Abstract
Interventional Oncology (IO) is a subspecialty field of Interventional Radiology bridging between diagnostic radiology and the clinical oncology team, addressing the diagnosis and treatment of cancer. There have been many exciting advancements in the field of IO in recent years; far too many to cover in a single paper. To give each topic sufficient attention, we have limited the scope of this review article to four topics which we feel have the potential to drastically change how cancer is treated managed in the immediate future.
Keywords: Interventional oncology, Immune checkpoint inhibitors, Breast ablation, Prostate ablation, Artificial intelligence
1. Introduction
Interventional Oncology (IO) is a subspecialty field of Interventional Radiology bridging between diagnostic radiology and the clinical oncology team, addressing the diagnosis and treatment of cancer. There have been many exciting advancements in the field of IO in recent years; far too many to cover in a single paper. To give each topic sufficient attention, we have limited the scope of this review article to four topics which we feel have the potential to drastically change how cancer is treated managed in the immediate future.
2. Immuno-oncology and interventional oncology
Immuno-oncology is a young area of research with a goal to develop treatments which enable the body’s immune system to fight cancer. Over the past decade, general interest in this field has shifted from cytokines and cancer vaccines to immune checkpoint inhibitors (ICI) due to their impressive clinical efficacy. In fact, research into immune checkpoints as potential targets for cancer treatments earned Drs. Tasuku Honjo and James Allison the Nobel Prize in Medicine 2018 [1]. There has also been a growing body of literature investigating the use of locoregional interventional oncology therapies in conjunction with ICI, offering exciting possibilities for interventional radiologists and patients.
The immune response against tumors is driven primarily by cytotoxic CD8 + T cells. Tumor antigens are presented to these cells by antigen-presenting cells, which establishes target specificity in the cytotoxic T cells. However, the activity of these cells against tumor cells is dependent on the dominance of either costimulatory or coinhibitory signals. Costimulatory signals are necessary for an immune response to foreign antigens, but coinhibitory signals are important for preventing autoimmune reactions. Coinhibitory signals depend on immune checkpoints, which are protein-protein interactions that control the type and magnitude of immune responses. Tumoral expression of checkpoint proteins protects the tumor cells from immune attack by inducing T cell clonal deletion and anergy. This has been found to have clinical significance. With hepatocellular carcinoma, for example, higher expression levels of the checkpoints programmed cell death protein 1 (PD-1) and programmed cell death ligand 1 (PD-L1) have been found to correlate with tumor stage, local recurrence and progression rates, and worse prognosis [2].
By blocking the checkpoint proteins which modulate cytotoxic T cell function, ICI permit the activation of the immune response against tumors. The checkpoint inhibitors which have been approved by the Food and Drug Administration are monoclonal antibodies against PD-1, PD-L1 and cytotoxic T-lymphocyte associated protein 4 (CTLA-4) (Fig. 1). A number of groundbreaking clinical trials have led to the widespread acceptance of ICI as first or second line treatments, either alone or in combination with chemotherapy, for approximately 50 types of cancer. In patients with advanced non-small cell lung cancer (NSCLC) and tumors with PD-L1 expression > 50 %, pembrolizumab demonstrated a superior progression-free survival than chemotherapy (10.3 months vs 6.0 months) [3]. In patients with metastatic melanoma, pembrolizumab and nivolumab have increased the median overall survival (OS) to > 2 years from the historical OS of < 1 year, with about 20 % of patients achieving a complete response of metastatic disease (i.e. complete disappearance of all visible metastases on imaging) [4]. In patients with nonresectable HCC, the IMBrave 150 trial has shown promising results with atezolizumab plus bevacizumab demonstrating superior 12-month OS compared to the standard of care sorafenib (67.2 % vs 54.6 %) [5]. Although these results are impressive, there is still a great deal of room for improvement.
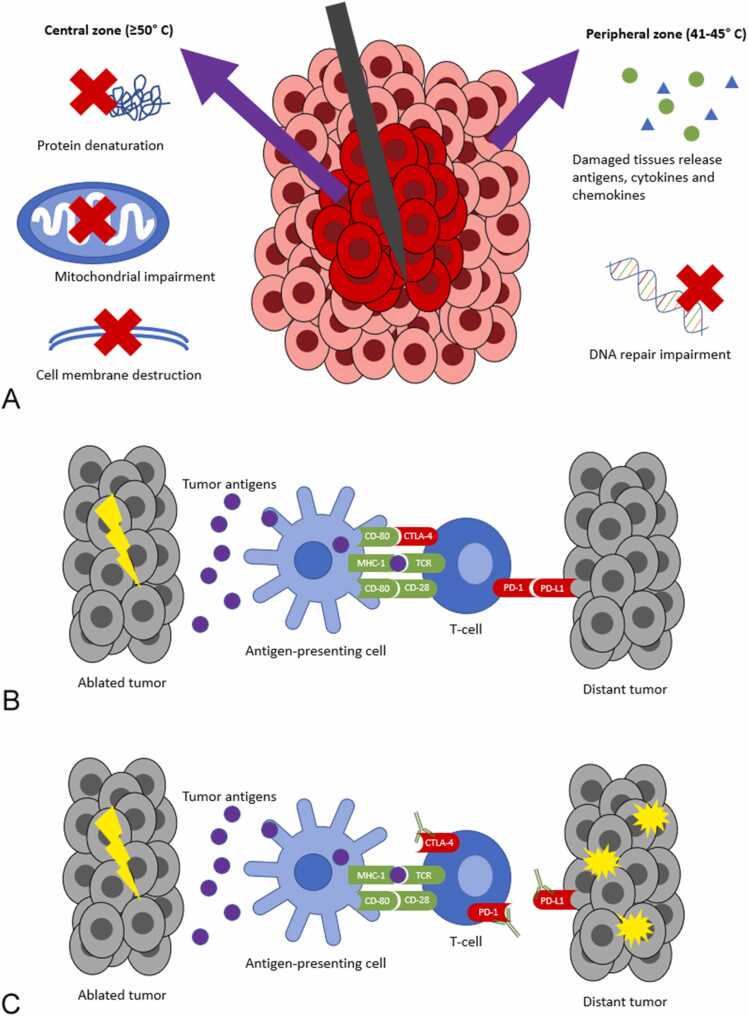
Fig. 1.
Thermal ablation systems have both local and systemic effects on tumors. A. Heat-based modalities (e.g. radiofrequency ablation and microwave ablation) damage tumor cells in a number of ways and lead to the release of tumor antigens. B. The antigens released by dead tumor cells are taken up by antigen-presenting cells, such as dendritic cells. These present the antigens to T-cells, and can activate them through costimulatory checkpoint proteins (green). However, coinhibitory checkpoint proteins (red) on T-cells or tumor cells can cause T-cell apoptosis. C. Immune checkpoint inhibitors are antibodies which bind and inactivate coinhibitory proteins on T-cells and tumor cells, allowing activated T-cells to attack distant tumor cells.
Interventional radiologists use a variety of locoregional ablation and embolization therapies to destroy tumors. These techniques lead to tumor cell death and the liberation of tumor antigens which incite a systemic immune response, similar to the immune response mounted against pathogenic organisms (Fig. 1). There are even a handful of case reports in which distant metastases spontaneously decreased in size following ablation of one lesion [6], [7], [8] (Fig. 2). This phenomenon has been called the abscopal effect. However, in the majority of cases the immune effect is transient, shown to last about 4 weeks in one preclinical study [9]. It has been shown that tumors increase expression of PD-L1 and tumor-infiltrating lymphocytes increase expression of PD-1 after thermal ablation, dampening the immune system’s anti-tumor activity [10]. There is also a growing concern that tumor ablation may stimulate growth of tumor cells at remote sites in some cases [11]. Clearly, the immunomodulating effect of locoregional therapy alone is inadequate as an immuno-monotherapy.
