Summary
Background
This study aims to evaluate the association between thromboembolic events and hemorrhagic stroke following BNT162b2 and CoronaVac vaccination.
Methods
Patients with incident thromboembolic events or hemorrhagic stroke within 28 days of covid-19 vaccination or SARS-CoV-2 positive test during 23 February to 30 September 2021 were included. The incidence per 100,000 covid-19 vaccine doses administered and SARS-CoV-2 test positive cases were estimated. A modified self-controlled case series (SCCS) analysis using the data from the Hong Kong territory-wide electronic health and vaccination records. Seasonal effect was adjusted by month.
Findings
A total of 5,526,547 doses of BNT162b2 and 3,146,741 doses of CoronaVac were administered. A total of 334 and 402 thromboembolic events, and 57 and 49 hemorrhagic stroke cases occurred within 28 days after BNT162b2 and CoronaVac vaccination, respectively. The crude incidence of thromboembolic events and hemorrhagic stroke per 100,000 doses administered for both covid-19 vaccines were smaller than that per 100,000 SARS-CoV-2 test positive cases. The modified SCCS detected an increased risk of hemorrhagic stroke in BNT162b2 14-27 days after first dose with adjusted IRR of 2.53 (95% CI 1.48-4.34), and 0-13 days after second dose with adjusted IRR 2.69 (95% CI 1.54-4.69). No statistically significant risk was observed for thromboembolic events for both vaccines.
Interpretation
We detected a possible safety signal for hemorrhagic stroke following BNT162b2 vaccination. The incidence of thromboembolic event or hemorrhagic stroke following vaccination is lower than that among SARS-CoV-2 test positive cases; therefore, vaccination against covid-19 remains an important public health intervention.
Funding
This study was funded by a research grant from the Food and Health Bureau, The Government of the Hong Kong Special Administrative Region (reference COVID19F01).
Keywords: Thromboembolic events; Hemorrhagic stroke; Adverse events, BNT162b2; CoronaVac
Research in context.
Evidence before this study
The combination between vaccine types (“CoronaVac”, “Comirnaty”, “BNT162b2”) and outcome of interests (“Thromboembolism”, “Thromboembolic”, “thrombosis”, “embolism”, “Hemorrhagic stroke”) were used as search terms in both PubMed and Embase on April 1, 2022, for all English articles without date restrictions. Nineteen analytical studies were found but there were inconsistent conclusions for BNT162b2 as two studies suggested a potentially increased risk of thromboembolic events after vaccination, one study suggested an increased risk of hemorrhagic stroke and the remainder reported no association between BNT162b2 and these outcomes. No published articles were found reporting significant risk of thromboembolic events nor hemorrhagic stroke after CoronaVac vaccination, and limited published articles explored the safety effects after both first and second doses.
Added value of this study
This is the first population-based study on the safety effect of thromboembolic events and hemorrhagic stroke after both doses of BNT162b2 and CoronaVac among the Chinese population. The modified self-control case series study showed an increased risk of hemorrhagic stroke after both first and second doses of BNT162b2 but not after CoronaVac. No increased risk was observed for thromboembolic events for both vaccines; whilst the incidences among those vaccinated are lower than that in SARS-CoV-2 test positive cases.
Implications of all the available evidence
An increased risk of hemorrhagic stroke was detected after BNT162b2 among a Chinese population. However, the incidence of thromboembolic events and hemorrhagic stroke after vaccination is lower than that among SARS-CoV-2 test positive cases. Hence, vaccination against covid-19 remains an important public health intervention.
Alt-text: Unlabelled box
Introduction
A recently published self-controlled case series (SCCS) and matched cohort study reported an increased risk of acute cardiovascular complications following SARS-CoV-2 infection in Sweden, in which the authors highlighted the need for vaccination against covid-19.1 Another SCCS study conducted in Scotland also reported an increased risk of thromboembolism a week after covid-19 positive result.2 The European Medicines Agency (EMA) reported 30 cases of thromboembolic events among five million people who received the Oxford-AstraZeneca covid-19 vaccine (Vaxzevria), which prompted the suspension of Vaxzevria in several western countries.3 It is hypothesized that the free DNA in the vaccine is a possible trigger of the PF4-reactive antibodies as part of the vaccine-induced thrombotic thrombocytopenia (VITT) cycle.4 Some case reports also showed occurrence of hemorrhagic stroke subsequent to Vaxzevria vaccination together with or without thrombocytopenia and ischemic stroke.5,6
A national cohort study in England found an increased risk of thrombotic episodes in adults under 65 years old within a month of a first dose of Vaxzevria, but not after the BNT162b2 mRNA vaccine.7 Another retrospective cohort study in Denmark did not identify statistically significant risk difference of thromboembolic events between vaccinated with BNT162b2 and unvaccinated people.8 A SCCS study conducted in the United Kingdom showed an increased risk of thromboembolism after first dose of both Vaxzevria and BNT162b2 vaccine.9 Other case reports of thrombosis or hemorrhage in the brain or leg were reported with Pfizer-BioNTech covid-19 mRNA vaccine (BNT162b2).10, 11, 12 Another SCCS, also conducted in the United Kingdom, found an increased risk of hemorrhagic stroke 1-28 days after BNT162b2 vaccination with an incidence rate ratio of 1.24 (95% confidence interval [CI] 1.07-1.43).13 However, a surveillance study of BNT162b2 in the US did not detect any increased risk for thromboembolic events nor hemorrhagic stroke.14 No increased risk of acute myocardial infarction, stroke, and pulmonary embolism was detected after both doses of BNT162b2 mRNA vaccine in a SCCS study among individuals aged 75 years or older in France.15 The authors recommended further measures to quantify these risks in younger populations and for other types of covid-19 vaccines. This safety signal was also not observed in previous analytical studies conducted in Israel16 and Scotland17; or clinical trials18 of BNT162b2 vaccine studies, which included hemorrhagic events as one of the safety outcomes. The pathophysiology between BNT162b2 and thromboembolic events or hemorrhagic stroke is not yet fully elucidated. It is unknown whether it shares the same mechanism hypothesized for Vaxzevria. To the best of our knowledge, no case reports were identified for inactivated vaccines such as CoronaVac. There is also no large-scale study on covid-19 vaccine safety on thromboembolic events and hemorrhagic stroke conducted in any Chinese population. With the emergency use of covid-19 vaccines to combat the global pandemic, continuous monitoring of vaccine safety is encouraged to ensure safe and rational use of covid-19 vaccines.19
In Hong Kong, two vaccines of different platforms are available to the public, the BNT162b2 mRNA vaccine (Comirnaty, distributed by BioNTech/Fosun Pharma in China, equivalent to the Pfizer-BioNTech vaccine outside China) and the inactivated vero cell vaccine (CoronaVac, distributed by Sinovac). With uncertainty of the risk for thromboembolic events and hemorrhagic stroke associated with BNT162b2 and limited published evidence on the risk for CoronaVac, safety concerns remain. Therefore, we assessed the association between thromboembolic events and hemorrhagic stroke and the two covid-19 vaccines available in Hong Kong using the SCCS study, a study design specifically designed to study the safety of vaccine.20 The incidence rate of outcome of interests among individuals vaccinated against covid-19 and had a positive test for SARS-CoV-2 were also compared to inform the risk and benefit of covid-19 vaccines.
