Abstract
Aim
In acute medicine, we occasionally treat life‐threatening conditions such as sepsis and trauma, which cause severe thrombocytopenia. Serum thrombopoietin levels have been reported to increase under the condition of thrombocytopenia related to severity. Collagen is a crucial activator of platelets, and Rho family members, such as Rho/Rho‐kinase and Rac, play roles as active molecules involved in the intracellular signaling pathways in platelet activation. The present study aimed to elucidate the effects of thrombopoietin (TPO) on subthreshold low‐dose collagen‐stimulated human platelets in terms of Rho/Rho‐kinase and Rac.
Methods
Platelet‐rich plasma donated from healthy volunteers was stimulated by the subthreshold low‐dose of collagen after pretreatment with TPO and/or NSC23766, an inhibitor of the Rac‐guanine nucleotide exchange factor interaction, or Y27632, an inhibitor of Rho‐kinase. Platelet aggregation was measured using an aggregometer based on laser‐scattering methods. Proteins involved in intracellular signaling were analyzed using western blotting, and the secretion of platelet‐derived growth factor‐AB from activated platelets was determined using an enzyme‐linked immunosorbent assay.
Results
Under the existence of TPO, the low dose of collagen remarkably elicited the aggregation and platelet‐derived growth factor‐AB secretion of platelets, which were suppressed by NSC23766 and Y27632. The combination of TPO and collagen considerably induced a transient increase of guanosine triphosphate (GTP)‐binding Rac and GTP‐binding Rho followed by an increase of phosphorylated cofilin, a Rho‐kinase substrate.
Conclusion
These results strongly suggest that TPO and collagen in low doses cooperatively potentiate human platelet activation through both Rac and Rho/Rho‐kinase mediated pathways.
Keywords: Coagulopathy, collagen, human platelets, thrombocytopenia, thrombopoetin
Thrombopoietin and collagen in low doses cooperatively potentiate human platelet activation via both Rac and Rho/Rho‐kinase mediated pathways. The effect of TPO on platelet activation under the stimulation of collagen, even though of which the levels are insufficient to trigger by itself, might indicate a compensatory mechanism to enhance platelet activation in thrombocytopenia.

INTRODUCTION
In acute medicine, we occasionally treat life‐threatening conditions such as sepsis and trauma, which cause severe thrombocytopenia. It has been reported that serum levels of thrombopoietin (TPO), a humoral growth factor that promotes the proliferation and differentiation of megakaryocytes, 1 increase under the condition of thrombocytopenia related to severity. 2 , 3 Thrombopoietin does not induce platelet aggregation by itself but promotes platelet activation in response to some agonists. 4 These findings let us speculate that TPO plays an important role in platelet activation, especially in the thrombocytopenic status caused by emergent severe diseases. Thrombopoietin produced by the liver and kidneys is physiologically eliminated from the circulation by binding to receptors expressed mainly on platelets and megakaryocytes. 5 In addition, the interaction of TPO with its receptor on platelets, c‐Mpl, activates various signaling pathways. 6 , 7
Collagen is a component of the subendothelial layer, which determines the thrombogenicity of the vessel wall. 8 Exposed at the sites of traumatic vascular injuries or endothelial damages by sepsis, collagen acts as a crucial activator of human platelets by binding to its receptors, glycoprotein VI (GPVI) and integrin‐α2ß1, expressed on platelets. 8 Activated integrin‐α2ß1 binds and adheres tightly to collagen, and the interaction between GPVI and collagen is enhanced, leading to integrated signaling, further upregulation of integrin activity, and enhancement of granule secretion and coagulation activity. 9 Thus, initial tethering to vascular injury sites by exposed subendothelial collagen causes platelet adhesion and aggregation, which trigger platelet activation, resulting in granule secretion composing positive feedback to amplify the activation. We previously reported that collagen activates platelets and promotes granule secretion, such as platelet‐derived growth factor (PDGF)‐AB, 10 which also potentiates platelet activation, known as a positive feedback mechanism. 11
Rho and Rac, which belong to a small‐molecular‐weight guanosine triphosphate (GTP)‐binding protein superfamily named the Rho family, are inactive when bound to guanosine diphosphate, but are activated upon the exchange of guanosine diphosphate for GTP, leading to downstream signaling. 12 Rho and the downstream effector Rho‐associated kinase (Rho‐kinase) are currently established to play crucial roles in various cellular functions. 12 In regard to platelet aggregation, we previously reported that both Rho and Rac are involved in human platelet activation, including PDGF‐AB secretion induced by thromboxane A2. 13 , 14 We also reported that collagen‐induced human platelet activation is regulated by Rac. 15 However, the exact mechanism behind the effects of TPO and collagen interactions on platelet activation has not yet been elucidated. The present study aimed to elucidate the effects of TPO on subthreshold low‐dose collagen‐stimulated human platelets in terms of Rho/Rho‐kinase and Rac.
