Abstract
Many SARS-CoV-2 studies have supported the theory that the Type II alveolar epithelial cells (AEC-2) are the primary portal of entry of the virus into the lung following its brief nasal occupation. However, the theory of inhalational transmission of the virus from the ciliated and goblet nasal cells to the lung parenchyma is not supported by the imaging findings on chest computerized tomography (CT), leading the authors to consider an alternative pathway from the nose to the lung parenchyma that could explain the peripheral, basilar predominant pattern of early disease. Imaging supports that the pulmonary capillaries may be an important vehicle for transmission of the virus and/or associated inflammatory mediators to the lung epithelium.
Keywords: COVID-19, Chest CT, Vascular endothelium
Many SARS-CoV-2 studies have supported the theory that the Type II alveolar epithelial cells (AEC-2) are the primary portal of entry of the virus into the lung following its brief nasal occupation.1., 2., 3., 4. However, the theory of inhalational transmission of the virus from the ciliated and goblet nasal cells to the lung parenchyma is not supported by the imaging findings on chest computerized tomography (CT), leading the authors to consider an alternative pathway from the nose to the lung parenchyma that could explain the peripheral, basilar predominant pattern of early disease. Imaging supports that the pulmonary capillaries may be an important vehicle for transmission of the virus and/or associated inflammatory mediators to the lung epithelium.
1. Distribution of pulmonary pathology in COVID-19 disease
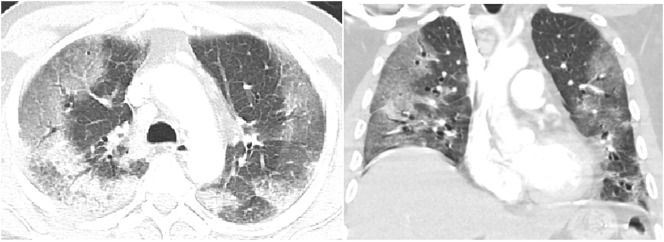
The chest CT pattern most frequently described in early COVID-19 infection is basilar and peripheral predominant ground-glass opacities (GGOs) and/or consolidation (Fig. 1 ). In a meta-analysis conducted by Ishfaq et al., a peripheral distribution was identified in 55% with the majority of disease in the lower lobes.5 Darwish also found peripheral, and right lower lobe predominance of ground-glass opacity progressing to consolidation.6 Al-Umairi included over 4000 patients in a meta-analysis with the most frequent finding of ground glass and consolidative pulmonary opacities in a lower lobe peripheral distribution.7 Karimian described peripheral opacities in 70% and peripheral and central opacities in 30% of 4598 COVID-19 infected patients.8
Fig. 1.
Chest CT with intravenous contrast on lung window settings in axial and coronal projection demonstrates peripheral ground glass opacities in a patient with early COVID-19 infection.
2. Distribution of pulmonary pathology in respiratory inhalation diseases
The pattern of disease on CT is related to the pathophysiology of the infection. If the primary entry of the virus were inhalational following the nasal infection, we would expect upper lung and airway-centered predominant distribution as is common with other inhalational lung diseases (Fig. 2 ). Centrilobular emphysema secondary to cigarette smoke inhalation has a predominantly central, upper lobe distribution. Additional smoking-related diseases including Langerhans cell histiocytosis and respiratory bronchiolitis interstitial lung disease are also upper lobe and central. Silicosis, berylliosis and hard metal diseases are inhaled and present with upper lung findings on chest CT. Hypersensitivity pneumonitis caused by an inhaled antigen has an upper lung predominant airway-centered distribution as well.9 If COVID-19 pulmonary disease was solely inhalational, we would expect that Hou et al. would have identified a high expression of ACE2 receptors in not only the respiratory mucosa in the nasal cavity but also throughout the upper and lower respiratory tract. In contrast, while elevated rates of cellular infection were noted in nasal mucosa, there was decrease in uptake along the lower bronchial trees making inhalation a less likely method for direct lung parenchymal infection.10 The hypothesis of viral microaspiration is also not supported by the imaging findings; aspiration typically causes opacification of the central lower lobes and not the periphery10 (Fig. 3 ).
Fig. 2.
