Abstract
A subset of dermal fibroblasts undergo rapid differentiation into adipocytes in response to infection and acutely produce the cathelicidin antimicrobial peptide gene Camp. Vitamin A and other retinoids inhibit adipogenesis, yet can show benefit to skin disorders such as cystic acne that are exacerbated by bacteria. We observed that retinoids potently increase and sustain the expression of Camp in preadipocytes undergoing adipogenesis despite inhibition of markers of adipogenesis such as Adipoq, Fabp4 and Rstn. Retinoids increase cathelicidin in both mouse and human preadipocytes, but this enhancement of antimicrobial peptide expression did not occur in keratinocytes or a sebocyte cell line. Preadipocytes undergoing adipogenesis more effectively inhibited growth of S. aureus when exposed to retinoic acid. Whole transcriptome analysis identified hypoxia-inducible factor 1α (HIF-1α) as a mechanism through which retinoids mediate this response. These observations uncouple the lipid accumulation element of adipogenesis from the innate immune response and uncover a previously unsuspected mechanism that may explain therapeutic benefits of retinoids in some skin disorders.
Introduction
Retinoids comprise a large group of chemical compounds that are vitamin A (retinol) analogues and/or derivatives, and can influence multiple physiological processes including vision, cell proliferation, differentiation, and immunity (1, 2). Within the skin, retinoids have been shown to affect multiple cell types, modulating several processes including wound healing, pigmentation, and immune defense (3–9) and have been used pharmacologically to treat chronological and photoaging, psoriasis, and acne (9–13). There are few cases in which retinoids have exhibited direct antimicrobial activity (14, 15), however under most circumstances immune modulation by retinoids is thought to only occur indirectly (5–7, 9, 16–18). For example, the mechanism by which retinoids are therapeutically useful in acne, a disorder that is driven in part by the bacterium Cutibacterium acnes, is thought to occur through their effect to decrease sebum production and alter epidermal function rather than through an influence on immune defense against bacterial growth (9).
It has been recently observed that dermal fibroblasts of the preadipocyte lineage will undergo rapid, local proliferation and differentiation in response to bacterial infection of the skin (19, 20). This process, dubbed reactive adipogenesis, results in an increase in the expression of the cathelicidin gene CAMP, an antimicrobial peptide (AMP) that peaks during this process as the local adipocytes mature (20). Reactive adipogenesis is essential for optimal innate immune defense since impairment of the adipogenic process decreases total skin cathelicidin expression and increases the severity of bacterial infections (19–21).
Retinoids have been shown to inhibit adipogenesis and promote lipid breakdown (22–27), and therefore would be predicted to decrease cutaneous antimicrobial defense capacity. In this study, we sought to understand the effects of retinoids on AMP production during adipogenesis in order to better understand the therapeutic mechanism of action of retinoids. Despite exhibiting the anticipated anti-adipogenic effects, we unexpectedly observed that retinoids enhanced and sustained cathelicidin levels in developing preadipocytes, ultimately translating to increased antimicrobial activity. This process was found to depend in part on hypoxia-inducible factor 1-alpha (HIF1α), a broadly active transcription factor within the CAMP gene promoter. These observations reveal an uncoupling between adipogenesis and antimicrobial expression that could be exploited as a novel means of modulating innate immune defense.
Materials and Methods
Antibodies, chemicals, and reagents
Vitamin A (retinol), all trans-retinal, all trans-retinoic acid, 9 cis-retinoic acid, 13 -cis-retinoic acid, 1α,25-Dihydroxyvitamin D3, L-Mimosine, tazarotene, IBMX (3-Isobutyl-1-methylxanthine), dexamethasone, indomethacin, and recombinant human insulin were purchased from Sigma-Aldrich (St. Louis, MO). Acryflavine hydrochloride was purchased from Thermo-Fisher Scientific (Pittsburgh, PA). AM580 was purchased from Abcam. MALP-2 was purchased from Enzo Life Sciences. Rabbit anti-CRAMP antibodies were made in our lab as previously described (28). Mouse anti-FABP4 was purchased from Santa Cruz Biotechnology (Santa Cruz, CA).
Cell Culture
3T3-L1 primary mouse preadipocytes were purchased from ATCC (CL-173). Neonatal primary dermal fibroblasts were isolated by our lab as previously described (19). Cells were grown in DMEM supplemented with 10% FBS, glutamax, and antibiotic-antimycotic (Thermo Fisher Scientific) in a humidified incubator at 5% CO2 and 37°C under sterile conditions. To induce differentiation, two-day post-confluent 3T3-L1 cells were switched to adipocyte differentiation medium containing 2 μM dexamethasone, 250 μM IBMX, 200 μM indomethacin, and 10 μg/mL recombinant human insulin. For timecourse experiments, differentiation media was refreshed at indicated times. Immortalized non-diabetic human preadipocytes (29) were a generous gift from Dr. Patrick M. Schliever (Carver College of Medicine, Iowa City, Iowa). Human preadipocytes were grown and differentiated as previously described (29) and differentiation media was refreshed on indicated days. Human epidermal keratinocytes (Life Technologies, #C-001–5C) were cultured in EpiLife medium supplemented with 60 μM CaCl2, EpiLife Defined Growth Supplement (Life Technologies), and antibiotic/antimycotic. SEB-1 sebocytes were cultured in Sebomed basal medium (Millipore) supplemented with recombinant human epidermal growth factor (5 ng/ml) (Sigma). To examine the effects of retinoids on cathelicidin expression, retinoids (1 μM unless otherwise noted) or vehicle (ethanol) were added to differentiation media or cell culture media on day 0 (unless indicated otherwise). Retinoid-containing media was subsequentially refreshed every 2–3 days throughout the course of the experiment. Treatment of cells with 1α,25-Dihydroxyvitamin D3 (Vitamin D) (10 nM) or vehicle (ethanol) was conducted identical to that of retinoids. To examine the role of HIF-1α, cells were cotreated with L-mimosine (400 μM) or acryflavine (5 μM) or vehicle (water) for 24 hours.
RNA isolation, cDNA synthesis, and RT-qPCR analysis
Cultured cells were lysed with Trizol reagent (Life Technologies) or PureLink Lysis Buffer (Ambion/Life Technologies) and RNA was isolated using the PureLink isolation kit. Up to 1 μg of RNA was reverse transcribed to cDNA using the iScript cDNA synthesis kit (Bio-Rad). Quantitative real-time PCR were performed the CFX96 Real-Time System (Bio-Rad) using SYBR Green Mix (Biomake, Houston, TX). The housekeeping gene, Tbp (TAT-binding box protein), was used to normalize gene expression in samples. Specific primer sequences are shown in supplemental table 1.
