Abstract
SARS-CoV-2 (Severe Acute Respiratory Syndrome-Coronavirus-2) is the most dangerous form of the coronavirus, which causes COVID-19. In patients with severe COVID-19, the immune system becomes markedly overactive. There is evidence that supplementation with select micronutrients may play a role in maintaining immune system function in this patient population. Throughout the COVID-19 pandemic, significant emphasis has been placed on the importance of supplementing critical micronutrients such as Vitamin C and Zinc (Zn) due to their immunomodulatory effects. Viral infections, like COVID-19, increase physiological demand for these micronutrients. Therefore, the purpose of this review was to provide comprehensive information regarding the potential effectiveness of Vitamin C and Zn supplementation during viral infection and specifically COVID-19. This review demonstrated a relation between Vitamin C and Zn deficiency and a reduction in the innate immune response, which can ultimately make patients with COVID-19 more vulnerable to viral infection. As such, adequate intake of Vitamin C and Zn, as an adjunctive therapeutic approach with any necessary pharmacological treatment(s), may be necessary to mitigate the adverse physiological effects of COVID-19. To truly clarify the role of Vitamin C and Zn supplementation in the management of COVID-19, we must wait for the results of ongoing randomized controlled trials. The toxicity of Vitamin C and Zn should also be considered to prevent over-supplementation. Over-supplementation of Vitamin C can lead to oxalate toxicity, while increased Zn intake can reduce immune system function. In summary, Vitamin C and Zn supplementation may be useful in mitigating COVID-19 symptomology.
Keywords: COVID-19, Vitamin C, Zn, Dietary supplement, Immune system
Abbreviations: SARS-CoV-2, Severe Acute Respiratory Syndrome-Coronavirus-2; Zn, Zinc; RCTs, Randomized controlled trials; RDA, Recommended Dietary Allowance; NK, Natural killer; PUFAs, Polyunsaturated fatty acids; HIF-1α, Hypoxia-inducible factor-1α; IFN-α, Intererferon alfa; INF-β, Interferon beta; TNF-α, Tumor necrosis factor alpha
Introduction
Since December 2019, the world has been plagued by a new viral disease, which has resulted in a pandemic. The novel coronavirus SARS-CoV-2 (Severe Acute Respiratory Syndrome-Coronavirus-2), causing COVID-19, is by far the most dangerous coronavirus ever identified [1]. The high pathogenicity and mortality associated with this virus have prompted many prevention- and treatment-related research efforts. In patients with severe COVID-19, the immune system becomes overactive, such that there is a marked increase in the secretion of pro-inflammatory cytokines, especially locally in the respiratory system [2]. Despite the multitude of medications prescribed (e.g., corticosteroids and cyclo-oxygenase 2 inhibitors) to mitigate COVID-19-related symptoms, many have proven to be ineffective [3,4]. Therefore, determining effective strategies for preventing and/or attenuating COVID-19-related symptoms is a biomedical research priority, as these therapeutic approaches could help to preserve immune function and lessen the release of pro-inflammatory cytokines in patients with COVID-19.
A patient's response to the virus is dependent on many factors, including age, lifestyle, and medical, socioeconomic and nutrient status [5]. Regarding nutrient status, serum levels of Vitamin C and Zinc (Zn) at the onset of infection have shown to influence the severity of COVID-19 symptomology [[6], [7], [8]]. Currently, there is a lack of evidence from randomized controlled trials (RCTs) on the preventative and/or therapeutic effects of Vitamin C and Zn supplementation for treating COVID-19-related symptoms. Therefore, in the present review, we discuss: 1) potential mechanisms by which Vitamin C and Zn supplementation may prevent or mitigate symptoms associated with COVID-19; and 2) potential toxicity concerns with Vitamin C and Zn supplementation.
The role of Vitamin C in immune system regulation
Vitamin C is a necessary water soluble nutrient for human health [9]. The normal serum concentration of Vitamin C is considered to be 30–90 mol/L. Serum concentrations between 11- 23 mol/L are qualified as marginal Vitamin C deficiency and levels below 11 mol/L are referred to as deficient [10]. The Recommended Dietary Allowance (RDA) for Vitamin C is approximately 75 mg/day for adult women and 90 mg/day for adult men [11]. Daily intake of 100–200 mg/day of Vitamin C from the diet appears to be adequate for maintaining normal serum levels in both men and women [12]. The immune system in general is a complex network of organs, tissues, and cells, which are designed to protect the body from foreign pathogens. The immune system can generally be divided into epithelial barriers and cellular and humoral immunity [13]. Many studies have shown that Vitamin C plays a critical role in maintaining immune system function, and it contributes to both innate and adaptive immunity, especially immune cell function (i.e., epithelial barrier integrity, chemotaxis and antimicrobial activities of phagocytes, natural killer [NK] cell activity, and proliferation and differentiation of lymphocytes) [14,15]. Vitamin C can accumulate in select populations of immune cells, such as phagocytes and T-cells, and these cell types are dependent on Vitamin C to maintain proper function [16]. Therefore, Vitamin C deficiency can result in reduced capacity of the immune system to combat foreign pathogens [17].
