausible. The pharmacokinetic properties of chemotherapy may be altered in people with obesity (33). Excess adiposity increases the volume of distribution (Vd) of lipophilic drugs, such as oxaliplatin (34), which correlates with maximum drug concentrations and predicts severe dose-limiting toxicity (15). Consistent with our findings, cancer survivors treated with platinum-based chemotherapy (e.g., oxaliplatin) with a larger waist circumference have an increased risk of chemotherapy induced neuropathy symptoms (35). Waist circumference is an anthropometric surrogate for visceral adipose tissue (36), and neuropathy symptoms are one of the most common dose-limiting toxicities of oxaliplatin-based chemotherapy (3). Skeletal muscle is a determinant of chemotherapy pharmacokinetics and toxicity risk in patients with cancer (16, 17). The association between adiposity and premature chemotherapy discontinuation in the current study was independent of skeletal muscle.
BMI was not statistically significantly associated with premature chemotherapy discontinuation. In correlational analyses, BMI explained more variation in subcutaneous adipose tissue (R2 = 0.739) than visceral adipose tissue (R2 = 0.381) or intramuscular adipose tissue (R2 = 0.179). These results are consistent with analyses among healthy adults that quantify adipose tissue using whole-body magnetic resonance imaging (37). Subcutaneous adipose tissue was not statistically significantly associated with premature chemotherapy discontinuation; we hypothesize that this lack of an association may be attributable to the reduced blood flow to subcutaneous adipose tissue in people with obesity (38). Excess visceral and intramuscular adipose tissue in women with breast cancer is associated with poorer chemotherapy tolerance (39). The data from this study contribute to a growing literature that BMI alone may be insufficient to predict important clinical outcomes in patients with cancer (26).
The American Society of Clinical Oncology recommends that full, weight-based cytotoxic chemotherapy doses be used to treat patients with cancer who have obesity (13). In our study, patients who prematurely discontinued chemotherapy had a body surface area and received first cycle relative dose intensities of 5-fluorouracil and oxaliplatin that were not statistically significantly different from patients who did not prematurely discontinue chemotherapy. This strengthens the clinical relevance of our findings that visceral and intramuscular adiposity independently predict premature chemotherapy discontinuation in patients with colon cancer.
Patients with colon cancer who have excess visceral and intramuscular adiposity at diagnosis should continue to receive full weight-based cytotoxic chemotherapy dosing, consistent with the current American Society of Clinical Oncology recommendations (13). However, our finding that patients with excess visceral or excess intramuscular adiposity may be more prone to prematurely discontinue chemotherapy suggests that this patient group may be more susceptible to develop severe treatment-related toxicities. Consequently, this subgroup of patients may benefit from more rigorous surveillance during therapy ― such as the Patient-Reported Outcomes of the Common Terminology Criteria for Adverse Events (PRO-CTCAE)―to enable prompt identification and treatment of emerging treatment-related toxicities (40).
It remains unknown if reducing visceral or intramuscular adiposity in patients with colon cancer will decrease the probability of premature chemotherapy discontinuation. Aerobic exercise reduces visceral adipose tissue and preserves lean tissue in patients with colon cancer (41). In 2019, the American College of Sports Medicine concluded that exercise is safe during chemotherapy, but the evidence that exercise improves chemotherapy tolerability is a significant research gap (42). To address this evidence gap, the National Cancer Institute launched the Exercise and Nutrition Interventions to Improve Cancer Treatment-Related Outcomes (ENICTO) in Cancer Survivors Consortium (https://grants.nih.gov/grants/guide/rfa-files/RFA-CA-21-031.html). The randomized trials of the ENICTO Consortium will quantify the efficacy of exercise and nutrition to modify body composition and improve treatment tolerability in cancer survivors. The totality of evidence generated from the ENICTO Consortium will provide definitive evidence to inform the future delivery of comprehensive evidence-based care to cancer survivors.
The principal limitation of this study is the observational design, which precludes our ability to rule out residual confounding. Body composition was measured at the third lumbar vertebrae at a solitary time point (e.g., diagnosis). The third lumbar region is strongly correlated with total body adipose tissue and skeletal muscle volumes (22, 23), however, whole-body measures may offer additional prognostic information and should be examined in future studies. We did not have comprehensive BMI and body composition measures before the diagnosis of colon cancer. Therefore, we cannot rule out that some patients may have experienced tumor-induced changes in BMI or body composition before cancer diagnosis. However, body composition at the time of diagnosis is most relevant to treatment planning and prediction of poor treatment tolerance.