Western Blots
Supernatants from treated cells were enriched for protein using Oasis HLB Plus Light Cartridges (Waters, Milford, MA) and lyophilized. Lyophilized protein from 500 μL of supernatant was separated on a Novex 10–20% Tricine Gel (Invitrogen) and transferred to a PVDF membrane using Trans-Blot Turbo Transfer Pack (Bio-Rad) according to the manufacturer’s instructions. Membranes were blocked with Odyssey blocking buffer (LI-COR) and incubated overnight at 4 °C with primary antibodies. Immunoblots were washed and incubated with fluorescent secondary antibodies (LI-COR) for 1 hour at room temperature and subsequentially imaged using the Odyssey Infrared Imager (LI-COR).
Immunofluorescence
For immunofluorescence analysis, 3T3-L1 cells were grown, differentiated, and treated in 8-well chamber slides as described earlier. Cells were fixed in 4% PFA for 10 minutes and subsequentially incubated with primary antibodies overnight at 4 °C. Cell were washed and incubated for 1 hour with secondary antibodies conjugated to Alexa Fluor 594 (Invitrogen) and nuclei were counterstained with DAPI. All images were taken with an Olympus BX41 microscope.
Histology and immunohistochemistry
Tissue biopsies were directly embedded in OCT compound. Frozen sections were fixed in 4% PFA for 10 minutes prior to Hematoxylin/Eosin (H&E) staining or immunofluorescence staining. For immunohistochemistry, fixed and permeabilized frozen tissue sections were stained with Bodipy solution to visualize lipid droplets and nuclei were counterstained with DAPI. All images were taken with an Olympus BX41 microscope.
In Vitro bacterial killing assay
Antibiotic free conditioned media was collected from differentiating dermal fibroblasts as previously described (20). Conditioned media (200 μL) was mixed with 105 colony forming units (CFU) of S. aureus stain USA300 in deep-well 96-well plates and incubated at 37° with shaking at 275 R.P.M. for 12 hours prior to plating for CFU counting.
Mice Experiments
All animal experiments were approved by the University of California, San Diego (UCSD), Institutional Animal Care and Use Committee. SKH-1 hairless mice were originally purchased from Charles River Laboratories and bred and maintained in animal facility of UCSD. Animals in all experimental models were age- and sex-matched. Skin infections were conducted as previously described (20) using S. aureus strain USA300 (MRSA) (S. aureus strain AH4807 was used for IVIS experiments) or PBS control. Mice were distally administered one subcutaneous injection with retinoic acid (10 mg/kg) or vehicle (DMSO) dissolved in olive oil on the day of infection and two additional injections on subsequent days. Mice were sacrificed on day 3 and a skin biopsy of the infected region was harvested. Skin biopsies were homogenized in PureLink Lysis Buffer (or PBS for CFU counting) with 2 mM zirconia beads in a mini-bead beater (Biospect, Bartleville, OK) for RNA extraction. To quantify CFU, homogenized skin samples were serially diluted and plated onto Baird-Parker agar and grown for 24 hrs. to quantify the CFU per gram of tissue. For in vivo live bacterial imaging, mice were imaged under isoflurane inhalation anesthesia (2%). Photons emitted from luminescent bacteria were collected during a one-minute exposure using the Xenogen IVIS Imaging System and living image software (Xenogen, Alameda, CA). Bioluminescent image data are presented on a pseudocolor scale overlaid onto a gray-scale photographic image. Using the image analysis tools in living image software, circular analysis windows (of uniform area) were overlaid onto regions of interest and the corresponding bioluminescence values (total flux) were measured.
RNA Sequencing
Purified RNA was submitted to the University of California, San Diego (UCSD) Institute for Genomic Medicine core facility for library preparation and high-throughput next-generation sequencing. Library preparation and subsequent analysis were conducted as previously described (30). Ven diagram was prepared using Venny (31).
Statistical analysis
Experiments conducted were performed at minimal performed in triplicate with at least three technical replicates. Statistical significance was calculated using a Student’s two-tailed t-test where *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001 as indicated in figure legends.
Results
Retinoids increase cathelicidin expression during adipogenesis
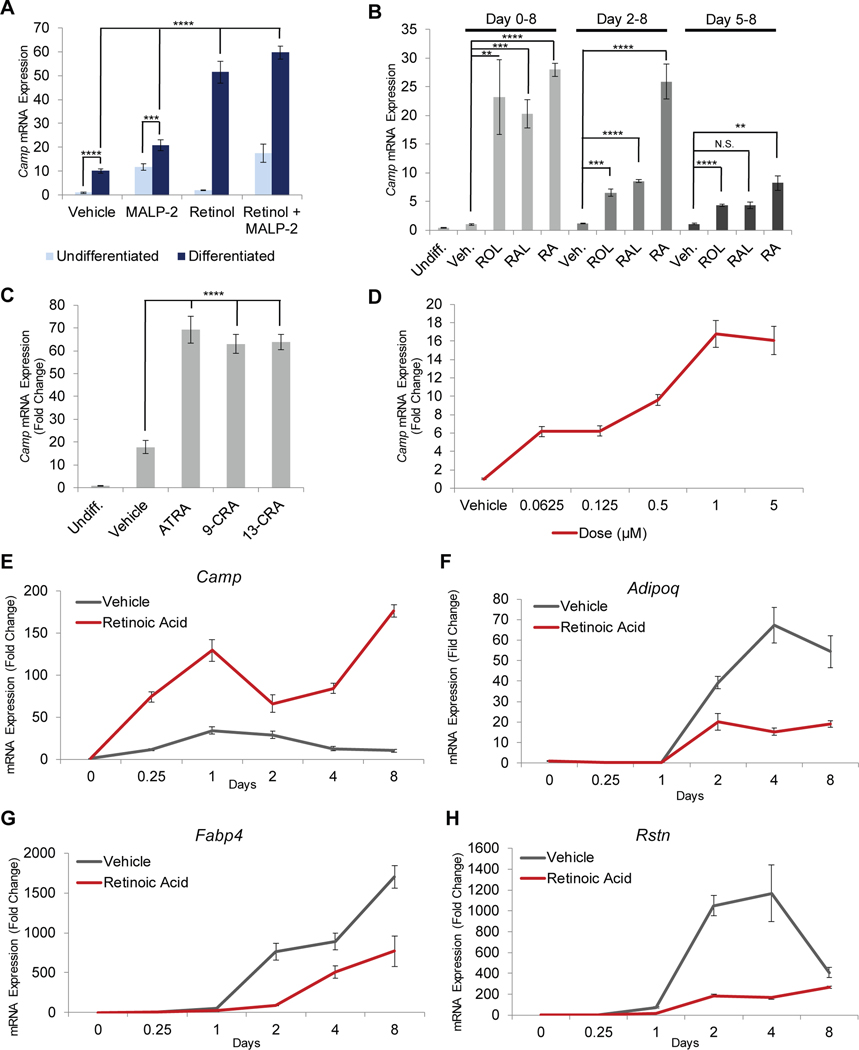
To determine the effects of retinoids on Camp expression during reactive adipogenesis, we first examined differentiation of preadipocytes in vitro under defined conditions. Differentiation was associated with increased Camp mRNA expression, and treatment with the TLR2/6 ligand, MALP-2, further stimulated Camp expression in both undifferentiated and differentiating 3T3-L1 preadipocytes (Figure 1A). The addition of vitamin A (retinol) greatly amplified the Camp response to differentiation, resulting in an increase in steady-state Camp mRNA levels compared to untreated 3T3-L1 cells with the greatest increase occurring with Malp-2 and retinol (Figure 1A).