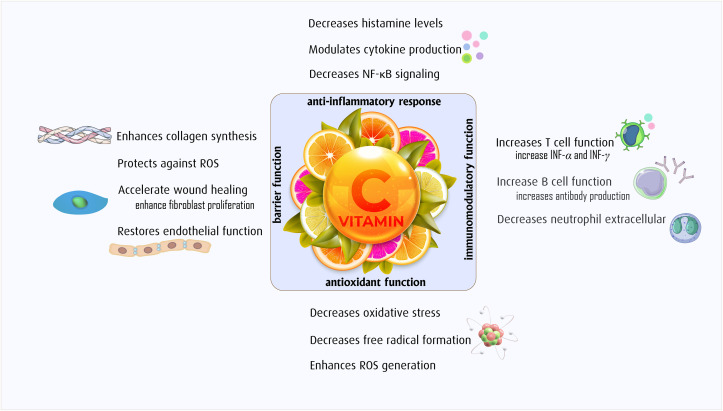
The membranes of immune cells contain an abundance of polyunsaturated fatty acids (PUFAs), making these cells sensitive to oxidative stress – i.e., PUFAs contain hydrogen atoms located near double bonds, and this location makes them highly susceptible to oxidation [18]. Vitamin C is a potent antioxidant due to its ability to donate electrons, which allows for protection against oxidant-related stress, which could take place as a result of exposure to foreign pathogens [19]. Vitamin C is also a co-factor for the lysyl and prolyl hydroxylases, which are involved in the stabilization of the fourth structure of collagen, which can help to maintain barrier function. Another critical role of Vitamin C is the regulation of DNA and histone methylation in immune cells, which highlights that Vitamin C may, in part, mediate epigenetic regulation of antioxidant defenses [20]. Recent research showed that Vitamin C is an important co-factor for the regulation of the transcription factor hypoxia-inducible factor-1α (HIF-1α) [21,22], and maintenance of HIF-1α has shown to be protective against COVID-19-related symptoms [23]. We have summarized the overall role of Vitamin C in boosting immune system function in Fig. 1 .
Fig. 1.
The role of Vitamin C supplement in viral infection.
The role of Zn in the immune system
Zn is a trace element and adequate intake is critical for human health [24]. After iron, the second-most abundant trace element in the body is Zn which is a crucial component of protein structure and function [25]. The total content of Zn in the human body is 2–4 g and serum concentration of Zn is 12–14 μM – 60% bound to albumin, 30% bound to α2-macroglobulin and 10% bound to transferrin [26]. At basal/physiological levels, the serum concentration of Zn is exceptionally low and daily intake of Zn (generally from the diet) is necessary to achieve optimal levels [26].
The RDA for Zn is approximately 11 mg/day for men and 8 mg/day for women [27]. Zn is necessary for adequate innate and adaptive immune system function [28]. According to the world health organization (WHO), approximately one-third of the world's population is deficient in Zn and Zn deficiency is responsible for nearly 16% of severe lung infections worldwide [29,30]. Zn deficiency may reduce immune system function, and in some cases, deficiency in Zn can cause several forms of infection (e.g., respiratory, inflammatory, and autoimmune) and in the most extreme case, could even increase mortality from infection [31,32]. Recent studies have shown that Zn has three important roles in immune system biology: 1) its impact on signal transduction within the immune system; 2) its role in immune cell function; and 3) it can enhance nutritional immunity – defined as limiting pathogenicity during an infection [33].
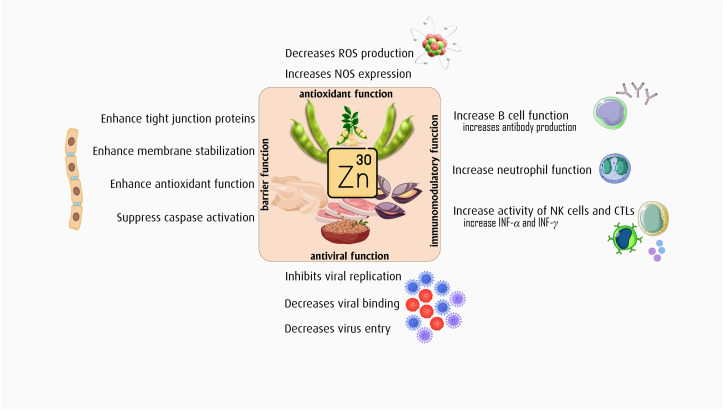
Zn is also a potent antioxidant as it can prevent the production of free radicals [34]. In addition, Zn can enhance immune system function by: 1) increasing the activity of T-helper 1 cells, which are primarily anti-inflammatory [35]; 2) suppressing the function of effector T cells, which are primarily pro-inflammatory [36,37]; and 3) regulating lymphocyte apoptosis [24]. Furthermore, Zn is a major co-factor for the activity of more than 300 enzymes such as Zn finger DNA binding proteins, DNA polymerase, thymidine kinase and DNA dependent RNA polymerase [26], which are all associated with immune system function. Moreover, thymulin, which is largely responsible for epithelial barrier function and in turn immune system function, is dependent on Zn for its biological activity [24]. Zn status and pro-inflammatory cytokine abundance may be mutually reinforcing, such that adequate levels of circulating Zn can support anti-inflammatory cytokine production and greater abundance of pro-inflammatory cytokines can reduce circulating levels of Zn [38]. Zn deficiency is common in the elderly and in individuals with malnutrition, autoimmune diseases, and certain pro-inflammatory conditions [39]. As such, maintaining adequate levels of Zn may be favorable for mitigating excess oxidative stress and inflammation, especially at the onset of exposure to a foreign pathogen. We have summarized the overall role of Zn in boosting immune system function in Fig. 2 .