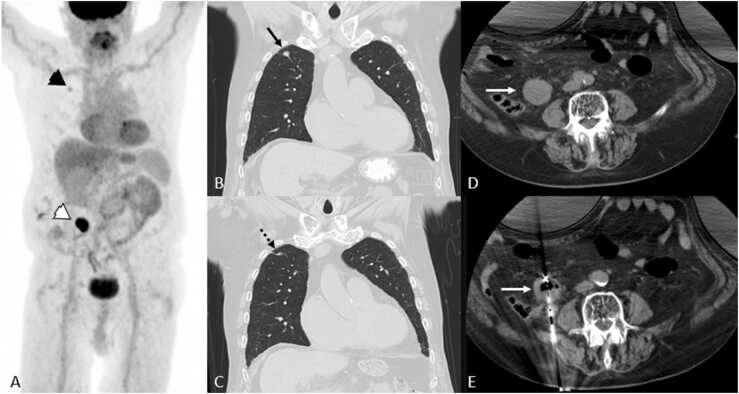
Fig. 2.
Abscopal effect in 77 year-old-man with metastatic renal cell carcinoma. A. 18FDG PET/CT MIP shows FDG-avid biopsy-proven right upper a right upper lobe pulmonary metastasis (black arrowhead) and right retroperitoneal recurrence (white arrowhead). B, C, D, and E. CT images show the pulmonary metastasis (black arrow) at the time of right retroperitoneal ablation (white arrows), and 1.5 years later which underwent gradual regression (dashed black arrow) in the absence of systemic treatment.
There have been several studies evaluating the combination of interventional oncology treatments with immunotherapies other than checkpoint inhibitors, such as natural killer cell injection, dendritic cell injection, CPG-oligodeoxynucleotides, IL-2 injection, and granulocyte macrophage colony stimulating factor (GM-CSF) [12], [13], [14], [15]. Interestingly, all of these combination therapies resulted in a stronger immune response than either ablation or immunotherapy alone. As research interest in immuno-oncology has now largely shifted to ICI, this will be the main focus of this manuscript.
A handful of case reports of cryoablation or transarterial radioembolization (TARE) combined with ICI have described decrease in size of distant tumors with few adverse events [16], [17], [18], [19]. Most of the larger available studies on interventional oncology therapies combined with ICI are focused on safety, with some reporting on immune changes or clinical efficacy. A pilot study of preoperative cryoablation and ipilimumab followed by mastectomy in women with breast cancer reported only grade 1 or 2 immune-related adverse event (irAE). In addition, serologic analysis found sustained elevations of Th1-type cytokines, activated CD4 + and CD8 + T cells, and T-effector cells relative to T-regulatory cells within tumor, suggesting a beneficial immunostimulatory effect [20].
A retrospective study of TARE combined with ICI (nivolumab +/- ipilimumab) in 26 patients with advanced HCC demonstrated no early grade 3 or 4 toxicities related to the TARE or ICI, with 2 patients (7.7 %) developing delayed grade 3 and 4 hepatobiliary toxicities in the setting of disease progression [21]. Although comparisons of outcomes between this relatively small study and larger studies can be problematic, the survival results in this study compared favorably with landmark studies, with median OS from time of TARE of 16.5 months compared to 11.3 months in the largest study of TARE alone [22]. The results of this study were also promising when compared to the CheckMate040 study of nivolumab [23] and the KEYNOTE-224 study of pembrolizumab [24], which showed median OS of 15.0 months and 12.9 months, respectively, while the median OS in this study was 17.2 months from the time of immunotherapy.
A similar retrospective study of TARE combined with ICI in 22 patients with metastatic disease to the liver from various primaries demonstrated a slightly higher rate of toxicities, with 3 patients (13.6 %) developing hepatobiliary toxicities and 2 patients (9.1 %) developing “clinical” toxicities (grade 4 colitis and hepatic abscess) [25]. Subgroup analysis of the most common primary malignancy, uveal melanoma, demonstrated a median OS of 17.0 months, which is favorable compared to an earlier study of TARE alone for metastatic uveal melanoma which demonstrated a median OS of 12.3 months [26].
A number of ongoing clinical trials are evaluating combination therapy of locoregional interventional oncology treatments with checkpoint inhibitors for the treatment of a variety of diseases [Table 1]. While the majority of this research is focused on HCC, there are also trials on NSCLC, renal cell carcinoma (RCC), breast cancer, melanoma, pancreatic cancer, neuroendocrine tumors, and non-Hodgkin lymphoma (NHL). Most of these studies evaluate a single locoregional therapy, however a handful of studies compare different modalities. NCT03949231 is a phase III trial comparing hepatic artery infusion with venous infusion of toripalimab (an anti-PD1 monoclonal antibody) in BCLC stage C HCC. NCT02821754 is a phase II trial comparing TACE, radiofrequency ablation (RFA) and cryoablation in patients with HCC and cholangiocarcinoma treated with tremelimumab and durvalumab. NCT03753659 is a phase II trial comparing RFA and microwave ablation (MWA) in patients with early HCC treated with pembrolizumab.
Table 1.
Ongoing clinical trials evaluating locoregional interventional oncology procedures combined with immune checkpoint inhibitors (ICI).
| NCT | Phase | Drug | Procedure | Malignancy |
|---|---|---|---|---|
| NCT03572582 | II | nivolumab | cTACE | intermediate-stage HCC |
| NCT04191889 | II | apatinib and camrelizumab | cTACE | stage C HCC |
| NCT04246177 | III | lenvatinib and pembrolizumab | cTACE | incurable/non-metastatic HCC |
| NCT03638141 | II | durvalumab and tremelimumab | DEB-TACE | intermediate-stage HCC |
| NCT03143270 | I | nivolumab | DEB-TACE | stage B HCC |
| NCT03259867 | IIA | nivolumab or pembrolizumab | trans-arterial tirapazamine embolization (TATE) | advanced HCC or other malignancies |
| NCT03033446 | II | nivolumab | TARE | advanced HCC |
| NCT02837029 | I | nivolumab | TARE | advanced HCC |
| NCT03099564 | I | pembrolizumab | TARE | high-risk HCC |
| NCT03949231 | III | toripalimab | hepatic artery or IV infusion of toripalimab | stage C HCC |
| NCT03753659 | II | pembrolizumab | RFA or MWA | early HCC |
| NCT04472767 | II | cabozantinib, ipilimumab, and nivolumab | TACE | unresectable HCC |
| NCT03778957 | III | durvalumab and bevacizumab | TACE | locoregional HCC |
| NCT04605731 | Ib | durvalumab and tremelimumab | TARE | unresectable locally-advanced HCC |
| NCT04541173 | II | atezolizumab and bevacizumab | TARE | unresectable HCC |
| NCT03630640 | II | nivolumab | electroporation | stage A HCC |
| NCT02821754 | II | tremelimumab and durvalumab | TACE, RFA, or cryoablation | stage B/C HCC, unresectable cholangiocarcinoma |
| NCT03937830 | II | durvalumab, bevacizumab, and tremelimumab | TACE | intermediate or advanced HCC and cholangiocarcinoma |
| NCT04301778 | II | durvalumab and SNDX-6352 | TACE or TARE | locally-advanced intrahepatic cholangiocarcinoma |
| NCT03457948 | II | pembrolizumab | IV peptide receptor radionuclide therapy, bland embolization or TARE | metastatic well-differentiated neuroendocrine tumor in liver |
| NCT04429321 | I | nivolumab and ipilimumab | lipiodol+ethanol embolization of primary or metastatic tumors | metastatic RCC |
| NCT03080974 | II | nivolumab | irreversible electroporation | stage III pancreatic cancer |
| NCT04339218 | III | pembrolizumab, pemetrexed, and carboplatin | cryoablation | metastatic lung adenocarcinoma |
| NCT04201990 | I/II | camrelizumab and apatinib | MWA | metastatic NSCLC |
| NCT04102982 | II | camrelizumab | MWA | advanced NSCLC |
| NCT03769129 | N/A | pembrolizumab | MWA | advanced NSCLC |
| NCT03290677 | II | not specified | cryoablation | metastatic lung cancer and metastatic melanoma |
| NCT03949153 | I/II | nivolumab | cryoablation and in situ ipilimumab injection | stage IIIB/C melanoma |
| NCT03546686 | II | ipilimumab and nivolumab | cryoablation | triple negative breast cancer |
| NCT02833233 | N/A | nivolumab and ipilimumab | cryoablation | early stage breast cancer |
| NCT03035331 | I/II | pembrolizumab and dendritic cell therapy | cryoablation | non-Hodgkins lymphoma |
The advent of ICI has already fundamentally changed the way that cancer is being treated and research on the combination with locoregional interventional oncology techniques are in the early stages. Many exciting trials investigating these combinations are underway, and preliminary results are promising. The field excitedly anticipates further results and development of future investigations as large randomized-controlled trials will be required for these treatment combinations to become the standard of care.