Figure 1. Retinoids significantly increase cathelicidin expression during adipogenesis.
3T3-L1 preadipocytes were differentiated in the presence of retinoids or control, and relative mRNA expression of Camp (A-E), Adipoq (F), Fabp4 (G), or Rstn (H) was assayed by RT-qPCR. (A) 3T3-L1 cells were treated for 24 h in the presence of 1 μM retinol and/or 100 ng/ml MALP-2 or vehicle control in the absence or presence of differentiation media (DM). (B) Cells were differentiated for 8 days and co-treatment was initiated on day 0, day 2, or day 5 in the presence of 1 μM retinol (ROL), all-trans retinal (RAL), retinoic acid (RA), or control. (C) Cells were differentiated for 24 h in the presence of ATRA, 9-cis retinoic acid (9-CRA), or 13-cis retinoic acid (13-CRA). (D) Cells were differentiated for 24 h in the presence of increasing concentrations of RA. (E-H) Cells were differentiated for 8 days in the presence of RA or vehicle control and mRNA expression was assayed at given timepoints. All data are mean ± S.D, *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001 using a Student’s paired T test.
Vitamin A, (AKA, retinol (ROL) is an inactive precursor that can be enzymatically converted to retinoic acid (RA) or retinal (RAL) to exhibit physiological activity. Addition of ROL, RAL or RA had similar effects on Camp induction when added to 3T3-L1 cultures at the start of an 8-day differentiation experiment. RA appeared most potent when added between day 2–8 after differentiation was initiated, and induction was least when added later during adipogenesis at day 5–8 (Figure 1B). These findings suggest adipocyte precursors can metabolize Vitamin A to active forms.
RA can isomerize between all-trans (ATRA), 9-cis retinoic acid (9CRA), and 13-cis retinoic acid (13CA) (32). Similar activity was observed by each of these isoforms on Camp expression (Figure 1C). Using RA analogues specific for the retinoid acid receptors (RAR), agonists for both RARα and RARβ/γ potently stimulated Camp expression to an extent similar to RA (Supplemental Figure 1A). RA demonstrated a dose-dependent induction of Camp at 24 hours (Figure 1D), but significantly induced Camp expression in as little as 6 hours and resulted in sustained high levels of expression at day 8, a prolonged increase which contrasted to the normal downregulation of Camp seen during late adipogenesis in the absence of a retinoid (Figure 1E). Previous observations have shown that Camp is the only antimicrobial peptide induced during adipogenesis whereas expression of α- and β-defensins was not altered (20). Our current studies are consistent with these observations as addition of RA was unable to induce expression of other AMPs (Supplemental Figure 1B). Contrary to the increase in AMP gene expression that was promoted by retinoids, but consistent with prior observations of the effect of RA to inhibit adipogenesis (27, 33), we observed that RA inhibited expression of adipocyte maturation markers Adipoq, Fab4 and Rstn during the time course of this experiment (Figure 1F–G). These observations suggest that RA promoted an uncoupling between AMP expression and classic markers of adipocyte differentiation that are associated with lipid accumulation.
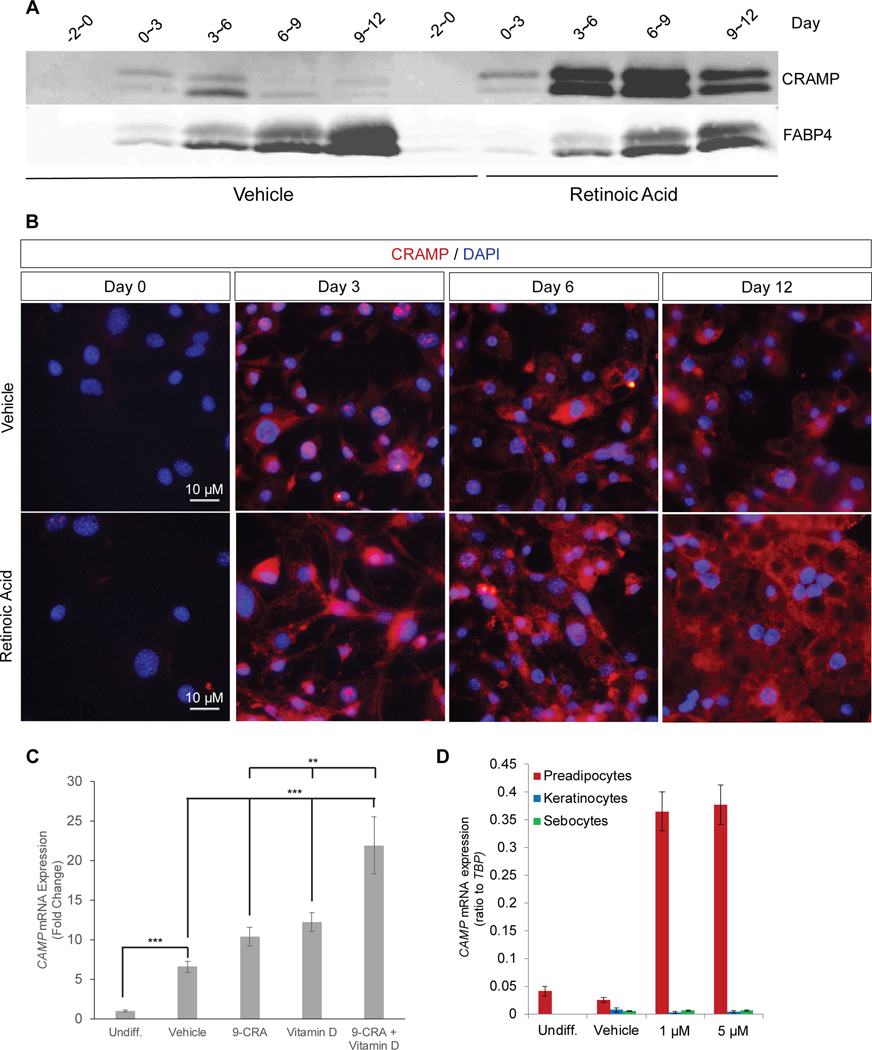
In agreement with our transcriptional studies, significantly elevated levels of the murine cathelicidin protein (Cramp) precursor protein were detected in RA treated 3T3-L1 cell supernatants (Figure 2A). Cramp immunostaining complimented these findings as more Cramp staining was seen in the cytoplasm of 3T3-L1 cells during differentiation into adipocytes in the presence of RA (Figure 2B). On day 12, numerous round areas in the cytosol that were unstained by DAPI or for Cramp are maturing lipid droplets.