Methods
Data source
We acquired data of medical and prescribing records directly from the electronic health record database in Hospital Authority (HA), a statutory body which serves as a major publicly funded healthcare provider and sole publicly funded acute care provider. It manages public hospitals, specialist out-patient clinics, general out-patient clinics and emergency rooms in Hong Kong with hospitalization coverage of more than 70%.21 All Hong Kong residents are eligible to have publicly subsidized healthcare services provided by the HA. The database has previously been used to evaluate the safety of different medications22, 23, 24 and it has recently been used to evaluate safety of BNT162b2 and CoronaVac vaccination including Bell's palsy, carditis and safety in patients with multi-morbidities and rheumatoid arthritis.25, 26, 27, 28, 29, 30 The covid-19 vaccination records are managed by the Department of Health (DH). A de-identified pseudo ID derived from the unique identifier for each Hong Kong citizen who received their vaccination or visited HA's service from 1 January, 2018 to 30 September, 2021 was used to link the medical and prescribing records from HA and the vaccination records from DH.
The mass covid-19 vaccination program in Hong Kong was launched on 23 February, 2021 for CoronaVac and 6 March, 2021 for BNT162b2. The rollout schedule of the vaccination program (Appendix 1) describes the initial priority groups. Currently, the recommended vaccination schedule after the first dose in Hong Kong is 28 days for CoronaVac and 21 days for BNT162b2.31 In Hong Kong, when eligible residents register for vaccination with their unique identity document number, the booking system managed by the Hong Kong Government automatically schedules vaccination of both doses. Participants can choose their preferred vaccine type; however, they are unable to switch vaccine type after the first dose. Recipients can amend their vaccination schedule if it is no shorter than the recommended schedule. Therefore, vaccine recipients may receive the second dose later than the recommended vaccination schedule.
Study design
Since the immunization program has broadened to include those previously not prioritized to be vaccinated, including healthier individuals, there is a possibility that the vaccine recipients are healthier than the unvaccinated cohort. This scenario has been demonstrated in previous literature, such as the mortality rate being lower among the covid-19 vaccinated population in Denmark and Norway32 and the risk of thromboembolic events being lower among those vaccinated in Scotland.17 In Hong Kong, a recent study also demonstrated that having underlying medical conditions is associated with higher vaccine hesitancy during our study period.33 To address the potential selection bias of relatively healthy vaccinated individuals compared to less healthy unvaccinated individuals, we conducted a SCCS study which relies on within-individual comparison to minimize unmeasured confounding.20 The SCCS study design was derived from the theory of cohort studies where the exposure times are fixed and the event occurrence is at random.34 It has been used to conduct high-quality studies, published in peer-reviewed journals.15,35, 36, 37 All patients aged 16 or above who visited any HA service between 1 January, 2018 and 30 September, 2021 where identified. We included patients who had their first diagnosis of thromboembolic events or hemorrhagic stroke as a principal diagnosis in the inpatient setting between 23 February and 30 September, 2021, the study period (Appendix 2). We excluded patients if they had a history of these events from 1 January, 2018 to 23 February, 2021 to minimize risk of misclassifying a recurrent event as incident case.
To compare the incidence rate of the outcomes of interest among individuals vaccinated against covid-19 and those with a positive SARS-CoV-2 test result, a separate cohort of SARS-CoV-2 test positive cases was also identified from the Hospital Authority dataset. The index date was defined as the date of the SARS-CoV-2 test. The first covid-19 case in Hong Kong was reported on 23 January 2020. Hence, we retrieved all cases tested positive for SARS-CoV-2 in real-time reverse transcription polymerase chain reaction tests from 23 January 2020 to 30 September 2021.
Patients were similarly identified in the SCCS analysis. We also excluded patients who had any positive SARS-CoV-2 test prior to and within the study periods. Thromboembolic events were further divided into subgroups of thrombotic cerebrovascular accident (CVA), MI, deep vein thrombosis (DVT), pulmonary embolism (PE) and others; whilst hemorrhagic stroke were divided into subgroups of subarachnoid hemorrhage and intracerebral hemorrhage. The index date of was defined as the date of hospital admission for thromboembolic events or hemorrhagic stroke.
Statistical analysis
Incidence rate of thromboembolic events and hemorrhagic stroke
The incidences per 100,000 doses for BNT162b2 and CoronaVac were calculated. The age-standardized incidence rates were estimated by using the Hong Kong population in 2021 as reference in 5-year age intervals. The incident was defined as new cases that occurred within 28 days after first dose (censored on event occurrence, date of death, date of second dose, or 30 September, 2021, whichever came first) or within 28 days after second dose (censored on event occurrence, date of death, or 30 September, 2021, whichever came first). We followed up the patients for 28 days after vaccination in line with the published literature on the related topic.17
The incidence of thromboembolic events and hemorrhagic stroke within 28 days after testing positive for SARS-CoV-2 was also calculated. Thromboembolic events and hemorrhagic stroke were reported as one of the complications of covid-19.38,39 Since only one diagnosis is recorded as the primary diagnosis in the database, patients who were tested positive for covid-19 likely had a primary diagnosis for covid-19. Therefore, we did not limit the case identification to primary diagnosis to capture any relevant complications. The 95% CI is estimated based on Poisson distribution.
As covid-19 vaccine related thromboembolic events is hypothesized to be associated with thrombocytopenia,17 we also reported the proportion of patients with concurrent thrombocytopenia among the post-vaccine thromboembolic event or hemorrhagic stroke cases. Blood platelet enumerated between first dose of covid-19 vaccine until discharge of thromboembolic event- or hemorrhagic stroke event-associated hospitalization were examined. Thrombocytopenia was defined as a platelet count of <150×109/L.40
Self-controlled case series study
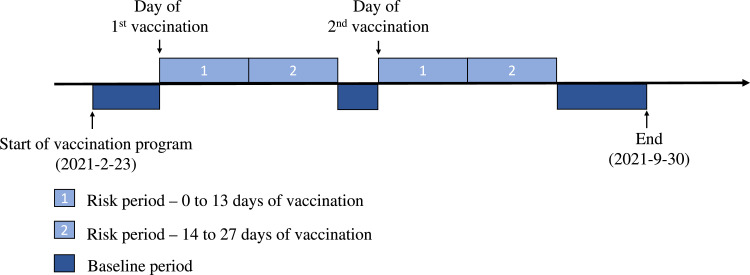
The exposure was defined as receiving the covid-19 vaccines with the vaccination date considered as Day 0 of the risk period. We defined the risk periods as 0-13 and 14-27 days from the vaccination date of both doses to observe the risk of the outcomes after vaccination. The observation period was from 23 February to 30 September, 2021. Any other non-risk periods within the observation period were considered baseline (control periods). If the patient only received one dose within the study period, the periods after the 28-day risk period were considered baseline. A typical observation period of a patient is illustrated in Figure 1. To ensure the application of SCCS is appropriate, there are three assumptions that should not be violated: 1) occurrence of events should be independent; 2) occurrence of event should not influence the probability of subsequent exposures and 3) events should not censor the observation period. We considered the incident event only, because any subsequent events within the study period are likely dependent, which violates assumption 1. It is unlikely for a person with a recent thromboembolic event or hemorrhagic stroke to receive any covid-19 vaccines. Similarly, the occurrence of an event after the first dose may affect the subsequent second dose exposure, which violates assumption 2.41 Therefore, we applied the modified SCCS “eventdepenexp” in the R-package “SCCS”, which is designed to account for event-dependent exposure,42 as our primary analysis. The modified SCCS is particularly useful in a two-dose vaccine scenario with the event of interest being a potential contraindication to vaccination.43 The modified SCCS requires inclusion of unexposed cases to account for the event-dependent exposure scenario. The modified SCCS has been used and was recommended in a published covid-19 vaccine safety study.15,44 The modified SCCS is based on a counterfactual that assumes exposures can never occur after an event, i.e. it will be able to take into account scenario of event-dependent exposure. If a patient dies for reasons related to the event, which follows into the scenario of event-dependent observation period, this potentially violates assumption 3. To account for deaths following the event, it is recommended to omit the date of death and set the planned observation end date for all subjects in the modified SCCS model.44 Patients who were tested positive for SARS-CoV-2 were removed from the SCCS analysis to minimize bias from misclassification. Monthly seasonal effect was adjusted. The study design was developed and independently checked by consulting senior statisticians (EYFW, CKHW, KKCM and BJC) and the developer of the SCCS R-package (YGW).
