Abstract
Aims
Treatment outcomes for methicillin-resistant Staphylococcus aureus (MRSA) periprosthetic joint infection (PJI) using systemic vancomycin and antibacterial cement spacers during two-stage revision arthroplasty remain unsatisfactory. This study explored the efficacy and safety of intra-articular vancomycin injections for PJI control after debridement and cement spacer implantation in a rat model.
Methods
Total knee arthroplasty (TKA), MRSA inoculation, debridement, and vancomycin-spacer implantation were performed successively in rats to mimic first-stage PJI during the two-stage revision arthroplasty procedure. Vancomycin was administered intraperitoneally or intra-articularly for two weeks to control the infection after debridement and spacer implantation.
Results
Rats receiving intra-articular vancomycin showed the best outcomes among the four treatment groups, with negative bacterial cultures, increased weight gain, increased capacity for weightbearing activities, increased residual bone volume preservation, and reduced inflammatory reactions in the joint tissues, indicating MRSA eradication in the knee. The vancomycin-spacer and/or systemic vancomycin failed to eliminate the MRSA infections following a two-week antibiotic course. Serum vancomycin levels did not reach nephrotoxic levels in any group. Mild renal histopathological changes, without changes in serum creatinine levels, were observed in the intraperitoneal vancomycin group compared with the intra-articular vancomycin group, but no changes in hepatic structure or serum alanine aminotransferase or aspartate aminotransferase levels were observed. No local complications were observed, such as sinus tract or non-healing surgical incisions.
Conclusion
Intra-articular vancomycin injection was effective and safe for PJI control following debridement and spacer implantation in a rat model during two-stage revision arthroplasties, with better outcomes than systemic vancomycin administration.
Cite this article: Bone Joint Res 2022;11(6):371–385.
Keywords: Periprosthetic joint infection, Two-stage revision, Methicillin-resistant S. aureus , Intra-articular injection, Vancomycin
Keywords: Debridement, vancomycin, rat model, Periprosthetic joint infection (PJI), methicillin-resistant Staphylococcus aureus (MRSA), antibiotics, knees, Serum, infections, joint tissues
Article focus
We tested whether the use of intra-articular vancomycin injections after debridement and spacer implantation provided better control of periprosthetic joint infection (PJI) than systemic intraperitoneal vancomycin administration.
We performed the in vivo evaluation of intra-articular vancomycin treatment compared with intraperitoneal systemic vancomycin treatment following debridement and spacer implantation in a rat model of total knee arthroplasty.
Key messages
Intra-articular injection of vancomycin performed better for the elimination of methicillin-resistant Staphylococcus aureus (MRSA) infection than vancomycin-spacer or systemic vancomycin administration for infections, on both the spacer and in joint tissues, after debridement and cement spacer implantation over a two-week course in a rat model.
Strengths and limitations
We demonstrated that intra-articular vancomycin injection is effective and safe for the eradication of MRSA PJI after debridement and cement spacer implantation, and performs better than systemic vancomycin in a rat model, which allowed for a reduction in selection bias and observation bias compared with existing retrospective clinical studies and case reports.
The current study is a rat-based study, which could not exactly mimic the PJI process in humans, as the absorption, distribution, metabolism, and excretion of drugs by rats differs from those in humans, and the rat knee presents with different biodynamics and biomechanical properties than the human knee, requiring caution when attempting to extrapolate these results directly to clinical PJI patients.
Our study compared the efficacy of different vancomycin administration approaches without adjuvant oral antibiotics, such as rifampicin, which may have affected the ability of the vancomycin spacer or systemic vancomycin to eliminate the infection, and further investigations into the efficacy and safety of vancomycin, combined with additional oral antibiotics, remain necessary.