Figure 2. Retinoic acid enhances adipogenic innate immune response in vitro.
(A)Western blot analysis of concentrated 3T3-L1 cell supernatants treated with 1 μM retinoic acid (RA) or vehicle (ethanol). (B) Immunofluorescence analysis of CRAMP expression (red) in cultured 3T3-L1 cells treated with 1 μM RA or vehicle. Nuclei are counterstained with DAPI. Scale bar 10 μm. (C) Human preadipocytes were differentiated for 16 days in presence of 1 μM 9-cis retinoic acid (9-CRA) and/or 10 nM 1α,25-Dihydroxyvitamin D3 (Vitamin D) or vehicle control and CAMP mRNA expression was assayed. (D) Differentiating 3T3-L1 preadipocytes, human keratinocytes, and human sebocytes were treated with 1 or 5 μM RA for 5 days and CAMP mRNA expression was measured relative to TBP. All data are mean ± S.D, *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001 using a Student’s paired T test.
Human and mouse cathelicidin genes exhibit several regulatory parallels, but also have important differences. The human CAMP promoter possesses an active vitamin D response element (VDR) that is lacking in mice (34, 35), and vitamin D can upregulate CAMP expression in several human cell types including human keratinocytes (34, 36). To determine if the observations made in murine-derived 3T3-L1 cells could be found in human preadipocytes, we investigated the effects of RA and vitamin D in a cultured human preadipocyte line (19, 20). Both 9-cis retinoic acid and vitamin D increased CAMP expression in human preadipocytes and exhibited an additive effect when cells were treated with both together (Figure 2C). However, RA was unable to stimulate CAMP expression under similar culture conditions in a human sebocyte cell line or in primary cultures of normal human keratinocytes (Figure 2D). Thus, we conclude that retinoids can increase expression of cathelicidin in both human and mouse preadipocytes but the effects of retinoic acid on this response are specific to cell type.
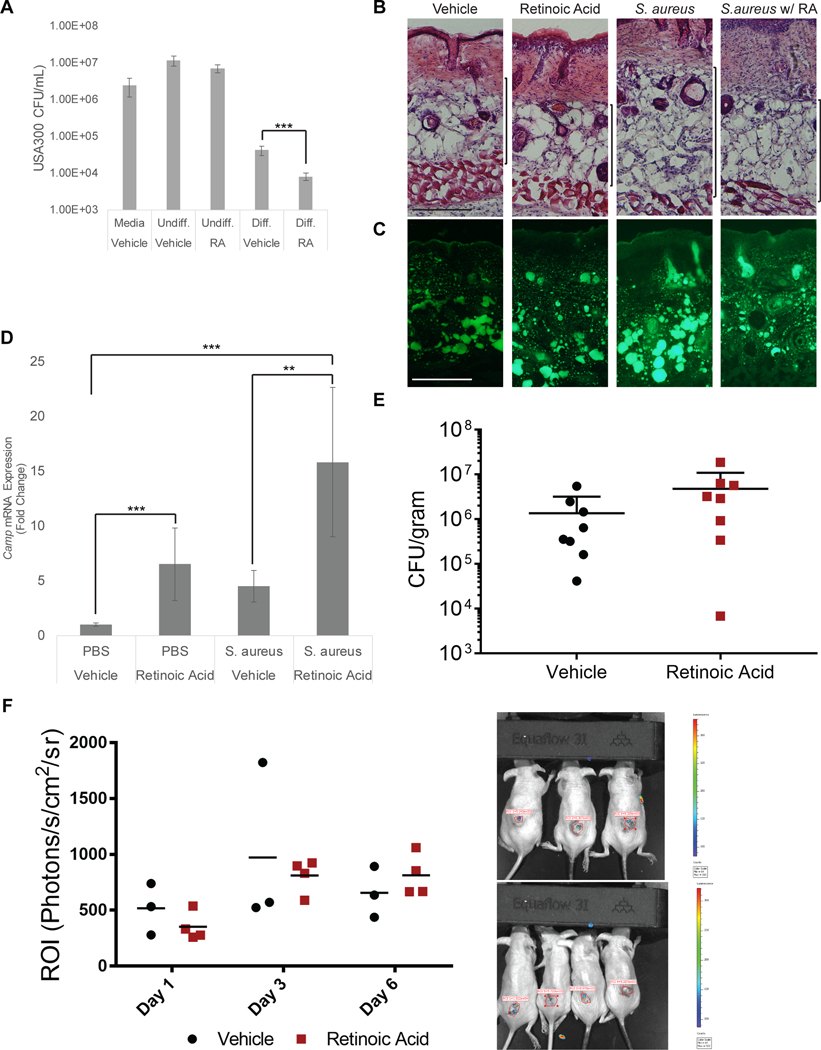
Given the significant cathelicidin upregulation observed with retinoid treatment, we next wished to determine whether these results correlate with antimicrobial activity. As our observations have shown that mouse neonatal dermal fibroblasts (MSFBs) express significantly more Cramp protein compared to 3T3-L1 cells (Supplemental Figure 2A) we selected to use MSFBs to determine antimicrobial activity. MSFB cells were stimulated to initiate adipogenesis in culture with in the presence of RA, and this culture supernatant then added to bacterial cultures of S. aureus (USA300). The growth of S. aureus was maximally inhibited from culture supernatants of cells treated with RA (Figure 3A). Despite promoting Camp expression and enhancing antimicrobial activity, RA’s effects on cytokine and chemokine expression were comparatively minor among under the conditions we examined (Supplemental Figure 2B).