Figure 1.
An observation period of a patient in the self-controlled case series study. We illustrated a typical follow-up of a patient included in the self-controlled case series study. The risk and baseline periods were defined.
Conditional Poisson regression was used to estimate the incidence rate ratio (IRR) and its corresponding 95% CI. The primary analysis examined the risk of thromboembolic events and hemorrhagic stroke. We also conducted a series of additional analyses to demonstrate the robustness of the analyses. 1) The IRRs of collapsed risk period 0-27 days was also estimated. 2) We conducted a negative control analysis with fracture (ICD-9: 800-829) as the outcome to ensure any detected signal did not occur by random using the same study setting of the primary SCCS analysis. Fracture was chosen because there is no known biological association with covid-19 nor covid-19 vaccines to-date. 3) Subgroup analyses were also conducted for different types of thromboembolic events and hemorrhagic stroke, age <60 and age ≥ 60, and if sample size was deemed sufficient (a sample size of 74 or above to detect an IRR of 2.0, Appendix 3). 4) There is uncertainty of the timing of events and vaccination on Day 0. Even if it is unlikely to develop thromboembolic events or hemorrhagic stroke on the same day as vaccination or vice versa, we specified Day 0 from the risk period to check the results’ robustness.44,45 5) Since the recommended vaccination schedule for BNT162b2 was 21 days (shorter than the 28-day risk period), the risk periods were stratified into 0-13 days, 14 to 20 days, and 21 to 28 days to examine the differences.
All statistical tests were two sided and p values of less than 0.05 were considered statistically significant. MF, MTYL and EC independently conducted the analyses. All analyses were performed in R version 4.1.0 (R Foundation for Statistical Computing, Vienna, Austria).
Ethical approval
Ethical approval for this study was granted by the Institutional Review Board of the University of HK/HA HK West Cluster (UW20-556, UW21-149 and UW21-138); and the DH Ethics Committee (LM21/2021). All clinical data were anonymized; therefore, the regulations in Hong Kong did not require us to obtain consent from participants.
Role of the funding source
This was a regulatory pharmacovigilance study initiated by the Department of Health and funded via the Food and Health Bureau of the Government of the Hong Kong Special Administrative Region. The sponsor of the study was involved in study design, data collection, data analysis, data interpretation and writing of the report via the Department of Health. CSLC, MF, EYFW, and ICKW have accessed and verified the data used in the study. All authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
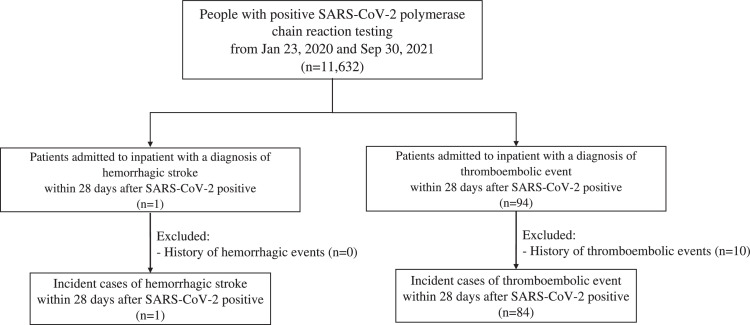
A total of 5,526,547 doses of BNT162b2 and 3,146,741 doses of CoronaVac were administered between 23 February and 30 September, 2021. After removing duplicated records, a total of 4,492,167 individuals (65.2%, out of 6,897,400 eligible population) received at least one dose of covid-19 vaccine in the study period. Around 4,181,121 individuals (60.1%, out of 6,897,400 eligible population) received two doses of covid-19 vaccine. A total of 2,862,245 and 1,629,922 individuals received the first dose of BNT162b2 and CoronaVac, respectively. Among them, 2,664,302 (93.1%, out of 2,862,245) and 1,516,819 (93.1%, out of 1,629,922) individuals received the second dose of BNT162b2 and CoronaVac, respectively. A total of 334 and 402 thromboembolic events occurred within 28 days after BNT162b2 and CoronaVac vaccination, respectively; whilst 57 and 49 hemorrhagic stroke cases occurred after BNT162b2 and CoronaVac vaccination, respectively (Appendix 4). There were 11,632 SARS-CoV-2 positive cases from 21 January, 2020 to 30 September, 2021. There were 84 incident thromboembolic events and 1 hemorrhagic stroke event identified within 28 days from the test date in the same period. The flowchart of inclusion and exclusion for calculation is illustrated in Figure 3.
Figure 3.
Flowchart of inclusion and exclusion criteria in calculating incidence among SARS-CoV-2 positive cases.
We detailed the inclusion and exclusion criteria in calculating incidence rate among SARS-CoV-2 positive cases and the corresponding number of patients at each step in this figure.
Incidence rate of vaccinated individuals and SARS-CoV-2 test positive cohorts
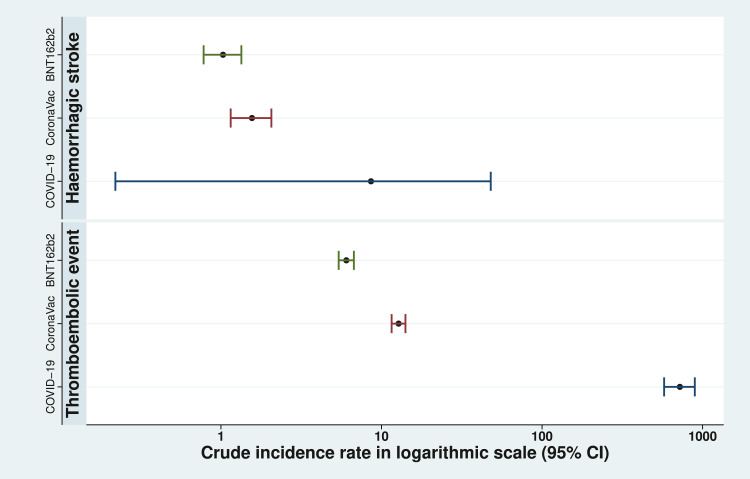
The crude incidence of thromboembolic events was 6.04 (95%CI: 5.41-6.73) for BNT162b2 and 12.78 (95% CI: 11.56-14.09) CoronaVac per 100,000 doses administered, within 28 days of vaccination. The age-standardized incidence rate within 28 days after vaccination per 100,000 person-year were 171.97 (95%CI: 159.85-184.76) and 183.83 (95%CI: 171.88-196.38) for BNT162b2 and CoronaVac respectively.