METHODS
Materials
Collagen was purchased from Takeda Austria (Linz, Austria). Recombinant human TPO and PDGF‐AB enzyme‐linked immunosorbent assay (ELISA) kits were purchased from R&D Systems, Inc. (Minneapolis, MN, USA). NSC23766 and Y27632 were purchased from Tocris Bioscience (Bristol, UK) and Calbiochem‐Novabiochem Co. (La Jolla, CA, USA), respectively. Control immunoglobulin G and glyceraldehyde‐3‐phosphate dehydrogenase (GAPDH) antibodies were purchased from Santa Cruz Biotechnology Inc. (Santa Cruz, CA, USA). Phospho‐specific cofilin antibodies were obtained from Cell Signaling Technology, Inc. (Beverly, MA, USA). Rac1 and Rho activation assay kits were obtained from Millipore Co. (Billerica, MA, USA).
Platelet preparation
Human blood was donated from healthy volunteers and immediately added to a 1/10 volume of 3.8% sodium citrate. Platelet‐rich plasma (PRP) was obtained by centrifugation at 155 g at room temperature for 12 min. Platelet‐poor plasma was obtained from the residual samples by centrifugation at 1,400 g at room temperature for 5 min. 15 The present study was composed of in vitro experiments. This study was approved by the Ethics Committee of Gifu University Graduate School of Medicine (Gifu, Japan). Written informed consent was obtained from all participants.
Platelet aggregation
Platelet aggregation was measured using PA‐200 aggregometer (Kowa Co. Ltd., Tokyo, Japan), which can analyze the size of platelet aggregates based on particle counting by laser scattering methods (small, 9–25 μm; medium, 25–50 μm; and large, 50–70 μm). Preincubation of PRP was carried out at 37°C for 1 min with a stirring speed of 800 rpm. PRP was pretreated with TPO for 15 min, and then stimulated by collagen. When indicated, PRP was pretreated with NSC23766 or Y27632 for 3 min before the stimulation by collagen. Platelet aggregation was monitored for 10 min. The doses of TPO and collagen were adjusted individually to achieve 80%–100% aggregation by a percentage transmittance stimulated by the combination, but not to induce considerable aggregation stimulated by alone. The percentage of transmittance of the isolated platelets was recorded as 0%, and that of platelet‐poor plasma was recorded as 100%. 15
Protein preparation after stimulation
After the pretreatment with TPO and the stimulation by collagen, platelet aggregation was terminated by adding ice‐cold ethylenediaminetetraacetic acid (EDTA; 10 mmol/L). The mixture was centrifuged at 10,000 g at 4°C for 2 min. The supernatant was collected for ELISA and stored at −80°C until the determination. The pellet was washed twice with phosphate‐buffered saline (PBS) and then lysed by boiling in a lysis buffer 62.5 mmol/L Tris–HCl, pH 6.8, 2% sodium dodecyl sulfate (SDS), 50 mmol/L dithiothreitol, and 10% glycerol for western blot analysis. 15
Western blot analysis