Figure 3. Retinoic acid modulates reactive adipogenesis.
(A) Supernatants from differentiated and undifferentiated dermal fibroblasts were grown in the presence of USA300 for 12 hrs. and CFU counts were determined. (B-D) SKH-1 wildtype mice (~8 weeks old) were distally intradermally infected with S. aureus on day 0 and simultaneously treated subcutaneously with RA or olive oil (control) for 3 days (n=4 per group). (B) Representative H&E histology of skin on day 3 of infection,scale bar 100 μm. (C) Lipid droplet staining of frozen sections, scale bar 100 μm. (D) Skin-lesions were collected and gene expression was analyzed by qPCR for Camp expression. (E-F) SKH-1 wildtype mice were distally intradermally infected with S. aureus or bioluminescent S. aureus as on day 0 and treated subcutaneously with RA or olive oil (control) for 3 days (Day 0, 1, and 2). (E) Measurement of bacterial CFU from skin lesion of the infected area on day 3 (N=8) (F) Representative day 3 images of vehicle (top) and RA treated (bottom) and quantification of fluorescence in region of interest (ROI) of skin from images taken by IVIS on days 1, 3, and 6 (N=3 or 4). All data are mean ± S.D., *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001 using a Student’s paired T test.
Given our in vitro findings, we next wished to determine how RA might contribute to antimicrobial activity during S. aureus infection. Systemic treatment of mice with 10 mg/kg RA for 3 days decreased dermal adipose in uninfected skin as well as in skin after challenge with S. aureus (Figure 3B,C). Camp mRNA expression was induced by S. aureus and RA (Figure 3D). However, no consistent change in the susceptibility to S. aureus survival was seen in mice treated with RA by manual CFU counting of live bacteria (Figure 3E) or as detected by IVIS analysis of a reporter strain of S. aureus (Figure 3F).
Transcriptomic response of preadipocytes to RA identifies HIF1α as mediator of antimicrobial response.
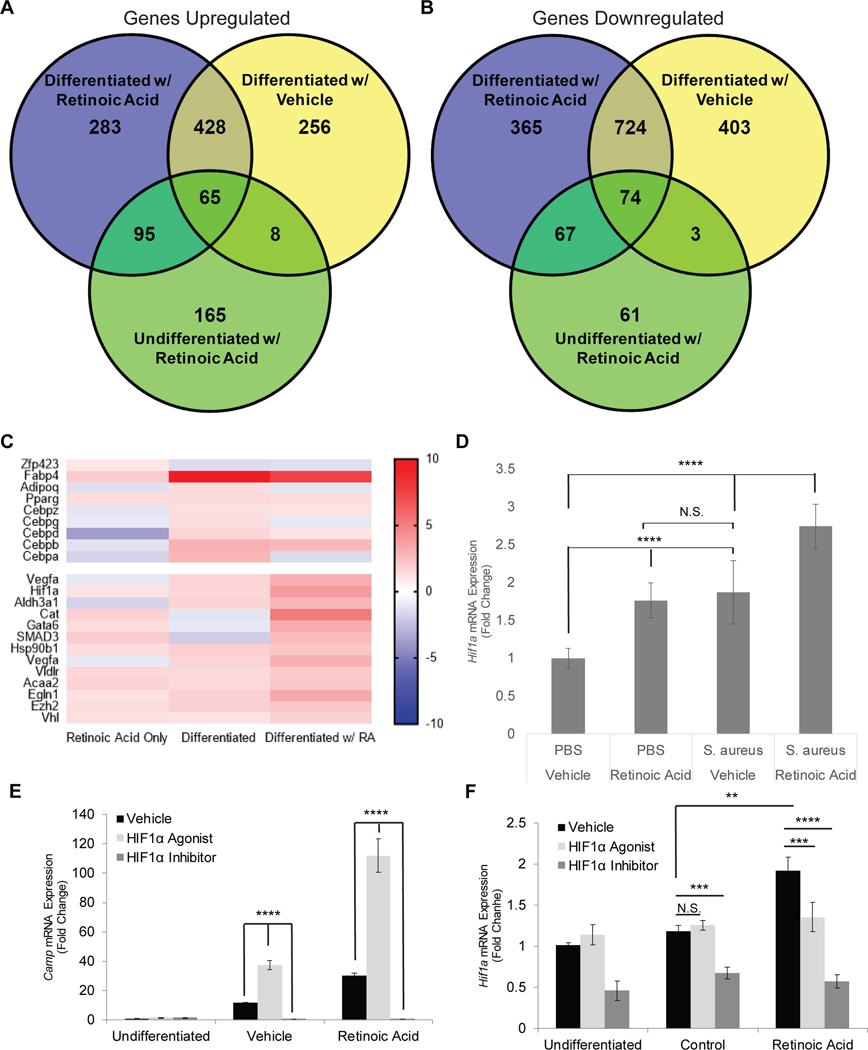
To gain further insight into the mechanism through which preadipocytes increase Camp in response to RA, we performed RNA-sequencing of 3T3-L1 cells. The expression of genes that were changed by more than 2-fold when 3T3-L1 cells were differentiated in the presence of retinoic acid were identified (Figure 4A–B). An increase in genes associated with adipogenesis (Zfp423, Fabp4, Adipoq, Cebpa) was evident with differentiation, but several genes involved in hypoxic responses were also observed to be upregulated by RA (Supplementary Figure 3A), particularly HIF1α (Figure 4C). Quantitative PCR validated that Hif1a expression was increased in both mouse and human preadipocytes treated with RA (Figures 4F and Supplemental Figure 3B). Hif1a mRNA expression was also increased in skin samples from mice treated with RA and infected with S. aureus (Figure 4D).
Figure 4. Retinoic acid and HIF1α coordinate to enhance innate immune defense.
Transcriptomic comparisons of genes (A) upregulated or (B) downregulated in 3T3-L1 cells differentiated and/or treated with RA for 24 hours. (C) Heat map clustering of adipogenic-related genes and hypoxia response genes. (D) SKH-1 wildtype mice (~8 weeks old) were distally intradermally infected with S. aureus on day 0 and simultaneously treated subcutaneously with RA or olive oil (control) for 3 days (n=4 per group). Skin-lesions were collected and gene expression was analyzed by qPCR. (E-F) 3T3-L1 cells were differentiated for 24 hours in the presence of RA supplemented with a HIF1α agonist (400 μM L-mimosine) or antagonist (5 μM acryflavine) along with appropriate controls and mRNA expression of (E) Camp or (F) Hif1a was determined by qPCR. and mRNA expression of (E) Camp or (F) Hif1a was determined by qPCR.