Introduction
Periprosthetic joint infection (PJI) is a serious complication following artificial joint arthroplasty, occurring in 1% to 2% of primary arthroplasty operations, 1 and the risk of revision increases three-fold for total hip arthroplasty in cases with PJI compared to cases without PJI. 2 PJI is difficult to control and prone to reinfection, due to the formation of bacterial biofilms, which protect the bacteria from antibiotics and the immune system. For some bacterial species, the minimum biofilm eradication concentration (MBEC) can be 100 to 1,000 times greater than the minimal inhibitory concentration (MIC) for the same antibiotic, 3 requiring much higher antibiotic concentrations for the effective eradication of bacterial biofilms. 4,5 However, systemic antibiotics administered intravenously (IV) can only reach two to three times the MIC in joints and infected tissues. 6 Increasing the IV antibiotic dose increases the incidence of antibiotic-associated adverse events, such as hepatic and renal toxicity, and longer courses of antibiotic therapy are associated with increased rates of antibiotic resistance without increasing cure rates. 7-11 Therefore, systemic antibiotic administration might not be the ideal approach to achieving local MBECs in the joint.
Two-stage revision is considered to be the gold standard for PJI treatment. 12 During the first stage, an antibiotic-loaded cement spacer (ALCS) is implanted in the joint after debridement, followed by a two-week or longer course of IV antibiotics. 13 However, the eradication rate of infections remains unsatisfactory, especially in cases associated with methicillin-resistant Staphylococcus aureus (MRSA). Up to 28% of the patients require repeated debridement due to infection recrudescence caused by MRSA. 14 Although up to 4 g of vancomycin can be added to 40 g cement powder when generating an antibiotic spacer, according to some clinical reports 15,16 and reviews, 17,18 less than 5% of the total vancomycin has been shown to be released from cement spacer, 19,20 and an effective vancomycin concentration was only detected during the first few days after spacer implantation. 21,22 Thus, the traditional approach of combining systemic vancomycin with an ALCS does not appear to be an optimal approach for achieving MBECs, due to the limited spacer antibiotic loading and release.
The local injection of antibiotics directly into the articular cavity, rather than the systemic administration of antibiotics or the use of an antibiotic spacer, might represent a promising approach to achieving a sufficient antibiotic MBEC in the joint. Several surgeons have administered IA vancomycin for infection control during one-stage revision arthroplasties after PJI, achieving satisfactory outcomes (infection eradication rates of 89%, 94%, and 95% have been reported). 23-25 However, IA applications of vancomycin during two-stage revision arthroplasty after PJI have not been investigated. This study explored the efficacy and safety of IA vancomycin injections for PJI control after debridement and cement spacer implantation during a two-stage revision arthroplasty in a rat model, to provide the experimental evidence to support clinical PJI treatment.
Methods
Animals and reagents
The study was designed and performed following the Animal Research: Reporting of In Vivo Experiments (ARRIVE) and the Institutional Animal Care and Use Committee (IACUC) guidelines. An ARRIVE checklist is included in the Supplementary Material to show that the ARRIVE guidelines were adhered to in this study. A total of 52 specific pathogen-free grade Wistar rats (male, 12 weeks old, weighing 304 g ± 7) were used in the current study. All rats were housed in ventilated and sterilized cages at 22 ± 2 °C (humidity: 55 ± 5%) on a 12 h light/dark cycle with free access to standard chow and water.Clinical-grade vancomycin hydrochloride for injection was obtained from Lilly (Japan).
