Highlights
-
•
We reviewed 12 studies that measured metabolites pre and post rTMS in MDD.
-
•
Frontal lobe Glu, Gln, NAA, and GABA increased after rTMS.
-
•
Increases in metabolites were often associated with MDD symptom improvement.
-
•
We propose novel intracellular mechanisms by which metabolites are altered by rTMS.
Keywords: Repetitive transcranial magnetic stimulation, Magnetic resonance spectroscopy, Major depressive disorder, Treatment resistant depression, GABA, Glutamate, Glx, Glutamine, NAA, Intracellular mechanisms, Neuroimaging
Abstract
Introduction
Repetitive Transcranial magnetic stimulation (rTMS) is an FDA approved treatment for major depressive disorder (MDD). However, neural mechanisms contributing to rTMS effects on depressive symptoms, cognition, and behavior are unclear. Proton magnetic resonance spectroscopy (MRS), a noninvasive neuroimaging technique measuring concentrations of biochemical compounds within the brain in vivo, may provide mechanistic insights.
Methods
This systematic review summarized published MRS findings from rTMS treatment trials to address potential neurometabolic mechanisms of its antidepressant action. Using PubMed, Google Scholar, Web of Science, and JSTOR, we identified twelve empirical studies that evaluated changes in MRS metabolites in a within-subjects, pre- vs. post-rTMS treatment design in patients with MDD.
Results
rTMS protocols ranged from four days to eight weeks duration, were applied at high frequency to the left dorsolateral prefrontal cortex (DLPFC) in most studies, and were conducted in patients aged 13-to-70. Most studies utilized MRS point resolved spectroscopy acquisitions at 3 Tesla in the bilateral anterior cingulate cortex and DLPFC. Symptom improvements were correlated with rTMS-related increases in the concentration of glutamatergic compounds (glutamate, Glu, and glutamine, Gln), GABA, and N-acetylated compounds (NAA), with some results trend-level.
Conclusions
This is the first in-depth systematic review of metabolic effects of rTMS in individuals with MDD. The extant literature suggests rTMS stimulation does not produce changes in neurometabolites independent of clinical response; increases in frontal lobe glutamatergic compounds, N-acetylated compounds and GABA following high frequency left DLPFC rTMS therapy were generally associated with clinical improvement. Glu, Gln, GABA, and NAA may mediate rTMS treatment effects on MDD symptomatology through intracellular mechanisms.
1. Introduction
Major depressive disorder (MDD) is the leading cause of medical disability worldwide, affecting approximately 322 million individuals per year, or four to six percent of the total global population (Murray et al., 2013, Lim et al., 2018, Friedrich, 2017, Bromet et al., 2011). MDD is characterized by persistent depressed mood, anhedonia, feelings of worthlessness, indecisiveness, behavioral dysfunction, and suicidal ideation (Otte et al., 2016). These symptoms place a substantial burden on individuals, families, national healthcare systems and the global economy (Wang et al., 2003). Sustained remission of depressive symptoms is currently considered the optimal outcome for clinical management and treatment (Reesal and Lam, 2001). Though gold standard antidepressant pharmacotherapy and psychotherapy are effective and well-established, symptoms persist after treatment in up to half of MDD patients. This form of MDD is referred to as treatment resistant depression (TRD) (Souery et al., 2006, Fava, 2003); for TRD patients, the development of effective, alternative treatments is imperative.
One treatment for MDD which is particularly relevant for TRD is repetitive transcranial magnetic stimulation (rTMS). rTMS is a type of noninvasive brain stimulation delivered to awake patients in ambulatory care settings. During rTMS for MDD, brief, high-intensity magnetic pulses are typically applied to the left dorsolateral prefrontal cortex (DLPFC) via a magnetic coil placed upon the surface of the head (Hallett, 2007). rTMS can be applied at excitatory frequencies (≥ 5 Hz) intended to evoke increased firing of cortical action potentials, or at low frequencies (≤ 1 Hz) which “inhibit” neural activity (Siebner and Rothwell, 2003). The first rTMS device for treating MDD was FDA-cleared in 2008 with a protocol for delivery of 3,000 pulses per session of excitatory rTMS (10 Hz) at 120% resting motor threshold; a series of 20–30 once-daily sessions are given over the course of four to six weeks (McClintock et al., 2018, O’Reardon et al., 2007). As glutamate (Glu) is the brain’s principle excitatory neurotransmitter, and γ-aminobutyric acid (GABA) is inhibitory (Godfrey et al., 2018), increases in Glu relative to GABA levels may follow excitatory rTMS, while increases in GABA relative to Glu may follow inhibitory rTMS. Effective rTMS protocols for depression also include low-frequency rTMS applied to the right DLPFC (Berlim et al., 2013), bilateral rTMS (Berlim et al., 2013), and intermittent theta burst stimulation (iTBS), which consists of patterned rTMS and is FDA approved for MDD (Bakker et al., 2015, Huang et al., 2005, Caulfield, 2020). rTMS has demonstrated efficacy in TRD (Gaynes et al., 2014) and has been widely adopted for treatment of MDD in clinical practice (Hutton, 2014). Despite its ubiquity and clinical utility, the biological mechanisms by which rTMS relieves symptoms of MDD and TRD remain unclear. Identification of the neural mechanisms through which rTMS acts to bring clinical benefit in MDD would thus represent a significant advance.
Proton magnetic resonance spectroscopy (MRS), an imaging technique that measures the concentration of specific biochemical compounds in the brain in vivo, may inform rTMS mechanisms (Burtscher and Holtås, 2001). Metabolites that can be evaluated using proton MRS include creatine (Cr), choline (Cho), myoinositol (Ins), N-acetylaspartate (NAA), γ-aminobutyric acid (GABA), and the glutamatergic compounds (Glx) glutamate (Glu) and glutamine (Gln). Lower levels of Glx, Glu, Gln, GABA, and NAA in the anterior cingulate cortex (ACC) and the prefrontal cortex have been reported in individuals with MDD when compared to healthy controls (Auer et al., 2000, Hasler et al., 2007, Moriguchi et al., 2019, Zhong et al., 2014). Moreover, several studies have reported associations between the extent of metabolite reductions and MDD severity (Auer et al., 2000, Moriguchi et al., 2019, Price et al., 2009, Mirza et al., 2004, Michael et al., 2003a). In a recent meta-analysis, reductions in whole brain and ACC GABA were consistently observed in individuals diagnosed with MDD vs. controls. The same meta-analysis also observed a trend-level association of low Glx in ACC with MDD diagnosis (Godfrey et al., 2018), but did not replicate whole brain reductions observed in previous meta-analyses (Luykx et al., 2012).
MRS studies of various antidepressant treatments have reported changes in metabolite levels. For example, restoration of the metabolites NAA, GABA, Glx, Glu and Gln to levels similar to those of non-depressed individuals have been observed following electroconvulsive therapy (ECT), ketamine, and citalopram (a selective serotonin reuptake inhibitor) (Michael et al., 2003a, Michael et al., 2003b, Bhagwagar et al., 2004, Milak et al., 2020, Lener et al., 2017, Erchinger et al., 2021). Depression improvement has been shown to positively correlate with post-treatment increases in glutamatergic and GABAergic compounds, though not consistently (Michael et al., 2003a, Michael et al., 2003b, Bhagwagar et al., 2004, Milak et al., 2020, Lener et al., 2017, Erchinger et al., 2021, Pfleiderer et al., 2003, Milak et al., 2016, Sanacora et al., 2003). Meta-analytic data suggests that at least in the case of GABA, inconsistencies may be state dependent with sub-analyses indicating that GABA reductions were specific to studies examining current, not remitted, depression (Godfrey et al., 2018).
GABA and glutamatergic reductions may underlie alterations in cell morphology and brain connectivity that are characteristic of depression and chronic stress (reviewed in Duman et al., 2019). As the brain’s principle inhibitory and excitatory neurotransmitters, the balance GABA and glutamatergic reductions may destabilize brain connectivity. Indeed, recent evidence links normalized connectivity and increased post-infusion GABA and glutamate, to responsiveness to ketamine treatment for MDD (Abdallah et al., 2017). GABA and glutamate changes are associated with multiple neuronal and glial processes implicated in MDD (reviewed in Duman et al., 2019), for example reductions in prefrontal glia and synaptic density in DLPFC are observed postmortem (Rajkowska and Stockmeier, 2013, Sanacora et al., 2008, Kang et al., 2012).
MRS has been used to measure neurometabolic response to rTMS in only a handful of MDD studies conducted over the past 15 years. As a clear evaluation of effective neurometabolic mechanisms will enable clinicians to better understand and optimize rTMS to treat MDD, the field would benefit from a summary of findings to date, in light of observable inconsistencies across the results of individual papers, potential lack of consensus, limitations of the small sample sizes used, and the specialized, technically precise nature of both rTMS and MRS techniques. We thus evaluate and discuss findings to date to further our understanding of mechanisms that may underlie rTMS efficacy in MDD, and to guide future research and treatment efforts. We therefore conducted a systematic review of published MRS studies which used a within-subjects, pre-post rTMS treatment design in patients with a primary diagnosis of MDD. The goal of the review is to summarize (a) baseline metabolic predictors of rTMS treatment outcomes, (b) rTMS-associated effects on brain metabolites irrespective of treatment outcome, i.e., all metabolic changes pre vs. post rTMS, and (c) rTMS-associated changes in brain metabolites which relate to clinical improvement. This review thus summarizes the body of MRS findings associated with rTMS therapy, metabolic mechanisms related to treatment efficacy, and future directions for the field.
2. Methods
2.1. Eligibility Criterion
Eligible studies were peer-reviewed empirical investigations during which MRS imaging was conducted in depressed subjects prior to and following a course of rTMS clinical treatment for MDD. Though status of depression treatment resistance was examined, TRD was not required for inclusion. We excluded: (1) review articles; (2) empirical studies that did not utilize a pre-post rTMS treatment design; and (3) empirical studies that did not evaluate individuals with a diagnosis of MDD. All forms of therapeutic rTMS protocols approved for MDD treatment (i.e., high frequency rTMS, low frequency rTMS, iTBS) were included in this review. Fig. 1 provides the PRISMA chart for study flow and inclusion. Information sources and searches were double verified by two independent researchers to ensure no potentially eligible articles were excluded.
Fig. 1.
PRISMA Flowchart of Included Studies.
2.2. Information Sources and Search
Databases of references and abstracts on biomedical topics (i.e., PubMed, Google Scholar, Web of Science, and JSTOR) were systematically searched with the following keyword strings: “TMS AND MRS AND Depression;” “Transcranial Magnetic Stimulation AND Magnetic Resonance Spectroscopy AND Depression;” “Theta Burst AND Magnetic Resonance Spectroscopy AND Depression;” “Transcranial Magnetic Stimulation AND MRS AND Depression;” and ”TMS AND Magnetic Resonance Spectroscopy AND Depression.” The earliest study identified using this search method was published in 2007 (see PRISMA chart, Fig. 1). Eligible studies were included through January 10th, 2022.
2.3. Data Collection Process
Information pertaining to experimental design, rTMS procedures, MRS methods, and treatment-induced changes in metabolites were extracted and double verified for accuracy (MAG, HEJ, AMF).
2.4. Data Items
The goal of the review was to provide detailed information on the metabolic response to standard-of-care rTMS treatment protocols for MDD, including high frequency 10 Hz rTMS and iTBS. Our overarching objective was to quantify the direction and magnitude of effects of rTMS on metabolites in specific regions of the brain in individuals with MDD. Following (Cuypers and Marsman, 2021), we compiled (1) demographic characteristics, depression diagnoses, medical and neuropsychiatric comorbidities, concurrent medications, MDD severity and treatment resistance, treatment response criteria, and experimental design; (2) rTMS treatment protocol (i.e., rTMS type, target, and basic stimulation parameters such as pulse frequency and number of sessions in the course of therapy, etc.); (3) MRS acquisition parameters (i.e., voxel localization; voxel size; MRS acquisition method; procedures); and three types of MRS findings: (4a) baseline metabolic predictors of rTMS treatment outcomes, (4b) direction and magnitude of changes associated with rTMS effects on brain metabolites, irrespective of treatment outcomes, i.e., pooled effects of all changes pre vs. post rTMS treatment, and (4c) changes in brain metabolites associated with rTMS treatment outcomes.
2.5. Evaluation of Metabolites.
Metabolites assessed were Glx (Glu + Gln together), Glu, Gln, GABA, NAA, Cho, Ins, and Cr (Blüml et al., 2013). Metabolite levels were quantified relative to both water and other compounds. Historically, Cr has been used as a reference as it has been assumed to be stable in neurological and psychiatric conditions (Li et al., 2003), however, more recently this assumption has been questioned. Cr-referencing does, however, assist to control for various confounds including radio-frequency field inhomogeneities, differences in voxel localization, drifts in instrumental gain, and partial volume cerebrospinal fluid contamination (Li et al., 2002). We discuss all results regardless of referencing and quantification approach (i.e., water- and Cr-referenced metabolites).
3. Results
Twelve studies, comprising a total of n = 280 participants with primarily unipolar depression receiving rTMS, met eligibility criteria and were included in the review.
3.1. Experimental Design
3.1.1. Study Design
Studies were small to moderate in size, with samples ranging from 6 to 55 participants, and an overall mean sample size of 23 participants (SD = 13) (Table 1). Across studies, 49.6 ± 17.3% (mean ± SD) of participants identified as female (range = 11.8%-69.6%). Participants’ mean age ranged from 15.5 to 53 years across studies, with an overall mean ± SD age of 36.8 ± 11.3. Age composition varied and included studies of adolescents (ages 13–17 (Croarkin et al., 2016)), adolescents to young adults (ages 15–21 (Yang et al., 2014)); and young adults (ages 18–40 (Zheng et al., 2015, Zheng et al., 2010)), with most studies (8/12) focusing on adult patients (ages 20–70 (Baeken et al., 2017, Dubin et al., 2016, Luborzewski et al., 2007, Erbay et al., 2019, Zavorotnyy et al., 2020, Godfrey et al., 2021, Levitt et al., 2019, Bhattacharyya et al., 2021).
Table 1.