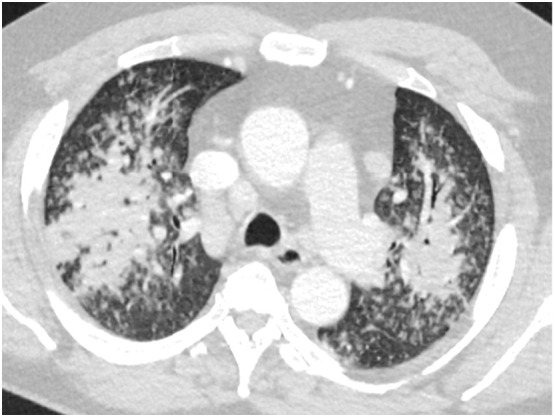
Non-COVID 19 bacterial pneumonia with airway-centered upper lobe predominance.\.
Fig. 3.
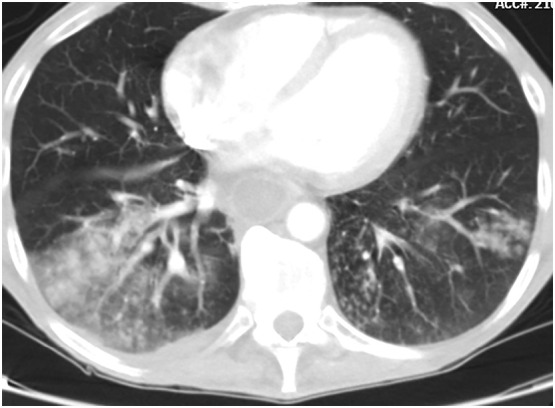
Bilateral lower lobe aspiration pneumonia with airway-centered consolidation and tree in bud opacities. Note the dilated, fluid-filled esophagus.
3. Distribution of pulmonary pathology in hematologic spread
Diseases related to pulmonary blood flow are typically lower lobe predominant and peripheral. They include metastatic disease which is spread randomly and located predominantly at the end of lower lung blood vessels. Pulmonary infarcts and septic emboli (Fig. 4 ) are also lower lung predominant and peripheral similar to COVID-19's earliest CT findings.11 If the alveolar epithelial type 2 cell (AEC-2) was the primary target of the virus then the majority of patients would be expected to have acute lung injury (ALI) as the predominant finding at autopsy. In a large autopsy series performed at our institution, 36/40 (90%) of patients had evidence of micro thrombotic disease compared to only 73% with ALI. 11/40 patients had only micro thrombotic disease and no ALI.12 The results of Chen et al. were concordant with 91.3% of 335 patients having micro thrombotic disease.13 In matched radiology pathology samples microthrombi were identified in areas of normal imaging supporting the idea that vascular disease precedes the ground glass opacities of alveolar disease.14 Ackermann found that capillary microthrombi in the lung were 9 times more prevalent in patients with Covid-19 compared to influenza (P < 0.001).15
Fig. 4.
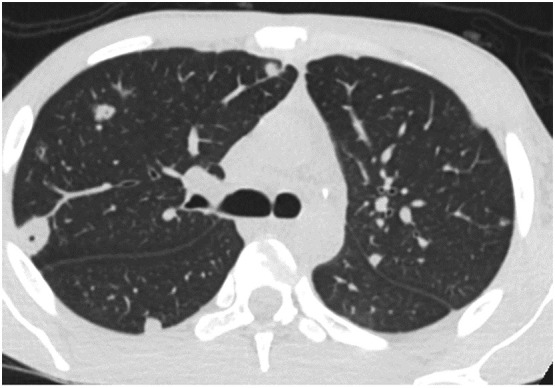
Peripheral septic emboli at the end of blood vessels are spread hematologically to the lungs.
The nasal cavity is a favorable site for early inoculation of SARS-CoV-2 infection due to high levels of ACE2 expression, the predominant receptor facilitating viral entry, that is highly expressed by ciliated respiratory epithelial cells.10 Air inspired through the nose passes the nasal turbinates causing a change in airflow from a laminar pattern to a protective “transitional” pattern that is not quite laminar, and not quite turbulent. Much of the aerosolized debris, including pathogens and dust, precipitate from the inspired air into the mucus blanket covering the epithelium.16 Humans inspire about 10-20 k L of air per day; about 80% of particles 3–5 μm in diameter and 60% of particles 2 μm in diameter are filtered by the nose. Anything <1 μm will probably end up in the lower airway.17., 18. SARS-CoV-2 is about 0.1 μm,19 therefore, the nose would do a poor job of filtering the virus alone, and if inspired it would probably reach the lower airway. However, respiratory droplets are generally bigger than 1 μm and droplets from sneezing are probably 5 μm. So overall, the nose would be highly effective at retaining respiratory droplets containing the virus.