Study design
The rats underwent total knee arthroplasty (TKA) surgery and MRSA inoculation (50 µl of 1.75 × 107 colony-forming units (CFUs)/ml; ATCC-43300; intra-articular injection), then rats were randomly assigned to four groups after debridement at two weeks postoperatively: 1) control (antibiotic-free cement spacer), n = 13; 2) vancomycin-cement spacer (4 g vancomycin per 40 g cement powder, 10%, (wt/wt)), n = 13; 3) vancomycin-cement spacer and intraperitoneal injection of vancomycin (88 mg/kg, 1 ml, every 12 hours (Q12h), equivalent to 1 g, IV, Q12h in a patient weighing 70 kg), n = 13; 4) vancomycin-cement spacer and intra-articular injection of vancomycin (44 mg/kg, 150 µl, once per day (QD), equivalent to 0.5 g, intra-articular, QD in a patient weighing 70 kg), n = 13. Doses for the weight-based intraperitoneal and intra-articular vancomycin administrations were based on routine therapeutic antibiotic doses used for skeletal infections in humans, 24,26 and correspond to vancomycin doses used in a prior rat model. 27-29 Table I reports the allocation of animals per group and the relative analysis.
Table I.
Allocation of animals per group and investigations.
| Analyses | Number of animals |
|---|---|
| Total animals (n = 13 per group) | 1 2 3 4 5 6 7 8 9 10 11 12 13 |
| TKA surgery + bacterial inoculation (Day 0) | x x x x x x x x x x x x x |
| Debridement and cement spacer implantation (Day 14) | x x x x x x x x x x x x x |
| Serum α1-AGP levels (Days 0, 1, 7, 14, and 28) | x x x x x x |
| General status (Days 0, 1, 4, 7, 11, 14, 21, and 28) | x x x x x x x x x x x x x |
| Incision examination (Day 28) | x x x x x x x x x x x x x |
| radiograph and micro-CT (Day 28) | x x x x x x |
| Knee histology (Day 28) | x x x x x x |
| Cement spacer SEM (Day 28) | x x x x x x |
| Microbiology (Day 28) | x x x x x x x |
| Serum levels of vancomycin (Day 28) | x x x x x x |
| Liver or kidney histology (Day 28) | x x x x x x x x x x x x x |
| Serum ALT, AST or Cr analysis (Day 28) | x x x x x x x x x x x x x |
ALT, alanine aminotransferase; AST, aspartate aminotransferase; Cr, creatinine; SEM, scanning electron microscopy; TKA, total knee arthroplasty; α1-AGP, alpha-1-acid glycoprotein.
Vancomycin-cement elution sample and cement spacers preparation
Following a protocol for the elution test sample preparation published in previous literature, 30 the vancomycin cement (4 g vancomycin per 40 g cement powder, 10 %, (wt/wt)) mixture was hand-mixed and then manually pressed into a metal mould to form uniform test cylindrical specimens (height: 12 mm ± 0.1, diameter: 6 mm ± 0.1). A 3D mould of the rat knee joint was printed based on available anatomical data (Supplementary Figure ah). Vancomycin cement spacers (4 g vancomycin per 40 g cement powder) and antibiotic-free acrylic cement (VERSABOND, UK) spacers were generated using the 3D-printed mould to form uniform spacers (height: 2 mm, diameter: 5 mm), which were sterilized with ethylene oxide (sterilization temperature: 55°C to 60°C, under negative pressure; sterilization time: one hour; residual removal time > 12 hours; the entire process lasted longer than 15 hours).
Bacteria
MRSA (ATCC-43300) was streaked onto Luria-Bertani (LB) agar plates (Solarbio, China) and incubated overnight at 37°C. Individual colonies were cultured in LB broth overnight at 37°C with shaking. After centrifugation (1,878 × g, 10°C, 10 mins), bacteria were rinsed in phosphate-buffered saline (PBS) and resuspended to an inoculum of (1.65 to 1.80) × 108 CFU/ml, as determined by the absorbance at 600 nm (absorbance value was approximately 0.5), and confirmed by overnight culture on plates.