To ascertain the role of HIF1α in the response of preadipocytes to RA we used an established HIF1α agonist (L-mimosine) and inhibitor (acryflavine). Camp expression was induced in preadipocytes treated with the HIF1α agonist and further enhanced by RA, and the HIF1α antagonist blocked Camp expression (Figure 4E,F). Vascular endothelial growth factor A (VEGFA), a regulatory target of HIF1α, responded as expected with an increase in expression after agonist exposure and a decrease after antagonist treatment, thus serving as a control for the response to these chemical modifiers of HIF1α activity (Supplemental Figure 3C). Given our observations that MALP-2 can stimulate Camp expression both in undifferentiated and differentiating 3T3-L1 cells, we validated the role HIF1α in Camp regulation by cotreating cells with both acryflavine and MALP-2 under both conditions. Similarly, HIF1α inhibition effectively blocked MALP-2 induction of Camp expression and further evidenced the regulatory role of HIF1α (Supplemental Figure 3D, E).
Discussion
Retinoids are an effective therapy in the treatment of acne and other disorders (9–13). However, the clinical response to retinoids is dependent on mode of delivery and the mechanisms responsible for the physiological effects of retinoids are incompletely understood. Retinoids indeed have been shown to exhibit both anti-inflammatory and pro-inflammatory effects on cellular immune responses of the skin (9, 37–39), but the activity of retinoids on innate host antimicrobial defense have not been well characterized. In this study we present an unexpected observation that retinol and its metabolites potently enhance cathelicidin antimicrobial peptide expression during reactive adipogenesis. These findings suggest an alternative and previously underappreciated mechanism for the action of retinoids in the skin.
Retinoids inhibit adipogenesis and promote lipid breakdown (22–27). Inhibition of adipogenesis by inhibition of the Pparγ system will also inhibit the antimicrobial activity of dermal preadipocytes (19, 20). However, and contrary to expectations, we show in the current study that retinoids increase Camp expression despite exhibiting anti-adipogenic activity. This observation demonstrates an uncoupling between the processes of adipogenesis and antimicrobial peptide expression.
Previous observations have shown that adipocytes produce the cathelicidin precursor protein and that is upregulated during adipogenesis that is processed into an active antimicrobial peptide (CRAMP), however the enzyme that catalyzes this reaction in adipocytes has not been identified (19, 20). Our studies have shown that an increase in Camp gene expression then results in a significant upregulation of the CRAMP preprotein, yet the increase in antimicrobial activity was modest in comparison to the change in protein abundance. Whether this was indicative of a saturation of the system or modulation of CRAMP processing from inactive precursor protein to active peptide is unclear. We hypothesize that the contribution of other cells in vivo during the complex response of reactive adipogenesis may contribute to the maturation of the cathelicidin precursor that is enhanced by retinoids in preadipocytes.
Excess Vitamin A results in epidermal hyperplasia and a reduction in white adipose tissue (WAT), while infection results in an expansion of dermal white adipose tissue (DWAT) (20, 27, 40). Interestingly, RA treatment attenuated the DWAT expansion associated with S. aureus infection, yet Camp was still significantly upregulated in comparison to the individual stimuli. RA improved the capacity of differentiating preadipocytes to inhibit S. aureus. However, despite these observations that RA was active in vivo and induced antimicrobial activity in vitro, there was no clear increase in the antimicrobial activity in vivo of mouse skin against S. aureus. This suggests that the immunomodulatory role of RA in the skin is complex and may be dependent on dose, timing or target organism. Additional work is needed to define the physiological significance of retinoid exposure on innate immune defense.
To gain further insight and understand the complexity of RA-mediated regulation, we examined the transcriptomic response to RA in the context of differentiating preadipocytes. RNA-seq analysis identified HIF1α as a potential regulator of Camp expression. The human CAMP promoter possesses a HIF1α-response element (HRE) and has been implicated in cutaneous immune defense as well as CRAMP expression in the colon (41). The Hif1a promoter possesses a retinoic acid-response element (RARE) (42) and RA has been reported to enhance HIF1α expression under normoxic conditions (42–44). These past observations are in agreement with our RNA-seq and qPCR data where we found that RA induced elevated Hif1a. Similarly, activation of HIF1α by the iron-chelator L-mimosine mimicked the effects of RA on Camp expression. The combinatorial effect of both activation and increased Hif1a expression exhibited a significant additive effect, thus providing further evidence that HIF1α and RA work in concert to promote Camp expression. Interestingly, HIF1α inhibition abolished Camp expression under all conditions. This may suggest Camp transcription is entirely dependent on HIF1α or that the global effects of HIF1α further attenuate other mechanisms involved in the expression of Camp. Support for this comes from observations that HIF1α inhibition also downregulates Cebpb transcription and inhibits initiation of adipogenesis (45). Inhibition by acryflavine similarly downregulated Cebpb expression while L-mimosine downregulated Cebpa, the latter of which has also been proposed to regulate CAMP expression. Taken together this could suggest that C/EBPβ indirectly regulates Camp expression, however our data do not define if either C/EBPβ or C/EBPα directly regulate this process.
Induction of adipogenesis is mediated by C/EBPβ, which in turn mediates the expression of the preadipogenic factors C/EBPα and PPARγ (46, 47). While RA does not inhibit the expression of C/EBPβ, it has been shown to inhibit its function (22). Our observations have shown that initiation of differentiation, yet not final maturation of adipocytes, is sufficient and necessary to enhance Camp expression by retinoids. However, there appears to be a limited timeframe during which this effect can be observed as the potency of retinoid stimulation was markedly reduced when treatment was initiated at later timepoints during differentiation. This suggests that access to the Camp promoter is limited during adipogenesis, such a limited access period may partially explain why acute, high dose RA administration to mice did not improve resistance to S. aureus infection. Under these conditions initiation of adipogenesis is also inhibited and may counteract the beneficial host defense function of RA that we have observed in vitro. Analysis of low dose Vitamin A exposure, as well as correlation of responses in human subjects on RA therapy, is needed.