The study cohort was restricted to patients who initiated FOLFOX within six months of surgical resection. Between 2006 and 2011, when cohort subjects were diagnosed, FOLFOX was the standard of care. Thus, we cannot comment on the association of adiposity with other chemotherapy regimens, such as capecitabine and oxaliplatin (CAPOX) or 5-fluorouracil and leucovorin (5-FU/LV). FOLFOX is used to treat various gastrointestinal malignancies. However, it is not known if our findings in patients with resected colon cancer can be generalized to patients with other types of malignancies or with advanced disease. Due to the real-world nature of our dataset, we were unable to determine why patients prematurely discontinued postoperative chemotherapy; nonetheless, interventions are needed to improve patient adherence to postoperative chemotherapy to maximize the efficacy and probability of cure.
The principal strengths of this study are the population-based sample and gold-standard measures of adipose tissue quantity and distribution. The use of real-world electric medical records and pharmacy data, combined with clinically acquired computed tomography images, enabled the identification of a novel and modifiable risk factor for the premature discontinuation of chemotherapy.
Conclusion
In conclusion, among patients with stage II and III colon cancer who initiate postoperative chemotherapy, excess visceral and intramuscular adiposity may be risk factors for the premature discontinuation of chemotherapy. Efforts to decrease the incidence of premature chemotherapy discontinuation are critical to maximize chemotherapy efficacy and reduce death attributable to colon cancer. If these findings are replicated, interventions to reduce excess adiposity may improve patient tolerance to evidence-based chemotherapy regimens and improve the probability of cure.
Supplementary Material
Funding Statement
The content is solely the authors’ responsibility and does not represent the official views of the National Institutes of Health. The funding agency had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Conflict of Interest
Dr. Brown reports receiving grants from the National Cancer Institute and the American Institute for Cancer Research during the conduct of the study. Dr. Cespedes Feliciano reports receiving grants from the National Cancer Institute during the conduct of the study. Dr. Meyerhardt reports receiving grants from the National Cancer Institute during the conduct of the study and reports receiving consulting fees from Cota Healthcare, and Taiho during the 36 months before publication (all fees <$5,000). Dr. Caan reports receiving grants from the National Cancer Institute during the conduct of the study. All other authors report no disclosures.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Gill S, Loprinzi CL, Sargent DJ, Thome SD, Alberts SR, Haller DG, et al. Pooled analysis of fluorouracil-based adjuvant therapy for stage II and III colon cancer: who benefits and by how much? J Clin Oncol. 2004;22(10):1797–806. [DOI] [PubMed] [Google Scholar]
- 2.Andre T, Boni C, Mounedji-Boudiaf L, Navarro M, Tabernero J, Hickish T, et al. Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med. 2004;350(23):2343–51. [DOI] [PubMed] [Google Scholar]
- 3.Grothey A, Sobrero AF, Shields AF, Yoshino T, Paul J, Taieb J, et al. Duration of Adjuvant Chemotherapy for Stage III Colon Cancer. N Engl J Med. 2018;378(13):1177–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andre T, Meyerhardt J, Iveson T, Sobrero A, Yoshino T, Souglakos I, et al. Effect of duration of adjuvant chemotherapy for patients with stage III colon cancer (IDEA collaboration): final results from a prospective, pooled analysis of six randomised, phase 3 trials. Lancet Oncol. 2020;21(12):1620–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Webster-Clark M, Keil AP, Sanoff HK, Sturmer T, Westreich D, Lund JL. Introducing longitudinal cumulative dose to describe chemotherapy patterns over time: Case study of a colon cancer trial. Int J Cancer. 2021;149(2):394–402. [DOI] [PubMed] [Google Scholar]
- 6.Neugut AI, Matasar M, Wang X, McBride R, Jacobson JS, Tsai WY, et al. Duration of adjuvant chemotherapy for colon cancer and survival among the elderly. J Clin Oncol. 2006;24(15):2368–75. [DOI] [PubMed] [Google Scholar]