Vancomycin elution and bioassay of vancomycin activity
Cylinders (n = 6) were submerged in 5 ml of PBS at 37°C with constant shaking at 60 rpm. Samples were removed from the fluid at specific times (12 hours, 1 day, 3 days, 5 days, 7 days, 10 days, 14 days, and 28 days), gently washed, and transferred to 5 ml of fresh PBS to maintain sink conditions. The concentrations of vancomycin released in the collected eluents were quantified by high-performance liquid chromatography mass spectrometry (HPLC-MS, Thermo TSQ Quantis, USA; external standard; filter: SRM MS2; 725.80 to 1,307.39 m/z; mass: 1,307.39 m/z; retention time: 3.50 mins; solvents: 1% formic acid water and pure acetonitrile; columns: Hypersil GOLD, Thermo Fisher, USA, 100 × 2.1 mm, 3 µm; flow rates: 0.2 ml/min; time: 6 mins). The biological activities of released vancomycin were studied by applying aliquots of each sample (n = 6) to a modified microtube dilution bioassay (10 µl elution sample at specific times was added to 100 µl of 105 CFU/ml tested bacterial broth in 96-well plates). 19 The strain of the current study (MRSA, ATCC 43300) was selected as the test organism. The in vitro samples were inoculated with 105 CFU/ml of MRSA in 96-well culture dishes and incubated at 37°C for 24 hours. The growth of MRSA associated with the collected vancomycin eluents of different times were compared visibly against the positive control (without antibiotic). The MIC of vancomycin against ATCC 43300 was determined by microtube dilution bioassay. 31
Animal surgery procedures
The rats (n = 13 per group) were anaesthetized using 2.5% isoflurane by inhalation delivered via nose cone, and preoperative analgesics, consisting of subcutaneous buprenorphine (0.1 mg/kg) and parecoxib (2 mg/kg), were administered. After sterile draping of the surgical site, a 2 cm midline longitudinal skin incision was made over the right knee. After knee joint exposure, the anterior cruciate ligament and medial and lateral menisci were removed. Most of the cartilaginous surfaces were removed and replaced with artificial joint prostheses (Supplementary Figures aa and ab). After closing the joint capsule, the joint cavity was injected with 50 µl of 1.75 × 107 CFU/ml MRSA (ATCC-43300). X-rays were performed immediately after the operation to confirm the position of the prosthesis (Supplementary Figure ac) (Bruker Xtreme BI, Germany; filter: 0.4 mm; 45 kVp; exposure time: 1.2 s; bin: 1 × 1 pixels; field of view (FOV): 10 cm; F-stop: 2). Pain was controlled with buprenorphine (0.1 mg/kg) for three days post-surgery. On day 14 after TKA surgery and bacterial inoculation, all incisions were healed. Prosthesis loosening and mild bone osteolysis were observed by radiograph (Supplementary Figure af), with ulceration and abscesses observed both extra-articularly and intra-articularly (Supplementary Figures ad and ae). The synovial tissues and prosthesis were removed for bacterial culture, as described under the microbiological analysis section below. Bacterial detection and identification were performed to confirm the successful establishment of the MRSA PJI model (catalase testing, Gram staining, rapid agglutination test of rabbit plasma coagulase, cefoxitin susceptibility test disk). Debridement was performed, and a cement spacer was implanted in the joint (Supplementary Figures ag to ai). Rats assigned to the intraperitoneal or intra-articular vancomycin groups were injected with vancomycin as described in the study design section for 14 consecutive days after the revision surgery. All animals were euthanized on day 28 (post-debridement day 14), and blood collection and tissue harvesting were performed in accordance with the IACUC-approved protocol.
General status
The body weights (n = 13), body temperatures (n = 13), and daytime weightbearing activities (n = 6; as described in the figure legends) of the rats were recorded preoperatively, and on postoperative days 1, 4, 7, 14, 21, and 28.