Sample Characteristics and Experimental Design.
| Study | Patient Population (N) | TRD Definition (where applicable) | Comorbid Psychiatric Diagnoses | Age (M (SD)), range (years) | Females N (%) | TMS Experimental Design | Treatment Outcome Measures & Definitions | Timing of Post- Treatment MRS Scan |
Age/Gender Matched Control Group for Baseline Comparisons? Yes/No (N) |
|---|---|---|---|---|---|---|---|---|---|
| Baeken et al. (2017) | Antidepressant-free adults with MDD (18) | Stage III or greater in current MDE; at least 2 failed trials with SSRI and/or SNRI and 1 failed TCA trial | Not described | 47.2 (12.5), N/A | 12 (66.7%) | Double-blind, sham-controlled, cross-over design | BDI-II; Absolute delta in BDI score | Within a week of final rTMS session (for both sham and active) | Yes (18) |
| Bhattacharyya et al. (2021) | Adults with MDD on stable doses of antidepressants in combination with neuroleptics (n = 2), mood stabilizers (n = 2), stimulants (n = 2), other augmentation agents (n = 2), and low dose anti-anxiety medication (n = 4) (12) | Inadequate response to at least one antidepressant despite an adequate dosage for at least 8 weeks | Not described | 53 (15), 23–74 | 8 (66.7%) | Open-label, all active rTMS | HAMD-17 Item; Percent change in HAMD score | After final rTMS session (timing not specified) | Yes (4); data not compared to patient group, used for test–retest reliability of Glx/Cr and GABA/Cr measurements |
| Croarkin et al. (2016) | Adolescents with MDD on stable dose of antidepressants (10) | At least 1 prior failed trial of antidepressant medication in the current MDE | Comorbid ADHD (n = 3) | 15.4 (1.2), 13–17 | 4 (40.0%) | Open-label, all active rTMS | CDRS-R; Responders defined by CGI-I of 1 or 2 and CDRS-R total < 40 | After final rTMS session (timing not specified) and at 6-month follow-up | No |
| Dubin et al. (2016) | Adults with MDD (n = 21) or bipolar II DO in a depressive episode (n = 2) on stable dose of antidepressants (23) | At least 2 previous antidepressant medication trials for at least 8 weeks during current MDE | Comorbid OCD (n = 2), ADHD (n = 1), and GAD (n = 1) | 41.7 (15.9), 23–68 | 16 (69.6%) | Open-label, all active rTMS | HAMD 24-Item; Response defined by 50% reduction in HAMD compared to baseline | Mean ± SD = 1.0 ± 1.1 days after final rTMS session | No |
| Erbay et al. (2019) | Antidepressant-free adults with MDD (18) | Sample not specifically characterized by treatment resistance | Comorbid anxiety disorder (n = 3) | 43.4 (11.1), N/A | 10 (55.6%) | Open-label, all active rTMS | HAMD; Did not classify by or define treatment responder | Within 3 days of final rTMS session | No |
| Godfrey et al. (2021) | Adults with MDD (n = 26) or bipolar disorder (n = 1) in a depressive episode on stable dose of antidepressants (27) | Insufficient response to at least 2 adequate courses of antidepressant treatments | Comorbid ADHD (n = 3), any anxiety disorder (n = 9), PTSD (n = 6), any comorbidity (n = 14) | 41.6 (N/A), 19–63 | 11 (40.7%) | Open-label, all active rTMS | MADRS; Response defined by 50% reduction in MADRS compared to baseline | After final rTMS session (timing not specified) | No |
| Levitt et al. (2019) | Adults with MDD on stable dose of antidepressants (26) | At least 2 failed trials of antidepressant medications in current MDE | None | 38.4 (13.8), 20–70 | 14 (53.9%) | Open-label, all active rTMS | IDS-SR30; Responders have 30% reduction in IDS-SR30 compared to baseline | After final rTMS session (timing not specified) | No |
| Luborzewski et al. (2007) | Adults with MDD on stable dose of SSRI antidepressants or medication-free (17) | Treatment resistance not defined for entire sample, but 9 of 17 were resistant to at least the current SSRI | Comorbid psychiatric disorders were exclusionary | 45 (11), 28–61 | 2 (11.8%) | Open-label, all active rTMS | HAMD 28-Item, MADRS, & BDI; Responders defined by 50% in HAMD compared to baseline; % change on each measure | After final rTMS session (timing not specified) | No |
| Yang et al. (2014) | Teenagers & young adults with MDD; medication status not reported (6) | Failure to respond to an appropriate SSRI trial (at least 8 weeks of a dose) in current MDE | Comorbid GAD (n = 5), social phobia (n = 2), and ADHD (n = 1) | 18.7 (2.0), 15–21 | 4 (66.7%) | Open-label, all active rTMS | HAMD 17-Item, HARS, & BDI; Responders defined by 50% reduction in HAMD | After final rTMS session (timing not specified) | No |
| Zavorotnyy et al. (2020) | Adult inpatients with MDD on stable dose of antidepressants and concurrent CBT (57) | Failure to remit on current regimen of medication and CBT | Not described | 43 (12.7), N/A | 29 (50.9%) | Single-blind (participants and raters), randomized, sham-controlled | HAMD 21-Item & BDI; %Change on HAMD and BDI; Response defined by 50% decrease on HAMD; Remission defined by HAMD < 7 | After final rTMS session (timing not specified) | Yes (15) |
| Zheng et al. (2010) | Young adults with MDD on stable dose of antidepressants (34) | At least 2 different adequate antidepressant trials (max dose taken > 4 weeks) in current MDE | None allowed | 27.2 (5.2), 18–37 | 12 (35.3%) | Double-blind, randomized, sham-controlled | HAMD 17-item & BDI; Responders defined by 50% reduction on HAMD | Within 24 hours of final rTMS session | Yes (28) |
| Zheng et al. (2015) | Young adults with MDD on stable dose of antidepressants (32) | At least 2 different adequate antidepressant trials (max dose taken > 4 weeks) in current MDE | None allowed | 26.9 (5.4), 18–40 | 12 (37.5%) | Double-blind, randomized, sham-controlled | HAMD 17-Item & BDI; Responders defined by 50% reduction in HAMD | Within 24 hours of final rTMS session | Yes (28) |
Abbreviations: TRD: treatment resistant depression; MDD: major depressive disorder; MDE: major depressive episode; SSRI: selective serotonin reuptake inhibitor; SNRI: serotonin norepinephrine reuptake inhibitor; TCA: tricyclic antidepressant; BDI-II: Beck Depression Inventory II; CDRS-R: Children's Depression Rating Scale-Revised; CGI-I: clinical global impressions scale-global improvement; DO: disorder; OCD: obsessive compulsive disorder; ADHD: attention-deficit hyperactivity disorder; GAD: generalized anxiety disorder; PTSD: post traumatic stress disorder; IDS-SR30: 30-item Inventory of Depressive Symptoms; HAMD: Hamilton Depression Rating Scale; MADRS: Montgomery Asberg Depression Rating Scale; BDI: Beck Depression Inventory; HARS: Hamilton Anxiety Rating Scale; CORE: CORE Measurement of Psychomotor Activity; QIDS-A17-SR: Quick Inventory of Depression Symptomatology—Adolescent (17 Item)—Self Report; EMG: electromyography; CBT: cognitive behavioral therapy.
3.1.2. Depressive Symptomology and Assessment
With the exception of two studies which included a small number of participants with a primary diagnosis of bipolar II or bipolar I disorder (n = 3) (Dubin et al., 2016, Godfrey et al., 2021), participants had a primary diagnosis of current MDD using standard Diagnostic and Statistical Manual of Mental Disorders (DSM) DSM-IV, DSM-IV-TR or DSM 5 criteria (American Psychiatric Association, 2013, American Psychiatric Association, 2000). Some level of pharmacoresistance characterized the participants in nine studies; one study did not specify participants’ past treatment history or pharmacoresistance (Zavorotnyy et al., 2020), and two did not require treatment resistance (Luborzewski et al., 2007, Erbay et al., 2019). Information on psychiatric comorbidities was available in five studies and included secondary diagnoses of attention deficit hyperactivity disorder, obsessive compulsive disorder, generalized anxiety disorder, social phobia, and post-traumatic stress disorder (Croarkin et al., 2016, Yang et al., 2014, Dubin et al., 2016, Erbay et al., 2019, Godfrey et al., 2021). Use of concurrent medication during rTMS varied across studies. The majority of studies (9/12) allowed continuation of stable doses of antidepressants throughout rTMS/iTBS treatment and MRS scanning (Croarkin et al., 2016, Zheng et al., 2015, Zheng et al., 2010, Dubin et al., 2016, Luborzewski et al., 2007, Zavorotnyy et al., 2020, Godfrey et al., 2021, Levitt et al., 2019, Bhattacharyya et al., 2021), however two studies required participants remain off antidepressant medications (Baeken et al., 2017, Erbay et al., 2019), and one study did not report concurrent medication status (Yang et al., 2014). For all 12 studies, depression symptom severity was assessed using self-report and/or clinician ratings including the Beck Depression Inventory (BDI), Hamilton Depression Rating Scale (HAMD), Montgomery-Asberg Depression Rating Scale (MADRS), and the 30-Item Inventory of Depression Symptomatology (IDS-SR30). Seven studies compared metabolite changes across subgroups defined by categorical treatment outcomes, i.e., rTMS responders vs. non-responders (see Table 1 for further details; Dubin et al., 2016, Luborzewski et al., 2007, Levitt et al., 2019, Croarkin et al., 2016, Yang et al., 2014, Zheng et al., 2015, Zheng et al., 2010).
3.2. rTMS Treatment Procedures for MDD
3.2.1. Study Design
rTMS treatment procedures appear in Table 2. Procedures for rTMS treatment and outcome assessment varied, with over half of studies (8/12) using an unblinded, open-label design in which rTMS was administered without a sham (control) condition (Croarkin et al., 2016, Yang et al., 2014, Dubin et al., 2016, Luborzewski et al., 2007, Erbay et al., 2019, Godfrey et al., 2021, Levitt et al., 2019, Bhattacharyya et al., 2021). Three studies used a randomized, controlled design in which participants were assigned to a series of active or sham treatments under double-blind conditions (Zheng et al., 2015, Zheng et al., 2010, Baeken et al., 2017). One study used a within-subjects, crossover design, with participants undergoing both sham and active iTBS treatment conditions in random order (Zavorotnyy et al., 2020). In addition to sham, these four studies included control groups comprised of age- and gender-matched healthy individuals not receiving either stimulation (sample size: n’s = 15–28; (Zavorotnyy et al., 2020, Zheng et al., 2015, Zheng et al., 2010, Baeken et al., 2017) for comparison of baseline metabolite levels between nondepressed participants and patients with MDD. One study included four healthy controls in order to obtain test–retest reliability of metabolite measurements; these metabolite levels were not compared to baseline metabolite levels in individuals with MDD (Bhattacharyya et al., 2021).
Table 2.
rTMS Treatment Protocol Features and Course Characteristics.
| Study | Treatment Target | Treatment Intensity relative to Resting Motor Threshold (MT) | Frequency | Number of Sessions/Course Duration | Number of rTMS Pulses per Session |
|---|---|---|---|---|---|
| Baeken et al. (2017) | Left DLPFC | 110% | 20 Hz | 20 sessions over 4 days (5 sessions per day); counterbalanced with 20 sham sessions at same schedule | 1560 |
| Bhattacharyya et al. (2021) | Left DLPFC | 120% | 10 Hz | 30 sessions over 6 weeks | 3000 |
| Croarkin et al. (2016) | Left DLPFC | 120% | 10 Hz | 30 sessions over 6–8 weeks | 3000 |
| Dubin et al. (2016) | Left DLPFC | 80%-120% | 10 Hz | 25 sessions over 5 weeks | 3000 |
| Erbay et al. (2019) | Left DLPFC | not reported | 10 Hz | 20 sessions over 2 weeks | 3000 |
| Godfrey et al. (2021) | Left DLPFC | 120% | 10 Hz | 20 sessions over 4 weeks | 4000 |
| Levitt et al. (2019) | Left DLPFC, right DLPFC, or sequential bilateral (left followed by right DLPFC) | 100%-120% | 10 Hz (left), 10 Hz (right), 1 Hz (sequential bilateral protocol) | 30 sessions over 6 weeks | 3000 (adjustments allowed; not reported for total on each side) |
| Luborzewski et al. (2007) | Left DLPFC | 100% | 20 Hz | 10 sessions over 2 weeks | 2000 |
| Yang et al. (2014) | Left DLPFC | 120% | 10 Hz | 15 sessions over 3 weeks | 3000 |
| Zavorotnyy et al. (2020)* | Left DLPFC | 90% | 50 Hz triplets at 5 Hz | 20 sessions over 4 weeks | 1200 |
| Zheng et al. (2010) | Left DLPFC | 110% | 15 Hz | 20 sessions over 4 weeks | 3000 |
| Zheng et al. (2015) | Left DLPFC | 110% | 15 Hz | 20 sessions over 4 weeks | 3000 |
*Intermittent theta burst (iTBS) stimulation protocol used.
Abbreviations: DLPFC: dorsolateral prefrontal cortex; ACC: anterior cingulate cortex; MPFC: medial prefrontal cortex; iTBS: intermittent theta burst stimulation; PFC: prefrontal cortex.
3.2.2. rTMS Protocols, Targets, and Course Features
10 of 12 studies used protocols targeting left DLPFC with high-frequency (≥ 10 Hz) once-daily stimulation sessions (Table 2). One used sequential bilateral or right-sided DLPFC stimulation in addition to left DLPFC stimulation (Levitt et al., 2019), and one used iTBS (Zavorotnyy et al., 2020). Excluding the iTBS protocol, pulse frequency ranged from 10 to 20 Hz (excitatory frequencies) for standard left-sided sessions. The sequential bilateral protocol used by Levitt et al. added 1 Hz stimulation (inhibitory frequency) to the right DLPFC for 19 participants who did not respond to left DLPFC rTMS after the 15th treatment session (Levitt et al., 2019). No other studies used low frequency stimulation. In Levitt et al., one participant was unable to tolerate left DLPFC stimulation, and thus only received rTMS to the right DLPFC (10 Hz); another participant switched from left to right DLPFC rTMS (10 Hz) after their fifth session (Levitt et al., 2019). The iTBS protocol included 50 Hz bursts with a 5 Hz carrier frequency (excitatory frequencies) (Zavorotnyy et al., 2020). A range of 1200 to 4000 pulses was administered per rTMS session. One study used an accelerated schedule which delivered multiple sessions per day (Baeken et al., 2017). The courses of active treatment lasted from 4 days to 8 weeks. 11 of 12 studies aimed to treat at 90%-120% intensity, relative to resting motor threshold (MT), except for one study that delivered intensity as low as 80% MT (Dubin et al., 2016).