Fig. 2.
The role of Zn supplement in viral infection.
The role of Vitamin C on antiviral function
Vitamin C has known antiviral properties and some studies have shown that Vitamin C may be useful for treating and preventing viral infections in the respiratory tract (i.e., influenza and the common cold) [40,41]. Furthermore, recent evidence suggests that Vitamin C supplementation may be an effective adjunctive treatment strategy for mitigating COVID-19-related symptoms [42]. The release of pro-inflammatory cytokines is a common side effect of bacterial and viral infections, and these cytokines can increase oxidative stress [43]; thus, an antioxidant such as Vitamin C could potentially mitigate excess inflammation and oxidative stress.
Previously, Vitamin C has shown to regulate antiviral cytokine production, such as with interferon alfa (IFN-α) production, which can protect against influenza-mediated lung injury [44]. Interferons are cytokines that are produced by infected cells and these cytokines are released in response to viruses [45]. To achieve a stronger immune response, interferons can act in a paracrine and/or endocrine manner, as these cytokines can activate other immune cells [46]. A majority of the antiviral properties of Vitamin C are attributed to improving the overall immune response [47]. Several in vitro studies have demonstrated that high dose Vitamin C has antiviral activity [48], which is thought to occur via the function of Vitamin C as an antioxidant [49,50]. Vitamin C can also modulate the immune system of patients with viral infections, which could be due to increased production of anti-inflammatory INF-α and INF-β and reduced production of pro-inflammatory cytokines [41,51]. Although Vitamin C may have beneficial effects on disease states associated with viral infections, there is still a need for placebo controlled RCTs to directly evaluate the influence of Vitamin C in mitigating symptoms related to viral infections.
The role of Zn on antiviral function
Zn has shown to have direct and indirect antiviral effects. Previous work has shown that Zn deficiency: 1) is associated with reduced antibody production in response to a virus; 2) can reduce the function of the innate immune system; 3) can lower cytokine production from monocytes [52]; and 4) increased susceptibility to infectious diseases that were brought about by bacterial and viral pathogens [53]. Furthermore, Zn can prevent viral replication via inhibition of RNA synthesis [54]. Furthermore, in vitro studies have shown that Zn can increase the production of IFN-α and IFN-γ, which have shown to elicit antiviral effects [55]. In vivo evidence suggests that Zn supplementation may enhance the production of IFN-α by leukocytes and decrease the production of tumor necrosis factor (TNF-α) by B lymphocytes [52,56].
The antiviral activity of IFNα occurs via up-regulation of antiviral enzymes and signaling through the JAK1/STAT1 pathway [57]. In healthy adults, supplementation with Zn has shown to reduce TNF-α and IL-1β production [58]. In addition, Zn can improve resistance of cells to apoptosis via inhibition of caspase-3, 6, and 9 [59]. The Anti-apoptotic actions of Zn in peripheral tissues (e.g., the thymus) could lead to an increase in the abundance of anti-inflammatory T-helper cells. The antiviral action of Zn may also occur via metallothioneins, cysteine-rich proteins, which have a role in transfer and storage of Zn [60]. Moreover, the common cold accounts for approximately 30% of the most prevalent respiratory infections caused by the coronavirus, and studies have shown that Zn supplementation can reduce the duration and severity of the common cold; therefore, it can be concluded that Zn supplementation may be an effective therapeutic strategy for strengthening the immune system in patients with COVID-19 [61,62].
Clinical trials: intervention with Vitamin C and Zn supplementation in patients with COVID-19
Currently, multiple RCTs in which patients with COVID-19 are being supplemented with Vitamin C and Zn have been registered at clinicaltrials.gov [63,64]. The details of previously published RCTs are summarized in Table1 . An intervention with 6 g/day of Vitamin C for 5 days in 30 patients with severe COVID-19, compared to a placebo control group, did not influence COVID-19-related symptoms, including body temperature, peripheral capillary oxygen saturations (SpO2), length of ICU stay and mortality [65]. Furthermore, another study which consisted of 4 groups over a 10 day supplementation period – 1) Zn gluconate (50 mg); 2) Vitamin C (800 mg); 3) Zn gluconate (50 mg) + Vitamin C (800 mg); and 4) a placebo control – assessed fever, cough, shortness of breath, fatigue, hospitalization and death, and found no differences in the outcome variables [66]. Moreover, supplementation with 220 mg Zn gluconate (contains 50 mg Zn) twice daily for 15 days did not improve any of the assessed biochemical, hematological and clinical outcomes [67]. Lastly, an RCT in which 0.24 mg/kg/day of elemental Zn was administered to patients for a maximum of 10 days, found that respiratory parameters did not differ between patients supplemented with Zinc or the placebo control [68]. Accordingly, the results published thus far regarding the influence of Vitamin C and Zn supplementation on COVID-19-related symptoms suggest that these strategies may not be effective; however, we must wait for the results of the ongoing RCTs.