Blood biochemical markers and serum levels of vancomycin
Serum samples were obtained by centrifugation (1,057 × g, 4°C, 15 mins). The serum levels of alpha-1-acid glycoprotein (α1-AGP; n = 6), creatinine (Cr; n = 13), alanine aminotransferase (ALT; n = 13), and aspartate aminotransferase (AST; n = 13) were measured by enzyme-linked immunosorbent assay (ELISA; CUABIO, China). The serum levels of vancomycin (n = 6) before and after the last vancomycin injection were detected by HPLC-MS (filter: SRM MS2; 725.80 to 1,307.30 m/z; mass: 1,307.30 m/z; retention time: 1.58 mins) on day 28 (pre-injection, 0.5, two, and four hours after injection).
Radiograph imaging
Radiographs were obtained for the right hind limbs (n = 6) on day 28 to confirm cement spacer positioning and bone destruction osteolysis using the Bruker Xtreme BI (Germany) general unit and digital detector plate (filter: 0.4 mm; 45 kVp; exposure time: 1.2 s; bin: 1 × 1 pixels; FOV: 10 cm; F-stop: 2).
Micro-CT imaging and data analysis
The prostheses were carefully removed from the joint, and micro-CT analysis (n = 6) was performed to evaluate the bone volume in the distal femoral and proximal tibial using a SkyScan 1276 scanner (Bruker, Germany; voltage: 70 kV; current: 200 µA; exposure time: 446 ms/projection; scanning position = 156.463 mm, camera binning = 1 × 1, rotation step (°) = 0.30, scan duration: 0 hr:6 mins:50 s, camera pixel size: 17.420 µm; isotropic resolution: 93 µm; image pixel size: 9.0338 µm; filter: Al 0.5 mm; 180° rotation). Scan images were reconstructed, and bone parameters surrounding the cement spacer were assessed using CT-viewer version 2.0.4.5 software (Materialise, Belgium). We analyzed the 3D bone reconstructed images with CT-An software (Bruker). After scan calibration, we created two identical box volumes of interest (VOIs), sized 1,327.446 mm3 (X: 13.55 mm; Y: 13.55 mm; Z: 7.23 mm) for the distal femoral and proximal tibial metaphysis (number of femur or tibia images inside VOI was 800, respectively). The bone volumes (BVs, mm3) within these two VOIs were quantitatively measured and reported as the residual bone volume in each group.
Scanning electron microscopy of cement spacers
Cement spacers (n = 6) were carefully removed, and their surfaces were examined by a single, experienced observer (YHW, see Acknowledgements) blinded to treatment. The samples were fixed (2.5% glutaraldehyde 4°C 24 hours, osmium acid two hours), dehydrated in an alcohol gradient (concentration of 50%, 60%, 80%, 95%, and 100%, for ten minutes per concentration), dried in an EM CPD300 Critical Point Dryer (Leica, Germany), coated with a conductive coating using a Q150R S Plus Sputter Coater (Quorumtech, UK), and observed using a Zeiss Auriga field emission scanning electron microscope (SEM) and Gatan digital camera system (SEM voltage: 20 kV; SEM magnification: 5,000× and 10,000×; Zeiss, Germany). MRSA was identified as spherical structures with the following features: no surface deformities, organized in pairs or clusters, and approximately 0.8 µm to 1.0 µm in diameter. Host leucocytes were identified as spherical objects larger than 2 µm proximal to bacteria, with or without extracellular material coverage, and not adherent to the surface of cement spacers. 32 Erythrocytes were identified as double concave disks with a mean diameter of 7 µm. 33
Microbiological analysis
On day 28, the surgical incision site was sterilized and then reopened under sterile conditions. Sterile instruments were used to harvest joint tissue (n = 7), including the muscles and soft-tissues around the knee, bone, and cement spacers. Tissue specimens were placed in 10 ml sterile PBS and homogenized with a fast tissue grinder (70 Hz; 10 min; JXFSTPRP, China). A 100 µl volume of supernatant was inoculated onto LB agar Petri dishes and incubated for 24 hours at 37°C. The retrieved cement spacer was placed in 2 ml sterile PBS solution (containing 0.3% Tween 20) and sonicated to stimulate bacterial biofilm release from the spacer. 34 A 100 µl aliquot of cement spacer supernatant was plated as described for tissue supernatants. 35 Bacterial colonies were quantified using the plate count method. 36,37 According to the results of bacterial culture for 24 hours, the initial tissue or cement supernatant was diluted gradiently and cultured until the colony counts ranged from 0 to 200 CFU/100 µl. The bacterial testing was conducted in triplicate repeatedly and the mean was calculated.