3.3. MRS Imaging Protocols
3.3.1. MRS Analysis
All 12 studies evaluated metabolites in one or more brain regions using single voxel MRS data collected pre- and post-rTMS treatment, i.e., at least two serial scans per participant (details in Table 3). Analyses compared post-active rTMS or post-sham treatment data compared with pre-treatment baseline. One study provided a six-month follow-up, informing long-term metabolic response to rTMS treatment in adolescents (Croarkin et al., 2016).
Table 3.
MRS parameters.
| Study | Voxel Location & Size | MRS Acquisition Type | Magnet Strength (Tesla) | Metabolites Analyzed | MRS Acquisition Details |
|---|---|---|---|---|---|
| Baeken et al. (2017) | Left DLPFC (15 × 15 × 15 mm); Right DLPFC (15 × 15 × 15 mm); Right ACC (30 × 30 × 15 mm) | PRESS | 3 T | GABA, Glx, tNAA/tCr | TR = 2000 ms, TE = 40 ms, 128 averages |
| Bhattacharyya et al. (2021) | Left DLPFC (2 × 2 × 2 cm) | MEGA-PRESS | 3 T | GABA/tCr, Glx/tCr | TR = 2700 ms, TE = 68 ms, 128 averages per session |
| Croarkin et al. (2016) | Bilateral (over midline) ACC (2 × 2 × 2 cm); Left DLPFC (2 × 2 × 2cm) | PRESS (Glu and Gln); 2-dimensional J-resolved averaged PRESS (2DJ; for Glu) | 3 T | Glu (PRESS, 2DJ), Gln (PRESS), Gln/Glu (PRESS, 2DJ) | PRESS: TR = 2000 ms, TE = 80 ms, 8 excitations, 128 averages; 2DJ PRESS: TR = 2000 ms, TE = 35–195 ms in 16 steps, 8 averages at each step |
| Dubin et al. (2016) | Bilateral (over midline) MPFC that includes a rostral component of the ACC (2.5 × 2.5 × 3.0 cm) | MEGA-PRESS (J-edited) | 3 T | GABA, Glx | TR = 1500 ms, TE = 68 ms, 256 interleaved averages (512 total) |
| Erbay et al. (2019) | Left DLPFC (15 × 20 × 15 mm) | PRESS | 3 T | NAA/Cr, Cho/Cr, Ins/Cr, Glu/Cr, Lac/Cr, GSH/Cr, Gln/Cr | TR = 2000 ms, TE = 30 ms |
| Godfrey et al. (2021) | Left DLPFC (25 × 35 × 30 mm); Right M1 (25 × 35 × 30 mm) | MEGA-PRESS | 3 T | GABA, Glx | TR = 2000 ms, TE = 68 ms, 96 interleaved excitations (192 total) |
| Levitt et al. (2019) | Left DLPFC (30 × 20 × 10 mm), with adjustments in size to maximize grey matter content | MEGA-PRESS | 3 T | GABA, Glx | TR = 2000 ms, TE = 68 ms, 192 excitations |
| Luborzewski et al. (2007) | Left DLPFC (2 × 2 × 2cm); Bilateral (over midline) ACC (2.5 × 4 × 2 cm) | PRESS | 3 T | tCho, tCr, NAA, Glu | TR = 3000 ms, TE = 80 ms, 128 averages (ACC) & 256 averages (DLPFC) |
| Yang et al. (2014) | Left DLPFC (4.5 cm3) | PRESS | 3 T | Glu | TR = 2000 ms, TE = 30 ms, 128 averages |
| Zavorotnyy et al. (2020) | Bilateral (over midline) ACC (20 × 20 × 20 mm) | PRESS | 3 T | NAA, Cho, Cho/NAA, NAA/Cr, Cho/Cr | TR = 1390 ms, TE = 135 ms, used CHESS pulses for water suppression |
| Zheng et al. (2010) | Left PFC (1 × 1 × 1.5 cm); Right PFC (1 × 1 × 1.5 cm) | PRESS | 3 T | NAA, Ins, Cho, Cr, Glx, Ins/Cr, Cho/Cr, NAA/Cr, Glx/Cr | TR = 1700 ms, TE = 30 ms, acquisition repeated 3 times |
| Zheng et al. (2015) | Right ACC (1 × 1 × 1.5 cm); Left ACC (1 × 1 × 1.5 cm) | PRESS | 3 T | NAA, Ins, Cho, Cr, Glx, Ins/Cr, Cho/Cr, NAA/Cr, Glx/Cr | TR = 1700 ms, TE = 30 ms, acquisition repeated 3 times |
Abbreviations: DLPFC: dorsolateral prefrontal cortex; ACC: anterior cingulate cortex; MPFC: medial prefrontal cortex; CHESS: chemical shift selective saturation; PFC: prefrontal cortex; GSH: glutathione; PRESS: point resolved spectroscopy; MEGA-PRESS: Meshcher-Garwood point resolved spectroscopy; TR: repetition time; TE: echo time; M1: primary motor cortex; T: Tesla.
3.3.2. MRS Acquisition Parameters
MRS parameters are in Table 3. All 12 studies acquired data from voxels placed in one to three discrete cortical areas per scan; one study imaged three separate areas (Baeken et al., 2017), five studies examined voxels in two areas (Croarkin et al., 2016, Zheng et al., 2015, Zheng et al., 2010, Luborzewski et al., 2007, Godfrey et al., 2021), and six acquired data from a single voxel (Yang et al., 2014, Dubin et al., 2016, Erbay et al., 2019, Zavorotnyy et al., 2020, Levitt et al., 2019, Bhattacharyya et al., 2021). Most studies (n = 8) included a voxel placed over the left DLPFC with dimensions ranging from 1.5 × 1.5 × 1.5 cm to 2.5 × 3.5 × 3.0 cm (Croarkin et al., 2016, Yang et al., 2014, Baeken et al., 2017, Luborzewski et al., 2007, Erbay et al., 2019, Godfrey et al., 2021, Levitt et al., 2019, Bhattacharyya et al., 2021). Other areas investigated were the right DLPFC (n = 1; (Baeken et al., 2017), the right anterior cingulate cortex (ACC; n = 2; (Zheng et al., 2015, Baeken et al., 2017)), the left ACC (n = 1; (Zheng et al., 2015)), the bilateral ACC placed over the midline (n = 3; (Croarkin et al., 2016, Luborzewski et al., 2007, Zavorotnyy et al., 2020)), the bilateral medial prefrontal cortex placed over the midline and incorporating the rostral ACC (n = 1; (Dubin et al., 2016)), the left prefrontal cortex (PFC; n = 1; (Zheng et al., 2010)), and the right PFC (n = 1; (Zheng et al., 2010)). One study investigated right-sided primary motor cortex (M1) as a control region for the left DLPFC (Godfrey et al., 2021). All studies obtained MRS data via point resolved spectroscopy (PRESS), with four using variations of PRESS including Meshcher-Garwood PRESS (MEGA-PRESS; (Dubin et al., 2016, Godfrey et al., 2021, Levitt et al., 2019, Bhattacharyya et al., 2021) to measure GABA and one using 2-dimensional J-resolved averaged PRESS (Croarkin et al., 2016) specifically to measure Glu. Full MRS acquisition details including repetition time (TR) and echo time (TE) are in Table 3.
3.4. rTMS treatment effects and brain metabolites
Findings are summarized regarding baseline predictors of rTMS treatment outcomes; overall effect of rTMS on metabolites irrespective of rTMS responder status, i.e., overall effects of changes pre vs. post rTMS procedures, pooled across treatment responders and non-responders; and metabolic changes associated with symptom improvement following rTMS, i.e., effects in treatment responders (details in Table 4). Findings are considered significant at an alpha of 0.05, with select results presented at p > .05 to reduce Type II error, which has significant adverse impact on scientific progress (see Amrhein et al., 2019 for discussion).
Table 4.
Summary of rTMS-Related Changes in MRS Compounds.
| Study | Pooled Findings | rTMS Treatment Responder Findings | rTMS Treatment Non-Responder Findings |
|---|---|---|---|
| Baeken et al., 2017, Baeken et al., 2017 |
|
N/A | N/A |
| Bhattacharyya et al. (2021) |
|
N/A | N/A |
| Croarkin et al. (2016) |
|
|
|
| Dubin et al. (2016) |
|
|
|
| Erbay et al. (2019) |
|
N/A | N/A |
| Godfrey et al., 2021, Godfrey et al., 2021 |
|
N/A | N/A |
| Levitt et al. (2019) |
|
|
|
| Luborzewski et al. (2007) |
|
|
|
| Yang et al. (2014) | N/A |
|
|
| Zavorotnyy et al., 2020, Zavorotnyy et al., 2020 |
|
N/A | N/A |
| Zheng et al. (2010) |
|
|
|
| Zheng et al. (2015) |
|
|
|
Abbreviations: BDI-II: Beck Depression Inventory II; CDRS-R: Children's Depression Rating Scale-Revised; IDS-SR30: 30-item Inventory of Depressive Symptoms; HAMD: Hamilton Depression Rating Scale; MADRS: Montgomery Asberg Depression Rating Scale; BDI: Beck Depression Inventory; CORE: CORE Measurement of Psychomotor Activity; QIDS-A17-SR: Quick Inventory of Depression Symptomatology-Adolescent (17 Item)-Self Report; M1: primary motor cortex; GSH: glutathione.
3.4.1. Glutamatergic Compounds (Glx)
Seven studies evaluated rTMS effects on Glx using water-referenced (Godfrey et al., 2021, Levitt et al., 2019, Zheng et al., 2015, Zheng et al., 2010, Baeken et al., 2017, Dubin et al., 2016) and Cr-referenced methods (Zheng et al., 2015, Zheng et al., 2010, Bhattacharyya et al., 2021). Baseline Predictors. In Godfrey et al., baseline concentrations of Glx in the M1 (control) region positively predicted percent change in MADRS scores following rTMS (r = 0.53, p = .01, n = 23), where lower levels of Glx were associated with greater antidepressant response (Godfrey et al., 2021). In comparison, Baeken et al. demonstrated a pattern of greater baseline Glx in the left DLPFC predicting degree of improvement in BDI scores following rTMS (τ = -0.34, p = .06, n = 16) (Baeken et al., 2017). Bhattacharyya et al. identified a similar pattern, where higher pre-treatment left DLPFC Glx/tCr predicted lower post-treatment HAMD scores (p < .0005, n = 7) and greater change in HAMD scores following rTMS (p = .001, n = 7) (Bhattacharyya et al., 2021). The other four studies did not evaluate Glx as a baseline predictor of rTMS outcomes (Zheng et al., 2015, Zheng et al., 2010, Dubin et al., 2016, Levitt et al., 2019). Overall Effects. Godfrey et al. demonstrated a significant post-rTMS increase in Glx in the left DLPFC and right M1 in a pooled sample (F(1,21) = 4.48, p = 0.046) which was unconnected to clinical outcome (Godfrey et al., 2021). Additionally, three studies did not find any significant differences in Glx post-rTMS at the group level (Baeken et al., 2017, Dubin et al., 2016, Bhattacharyya et al., 2021), and three studies did not examine such relationship (Zheng et al., 2015, Zheng et al., 2010, Levitt et al., 2019). Symptom Improvement. rTMS effects on Glx or Glx/Cr in voxels in the left ACC, right ACC, left PFC, right PFC, or bilateral MPFC did not differ as a function of clinical response to rTMS in three studies (Zheng et al., 2015, Zheng et al., 2010, Dubin et al., 2016); four studies did not analyze this relationship by responder status (Baeken et al., 2017, Godfrey et al., 2021, Levitt et al., 2019, Bhattacharyya et al., 2021). However, Bhattacharyya et al. found a significant positive correlation between change in left DLPFC Glx/tCr and change in HAMD score (n = 6, p = .02) (Bhattacharyya et al., 2021). Interpretation. These data indicate baseline Glx may predict rTMS treatment outcomes, though the directionality of results was inconsistent between studies; currently, there is mixed evidence for significant rTMS-associated changes on Glx at the group level and their relationship with symptom improvement.
3.4.2. Glutamate (Glu)
Four studies evaluated rTMS effects on Glu, a component of Glx, using water-, Gln-, and Cr-referenced approaches (Glu, Glu/Gln, Glu/Cr, (Croarkin et al., 2016, Yang et al., 2014, Luborzewski et al., 2007, Erbay et al., 2019). Baseline Predictors. One study showed treatment responders had significantly lower baseline level of Glu compared to non-responders (p = .035, n = 17) (Luborzewski et al., 2007); another study had similar findings in the left DLPFC, though significance was not calculated (Yang et al., 2014). The two other studies did not evaluate Glu as a baseline predictor of rTMS outcomes (Croarkin et al., 2016, Erbay et al., 2019). Overall Effects. Water-referenced Glu rose with stimulation intensity in one pooled sample (r = 0.47, p = .064, n = 15) (Luborzewski et al., 2007), and rTMS effects were not identified for Gln- or Cr-referenced Glu in two other pooled samples, despite rTMS-related symptom improvement (Croarkin et al., 2016, Erbay et al., 2019). One study did not calculate changes in Glu post-rTMS by pooled sample (Yang et al., 2014). Symptom Improvement. rTMS-associated increases in Glu in the left DLPFC significantly correlated with symptom improvement on the HAMD (r = -0.89, p < .001), MADRS (r = -0.80, p < .001), and BDI (r = -0.64, p =.008) in Luborzewski et al. (Luborzewski et al., 2007). rTMS was associated with increased Glu in the left DLPFC of treatment responders (M = +1.19 mmol/l (Luborzewski et al., 2007); M = +10.37 mmol/kg ((Yang et al., 2014); significance not calculated) in two pilot samples (n = 6 (Luborzewski et al., 2007); n = 3 (Yang et al., 2014)). In non-responders, rTMS was associated with decreases in Glu in the left DLPFC (M = -1.16 mmol/l, p = .007, n = 11 (Luborzewski et al., 2007)); significance not calculated) in one study (Yang et al., 2014). Two studies did not analyze the relationship between changes in Glu and depression scores (Croarkin et al., 2016, Erbay et al., 2019). Interpretation. These data indicate baseline Glu predicts rTMS treatment outcomes in studies that analyzed this relationship; rTMS-associated increases in Glu characterizes treatment responders and is associated with symptom improvement; according to one study, rTMS-associated reduction in Glu characterizes non-responders.
