Abstract
C-peptide declines in type 1 diabetes, although many long-duration patients retain low, but detectable levels. Histological analyses confirm that β-cells can remain following type 1 diabetes onset. We explored the trends observed in C-peptide decline in the UK Genetic Resource Investigating Diabetes (UK GRID) cohort (N = 4,079), with β-cell loss in pancreas donors from the network for Pancreatic Organ donors with Diabetes (nPOD) biobank and the Exeter Archival Diabetes Biobank (EADB) (combined N = 235), stratified by recently reported age at diagnosis endotypes (<7, 7–12, ≥13 years) across increasing diabetes durations. The proportion of individuals with detectable C-peptide declined beyond the first year after diagnosis, but this was most marked in the youngest age group (<1-year duration: age <7 years: 18 of 20 [90%], 7–12 years: 107 of 110 [97%], ≥13 years: 58 of 61 [95%] vs. 1–5 years postdiagnosis: <7 years: 172 of 522 [33%], 7–12 years: 604 of 995 [61%], ≥13 years: 225 of 289 [78%]). A similar profile was observed in β-cell loss, with those diagnosed at younger ages experiencing more rapid loss of islets containing insulin-positive (insulin+) β-cells <1 year postdiagnosis: age <7 years: 23 of 26 (88%), 7–12 years: 32 of 33 (97%), ≥13 years: 22 of 25 (88%) vs. 1–5 years postdiagnosis: <7 years: 1 of 12 (8.3%), 7–12 years: 7 of 13 (54%), ≥13 years: 7 of 8 (88%). These data should be considered in the planning and interpretation of intervention trials designed to promote β-cell retention and function.
Introduction
Circulating C-peptide, a marker of endogenous insulin secretion from pancreatic β-cells, is known to decline following a diagnosis of type 1 diabetes, but can persist for many years (1–8). It is frequently observed, however, that those diagnosed at the youngest ages have lower levels of C-peptide at diagnosis (2,3,5,6,9). Histological analyses of donor pancreata provide evidence for persistent immunoreactive insulin-positive (insulin+) β-cells; sometimes for many years after diagnosis (7,8,10,11). These findings challenge the dogma that all β-cells are destroyed at the onset of type 1 diabetes or soon after. The center piece of many disease-modifying intervention trials is to augment the survival of these residual β-cells, assessed via measures of preserved C-peptide secretion. However, currently there is little understanding of how C-peptide levels relate to absolute β-cell mass, as residual C-peptide alone cannot distinguish between loss of β-cell mass and reduced functionality. It is known that there are clear differences in disease progression between children and adults (3,5), but few studies have explored how this progression varies within children, particularly young children diagnosed <7 years compared with those diagnosed around puberty (at or >13 years) (6,11). In this study, we questioned whether trends of C-peptide decline observed in children and young people with type 1 diabetes from the UK Genetic Resource Investigating Diabetes (UK GRID) cohort were similar to trends of β-cell loss in pancreatic donors from the Network of Pancreatic Organ Donors (nPOD) and the Exeter Archival Diabetes Biobank (EADB), across wide ranges of age at diagnosis and durations.
Research Design and Methods
Three independent resources were used to assess C-peptide levels in the plasma and β-cell loss within the pancreas, respectively: 1) plasma samples from the GRID collection (12), and 2) type 1 diabetes pancreas samples from the EADB (11,13) and nPOD biobank (14). We stratified subjects by age at diagnosis (<7, 7–12, ≥13 years) (6), and grouped them by diabetes duration (<1, 1–5, 5–10, ≥10 years).
We report the proportion of individuals from the GRID collection with detectable C-peptide (>9 pmol/L) and distribution of these levels. We report the proportion of donors from the combined EADB and nPOD biobanks, retaining islets containing insulin+ β-cells and distribution of β-cell area, expressed as insulin+ area with respect to the sum of insulin+ and glucagon+ area.
Study Cohorts
We analyzed 4,079 random nonfasting plasma C-peptide measurements from people with clinically defined type 1 diabetes (on insulin from diagnosis) from the GRID collection, diagnosed ≤16 years (12), and 235 native pancreas samples recovered from people with type 1 diabetes, diagnosed <18 years, from the nPOD biobank (n = 111) and EADB (n = 124) (Table 1 and Supplementary Table 1). Histopathology notes and slide digitization were available through nPOD, as previously described (14).