Histopathology
Histopathological analyses (knee joint bone and capsule, n = 6) were performed to assess inflammation, bone necrosis, and osteomyelitis, and verify the presence of tissue degeneration or necrosis in the liver (n = 13) and kidney (n = 13). After decalcification (bone; 0.3 M ethylenediaminetetraacetic acid, 28 days), dehydration (ethanol, xylene, and paraffin), and paraffin-embedding (all samples), the samples were sectioned (4 µm) and stained with haematoxylin and eosin (H&E). All the slices were observed and photographed using a H550S Photo Imaging System (Nikon, Japan).
Statistical analysis
According to prior rat PJI studies, 37-42 we chose an appropriate sample size to reduce Type 1 errors using the G-power program (version 3.1; Germany; serum α1-AGP levels, n = 6; serum vancomycin levels, n = 6; microbiological analysis of joint tissue, n = 7; micro-CT analysis of the knee joint, n = 6; SEM of the cement spacer, n = 6; histopathological analysis of the knee, n = 6). Statistical analyses were performed using SPSS software (version 22.0; IBM, USA) and are presented as the means and standard error of the means (SEM). Normality was assessed using the Shapiro-Wilks test, with Levene’s test used to determine the equality of variances. Independent-samples t-tests were used for between-groups comparison of normally distributed data. Within-group differences were analyzed with paired t-tests or, where appropriate, repeated measures analysis of variance (ANOVA) with Dunnett’s post-hoc analysis. Non-normally distributed data were compared using a Mann–Whitney U test or Kruskal–Wallis test with Dunn’s post-hoc analysis. A p-value < 0.05 was considered significant.
Results
Vancomycin elution and bioassay of vancomycin activity
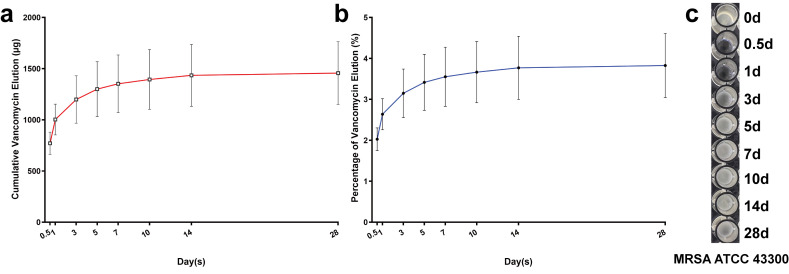
Over the entire 28-day period, the cumulative release of vancomycin from cement cylinders was 3.833% (SEM 0.806%; (1,456.64 µg (SEM 306.27))). The released proportion of vancomycin in the total cumulative elution was 82.52%, 92.86%, and 98.48% on days 3, 7, and 14, respectively (Figures 1a and 1b). The antibacterial effects against MRSA (ATCC 43300) lasted for less than three days (Figure 1c). The vancomycin MIC of ATCC 43300 used in the current study was detected as 2 µg/ml.
Fig. 1.
Vancomycin elution from cement specimens and biological activity assays within the 28-day period for in vitro samples. a) The cumulative release of vancomycin (µg) from acrylic cement cylinders specimens measured with elution assays over 28 days (12 hours, 1, 3, 5, 7, 10, 14, and 28 days); n = 6. b) The overall released percentage (%) of vancomycin in the total cumulative elution from the cylinders over 28 days (12 hours, 1, 3, 5, 7, 10, 14, and 28 days); n = 6. c) The in vitro biological activity using a modified microtube dilution bioassay against methicillin-resistant S. aureus in the current study (MRSA; ATCC 43300) of released vancomycin from cement specimens over 28 days (12 hours, 1, 3, 5, 7, 10, 14, and 28 days). The data in the figures represent the means and standard error of the means.