3. Breast ablation
Improvements in diagnostic and therapeutic modalities for breast malignancies allow for earlier detection of breast cancers and have driven a shift towards using surgically conservative and targeted treatments. In addition, our deeper understanding of post treatment disease progression, recurrence rates, and impact of conservative treatment on disease free survival, encourages further consideration of minimally invasive approaches, such as thermal ablation, for early breast cancer treatment. Although accepted for the treatment of many malignancies, thermal ablation has not been widely studied in breast malignancies. Here, we disuses discuss thermal ablation techniques and summarize the current literature on efficacy and safety of thermal ablation for breast cancer. Thermal ablation may effectively treat benign breast masses, early-stage tumors, and reduce tumor burden in more extensive disease; however, well- controlled studies are needed to fully understand the impact on disease recurrence and disease-free survival.
Breast carcinoma represents a variety of malignant pathologies. It is the most common cancer diagnosis in women and the second leading cause of cancer death worldwide [27], [28]. The traditional treatment for most malignant breast masses included surgical therapy with complete mastectomy and axillary lymph node dissection, which often results in significant morbidity and reduced quality of life. Recent advancements in screening and imaging modalities enable the detection of smaller, earlier stage tumors that are ideal candidates for breast conserving therapy (BCT) [29]. However, even with implementation of BCT, cosmetic and functional sequalae persist, and patients with non-malignant masses may undergo unnecessary surgical procedures [30]. In addition, many patients with comorbidities or advanced staged disease may not be appropriate surgical candidates. The application of nonminimally-invasive ablation techniques provides opportunities to effectively treat benign and malignant breast masses, while minimizing reducing sequalae of surgical approaches.
Thermal ablation techniques, including radiofrequency ablation (RFA), microwave ablation (MVA), and cryoablation, use energy generated around an inserted device to create temperature differentials that induce cellular injury and death [31]. There is a multiphasic effect on neoplastic growth, with immediate and delayed cell death, and possible immune activation [32]. Thermal ablation is already utilized for many malignancies including liver, lung [33], kidney, and thyroid, providing excellent proof of concept for its use in breast neoplasms [31].
Although many studies have investigated the use of thermal ablation in treating malignant breast masses, non-malignant breast masses, and metastatic disease as palliative or debulking therapy, few randomized controlled studies have been completed to date. Thus, the efficacy of thermal ablation in primary breast lesions varies between studies, likely due to the large range of tumor burden, varying technical approaches, recent advances in available tools, and varying primary outcomes [34], [35].
Studies evaluating RFA for invasive ductal carcinomas measuring less than 2 cm demonstrated feasibility [36], with effective coagulative necrosis of masses, low complication rates [37], [38], efficacy in reducing tumor size [39], [40], [41], and efficacy in reducing positive margin rate when compared with lumpectomy [42]. In 2021, two meta analyses of studies using RFA for small invasive ductal carcinomas (<2 cm) reported high technical success of RFA, complete ablation rates of 88.6 % [34] and 99 % [35], and low complication rates of 9.4 % [34] and 2 % [35]. Unfortunately, due to the small sample sizes, short duration of follow up, and lack of standard of care control groups, the impact on local and distant recurrence has not been well established.
Similarly, MWA and cryoablation demonstrated technical feasibility, safety, and efficacy in initial treatment of early-stage breast masses [43], [44], [45]. Prospective studies with long-term follow up are necessary to clearly evaluate long term efficacy and impact on recurrence of early-stage breast cancers and survival in patients undergoing thermal ablation compared to breast conserving therapy.
Minimally invasive thermal ablative techniques are non-surgical and can be performed in the outpatient or ambulatory settings making them ideal for the treatment of benign breast lesions, including fibroadenoma and mammary adenosis [46]. Most studied in the context of non-malignant masses is cryoablation. A large prospective study showed that cryoablation of fibroadenomas achieved complete resolution of masses in up to 75 % of patients at 12 months, and patient satisfaction rates of 88 % [47]. In a recent study, MWA showed promising reductions in frequency of palpable mass, breast pain, and low rates of cosmetic complications (<1 %) for non-malignant breast masses [48]. Studies using cryoablation serve as excellent proof of concept for the use of other thermal ablation techniques, including RFA and MWA, for non-malignant masses.
Although single-probe thermal ablation is particularly useful in masses less than 2–3 centimeters, there is an interesting role for ablative techniques in metastatic disease for palliative or debulking therapy. Multiple studies validated the feasibility of thermal ablation for metastatic breast cancer [49], [50], [51], [52]. More recently, Li et al., evaluated the efficacy of MRI-guided RFA in patients with either metastatic disease or those who were poor surgical candidates and demonstrated significant reduction of tumor volume, only two treatment related complications (breast pain and perspiration), and no new local or distant tumor recurrence [53]. In a retrospective study, Ridouani et al. evaluated progression free survival after RFA in patients with oligometastatic breast cancer and demonstrated median progression free survival of 10 months [54]. Furthermore, laboratory studies confirm feasibility and physiology of combinatorial approaches that include both thermal ablation and immunotherapy for the treatment of metastatic breast cancer [55], [56], with other trials ongoing (NCT03546686 and NCT02833233). Moving forward, such combinatorial approaches for metastatic breast cancer need to be thoroughly evaluated in clinical settings.
As diagnostic and treatment modalities for breast masses improve, focus shifts to minimally invasive therapeutic techniques that achieve disease control without sacrificing functionality, cosmetic appearance, and quality of life. Image guided, thermal ablative techniques including ultrasound or MRI guided RFA, MWA, and cryoablation, are ideal modalities for the treatment of small, early-stage breast cancers, non-malignant masses, and in non-surgical candidates. The physiologic efficacy in inducing coagulative necrosis of breast masses has been well established. However, well designed and well controlled studies comparing ablative techniques to the standard of care are truly necessary to determine the impact on local and distant disease recurrence and quality of life outcomes.
4. Prostate ablation
Prostate cancer is the most common, noncutaneous malignancy in men, affecting approximately 1 in 8 men during their lifetime. It is also the second leading cause of cancer death in American men, killing approximately 1 in 41 men [57]. With the adoption of serum prostate-specific antigen (PSA) screening, the majority of prostate cancers today are discovered at a localized stage. Many of these cancers are indolent, and are unlikely to pose a significant threat to the patient’s health or life. Approximately three quarters of all prostate cancer patients have low- or intermediate-risk prostate cancer [58]. Traditional management for prostate cancer confined to the gland can be divided into definitive therapy and active surveillance or watchful waiting.
The standard definitive therapy options include radical prostatectomy and whole gland radiation therapy. However, these can carry significant morbidity affecting quality of life. In a study published in the New England Journal of Medicine in 2008, poor sexual function was reported in 53 % of patients who had a prostatectomy, 58 % of patient who had undergone external beam radiotherapy, and 46 % of patients who had undergone brachytherapy at 24 months. Urinary incontinence was reported in 14 % of patients who had a prostatectomy, 7 % of patients who had undergone external beam radiotherapy, and 10 % of patients who had undergone brachytherapy at 24 months. While bowel function was not significantly affected by prostatectomy, some bowel problem was reported in 11 % of patients who had undergone external beam radiotherapy and 8 % of patients who had undergone brachytherapy at 24 months [59].
Active surveillance for diagnosed prostate cancers can include periodic digital rectal examinations and/or PSA testing or periodically repeated prostate biopsies. While active surveillance can be an acceptable option in appropriately-selected patients, it is variably utilized [60]. The obvious advantage over definitive therapies is avoidance of treatment-related morbidity. However, several studies have shown a significant decrease in sexual function and psychological morbidity [61], [62], even without localized surgery or radiation. Furthermore, there is a risk of disease progression and subsequent death.
As imaging of the prostate has advanced, minimally-invasive image-guided focal therapies for very low to intermediate risk prostate cancer have emerged. The theoretical advantage is that only the portion of the prostate with known cancer could be treated while minimizing damage to surrounding structures, decreasing the incidence of side effects. These focal therapies include cryotherapy, high-intensity focused ultrasound (HIFU), irreversible electroporation (IRE), photodynamic therapy, and focal laser ablation (FLA). In this article, we will discuss FLA which is one of the newest modalities.