The crude incidence of hemorrhagic stroke within 28 days after vaccination for BNT162b2 and CoronaVac are 1.03 (95%CI: 0.78-1.34) and 1.56 (95%CI: 1.15-2.06) per 100,000 doses, respectively. The age-standardized incidence rate within 28 days after vaccination per 100,000 person-year were 30.73 (95%CI: 25.74-36.42) and 21.62 (95%CI: 17.66-26.21) for BNT162b2 and CoronaVac, respectively.
The incidence of thromboembolic events and hemorrhagic stroke were 722.15 (95% CI: 576.01-894.07) and 8.60 (95% CI: 0.22-47.90) per 100,000 SARS-CoV-2 test positive cases, respectively. A comparison of incidence rate of cases among different types of vaccines and COVID-19 positive patients is illustrated in Figure 4.
Figure 4.
Incidence of events within 28 days after covid-19 vaccination per 100,000 doses or SARS-CoV-2 test positive per 100,000 SARS-CoV-2 test positive cases.
The crude incidence with 95% confidence intervals in logarithmic scale of thromboembolic events and hemorrhagic stroke per 100,000 doses administered for both covid-19 vaccines, and per 100,000 SARS-CoV-2 test positive cases are illustrated.
Concurrent thrombocytopenia among post-vaccination cases
The concurrent thrombocytopenia information is presented in Appendix 5. Among people with thromboembolic events or hemorrhagic stroke after vaccination, almost all of them (99.2-100%) have received platelet enumeration within the hospitalization. Among the hemorrhagic stroke cases, 16.4% (n = 12) patients with CoronaVac and 7.7% (n = 6) patients with BNT162b2 were identified with thrombocytopenia. In thromboembolic events cases, 8.5% (n = 71) patients with CoronaVac and 7.8% (n = 48) patients with BNT162b2 were identified with thrombocytopenia.
Self-controlled case series study
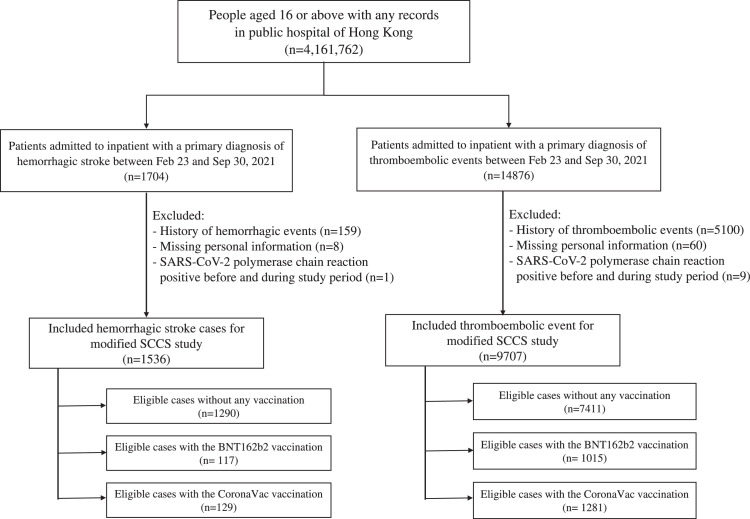
A total of 9,707 thromboembolic events and 1,536 cases of hemorrhagic stroke were included in the SCCS primary analysis. Among cases of thromboembolic events, 1,015 patients received BNT162b2 and 1,281 patients received CoronaVac; whilst 117 received BNT162b2 and 129 received CoronaVac among cases with hemorrhagic stroke (Figure 2). The median gap for vaccinated people between 1st dose and 2nd dose is 21 [interquartile range (IQR): 21-23] days and 28 (IQR: 28-29) days for BNT162b2 and CoronaVac. The patients’ characteristics are presented in Appendix 6. In vaccinated patients with thromboembolic events, a total of 805 (79.3%, out of 1,015) and 982 (76.7%, out of 1,281) patients received two doses of BNT162b2 and CoronaVac respectively within the observation period; 43 (4.2%, out of 1,015) and 63 (4.9%, out of 1,281) patients had thromboembolic events between the first dose and the second dose of BNT162b2 and CoronaVac. In vaccinated patients with hemorrhagic stroke, a total of 80 (68.4%, out of 117) and 91 (70.5%, out of 129) patients received two doses of BNT162b2 and CoronaVac respectively within the observation period; 4 (3.4%, out of 117) and 4 (3.1%, out of 129) patients had hemorrhagic stroke between the first dose and the second dose of BNT1462b2 and CoronaVac.
Figure 2.
Flowchart of inclusion and exclusion criteria in self-controlled case series study.
We detailed the inclusion and exclusion criteria of self-controlled case series study and the corresponding number of patients at each step in this figure.
The modified SCCS model detected an increased risk of hemorrhagic event in BNT162b2 14-27 days after first dose with adjusted IRR of 2.53 (95% CI 1.48-4.34), and 0-13 days after second dose 2.69 (95% CI 1.54-4.69). No risk of thromboembolic events was detected for either vaccines. The IRRs, number of events and follow-up time for each risk period are summarized in Table 1. The duration between event occurrence and vaccination date is illustrated in Appendix 7. An increase in event occurrence for both thromboembolic events and hemorrhagic stroke was observed shortly after vaccination. For the negative control outcome analysis, no increased risk was observed for fracture in any risk periods for both covid-19 vaccines (Appendix 8). Subgroup analysis by types of thromboembolic event (Appendix 9) did not result in any significant IRR except 14 to 27 days after second dose of CoronaVac for CVA [adjusted IRR: 1.36 (95% CI: 1.01-1.83)], which was marginally significant. Subgroup analysis by types of hemorrhagic stroke (Appendix 10) and by age (Appendix 11) remain largely similar as the primary analyses. The results of the analyses with Day 0 separated risk period, and further stratifying risk periods to 0-13 days, 14 to 20 days, and 21 to 28 days were similar to that of the primary analysis (Appendix 12 and Appendix 13).
Table 1.