General status and serum infection biomarkers
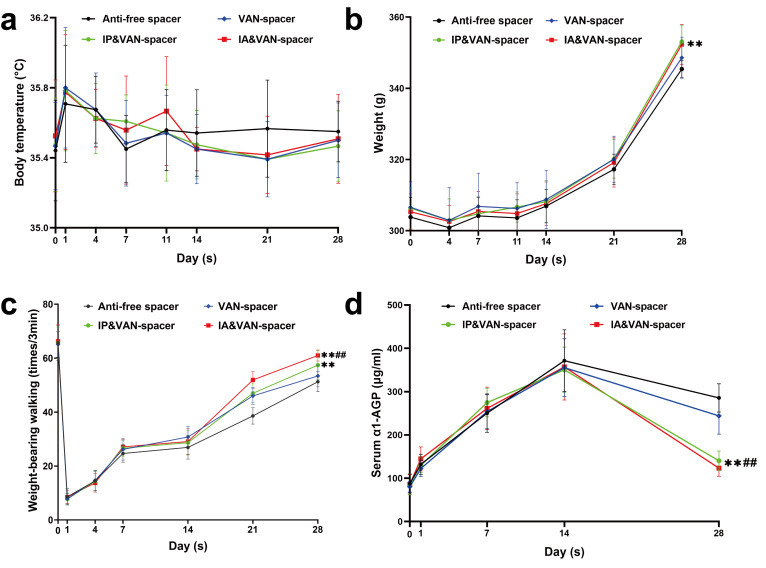
No significant differences among the four groups were observed for body temperature throughout the entire experiment (Figure 2a). No significant differences in body weight were detected among the vancomycin spacer only (Van-spacer), intraperitoneal vancomycin and vancomycin spacer (IP & Van-spacer), or intra-articular vancomycin and vancomycin spacer (IA & Van-spacer) groups on day 28 (Figure 2b, p = 0.065, 0.161, and 0.974, respectively), whereas the body weights in the IP & Van-spacer and IA & Van-spacer groups were greater than that of the antibiotic-free spacer (Control) group (p = 0.002 and 0.001, respectively). More weightbearing activities were observed at day 28 in the IP & Van-spacer and IA & Van-spacer groups, especially in the IA & Van-spacer group (Figure 2c), than in the other groups. In addition, serum alpha-1-acid glycoprotein (α1-AGP), a biomarker for infection in rats, was also reduced in the IP & Van-spacer and IA & Van-spacer groups at day 28 (no significant difference between these two groups) compared with the Control and Van-spacer groups (Figure 2d).
Fig. 2.
Changes in the general status and serum inflammatory markers of rats from different treatment groups throughout the experiment. a) Changes in body temperature of rats were detected from four treatment groups throughout the experiment (within 28 days). An electronic animal thermometer and infrared thermometer were used to measure the anal and rectal temperatures of rats preoperatively (day 0) and on postoperative days 1, 4, 7, 11, 14, 21, and 28. n = 13. b) The body weights of rats in each group were recorded using an electronic scale preoperatively (day 0) and on postoperative days 4, 7, 11, 14, 21, and 28; n = 13. c) Changes in weightbearing activities for the right knee of rats were observed via their daytime weightbearing activities, which were monitored in a large carton using video software on an iPhone 10 (Apple, USA). The weightbearing incidences, when the right foot made contact with the ground during a three-minute period were recorded and analyzed preoperatively (day 0) and on postoperative days 1, 4, 7, 14, 21, and 28; n = 6. d) Changes in serum alpha-1-acid glycoprotein (α1-AGP) levels were detected in each treatment group throughout the experiment, measured on days 0, 1, 7, 14, and 28, n = 6. The four treatment groups were as follows: Control (antibiotic-free cement spacer); Van-Cement spacer (4 g vancomycin per 40 g cement powder); IP & Van-Cement spacer (4 g vancomycin per 40 g cement powder and intraperitoneal (IP) injection of vancomycin, 88 mg/kg, 1 ml, every 12 hours); IA & Van-Cement spacer (4 g vancomycin per 40 g cement powder and intra-articular (IA) injection of vancomycin, 44 mg/kg, 150 µl, once per day). The data in the figures represent the means and standard error of the means. Significance was evaluated using a two-way analysis of variance (ANOVA). **p < 0.01 (compared with Control group), ##p < 0.01 (compared with Van-spacer group).