Table 1.
Published RCTs on intervention with Vitamin C and Zn supplement in COVID-19 patients.
| Author, year (ref) | Country | Type of study | Participants | Number of I/C | Intervention | Control | Duration | Results |
|---|---|---|---|---|---|---|---|---|
| JamaliMoghadamSiahkali, et al., 2021 [65] | Iran | RCT | Severe COVID-19 | 30/30 | High dose Vitamin C (6 g/daily) | lopinavir/ritonavir and hydroxychloroquine | 5 days | Body temperature ↔ SpO2 ↔ Length of hospitalization ↑ Length of ICU stay ↔ Mortality ↔ |
| Thomas et al., 2021 [66] | USA | RCT | COVID-19 | Group 1: 58, group 2: 48, group 3: 58/50 | Group 1: Zn gluconate (50 mg) Group 2: ascorbic acid (8000 mg) Group 3: both supplement |
Standard of care | 10 days | Fever ↔ Cough ↔ Shortness of breath ↔ Fatigue ↔ Hospitalization ↔ Death ↔ |
| Abd-Elsalam et al., 2021 [67] | Egypt | RCT | COVID-19 | 96/95 | Chloroquine/hydroxychloroquine and Zn sulfate (220 mg contains 50 mg Zn/twice daily) | Chloroquine/hydroxychloroquine | 15 days | Hemoglobin ↔ Platelets ↔ WBCs ↔ Direct bilirubin ↔ Indirect bilirubin ↔ Albumin ↔ ALT ↔ AST ↔ D-dimer ↔ Ferritin ↔ Creatinine ↔ CRP ↔ Duration of hospital stay in days ↔ Recovery after 28 days ↔ Need for mechanical ventilation ↔ Survived ↔ |
| Patel et al., 2021 [68] | Australia | RCT | COVID-19 | 15/18 | Elemental Zn concentration, 0.24 mg/kg/day | Saline placebo | 7 days | Serum Zn ↑ Mean daily peak oxygenation requirement ↔ Number of patients with supplemental oxygen ↔ Number of patients with NIV, and/or HFNC ↔ Number of patients with mechanical ventilation ↔ |
RCT, randomized controlled trial; Spo2, Peripheral capillary oxygen saturations; ICU, intensive care unit; WBC, white blood cell; ALT, alanine aminotransferase; AST, aspartate aminotransferase; CRP, C-reactive protein; HFNC, high-flow nasal cannula, NIV, noninvasive ventilation.
Concern about toxicity with Vitamin C and Zn
Vitamin C is a water-soluble vitamin, and excess can be excreted by the kidneys; however, in high doses Vitamin C can increase the formation of oxalate stones. Currently, no Vitamin C-related toxicity has been reported in RCTs in patients with COVID-19 [65,66]. However, a case report of two patients with COVID-19 reported oxalate nephropathy due to high doses of Vitamin C (>100 g total) [69]. Critically ill patients with COVID-19 are at increased risk of acute kidney injury due to multiple mechanisms, including: i) renal hypo-perfusion which occurs as a result of decreased blood circulation and sepsis [70]; ii) activation of the inflammatory cascade that damages renal epithelial cells [70]; iii) potential mechanisms associated with acute renal impairment in patients with acute respiratory distress syndrome (e.g., high intrathoracic pressures due to mechanical ventilation, hypoxemia, and systemic acidosis that affect renal vascular resistance and alter renal blood pressure; and facilitates the release of proinflammatory cytokines [71]); iv) and drug toxicity. In addition, Vitamin C increases the likelihood of hyperoxaluria through endogenous conversion of ascorbic acid to oxalate [72]. Oxalate crystals are excreted rapidly in healthy individuals but may be retained in patients whose renal epithelial cells are damaged [73]. Therefore, oxalate nephropathy may be present in patients with COVID-19 for the reasons mentioned previously. Currently, there is no known toxic dose of Vitamin C, but the dose of 1000 mg per day can increase oxalate excretion up to 13–16 mg/day and may cause calcium oxalate stones [74].
Acute high dose Zn supplementation (300 mg/day for 6 weeks) [75], as well as long-term treatment [64], can have adverse effects on immune system function. Furthermore, a study in older adults showed that high doses of Zn increased circulating levels of IL-1α [58]. As well, an in vitro study has shown that high concentrations of Zn reduced IFN regulatory factor 1 expression in regulatory T cells and consequently reduced IFN- γ [76]. Therefore, we need further studies to establish the therapeutic efficacy (or lack thereof) of Zn supplementation.
Conclusion
Adequate intake of immune-enhancing nutrients (Vitamin C and Zn) in conjunction with pharmacological agents is necessary during the COVID-19 pandemic. To specifically clarify the role of supplementation with Vitamin C and Zn in the management of coronavirus we must wait for the completion of the ongoing RCTs. The effects of the toxicity of these micronutrients should also be considered to prevent over-supplementation.