Western blot analysis was carried out as described previously. 16 Briefly, SDS–polyacrylamide gel electrophoresis (SDS‐PAGE) was carried out as described by Laemmli 17 using 10% or 12.5% polyacrylamide gel. The proteins in the gel were transferred onto a polyvinylidene difluoride (PVDF) membrane and blocked with 5% fat‐free dry milk in PBS containing 0.1% Tween 20 (PBS‐T; 10 mmol/L Na2HPO4, 1.8 mmol/L KH2PO4, pH 7.4, 137 mmol/L NaCl, 2.7 mmol/L KCl, 0.1% Tween 20) for 2 h and then incubated with the indicated primary antibodies. Peroxidase‐labeled anti‐rabbit IgG antibodies were used as secondary antibodies. The primary and secondary antibodies were diluted to optimal concentrations with 5% fat‐free dry milk in PBS‐T. The peroxidase activity on the PVDF membrane was visualized on an X‐ray film using an ECL western blot detection system (GE Healthcare, Chalfont St. Giles, UK) as described in the manufacturer's protocol. A densitometric analysis was undertaken using a scanner and imaging software program (Image J version 1.50; NIH, Bethesda, MD, USA). The phosphorylation levels were calculated as follows: the background‐subtracted intensity of each signal was normalized to the respective intensity of total protein or GAPDH and plotted as the fold increase compared with that of the control cells. 15
Measurement of Rac and Rho activities
Following the stimulation by 0.2 μg collagen after the pretreatment with 60 ng/mL TPO, the reaction was terminated by adding ice‐cold EDTA (10 mmol/L). The mixture was centrifuged at 10,000 g at 4°C for 2 min. The pellet was washed twice with ice‐cold Tris‐buffered saline, and the activities of Rac1 and Rho were determined using the responding activation kit. 15
Measurement of plasma PDGF‐AB level
The PDGF‐AB levels in the supernatant of the conditioned mixture were determined using an ELISA kit for PDGF‐AB according to the manufacturer's instructions. 15
RESULTS
Effect of TPO and low‐dose collagen on the platelet aggregation
We first examined the effect of TPO pretreatment at a fixed dose (10 ng/mL) on the platelet aggregation stimulated by various doses (0.1, 0.2, and 0.3 μg/mL) of collagen. Thrombopoietin alone (10 ng/mL) had no effect, whereas the dose of TPO significantly potentiated the platelet aggregation in combination with collagen at a dose of 0.3 μg/mL, which caused the increase of large aggregate ratio up to approximately 60% (Fig. 1A). Indeed, a slight decrease of small aggregate ratio was observed with stimulation at a dose of 0.2 μg/mL, suggesting the dose‐dependent effect. We next examined the effect of TPO pretreatment at various doses (1, 3, and 10 ng/mL) on the platelet aggregation stimulated by a fixed dose (0.3 μg/mL) of collagen. Neither 0.3 μg/mL of collagen alone nor the combination with TPO up to 3 ng/mL affected platelet aggregation, whereas the subthreshold dose of collagen in combination with 10 ng/mL TPO significantly induced platelet aggregation with an increase of the large aggregate ratio (Fig. 1B).
Fig. 1.

Effect of thrombopoietin (TPO) and low‐dose collagen on the aggregation of platelets. (A) Platelet‐rich plasma (PRP) was pretreated with 10 ng/mL TPO or vehicle for 15 min and then stimulated by 0, 0.1, 0.2, or 0.3 μg/mL of collagen for 10 min. (B) PRP was pretreated with 0, 1, 3, or 10 ng/mL TPO for 15 min and then stimulated by 0.3 μg/mL collagen or vehicle for 10 min. Black line, percentage of transmittance of each sample (isolated platelets recorded as 0%, and platelet‐poor plasma recorded as 100%; blue line, small aggregates (9–25 μm); green line, medium aggregates (25–50 μm); red line, large aggregates (50–70 μm). The lower panel presents the distribution (%) of aggregated particle size by area under the curve of each line. Representative results obtained from 10 healthy donors are shown.