The virus multiplies in the ciliated nasal cells and via transcellular and paracellular migration could traverse the disrupted basilar lamina and infect immediately adjacent nasal capillary endothelial cells and/or lymphatics.20 Monteil et al. demonstrated that SARS-CoV-2 can directly infect engineered human blood vessel organoids in vitro.21 Following vascular access, the virus and associated inflammatory mediators could then travel to the superior vena cava, right atrium, right ventricle and pulmonary capillaries where it could have its first opportunity to infect pulmonary capillary endothelial cells.22., 23. Endothelial cells are polarized with their luminal membrane directly exposed to the blood and its components, the basolateral surface is separated from tissues by a basement membrane anchored to the cell membrane.24 Viral infection of the endothelium leads to endotheliitis with associated disruption of the capillary basement membrane and leakage of fluid and virus into the interstitium and alveolus. The injured endothelial cell causes activation of von Willebrand factor with clot formation. The endothelial cell itself can increase in height and narrow a capillary lumen further increasing the risk of organ ischemia.24
Hough et al. describe how reverse crosstalk could occur with blood-borne infections; proinflammatory factors in the blood cause injury to the alveolar-capillary barrier likely via endothelium-activated neutrophils which travel to the epithelium causing inflammation.25 To support reverse crosstalk as a mechanism for viral entry into an organ, the virus needs to be identified in cells of organs other than the lung that could have only been accessed via the blood. Mondello's systematic review described viral particles in the interstitial cells of the myocardium on electron microscopy (EM). Coronavirus was identified in membrane-bound vesicles in hepatocytes and in the tubular epithelial cells of the kidney.26 Livanos found SARS-CoV-2 in small intestinal epithelial cells by immunofluorescence staining or EM in 15 of 17 patients.27 Eymieux et al. used EM to demonstrate the existence of exit tunnels that link cellular compartments with secretory vesicles which can fuse with the plasma membrane and release virus into the extracellular space.28
The peripheral opacities that are the characteristic imaging finding likely reflect fluid in the peripheral alveoli caused by the disruption of capillary basement membranes; they first increase in density and then decrease in density over time as is characteristic of fluid filling alveoli. Inflammatory mediators and viral particles could access the lung via the damaged basement membrane. This concept of a spread of virus from endothelium to epithelium explains the vulnerability of those with endothelial instability29 to worse outcomes and supports interventions to stabilize the vascular endothelium.30., 31.
References
- 1.Carcaterra M., Caruso C. Alveolar epithelial cell type II as main target of SARS-CoV-2 virus and COVID-19 development via NF-kb pathway deregulation: a physio-pathological theory. Med Hypotheses. 2021;146 doi: 10.1016/j.mehy.2020.110412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Morris G., Bortolasci C.C., Puri B.K., Olive L., Marx W., O'Neil A., Athan E., Carvalho A.F., Maes M., Walder K., Berk M. The pathophysiology of SARS-CoV-2: a suggested model and therapeutic approach. Life Sci. 2020;1(258) doi: 10.1016/j.lfs.2020.118166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang J., Hume A.J., Abo K.M., Werder R.B., Villacorta-Martin C., Alysandratos K.D., Beermann M.L., Simone-Roach C., Lindstrom-Vautrin J., Olejnik J., Suder E.L., Bullitt E., Hinds A., Sharma A., Bosmann M., Wang R., Hawkins F., Burks E.J., Saeed M., Wilson A.A., Mühlberger E., Kotton D.N. SARS-CoV-2 infection of pluripotent stem cell-derived human lung alveolar type 2 cells elicits a rapid epithelial-intrinsic inflammatory response. Cell Stem Cell. 2020;27(6):962–973. doi: 10.1016/j.stem.2020.09.013. e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Batah