Author contributions
The authors' responsibilities were as follows. SF, MM and NP, Contributed to conception and design. SF, MTB, MM, NP, and JGN: conducted the library search and wrote the manuscript; JGN designed table and figures; and ZSC and MM participated in the drafting and editing of the manuscript. All of the authors read and approved the final manuscript.
Conflict of interest
The authors declare that there is no conflict of interest.
Acknowledgments
We are very thankful to all colleagues with whom we have shared our research on Vitamin C and Zn and their mechanism of action in Covid-19 and who have helped us with valuable comments. We did not receive any funding for this research project.
References
- 1.Chen Y., Liu Q., Guo D. Emerging coronaviruses: genome structure, replication, and pathogenesis. J Med Virol. 2020;92(4):418–423. doi: 10.1002/jmv.25681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Conti P., Ronconi G., Caraffa A., Gallenga C., Ross R., Frydas I., et al. Induction of pro-inflammatory cytokines (IL-1 and IL-6) and lung inflammation by Coronavirus-19 (COVI-19 or SARS-CoV-2): anti-inflammatory strategies. J Biol Regul Homeost Agents. 2020;34(2):1. doi: 10.23812/CONTI-E. [DOI] [PubMed] [Google Scholar]
- 3.Arabi Y.M., Fowler R., Hayden F.G. Critical care management of adults with community-acquired severe respiratory viral infection. Int Care Med. 2020;46(2):315–328. doi: 10.1007/s00134-020-05943-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Russell C.D., Millar J.E., Baillie J.K. Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. The Lancet. 2020;395(10223):473–475. doi: 10.1016/S0140-6736(20)30317-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weiss P., Murdoch D.R. Clinical course and mortality risk of severe COVID-19. The Lancet. 2020;395(10229):1014–1015. doi: 10.1016/S0140-6736(20)30633-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alexander J., Tinkov A., Strand T.A., Alehagen U., Skalny A., Aaseth J. Early nutritional interventions with zinc, selenium and vitamin D for raising anti-viral resistance against progressive COVID-19. Nutrients. 2020;12(8):2358. doi: 10.3390/nu12082358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Simonson W. Vitamin C and coronavirus. Geriatr Nurs (New York, Ny) 2020;41(3):331. doi: 10.1016/j.gerinurse.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pahlavani N., Navashenaq J. Current Nutritional Support in Critical Ill Covid-19 Patients. A Brief Review. 2021;8:1–5. [Google Scholar]
- 9.Pavlovic V., Sarac M. A short overview of vitamin C and selected cells of the immune system. Central Eur J Med. 2011;6(1):1–10. [Google Scholar]
- 10.Aditi A., Graham D.Y. Vitamin C, Gastritis, and Gastric Disease: A Historical Review and Update. Dig Dis Sci. 2012;57(10):2504–2515. doi: 10.1007/s10620-012-2203-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bendich A. National Academy Press; washington, DC: 2000. Dietary reference intakes for vitamin C, vitamin E, selenium, and carotenoids institute of medicine. ISBN: 0-309-06935-1. Nutrition. 2001;vol. 4(17):364. [PubMed] [Google Scholar]
- 12.Naidu K.A. Vitamin C in human health and disease is still a mystery? An overview. Nutr J. 2003;2(1):1–10. doi: 10.1186/1475-2891-2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parkin J., Cohen B. An overview of the immune system. The Lancet. 2001;357(9270):1777–1789. doi: 10.1016/S0140-6736(00)04904-7. [DOI] [PubMed] [Google Scholar]
- 14.Webb A.L., Villamor E. Update: effects of antioxidant and non-antioxidant vitamin supplementation on immune function. Nutrition Rev. 2007;65(5):181–217. doi: 10.1111/j.1753-4887.2007.tb00298.x. [DOI] [PubMed] [Google Scholar]
- 15.Jafari D., Esmaeilzadeh A., Mohammadi-Kordkhayli M., Rezaei N. Nutrition and immunity. Springer; 2019. Vitamin C and the immune system; pp. 81–102. [Google Scholar]
- 16.Hong J.-M., Kim J.-H., Kang J.S., Lee W.J., Hwang Y-i. Vitamin C is taken up by human T cells via sodium-dependent vitamin C transporter 2 (SVCT2) and exerts inhibitory effects on the activation of these cells in vitro. Anatomy & Cell Biol. 2016;49(2):88–98. doi: 10.5115/acb.2016.49.2.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ströhle A., Hahn A. [Vitamin C and immune function] Med Monatsschr Pharm. 2009;32(2):49–54. quiz 5-6. [PubMed] [Google Scholar]
- 18.Víctor V.M., Guayerbas N., De la Fuente M. Changes in the antioxidant content of mononuclear leukocytes from mice with endotoxin-induced oxidative stress. Molecular and Cell Biochem. 2002;229(1):107–111. doi: 10.1023/a:1017976629018. [DOI] [PubMed] [Google Scholar]