Effect of TPO and low‐dose collagen on Rac activation in platelets
Based on our previous finding that Rac1 is involved in collagen‐induced platelet activation, we investigated whether the combination of TPO and low‐dose collagen induces the activation of Rac in human platelets. The combination of TPO (60 ng/mL) and collagen (0.2 μg/mL) increased GTP‐binding Rac for up to 3 min, and decreased almost to the basal levels at 5 min after the stimulation (Fig. 2).
Fig. 2.

Effect of thrombopoietin (TPO) and low‐dose collagen on the activation of Rac in platelets. Platelet‐rich plasma (PRP) was pretreated with 60 ng/mL TPO for 15 min and then stimulated by 0.2 μg/mL collagen for the indicated periods. The reaction was terminated by the addition of ice‐cold ethylenediaminetetraacetic acid (10 mmol/L) solution. The protein extracts were harvested as described in Methods and then guanosine triphosphate (GTP)‐Rac was immunoprecipitated using the Rac1 Activation Assay kit. The immunoprecipitated GTP‐Rac and pre‐immunoprecipitated lysates (Rac) were subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis using antibodies against Rac. The histogram shows the fold increase from unstimulated cells; data were obtained with laser densitometric analysis.
Effect of TPO and low‐dose collagen on activation of Rho/Rho‐kinase in platelets
We investigated whether the combination of TPO and a low dose of collagen induced the activation of Rho/Rho‐kinase in human platelets. The combination of TPO (60 ng/mL) and collagen (0.2 μg/mL) increased GTP‐binding Rho at 1 min after the stimulation and decreased to less than the basal levels thereafter (Fig. 3A). The phosphorylation of cofilin, a substrate of Rho‐kinase, 18 gradually increased up to 5 min after the stimulation (Fig. 3B).
Fig. 3.
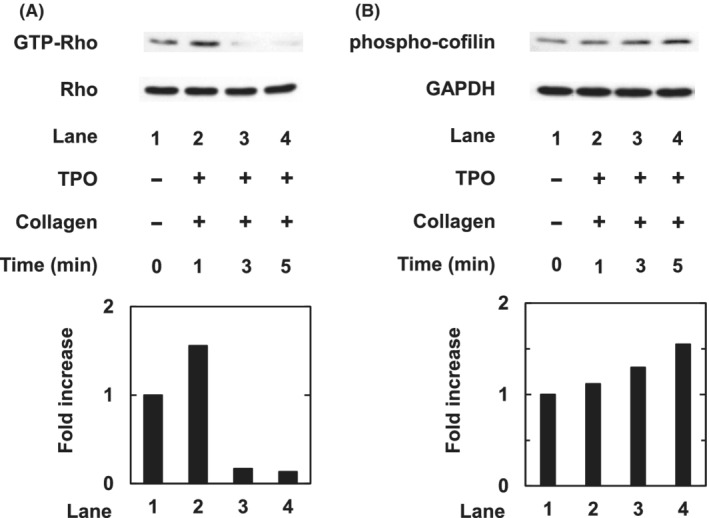
Effect of thrombopoietin (TPO) and low‐dose collagen on the activation of Rho/Rho‐kinase in platelets. Platelet‐rich plasma (PRP) was pretreated with 60 ng/mL TPO for 15 min and then stimulated by 0.2 μg/mL collagen for indicated periods. The reaction was terminated by the addition of ice‐cold ethylenediaminetetraacetic acid (10 mmol/L) solution. protein extracts were harvested as described in Methods. (A) Guanosine triphosphate (GTP)‐Rho was then immunoprecipitated using the Rho Activation Assay kit. The immunoprecipitated GTP‐Rho and pre‐immunoprecipitated lysates (Rho) were subjected to sodium dodecyl sulfate– polyacrylamide gel electrophoresis (SDS‐PAGE) using antibodies against Rho. (B) Protein extracts were subjected to SDS‐PAGE using antibodies of phosphorylated cofilin or GAPDH. The histogram shows the fold increase from unstimulated cells; data were obtained with laser densitometric analysis.