- 7.Dobie SA, Baldwin LM, Dominitz JA, Matthews B, Billingsley K, Barlow W. Completion of therapy by Medicare patients with stage III colon cancer. J Natl Cancer Inst. 2006;98(9):610–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morris M, Platell C, Fritschi L, Iacopetta B. Failure to complete adjuvant chemotherapy is associated with adverse survival in stage III colon cancer patients. Br J Cancer. 2007;96(5):701–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van der Geest LG, Portielje JE, Wouters MW, Weijl NI, Tanis BC, Tollenaar RA, et al. Complicated postoperative recovery increases omission, delay and discontinuation of adjuvant chemotherapy in patients with Stage III colon cancer. Colorectal Dis. 2013;15(10):e582–91. [DOI] [PubMed] [Google Scholar]
- 10.Hu CY, Delclos GL, Chan W, Du XL. Assessing the initiation and completion of adjuvant chemotherapy in a large nationwide and population-based cohort of elderly patients with stage-III colon cancer. Med Oncol. 2011;28(4):1062–74. [DOI] [PubMed] [Google Scholar]
- 11.Bray GA, Kim KK, Wilding JPH, World Obesity F. Obesity: a chronic relapsing progressive disease process. A position statement of the World Obesity Federation. Obes Rev. 2017;18(7):715–23. [DOI] [PubMed] [Google Scholar]
- 12.Greenlee H, Shi Z, Sardo Molmenti CL, Rundle A, Tsai WY. Trends in Obesity Prevalence in Adults With a History of Cancer: Results From the US National Health Interview Survey, 1997 to 2014. J Clin Oncol. 2016;34(26):3133–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Griggs JJ, Bohlke K, Balaban EP, Dignam JJ, Hall ET, Harvey RD, et al. Appropriate Systemic Therapy Dosing for Obese Adult Patients With Cancer: ASCO Guideline Update. J Clin Oncol. 2021;39(18):2037–48. [DOI] [PubMed] [Google Scholar]
- 14.Brown JC, Heymsfield SB, Caan BJ. Scaling of computed tomography body composition to height: relevance of height-normalized indices in patients with colorectal cancer. J Cachexia Sarcopenia Muscle. 2022;13(1):203–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Williams GR, Al-Obaidi M, Rower J, Harmon C, Dai C, Acosta E, et al. Does oxaliplatin pharmacokinetics (PKs) explain associations between body composition and chemotherapy toxicity risk in older adults with gastrointestinal (GI) cancers? J Clin Oncol. 2021;39(15_suppl):3095-. [Google Scholar]
- 16.Surov A, Pech M, Gessner D, Mikusko M, Fischer T, Alter M, et al. Low skeletal muscle mass is a predictor of treatment related toxicity in oncologic patients. A meta-analysis. Clin Nutr. 2021;40(10):5298–310. [DOI] [PubMed] [Google Scholar]
- 17.Williams GR, Deal AM, Shachar SS, Walko CM, Patel JN, O’Neil B, et al. The impact of skeletal muscle on the pharmacokinetics and toxicity of 5-fluorouracil in colorectal cancer. Cancer Chemother Pharmacol. 2018;81(2):413–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Forbes GB. Longitudinal changes in adult fat-free mass: influence of body weight. Am J Clin Nutr. 1999;70(6):1025–31. [DOI] [PubMed] [Google Scholar]
- 19.Cespedes Feliciano EM, Lee VS, Prado CM, Meyerhardt JA, Alexeeff S, Kroenke CH, et al. Muscle mass at the time of diagnosis of nonmetastatic colon cancer and early discontinuation of chemotherapy, delays, and dose reductions on adjuvant FOLFOX: The C-SCANS study. Cancer. 2017;123(24):4868–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Caan BJ, Meyerhardt JA, Kroenke CH, Alexeeff S, Xiao J, Weltzien E, et al. Explaining the Obesity Paradox: The Association between Body Composition and Colorectal Cancer Survival (C-SCANS Study). Cancer Epidemiol Biomarkers Prev. 2017;26(7):1008–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Biagi JJ, Raphael MJ, Mackillop WJ, Kong W, King WD, Booth CM. Association between time to initiation of adjuvant chemotherapy and survival in colorectal cancer: a systematic review and meta-analysis. JAMA. 2011;305(22):2335–42. [DOI] [PubMed] [Google Scholar]
- 22.Shen W, Punyanitya M, Wang Z, Gallagher D, St-Onge MP, Albu J, et al. Total body skeletal muscle and adipose tissue volumes: estimation from a single abdominal cross-sectional image. J Appl Physiol (1985). 2004;97(6):2333–8. [DOI] [PubMed] [Google Scholar]
- 23.Schweitzer L, Geisler C, Pourhassan M, Braun W, Gluer CC, Bosy-Westphal A, et al. What is the best reference site for a single MRI slice to assess whole-body skeletal muscle and adipose tissue volumes in healthy adults? Am J Clin Nutr. 2015;102(1):58–65. [DOI] [PubMed] [Google Scholar]