S.S., Fabro A.T. Pulmonary pathology of ARDS in COVID-19: a pathological review for clinicians. Respir Med. 2021;176 doi: 10.1016/j.rmed.2020.106239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ishfaq A., Yousaf Farooq S.M., Goraya A., Yousaf M., Gilani S.A., Kiran A., Ayoub M., Javed A., Bacha R. Role of high-resolution computed tomography chest in the diagnosis and evaluation of COVID -19 patients -a systematic review and meta-analysis. Eur J Radiol Open. 2021;8 doi: 10.1016/j.ejro.2021.100350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Darwish H.S., Habash M.Y., Habash W.Y. Chest computed tomography imaging features in patients with coronavirus disease 2019 (COVID-19) J Int Med Res. 2021;49(5) doi: 10.1177/03000605211010631. 3000605211010631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Al-Umairi R.S., Al-Kalbani J., Al-Tai S., Al-Abri A., Al-Kindi F., Kamona A. COVID-19 associated pneumonia: a review of chest radiograph and computed tomography findings. Sultan Qaboos Univ Med J. 2021;21(1):e4–e11. doi: 10.18295/squmj.2021.21.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Karimian M., Azami M. Chest computed tomography scan findings of coronavirus disease 2019 (COVID-19) patients: a comprehensive systematic review and meta-analysis. Pol J Radiol. 2021 Jan;14(86):e31–e49. doi: 10.5114/pjr.2021.103379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nemec Stefan F., Bankier Alexander A., Eisenberg Ronald L. Upper lobe-predominant diseases of the lung. Am J Roentgenol. 2013;200(3):W222–W237. doi: 10.2214/AJR.12.8961. [DOI] [PubMed] [Google Scholar]
- 10.Hou Y.J., Okuda K., Edwards C.E., et al. SARS-CoV-2 reverse genetics reveals a variable infection gradient in the respiratory tract. Cell. 2020;182(2):429–446. doi: 10.1016/j.cell.2020.05.042. e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nemec Stefan F., Bankier Alexander A., Eisenberg Ronald L. Manifestation lower lobe—predominant diseases of the lung. Am J Roentgenol. 2013;200(4):712–728. doi: 10.2214/AJR.12.9253. [DOI] [PubMed] [Google Scholar]
- 12.De Michele S., Sun Y., Yilmaz M.M., Katsyv I., Salvatore M., Dzierba A.L., Marboe C.C., Brodie D., Patel N.M., Garcia C.K., Saqi A. Forty postmortem examinations in COVID-19 patients. Am J Clin Pathol. 2020;154(6):748–760. doi: 10.1093/ajcp/aqaa156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen W., Pan J.Y. Anatomical and pathological observation and analysis of SARS and COVID-19: microthrombosis is the main cause of death. Biol Proced Online. 2021;23:4. doi: 10.1186/s12575-021-00142-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kianzad A., Meijboom L.J., Nossent E.J., et al. COVID-19: histopathological correlates of imaging patterns on chest computed tomography. Respirology. 2021;1–9 doi: 10.1111/resp.14101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ackermann M., Verleden S.E., Kuehnel M., Haverich A., Welte T., Laenger F., Vanstapel A., Werlein C., Stark H., Tzankov A., Li W.W., Li V.W., Mentzer S.J., Jonigk D. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med. 2020;383(2):120–128. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mirza N., Lanza D.C. The nasal airway and obstructed breathing during sleep. Otolaryngol Clin North Am. 1999;32(2):243–262. doi: 10.1016/s0030-6665(05)70128-6. [DOI] [PubMed] [Google Scholar]
- 17.Schwab J.A., Zenkel M. Filtration of particulates in the human nose. Laryngoscope. 1998 Jan;108(1 Pt 1):120–124. doi: 10.1097/00005537-199801000-00023. [DOI] [PubMed] [Google Scholar]
- 18.Heyder J. Deposition of inhaled particles in the human respiratory tract and consequences for regional targeting in respiratory drug delivery. Proc Am Thorac Soc. 2004;1(4):315–320. doi: 10.1513/pats.200409-046TA. [DOI] [PubMed] [Google Scholar]