Table 1.
Characteristics of the UK GRID cohort and the cohort from combined EADB and nPOD pancreas biobanks
| UK GRID | EADB (n = 124) and nPOD (n = 111) | |
|---|---|---|
| N = 4,079 | N = 235 | |
| Age (years) | 13 (10; 16) | 15 (10; 22) |
| Diabetes duration (years) | 5 (2; 8) | 5 (0.08; 12) |
| Age at diagnosis (years) | 8 (4; 11) | 8 (4.9; 13) |
| Male sex | 2149* (52.7) | 102 (43.4) |
| C-peptide (pmol/L) | <9‡ (<9‡; 31) | 16.4†‡ (16.4†‡; 16.4†‡) |
| Donors with islets containing insulin+ β-cells | ||
| None | — | 115 (48.1) |
| Present | — | 120 (51.9) |
Data are presented as median (25th; 75th) or as n (%).
Missing data, n = 2.
nPOD only, n = 109.
Limit of detection for UK GRID: 9 pmol/L, and for nPOD: 16.4 pmol/L.
Histological Analyses
We studied 235 (nontransplant) donors with type 1 diabetes diagnosed <18 years from the combined nPOD and EADB biobanks with native pancreas available or complete nPOD online pathology and age-at-disease diagnosis information. We examined pancreas sections using digitized slides via the nPOD online pathology database or pancreas material, which was stained for the presence of insulin and/or glucagon using standard immunohistochemical approaches (14). Sections were double-stained for insulin/glucagon, or serial sections were stained for insulin and glucagon, respectively, where alignment of the two stains allowed for identification of the insulin-negative (insulin−) islets. We defined type 1 diabetes histologically by the lobular loss of islets containing insulin+ β-cells with the presence of multiple (>10) insulin− islets in the section(s) studied. Insulin+ and insulin− islet counts were completed by light microscopy or using high-resolution digitized slides (via the Vectra Polaris Automated Quantitative Pathology Imagining system [Akoya]) when appropriately stained sections were available. In some (n = 12) older samples from the EADB collection, islet count information was collated from historical studies (6,11,13,15). For light microscopy, the total number of islets was quantified using the glucagon-stained section with the number of islets with residual β-cells assessed using the serially stained insulin section. Islets in such slides were defined as comprising >10 insulin+ and/or glucagon+ cells. When digitized slides were available, islets were identified using the random forest tissue classifier module of HALO V3.0 image analysis software (Indica Laboratories) and assessed for insulin positivity. Islets in slides assessed by the HALO V3.0 image analysis software were defined as groups of endocrine cells covering an area of ≥1,000 µm2. We identified 120 donors with islets containing insulin+ β-cells from collated recent and historical analyses and expressed the proportions of donors with islets containing insulin+ β-cells across diabetes duration, stratified by age at diagnosis.
Of the 120 donors with islets containing insulin+ β-cells, 100 had slides of appropriate quality available for digitization. The Random Forest Classifier Module (version 3.2.1851.354) was applied to tissue double-stained for insulin/glucagon or DenseNet AI V2 modules on serial single-stained tissue, within the Indica Laboratories HALO Image analysis platform (version 3.2.1851.354), to identify insulin+ area and glucagon+ area for the sections per donor analyzed across a total of 38,322 identified islets. We define insulin+ area relative to the sum of the insulin+ and glucagon+ area in the total section as β-cell area with respect to total islet area. We assumed that the insulin+ and glucagon+ areas represent islet area. We report the distribution of β-cell area for these 100 donors across diabetes duration stratified by age at diagnosis.
In an additional subanalysis, we selected 87 donors from nPOD that had been processed using the HALO Image analysis platform to identify β-cell area and who had random C-peptide measurements taken at the time of organ donation, without documented renal disease/failure or on dialysis, to assess whether those with detectable C-peptide also had islets containing insulin+ β-cells.