Laser ablation is either performed under MR guidance or MR fusion over ultrasound image guidance. It can be performed via either transrectal or transperineal approaches. Under image guidance, a laser is introduced into the prostate. During the ablation, the temperature within and around the treatment region is monitored to ensure lethal heat is applied to the tumor and not to normal structures. The treatment creates an identifiable hypovascular defect within the prostate on post treatment MRI (Fig. 3).
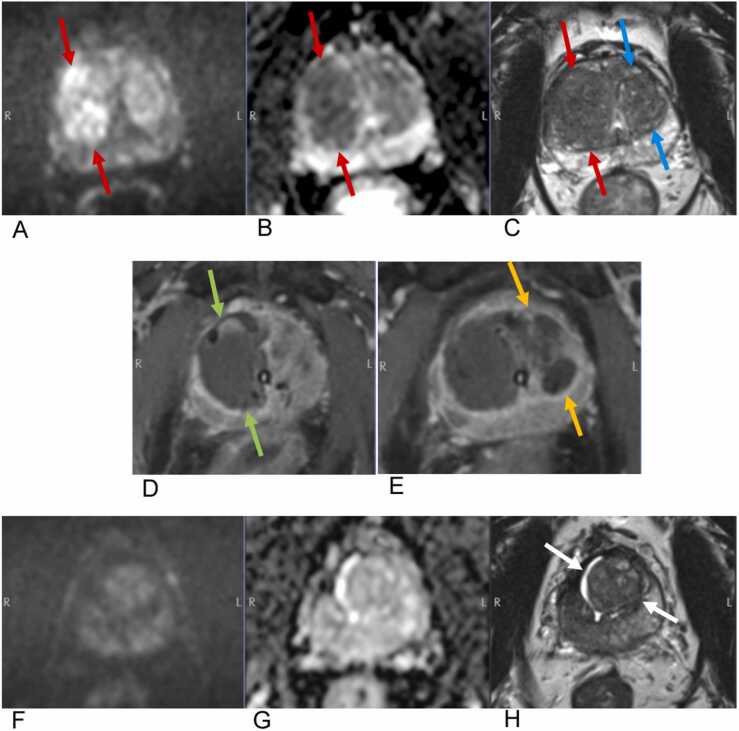
Fig. 3.
MR-guided focal laser ablation of prostate cancer in a 59-year-old man with biopsy-proven Gleason 3 + 4 disease. On pre-procedure MRI, cancer in the right midgland transitional zone (red arrows) appears hyperintense on axial high b value DWI (A), hypointense on ADC (B), and hypointense with “erased charcoal” sign on the T2-weighted sequence (C). Note the BPH changes in the left midgland transitional zone (blue arrows). Intraprocedural T1-weighted axial images with Dotarem demonstrate non-enhancing ablation defects in the right midgland (green arrows) after treatment of the cancer (D) and subsequently a second ablation defect in the left midgland (yellow arrows) after treatment of BPH (E). Surveillance MRI 1.5 years later demonstrates marked shrinkage of the transitional zone (white arrows) with no residual or recurrent signal abnormality on high b value DWI (F), ADC (G), or T2-weighted images. At this time, the patient’s serum PSA had decreased from 6.1 preprocedurally to 1.3, and he reported a dramatic reduction in symptoms related to BPH with no change in erectile function.
The side affect profile of focal laser ablation has been shown to be favorable in multiple studies. One phase I trial of 12 men found only mild symptoms of hematuria, hematospermia, and urinary retention in the perioperative period, but no significant decrease in International Prostate Symptom Score (IPSS) or International Index of Erectile Function (IIEF-5) scores at 1, 3, or 6 months postoperatively [63]. Another phase I trial of 9 men demonstrated no significant change in the average IPSS or Sexual Health Inventory for Men (SHIM) scores at 1 or 6 months [64]. A self-resolving perineal abrasion and focal paresthesia of the glans penis occurred in one patient each.
The longest follow-up to date was reported by Feller et. Al. in 2020 as 10-year interim results from an ongoing phase II clinical trial. One hundred and fifty-eight men and 248 cancer foci were treated with transrectal, MR-guided laser focal therapy. Of the 122 men who underwent biopsy of the treatment site at 6 months, 71 (59 %) were negative, 18 (15 %) were positive but clinically insignificant, and 32 (26 %) were positive and clinically significant. The metastasis-free survival rate was 99 % and the prostate cancer specific survival rate was 100 %. These results are promising, as they demonstrate similar oncologic control as whole-gland therapy. Furthermore, there was no significant change in the IPSS or SHIM scores [65]. These patients will be followed for twenty years.
However, a large 2020 study cast focal laser ablation of the prostate in a less flattering light. Zhou et al. conducted an analysis on data from the SEER database comparing survival outcomes between radiation therapy and focal laser ablation for the treatment of prostate cancer. A total of 93,469 patients were included, of which 428 were treated with laser ablation and the remainder received radiotherapy. Patients treated with laser ablation had worse overall survival (OS) in the adjusted multivariate regression, the propensity score matched analysis, and the instrumental variate (IV)-adjusted analysis. Interestingly, there was no significant difference in the cancer-specific mortality (CSM) between the two groups [66]. The main strength of this study, the size of the patient cohort, is also its most glaring limitation. With an aggregated patient population of this size, there is almost certainly significant heterogeneity in the specific laser devices, imaging used for guidance (in-bore MR versus MR-ultrasound fusion), and the specialty of the proceduralists (interventional radiologists versus urologists). This makes it difficult to generalize the results, when one combination of elements may perform drastically differently than another.
In the United States, FLA and other focal therapies are still considered investigational and are therefore not covered by Medicare or private insurance. In the American Urologic Association (AUA) guideline on clinically localized prostate cancer, focal therapies are not recommended as the standard of care outside of clinical trials due to insufficient evidence [67]. While some evidence suggests inferior outcomes of FLA compared with radiotherapy, the side effect profile seems to be more favorable in multiple studies. Proponents of FLA might argue that FLA does not have to demonstrate superiority or even non-inferiority in outcomes for it to become adopted as an option for patients who are mostly concerned with the impact of side effects on quality of life. Either way, prospective randomized trials comparing focal prostate therapies to prostatectomy, radiation therapy, and active surveillance are needed.
5. Artificial intelligence
Artificial intelligence (AI) within interventional radiology is a rapidly evolving field with new technologic and algorithmic advancements every year. Globally, AI can be thought of as intelligence demonstrated by machines in which the system can perceive its own environment using statistical and probabilistic techniques and take actions to improve the chance of achieving a set goal or parameter [68]. Within the field of radiology and especially within the field of interventional oncology, artificial intelligence has demonstrated many useful applications such as pattern recognition, procedural planning, and patient outcome prediction. As technology advances and clinical data management improves, utilizing artificial intelligence in everyday patient care is quickly becoming more commonplace. While the complexities of AI, machine learning, and deep neural networks are beyond the scope of this article, there are some initial specifics that should be addressed prior to further discussion.
AI is a larger generalizable term that includes both machine learning and deep learning. Machine learning is a specific form of AI in which the system learns to perform a task from available data. This data is initially taught to the system and the algorithm then estimates a specific relationship between the data provided (input) and the variable of interest (output). Examples of specific to interventional oncology inputs include age, sex, medical comorbidities, and tumor markers whereas the outputs include survival estimates or treatment response. There are multiple forms of machine learning and one such application is the use of neural networks such as artificial neural networks (ANN) and convolutional neural networks (CNN). These techniques are modeled after biologic neuronal networks and simulate action potentials as they pass through multiple layers, allowing for intricate relationships to be learned through inherent probabilistic weights [69]. CNNs are a type of deep ANNs specifically attuned for diagnostic imaging as they are initially inspired from the connectivity pattern of visual cortex neurons with deep receptive fields serving as the input to a deep neural network [70]. This type of learned model makes ANNs ideal for applications such as image recognition and intraprocedural needle localization. For additional specific and technical information, refer to the excellent AI in interventional oncology primer by Letzen et al. [69].