Risk of thromboembolic events and hemorrhagic stroke among participants in the self-controlled case series study.
| Risk periods | Number of events | Patient-years | Adjusted IRR (95% CI) |
|---|---|---|---|
| Thromboembolic events | |||
| BNT162b2 (n = 8426) | |||
| First dose | |||
| 0 to 13 days after | 110 | 38.08 | 1.15 (0.91-1.46) |
| 14 to 27 days after | 69 | 24.25 | 1.12 (0.86-1.48) |
| 0 to 27 days after | 179 | 62.33 | 1.08 (0.89-1.31) |
| Second dose | |||
| 0 to 13 days after | 94 | 29.25 | 1.20 (0.93-1.54) |
| 14 to 27 days after | 61 | 26.64 | 0.85 (0.63-1.15) |
| 0 to 27 days after | 155 | 55.89 | 1.03 (0.83-1.27) |
| Baseline | 8092 | 4956.99 | - |
| CoronaVac (n = 8692) | |||
| First dose | |||
| 0 to 13 days after | 105 | 48.02 | 0.82 (0.65-1.03) |
| 14 to 27 days after | 104 | 45.88 | 0.84 (0.67-1.06) |
| 0 to 27 days after | 209 | 93.90 | 0.84 (0.70-1.01) |
| Second dose | |||
| 0 to 13 days after | 92 | 36.04 | 0.89 (0.70-1.13) |
| 14 to 27 days after | 101 | 32.76 | 1.14 (0.90-1.44) |
| 0 to 27 days after | 193 | 68.79 | 0.99 (0.82-1.19) |
| Baseline | 8290 | 5072.73 | - |
| Hemorrhagic stroke | |||
| BNT162b2 (n = 1407) | |||
| First dose | |||
| 0 to 13 days after | 13 | 4.34 | 1.30 (0.68-2.48) |
| 14 to 27 days after | 18 | 3.00 | 2.53 (1.48-4.34) |
| 0 to 27 days after | 31 | 7.34 | 1.67 (1.04-2.69) |
| Second dose | |||
| 0 to 13 days after | 21 | 2.98 | 2.69 (1.54-4.69) |
| 14 to 27 days after | 5 | 2.84 | 0.73 (0.29-1.86) |
| 0 to 27 days after | 26 | 5.82 | 1.68 (0.99-2.84) |
| Baseline | 1350 | 834.31 | - |
| CoronaVac (n = 1419) | |||
| First dose | |||
| 0 to 13 days after | 18 | 4.84 | 1.24 (0.73-2.09) |
| 14 to 27 days after | 13 | 4.67 | 1.06 (0.58-1.93) |
| 0 to 27 days after | 31 | 9.51 | 1.07 (0.68-1.67) |
| Second dose | |||
| 0 to 13 days after | 7 | 3.32 | 0.61 (0.27-1.40) |
| 14 to 27 days after | 11 | 3.09 | 1.00 (0.51-1.96) |
| 0 to 27 days after | 18 | 6.41 | 0.82 (0.48-1.41) |
| Baseline | 1370 | 838.79 | - |
Adjusted for seasonality by month. CI denotes confidence interval. The primary analysis was based on event dependent exposure SCCS extension. IRR denotes incidence rate ratio.
Discussion
We detected a signal for hemorrhagic stroke within a month after exposure to the first and second dose of BNT162b2 in the SCCS analysis. No statistically significant signal was observed following either dose of CoronaVac for both thromboembolic events and hemorrhagic stroke. Similar to our study, Patone et al.13 detected an increased risk of hemorrhagic stroke within 28 days after vaccination with the first dose of BNT162b2 in a SCCS analysis conducted with the pooled UK dataset. They did not investigate the risk after second dose because outcomes following second dose was limited at the time of the study. On the contrary, Simpson et al.17 reported no positive association between hemorrhagic events and exposure to first dose BNT162b2 within and after 28 days also in Scotland. The possible reason for the difference from our study and Patone et al.’s13 despite having a similar study population is that Simpson et al.17 included hemorrhage of various body sites whilst this study and Patone et al.’s34 only focused on hemorrhage in the brain. This supports that the signal between BNT162b2 and hemorrhage is specific to the brain but not to other body sites. The observed signal in this study was also not reported in the clinical trial of BNT162b218 probably because of its relatively small sample size with stringent inclusion criteria. Such a signal was also not observed in the phase 3 trial of CoronaVac in Turkey,46 post-marketing surveillance study on the effectiveness (without data on safety) of CoronaVac in Chile,47 and a population-based safety study on BNT162b2 in Israel.16
The evidence on the association between BNT162b2 and thromboembolic events in the literature were inconsistent. In this study, we did not find any statistically significant risk of thromboembolic events after vaccination of CoronaVac or BNT162b2. Simpson et al.17 also reported no positive association between thromboembolic events (venous and arterial) and exposure to first dose BNT162b2 within and after 28 days. Jabagi et al.’s15 also reported no statistically significant risk for thromboembolic events after vaccination of BNT162b2. However, Hippisley-Cox et al.9 reports increased risk of thromboembolic events after vaccination of BNT162b2 in a SCCS analysis with consideration of event-dependent exposure in the UK. The possible reason for the difference between Hippisley-Cox et al.9 and our study is the ethnicity difference. Our cohort represents a predominantly Chinese population whilst theirs was predominantly Caucasian. Given that the risk of thromboembolism among Chinese was lower than that among the Caucasians48,49 possibly due to genetic differences,50 the ethnicity difference could be attributed to the lower risk found in this study.
The mechanism between the potential risk of hemorrhagic stroke and BNT162b2 is unclear. This could be due to the interaction between the spike protein of SARS-CoV-2 and platelets, increasing the risk of thromboembolic events in patients with SARS-CoV-2 infection51 which may contribute to major bleeding events. Spike protein is the target that is encoded by the mRNA- and vector-based vaccines, which may lead to the thrombosis and thrombocytopenia syndrome in the vaccine recipients that is similar to heparin-induced thrombocytopenia in patients.52 This is similar to the current understanding of the observed risk of thrombocytopenic thrombosis in Vaxzevria.4 However, we only detected risk of hemorrhagic stroke but not thromboembolic events in this study. It is also worth noting that almost all of the post-vaccination thromboembolic events or hemorrhagic stroke cases in our study received a blood platelet enumeration but only 7-16% of them had concurrent thrombocytopenia, which is different from the hypothesized mechanism of thrombosis accompanied with thrombocytopenia after Vaxzevria vaccination. The signal detected between hemorrhagic stroke and BNT162b2 in this study may not share the same mechanism as that hypothesized for Vaxzevria. The association between thrombocytopenia, thromboembolic event and hemorrhagic stroke and different types of vaccines requires further investigation.
Given the potential signal on hemorrhagic stroke after BNT162b2 vaccination but not CoronaVac, the safety consideration of covid-19 vaccines may be platform-related. CoronaVac is developed using inactivated SARS-CoV-2 virus platform, whilst BNT162b2 contains mRNA that specifically encodes the spike protein of SARS-CoV-2 virus. A recently published correspondence53 reported that antibody concentrations were lower after CoronaVac vaccination as compared to the BNT162b2 vaccination. This could be potentially reflecting the relative amount of spike protein from the two vaccines, the extent of cell signaling stimulation,54 and hence the differences in the risk of hemorrhagic stroke detected among the two vaccines.
In our study, the incidence of hemorrhagic stroke and thromboembolic events within 28 days among people tested positive for SARS-CoV-2 is much greater than the incidences among people administered either covid-19 vaccines. A recently published study reported an increased risk of acute MI and ischemic stroke following covid-19 infection in Sweden.1 The authors reported an IRR of 2.89 for acute MI and 2.97 for ischemic stroke within the first week of covid-19 infection, indicating a high risk for thromboembolic complication if a patient has covid-19 infection. Hippisley-Cox et al.9 and Patone et al.13 also demonstrated that the risks of thromboembolic outcomes and hemorrhagic stroke, respectively, were much higher after SARS-CoV-2 infection than after vaccination against covid-19, respectively.