C-Peptide Measurement
Plasma was obtained from 5,565 nonfasted blood samples from UK GRID patients, collected using the anticoagulant acid citrate dextrose. Samples were excluded with C-peptide >500 pmol/L (n = 75), if time from blood draw to freeze >72 h (n = 1,378), or data were incomplete (n = 33). Samples were stored at −80°C. C-peptide was measured using the DiaSorin Liaison C-peptide kit insert (product 316171, issued 24 February 2012), where the lower limit of the assay is 9 pmol/L, with a coefficient of variation of <20%. C-peptide levels in nPOD donors were measured as described (14). Owing to the variable limits of detection of C-peptide in nPOD, we chose the minimum limit of detection (16.4 pmol/L) as the limit of detection for nPOD C-peptide in our subanalysis, where detectable C-peptide is defined as ≥16.4 pmol/L. C-peptide levels are reported in pmol/L (1,000 pmol/L = 3 ng/mL).
Ethics Statement
All procedures in nPOD were in accordance with federal guidelines for organ donation and the University of Florida Institutional Review Board. All EADB samples were used with ethical permission from the West of Scotland Research Ethics Committee, Paisley, U.K. (ref: 20/WS/0074, IRAS project ID: 283620). Plasma samples were obtained from the Genetics Resource Investigating Diabetes or GRID Study (Rec Reference 00/5/44), which encompassed the UK Nephropathy Family Study (Rec Reference 00/5/65).
Data and Resource Availability
Further information about the data is available from the corresponding author upon request.
Results
Patterns of β-Cell Loss Mirror Patterns of C-Peptide Decline in Children and Young People
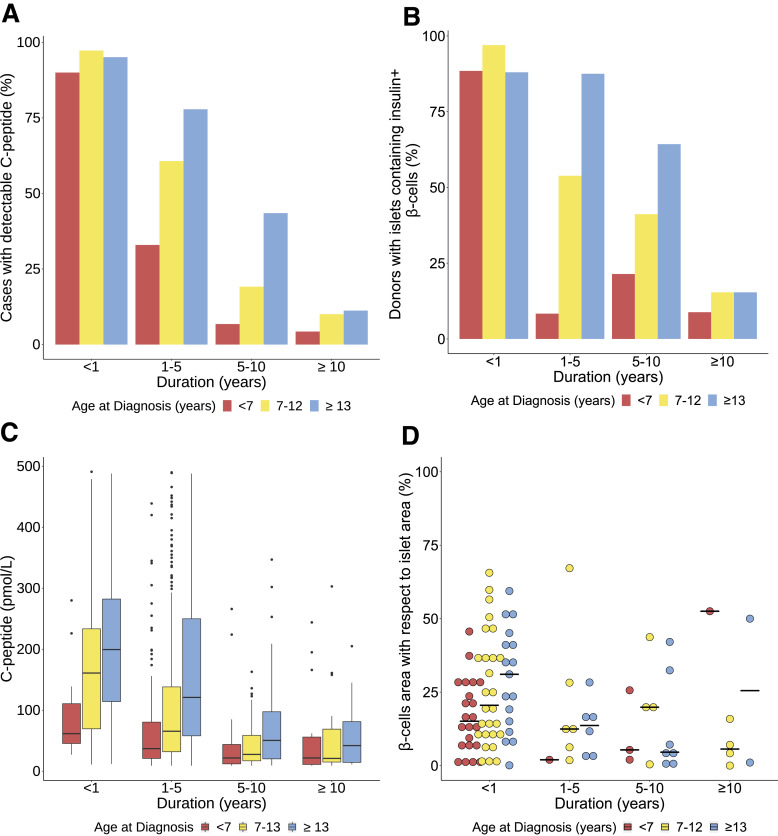
C-peptide levels were detectable in some individuals across all age-at-diagnosis groups and diabetes durations. This was least common in those diagnosed <7 years (detectable C-peptide: age <7 years: 254 of 1,666 [15%], 7–12 years: 838 of 1,887 [44%], ≥13 years: 325 of 526 [62%]) (Fig. 1A, Supplementary Table 2). In all age-at-diagnosis groups, the number of individuals with detectable C-peptide declined beyond the first year after diagnosis, but this trend was most marked in those diagnosed at younger ages (detectable C-peptide <1-year duration: age <7 years: 18 of 20 [90%], 7–12 years: 107 of 110 [97%], ≥13 years: 58 of 61 [95%] vs. detectable C-peptide 1–5 years postdiagnosis: <7 years: 172 of 522 [33%], 7–12 years: 604 of 995 (61%), ≥13 years: 225 of 289 [78%]).
Figure 1.