As image quality, acquisition, and data management improve, it is clear to see why radiology is one of the ideal fields for adapting ANNs and CNNs into daily practice. Further, the field of interventional oncology additionally benefits from the treatment and procedural planning that these algorithms can provide. In regard to improving and augmenting diagnostic skills, multiple groups have demonstrated the importance of utilizing AI in the oncologic realm. For example, Antonelli et. al. utilized machine learning to assist with identifying and stratifying aggressiveness of MR-detected prostate cancer. When the results were compared against radiologists, the machine learning model was able to outperform the radiologist in detecting Gleason pattern 4 prostate cancer [71]. Other groups have studied utilizing machine learning in the identification and classification of pulmonary nodules using computed tomography, demonstrating high AUC for positive case identification and exceeding the average performance of radiologists [72]. Another interesting application of machine learning in interventional oncology is the ability to predict tumor response particularly in the setting of transarterial chemoembolization (TACE) in patients with hepatocellular carcinoma (HCC). Prior studies have demonstrated that patients with moderate to severe cases of HCC have variable or unresponsive outcomes after a TACE procedure [73]. As a result, several groups have utilized machine learning to predict treatment response before attempting a TACE. Combining the Barcelona Clinic Liver Cancer (BCLC) staging system as an input data set along with CT image analysis, one group was able to accurately predict TACE response in approximately 74% of their cases compared to 61% with BCLC data alone in a retrospective study [74]. This data highlights how useful artificial intelligence can be to the interventional radiologist, especially at the initial pre planning assessment in an interdisciplinary setting.
While artificial intelligence can certainly be integrated into the diagnostic realm, intraprocedural applications are actively being tested, although the vast majority are still within the pre-clinical stages. One such application is utilizing image fusion to assist when attempting to target a tumor for biopsy or ablation. Using noncontrast CT can often provide limitations for needle guiding and planning due to the inherent lack of structural contrast. To overcome this challenge, machine learning is being used to improve lesion detection with the fusion of intraprocedural images with diagnostic scans. Liu et al. designed a proof-of-concept machine learning model that took advantage of multilayer CT liver segmentation which utilized CT overlay models combined with MRI, contrast enhanced CT and PET/CT [75]. This application would allow operators to have a flexible and fluid multi-modality approach to tumor localization in real time.
Other proof-of-concept algorithms being developed using machine learning include applications for accurate and safe needle localization are being developed utilizing machine learning. For example, MR-guided procedures provide the advantage of high soft tissue contrast without the need for ionizing radiation. However, procedures that require the placement and manipulation of a needle fall victim to visualization challenges depending on what sequence is implanted and to variations in signal void [76]. To overcome these challenges, one group successfully trained a CNN with in vivo intraprocedural images and ex vivo MRI data sets to accurately track needle tips during MR-guided prostate biopsies [77].
Similarly, ultrasound-guided procedures do not require ionizing radiation and comprise a large segment of interventional oncology volume. However, limitations to ultrasound include needle visibility, deep lesions, and artifact from adjacent structures. Using a combination of several CNNs, Mwikirize et al. were able to apply machine learning using curvilinear 2D ultrasound to refine the image analysis. After training their neural network utilizing bovine/porcine lumbosacral spine phantoms, their proof-of-concept algorithm was able to improve the overall needle tip localization, with 99.6 % precision, 99.8 % recall rate, and detection time of 0.04 s [78].
The role of artificial intelligence in interventional oncology is an exciting and constantly evolving field. While this is by no means an exhaustive list, it is clear that the applications are far reaching and can be applied to both the diagnostic and interventional fields of oncology. Additionally, the studies discussed in this article highlight the importance of developing a close collaboration between artificial intelligence researchers, engineers, and clinicians to develop the test tools to approach a specific case or diagnostic dilemma.
6. Conclusion
Interventional Oncology (IO) has already revolutionized the management of cancer, offering minimally-invasive therapies often with more tolerable side effects than conventional surgical or medical treatments. The advent of immune checkpoint inhibitors has inspired many investigators to look for synergistic combinations with locoregional IO therapies. Promising trials are also being done with percutaneous ablation of breast and prostate cancer, the most common noncutaneous cancers in women and men respectively. And artificial intelligence is being incorporated into many aspects of locoregional treatments to improve outcomes. IO was founded by some of the most creative minds in medicine, and today a new generation of interventional radiologists is boldly leading the field into an exciting future.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Huang P.W., Chang J.W.C. Immune checkpoint inhibitors win the 2018 Nobel Prize. Biomed. J. 2019;42:299–306. doi: 10.1016/j.bj.2019.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hato T., Goyal L., Greten T.F., Duda D.G., Zhu A.X. Immune checkpoint blockade in hepatocellular carcinoma: current progress and future directions. Hepatology. 2014;60:1776–1782. doi: 10.1002/hep.27246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reck M., Rodríguez-Abreu D., Robinson A.G., Hui R., Csőszi T., Fülöp A., Gottfried M., Peled N., Tafreshi A., Cuffe S., O’Brien M., Rao S., Hotta K., Leiby M.A., Lubiniecki G.M., Shentu Y., Rangwala R., Brahmer J.R. Pembrolizumab versus chemotherapy for PD-L1–positive non–small-cell lung cancer. N. Engl. J. Med. 2016;375:1823–1833. doi: 10.1056/nejmoa1606774. [DOI] [PubMed] [Google Scholar]
- 4.Robert C., Ribas A., Hamid O., Daud A., Wolchok J.D., Joshua A.M., Hwu W.J., Weber J.S., Gangadhar T.C., Joseph R.W., Dronca R., Patnaik A., Zarour H., Kefford R., Hersey P., Zhang J., Anderson J., Diede S.J., Ebbinghaus S., Hodi F.S. Durable complete response after discontinuation of pembrolizumab in patients with metastatic melanoma. J. Clin. Oncol. 2018;36:1668–1674. doi: 10.1200/JCO.2017.75.6270. [DOI] [PubMed] [Google Scholar]
- 5.Finn R.S., Qin S., Ikeda M., Galle P.R., Ducreux M., Kim T.-Y., Kudo M., Breder V., Merle P., Kaseb A.O., Li D., Verret W., Xu D.-Z., Hernandez S., Liu J., Huang C., Mulla S., Wang Y., Lim H.Y., Zhu A.X., Cheng A.-L. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N. Engl. J. Med. 2020;382:1894–1905. doi: 10.1056/nejmoa1915745. [DOI] [PubMed] [Google Scholar]
- 6.Soanes W.A., Ablin R.J., Gonder M.J. Remission of metastatic lesions following cryosurgery in prostatic cancer: immunologic considerations. J. Urol. 1970;104:154–159. doi: 10.1016/S0022-5347(17)61690-2. [DOI] [PubMed] [Google Scholar]