Effect of NSC23766 on platelet aggregation induced by combined TPO and low‐dose collagen
To clarify the involvement of Rac activation, we examined the effect of NSC23766, a selective inhibitor of Rac1‐guanine nucleotide exchange factor interaction, 19 on platelet aggregation induced by the combination of TPO and low‐dose collagen. Although NSC23766 at 10 μmol/L hardly affected the platelet aggregation induced by TPO and low‐dose collagen, NSC23766 at 20 and 30 μmol/L almost completely inhibited platelet aggregation, recorded as changes in the aggregate size ratio, decrease of large aggregates, and increase of small aggregates (Fig. 4).
Fig. 4.
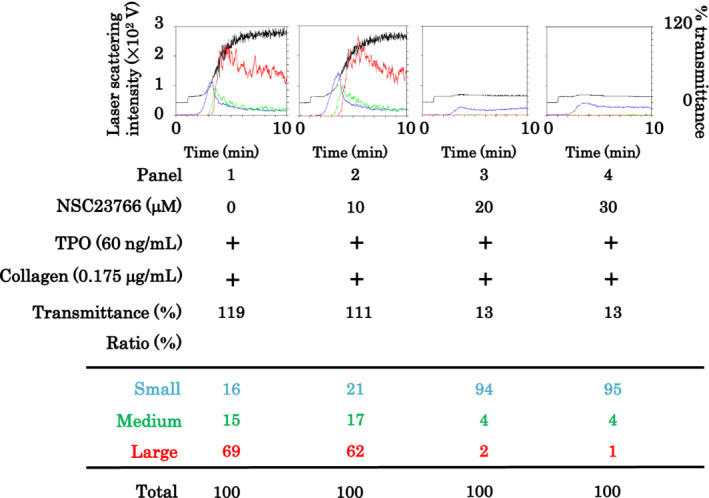
Effect of NSC23766 on platelet aggregation induced by the combination of thrombopoietin (TPO) and low‐dose collagen. Platelet‐rich plasma was pretreated with 0, 10, 20, or 30 μmol/L NSC23766 and 60 ng/mL TPO for 15 min and then stimulated by 0.175 μg/mL collagen for 10 min. Black line, percentage of transmittance of each sample (isolated platelets recorded as 0%, and platelet‐poor plasma recorded as 100%); blue line, small aggregates (9–25 μm); green line, medium aggregates (25–50 μm); red line, large aggregates (50–70 μm). The lower panel presents the distribution (%) of aggregated particle size by area under the curve of each line. Representative results obtained from 10 healthy donors are shown.
Effect of Y27632 on platelet aggregation induced by combined TPO and low‐dose collagen
To investigate the involvement of Rho activation, we examined the effect of Y27632, a Rho‐kinase inhibitor, 20 on platelet aggregation induced by the combination of TPO and low‐dose collagen. Although Y27632 at doses up to 20 μmol/L hardly affected platelet aggregation induced by TPO and low‐dose collagen, Y27632 at a dose of 30 μmol/L markedly suppressed the platelet aggregation, recorded as the change of aggregate size ratio, decrease of large aggregates, and increase of small aggregates (Fig. 5). The increase of small aggregates without the following increase of medium or large aggregates indicates the suppression of platelet aggregation.
Fig. 5.