Comparison of proportions of individuals with detectable C-peptide (n = 1,417 of 4,079) (A), proportions of donors retaining islets containing insulin+ β-cells (n = 120 of 235) (B), absolute levels of detectable C-peptide (n = 1,417) (C), and within-donor β-cell area, expressed as insulin+ area relative to the sum of the insulin+ and glucagon+ area (n = 100) (D) stratified by age at diagnosis (<7, 7–12, ≥13 years) and grouped by diabetes duration (<1, 1–5, 5–10, ≥10 years). Lines in the center represent median in C and D. Top and bottom borders of the box represent 75th and 25th quartiles, respectively, and whiskers represent range (C). Proportions of donors with detectable C-peptide from UK GRID cohort (A) and donors with insulin+ β-cells from nPOD and EADB (B) are outlined in more detail in Supplementary Table 2. A summary of donors with an available β-cell area (D) is outlined in Supplementary Table 3.
Across all diabetes durations, similar trends were observed in the proportions of individuals retaining islets containing insulin+ β-cells in the sections of pancreas studied. Although present in all groups irrespective of age at diagnosis or disease duration, fewer individuals diagnosed <7 years retained islets containing insulin+ β-cells (retaining islets containing insulin+ β-cells: < 7 years: 30 of 86 [35%], 7–12 years: 50 of 89 [56%], ≥13 years: 41 of 61 [67%]) (Fig. 1B and Supplementary Table 2). There was a more precipitous drop-off in the number of individuals retaining islets containing insulin+ β-cells post 1-year diagnosis in those diagnosed at younger ages compared with those diagnosed older (retaining islets containing insulin+ β-cells <1 year postdiagnosis: age <7 years: 23 of 26 [88%], 7–12 years: 32 of 33 [97%], ≥13 years: 22 of 25 [88%] vs. retaining islets containing insulin+ β-cells 1–5 years postdiagnosis: <7 years: 1 of 12 [8.3%], 7–12 years: 7 of 13 (54%), ≥13 years: 7 of 8 [88%]).
The absolute levels of detectable C-peptide declined in all age groups across all diabetes durations (Fig. 1C), and this mirrored the decline in β-cell area (as a fraction of insulin+ and glucagon+ area), across the groups (Fig. 1D and Supplementary Table 3).
Children Diagnosed <7 Years Had Lower Absolute Levels of C-Peptide and Fewer Insulin+ β-Cells Close to Diagnosis
C-peptide decreased in all age groups over time (Supplementary Table 4). In those with detectable levels, C-peptide was markedly lower soon after diagnosis in children diagnosed <7 years compared with those diagnosed ≥13 years (<1 year postdiagnosis: median [interquartile range] <7 years: 61.5 [45.4–110.8] pmol/L vs. ≥13 years: 199.5 [114.3–282.3] pmol/L; P = 1 × 10−4) (Fig. 1C). Similarly, among children diagnosed <7 years who retained islets containing insulin+ β-cells close to diagnosis, as judged by β-cell area, was lower (<1 year postdiagnosis: median [interquartile range] <7 years: 15% [6.7–27] vs. ≥13 years: 31% [12–42]; P = 0.025) (Fig. 1D and Supplementary Table 3). This compares with a median β-cell area of 70.4% (64.0–79.1) in 44 <18-year donors without diabetes (median age of donors 9 years [4.6–12.9]).
Approximately 5% of Children Diagnosed <7 Years Retained Detectable C-Peptide 10 Years Postdiagnosis
Across all age-groups, a proportion of children retained C-peptide >10 years postdiagnosis, and a similar proportion retained islets containing insulin+ β-cells over this time (Fig. 1A and Supplementary Table 2). In long-duration disease (≥10 years), children originally diagnosed <7 years were more likely to be insulin deficient at the time of organ donation than those who were older at diagnosis (detectable C-peptide ≥10 years postdiagnosis: <7 years: 21 of 489 [4.3%], 7–12 years: 25 of 249 [10%], ≥13 years: 12 of 107 [11%]), and they were also less likely to retain islets with insulin+ β-cells (retaining islets containing insulin+ β-cells ≥10 years postdiagnosis: <7 years: 3 of 34 [8.8%], 7–12 years: 4 of 26 [15%], ≥13 years: 2 of 13 [15%]) (Fig. 1B and Supplementary Table 2).