- 19.Carr A., Frei B. Does vitamin C act as a pro-oxidant under physiological conditions? The FASEB J. 1999;13(9):1007–1024. doi: 10.1096/fasebj.13.9.1007. [DOI] [PubMed] [Google Scholar]
- 20.Elmadfa I., Meyer A.L. The role of the status of selected micronutrients in shaping the immune function. Endocr, Metab Immune Disorders-Drug Targets (Formerly Current Drug Targets-Immune, Endocrine & Metabolic Disorders) 2019;19(8):1100–1115. doi: 10.2174/1871530319666190529101816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kuiper C., Vissers M.C. Ascorbate as a co-factor for fe- and 2-oxoglutarate dependent dioxygenases: physiological activity in tumor growth and progression. Frontiers in Oncol. 2014;4:359. doi: 10.3389/fonc.2014.00359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Young J.I., Züchner S., Wang G. Regulation of the epigenome by vitamin C. Annu Rev Nutr. 2015;35:545–564. doi: 10.1146/annurev-nutr-071714-034228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jahani M., Dokaneheifard S., Mansouri K. Hypoxia: A key feature of COVID-19 launching activation of HIF-1 and cytokine storm. J Inflammation. 2020;17(1):1–10. doi: 10.1186/s12950-020-00263-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dardenne M. Zinc and immune function. Eur J Clinical Nutr. 2002;56(3):S20–S23. doi: 10.1038/sj.ejcn.1601479. [DOI] [PubMed] [Google Scholar]
- 25.Lambert S.A., Jolma A., Campitelli L.F., Das P.K., Yin Y., Albu M., et al. The human transcription factors. Cell. 2018;172(4):650–665. doi: 10.1016/j.cell.2018.01.029. [DOI] [PubMed] [Google Scholar]
- 26.Rink L. Zinc and the immune system. Proceedings of the Nutr Soc. 2000;59(4):541–552. doi: 10.1017/s0029665100000781. [DOI] [PubMed] [Google Scholar]
- 27.Meyers L.D., Hellwig J.P., Otten J.J. National Academies Press; 2006. Dietary reference intakes: the essential guide to nutrient requirements. [Google Scholar]
- 28.Gammoh N.Z., Rink L. Springer; 2019. Zinc and the immune system. Nutrition and immunity; pp. 127–158. [Google Scholar]
- 29.Tsianakas V., Liamputtong P. What women from an Islamic background in Australia say about care in pregnancy and prenatal testing. Midwifery. 2002;18(1):25–34. doi: 10.1054/midw.2002.0296. [DOI] [PubMed] [Google Scholar]
- 30.Wessels I., Rolles B., Rink L. The potential impact of zinc supplementation on COVID-19 pathogenesis. Frontiers in Immunol. 2020;11:1712. doi: 10.3389/fimmu.2020.01712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wessels I., Rolles B., Slusarenko A.J., Rink L. Zinc deficiency as a possible risk factor for increased susceptibility and severe progression of COVID-19. British J Nutr. 2021:1–42. doi: 10.1017/S0007114521000738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gombart A.F., Pierre A., Maggini S. A review of micronutrients and the immune system–working in harmony to reduce the risk of infection. Nutrients. 2020;12(1):236. doi: 10.3390/nu12010236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Haase H., Rink L. Multiple impacts of zinc on immune function. Metallomics. 2014;6(7):1175–1180. doi: 10.1039/c3mt00353a. [DOI] [PubMed] [Google Scholar]
- 34.Prasad A.S. Zinc in Human Health: Effect of Zinc on Immune Cells. Molecular Med. 2008;14(5):353–357. doi: 10.2119/2008-00033.Prasad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bao B., Prasad A.S., Beck F.W., Bao G.W., Singh T., Ali S., et al. Intracellular free zinc up-regulates IFN-γ and T-bet essential for Th1 differentiation in Con-A stimulated HUT-78 cells. Biochem Biophysical Res Communications. 2011;407(4):703–707. doi: 10.1016/j.bbrc.2011.03.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Faber C., Gabriel P., Ibs K.H., Rink L. Zinc in pharmacological doses suppresses allogeneic reaction without affecting the antigenic response. Bone Marrow Transplantation. 2004;33(12):1241–1246. doi: 10.1038/sj.bmt.1704509. [DOI] [PubMed] [Google Scholar]
- 37.Kitabayashi C., Fukada T., Kanamoto M., Ohashi W., Hojyo S., Atsumi T., et al. Zinc suppresses Th17 development via inhibition of STAT3 activation. International Immunology. 2010;22(5):375–386. doi: 10.1093/intimm/dxq017. [DOI] [PubMed] [Google Scholar]
- 38.Prasad A.S. Effects of zinc deficiency on immune functions. The J Trace Elem in Experimental Med: The Official Publication of the Int Soc for Trace Element Res in Humans. 2000;13(1):1–20. [Google Scholar]
- 39.Hotz C. Identifying populations at risk of zinc deficiency: the use of supplementation trials. Nutr Rev. 2001;59(3):80–84. doi: 10.1111/j.1753-4887.2001.tb06992.x. [DOI] [PubMed] [Google Scholar]