Effect of Y27632 on platelet aggregation induced by the combination of thrombopoietin (TPO) and low‐dose collagen. Platelet‐rich plasma (PRP) was pretreated with 0, 10, 20, or 30 μmol/L Y27632 and 60 ng/mL TPO for 15 min and then stimulated by 0.05 μg/mL collagen for 10 min. Black line, percentage of transmittance of each sample (isolated platelets recorded as 0%, and PRP recorded as 100%); blue line, small aggregates (9–25 μm); green line, medium aggregates (25–50 μm); red line, large aggregates (50–70 μm). The lower panel presents the distribution (%) of aggregated particle size by area under the curve of each line. Representative results obtained from two healthy donors are shown.
Effects of NSC23766 and Y27632 on secretion of PDGF‐AB from platelets induced by combined TPO and low‐dose collagen
We further examined the effects of NSC23766 and Y27632 on PDGF‐AB secretion from platelets stimulated by the combination of TPO and low‐dose collagen. NSC23766 (20 μmol/L) remarkably suppressed PDGF‐AB secretion from the platelets stimulated by TPO and low‐dose collagen (Fig. 6A). In addition, Y27632 (30 μmol/L) partially reduced PDGF‐AB secretion from the platelets stimulated by TPO and low‐dose collagen (Fig. 6B).
Fig. 6.

Effects of NSC23766 and Y27632 on the secretion of platelet‐derived growth factor (PDGF)‐AB from platelets stimulated by the combination of thrombopoietin (TPO) and low dose of collagen. (A) Platelet‐rich plasma (PRP) was pretreated with 20 μmol/L NSC23766 or vehicle and 60 ng/mL TPO or vehicle for 15 min, and then stimulated by 0.175 μg/mL collagen or vehicle for 30 min. (B) PRP was pretreated with 30 μmol/L Y27632 or vehicle and 60 ng/mL TPO or vehicle for 15 min, and then stimulated by 0.05 μg/mL collagen or vehicle for 30 min. The reactions were terminated by the addition of ice‐cold ethylenediaminetetraacetic acid (10 mmol/L) solution. The conditioned mixture was centrifuged at 10,000 g at 4°C for 2 min, and the supernatant was then subjected to an enzyme‐linked immunosorbent assay for PDGF‐AB. Representative results obtained from 10 (A) or two (B) healthy donors are shown.
DISCUSSION
In the present study, we investigated the effects of TPO in combination with subthreshold low‐dose collagen on platelet activation. We found that pretreatment with TPO, which by itself resulted in very little effect, potentiated platelet aggregation under low‐dose collagen stimulation. Regarding the size of platelet aggregates, we confirmed that the combination of TPO with low‐dose collagen stimulation caused an increase in the ratio of large aggregates but a decrease in small aggregates, suggesting considerable augmentation of platelet aggregation. It is likely that the combination of TPO and collagen, although at the subthreshold level, remarkably triggers platelet activation, resulting in the aggregation.
To clarify the underlying mechanism, we examined the effect of TPO with low‐dose collagen stimulation on the levels of GTP‐binding Rac based on previous findings 15 and found that the levels increased transiently after stimulation. It is likely that Rac activation is considerably elicited by the combination of TPO and low‐dose collagen in human platelets. We also found that levels of GTP‐binding Rho increased after stimulation by TPO and low‐dose collagen in human platelets but decreased more rapidly than the levels of GTP‐binding Rac, which was to less than basal levels. Guanosine triphosphate‐binding Rho is considered to exist at basal levels to maintain cell function even in unstimulated cells. Once stimulated by TPO and low‐dose collagen, the rapid increase and subsequent decrease might cause the oversuppression of GTP‐binding Rho formation due to the negative feedback mechanism to recover from activation. We confirmed that the levels of phosphorylated coffilin 18 gradually increased after stimulation, suggesting that Rho/Rho kinase activation functions under stimulation in human platelets. Therefore, it seems that both Rac and Rho/Rho kinase pathways could be involved in platelet activation stimulated by the combination of TPO with low‐dose collagen, and the former might be more dominant than the latter. Furthermore, we found that both NSC23766 19 and Y27632 20 suppressed platelet aggregation under the stimulation of the combination of TPO and low‐dose collagen, but the inhibitory effect of Y27632 seems to be weaker than that of NSC23766. In addition, we also showed that NSC23766 suppressed the secretion of PDGF‐AB stimulated by the combination more potently than Y27632. Taking our present findings as whole, it is most likely that the combination of TPO and the subthreshold low‐dose collagen cooperatively potentiates the activation of human platelets through both Rac and Rho/Rho‐kinase mediated pathways.