In nPOD Pancreas Donors With Detectable C-Peptide, the Majority Also Had Presence of Insulin+ Islets
Among a subset of nPOD donors (n = 87), 17 had detectable C-peptide, with 13 of these donors (76%) having presence of insulin+ β-cells, as determined by a >0% β-cell area (Supplementary Table 5). There was a significant difference in the presence or absence of insulin+ islets between the detectable/undetectable C-peptide groups (81.6% agreement, P = 1.5 × 10−6). The characteristics of four donors with detectable C-peptide but with no insulin+ β-cells in sections analyzed are outlined in Supplementary Table 6. In these four donors, the C-peptide level was low (<100 pmol/L), and in two of the donors, the histopathology notes state that in some curated sections, islets containing insulin+ β-cells were seen but were rare (Supplementary Table 6).
Discussion
We report that trends in C-peptide decline in living children and young people with type 1 diabetes are similar to the trends of loss of islets containing insulin+ β-cells within sections of donor pancreata, across all ages and disease durations. Our results support the proposition that C-peptide levels are a reliable, inexpensive, and practical marker of retention of islets containing insulin+ β-cells in children and young adults with type 1 diabetes. Our results are consistent with those of other studies showing higher C-peptide levels in people diagnosed at older ages, but decline over time (2,3,5,9). Our study also supports the findings of Aida et al. (16), who demonstrated a significant correlation between β-cell volume and fasting serum C-peptide levels in Japanese patients with adult-onset type 1 diabetes. Our study is the first to provide a comparison of pancreatic histology with an independent clinical cohort, examining patterns of C-peptide loss according to age at diagnosis and duration in children with type 1 diabetes. Our study is also the largest to assess such disease progression trends in very young children (<7 years). We demonstrate that compared with those who are older at diagnosis, children diagnosed <7 years progress more rapidly toward total C-peptide loss and have minimal β-cell retention.
These data confirm that trialing a safe immunotherapy close to diagnosis to inhibit or halt the autoimmune destruction, as in recent clinical trials (17), is worthwhile to preserve pancreatic mass. The rapid depletion of C-peptide and β-cells in children diagnosed <7 years, when comparing <1 years’ and 1–5 years’ duration, emphasizes that early intervention close to (or before) diagnosis may be most time critical in those progressing to disease in very early life. Our results highlight that among children, there are differences in progression that should be considered in the planning and interpretation of intervention trials designed to promote β-cell retention and function.
We find that a small proportion of children retain some residual β-cells over >10 years’ duration and that a similar proportion retain C-peptide over this period. These proportions do not change markedly between disease durations of 5 or 10 years, in keeping with the concept that there are two phases of C-peptide decline: a rapid fall in the first 7 years after diagnosis, followed by a more stable phase (2).Our results are likely to be an underestimate given a higher limit of detection of C-peptide (9 pmol/L) compared with contemporary assays (2). It must be noted that the UK GRID cohort included only those individuals with type 1 diabetes, and as such, in this study we do not have access to a population without type 1 diabetes for comparison of C-peptide levels. However, it is well established that levels of residual C-peptide in long-duration type 1 diabetes are low and detectable using ultrasensitive assays (18,19).
We acknowledge that in histological analyses, we were not able to assess β-cell area for all 120 donors with islets containing insulin+ β-cells, calculating this for 100 such samples. Of the 20 samples in which we were unable to calculate β-cell area, 12 were derived from the EADB biobank, a 50-year-old archival biobank mainly comprising nonsystematically collected autopsy samples from younger children very close to diagnosis. We were unable to include these archival sections due to deterioration of glass slides/staining intensity, which impacted on scan quality, and the rarity of material available from these donors precluded restaining. In addition, we must emphasize that the SE around the proportion estimates in the histological analyses are large, as influenced by the sample numbers. We also acknowledge that there is little information on the anatomical location of the sampled pancreas in the histological analyses of the EADB donors. However, as sampling was random across the 235 donors, we think it is very unlikely that systematic sampling bias might explain our observations.