This study has several strengths. As the covid-19 vaccine coverage is increasing, SCCS remains the most appropriate method to study the safety of covid-19 vaccines.43 This study applied the modified SCCS method to avoid violation of assumption on the event-dependent exposure as well as event-dependent censoring of observation of SCCS. The numerous sensitivity and subgroup analyses conducted in this study also supports the robustness of the findings. The lower risk of fracture observed in the negative control analysis is likely because once people received the vaccine, they may experience mild adverse effect such as fatigue or tiredness55 and stayed home which reduces their risk of accidental injury, accounting for the lower risk of fracture. The absence of increased risk observed in fracture further supported the validity of the main analysis. This is one of the first population-based BNT162b2 and CoronaVac safety studies on the risk of thromboembolic events and hemorrhagic stroke conducted in the Chinese population of more than 8.5 million doses of vaccines administered. To the best of our knowledge, most of the published literature were conducted in Europe or North America where Caucasian is the predominant ethnic group. This study serves as an important piece of evidence to fill the knowledge gap on the safety profile of covid-19 vaccines among the Chinese population.
This study is subject to several limitations. Potential time-varying covariates such as body weight, lifestyle factors, and covid-19 infection cannot be adjusted in the SCCS model. However, with the short observation time of approximately seven months, it is unlikely that these conditions would drastically change and introduce bias. Since only incident cases were included, the risk of recurrent thromboembolic events and hemorrhagic stroke were not examined. Further studies are warranted to examine such risk among patients with prior history of cardiovascular diseases. However, a recent study reported that patients with previous history of major adverse cardiac events (MACE) are not at higher risk of further MACE after mRNA (BNT162b2) and inactivated (CoronaVac) vaccinations.56 We also excluded cases with a positive SARS-CoV-2 test before and within the study period to minimize bias. Both hemorrhagic stroke and thromboembolic event cases such as ischemic stroke could potentially be an result from SARS-CoV-2 infection.1,57 However, only 10 patients out of 16,580 cases of thromboembolic events (n = 14,876) or hemorrhagic stroke (n = 1704) had positive SARS-CoV-2 PCR or antigen laboratory tests prior to the event date were identified and we excluded these cases from the analysis. The potential bias from misclassifying a case related to SARS-CoV-2 infection is minimized. In addition, infection cases in Hong Kong is also relatively low with less than 13,000 confirmed cases out of 7.6 million population by September 2021 (including imported and asymptomatic cases inclusive). Therefore, it is unlikely that the occurrence of the included cases was due to SARS-CoV-2 infections.
We detected a possible safety signal for hemorrhagic stroke in the SCCS after BNT162b2 vaccination but not CoronaVac. This signal should be further investigated and monitoring of hemorrhagic stroke after BNT162b2 vaccination is needed. However, the occurrence of this event was estimated to be very rare. The observed incidence of thromboembolic events and hemorrhagic stroke among SARS-CoV-2 positive patients are higher than that in the covid-19 vaccination population. Although a possible safety signal after vaccination is detected, there are considerable benefits of vaccination when compared to SARS-CoV-2 infection in this Chinese predominant cohort of almost 4.5 million vaccinated individuals.
Contributors
CSLC, MF, EYFW and ICKW had the original idea for the study, contributed to the development of the study, extracted data from the source database, constructed the study design and the statistical model, reviewed the literature, and act as guarantors for the study. MF, MTYL, and EC undertook the statistical analysis. CSLC, MF, and ICKW wrote the first draft of the manuscript. CSLC, MF, MTYL, EC, VKCY, LG, ICHL extracted data from the source database. ICKW is the principal investigator and provided oversight for all aspects of this project. YG, KKCM, KKL, ICHL, FTTL, XL, CKHW, EWYC, CLC, CWS, CKL, IFNH, CSL, JYSC, MKYL, VCTM, CWS, LSTC, TC, FLFC, AYHL, BJC and GML provided critical input to the analyses, design and discussion. All authors contributed to the interpretation of the analysis, critically reviewed and revised the manuscript, and approved the final manuscript as submitted.
Data sharing statement
Data will not be available for others as the data custodians have not given permission. Any request will be subjected to approval from the data custodians.
Declaration of interests
CSLC has received grants from the Food and Health Bureau of the Hong Kong Government, Hong Kong Research Grant Council, Hong Kong Innovation and Technology Commission, Pfizer, IQVIA, and Amgen; personal fees from Primevigilance Ltd.; outside the submitted work. EYFW has received research grants from the Food and Health Bureau of the Government of the Hong Kong SAR, and the Hong Kong Research Grants Council, outside the submitted work. KKL received grants from the Research Fund Secretariat of the Food and Health Bureau, Innovation and Technology Bureau, Research Grants Council, Amgen, Boehringer Ingelheim, Eisai and Pfizer; and consultation fees from Amgen, Boehringer Ingelheim, Daiichi Sankyo and Sanofi, all outside the submitted work. FTTL has been supported by the RGC Postdoctoral Fellowship under the Hong Kong Research Grants Council and has received research grants from Food and Health Bureau of the Government of the Hong Kong SAR, outside the submitted work. XL received research grants from Research Fund Secretariat of the Food and Health Bureau (HMRF, HKSAR), Research Grants Council Early Career Scheme (RGC/ECS, HKSAR), Janssen and Pfizer; internal funding from the University of Hong Kong; consultancy fee from Merck Sharp & Dohme, unrelated to this work. EWYC reports honorarium from Hospital Authority, grants from Research Grants Council (RGC, Hong Kong), grants from Research Fund Secretariat of the Food and Health Bureau, National Natural Science Fund of China, Wellcome Trust, Bayer, Bristol-Myers Squibb, Pfizer, Janssen, Amgen, Takeda, Narcotics Division of the Security Bureau of HKSAR, outside the submitted work. CLC has received grants from Hong Kong RGC, HMRF, the Narcotics Division of the Security Bureau of HKSAR, Amgen, outside the submitted work. CWS has received grants from the Hong Kong Health and Medical Research Fund (HMRF, HKSARS) outside the submitted work. ICKW reports research funding outside the submitted work from Amgen, Bristol-Myers Squibb, Pfizer, Janssen, Bayer, GSK, Novartis, the Hong Kong RGC, and the Hong Kong Health and Medical Research Fund, National Institute for Health Research in England, European Commission, National Health and Medical Research Council in Australia, and also received speaker fees from Janssen and Medice in the previous 3 years. He is also an independent non-executive director of Jacobson Medical in Hong Kong.
Acknowledgements
We thank members of the Expert Committee on Clinical Events Assessment Following COVID-19 Immunization for case assessment and colleagues from the Drug Office of the Department of Health and from the Hospital Authority for providing vaccination and clinical data. We thank Professor Ian Douglas, Dr Bernard Chan, Mr Yuan Wang, and Ms Natalie Tsie for their technical support.
Funding
This was a regulatory pharmacovigilance study initiated by the Department of Health and funded via the Food and Health Bureau of the Government of the Hong Kong Special Administrative Region. This study was funded by a research grant from the Food and Health Bureau, The Government of the Hong Kong Special Administrative Region (reference COVID19F01).