The serum levels of TPO are known to increase in the condition of thrombocytopenia, which is inversely related to platelet counts. 2 , 3 It is established that collagen is a potent physiological platelet activator‐in‐chief. 8 Thus, our present findings on the effect of TPO on platelet activation under the stimulation of collagen indicate that, as the levels alone are insufficient to trigger platelet activation, a compensatory mechanism might exist to enhance platelet activation in thrombocytopenia. In addition, it is possible that these findings provide a novel therapeutic strategy for the promotion of hemostasis under thrombocytopenic conditions. It has been reported that a rescue therapy with recombinant human TPO (rhTPO) could quickly recover platelet counts of thrombocytopenia and improve the prognosis in sepsis patients. 21 The improvement of prognosis with rhTPO might be due not only to the recovery of platelet counts but also to the enhancement of platelet activation, which we demonstrated herein. In addition, TPO receptor agonists, accepted as second‐line therapy for idiopathic thrombocytopenia, 22 could be potentially useful therapeutic tools for thrombocytopenia caused by acute medicine‐handled emergent diseases such as sepsis and trauma. Further studies would be required to clarify the details.
CONCLUSION
Our findings suggest that TPO and collagen in low doses cooperatively induce human platelet activation through both Rac and Rho/Rho‐kinase mediated pathways.
DISCLOSURE
Approval of the research protocol with approval number and committee name: The protocol for the research project was approved by a suitably constituted Ethics Committee of the Gifu University Graduate School of Medicine (approval no. 2020–133) and conformed to the provisions of the Declaration of Helsinki.
Informed consent: Informed consent was obtained from the participants.
Registry and registration no. of the study/trial: N/A.
Animal studies: N/A.
Conflict of interest: None.
ACKNOWLEDGMENTS
We are very grateful to Yumiko Kurokawa for her skillful technical assistance.
Funding information
No funding information provided.
REFERENCES
- 1. Kaushansky K. Thrombopoietin: a tool for understanding thrombopoiesis. J. Thromb. Haemost. 2003; 1: 1587–92. [DOI] [PubMed] [Google Scholar]
- 2. Segre E, Pigozzi L, Lison D et al. May thrombopoietin be a useful marker of sepsis severity assessment in patients with SIRS entering the emergency department? Clin. Chem. Lab. Med. 2014; 52: 1479–83. [DOI] [PubMed] [Google Scholar]
- 3. Hobisch‐Hagen P, Jelkmann W, Mayr A et al. Low platelet count and elevated serum thrombopoietin after severe trauma. Eur. J. Haematol. 2000; 64: 157–63. [DOI] [PubMed] [Google Scholar]
- 4. Oda A, Miyakawa Y, Druker BJ et al. Thrombopoietin primes human platelet aggregation induced by shear stress and by multiple agonists. Blood 1996; 87: 4664–70. [PubMed] [Google Scholar]
- 5. Sungaran R, Markovic B, Chong BH. Localization and regulation of thrombopoietin mRNa expression in human kidney, liver, bone marrow, and spleen using in situ hybridization. Blood 1997; 89: 101–7. [PubMed] [Google Scholar]