3.4.3. Glutamine (Gln)
Two studies evaluated specific rTMS effects on Gln, the other subcomponent of Glx, using water-, Cr-, and Glu-referenced methods (Gln, Gln/Cr, Gln/Glu (Croarkin et al., 2016, Erbay et al., 2019)). Baseline Predictors. Gln at baseline was not evaluated as a predictor of rTMS outcomes (Croarkin et al., 2016, Erbay et al., 2019). Overall Effects. Croarkin et al. found rTMS in teens was associated with increased Gln/Glu in the bilateral ACC immediately after treatment, with continued increases observed at six months post treatment (F(2,10) = 5.32, p =.02) (Croarkin et al., 2016). Furthermore, Gln/Glu effects from baseline to post-treatment (hedges’ g = 1.064), post-treatment to six-month follow-up (hedges’ g = 1.242), and baseline to six-month follow-up (hedges’ g = 1.474) were large in size (Croarkin et al., 2016). In adults, Erbay et al. found an increase in Gln/Cr in the left DLPFC following rTMS (p = .008, n = 18) (Erbay et al., 2019). Symptom Improvement. rTMS effects on mean change in Gln/Glu (above) increased as depression severity decreased, as measured by the CDRS-R in teen patients (p = .003) in Croarkin et al. (Croarkin et al., 2016). Increases in Gln/Cr in the left DLPFC were unrelated to HAMD improvement in Erbay et al. (Erbay et al., 2019). Interpretation. These data indicate a dearth of information on predictive features of baseline Gln; rTMS is associated with increases in various referenced forms of Gln, though there is conflicting evidence on if this increase is related to symptom improvement.
3.4.4. γ-Aminobutyric Acid (GABA)
Five studies evaluated rTMS-associated effects on water-referenced GABA (Baeken et al., 2017, Dubin et al., 2016, Godfrey et al., 2021, Levitt et al., 2019) and tCr-referenced GABA (Bhattacharyya et al., 2021). Baseline Predictors. Baeken et. al., Godfrey et. al., and Bhattacharyya et al. found that baseline GABA and GABA/tCr concentrations did not significantly predict clinical outcomes (Baeken et al., 2017, Godfrey et al., 2021, Bhattacharyya et al., 2021); the other two studies did not investigate GABA as a baseline predictor of depression improvement (Dubin et al., 2016, Levitt et al., 2019). Overall Effects. Levitt et al. found GABA increased post-rTMS in the left DLPFC (M = 10%, F(1,20) = 6.8, p = .017) in the pooled sample (Levitt et al., 2019). Dubin et. al. (2016) also found increased MPFC GABA following rTMS in a pooled sample (M = 13.8%, t(22) = 2.69, p = .013 (Dubin et al., 2016). Godfrey et. al. (2021) did not observe significant post-treatment changes in GABA in the left DLPFC or right M1 (Godfrey et al., 2021), nor did Baeken et. al. in the left DLPFC or bilateral ACC (Baeken et al., 2017). Bhattacharyya et al. also did not observe any significant post-treatment changes in left DLPFC GABA/tCr (Bhattacharyya et al., 2021). Symptom Improvement. Levitt et. al. found larger rTMS-associated increases in GABA in responders (M = 23.6%, p = .015, n = 12) than non-responders (M = 4.1%, n = 14, not significant) (Levitt et al., 2019). Dubin et al. found post-rTMS increase in MPFC GABA was significantly greater (M = 17.4%) in responders than non-responders (M = 11.9%) (Dubin et al., 2016). Baeken et al. found rTMS-associated changes in GABA related to clinical outcome, with greater degree of clinical improvement (BDI-II) associated with greater increase in left DLPFC GABA (τ = -0.44, p = 0.04) (Baeken et al., 2017). Godfrey et al. and Bhattacharyya et al. did not find any significant correlations between changes in metabolite levels and antidepressant response (Godfrey et al., 2021, Bhattacharyya et al., 2021). Interpretation. These data indicate baseline GABA may not predict rTMS-associated clinical improvement; rTMS is associated with mixed effects (i.e., increase or no change in GABA) in pooled samples of responders and non-responders; and rTMS-associated increases in GABA relate to symptom improvement in treatment responders.
3.4.5. N-Acetylaspartate (NAA)
Six studies evaluated rTMS/iTBS effects on NAA using water- and Cr-referenced methods (NAA, NAA/Cr, tNAA/tCR; (Zheng et al., 2015, Zheng et al., 2010, Baeken et al., 2017, Luborzewski et al., 2007, Erbay et al., 2019, Zavorotnyy et al., 2020). Baseline Predictors. Luborzewski et. al. did not detect a significant relationship between baseline NAA and treatment outcome (Luborzewski et al., 2007). The other five studies did not investigate NAA as a baseline predictor of post-rTMS/iTBS depression improvement (Erbay et al., 2019, Zavorotnyy et al., 2020, Zheng et al., 2015, Zheng et al., 2010, Baeken et al., 2017). Overall Effects. Erbay et. al. demonstrated rTMS-associated increases of NAA/Cr in the left DLPFC in a pooled sample (p = .016, n = 18) (Erbay et al., 2019). In comparison to participants receiving sham, participants receiving iTBS had an increase in NAA post-treatment in Zavorotnyy et al. (Zavorotnyy et al., 2020). Three studies did not identify overall rTMS-associated treatment effects on frontal NAA, tNAA/tCr, or NAA in pooled samples (Zheng et al., 2010, Baeken et al., 2017, Luborzewski et al., 2007); one study did not calculate changes in NAA post-rTMS by pooled sample (Zheng et al., 2015). Symptom Improvement. Two studies showed differential NAA changes as a function of clinical outcomes (Zheng et al., 2015, Zavorotnyy et al., 2020). Zheng et. al. (2015) demonstrated significant increases in left ACC NAA only in rTMS responders (p = .004, n = 11) and not in non-responders (n = 7) (Zheng et al., 2015). In this study, increases in NAA significantly correlated with percent improvement in perseverative errors on the Wisconsin Card Sorting Task (r = 0.835, p < .001, n = 18) a behavioral measure of inhibitory control. Zavorotnyy et al. observed a significant interaction revealing increases in NAA were associated with clinical improvement for the active iTBS group, but not the sham iTBS group (Zavorotnyy et al., 2020): a 10% increase in anterior cingulate NAA predicted BDI score improvement of 73.9 ± 1.77% (R2 = 0.221, n = 57); in contrast NAA did not predict outcomes on the HAMD scale (Zavorotnyy et al., 2020). Erbay et al. did not observe a significant relationship between increases in NAA/Cr and changes in HAMD scores (Erbay et al., 2019). Zheng et al. (2010) and Luborzewski et al. did not observe a change in NAA in active responders (Zheng et al., 2010, Luborzewski et al., 2007) and Baeken et al. did not find a significant correlation between changes in tNAA/tCr and depressive symptoms (Baeken et al., 2017). Interpretation. These data indicate limited information on predictive features of baseline NAA, and that NAA might not predict rTMS outcomes; rTMS was associated with mixed effects on NAA (i.e., increase or no change in NAA) at the group level; and rTMS-associated increases in NAA related to symptom improvement in treatment responders for a handful of studies.
3.4.6. Choline (Cho)
Five studies evaluated rTMS/iTBS effects on Cho using Cr and NAA-referencing (Cho/Cr, Cho/NAA) and water-referencing (Cho, total choline (tCho)) (Zheng et al., 2015, Zheng et al., 2010, Luborzewski et al., 2007, Erbay et al., 2019, Zavorotnyy et al., 2020). Baseline Predictors. At baseline, tCho was significantly decreased in the left DLPFC of rTMS responders in comparison to non-responders in one study (p = .044, n = 17) (Luborzewski et al., 2007). The relationship of baseline Cho and treatment outcomes was not analyzed in the four other studies (Zheng et al., 2015, Zheng et al., 2010, Erbay et al., 2019, Zavorotnyy et al., 2020). Overall Effects. In two studies, no overall rTMS treatment effects were identified for Cho or Cho/Cr in the left DLPFC (n = 18 (Erbay et al., 2019)) or bilateral ACC (n = 18 (Zheng et al., 2015)) in pooled samples. Three studies did not analyze post-rTMS changes in Cho, Cho/Cr, or Cho/NAA in pooled samples (Zheng et al., 2010, Luborzewski et al., 2007, Zavorotnyy et al., 2020). Symptom Improvement. Three studies reported changes in Cho associated with symptom improvement. Zavorotnyy et. al. (2020) found that iTBS-associated increases in Cho/NAA in the bilateral ACC are a significant predictor of improvement on the BDI (F(1,38) = 5.190, p < .028, R2 = 0.12) (Zavorotnyy et al., 2020). Though groups were relatively small in other studies, treatment responders showed significant rTMS-associated increases in left DLPFC tCho post treatment in one study (p = .028, n = 6) (Luborzewski et al., 2007) and a marginally significant increase of Cho/Cr in the left PFC in another study (p = .056, n = 12 (Zheng et al., 2010). Zheng et al. (2015) did not find a significant change in Cho in rTMS treatment responders or non-responders (Zheng et al., 2015). One study did not analyze the relationship between changes in Cho/Cr and depression symptoms (Erbay et al., 2019). Interpretation. These data indicate a relative dearth of information on the predictive features of baseline Cho, though one study identified predictive features of low baseline Cho; rTMS-associated null effects on Cho and Cho/Cr at the group level; and rTMS-associated increases in Cho related to symptom improvement in treatment responders for the majority of studies that analyzed such relationship (3/4).
3.4.7. Myoinositol (Ins)
Three studies evaluated rTMS effects on water- and Cr- referenced Ins (Zheng et al., 2015, Zheng et al., 2010, Erbay et al., 2019). Baseline Predictors. Ins at baseline was not investigated as a predictor of rTMS outcomes in all three studies (Zheng et al., 2015, Zheng et al., 2010, Erbay et al., 2019). Overall Effects. All three studies did not report any significant changes in Ins at the group-level post-rTMS (Zheng et al., 2015, Zheng et al., 2010, Erbay et al., 2019). Symptom Improvement. Zheng et. al. (2010) identified a significant increase in Ins in the left PFC (p < .001, n = 12) and a marginally significant increase in the right PFC (p = .062, n = 12) in treatment responders; no significant differences in Ins were found in non-responders following rTMS (Zheng et al., 2010). Zheng et. al. (2010) also found a significant positive relationship of post-treatment change in Ins and change in HAMD scores (r = 0.86, p < .0001, n = 19) (Zheng et al., 2010), consistent with increased Ins in patients with symptom reduction. The other two studies did not identify any significant relationships between changes in Ins and depression symptoms (Zheng et al., 2015, Erbay et al., 2019). Interpretation. These data indicate a dearth of information on predictive features of baseline Ins; rTMS-associated increases in Ins in treatment responders; and rTMS-associated increases in Ins which correlate with MDD symptom improvement in one of three studies.
3.4.8. Creatine (Cr)
Four studies evaluated rTMS effects on Cr using water-referenced measures (Cr, total creatine (tCr); (Zheng et al., 2015, Zheng et al., 2010, Luborzewski et al., 2007, Erbay et al., 2019)). Baseline Predictors. Cr at baseline was not investigated as a predictor of rTMS outcomes (Zheng et al., 2015, Zheng et al., 2010, Luborzewski et al., 2007, Erbay et al., 2019). Overall Effects. Cr and tCr were not significantly altered by rTMS at the group level in any of the four studies (Zheng et al., 2015, Zheng et al., 2010, Luborzewski et al., 2007, Erbay et al., 2019). Symptom Improvement. Additionally, Cr and tCr did not relate to rTMS-associated depression improvement in these studies (Zheng et al., 2015, Zheng et al., 2010, Luborzewski et al., 2007, Erbay et al., 2019). Interpretation. These data indicate a dearth of information on predictive features of baseline Cr; and rTMS is not associated with change in Cr, irrespective of symptom improvement or treatment outcome.
4. Discussion
We conducted an in-depth systematic review of metabolic effects of rTMS in individuals with clinical depression. Open-label designs and small samples were common, as is typical in an emerging field. In consequence, variability between studies was considerable. Differences in the types of stimulation used, MRS imaging and analysis methods, sample sizes, clinical demographics, depression severity, and inclusion or exclusion criteria related to treatment resistance, concurrent psychotropic medication use, and psychiatric comorbidities likely contribute to relative variation in results. These caveats aside, collectively, the findings generally indicate there are no rTMS-associated metabolite changes independent of clinical response, with antidepressant response to rTMS associated with relative increases in mean levels of metabolites (Glu in 2 of 4 studies; Gln in 1 of 2 studies; GABA in 3 of 5 studies; NAA in 2 of 6 studies; Cho in 3 of 5 studies) though findings were trend-level in some instances. Evidence suggesting a role of increased Ins (1 of 3 studies) and Glx (1 of 7 studies) in treatment response was less consistent. The present studies do not suggest a role of Cr in treatment response (0 of 4 studies). Most findings arise from imaging voxels in bilateral DLPFC and ACC, with results from a single study showing significant increase of GABA in MPFC. Interestingly, baseline Glx levels predicted rTMS treatment outcomes, but the directionality of Glx levels was inconsistent across studies (i.e., one study showed lower levels leading to better outcomes (Godfrey et al., 2021), while two studies showed higher levels leading to depression symptom reduction (Baeken et al., 2017, Bhattacharyya et al., 2021). Despite variability, neurometabolic changes observed with rTMS are generally consistent with the neurometabolic responses reported for these same frontal brain regions following other successful treatments for depression such as ECT and antidepressant medications. Of note, while increases in frontal metabolite concentrations were found to be associated with clinical improvement in these 12 rTMS studies, none of the rTMS MRS studies we reviewed imaged the occipital regions, where decreased GABA has been shown to be a marker of antidepressant effects following psychotherapy and pharmacotherapy (Sanacora et al., 2002). All but two of the 12 studies we reviewed included patients who were receiving psychotropic medications in addition to rTMS therapy, inherently complicating findings. This said, stability of ongoing psychotropic medication agents and doses prior to and during rTMS therapy in the study participants lends confidence to the conclusions drawn by the individual studies evaluated in this review.