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.eclinm.2022.101504.
Appendix. Supplementary materials
References
- 1.Katsoularis I, Fonseca-Rodriguez O, Farrington P, Lindmark K, Fors Connolly AM. Risk of acute myocardial infarction and ischaemic stroke following COVID-19 in Sweden: a self-controlled case series and matched cohort study. Lancet. 2021;398(10300):599–607. doi: 10.1016/S0140-6736(21)00896-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ho F, Man K, Toshner M, et al. Thromboembolic risk in hospitalised and non-hospitalised Covid-19 patients: a selfcontrolled case series analysis of a nation-wide cohort. Mayo Clin Proc. 2021 doi: 10.1016/j.mayocp.2021.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wise J. Covid-19: European countries suspend use of Oxford-AstraZeneca vaccine after reports of blood clots. BMJ. 2021;372:n699. doi: 10.1136/bmj.n699. [DOI] [PubMed] [Google Scholar]
- 4.Greinacher A, Thiele T, Warkentin TE, Weisser K, Kyrle PA, Eichinger S. Thrombotic thrombocytopenia after ChAdOx1 nCov-19 vaccination. N Engl J Med. 2021;384(22):2092–2101. doi: 10.1056/NEJMoa2104840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blauenfeldt RA, Kristensen SR, Ernstsen SL, Kristensen CCH, Simonsen CZ, Hvas AM. Thrombocytopenia with acute ischemic stroke and bleeding in a patient newly vaccinated with an adenoviral vector-based COVID-19 vaccine. J Thromb Haemost. 2021;19(7):1771–1775. doi: 10.1111/jth.15347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Melo Silva ML, Jr., Lopes DP. Large hemorrhagic stroke after ChAdOx1 nCoV-19 vaccination: a case report. Acta Neurol Scand. 2021;144(6):717–718. doi: 10.1111/ane.13505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Andrews NJ, Stowe J, Ramsay ME, Miller E. Risk of venous thrombotic events and thrombocytopenia in sequential time periods after ChAdOx1 and BNT162b2 COVID-19 vaccines: a national cohort study in England. Lancet Reg Health Eur. 2022;13 doi: 10.1016/j.lanepe.2021.100260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hviid A, Hansen JV, Thiesson EM, Wohlfahrt J. Association of AZD1222 and BNT162b2 COVID-19 vaccination with thromboembolic and thrombocytopenic events in frontline personnel: a retrospective cohort study. Ann Intern Med. 2022 doi: 10.7326/m21-2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hippisley-Cox J, Patone M, Mei XW, et al. Risk of thrombocytopenia and thromboembolism after covid-19 vaccination and SARS-CoV-2 positive testing: self-controlled case series study. BMJ. 2021;374:n1931. doi: 10.1136/bmj.n1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zakaria Z, Sapiai NA, Ghani ARI. Cerebral venous sinus thrombosis 2 weeks after the first dose of mRNA SARS-CoV-2 vaccine. Acta Neurochir. 2021:1–4. doi: 10.1007/s00701-021-04860-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shimazawa R, Ikeda M. Potential adverse events in Japanese women who received tozinameran (BNT162b2, Pfizer-BioNTech) J Pharma Policy Pract. 2021;14(1):46. doi: 10.1186/s40545-021-00326-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carli G, Nichele I, Ruggeri M, Barra S, Tosetto A. Deep vein thrombosis (DVT) occurring shortly after the second dose of mRNA SARS-CoV-2 vaccine. Intern Emerg Med. 2021;16(3):803–804. doi: 10.1007/s11739-021-02685-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Patone M, Handunnetthi L, Saatci D, et al. Neurological complications after first dose of COVID-19 vaccines and SARS-CoV-2 infection. Nat Med. 2021 doi: 10.1038/s41591-021-01556-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klein NP, Lewis N, Goddard K, et al. Surveillance for adverse events after COVID-19 mRNA vaccination. JAMA. 2021 doi: 10.1001/jama.2021.15072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jabagi MJ, Botton J, Bertrand M, et al. Myocardial infarction, stroke, and pulmonary embolism after BNT162b2 mRNA COVID-19 vaccine in people aged 75 years or older. JAMA. 2021 doi: 10.1001/jama.2021.21699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barda N, Dagan N, Ben-Shlomo Y, et al. Safety of the BNT162b2 mRNA Covid-19 vaccine in a nationwide setting. N Engl J Med. 2021 doi: 10.1056/NEJMoa2110475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Simpson CR, Shi T, Vasileiou E, et al. First-dose ChAdOx1 and BNT162b2 COVID-19 vaccines and thrombocytopenic, thromboembolic and hemorrhagic events in Scotland. Nat Med. 2021;27(7):1290–1297. doi: 10.1038/s41591-021-01408-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Klungel OH, Pottegård A. Strengthening international surveillance of vaccine safety. BMJ. 2021;374:n1994. doi: 10.1136/bmj.n1994. [DOI] [PubMed] [Google Scholar]
- 20.Whitaker H, Farrington CP, Spiessens B, Musonda P. Tutorial in biostatistics: the self-controlled case series method. Stat Med. 2005;0:1–31. doi: 10.1002/sim.2302. [DOI] [PubMed] [Google Scholar]
- 21.Census and Statistics Department . 2015. Thematic Household Survey Report No. 58. [Google Scholar]
- 22.Lau WC, Chan EW, Cheung CL, et al. Association between Dabigatran vs Warfarin and risk of osteoporotic fractures among patients with nonvalvular atrial fibrillation. JAMA. 2017;317(11):1151–1158. doi: 10.1001/jama.2017.1363. [DOI] [PubMed] [Google Scholar]
- 23.Lau WCY, Cheung CL, Man KKC, et al. Association between treatment with apixaban, dabigatran, rivaroxaban, or warfarin and risk for osteoporotic fractures among patients with atrial fibrillation: a population-based cohort study. Ann Intern Med. 2020;173(1):1–9. doi: 10.7326/M19-3671. [DOI] [PubMed] [Google Scholar]
- 24.Wong AY, Root A, Douglas IJ, et al. Cardiovascular outcomes associated with use of clarithromycin: population based study. BMJ. 2016;352:h6926. doi: 10.1136/bmj.h6926. [DOI] [PubMed] [Google Scholar]
- 25.Chua GT, Kwan MYW, Chui CS, et al. Epidemiology of acute myocarditis/pericarditis in Hong Kong adolescents following comirnaty vaccination. Clin Infect Dis. 2021 doi: 10.1093/cid/ciab989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lai FTT, Huang L, Chui CSL, et al. Multimorbidity and adverse events of special interest associated with Covid-19 vaccines in Hong Kong. Nat Commun. 2022;13(1):411. doi: 10.1038/s41467-022-28068-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lai FTT, Li X, Peng K, et al. Carditis after COVID-19 vaccination with a messenger rna vaccine and an inactivated virus vaccine: a case-control study. Ann Intern Med. 2022;175(3):362–370. doi: 10.7326/M21-3700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li X, Lai FTT, Chua GT, et al. Myocarditis following COVID-19 BNT162b2 vaccination among adolescents in Hong Kong. JAMA Pediatrics. 2022 doi: 10.1001/jamapediatrics.2022.0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li X, Tong X, Yeung WWY, et al. Two-dose COVID-19 vaccination and possible arthritis flare among patients with rheumatoid arthritis in Hong Kong. Ann Rheum Dis. 2021 doi: 10.1136/annrheumdis-2021-221571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wan EYF, Chui CSL, Lai FTT, et al. Bell's palsy following vaccination with mRNA (BNT162b2) and inactivated (CoronaVac) SARS-CoV-2 vaccines: a case series and nested case-control study. Lancet Infect Dis. 2021 doi: 10.1016/S1473-3099(21)00451-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hong Kong Government . 2021. COVID-19 Vaccination Programme.https://www.covidvaccine.gov.hk/en/faq Accessed. [Google Scholar]