- 19.Bar-On Y.M., Flamholz A., Phillips R., Milo R. SARS-CoV-2 (COVID-19) by the numbers. Elife. 2020 Apr;2(9) doi: 10.7554/eLife.57309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Keller L.A., Merkel O., Popp A. Intranasal drug delivery: opportunities and toxicologic challenges during drug development. Drug Deliv Transl Res. 2022 Apr;12(4):735–757. doi: 10.1007/s13346-020-00891-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Montiel K.H., V, Prado P., Hagelkrüys A., et al. Inhibition of SARS-CoV-2 infections in engineered human tissues using clinical-grade soluble human ACE2. Cell. 2020 May 14;181(4):905–913. doi: 10.1016/j.cell.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guney C., Akar F. Epithelial and endothelial expressions of ACE2: SARS-CoV-2 entry routes. J Pharm Pharm Sci. 2021;24:84–93. doi: 10.18433/jpps31455. [DOI] [PubMed] [Google Scholar]
- 23.Kumar A., Narayan R.K., Kumari C., Faiq M.A., Kulandhasamy M., Kant K., Pareek V. SARS-CoV-2 cell entry receptor ACE2 mediated endothelial dysfunction leads to vascular thrombosis in COVID-19 patients. Med Hypotheses. 2020;145 doi: 10.1016/j.mehy.2020.110320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krüger-Genge A., Blocki A., Franke R.P., Jung F. Vascular endothelial cell biology: an update. Int J Mol Sci. 2019;20(18):4411. doi: 10.3390/ijms20184411. Published 2019 Sep 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hough R.F., Bhattacharya S., Bhattacharya J. Crosstalk signaling between alveoli and capillaries. Pulm Circ. 2018;8(3) doi: 10.1177/2045894018783735. 2045894018783735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mondello C., Roccuzzo S., Malfa O., Sapienza D., Gualniera P., Ventura Spagnolo E., Di Nunno N., Salerno M., Pomara C., Asmundo A. Pathological findings in COVID-19 as a tool to define SARS-CoV-2 pathogenesis. A systematic review. Front Pharmacol. 2021;12:614586. doi: 10.3389/fphar.2021.614586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Livanos A.E., Jha D., Cossarini F., Gonzalez-Reiche A.S., Tokuyama M., Aydillo T., Parigi T.L., Ladinsky M.S., Ramos I., Dunleavy K., Lee B., Dixon R.E., Chen S.T., Martinez-Delgado G., Nagula S., Bruce E.A., Ko H.M., Glicksberg B.S., Nadkarni G., Pujadas E., Reidy J., Naymagon S., Grinspan A., Ahmad J., Tankelevich M., Bram Y., Gordon R., Sharma K., Houldsworth J., Britton G.J., Chen-Liaw A., Spindler M.P., Plitt T., Wang P., Cerutti A., Faith J.J., Colombel J.F., Kenigsberg E., Argmann C., Merad M., Gnjatic S., Harpaz N., Danese S., Cordon-Cardo C., Rahman A., Schwartz R.E., Kumta N.A., Aghemo A., Bjorkman P.J., Petralia F., van Bakel H., Garcia-Sastre A., Mehandru S. Intestinal host response to SARS-CoV-2 infection and COVID-19 outcomes in patients with gastrointestinal symptoms. Gastroenterology. 2021;160(7):2435–2450. doi: 10.1053/j.gastro.2021.02.056. e34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eymieux S., Uzbekov R., Rouillé Y., Blanchard E., Hourioux C., Dubuisson J., Belouzard S., Roingeard P. Secretory vesicles are the principal means of SARS-CoV-2 egress. Cells. 2021;10(8):2047. doi: 10.3390/cells10082047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vrints C.J.M., Krychtiuk K.A., Van Craenenbroeck E.M., Segers V.F., Price S., Heidbuchel H. Endothelialitis plays a central role in the pathophysiology of severe COVID-19 and its cardiovascular complications. Acta Cardiol. 2021;76(2):109–124. doi: 10.1080/00015385.2020.1846921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ko J.Y., Danielson M.L., Town M., Derado G., Greenlund K.J., Kirley P.D., Alden N.B., Yousey-Hindes K., Anderson E.J., Ryan P.A., Kim S., Lynfield R., Torres S.M., Barney G.R., Bennett N.M., Sutton M., Talbot H.K., Hill M., Hall A.J., Fry A.M., Garg S., Kim L. COVID-NET surveillance team. Risk factors for coronavirus disease 2019 (COVID-19)-associated hospitalization: COVID-19-associated hospitalization surveillance network and behavioral risk factor surveillance system. Clin Infect Dis. 2021;72(11):e695–e703. doi: 10.1093/cid/ciaa1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Anderson T.J. The effect of cholesterol-lowering and antioxidant therapy on endothelium-dependent coronary vasomotion. N Engl J Med. 1995;332:488–493. doi: 10.1056/NEJM199502233320802. [DOI] [PubMed] [Google Scholar]