A further limitation of this study is its cross-sectional design and the dissociated biobanks used. Extensive, within-donor analysis is difficult in this setting, since there are no large systematic studies of C-peptide in clinical cohorts with type 1 diabetes in whom postdeath pancreas samples are available. Despite this, we are able to demonstrate that 81% (17 of 21) of donors from the nPOD biobank with detectable C-peptide also had islets containing insulin+ β-cells in the sections studied. Four donors had detectable C-peptide and no islets containing insulin+ β-cells in the sections we were able to assess. It is reasonable to assume that due to the nature of sampling, such islets could be present elsewhere in the pancreas. This is illustrated in two of the four donors studied, since the histopathology reports held by nPOD describe rare islets containing insulin+ β-cells in the sections they curate. In addition, it should be noted that C-peptide levels in nPOD organ donors may be influenced by end-of-life circumstances and must therefore be interpreted with caution. In donors with undetectable C-peptide but who retain insulin+ β-cells, acute glucotoxicity (20) and sample degradation may have contributed to false-negative C-peptide results. Additionally, we accept that limited clinical data were available and that, in particular, no information was accessible on rates of diabetic ketoacidosis in the UK GRID cohort, which is known to be an independent predictor of C-peptide decline (20).
Despite these caveats, our data suggest that progressive loss of β-cells is the main contributory factor to the decline in endogenous insulin secretion observed in children and young people diagnosed with type 1 diabetes. Our results add weight to the proposal that intervention trials should be powered separately for each age-at-diagnosis group and highlight that consideration of age at diagnosis is very important in the interpretation of outcomes. Interventions that delay diagnosis in “at-risk” individuals are likely to improve clinical outcomes by promoting the retention of β-cells and maintaining a higher C-peptide secretion rate.
Article Information
Acknowledgments. The authors would like to thank Dr. Irina Kusmartseva and Maria Beery (Department of Pathology, Immunology and Laboratory Medicine, University of Florida Diabetes Institute, Gainesville, FL) for assistance in providing nPOD images for analysis.
Funding. The research was supported by the National Institute for Health Research (NIHR) Oxford Biomedical Research Centre (BRC), through funding of R.E.J.B. R.E.J.B. is also funded through the JDRF/Wellcome’s Strategic Award. The study received financial support from Diabetes UK via project grant 16/0005480 (P.L., N.G.M., S.J.R.) and Diabetes UK Harry Keen Fellowship (16/0005529 to R.A.O.), and from a JDRF Career Development Award (5-CDA-2014-221-A-N). This research was performed with the support of the Network for Pancreatic Organ donors with Diabetes (nPOD; RRID:SCR_014641), a collaborative type 1 diabetes research project sponsored by JDRF (nPOD: 5-SRA-2018-557-Q-R) and The Leona M. & Harry B. Helmsley Charitable Trust (grant no. 2018PG-T1D053), a strategic award to the Diabetes and Inflammation Laboratory from the JDRF (4-SRA-2017-473-A-A), and Wellcome (107212/A/15/Z). Organ Procurement Organizations (OPO) partnering with nPOD to provide research resources are listed at https://www.jdrfnpod.org/for-partners/npod-partners/.
The content and views expressed are those of the author(s) and not necessarily those of the National Health Service, the NIHR, and the Department of Health, and do not necessarily reflect the official view of nPOD.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. A.L.J.C. performed analysis of histological data and wrote the first draft. A.L.J.C., J.R.J.I., S.J.R., and R.E.J.B. designed the study. A.L.J.C., C.S.F., P.L., R.C.W., L.A.R., M.P., D.P., T.W., B.H., and S.J.R. performed histological assessments and analysis. J.R.J.I. and R.E.J.B. performed analysis of C-peptide data. L.S.W., D.B.D., and J.A.T., provided access to the data from the GRID study and contributed to scientific discussion. R.A.O. and N.G.M. helped with data interpretation and revision of manuscript. All authors reviewed analysis and reviewed and contributed to final draft. R.E.J.B. and S.J.R. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in abstract form at the 56th Annual Meeting of the European Association for the Study of Diabetes Conference, virtual meeting, 21–25 September 2020, and at the 13th Annual Scientific Meeting of the Network for Pancreatic Organ Donors Conference, virtual meeting, 22–24 February 2021.
Footnotes
A.L.J.C. and J.R.J.I. share first authorship. S.J.R. and R.E.J.B. share senior authorship.
This article contains supplementary material online at https://doi.org/10.2337/figshare.19610127.