- 40.Colunga Biancatelli R.M.L., Berrill M., Marik P.E. The antiviral properties of vitamin C. Expert Rev of Anti-infective Ther. 2020;18(2):99–101. doi: 10.1080/14787210.2020.1706483. [DOI] [PubMed] [Google Scholar]
- 41.Hemilä H., Chalker E. Vitamin C for preventing and treating the common cold. Cochrane Database of Systematic Rev. 2013;(1) doi: 10.1002/14651858.CD000980.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang J., Rao X., Li Y., Zhu Y., Liu F., Guo G., et al. Pilot trial of high-dose vitamin C in critically ill COVID-19 patients. Ann Intensive Care. 2021;11(1):1–12. doi: 10.1186/s13613-020-00792-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fowler A.A., III, Kim C., Lepler L., Malhotra R., Debesa O., Natarajan R., et al. Intravenous vitamin C as adjunctive therapy for enterovirus/rhinovirus induced acute respiratory distress syndrome. World J Critical Care Med. 2017;6(1):85. doi: 10.5492/wjccm.v6.i1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim Y., Kim H., Bae S., Choi J., Lim S.Y., Lee N., et al. Vitamin C is an essential factor on the anti-viral immune responses through the production of interferon-α/β at the initial stage of influenza A virus (H3N2) infection. Immune Network. 2013;13(2):70–74. doi: 10.4110/in.2013.13.2.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ferreira V.L., Borba H.H., Bonetti AdF., Leonart L., Pontarolo R. Cytokines and interferons: types and functions. Autoantibodies and Cytokines. 2018;13 [Google Scholar]
- 46.Boretti A., Banik B.K. Intravenous vitamin C for reduction of cytokines storm in acute respiratory distress syndrome. PharmaNutrition. 2020;12 doi: 10.1016/j.phanu.2020.100190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Carr A.C., Maggini S. Vitamin C and immune function. Nutrients. 2017;9(11):1211. doi: 10.3390/nu9111211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pauling L. The Significance of the Evidence about Ascorbic Acid and the Common Cold. Proceedings of the Nation Academy of Sci. 1971;68(11):2678–2681. doi: 10.1073/pnas.68.11.2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.White L.A., Freeman C.Y., Forrester B.D., Chappell W.A. In vitro effect of ascorbic acid on infectivity of herpesviruses and paramyxoviruses. J Clinical Microbiol. 1986;24(4):527–531. doi: 10.1128/jcm.24.4.527-531.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Klein M. The mechanism of the virucidal action of ascorbic acid. Science. 1945;101(2632):587–589. doi: 10.1126/science.101.2632.587. [DOI] [PubMed] [Google Scholar]
- 51.Dehghani-Samani A., Kamali M., Hoseinzadeh-Chahkandak F. The Role of vitamins on the prevention and/or treatment of COVID-19 infection; A Systematic Review. Modern Care J. 2020;17(3) [Google Scholar]
- 52.Ibs K.-H., Rink L. Zinc-Altered Immune function. The J Nutr. 2003;133(5):1452S. doi: 10.1093/jn/133.5.1452S. 6S. [DOI] [PubMed] [Google Scholar]
- 53.Himoto T., Masaki T. Associations between zinc deficiency and metabolic abnormalities in patients with chronic liver disease. Nutrients. 2018;10(1):88. doi: 10.3390/nu10010088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Te Velthuis A.J., van den Worm S.H., Sims A.C., Baric R.S., Snijder E.J., van Hemert M.J. Zn2+ inhibits coronavirus and arterivirus RNA polymerase activity in vitro and zinc ionophores block the replication of these viruses in cell culture. PLoS Pathogens. 2010;6(11) doi: 10.1371/journal.ppat.1001176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nabi-Afjadi M., Karami H., Goudarzi K., Alipourfard I., Bahreini E. The effect of vitamin D, magnesium and zinc supplements on interferon signaling pathways and their relationship to control SARS-CoV-2 infection. Clin Mol Allergy. 2021;19(1):1–10. doi: 10.1186/s12948-021-00161-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pala O., Diaz A., Blomberg B.B., Frasca D. B lymphocytes in rheumatoid arthritis and the effects of anti–TNF-α agents on B lymphocytes: a review of the literature. Clin Therapeutics. 2018;40(6):1034–1045. doi: 10.1016/j.clinthera.2018.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lin F-c, Young H.A. Interferons: success in anti-viral immunotherapy. Cytokine & Growth Factor Rev. 2014;25(4):369–376. doi: 10.1016/j.cytogfr.2014.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cakman I., Kirchner H., Rink L. Zinc supplementation reconstitutes the production of interferon-α by leukocytes from elderly persons. J Interferon & Cytokine Res. 1997;17(8):469–472. doi: 10.1089/jir.1997.17.469. [DOI] [PubMed] [Google Scholar]
- 59.Perry D.K., Smyth M.J., Stennicke H.R., Salvesen G.S., Duriez P., Poirier G.G., et al. Zinc Is a Potent Inhibitor of the Apoptotic Protease, Caspase-3: A NOVEL TARGET FOR ZINC IN THE INHIBITION OF APOPTOSIS. J Biol Chem. 1997;272(30):18530–18533. doi: 10.1074/jbc.272.30.18530. [DOI] [PubMed] [Google Scholar]