- 24.Goodpaster BH, Carlson CL, Visser M, Kelley DE, Scherzinger A, Harris TB, et al. Attenuation of skeletal muscle and strength in the elderly: The Health ABC Study. J Appl Physiol (1985). 2001;90(6):2157–65. [DOI] [PubMed] [Google Scholar]
- 25.Goodpaster BH, Kelley DE, Thaete FL, He J, Ross R. Skeletal muscle attenuation determined by computed tomography is associated with skeletal muscle lipid content. J Appl Physiol (1985). 2000;89(1):104–10. [DOI] [PubMed] [Google Scholar]
- 26.Brown JC, Caan BJ, Prado CM, Weltzien E, Xiao J, Cespedes Feliciano EM, et al. Body Composition and Cardiovascular Events in Patients With Colorectal Cancer: A Population-Based Retrospective Cohort Study. JAMA Oncol. 2019;5(7):967–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17(6):1471–4. [DOI] [PubMed] [Google Scholar]
- 28.Mosteller RD. Simplified calculation of body-surface area. N Engl J Med. 1987;317(17):1098. [DOI] [PubMed] [Google Scholar]
- 29.Du Bois D, Du Bois EF. A formula to estimate the approximate surface area if height and weight be known. 1916. Nutrition. 1989;5(5):303–11; discussion 12–3. [PubMed] [Google Scholar]
- 30.Harrell F Regression modeling strategies: with applications to linear models, logistic and ordinal regression, and survival analysis: Springer; 2015. [Google Scholar]
- 31.Kleinman LC, Norton EC. What’s the Risk? A simple approach for estimating adjusted risk measures from nonlinear models including logistic regression. Health Serv Res. 2009;44(1):288–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Andre T, Boni C, Navarro M, Tabernero J, Hickish T, Topham C, et al. Improved overall survival with oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment in stage II or III colon cancer in the MOSAIC trial. J Clin Oncol. 2009;27(19):3109–16. [DOI] [PubMed] [Google Scholar]
- 33.Pai MP. Drug dosing based on weight and body surface area: mathematical assumptions and limitations in obese adults. Pharmacotherapy. 2012;32(9):856–68. [DOI] [PubMed] [Google Scholar]
- 34.Levi F, Metzger G, Massari C, Milano G. Oxaliplatin: pharmacokinetics and chronopharmacological aspects. Clin Pharmacokinet. 2000;38(1):1–21. [DOI] [PubMed] [Google Scholar]
- 35.Timmins HC, Li T, Goldstein D, Trinh T, Mizrahi D, Harrison M, et al. The impact of obesity on neuropathy outcomes for paclitaxel- and oxaliplatin-treated cancer survivors. J Cancer Surviv. 2022;16(2):223–32. [DOI] [PubMed] [Google Scholar]
- 36.Tchernof A, Despres JP. Pathophysiology of human visceral obesity: an update. Physiol Rev. 2013;93(1):359–404. [DOI] [PubMed] [Google Scholar]
- 37.Neamat-Allah J, Wald D, Husing A, Teucher B, Wendt A, Delorme S, et al. Validation of anthropometric indices of adiposity against whole-body magnetic resonance imaging--a study within the German European Prospective Investigation into Cancer and Nutrition (EPIC) cohorts. PLoS One. 2014;9(3):e91586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Frayn KN, Karpe F. Regulation of human subcutaneous adipose tissue blood flow. Int J Obes (Lond). 2014;38(8):1019–26. [DOI] [PubMed] [Google Scholar]
- 39.Cespedes Feliciano EM, Chen WY, Lee V, Albers KB, Prado CM, Alexeeff S, et al. Body Composition, Adherence to Anthracycline and Taxane-Based Chemotherapy, and Survival After Nonmetastatic Breast Cancer. JAMA Oncol. 2020;6(2):264–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Basch E, Deal AM, Dueck AC, Scher HI, Kris MG, Hudis C, et al. Overall Survival Results of a Trial Assessing Patient-Reported Outcomes for Symptom Monitoring During Routine Cancer Treatment. JAMA. 2017;318(2):197–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brown JC, Zemel BS, Troxel AB, Rickels MR, Damjanov N, Ky B, et al. Dose-response effects of aerobic exercise on body composition among colon cancer survivors: a randomised controlled trial. Br J Cancer. 2017;117(11):1614–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Campbell KL, Winters-Stone KM, Wiskemann J, May AM, Schwartz AL, Courneya KS, et al. Exercise Guidelines for Cancer Survivors: Consensus Statement from International Multidisciplinary Roundtable. Med Sci Sports Exerc. 2019;51(11):2375–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