- 7.Kim H., Byung K.P., Chan K.K. Spontaneous regression of pulmonary and adrenal metastases following percutaneous radiofrequency ablation of a recurrent renal cell carcinoma. Korean J. Radiol. 2008;9:470–472. doi: 10.3348/kjr.2008.9.5.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ghodadra A., Bhatt S., Camacho J.C., Kim H.S. Abscopal effects and Yttrium-90 radioembolization. Cardiovasc. Interv. Radiol. 2016;39:1076–1080. doi: 10.1007/s00270-015-1259-0. [DOI] [PubMed] [Google Scholar]
- 9.Si T., Guo Z., Hao X. Immunologic response to primary cryoablation of high-risk prostate cancer. Cryobiology. 2008;57:66–71. doi: 10.1016/j.cryobiol.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 10.Shi L., Chen L., Wu C., Zhu Y., Xu B., Zheng X., Sun M., Wen W., Dai X., Yang M., Lv Q., Lu B., Jiang J. PD-1 blockade boosts radiofrequency ablation-elicited adaptive immune responses against tumor. Clin. Cancer Res. 2016;22:1173–1184. doi: 10.1158/1078-0432.CCR-15-1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rozenblum N., Zeira E., Scaiewicz V., Bulvik B., Gourevitch S., Yotvat H., Galun E., Goldberg S.N. Oncogenesis: an “off-target” effect of radiofrequency ablation. Radiology. 2015;276:426–432. doi: 10.1148/radiol.2015141695. [DOI] [PubMed] [Google Scholar]
- 12.Domingo-Musibay E., Heun J.M., Nevala W.K., Callstrom M., Atwell T., Galanis E., Erickson L.A., Markovic S.N. Endogenous heat-shock protein induction with or without radiofrequency ablation or cryoablation in patients with stage IV melanoma. Oncologist. 2017;22:1026–e93. doi: 10.1634/theoncologist.2017-0060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nakagawa H., Mizukoshi E., Iida N., Terashima T., Kitahara M., Marukawa Y., Kitamura K., Nakamoto Y., Hiroishi K., Imawari M., Kaneko S. In vivo immunological antitumor effect of OK-432-stimulated dendritic cell transfer after radiofrequency ablation. Cancer Immunol. Immunother. 2014;63:347–356. doi: 10.1007/s00262-013-1514-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kroeze S.G.C., Daenen L.G.M., Nijkamp M.W., Roodhart J.M.L., De Gast G.C., Bosch J.L.H.R., Jans J.J.M. Radio frequency ablation combined with interleukin-2 induces an antitumor immune response to renal cell carcinoma in a murine model. J. Urol. 2012;188:607–614. doi: 10.1016/j.juro.2012.03.116. [DOI] [PubMed] [Google Scholar]
- 15.Liang S., Niu L., Xu K., Wang X., Liang Y., Zhang M., Chen J., Lin M. Tumor cryoablation in combination with natural killer cells therapy and Herceptin in patients with HER2-overexpressing recurrent breast cancer. Mol. Immunol. 2017;92:45–53. doi: 10.1016/j.molimm.2017.10.003. [DOI] [PubMed] [Google Scholar]
- 16.Deipolyi A.R., Bromberg J.F., Erinjeri J.P., Solomon S.B., Brody L.A., Riedl C.C. Abscopal effect after radioembolization for metastatic breast cancer in the setting of immunotherapy. J. Vasc. Interv. Radiol. 2018;29:432–433. doi: 10.1016/j.jvir.2017.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Soule E., Bandyk M., Matteo J. Percutaneous ablative cryoimmunotherapy for micrometastaic abscopal effect: No complications. Cryobiology. 2018;82:22–26. doi: 10.1016/j.cryobiol.2018.04.013. [DOI] [PubMed] [Google Scholar]
- 18.Adam L.C., Raja J., Ludwig J.M., Adeniran A., Gettinger S.N., Kim H.S. Cryotherapy for nodal metastasis in NSCLC with acquired resistance to immunotherapy. J. Immunother. Cancer. 2018;6:1–5. doi: 10.1186/s40425-018-0468-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Adashek J.J., Salgia M., Dizman N., Kessler J., Pal S.K. Concomitant radioembolization and immune checkpoint inhibition in metastatic renal cell carcinoma. Case Rep. Oncol. 2018;11:276–280. doi: 10.1159/000489995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McArthur H.L., Diab A., Page D.B., Yuan J., Solomon S.B., Sacchini V., Comstock C., Durack J.C., Maybody M., Sung J., Ginsberg A., Wong P., Barlas A., Dong Z., Zhao C., Blum B., Patil S., Neville D., Comen E.A., Morris E.A., Kotin A., Brogi E., Wen Y.H., Morrow M., Lacouture M.E., Sharma P., Allison J.P., Hudis C.A., Wolchok J.D., Norton L. A pilot study of preoperative single-dose ipilimumab and/or cryoablation in women with early-stage breast cancer with comprehensive immune profiling. Clin. Cancer Res. 2016;22:5729–5737. doi: 10.1158/1078-0432.CCR-16-0190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhan C., Ruohoniemi D., Shanbhogue K.P., Wei J., Welling T.H., Gu P., Park J.S., Dagher N.N., Taslakian B., Hickey R.M. Safety of combined yttrium-90 radioembolization and immune checkpoint inhibitor immunotherapy for hepatocellular carcinoma. J. Vasc. Interv. Radiol. 2020;31:25–34. doi: 10.1016/j.jvir.2019.05.023. [DOI] [PubMed] [Google Scholar]
- 22.Salem R., Gabr A., Riaz A., Mora R., Ali R., Abecassis M., Hickey R., Kulik L., Ganger D., Flamm S., Atassi R., Atassi B., Sato K., Benson A.B., Mulcahy M.F., Abouchaleh N., Al Asadi A., Desai K., Thornburg B., Vouche M., Habib A., Caicedo J., Miller F.H., Yaghmai V., Kallini J.R., Mouli S., Lewandowski R.J. Institutional decision to adopt Y90 as primary treatment for hepatocellular carcinoma informed by a 1,000-patient 15-year experience. Hepatology. 2018;68:1429–1440. doi: 10.1002/hep.29691. [DOI] [PubMed] [Google Scholar]
- 23.El-Khoueiry A.B., Sangro B., Yau T., Crocenzi T.S., Kudo M., Hsu C., Kim T.-Y., Choo S.-P., Trojan J., Welling T.H., Meyer T., Kang Y.-K., Yeo W. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. 2017;389:2492–2502. doi: 10.1016/S0140-6736(17)31046-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhu A.X., Finn R.S., Edeline J., Cattan S., Ogasawara S., Palmer D., Verslype C., Zagonel V., Fartoux L., Vogel A., Sarker D., Verset G., Chan S.L., Knox J., Daniele B., Webber A.L., Ebbinghaus S.W., Ma J., Siegel A.B., Cheng A.L., Kudo M., Alistar A., Asselah J., Blanc J.F., Borbath I., Cannon T., Chung K., Cohn A., Cosgrove D.P., Damjanov N., Gupta M., Karino Y., Karwal M., Kaubisch A., Kelley R., Van Laethem J.L., Larson T., Lee J., Li D., Manhas A., Manji G.A., Numata K., Parsons B., Paulson A.S., Pinto C., Ramirez R., Ratnam S., Rizell M., Rosmorduc O., Sada Y., Sasaki Y., Stal P.I., Strasser S., Trojan J., Vaccaro G., Van Vlierberghe H., Weiss A., Weiss K.H., Yamashita T. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label phase 2 trial. Lancet Oncol. 2018;19:940–952. doi: 10.1016/S1470-2045(18)30351-6. [DOI] [PubMed] [Google Scholar]
- 25.Ruohoniemi D.M., Zhan C., Wei J., Kulkarni K., Aaltonen E.T., Horn J.C., Hickey R.M., Taslakian B. Safety and effectiveness of Yttrium-90 radioembolization around the time of immune checkpoint inhibitors for unresectable hepatic metastases. J. Vasc. Interv. Radiol. 2020;31:1233–1241. doi: 10.1016/j.jvir.2020.04.029. [DOI] [PubMed] [Google Scholar]
- 26.Eldredge-Hindy H., Ohri N., Anne P.R., Eschelman D., Gonsalves C., Intenzo C., Bar-Ad V., Dicker A., Doyle L., Li J., Sato T. Yttrium-90 microsphere brachytherapy for liver metastases from uveal melanoma. Am. J. Clin. Oncol. 2016;39:189–195. doi: 10.1097/coc.0000000000000033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ferlay J., Colombet M., Soerjomataram I., Mathers C., Parkin D.M., Piñeros M., Znaor A., Bray F. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int. J. Cancer. 2019;144:1941–1953. doi: 10.1002/ijc.31937. [DOI] [PubMed] [Google Scholar]
- 28.Siegel R., Miller K., Fuchs H., Jemal A. Cancer statistics, 2021. CA Cancer J. Clin. 2021;71:7–33. doi: 10.3322/caac.21654. [DOI] [PubMed] [Google Scholar]
- 29.Fisher B., Anderson S., Bryant J., Margolese R.G., Deutsch M., Fisher E.R., Jeong J.-H., Wolmark N. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N. Engl. J. Med. 2002;347:1233–1241. doi: 10.1056/nejmoa022152. [DOI] [PubMed] [Google Scholar]
- 30.De La Cruz L., Blankenship S.A., Chatterjee A., Geha R., Nocera N., Czerniecki B.J., Tchou J., Fisher C.S. Outcomes after oncoplastic breast-conserving surgery in breast cancer patients: a systematic literature review. Ann. Surg. Oncol. 2016;23:3247–3258. doi: 10.1245/s10434-016-5313-1. [DOI] [PubMed] [Google Scholar]