- 32.Pottegård A, Lund LC, Karlstad Ø, et al. Arterial events, venous thromboembolism, thrombocytopenia, and bleeding after vaccination with Oxford-AstraZeneca ChAdOx1-S in Denmark and Norway: population based cohort study. BMJ. 2021;373:n1114. doi: 10.1136/bmj.n1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xiao J, Cheung JK, Wu P, Ni MY, Cowling BJ, Liao Q. Temporal changes in factors associated with COVID-19 vaccine hesitancy and uptake among adults in Hong Kong: Serial cross-sectional surveys. Lancet Reg Health. 2022;23 doi: 10.1016/j.lanwpc.2022.100441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Farrington CP, Anaya K, Whitaker H, Hocine MN, Douglas IJ, Smeeth L. Self-controlled case series analysis with event-dependent observation periods. J Am Stat Assoc. 2010;106(494):417–426. [Google Scholar]
- 35.Smeeth L, Thomas SL, Hall AJ, Hubbard R, Farrington P, Vallance P. Risk of myocardial infarction and stroke after acute infection or vaccination. N Engl J Med. 2004;351(25):2611–2618. doi: 10.1056/NEJMoa041747. [DOI] [PubMed] [Google Scholar]
- 36.Forbes H, Douglas I, Finn A, et al. Risk of herpes zoster after exposure to varicella to explore the exogenous boosting hypothesis: self controlled case series study using UK electronic healthcare data. BMJ. 2020;368:l6987. doi: 10.1136/bmj.l6987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wong AY, Wong IC, Chui CS, et al. Association between acute neuropsychiatric events and helicobacter pylori therapy containing clarithromycin. JAMA Intern Med. 2016;176(6):828–834. doi: 10.1001/jamainternmed.2016.1586. [DOI] [PubMed] [Google Scholar]
- 38.Lodigiani C, Iapichino G, Carenzo L, et al. Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thromb Res. 2020;191:9–14. doi: 10.1016/j.thromres.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Varatharaj A, Thomas N, Ellul MA, et al. Neurological and neuropsychiatric complications of COVID-19 in 153 patients: a UK-wide surveillance study. Lancet Psychiatry. 2020;7(10):875–882. doi: 10.1016/S2215-0366(20)30287-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Farrington CP, Whitaker HJ, Hocine MN. Case series analysis for censored, perturbed, or curtailed post-event exposures. Biostatistics. 2009;10(1):3–16. doi: 10.1093/biostatistics/kxn013. [DOI] [PubMed] [Google Scholar]
- 42.Ghebremichael-Weldeselassie Y. 2021. The Self-Controlled Case Series Method.https://cran.r-project.org/web/packages/SCCS/SCCS. pdf [Google Scholar]
- 43.Farrington P, Whitaker H, Ghebremichael-Weldeselassie Y., Press CHC, editors. Self-Controlled Case Series Studies: A Modelling Guide with R. 2018. Boca Raton. [Google Scholar]
- 44.Ghebremichael-Weldeselassie Y, Jabagi MJ, Botton J, et al. A modified self-controlled case series method for event-dependent exposures and high event-related mortality, with application to COVID-19 vaccine safety. Stat Med. 2022 doi: 10.1002/sim.9325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fonseca-Rodriguez O, Fors Connolly AM, Katsoularis I, Lindmark K, Farrington P. Avoiding bias in self-controlled case series studies of coronavirus disease 2019. Stat Med. 2021;40(27):6197–6208. doi: 10.1002/sim.9179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tanriover MD, Doganay HL, Akova M, et al. Efficacy and safety of an inactivated whole-virion SARS-CoV-2 vaccine (CoronaVac): interim results of a double-blind, randomised, placebo-controlled, phase 3 trial in Turkey. Lancet. 2021;398(10296):213–222. doi: 10.1016/S0140-6736(21)01429-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jara A, Undurraga EA, Gonzalez C, et al. Effectiveness of an inactivated SARS-CoV-2 vaccine in Chile. N Engl J Med. 2021 doi: 10.1056/NEJMoa2107715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Joynt GM, Li TS, Griffith JF, et al. The incidence of deep venous thrombosis in Chinese medical Intensive Care Unit patients. Hong Kong Med J. 2009;15(1):24–30. [PubMed] [Google Scholar]
- 49.White RH, Keenan CR. Effects of race and ethnicity on the incidence of venous thromboembolism. Thromb Res. 2009;123(Suppl 4):S11–S17. doi: 10.1016/S0049-3848(09)70136-7. [DOI] [PubMed] [Google Scholar]
- 50.Ridker PM, Miletich JP, Hennekens CH, Buring JE. Ethnic distribution of factor V Leiden in 4047 men and women. Implications for venous thromboembolism screening. JAMA. 1997;277(16):1305–1307. [PubMed] [Google Scholar]
- 51.Zhang S, Liu Y, Wang X, et al. SARS-CoV-2 binds platelet ACE2 to enhance thrombosis in COVID-19. J Hematol Oncol. 2020;13(1):120. doi: 10.1186/s13045-020-00954-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fan BE, Shen JY, Lim XR, et al. Cerebral venous thrombosis post BNT162b2 mRNA SARS-CoV-2 vaccination: a Black Swan event. Am J Hematol. 2021 doi: 10.1002/ajh.26272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lim WW, Mak L, Leung GM, Cowling BJ, Peiris M. Comparative immunogenicity of mRNA and inactivated vaccines against COVID-19. Correspondence. Lancet Microbe. 2021 doi: 10.1016/S2666-5247(21)00177-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Suzuki YJ, Gychka SG. SARS-CoV-2 spike protein elicits cell signaling in human host cells: implications for possible consequences of COVID-19 vaccines. Vaccines. 2021;9(1) doi: 10.3390/vaccines9010036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chan EWW, Leung MTY, Lau LKW, et al. Comparing self-reported reactogenicity between adolescents and adults following the use of BNT162b2 (Pfizer-BioNTech) messenger RNA COVID-19 vaccine: a prospective cohort study. Int J Infect Dis. 2022;116:47–50. doi: 10.1016/j.ijid.2021.12.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ye X, Ma T, Blais JE, et al. Association between BNT162b2 or CoronaVac COVID-19 vaccines and major adverse cardiovascular events among individuals with cardiovascular disease. Cardiovasc Res. 2020 doi: 10.1093/cvr/cvac068. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mowla A, Shakibajahromi B, Shahjouei S, et al. Cerebral venous sinus thrombosis associated with SARS-CoV-2; a multinational case series. J Neurol Sci. 2020;419 doi: 10.1016/j.jns.2020.117183. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.