References
- 1. Dabelea D, Mayer-Davis EJ, Andrews JS, et al. Clinical evolution of beta cell function in youth with diabetes: the SEARCH for Diabetes in Youth study. Diabetologia 2012;55:3359–3368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Shields BM, McDonald TJ, Oram R, et al. C-peptide decline in type 1 diabetes has two phases: an initial exponential fall and a subsequent stable phase. Diabetes Care 2018;41:1486–1492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Davis AK, DuBose SN, Haller MJ, et al. Prevalence of detectable C-peptide according to age at diagnosis and duration of type 1 diabetes. Diabetes Care 2015;38:476–481 [DOI] [PubMed] [Google Scholar]
- 4. Hao W, Gitelman S, DiMeglio LA, Boulware D, Greenbaum CJ. Fall in C-peptide during first 4 years from diagnosis of type 1 diabetes: variable relation to age, HbA1c, and insulin dose. Diabetes Care 2016;39:1664–1670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Greenbaum CJ, Beam CA, Boulware D, et al. Fall in C-peptide during first 2 years from diagnosis: evidence of at least two distinct phases from composite type 1 Diabetes TrialNet Data. Diabetes 2012;61:2066–2073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Leete P, Oram RA, McDonald TJ, et al. Studies of insulin and proinsulin in pancreas and serum support the existence of aetiopathological endotypes of type 1 diabetes associated with age at diagnosis. Diabetologia 2020;63:1258–1267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Keenan HA, Sun JK, Levine J, et al. Residual insulin production and pancreatic β-cell turnover after 50 years of diabetes: Joslin Medalist Study. Diabetes 2010;59:2846–2853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yu MG, Keenan HA, Shah HS, et al. Residual β cell function and monogenic variants in long-duration type 1 diabetes patients. J Clin Invest 2019;129:3252–3263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Besser REJ, Shields BM, Casas R, Hattersley AT, Ludvigsson J. Lessons from the mixed-meal tolerance test. Diabetes Care 2013;36:195–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rodriguez-Calvo T, Richardson SJ, Pugliese A. Pancreas pathology during the natural history of type 1 diabetes. Curr Diab Rep 2018;18:124. [DOI] [PubMed] [Google Scholar]
- 11. Leete P, Willcox A, Krogvold L, et al. Differential insulitic profiles determine the extent of β-cell destruction and the age at onset of type 1 diabetes. Diabetes 2016;65:1362–1369 [DOI] [PubMed] [Google Scholar]
- 12. Barrett JC, Clayton DG, Concannon P, et al. Genome-wide association study and meta-analysis find that over 40 loci affect risk of type 1 diabetes. Nat Genet 2009;41:703–707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Foulis AK, Liddle CN, Farquharson MA, Richmond JA, Weir RS. The histopathology of the pancreas in type 1 (insulin-dependent) diabetes mellitus: a 25-year review of deaths in patients under 20 years of age in the United Kingdom. Diabetologia 1986;29:267–274 [DOI] [PubMed] [Google Scholar]
- 14. Campbell-Thompson M, Wasserfall C, Kaddis J, et al. Network for Pancreatic Organ Donors with Diabetes (nPOD): developing a tissue biobank for type 1 diabetes. Diabetes Metab Res Rev 2012;28:608–617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Willcox A, Richardson SJ, Bone AJ, Foulis AK, Morgan NG. Analysis of islet inflammation in human type 1 diabetes. Clin Exp Immunol 2009;155:173–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Aida K, Fukui T, Jimbo E, et al. Distinct inflammatory changes of the pancreas of slowly progressive insulin-dependent (type 1) diabetes. Pancreas 2018;47:1101–1109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Herold KC, Bundy BN, Long SA, et al. An anti-CD3 antibody, teplizumab, in relatives at risk for type 1 diabetes. N Engl J Med 2019;381:603–613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Oram RA, Jones AG, Besser REJ, et al. The majority of patients with long-duration type 1 diabetes are insulin microsecretors and have functioning beta cells. Diabetologia 2014;57:187–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Oram RA, McDonald TJ, Shields BM, et al. Most people with long-duration type 1 diabetes in a large population-based study are insulin microsecretors. Diabetes Care 2015;38:323–328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Komulainen J, Lounamaa R, Knip M, Kaprio EA, Akerblom HK. Ketoacidosis at the diagnosis of type 1 (insulin dependent) diabetes mellitus is related to poor residual beta cell function. Childhood Diabetes in Finland Study Group. Arch Dis Child 1996;75:410–415 [DOI] [PMC free article] [PubMed] [Google Scholar]