- 31.Ahmed M., Brace C.L., Lee F.T., Goldberg S.N. Principles of and advances in percutaneous ablation. Radiology. 2011;258:351–369. doi: 10.1148/radiol.10081634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chu K.F., Dupuy D.E. Thermal ablation of tumours: biological mechanisms and advances in therapy. Nat. Rev. Cancer. 2014;14:199–208. doi: 10.1038/nrc3672. [DOI] [PubMed] [Google Scholar]
- 33.Alexander E.S., Xiong L., Baird G.L., Fernando H., Dupuy D.E. Computed tomography densitometry and morphology of radiofrequency ablated Stage IA non-small cell lung cancer: results from the american college of surgeons oncology group Z4033 (Alliance) trial. J. Vasc. Inter. Radio. 2020;31:286–293. doi: 10.1016/j.jvir.2019.09.010.Computed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van de Voort E., Struik G., Birnie A., Moelker C., Verhoef C., Klem T. Thermal ablation as an alternative for surgical resection of small (</= 2 cm) breast cancers: a meta-analysis. Clin. Breast Cancer. 2021;21:e715–e730. doi: 10.1016/j.clbc.2021.03.004. [DOI] [PubMed] [Google Scholar]
- 35.Xia L.Y., Hu Q.L., Xu W.Y. Efficacy and safety of radiofrequency ablation for breast cancer smaller than 2 cm: a systematic review and meta-analysis. Front. Oncol. 2021;11:1–11. doi: 10.3389/fonc.2021.651646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jeffrey S. Radiofrequency ablation of breast cancer. Arch. Surg. 1999;134:1064. doi: 10.1001/archsurg.134.10.1064. [DOI] [PubMed] [Google Scholar]
- 37.Izzo F., Thomas R., Delrio P., Rinaldo M., Vallone P., DeChiara A., Botti G., D’Aiuto G., Cortino P., Curley S. Radiofrequency ablation in patients with primary breast carcinoma: a pilot study in 26 patients. Cancer. 2001;92:2036–2044. doi: 10.1002/1097-0142(20011015)92:8<2036::aid-cncr1542>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 38.Burak W.E., Agnese D.M., Povoski S.P., Yanssens T.L., Bloom K.J., Wakely P.E., Spigos D.G. Radiofrequency ablation of invasive breast carcinoma followed by delayed surgical excision. Cancer. 2003;98:1369–1376. doi: 10.1002/cncr.11642. [DOI] [PubMed] [Google Scholar]
- 39.Oura S., Tamaki T., Hirai I., Yoshimasu T., Ohta F., Nakamura R., Okamura Y. Radiofrequency ablation therapy in patients with breast cancers two centimeters or less in size. Breast Cancer. 2007;14:48–54. doi: 10.2325/jbcs.14.48. [DOI] [PubMed] [Google Scholar]
- 40.Yamamoto N., Fujimoto H., Nakamura R., Arai M., Yoshii A., Kaji S., Itami M. Pilot study of radiofrequency ablation therapy without surgical excision for T1 breast cancer: evaluation with MRI and vacuum-assisted core needle biopsy and safety management. Breast Cancer. 2011;18:3–9. doi: 10.1007/s12282-010-0197-6. [DOI] [PubMed] [Google Scholar]
- 41.Yoshinaga Y., Enomoto Y., Fujimitsu R., Shimakura M., Nabeshima K., Iwasaki A. Image and pathological changes after radiofrequency ablation of invasive breast cancer: a pilot study of nonsurgical therapy of early breast cancer. World J. Surg. 2013;37:356–363. doi: 10.1007/s00268-012-1820-9. [DOI] [PubMed] [Google Scholar]
- 42.García-Tejedor A., Guma A., Soler T., Valdivieso A., Petit A., Contreras N., Chappuis C.G., Falo C., Pernas S., Amselem A., Plà M.J., Fernández-Montolí E., Burdío F., Ponce J. Radiofrequency ablation followed by surgical excision versus lumpectomy for early stage breast cancer: a randomized phase II clinical trial. Radiology. 2018;289:317–324. doi: 10.1148/radiol.2018180235. [DOI] [PubMed] [Google Scholar]
- 43.Sabel M.S., Kaufman C.S., Whitworth P., Chang H., Stocks L.H., Simmons R., Schultz M. Cryoablation of early-stage breast cancer: work-in-progress report of a multi-institutional trial. Ann. Surg. Oncol. 2004;11:542–549. doi: 10.1245/ASO.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 44.Mauri G., Sconfienza L.M., Pescatori L.C., Fedeli M.P., Alì M., Di Leo G., Sardanelli F. Technical success, technique efficacy and complications of minimally-invasive imaging-guided percutaneous ablation procedures of breast cancer: a systematic review and meta-analysis. Eur. Radiol. 2017;27:3199–3210. doi: 10.1007/s00330-016-4668-9. [DOI] [PubMed] [Google Scholar]
- 45.Takada M., Toi M. Cryosurgery for primary breast cancers, its biological impact, and clinical outcomes. Int. J. Clin. Oncol. 2019;24:608–613. doi: 10.1007/s10147-019-01448-4. [DOI] [PubMed] [Google Scholar]
- 46.Lakoma A., Kim E.S. Minimally invasive surgical management of benign breast lesions. Gland Surg. 2014;3:142–148. doi: 10.3978/j.issn.2227-684X.2014.04.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nurko J., Mabry C., Whitworth P., Jarowenko D., Oetting L., Potruch T., Han L., Edwards M. Interim results from the fibroadenoma cryoablation treatment registry. Am. J. Surg. 2005;190:647–651. doi: 10.1016/j.amjsurg.2005.06.033. discussion 651-642. [DOI] [PubMed] [Google Scholar]
- 48.Zhang W., Jin Z.Q., Baikpour M., Li J.M., Zhang H., Liang T., Pan X.M., He W. Clinical application of ultrasound-guided percutaneous microwave ablation for benign breast lesions: A prospective study. BMC Cancer. 2019;19:1–10. doi: 10.1186/s12885-019-5523-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Livraghi T., Goldberg S.N., Solbiati L., Meloni F., Ierace T., Gazelle G.S. Percutaneous radio-frequency ablation of liver metastases from breast cancer: Initial experience in 24 patients. Radiology. 2001;220:145–149. doi: 10.1148/radiology.220.1.r01jl01145. [DOI] [PubMed] [Google Scholar]
- 50.Jakobs T., Hoffmann R., Schrader A., Stemmler H., Trumm C., Lubienski A., Murthy R., Helmberger T., Reiser M. CT-guided radiofrequency ablation in patients with hepatic metastases from breast cancer. Cardiovasc. Interv. Radiol. 2008;32:38–46. doi: 10.1007/s00270-008-9384-7. [DOI] [PubMed] [Google Scholar]
- 51.Meloni M.F., Andreano A., Laeseke P.F., Livraghi T., Sironi S., Lee F.T. Percutaneous, us-guided breast cancer liver metastases. Radiology. 2009;253:861–869. doi: 10.1148/radiol.2533081968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Barral M., Auperin A., Hakime A., Cartier V., Tacher V., Otmezguine Y., Tselikas L., de Baere T., Deschamps F. Percutaneous thermal ablation of breast cancer metastases in oligometastatic patients. Cardiovasc. Interv. Radiol. 2016;39:885–893. doi: 10.1007/s00270-016-1301-x. [DOI] [PubMed] [Google Scholar]
- 53.Li J., Wang D.D., Zhao Y.N., Zhou J.W., Tang J.H. Clinical assessment of magnetic resonance imaging-guided radiofrequency ablation for breast cancer. Mol. Clin. Oncol. 2019;11:411–415. doi: 10.3892/mco.2019.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ridouani F., Solomon S.B., Bryce Y., Bromberg J.F., Sofocleous C.T., Deipolyi A.R. Predictors of progression-free survival and local tumor control after percutaneous thermal ablation of oligometastatic breast cancer: retrospective study. J. Vasc. Interv. Radiol. 2020;31:1201–1209. doi: 10.1016/j.jvir.2020.02.016. [DOI] [PubMed] [Google Scholar]
- 55.M. Habibi, M. Kmieciak, L. Graham, J.K. Morales, D. Harry, Radiofrequency thermal ablation of breast tumors combined with intralesional administration of IL-7 and IL-15 augments anti-tumor immune responses and inhibits tumor development and metastasis, 114 (2009) 423–431. 10.1007/s10549-008-0024-3.Radiofrequency. [DOI] [PMC free article] [PubMed]
- 56.Sheybani N.D., Witter A.R., Thim E.A., Yagita H., Bullock T.N.J., Price R.J. Combination of thermally ablative focused ultrasound with gemcitabine controls breast cancer via adaptive immunity. J. Immunother. Cancer. 2020;8:1–15. doi: 10.1136/jitc-2020-001008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Www.Cancer.Org, (n.d.). 〈https://www.cancer.org/cancer/prostate-cancer/about/key-statistics.html〉 (accessed October 11, 2021).