- 6. Kojima H, Shinagawa A, Shimizu S et al. Role of phosphatidylinositol‐3 kinase and its association with Gab1 in thrombopoietin‐mediated up‐regulation of platelet function. Exp. Hematol. 2001; 29: 616–22. [DOI] [PubMed] [Google Scholar]
- 7. Cuong NT, Doi T, Matsushima‐Nishiwaki R et al. Thrombopoietin amplifies ADP‐induced HSP27 phosphorylation in human platelets: importance of pre‐treatment. Int. J. Mol. Med. 2013; 31: 1291–7. [DOI] [PubMed] [Google Scholar]
- 8. Kahn ML. Platelet‐collagen responses: molecular basis and therapeutic promise. Semin. Thromb. Hemost. 2004; 30: 419–25. [DOI] [PubMed] [Google Scholar]
- 9. Nieswandt B, Watson SP. Platelet‐collagen interaction: is GPVI the central receptor? Blood 2003; 102: 449–61. [DOI] [PubMed] [Google Scholar]
- 10. Kato H, Adachi S, Doi T et al. Mechanism of collagen‐induced release of 5‐HT, PDGF‐AB and sCD40L from human platelets: role of HSP27 phosphorylation via p44/p42 MAPK. Thromb. Res. 2010; 126: 39–43. [DOI] [PubMed] [Google Scholar]
- 11. Li Z, Delamey MK, O'Brien KA, Du X. Signaling during platelet adhesion and activation. Arterioscler. Thromb. Vasc. Biol. 2010; 30: 2341–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Takai Y, Sasaki T, Matozaki T. Small GTP‐binding proteins. Physiol. Rev. 2001; 81: 153–208. [DOI] [PubMed] [Google Scholar]
- 13. Iida Y, Doi T, Tokuda H et al. Rho‐kinase regulates human platelet activation induced by thromboxane A2 independently of p38 MAP kinase. Prostaglandins Leukot. Essent. Fatty Acids 2015; 94: 73–81. [DOI] [PubMed] [Google Scholar]
- 14. Kageyama Y, Doi T, Matsushima‐Nishiwaki R et al. Involvement of Rac in thromboxane A2‐induced human platelet activation: regulation of sCD40 ligand release and PDGF‐AB secretion. Mol. Med. Rep. 2014; 10: 107–12. [DOI] [PubMed] [Google Scholar]
- 15. Kageyama Y, Doi T, Akamatsu S et al. Rac regulates collagen‐induced HSP27 phosphorylation via p44/p42 MAP kinase in human platelets. Intern. J. Mol. Med. 2013; 32: 813–8. [DOI] [PubMed] [Google Scholar]
- 16. Kato K, Ito H, Hasegawa K, Inagawa Y, Kozawa O, Asano T. Modulation of the stress‐induced synthesis of hsp27 and alpha B‐crystallin by cyclic AMP in C6 rat glioma cells. J. Neurochem. 1996; 66: 946–50. [DOI] [PubMed] [Google Scholar]
- 17. Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970; 227: 680–5. [DOI] [PubMed] [Google Scholar]
- 18. Arber S, Barbayannis FA, Hanser H et al. Regulation of Actin dynamics through phosphorylation of cofilin by LIM‐kinase. Nature 1998; 393: 805–9. [DOI] [PubMed] [Google Scholar]
- 19. Gao Y, Dickerson JB, Guo F, Zheng J, Zheng Y. Rational design and characterization of a Rac GTPase‐specific small molecule inhibitor. Proc. Natl. Acad. Sci. U. S. A. 2004; 101: 7618–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shimokawa H, Rashid M. Development of Rho‐kinase inhibitors for cardiovascular medicine. Trends Pharmacol. Sci. 2007; 28: 296–302. [DOI] [PubMed] [Google Scholar]
- 21. Zhou Z, Feng T, Xie Y et al. Prognosis and rescue therapy for sepsis‐related severe thrombocytopenia in critically ill patients. Cytokine 2020; 136: 155227. [DOI] [PubMed] [Google Scholar]
- 22. Neunert C, Terrell DR, Arnold DM et al. American society of hematology 2019 guidelines for immune thrombocytopenia. Blood Adv. 2019; 3: 3829–66. [DOI] [PMC free article] [PubMed] [Google Scholar]