4.1. rTMS mechanisms via the glutamate/GABA-glutamine pathway
MRS is limited to detecting signals at mM concentrations. Thus it is primarily sensitive to intracellular signals (Blüml et al., 2013, White et al., 2018, Savtchenko and Rusakov, 2007), and cannot detect metabolic changes that occur on a nm scale, such as exocytosis, transport, and reuptake (Burtscher and Holtås, 2001). Changes in the latter, extracellular processes thus cannot explain the present findings, which occur at a mM scale. Potential intracellular mechanisms involved in these rTMS-related increases in Glu, Gln and GABA may include increases in rate of synthesis and/or reduction in catabolism within the examined voxels. Changes in these processes in the Glu/GABA-Gln pathway affect the rate of Glu-Gln cycling in excitatory neurons, GABA-Gln cycling in inhibitory neurons, and Glu/GABA-Gln cycling in glia (Bak et al., 2006).
4.1.1. rTMS Effects on Glu and Gln
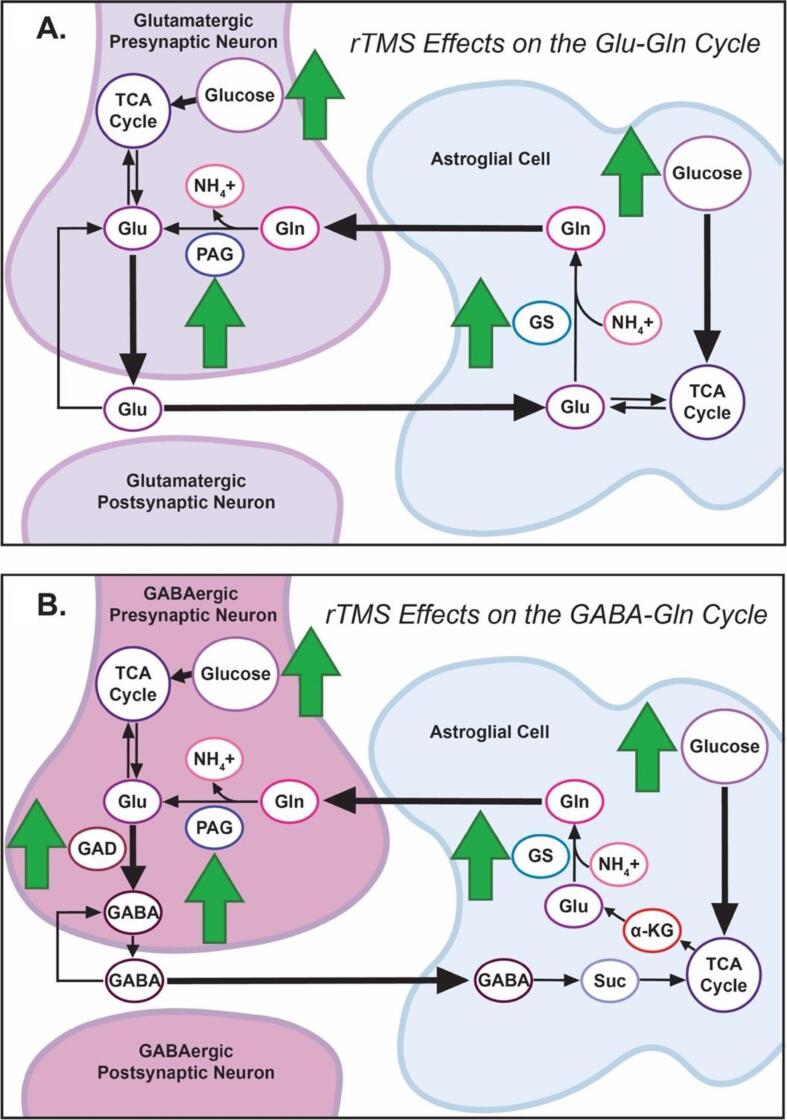
Changes in Glu and Gln volume observed following successful treatment suggest increased glucose utilization and upregulation of the tricarboxylic acid (TCA, or “Krebs”) cycle are key components of depression reduction (Fig. 2A; see (Bak et al., 2006, Flanagan et al., 2018) for details). Regional cerebral glucose metabolism (rCMRglu) and cerebral blood flow (rCBF) in the frontal cortex are altered in depression, indicating dysregulation in frontal utilization of glucose, a precursor for Glu and Gln (Baxter et al., 1989, Kimbrell et al., 2002, Bench et al., 1995, Hyder et al., 2006, Kimbrell et al., 1999, Conca et al., 2002, Li et al., 2010). Chronic unpredictable stress, which is associated with depression (Katz, 1982), decreases TCA cycle activity, thereby also reducing Glu and Gln synthesis (Kim et al., 2014, Rae, 2014). Recent meta-analyses link unmedicated depression with low Glx in the mPFC (Moriguchi et al., 2019). Successful treatment with antidepressants (Chen et al., 2014) and ECT (Njau et al., 2017) has been associated with increased Glx in medial PFC (inclusive of rostral ACC). Augmenting glucose utilization and/or upregulation of phosphate-activated glutaminase (PAG) and glutamine synthetase (GS; green arrows, Fig. 2A) may accelerate Glu and Gln synthesis. Together these data suggest rTMS may achieve its therapeutic effects via an increase in glucose utilization and generation of new Glu and Gln in frontal region voxels through an upregulation of the glycolytic pathway and TCA cycle (Blüml et al., 2002, Van Den Berg et al., 1969, Hertz and Chen, 2018, Gibbs, 2015, Jueptner and Weiller, 1995, Yelamanchi et al., 2016). Notably, in two studies reviewed here, decreases in Glu and Gln were linked to treatment non-response (Yang et al., 2014, Luborzewski et al., 2007), lending ancillary support to this hypothesis.
Fig. 2.
Hypothesized rTMS Intracellular Effects on the Glu/GABA-Gln Pathway. A schematic representation of hypothesized rTMS intracellular effects on the Glu/GABA-Gln cycle, overlaid on neural-astrocyte Glu/GABA-Gln pathway of Bak et al. (2006). Fig. 2A: In the Glu-Gln pathway, green arrows represent rTMS-induced increases in glucose, phosphate-activated glutaminase (PAG), and glutamine synthetase (GS), leading to tricarboxylic acid (TCA) cycle upregulation and increased intracellular glutamate (Glu) and glutamine (Gln) production. Fig. 2B: In the GABA-Gln pathway, green arrows represent rTMS-induced increases in glucose, phosphate-activated glutaminase (PAG), glutamine synthetase (GS), and glutamate decarboxylase (GAD) leading to tricarboxylic acid (TCA) cycle upregulation and increased intracellular GABA and glutamine (Gln) production. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
4.1.2. rTMS Effects on GABA
GABA is the major inhibitory neurotransmitter in the human brain and plays an important role in depression (Petroff, 2002, Kalueff and Nutt, 2007). GABA is powered by glucose in the TCA cycle, is synthesized by glutamate decarboxylase (GAD), and is stored intracellularly (Bak et al., 2006, Flanagan et al., 2018, Waller and Sampson, 2018, Jin et al., 2004) (Fig. 2B). In rats, rTMS-administered theta burst stimulation upregulates GAD65, an isoform of GAD (green arrows, Fig. 2B), preceding increases in intracellular GABA production (Peng et al., 2018, Mix et al., 2010, Trippe et al., 2009). The findings summarized in this review are consistent with upregulation of GS and PAG and increased glucose utilization in neurons and glia when there is clinical response to rTMS (green arrows, Fig. 2B). Another possibility is that this occurs in the context of decreases in catabolic compounds, such as succinate (Suc), which could contribute to net increases in GABA. However, because this proposed mechanism would selectively decrease astrocytic Glu (Savtchenko and Rusakov, 2007), a pattern discordant with the coupled nature of Glu and GABA fluctuations, PAG or GS upregulation is the more likely mechanistic pathway. While promising, it bears reminding that sample size limitations may be especially germane to interpretation of GABA findings; results from an evaluation of power in MRS studies suggest that with a MEGA-PRESS acquisition, > 20 subjects are needed to detect a 20% within-subjects change in GABA concentrations (Sanaei Nezhad et al., 2020). Indeed, issues of power and effect size may help to explain heterogeneity between an initial study (n = 10) reporting increased GABA post-ECT (Knudsen et al., 2019) and several failed replications (Yue et al., 2009).
4.1.3. Downstream effects
rTMS treatment-related increases in Glu, Gln and GABA likely influence downstream cellular signaling. In rats, rTMS-induced membrane depolarizations modulate synaptic transmission via ionotropic receptors, facilitating an increase in Glu (Gallo and Ghiani, 2000). Glutamate is removed from synapses by glia, affecting function in astrocytes (Volz et al., 2013). Theta burst stimulation also increases vesicular glutamate transporter 1 (VGLUT1) expression (Rajkowska, 2000), which is consistent with overall upregulation of Glu. Depressed patients show decreased glia in the PFC (Kempermann et al., 2018), suggesting Glu-mediated rTMS changes in glial activity contribute to clinical improvement (Croarkin et al., 2016, Zheng et al., 2010).
4.2. rTMS mechanisms via NAA-related adult neurogenesis
Several lines of evidence potentially suggest that rTMS-related increases in NAA may reflect changes in the rate or efficacy of adult neurogenesis, with downstream effects on functional activity, monoaminergic processes, cerebral glucose utilization and neurometabolism. In humans, adult neurogenesis occurs in the hippocampus and the striatum, regions involved in the etiology and treatment of MDD (Ernst and Frisen, 2015, Spalding et al., 2005, Ernst et al., 2014, White and Gonsalves, 2021, White et al., 2021). In healthy volunteers, the level of NAA in the dACC relates to an aspect of emotional functioning termed neuroaffective reserves, which encompasses capacities for emotional fluidity, positive agency, and resilience (Ueyama et al., 2011). Increased NAA observed following successful rTMS treatment of MDD is thus possibly consistent with treatment-related increases in the rate and/or uptake of adult neurogenesis in treatment-responders. In animal models, high-frequency rTMS increases hippocampal neurogenesis (Piatti et al., 2011). Adult neurogenesis takes approximately 3–6 weeks for new neurons to generate, mature and integrate into existing circuitry (Inta et al., 2013). This timeframe is similar to the time course of clinical rTMS therapy, which when effective, requires daily treatments for 2–6 weeks to produce remission (McClintock et al., 2018). In animal models, the rate and efficacy of adult neurogenesis is modulated by neural activity and physical activity (Inta et al., 2013); in humans with depression, stimulation alters local neural activity and regional cerebral blood flow in those responsive to clinical rTMS treatment (McClintock et al., 2018, O’Reardon et al., 2007). Neurogenic effects are also thought to play a role in the clinical improvement to other treatment modalities, including ECT (Madsen et al., 2000, Ohira et al., 2013) and SSRIs (Samuels and Hen, 2011) (see (Zhang et al., 2017) for a review). Direct evidence of a neurogenesis-NAA relationship is found in animals, where successful neural stem cell implants produce an increase in NAA in hippocampus (Baslow, 2010). Neurons display a > 200-fold intracellular/extracellular NAA gradient, allowing NAA to provide a marker for neurons, neuronal health and neuronal integrity in regions assessed by MRS (Chang et al., 2009, Dager et al., 2008). Moreover, as the MRS peak for tNAA typically contains two metabolites, NAA and N-acetylaspartyl-glutamate (NAAG), increases in tNAA following successful rTMS therapy suggest several downstream effects. These effects are not mutually exclusive and include (1) NAA-related contributions to functional activity, facilitating patients’ capacity to respond to low-intensity positive stimuli, (2) NAAG-related modulation of monoamines, ACh and GABA, lowering neural thresholds for perception, experience and action, and (3) NAAG-related potentiation of local glucose utilization in neurons, which improves continuity and depth of recurrent processing (Ueyama et al., 2011). These sequelae are discussed in detail in White et al. (2021) and Ueyama et al. (2011) and are potentially relevant to proximal mechanisms of clinical improvement in MDD after rTMS treatment. To validate such hypotheses, future research measuring NAA levels in the hippocampus and/or striatum prior to and following rTMS treatment is required. This has not yet been collected, as existing rTMS studies have restricted MRS data collection to voxels in the frontal lobe. Potential rTMS-related effects on neurogenesis in these two areas, indexed by NAA, is thus worthy of evaluation in future work.
4.3. Strengths and limitations
This review has several limitations. First, only a small number of studies (N = 12) have been conducted, and most (n = 8) evaluated effects in samples of < 30 participants. Power estimates vary by metabolite and voxel location, but detection of change may require at least 50 subjects (Sanaei Nezhad et al., 2020), perhaps more in older adult samples (Fitzgerald, 2021). The included studies are thus underpowered to detect small and medium effect sizes of successful rTMS on MRS metabolites. Second, the included studies focused upon metabolic effects predominantly in the frontal lobe (i.e., MPFC, PFC, ACC, M1, and DLPFC), excluding other regions implicated in depression, including temporal, parietal, and occipital structures. Addressing this gap in our knowledge will be important for understanding the full impact of rTMS, as metabolic alterations have been observed throughout the brain in MDD (Near et al., 2021). Third, all reviewed studies applied rTMS to the left DLPFC, with one study also using sequential bilateral protocol and right DLPFC rTMS (Levitt et al., 2019). A left sided coil placement is common in clinical practice and effectively reduces depression symptoms (Mullins et al., 2014). As such, the present conclusions are mainly restricted to rTMS applied to the left DLPFC. Additional studies evaluating sequential bilateral protocol and right DLPFC on metabolites are warranted. Finally, we note that limitations inherent to MRS may contribute to the lack of cohesion across studies. Few studies report findings for both GABA and Glu, making it difficult to examine each metabolite’s differential contributions to clinical recovery. Different pulse sequences and acquisition parameters (i.e., TR, TE, number of averages, editing pulses, quantification of compounds independently or derived from co-edited signals) are optimal for the quantification of GABA versus Glu. Moreover, quantifying metabolic peaks from GABA is more difficult due to the degree of spectral overlap (Sanaei Nezhad et al., 2020). Post-acquisition techniques and workflows vary considerably between the reviewed studies and may have contributed to differences in results and interpretation. Expert consensus and emerging standards regarding preprocessing, quantification, and analysis (Near et al., 2021) will be helpful for the MRS field going forward. Techniques that improve signal-to-noise ratio, such as PRESS or MEGA-PRESS acquisitions at a higher field strength (i.e., 7 Tesla) may improve quantification of GABA and other compounds (Mullins et al., 2019) in future work.