- 58.Fletcher S.A., von Landenberg N., Cole A.P., Gild P., Choueiri T.K., Lipsitz S.R., Trinh Q.-D., Kibel A.S. Contemporary national trends in prostate cancer risk profile at diagnosis. Prostate Cancer Prostatic Dis. 2020;23:81–87. doi: 10.1038/s41391-019-0157-y. [DOI] [PubMed] [Google Scholar]
- 59.Sanda M.G., Dunn R.L., Michalski J., Sandler H.M., Northouse L., Hembroff L., Lin X., Greenfield T.K., Litwin M.S., Saigal C.S., Mahadevan A., Klein E., Kibel A., Pisters L.L., Kuban D., Kaplan I., Wood D., Ciezki J., Shah N., Wei J.T. Quality of life and satisfaction with outcome among prostate-cancer survivors. NEJM. 2008;358:1250–1261. doi: 10.1056/NEJMoa074311. [DOI] [PubMed] [Google Scholar]
- 60.Tyson M.D., Graves A.J., O’neil B., Barocas D.A., Chang S.S., Penson D.F., Resnick M.J. Urologist-level correlation in the use of observation for low-& high-risk prostate cancer. JAMA Surg. 2017;152:27–34. doi: 10.1001/jamasurg.2016.2907. [DOI] [PubMed] [Google Scholar]
- 61.Arredondo S.A., Downs T.M., Lubeck D.P., Pasta D.J., Silva S.J., Wallace K.L., Carroll P.R. Watchful waiting and health related quality of life for patients with localized prostate cancer: Data from CaPSURE. J. Urol. 2004;172:1830–1834. doi: 10.1097/01.ju.0000140758.04424.77. [DOI] [PubMed] [Google Scholar]
- 62.Pickles T., Ruether J.D., Weir L., Carlson L., Jakulj F., Hack T.F., Butler L., Degner L.F. Psychosocial barriers to active surveillance for the management of early prostate cancer and a strategy for increased acceptance. BJU Int. 2007;100:544–551. doi: 10.1111/j.1464-410X.2007.06981.x. [DOI] [PubMed] [Google Scholar]
- 63.Lindner U., Weersink R.A., Haider M.A., Gertner M.R., Davidson S.R.H., Atri M., Wilson B.C., Fenster A., Trachtenberg J. Image guided photothermal focal therapy for localized prostate cancer: phase I trial. J. Urol. 2009;182:1371–1377. doi: 10.1016/j.juro.2009.06.035. [DOI] [PubMed] [Google Scholar]
- 64.Oto A., Sethi I., Karczmar G., McNichols R., Ivancevic M.K., Stadler W.M., Watson S., Eggener S. MR imaging-guided focal laser ablation for prostate cancer: phase I trial. Radiology. 2013;267:932–940. doi: 10.1148/radiol.13121652. [DOI] [PubMed] [Google Scholar]
- 65.Feller J., Greenwood B., Jones W., Toth R., Gunberg S., Herz J., Wells I. Outpatient trans-rectal MR-guided laser focal therapy phase II clinical trial: ten-year interim results. J. Urol. 2020;203 [Google Scholar]
- 66.Zhou X., Jin K., Qiu S., Jin D., Liao X., Tu X., Zheng X., Li J., Yang L., Wei Q. Comparative effectiveness of radiotherapy versus focal laser ablation in patients with low and intermediate risk localized prostate cancer. Sci. Rep. 2020;10:1–8. doi: 10.1038/s41598-020-65863-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sanda M.G., Cadeddu J.A., Kirkby E., Chen R.C., Crispino T., Fontanarosa J., Freedland S.J., Greene K., Klotz L.H., Makarov D.V., Nelson J.B., Rodrigues G., Sandler H.M., Taplin M.E., Treadwell J.R. Clinically localized prostate cancer: AUA/ASTRO/SUO guideline. J. Urol. 2017;199:683–690. doi: 10.1016/j.juro.2017.11.095. [DOI] [PubMed] [Google Scholar]
- 68.Cruz J., Wishart D. Applications of machine learning in cancer prediction and prognosis. Cancer Inf. 2007;2:59–77. [PMC free article] [PubMed] [Google Scholar]
- 69.Letzen B., Wang C., Chapiro J. The role of artificial intelligence in interventional oncology: a primer. J. Vasc. Interv. Radiol. 2019;30:38–41. doi: 10.1016/j.jvir.2018.08.032. [DOI] [PubMed] [Google Scholar]
- 70.Kriegeskorte N. Deep neural networks: a new framework for modeling biological vision and brain information processing. Annu. Rev. Vis. Sci. 2015;1:417–446. doi: 10.1146/annurev-vision-082114-035447. [DOI] [PubMed] [Google Scholar]
- 71.Antonelli M., Johnston E.W., Dikaios N., Cheung K.K., Sidhu H.S., Appayya M.B., Giganti F., Simmons L.A.M., Freeman A., Allen C., Ahmed H.U., Atkinson D., Ourselin S., Punwani S. Correction to: Machine learning classifiers can predict Gleason pattern 4 prostate cancer with greater accuracy than experienced radiologists (European Radiology, (2019), 29, 9, (4754-4764), 10.1007/s00330-019-06244-2) Eur. Radiol. 2020;30:1295. doi: 10.1007/s00330-019-06429-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cui S., Ming S., Lin Y., Chen F., Shen Q., Li H., Chen G., Gong X., Wang H. Development and clinical application of deep learning model for lung nodules screening on CT images. Sci. Rep. 2020;10:1–10. doi: 10.1038/s41598-020-70629-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Izumoto H., Hiraoka A., Ishimaru Y., Murakami T., Kitahata S., Ueki H., Aibiki T., Okudaira T., Miyamoto Y., Yamago H., Iwasaki R., Tomida H., Mori K., Kishida M., Tsubouchi E., Miyata H., Ninomiya T., Kawasaki H., Hirooka M., Matsuura B., Abe M., Hiasa Y., Michitaka K., Kudo M. Validation of newly proposed time to transarterial chemoembolization progression in intermediate-stage hepatocellular carcinoma cases. Oncol. 2017;93:120–126. doi: 10.1159/000481242. [DOI] [PubMed] [Google Scholar]
- 74.Morshid A., Elsayes K.M., Khalaf A.M., Elmohr M.M., Yu J., Kaseb A.O., Hassan M., Mahvash A., Wang Z., Hazle J.D., Fuentes D. A machine learning model to predict hepatocellular carcinoma response to transcatheter arterial chemoembolization. Radiol. Artif. Intell. 2019;1 doi: 10.1148/ryai.2019180021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Liu Y., Chen X., Wang Z., Jane Wang Z., Ward R., Wang X. Deep learning for pixel-level image fusion: recent advances and future prospects. Inf. Fusion. 2018;42:158–173. [Google Scholar]
- 76.Moore C., Robertson N., Arsanious N., Middleton T., Villers A., Klotz L., Taneja S., Emberton M. Image-guided prostate biopsy using magnetic resonance imaging–derived targets: a systematic review. Eur. Urol. 2013;63:125–140. doi: 10.1016/j.eururo.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 77.Li X., Young A., Raman S., Lu D., Lee Y., Ysao T., Wu H. Automatic needle tracking using Mask R-CNN for MRI-guided percutaneous interventions. Int J. Comput. Assist Radio. Surg. 2020;15:1673–1684. doi: 10.1007/s11548-020-02226-8. [DOI] [PubMed] [Google Scholar]
- 78.Mwikirize C., Nosher J., Hacihaliloglu I. Convolution neural networks for real-time needle detection and localization in 2D ultrasound. Int J. Comput. Assist Radio. Surg. 2018;13:647–657. doi: 10.1007/s11548-018-1721-y. [DOI] [PubMed] [Google Scholar]