In summary, these observations have revealed a novel disassociation between adipogenesis and the process through which fibroblasts in the dermis that become adipocytes can produce cathelicidin. Reactive adipogenesis is a recently discovered and important physiological response to infection of skin and gut (19–21, 48). These findings provide further insight into how Camp is regulated in adipocytes, a mechanism with important therapeutic implications.
Supplementary Material
Key Points.
Retinoids enhance cathelicidin antimicrobial peptide expression during adipogenesis
The action of retinoids is dependent on hypoxia-inducible factor 1α
The capacity to induce cathelicidin may explain some therapeutic effects of retinoids
Acknowledgements
We thank members of the Gallo lab for helpful advice pertaining to experimental procedures and design. We also thank Dr. Patrick M. Schliever (Carver College of Medicine, Iowa City, Iowa) for the immortalized human adipocyte cell line.
This study was funded by NIH R01 grant R01AR069653.
Footnotes
Disclosures
RLG is a consultant for and has equity interest in MatriSys bioscience and Sente Inc.
References
- 1.Morriss-Kay GM. Treatment of mice with retinoids in vivo and in vitro. Skeletal staining. Methods Mol Biol. 1999;97:33–9. [DOI] [PubMed] [Google Scholar]
- 2.Kane MA, Napoli JL. Quantification of endogenous retinoids. Methods Mol Biol. 2010;652:1–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gendimenico GJ, Mezick JA. Pharmacological effects of retinoids on skin cells. Skin Pharmacol. 1993;6 Suppl 1:24–34. [DOI] [PubMed] [Google Scholar]
- 4.Paterson EK, Ho H, Kapadia R, Ganesan AK. 9-cis retinoic acid is the ALDH1A1 product that stimulates melanogenesis. Exp Dermatol. 2013;22(3):202–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morizane S, Yamasaki K, Kabigting FD, Gallo RL. Kallikrein expression and cathelicidin processing are independently controlled in keratinocytes by calcium, vitamin D(3), and retinoic acid. J Invest Dermatol. 2010;130(5):1297–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kligman LH. Effects of all-trans-retinoic acid on the dermis of hairless mice. J Am Acad Dermatol. 1986;15(4 Pt 2):779–85, 884–7. [DOI] [PubMed] [Google Scholar]
- 7.Hunt TK. Vitamin A and wound healing. J Am Acad Dermatol. 1986;15(4 Pt 2):817–21. [DOI] [PubMed] [Google Scholar]
- 8.Kitano Y, Yoshimura K, Uchida G, Sato K, Harii K. Pretreatment with topical all-trans-retinoic acid is beneficial for wound healing in genetically diabetic mice. Arch Dermatol Res. 2001;293(10):515–21. [DOI] [PubMed] [Google Scholar]
- 9.Chivot M. Retinoid therapy for acne. A comparative review. Am J Clin Dermatol. 2005;6(1):13–9. [DOI] [PubMed] [Google Scholar]
- 10.Kafi R, Kwak HS, Schumacher WE, Cho S, Hanft VN, Hamilton TA, et al. Improvement of naturally aged skin with vitamin A (retinol). Arch Dermatol. 2007;143(5):606–12. [DOI] [PubMed] [Google Scholar]
- 11.Mukherjee S, Date A, Patravale V, Korting HC, Roeder A, Weindl G. Retinoids in the treatment of skin aging: an overview of clinical efficacy and safety. Clin Interv Aging. 2006;1(4):327–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roeder A, Schaller M, Schafer-Korting M, Korting HC. Tazarotene: therapeutic strategies in the treatment of psoriasis, acne and photoaging. Skin Pharmacol Physiol. 2004;17(3):111–8. [DOI] [PubMed] [Google Scholar]
- 13.Hoegler KM, John AM, Handler MZ, Schwartz RA. Generalized pustular psoriasis: a review and update on treatment. J Eur Acad Dermatol Venereol. 2018;32(10):1645–51. [DOI] [PubMed] [Google Scholar]
- 14.Pechere M, Germanier L, Siegenthaler G, Pechere JC, Saurat JH. The antibacterial activity of topical retinoids: the case of retinaldehyde. Dermatology. 2002;205(2):153–8. [DOI] [PubMed] [Google Scholar]
- 15.Kim W, Zhu W, Hendricks GL, Van Tyne D, Steele AD, Keohane CE, et al. A new class of synthetic retinoid antibiotics effective against bacterial persisters. Nature. 2018;556(7699):103–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wheelwright M, Kim EW, Inkeles MS, De Leon A, Pellegrini M, Krutzik SR, et al. All-trans retinoic acid-triggered antimicrobial activity against Mycobacterium tuberculosis is dependent on NPC2. J Immunol. 2014;192(5):2280–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.le Maire A, Alvarez S, Shankaranarayanan P, Lera AR, Bourguet W, Gronemeyer H. Retinoid receptors and therapeutic applications of RAR/RXR modulators. Curr Top Med Chem. 2012;12(6):505–27. [DOI] [PubMed] [Google Scholar]
- 18.Hammerschmidt SI, Friedrichsen M, Boelter J, Lyszkiewicz M, Kremmer E, Pabst O, et al. Retinoic acid induces homing of protective T and B cells to the gut after subcutaneous immunization in mice. J Clin Invest. 2011;121(8):3051–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang LJ, Chen SX, Guerrero-Juarez CF, Li F, Tong Y, Liang Y, et al. Age-Related Loss of Innate Immune Antimicrobial Function of Dermal Fat Is Mediated by Transforming Growth Factor Beta. Immunity. 2019;50(1):121–36 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang LJ, Guerrero-Juarez CF, Hata T, Bapat SP, Ramos R, Plikus MV, et al. Innate immunity. Dermal adipocytes protect against invasive Staphylococcus aureus skin infection. Science. 2015;347(6217):67–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dokoshi T, Zhang LJ, Nakatsuji T, Adase CA, Sanford JA, Paladini RD, et al. Hyaluronidase inhibits reactive adipogenesis and inflammation of colon and skin. JCI Insight. 2018;3(21). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schwarz EJ, Reginato MJ, Shao D, Krakow SL, Lazar MA. Retinoic acid blocks adipogenesis by inhibiting C/EBPbeta-mediated transcription. Mol Cell Biol. 1997;17(3):1552–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Berry DC, DeSantis D, Soltanian H, Croniger CM, Noy N. Retinoic acid upregulates preadipocyte genes to block adipogenesis and suppress diet-induced obesity. Diabetes. 2012;61(5):1112–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ziouzenkova O, Orasanu G, Sharlach M, Akiyama TE, Berger JP, Viereck J, et al. Retinaldehyde represses adipogenesis and diet-induced obesity. Nat Med. 2007;13(6):695–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Garcia E, Lacasa D, Agli B, Giudicelli Y, Castelli D. Antiadipogenic properties of retinol in primary cultured differentiating human adipocyte precursor cells. Int J Cosmet Sci. 2000;22(2):95–103. [DOI] [PubMed] [Google Scholar]