Strengths of the review include the consistency of the directionality of rTMS effects on metabolites, and the timeliness of examining understudied molecular mechanisms of rTMS, a relatively new depression treatment. While the reviewed studies are small and underpowered, the alignment of metabolite effects of therapeutic rTMS with metabolic responses observed following other effective MDD treatments (i.e., ECT and antidepressants (Michael et al., 2003a, Michael et al., 2003b, Bhagwagar et al., 2004, Milak et al., 2016)), makes it unlikely that the changes described following rTMS are specific to this treatment modality. Rather, the findings provide convergent support for the observed neurometabolic effects associated with recovery from depression across multiple treatment types. Furthermore, the effects summarized in this review were generally consistent across age groups, indicating a common pathway through which rTMS and other clinical treatments achieve efficacy against MDD.
4.4. Future directions
Future work should recruit larger sample sizes, evaluate metabolites in regions beyond the frontal lobe and in the context of depressive relapse following a treatment-induced remission. Future studies should also identify relationships between metabolites and symptom response within dimensions of depression (e.g., mood, appetite, lethargy, anhedonia, and psychomotor agitation). To date, existing studies typically evaluate only the change in overall depression scores. Particularly in the setting of rTMS treatment for depression where patients are seen daily and frequently assessed with standardized measures, there is substantial opportunity to explore the specific ways in which metabolic changes relate to improvement in cognitive, affective, and behavioral outcomes in MDD. rTMS is a large commitment in terms of patients’ time (4–6 weeks) and financial cost ($6,000-$12,000) (McClintock et al., 2018). Identification of metabolites that serve as readouts of clinical progress could be used to optimize treatment course length for individual patients. Though more research on right DLPFC applications is needed, hemispheric differences in metabolite concentrations may be useful for matching coil applications to specific patients. The potential utility of neuroimaging predictors of rTMS response has been demonstrated using imaging modalities (Corlier et al., 2019, Drysdale et al., 2017), Based on the reviewed evidence, MRS shows promise as a potential source of novel predictors of rTMS treatment efficacy for depression. Future work using machine learning tools and baseline metabolite levels may assist in a personalized medicine approach of determining which patients will most benefit from rTMS treatment for MDD.
4.5. Conclusions and implications
This systematic review identifies rTMS treatment-related effects on MRS compounds in individuals with depression. Several metabolites present preliminary reasonable evidence for a specific metabolic change in response to left DLPFC rTMS (i.e., Glu, Gln, GABA, and NAA), and not all demonstrate a correlation with symptom severity, either before or after treatment (i.e., Cr). Several of these relationships are similar to findings with other depression treatments, such as ECT and citalopram, which indicate treatment effects on Glu, Gln, GABA, and NAA that relates to improvement in depression symptoms. These findings are generally consistent with those of rTMS and suggest common metabolic mechanisms by which patients’ depression resolves. The body of MRS data we review here extends and informs our understanding of the mechanisms of improvement in individuals with severe depression and has potential utility as a future clinical tool for guiding clinical care.
Author contributions
This review was conceptualized, initiated, and designed by MAG, TLW, and LLC. MAG wrote the first draft of the manuscript, MAG and TLW edited and revised the initial draft, MAG, TLW, and JB edited and revised the final draft. MAG and AMF tailored the scope of the literature search. MAG completed the literature search, determined which articles were eligible, and compiled the included articles. MAG, HJ, AMF extracted and verified the relevant data from the articles. MAG performed the data analyses. MAG created the tables and figures. MAG and TLW created the model. MAG, TLW, HEJ, AMF, ADH, JB, and LLC provided input on data analysis and results and revised the manuscript. All authors read and approved the final manuscript.
5. Financial support
MAG is supported by the Brown University Department of Neuroscience and the Hanlon Foundation. TLW is supported by the Hanlon Foundation and the Brown University Carney Institute for Brain Science Zimmerman Innovation Award. JB was supported by grants from the U.S. Veteran’s Heath Association (IK2 CX001824) and Brain Behavior Research Foundation. ADH holds a Canada Research Chair in Magnetic Resonance Spectroscopy in Brain Injury. AMF is supported by NIMH R25 MH101076 and L30MH127637. Research was facilitated by the National Institute of General Medical Sciences of the National Institutes of Health under Award Number P20GM130452 (Core Resources of the Butler Hospital COBRE Center for Neuromodulation).
6. Disclosures
Ms. Gonsalves, Dr. White, Ms. Joyce, Dr. Fukuda, Dr. Harris, and Dr. Barredo declare no conflicts of interest. Dr. Carpenter has served as scientific advisor or consultant to Neuronetics Inc, Nexstim PLC, AffectNeuro Inc, Neurolief LTD, Sage Therapeutics, Otsuka, Sunovion, and Janssen Pharmaceuticals Inc. Dr. Carpenter and her staff at Butler Hospital have received research support from Neuronetics Inc, Neosync Inc, Nexstim PLC, Affect Neuro Inc, and Janssen Pharmaceuticals Inc.
References
- Abdallah C.G., Averill L.A., Collins K.A., Geha P., Schwartz J., Averill C., DeWilde K.E., Wong E., Anticevic A., Tang C.Y., Iosifescu D.V., Charney D.S., Murrough J.W. Ketamine treatment and global brain connectivity in major depression. Neuropsychopharmacology. 2017;42(6):1210–1219. doi: 10.1038/npp.2016.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. (2000). Diagnostic and statistical manual of mental disorders (4th ed., text rev.). 10.1176/appi.books.9780890420249.dsm-iv-tr. [DOI]
- American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders (5th ed.). https://dsm.psychiatryonline.org/doi/book/10.1176/appi.books.9780890425596.
- Amrhein V., Greenland S., McShane B. Retire statistical significance. Nature. 2019;567(7748):305–307. doi: 10.1038/d41586-019-00857-9. [DOI] [PubMed] [Google Scholar]
- Auer D.P., Pütz B., Kraft E., Lipinski B., Schill J., Holsboer F. Reduced glutamate in the anterior cingulate cortex in depression: an in vivo proton magnetic resonance spectroscopy study. Biol. Psychiatry. 2000;47(4):305–313. doi: 10.1016/s0006-3223(99)00159-6. [DOI] [PubMed] [Google Scholar]
- Baeken C., Lefaucheur J.P., Van Schuerbeek P. The impact of accelerated high frequency rTMS on brain neurochemicals in treatment-resistant depression: Insights from (1)H MR spectroscopy. Clin. Neurophysiol. 2017;128(9):1664–1672. doi: 10.1016/j.clinph.2017.06.243. [DOI] [PubMed] [Google Scholar]
- Bak L.K., Schousboe A., Waagepetersen H.S. The glutamate/GABA-glutamine cycle: aspects of transport, neurotransmitter homeostasis and ammonia transfer. J. Neurochem. 2006;98(3):641–653. doi: 10.1111/j.1471-4159.2006.03913.x. [DOI] [PubMed] [Google Scholar]
- Bakker N., Shahab S., Giacobbe P., Blumberger D.M., Daskalakis Z.J., Kennedy S.H., Downar J. rTMS of the dorsomedial prefrontal cortex for major depression: safety, tolerability, effectiveness, and outcome predictors for 10 Hz versus intermittent theta-burst stimulation. Brain Stimulat. 2015;8(2):208–215. doi: 10.1016/j.brs.2014.11.002. [DOI] [PubMed] [Google Scholar]
- Baslow M.H. Evidence that the tri-cellular metabolism of N-acetylaspartate functions as the brain's “operating system”: how NAA metabolism supports meaningful intercellular frequency-encoded communications. Amino Acids. 2010;39(5):1139–1145. doi: 10.1007/s00726-010-0656-6. [DOI] [PubMed] [Google Scholar]
- Baxter L.R., Jr, et al. Reduction of prefrontal cortex glucose metabolism common to three types of depression. Arch. Gen. Psychiatry. 1989;46(3):243–250. doi: 10.1001/archpsyc.1989.01810030049007. [DOI] [PubMed] [Google Scholar]
- Bench C.J., Frackowiak R.S.J., Dolan R.J. Changes in regional cerebral blood flow on recovery from depression. Psychol. Med. 1995;25(2):247–261. doi: 10.1017/s0033291700036151. [DOI] [PubMed] [Google Scholar]
- Berlim M.T., Van den Eynde F., Jeff Daskalakis Z. Clinically meaningful efficacy and acceptability of low-frequency repetitive transcranial magnetic stimulation (rTMS) for treating primary major depression: a meta-analysis of randomized, double-blind and sham-controlled trials. Neuropsychopharmacology. 2013;38(4):543–551. doi: 10.1038/npp.2012.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berlim M.T., Van den Eynde F., Daskalakis Z.J. A systematic review and meta-analysis on the efficacy and acceptability of bilateral repetitive transcranial magnetic stimulation (rTMS) for treating major depression. Psychol. Med. 2013;43(11):2245–2254. doi: 10.1017/S0033291712002802. [DOI] [PubMed] [Google Scholar]
- Bhagwagar Z., Wylezinska M., Taylor M., Jezzard P., Matthews P.M., Cowen P.J. Increased brain GABA concentrations following acute administration of a selective serotonin reuptake inhibitor. Am. J. Psychiatry. 2004;161(2):368–370. doi: 10.1176/appi.ajp.161.2.368. [DOI] [PubMed] [Google Scholar]
- Bhattacharyya P., et al. Left dorsolateral prefrontal cortex Glx/tCr predicts efficacy of high frequency 4- to 6-Week rTMS treatment and is associated with symptom improvement in adults with major depressive disorder: findings from a pilot study. Front. Psychiatry. 2021;12 doi: 10.3389/fpsyt.2021.665347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blüml S., Moreno-Torres A., Shic F., Nguy C.-H., Ross B.D. Tricarboxylic acid cycle of glia in the in vivo human brain. NMR Biomed. 2002;15(1):1–5. doi: 10.1002/nbm.725. [DOI] [PubMed] [Google Scholar]
- Blüml, S., Magnetic Resonance Spectroscopy: Basics, in MR Spectroscopy of Pediatric Brain Disorders, S. Blüml and A. Panigrahy, Editors. 2013, Springer New York: New York, NY. p. 11-23.
- Bromet E., Andrade L.H., Hwang I., Sampson N.A., Alonso J., de Girolamo G., de Graaf R., Demyttenaere K., Hu C., Iwata N., Karam A.N., Kaur J., Kostyuchenko S., Lépine J.-P., Levinson D., Matschinger H., Mora M.E.M., Browne M.O., Posada-Villa J., Viana M.C., Williams D.R., Kessler R.C. Cross-national epidemiology of DSM-IV major depressive episode. BMC Medicine. 2011;9(1) doi: 10.1186/1741-7015-9-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burtscher I.M., Holtås S. Proton MR spectroscopy in clinical routine. J. Magn. Reson. Imaging. 2001;13(4):560–567. doi: 10.1002/jmri.1079. [DOI] [PubMed] [Google Scholar]
- Caulfield K.A. Is accelerated, high-dose theta burst stimulation a panacea for treatment-resistant depression? J. Neurophysiol. 2020;123(1):1–3. doi: 10.1152/jn.00537.2019. [DOI] [PubMed] [Google Scholar]
- Chen L.-P., Dai H.-Y., Dai Z.-Z., Xu C.-T., Wu R.-H. Anterior cingulate cortex and cerebellar hemisphere neurometabolite changes in depression treatment: A 1H magnetic resonance spectroscopy study. Psychiatry Clin. Neurosci. 2014;68(5):357–364. doi: 10.1111/pcn.12138. [DOI] [PubMed] [Google Scholar]
- Chang L., Jiang C.S., Ernst T. Effects of age and sex on brain glutamate and other metabolites. Magn. Reson. Imaging. 2009;27(1):142–145. doi: 10.1016/j.mri.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conca A., Peschina W., König P., Fritzsche H., Hausmann A. Effect of chronic repetitive transcranial magnetic stimulation on regional cerebral blood flow and regional cerebral glucose uptake in drug treatment-resistant depressives. A brief report. Neuropsychobiology. 2002;45(1):27–31. doi: 10.1159/000048669. [DOI] [PubMed] [Google Scholar]
- Corlier J., et al. Changes in Functional Connectivity Predict Outcome of Repetitive Transcranial Magnetic Stimulation Treatment of Major Depressive Disorder. Cerebral Cortex. 2019;29(12):4958–4967. doi: 10.1093/cercor/bhz035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croarkin P.E., Nakonezny P.A., Wall C.A., Murphy L.L., Sampson S.M., Frye M.A., Port J.D. Transcranial magnetic stimulation potentiates glutamatergic neurotransmission in depressed adolescents. Psychiatry Res. Neuroimag. 2016;247:25–33. doi: 10.1016/j.pscychresns.2015.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuypers K., Marsman A. Transcranial magnetic stimulation and magnetic resonance spectroscopy: opportunities for a bimodal approach in human neuroscience. NeuroImage. 2021;224 doi: 10.1016/j.neuroimage.2020.117394. [DOI] [PubMed] [Google Scholar]