- 60.Schoggins J.W., Wilson S.J., Panis M., Murphy M.Y., Jones C.T., Bieniasz P., et al. A diverse range of gene products are effectors of the type I interferon antiviral response. Nature. 2011;472(7344):481–485. doi: 10.1038/nature09907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hemilä H. Zinc lozenges and the common cold: a meta-analysis comparing zinc acetate and zinc gluconate, and the role of zinc dosage. JRSM Open. 2017;8(5) doi: 10.1177/2054270417694291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Martinez-Estevez N., Alvarez-Guevara A., Rodriguez-Martinez C. Effects of zinc supplementation in the prevention of respiratory tract infections and diarrheal disease in Colombian children: A 12-month randomised controlled trial. Allergologia et immunopathologia. 2016;44(4):368–375. doi: 10.1016/j.aller.2015.12.006. [DOI] [PubMed] [Google Scholar]
- 63.Baladia E., Pizarro A.B., Ortiz-Muñoz L., Rada G. Group C-LOW. Vitamin C for COVID-19: A living systematic review. Medwave. 2020;20(6) doi: 10.5867/medwave.2020.06.7978. [DOI] [PubMed] [Google Scholar]
- 64.Pal A., Squitti R., Picozza M., Pawar A., Rongioletti M., Dutta A.K., et al. Zinc and COVID-19: basis of current clinical trials. Biol Trace Element Res. 2021;199(8):2882–2892. doi: 10.1007/s12011-020-02437-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.JamaliMoghadamSiahkali S., Zarezade B., Koolaji S., SeyedAlinaghi S., Zendehdel A., Tabarestani M., et al. Safety and effectiveness of high-dose vitamin C in patients with COVID-19: a randomized open-label clinical trial. Eur J Med Res. 2021;26(1):1–9. doi: 10.1186/s40001-021-00490-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Thomas S., Patel D., Bittel B., Wolski K., Wang Q., Kumar A., et al. Effect of high-dose zinc and ascorbic acid supplementation vs usual care on symptom length and reduction among ambulatory patients with SARS-CoV-2 infection: the COVID A to Z randomized clinical trial. JAMA Network Open. 2021;4(2) doi: 10.1001/jamanetworkopen.2021.0369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Abd-Elsalam S., Soliman S., Esmail E.S., Khalaf M., Mostafa E.F., Medhat M.A., et al. Do zinc supplements enhance the clinical efficacy of hydroxychloroquine?: a randomized, multicenter trial. Biol Trace Element Res. 2021;199(10):3642–3646. doi: 10.1007/s12011-020-02512-1. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 68.Patel O., Chinni V., El-Khoury J., Perera M., Neto A.S., McDonald C., et al. A pilot double-blind safety and feasibility randomized controlled trial of high-dose intravenous zinc in hospitalized COVID-19 patients. J Med Virol. 2021;93(5):3261–3267. doi: 10.1002/jmv.26895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fontana F., Cazzato S., Giovanella S., Ballestri M., Leonelli M., Mori G., et al. Oxalate nephropathy caused by excessive vitamin C administration in 2 patients with COVID-19. Kidney Int Reports. 2020;5(10):1815–1822. doi: 10.1016/j.ekir.2020.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zarbock A., Gomez H., Kellum J.A. Sepsis-induced AKI revisited: pathophysiology, prevention and future therapies. Curr Opin Crit Care. 2014;20(6):588. doi: 10.1097/MCC.0000000000000153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Panitchote A., Mehkri O., Hastings A., Hanane T., Demirjian S., Torbic H., et al. Factors associated with acute kidney injury in acute respiratory distress syndrome. Annals of Intensive Care. 2019;9(1):1–10. doi: 10.1186/s13613-019-0552-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cossey L.N., Rahim F., Larsen C.P. Oxalate nephropathy and intravenous vitamin C. Am J Kidney Dis. 2013;61(6):1032–1035. doi: 10.1053/j.ajkd.2013.01.025. [DOI] [PubMed] [Google Scholar]
- 73.Verkoelen C., Verhulst A. Proposed mechanisms in renal tubular crystal retention. Kidney Int. 2007;72(1):13–18. doi: 10.1038/sj.ki.5002272. [DOI] [PubMed] [Google Scholar]
- 74.Daudon M., Jungers P. Drug-induced renal calculi. Drugs. 2004;64(3):245–275. doi: 10.2165/00003495-200464030-00003. [DOI] [PubMed] [Google Scholar]
- 75.Chandra R.K. Excessive intake of zinc impairs immune responses. Jama. 1984;252(11):1443–1446. [PubMed] [Google Scholar]
- 76.Maywald M., Rink L. Zinc supplementation induces CD4+ CD25+ Foxp3+ antigen-specific regulatory T cells and suppresses IFN-γ production by upregulation of Foxp3 and KLF-10 and downregulation of IRF-1. Eur JNutr. 2017;56(5):1859–1869. doi: 10.1007/s00394-016-1228-7. [DOI] [PubMed] [Google Scholar]