- 26.Sagara C, Takahashi K, Kagechika H, Takahashi N. Molecular mechanism of 9-cis-retinoic acid inhibition of adipogenesis in 3T3-L1 cells. Biochem Biophys Res Commun. 2013;433(1):102–7. [DOI] [PubMed] [Google Scholar]
- 27.Bonet ML, Canas JA, Ribot J, Palou A. Carotenoids and their conversion products in the control of adipocyte function, adiposity and obesity. Arch Biochem Biophys. 2015;572:112–25. [DOI] [PubMed] [Google Scholar]
- 28.Dorschner RA, Pestonjamasp VK, Tamakuwala S, Ohtake T, Rudisill J, Nizet V, et al. Cutaneous injury induces the release of cathelicidin anti-microbial peptides active against group A Streptococcus. J Invest Dermatol. 2001;117(1):91–7. [DOI] [PubMed] [Google Scholar]
- 29.Vu BG, Gourronc FA, Bernlohr DA, Schlievert PM, Klingelhutz AJ. Staphylococcal superantigens stimulate immortalized human adipocytes to produce chemokines. PLoS One. 2013;8(10):e77988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sanford JA, Zhang LJ, Williams MR, Gangoiti JA, Huang CM, Gallo RL. Inhibition of HDAC8 and HDAC9 by microbial short-chain fatty acids breaks immune tolerance of the epidermis to TLR ligands. Sci Immunol. 2016;1(4). [DOI] [PubMed] [Google Scholar]
- 31.Oliveros JC. Venny. An interactive tool for comparing lists with Venn’s diagrams. 2007-2015. [Available from: http://bioinfogp.cnb.csic.es/tools/venny/.
- 32.O’Byrne SM, Blaner WS. Retinol and retinyl esters: biochemistry and physiology. J Lipid Res. 2013;54(7):1731–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bonet ML, Canas JA, Ribot J, Palou A. Carotenoids in Adipose Tissue Biology and Obesity. Subcell Biochem. 2016;79:377–414. [DOI] [PubMed] [Google Scholar]
- 34.Wang TT, Nestel FP, Bourdeau V, Nagai Y, Wang Q, Liao J, et al. Cutting edge: 1,25-dihydroxyvitamin D3 is a direct inducer of antimicrobial peptide gene expression. J Immunol. 2004;173(5):2909–12. [DOI] [PubMed] [Google Scholar]
- 35.Gombart AF. The vitamin D-antimicrobial peptide pathway and its role in protection against infection. Future Microbiol. 2009;4(9):1151–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schauber J, Dorschner RA, Yamasaki K, Brouha B, Gallo RL. Control of the innate epithelial antimicrobial response is cell-type specific and dependent on relevant microenvironmental stimuli. Immunology. 2006;118(4):509–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Babina M, Guhl S, Motakis E, Artuc M, Hazzan T, Worm M, et al. Retinoic acid potentiates inflammatory cytokines in human mast cells: identification of mast cells as prominent constituents of the skin retinoid network. Mol Cell Endocrinol. 2015;406:49–59. [DOI] [PubMed] [Google Scholar]
- 38.Tsukada M, Schroder M, Roos TC, Chandraratna RA, Reichert U, Merk HF, et al. 13-cis retinoic acid exerts its specific activity on human sebocytes through selective intracellular isomerization to all-trans retinoic acid and binding to retinoid acid receptors. J Invest Dermatol. 2000;115(2):321–7. [DOI] [PubMed] [Google Scholar]
- 39.Oliveira LM, Teixeira FME, Sato MN. Impact of Retinoic Acid on Immune Cells and Inflammatory Diseases. Mediators Inflamm. 2018;2018:3067126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rittie L, Varani J, Kang S, Voorhees JJ, Fisher GJ. Retinoid-induced epidermal hyperplasia is mediated by epidermal growth factor receptor activation via specific induction of its ligands heparin-binding EGF and amphiregulin in human skin in vivo. J Invest Dermatol. 2006;126(4):732–9. [DOI] [PubMed] [Google Scholar]
- 41.Elloumi HZ, Holland SM. Complex regulation of human cathelicidin gene expression: novel splice variants and 5’UTR negative regulatory element. Mol Immunol. 2008;45(1):204–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fernandez-Martinez AB, Jimenez MI, Hernandez IS, Garcia-Bermejo ML, Manzano VM, Fraile EA, et al. Mutual regulation of hypoxic and retinoic acid related signalling in tubular proximal cells. Int J Biochem Cell Biol. 2011;43(8):1198–207. [DOI] [PubMed] [Google Scholar]
- 43.Liang C, Guo S, Yang L. Effects of alltrans retinoic acid on VEGF and HIF1alpha expression in glioma cells under normoxia and hypoxia and its antiangiogenic effect in an intracerebral glioma model. Mol Med Rep. 2014;10(5):2713–9. [DOI] [PubMed] [Google Scholar]
- 44.Fernandez-Martinez AB, Arenas Jimenez MI, Lucio Cazana FJ. Retinoic acid increases hypoxia-inducible factor-1alpha through intracrine prostaglandin E(2) signaling in human renal proximal tubular cells HK-2. Biochim Biophys Acta. 2012;1821(4):672–83. [DOI] [PubMed] [Google Scholar]
- 45.Yamaguchi J, Tanaka T, Saito H, Nomura S, Aburatani H, Waki H, et al. Echinomycin inhibits adipogenesis in 3T3-L1 cells in a HIF-independent manner. Sci Rep. 2017;7(1):6516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lefterova MI, Haakonsson AK, Lazar MA, Mandrup S. PPARgamma and the global map of adipogenesis and beyond. Trends Endocrinol Metab. 2014;25(6):293–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Darlington GJ, Ross SE, MacDougald OA. The role of C/EBP genes in adipocyte differentiation. J Biol Chem. 1998;273(46):30057–60. [DOI] [PubMed] [Google Scholar]
- 48.Chen SX, Zhang LJ, Gallo RL. Dermal White Adipose Tissue: A Newly Recognized Layer of Skin Innate Defense. J Invest Dermatol. 2019;139(5):1002–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.