- Dager S.R., Corrigan N.M., Richards T.L., Posse S. Research applications of magnetic resonance spectroscopy to investigate psychiatric disorders. Topics Magn. Reson. Imag. TMRI. 2008;19(2):81–96. doi: 10.1097/RMR.0b013e318181e0be. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubin M.J., et al. Elevated prefrontal cortex GABA in patients with major depressive disorder after TMS treatment measured with proton magnetic resonance spectroscopy. J. Psychiatry Neurosci. 2016;41(3):E37–E45. doi: 10.1503/jpn.150223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman R.S., Sanacora G., Krystal J.H. Altered connectivity in depression: GABA and glutamate neurotransmitter deficits and reversal by novel treatments. Neuron. 2019;102(1):75–90. doi: 10.1016/j.neuron.2019.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drysdale A.T., Grosenick L., Downar J., Dunlop K., Mansouri F., Meng Y., Fetcho R.N., Zebley B., Oathes D.J., Etkin A., Schatzberg A.F., Sudheimer K., Keller J., Mayberg H.S., Gunning F.M., Alexopoulos G.S., Fox M.D., Pascual-Leone A., Voss H.U., Casey B.J., Dubin M.J., Liston C. Resting-state connectivity biomarkers define neurophysiological subtypes of depression. Nat. Med. 2017;23(1):28–38. doi: 10.1038/nm.4246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst A., Frisen J. Adult neurogenesis in humans- common and unique traits in mammals. PLoS Biol. 2015;13(1):e1002045. doi: 10.1371/journal.pbio.1002045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst A., Alkass K., Bernard S., Salehpour M., Perl S., Tisdale J., Possnert G., Druid H., Frisén J. Neurogenesis in the striatum of the adult human brain. Cell. 2014;156(5):1072–1083. doi: 10.1016/j.cell.2014.01.044. [DOI] [PubMed] [Google Scholar]
- Erbay M.F., Zayman E.P., Erbay L.G., Ünal S. Evaluation of transcranial magnetic stimulation efficiency in major depressive disorder patients: a magnetic resonance spectroscopy study. Psychiatry Investig. 2019;16(10):745–750. doi: 10.30773/pi.2019.07.17.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erchinger V.J., Ersland L., Aukland S.M., Abbott C.C., Oltedal L. Magnetic resonance spectroscopy in depressed subjects treated with electroconvulsive therapy—A systematic review of literature. Front. Psychiatry. 2021;12 doi: 10.3389/fpsyt.2021.608857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fava M. Diagnosis and definition of treatment-resistant depression. Biol. Psychiatry. 2003;53(8):649–659. doi: 10.1016/s0006-3223(03)00231-2. [DOI] [PubMed] [Google Scholar]
- Flanagan B., et al. A computational study of astrocytic glutamate influence on post-synaptic neuronal excitability. PLOS Computational Biology. 2018;14(4) doi: 10.1371/journal.pcbi.1006040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald P.B. Targeting repetitive transcranial magnetic stimulation in depression: do we really know what we are stimulating and how best to do it? Brain Stimulat. 2021;14(3):730–736. doi: 10.1016/j.brs.2021.04.018. [DOI] [PubMed] [Google Scholar]
- Friedrich M.J. Depression Is the Leading Cause of Disability Around the World. JAMA. 2017;317(15) doi: 10.1001/jama.2017.3826. p. 1517-1517. [DOI] [PubMed] [Google Scholar]
- Gallo V., Ghiani C.A. Glutamate receptors in glia: new cells, new inputs and new functions. Trends Pharmacol. Sci. 2000;21(7):252–258. doi: 10.1016/s0165-6147(00)01494-2. [DOI] [PubMed] [Google Scholar]
- Gaynes B.N., Lloyd S.W., Lux L., Gartlehner G., Hansen R.A., Brode S., Jonas D.E., Evans T.S., Viswanathan M., Lohr K.N. Repetitive transcranial magnetic stimulation for treatment-resistant depression: a systematic review and meta-analysis. J. Clin. Psychiatry. 2014;75(05):477–489. doi: 10.4088/JCP.13r08815. [DOI] [PubMed] [Google Scholar]
- Gibbs M.E. Role of glycogenolysis in memory and learning: regulation by noradrenaline, serotonin and ATP. Front. Integr Neurosci. 2015;9:70. doi: 10.3389/fnint.2015.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godfrey K.E.M., et al. Effect of rTMS on GABA and glutamate levels in treatment-resistant depression: an MR spectroscopy study. Psychiatry Res. Neuroimag. 2021;317 doi: 10.1016/j.pscychresns.2021.111377. [DOI] [PubMed] [Google Scholar]
- Godfrey K.E.M., Gardner A.C., Kwon S., Chea W., Muthukumaraswamy S.D. Differences in excitatory and inhibitory neurotransmitter levels between depressed patients and healthy controls: a systematic review and meta-analysis. J. Psychiatr. Res. 2018;105:33–44. doi: 10.1016/j.jpsychires.2018.08.015. [DOI] [PubMed] [Google Scholar]
- Hallett M. Transcranial magnetic stimulation: a primer. Neuron. 2007;55(2):187–199. doi: 10.1016/j.neuron.2007.06.026. [DOI] [PubMed] [Google Scholar]
- Hasler G., van der Veen J.W., Tumonis T., Meyers N., Shen J., Drevets W.C. Reduced prefrontal glutamate/glutamine and γ-aminobutyric acid levels in major depression determined using proton magnetic resonance spectroscopy. Arch. Gen. Psychiatry. 2007;64(2):193. doi: 10.1001/archpsyc.64.2.193. [DOI] [PubMed] [Google Scholar]
- Hertz L., Chen Y.e. Additional mechanisms for brain activation failure due to reduced glucose metabolism-a commentary on Zilberter and Zilberter: the vicious circle of hypometabolism in neurodegenerative diseases. J. Neurosci. Res. 2018;96(5):757–761. doi: 10.1002/jnr.24192. [DOI] [PubMed] [Google Scholar]
- Huang Y.-Z., Edwards M.J., Rounis E., Bhatia K.P., Rothwell J.C. Theta burst stimulation of the human motor cortex. Neuron. 2005;45(2):201–206. doi: 10.1016/j.neuron.2004.12.033. [DOI] [PubMed] [Google Scholar]
- Hutton T.M. The clinical application of transcranial magnetic stimulation. Psychiatric Ann. 2014;44(6):305–309. [Google Scholar]
- Hyder F., Patel A.B., Gjedde A., Rothman D.L., Behar K.L., Shulman R.G. Neuronal-glial glucose oxidation and glutamatergic-GABAergic function. J. Cereb. Blood Flow Metab. 2006;26(7):865–877. doi: 10.1038/sj.jcbfm.9600263. [DOI] [PubMed] [Google Scholar]
- Inta D., et al. Electroconvulsive Therapy Induces Neurogenesis in Frontal Rat Brain Areas. PLOS ONE. 2013;8(7) doi: 10.1371/journal.pone.0069869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin E.S., Jones J.G., Merritt M., Burgess S.C., Malloy C.R., Sherry A.D. Glucose production, gluconeogenesis, and hepatic tricarboxylic acid cycle fluxes measured by nuclear magnetic resonance analysis of a single glucose derivative. Anal. Biochem. 2004;327(2):149–155. doi: 10.1016/j.ab.2003.12.036. [DOI] [PubMed] [Google Scholar]
- Jueptner M., Weiller C. Review: does measurement of regional cerebral blood flow reflect synaptic activity? Implications for PET and fMRI. Neuroimage. 1995;2(2):148–156. doi: 10.1006/nimg.1995.1017. [DOI] [PubMed] [Google Scholar]
- Kang H.J., Voleti B., Hajszan T., Rajkowska G., Stockmeier C.A., Licznerski P., Lepack A., Majik M.S., Jeong L.S., Banasr M., Son H., Duman R.S. Decreased expression of synapse-related genes and loss of synapses in major depressive disorder. Nat. Med. 2012;18(9):1413–1417. doi: 10.1038/nm.2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalueff A.V., Nutt D.J. Role of GABA in anxiety and depression. Depression and Anxiety. 2007;24(7):495–517. doi: 10.1002/da.20262. [DOI] [PubMed] [Google Scholar]
- Katz R.J. Animal model of depression: Pharmacological sensitivity of a hedonic deficit. Pharmacol. Biochem. Behav. 1982;16(6):965–968. doi: 10.1016/0091-3057(82)90053-3. [DOI] [PubMed] [Google Scholar]
- Kempermann G., Gage F.H., Aigner L., Song H., Curtis M.A., Thuret S., Kuhn H.G., Jessberger S., Frankland P.W., Cameron H.A., Gould E., Hen R., Abrous D.N., Toni N., Schinder A.F., Zhao X., Lucassen P.J., Frisén J. Human adult neurogenesis: evidence and remaining questions. Cell Stem Cell. 2018;23(1):25–30. doi: 10.1016/j.stem.2018.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S.-Y., Lee D.-W., -Kim H., Bang E., Chae J.-H., Choe B.-Y. Chronic repetitive transcranial magnetic stimulation enhances GABAergic and cholinergic metabolism in chronic unpredictable mild stress rat model: 1H-NMR spectroscopy study at 11.7T. Neurosci. Lett. 2014;572:32–37. doi: 10.1016/j.neulet.2014.04.033. [DOI] [PubMed] [Google Scholar]
- Kimbrell T.A., Little J.T., Dunn R.T., Frye M.A., Greenberg B.D., Wassermann E.M., Repella J.D., Danielson A.L., Willis M.W., Benson B.E., Speer A.M., Osuch E., George M.S., Post R.M. Frequency dependence of antidepressant response to left prefrontal repetitive transcranial magnetic stimulation (rTMS) as a function of baseline cerebral glucose metabolism. Biol. Psychiatry. 1999;46(12):1603–1613. doi: 10.1016/s0006-3223(99)00195-x. [DOI] [PubMed] [Google Scholar]
- Kimbrell T.A., Ketter T.A., George M.S., Little J.T., Benson B.E., Willis M.W., Herscovitch P., Post R.M. Regional cerebral glucose utilization in patients with a range of severities of unipolar depression. Biol. Psychiatry. 2002;51(3):237–252. doi: 10.1016/s0006-3223(01)01216-1. [DOI] [PubMed] [Google Scholar]
- Knudsen M.K., Near J., Blicher A.B., Videbech P., Blicher J.U. Magnetic resonance (MR) spectroscopic measurement of γ-aminobutyric acid (GABA) in major depression before and after electroconvulsive therapy. Acta Neuropsychiatry. 2019;31(1):17–26. doi: 10.1017/neu.2018.22. [DOI] [PubMed] [Google Scholar]
- Lener M.S., Niciu M.J., Ballard E.D., Park M., Park L.T., Nugent A.C., Zarate C.A. Glutamate and gamma-aminobutyric acid systems in the pathophysiology of major depression and antidepressant response to ketamine. Biol. Psychiatry. 2017;81(10):886–897. doi: 10.1016/j.biopsych.2016.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitt J.G., Kalender G., O’Neill J., Diaz J.P., Cook I.A., Ginder N., Krantz D., Minzenberg M.J., Vince-Cruz N., Nguyen L.D., Alger J.R., Leuchter A.F. Dorsolateral prefrontal γ-aminobutyric acid in patients with treatment-resistant depression after transcranial magnetic stimulation measured with magnetic resonance spectroscopy. J .Psychiatry Neurosci. JPN. 2019;44(6):386–394. doi: 10.1503/jpn.180230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B.S.Y., Wang H., Gonen O. Metabolite ratios to assumed stable creatine level may confound the quantification of proton brain MR spectroscopy. Magn. Reson. Imaging. 2003;21(8):923–928. doi: 10.1016/s0730-725x(03)00181-4. [DOI] [PubMed] [Google Scholar]
- Li C.-T., Wang S.-J., Hirvonen J., Hsieh J.-C., Bai Y.-M., Hong C.-J., Liou Y.-J., Su T.-P. Antidepressant mechanism of add-on repetitive transcranial magnetic stimulation in medication-resistant depression using cerebral glucose metabolism. J. Affect. Disord. 2010;127(1-3):219–229. doi: 10.1016/j.jad.2010.05.028. [DOI] [PubMed] [Google Scholar]
- Li B.S.Y., Babb J.S., Soher B.J., Maudsley A.A., Gonen O. Reproducibility of 3D proton spectroscopy in the human brain. Magn. Reson. Med. 2002;47(3):439–446. doi: 10.1002/mrm.10081. [DOI] [PubMed] [Google Scholar]
- Lim G.Y., Tam W.W., Lu Y., Ho C.S., Zhang M.W., Ho R.C. Prevalence of depression in the community from 30 countries between 1994 and 2014. Sci. Rep. 2018;8(1) doi: 10.1038/s41598-018-21243-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luborzewski A., Schubert F., Seifert F., Danker-Hopfe H., Brakemeier E.-L., Schlattmann P., Anghelescu I., Colla M., Bajbouj M. Metabolic alterations in the dorsolateral prefrontal cortex after treatment with high-frequency repetitive transcranial magnetic stimulation in patients with unipolar major depression. J. Psychiatr. Res. 2007;41(7):606–615. doi: 10.1016/j.jpsychires.2006.02.003. [DOI] [PubMed] [Google Scholar]
- Luykx J.J., Laban K.G., van den Heuvel M.P., Boks M.P.M., Mandl R.C.W., Kahn R.S., Bakker S.C. Region and state specific glutamate downregulation in major depressive disorder: a meta-analysis of (1)H-MRS findings. Neurosci. Biobehav. Rev. 2012;36(1):198–205. doi: 10.1016/j.neubiorev.2011.05.014. [DOI] [PubMed] [Google Scholar]
- Madsen T.M., Treschow A., Bengzon J., Bolwig T.G., Lindvall O., Tingström A. Increased neurogenesis in a model of electroconvulsive therapy. Biol. Psychiatry. 2000;47(12):1043–1049. doi: 10.1016/s0006-3223(00)00228-6. [DOI] [PubMed] [Google Scholar]
- McClintock S.M., Reti I.M., Carpenter L.L., McDonald W.M., Dubin M., Taylor S.F., Cook I.A., O’Reardon J., Husain M.M., Wall C., Krystal A.D., Sampson S.M., Morales O., Nelson B.G., Latoussakis V., George M.S., Lisanby S.H. Consensus recommendations for the clinical application of repetitive transcranial magnetic stimulation (rTMS) in the treatment of depression. J. Clin. Psychiatry. 2018;79(1):35–48. doi: 10.4088/JCP.16cs10905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael N., Erfurth A., Ohrmann P., Arolt V., Heindel W., Pfleiderer B. Neurotrophic effects of electroconvulsive therapy: a proton magnetic resonance study of the left amygdalar region in patients with treatment-resistant depression. Neuropsychopharmacology. 2003;28(4):720–725. doi: 10.1038/sj.npp.1300085. [DOI] [PubMed] [Google Scholar]
- Michael N., Erfurth A., Ohrmann P., Arolt V., Heindel W., Pfleiderer B. Metabolic changes within the left dorsolateral prefrontal cortex occurring with electroconvulsive therapy in patients with treatment resistant unipolar depression. Psychol. Med. 2003;33(7):1277–1284. doi: 10.1017/s0033291703007931. [DOI] [PubMed] [Google Scholar]
- Milak M.S., Proper C.J., Mulhern S.T., Parter A.L., Kegeles L.S., Ogden R.T., Mao X., Rodriguez C.I., Oquendo M.A., Suckow R.F., Cooper T.B., Keilp J.G., Shungu D.C., Mann J.J. A pilot in vivo proton magnetic resonance spectroscopy study of amino acid neurotransmitter response to ketamine treatment of major depressive disorder. Mol. Psychiatry. 2016;21(3):320–327. doi: 10.1038/mp.2015.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milak M.S., Rashid R., Dong Z., Kegeles L.S., Grunebaum M.F., Ogden R.T., Lin X., Mulhern S.T., Suckow R.F., Cooper T.B., Keilp J.G., Mao X., Shungu D.C., Mann J.J. Assessment of relationship of ketamine dose with magnetic resonance spectroscopy of glx and GABA responses in adults with major depression: a randomized clinical trial. JAMA Network Open. 2020;3(8):e2013211. doi: 10.1001/jamanetworkopen.2020.13211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirza Y., Tang J., Russell A., Banerjee S.P., Bhandari R., Ivey J., Rose M., Moore G.J., Rosenberg D.R. Reduced anterior cingulate cortex glutamatergic concentrations in childhood major depression. J. Am. Acad. Child Adolesc. Psychiatry. 2004;43(3):341–348. doi: 10.1097/00004583-200403000-00017. [DOI] [PubMed] [Google Scholar]
- Mix A., Benali A., Eysel U.T., Funke K. Continuous and intermittent transcranial magnetic theta burst stimulation modify tactile learning performance and cortical protein expression in the rat differently. Eur. J. Neurosci. 2010;32(9):1575–1586. doi: 10.1111/j.1460-9568.2010.07425.x. [DOI] [PubMed] [Google Scholar]
- Moriguchi S., Takamiya A., Noda Y., Horita N., Wada M., Tsugawa S., Plitman E., Sano Y., Tarumi R., ElSalhy M., Katayama N., Ogyu K., Miyazaki T., Kishimoto T., Graff-Guerrero A., Meyer J.H., Blumberger D.M., Daskalakis Z.J., Mimura M., Nakajima S. Glutamatergic neurometabolite levels in major depressive disorder: a systematic review and meta-analysis of proton magnetic resonance spectroscopy studies. Mol. Psychiatry. 2019;24(7):952–964. doi: 10.1038/s41380-018-0252-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullins P.G., McGonigle D.J., O'Gorman R.L., Puts N.A.J., Vidyasagar R., Evans C.J., Edden R.A.E. Current practice in the use of MEGA-PRESS spectroscopy for the detection of GABA. NeuroImage. 2014;86:43–52. doi: 10.1016/j.neuroimage.2012.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray C.J., et al. The state of US health, 1990–2010: burden of diseases, injuries, and risk factors. JAMA. 2013;310(6):591–608. doi: 10.1001/jama.2013.13805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Near J., Harris A.D., Juchem C., Kreis R., Marjańska M., Öz G., Slotboom J., Wilson M., Gasparovic C. Preprocessing, analysis and quantification in single-voxel magnetic resonance spectroscopy: experts' consensus recommendations. NMR Biomed. 2021;34(5):e4257. doi: 10.1002/nbm.4257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Njau S., Joshi S.H., Espinoza R., Leaver A.M., Vasavada M., Marquina A., Woods R.P., Narr K.L. Neurochemical correlates of rapid treatment response to electroconvulsive therapy in patients with major depression. J. Psychiatry Neurosci. 2017;42(1):6–16. doi: 10.1503/jpn.150177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohira K., Takeuchi R., Shoji H., Miyakawa T. Fluoxetine-induced cortical adult neurogenesis. Neuropsychopharmacology. 2013;38(6):909–920. doi: 10.1038/npp.2013.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otte C., Gold S.M., Penninx B.W., Pariante C.M., Etkin A., Fava M., Mohr D.C., Schatzberg A.F. Major depressive disorder. Nat. Rev. Dis. Primers. 2016;2(1) doi: 10.1038/nrdp.2016.65. [DOI] [PubMed] [Google Scholar]
- O’Reardon J.P., Solvason H.B., Janicak P.G., Sampson S., Isenberg K.E., Nahas Z., McDonald W.M., Avery D., Fitzgerald P.B., Loo C., Demitrack M.A., George M.S., Sackeim H.A. Efficacy and safety of transcranial magnetic stimulation in the acute treatment of major depression: a multisite randomized controlled trial. Biol. Psychiatry. 2007;62(11):1208–1216. doi: 10.1016/j.biopsych.2007.01.018. [DOI] [PubMed] [Google Scholar]
- Petroff O.A.C. Book review: GABA and glutamate in the human brain. Neuroscientist. 2002;8(6):562–573. doi: 10.1177/1073858402238515. [DOI] [PubMed] [Google Scholar]
- Peng Z., et al. Mechanism of repetitive transcranial magnetic stimulation for depression. Shanghai Archives of Psychiatry. 2018;30(2):84–92. doi: 10.11919/j.issn.1002-0829.217047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfleiderer B., Michael N., Erfurth A., Ohrmann P., Hohmann U., Wolgast M., Fiebich M., Arolt V., Heindel W. Effective electroconvulsive therapy reverses glutamate/glutamine deficit in the left anterior cingulum of unipolar depressed patients. Psychiatry Res. 2003;122(3):185–192. doi: 10.1016/s0925-4927(03)00003-9. [DOI] [PubMed] [Google Scholar]
- Piatti V.C., Davies-Sala M.G., Esposito M.S., Mongiat L.A., Trinchero M.F., Schinder A.F. The timing for neuronal maturation in the adult hippocampus is modulated by local network activity. J. Neurosci. 2011;31(21):7715–7728. doi: 10.1523/JNEUROSCI.1380-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price R.B., Shungu D.C., Mao X., Nestadt P., Kelly C., Collins K.A., Murrough J.W., Charney D.S., Mathew S.J. Amino acid neurotransmitters assessed by proton magnetic resonance spectroscopy: relationship to treatment resistance in major depressive disorder. Biol. Psychiatry. 2009;65(9):792–800. doi: 10.1016/j.biopsych.2008.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rae C.D. A guide to the metabolic pathways and function of metabolites observed in human brain 1H magnetic resonance spectra. Neurochem. Res. 2014;39(1):1–36. doi: 10.1007/s11064-013-1199-5. [DOI] [PubMed] [Google Scholar]
- Rajkowska G. Postmortem studies in mood disorders indicate altered numbers of neurons and glial cells. Biol. Psychiatry. 2000;48(8):766–777. doi: 10.1016/s0006-3223(00)00950-1. [DOI] [PubMed] [Google Scholar]
- Rajkowska G., Stockmeier C.A. Astrocyte pathology in major depressive disorder: insights from human postmortem brain tissue. Curr. Drug Targets. 2013;14(11):1225–1236. doi: 10.2174/13894501113149990156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reesal R.T., Lam R.W. Clinical guidelines for the treatment of depressive disorders. II. Principles of management. Can J Psychiatry. 2001;46(Suppl 1):21s–28s. [PubMed] [Google Scholar]
- Sanacora G., Mason G.F., Rothman D.L., Krystal J.H. Increased occipital cortex GABA concentrations in depressed patients after therapy with selective serotonin reuptake inhibitors. Am. J. Psychiatry. 2002;159(4):663–665. doi: 10.1176/appi.ajp.159.4.663. [DOI] [PubMed] [Google Scholar]
- Sanacora G., Mason G.F., Rothman D.L., Hyder F., Ciarcia J.J., Ostroff R.B., Berman R.M., Krystal J.H. Increased cortical GABA concentrations in depressed patients receiving ECT. Am. J. Psychiatry. 2003;160(3):577–579. doi: 10.1176/appi.ajp.160.3.577. [DOI] [PubMed] [Google Scholar]
- Sanacora G., Zarate C.A., Krystal J.H., Manji H.K. Targeting the glutamatergic system to develop novel, improved therapeutics for mood disorders. Nat. Rev. Drug Discovery. 2008;7(5):426–437. doi: 10.1038/nrd2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanaei Nezhad F., Lea‐Carnall C.A., Anton A., Jung JeYoung, Michou E., Williams S.R., Parkes L.M. Number of subjects required in common study designs for functional GABA magnetic resonance spectroscopy in the human brain at 3 Tesla. Eur. J. Neurosci. 2020;51(8):1784–1793. doi: 10.1111/ejn.14618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuels B.A., Hen R. Neurogenesis and affective disorders. Eur. J. Neurosci. 2011;33(6):1152–1159. doi: 10.1111/j.1460-9568.2011.07614.x. [DOI] [PubMed] [Google Scholar]
- Savtchenko L.P., Rusakov D.A. The optimal height of the synaptic cleft. Proc. Natl. Acad. Sci. 2007;104(6):1823–1828. doi: 10.1073/pnas.0606636104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebner H., Rothwell J. Transcranial magnetic stimulation: new insights into representational cortical plasticity. Exp. Brain Res. 2003;148(1):1–16. doi: 10.1007/s00221-002-1234-2. [DOI] [PubMed] [Google Scholar]
- Spalding K.L., Bhardwaj R.D., Buchholz B.A., Druid H., Frisén J. Retrospective birth dating of cells in humans. Cell. 2005;122(1):133–143. doi: 10.1016/j.cell.2005.04.028. [DOI] [PubMed] [Google Scholar]
- Souery D., Papakostas G.I., Trivedi M.H. Treatment-resistant depression. J. Clin. Psychiatry. 2006;67(Suppl 6):16–22. [PubMed] [Google Scholar]
- Trippe J., Mix A., Aydin-Abidin S., Funke K., Benali A. Theta burst and conventional low-frequency rTMS differentially affect GABAergic neurotransmission in the rat cortex. Exp. Brain Res. 2009;199(3-4):411–421. doi: 10.1007/s00221-009-1961-8. [DOI] [PubMed] [Google Scholar]
- Ueyama E., Ukai S., Ogawa A., Yamamoto M., Kawaguchi S., Ishii R., Shinosaki K. Chronic repetitive transcranial magnetic stimulation increases hippocampal neurogenesis in rats. Psychiatry Clin. Neurosci. 2011;65(1):77–81. doi: 10.1111/j.1440-1819.2010.02170.x. [DOI] [PubMed] [Google Scholar]
- Van Den Berg C.J., Kržalić L.J., Mela P., Waelsch H. Compartmentation of glutamate metabolism in brain. Evidence for the existence of two different tricarboxylic acid cycles in brain. Biochem. J. 1969;113(2):281–290. doi: 10.1042/bj1130281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volz L.J., Benali A., Mix A., Neubacher U., Funke K. Dose-dependence of changes in cortical protein expression induced with repeated transcranial magnetic theta-burst stimulation in the rat. Brain Stimulation. 2013;6(4):598–606. doi: 10.1016/j.brs.2013.01.008. [DOI] [PubMed] [Google Scholar]
- Waller D.G., Sampson A.P. Medical Pharmacology and Therapeutics. Elsevier; 2018. pp. 73–90. [Google Scholar]
- Wang P.S., Simon G., Kessler R.C. The economic burden of depression and the cost-effectiveness of treatment. Int. J. Methods Psychiatric Res. 2003;12(1):22–33. doi: 10.1002/mpr.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White T.L., Gonsalves M.A. Dignity Neuroscience: universal human rights are rooted in human brain science. Annals New York Academy Sci. 2021;1505(1):40–54. doi: 10.1111/nyas.14670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White T.L., et al. The neurobiology of wellness: (1)H-MRS correlates of agency, flexibility and neuroaffective reserves in healthy young adults. Neuroimage. 2021;225 doi: 10.1016/j.neuroimage.2020.117509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White T.L., Monnig M.A., Walsh E.G., Nitenson A.Z., Harris A.D., Cohen R.A., Porges E.C., Woods A.J., Lamb D.G., Boyd C.A., Fekir S. Psychostimulant drug effects on glutamate, Glx, and creatine in the anterior cingulate cortex and subjective response in healthy humans. Neuropsychopharmacology. 2018;43(7):1498–1509. doi: 10.1038/s41386-018-0027-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X.-R., Kirton A., Wilkes T.C., Pradhan S., Liu I., Jaworska N., Damji O., Keess J., Langevin L.M., Rajapakse T., Lebel R.M., Sembo M., Fife M., MacMaster F.P. Glutamate alterations associated with transcranial magnetic stimulation in youth depression: a case series. J ect. 2014;30(3):242–247. doi: 10.1097/YCT.0000000000000094. [DOI] [PubMed] [Google Scholar]
- Yelamanchi S.D., Jayaram S., Thomas J.K., Gundimeda S., Khan A.A., Singhal A., Keshava Prasad T.S., Pandey A., Somani B.L., Gowda H. A pathway map of glutamate metabolism. J Cell Commun Signal. 2016;10(1):69–75. doi: 10.1007/s12079-015-0315-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue L., Xiao-lin H., Tao S. The effects of chronic repetitive transcranial magnetic stimulation on glutamate and gamma-aminobutyric acid in rat brain. Brain Res. 2009;1260:94–99. doi: 10.1016/j.brainres.2009.01.009. [DOI] [PubMed] [Google Scholar]
- Zavorotnyy M., Zöllner R., Rekate H., Dietsche P., Bopp M., Sommer J., Meller T., Krug A., Nenadić I. Intermittent theta-burst stimulation moderates interaction between increment of N-Acetyl-Aspartate in anterior cingulate and improvement of unipolar depression. Brain Stimul. 2020;13(4):943–952. doi: 10.1016/j.brs.2020.03.015. [DOI] [PubMed] [Google Scholar]
- Zheng H., Zhang L.i., Li L., Liu P., Gao J., Liu X., Zou J., Zhang Y., Liu J., Zhang Z., Li Z., Men W. High-frequency rTMS treatment increases left prefrontal myo-inositol in young patients with treatment-resistant depression. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2010;34(7):1189–1195. doi: 10.1016/j.pnpbp.2010.06.009. [DOI] [PubMed] [Google Scholar]
- Zheng H., Jia F., Guo G., Quan D., Li G., Wu H., Zhang B., Fan C., He X., Huang H. Abnormal anterior cingulate N-acetylaspartate and executive functioning in treatment-resistant depression after rTMS therapy. Int. J. Neuropsychopharmacol. 2015;18(11):pyv059. doi: 10.1093/ijnp/pyv059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W., Gu G.-J., Zhang Q.i., Liu J.-H., Zhang B.o., Guo Y.i., Wang M.-Y., Gong Q.-Y., Xu J.-R. NSCs promote hippocampal neurogenesis, metabolic changes and synaptogenesis in APP/PS1 transgenic mice. Hippocampus. 2017;27(12):1250–1263. doi: 10.1002/hipo.22794. [DOI] [PubMed] [Google Scholar]
- Zhong S., Wang Y., Zhao G., Xiang Q.i., Ling X., Liu S., Huang L.i., Jia Y. Similarities of biochemical abnormalities between major depressive disorder and bipolar depression: a proton magnetic resonance spectroscopy study. J. Affect. Disord. 2014;168:380–386. doi: 10.1016/j.jad.2014.07.024. [DOI] [PubMed] [Google Scholar]