Radiological evaluation
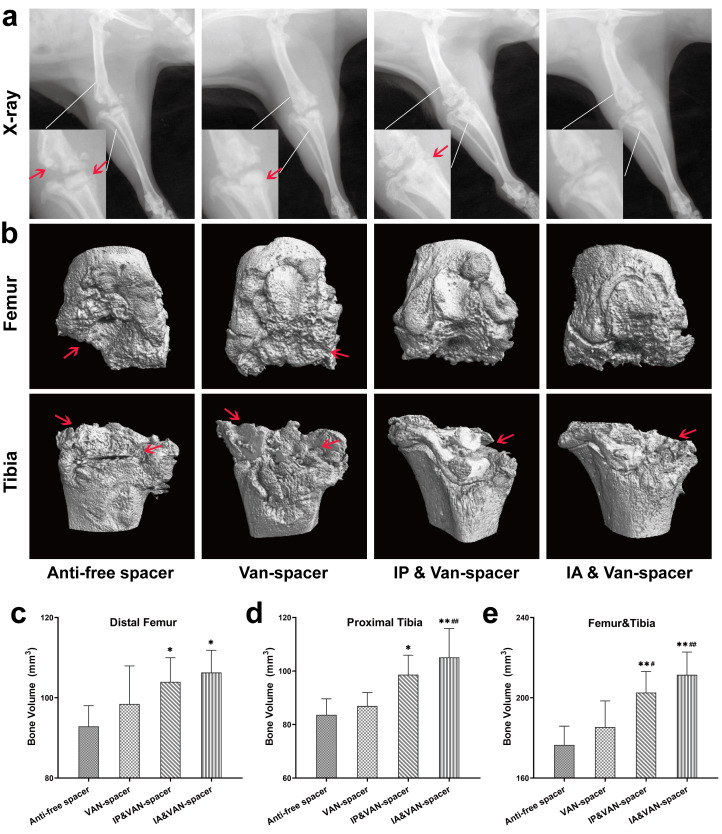
On day 28, radiographs showed that the cement spacers were still in the joints, but all of them were accompanied by signs of bone destruction around the joint, especially the proximal tibia (Figure 3a). Control group showed obvious osteolysis and destruction of the proximal tibia and distal femur. However, the radiograph results for the IP & Van-spacer and IA & Van-spacer groups showed milder osteolysis of the knee joint compared with the Control group (Figure 3a). Moreover, the BV of the distal femur, proximal tibia, and total knee in the Control and Van-spacer groups were reduced compared with those of the IP & Van-spacer and IA & Van-spacer groups (Figures 3b to 3e, p < 0.05).
Fig. 3.
Radiological evaluation of the knee joint in four treatment groups of rats on day 28. a) Radiograph of the right hind limb. b) 3D CT scan and bone reconstruction of the distal femur and proximal tibia. c) Bone volume (BV) analysis of the distal femur of micro-CT. d) BV analysis of the proximal tibia of micro-CT. e) Total BV analysis of the distal femur and proximal tibia of micro-CT. The four treatment groups were as follows: Control (antibiotic-free cement spacer); Van-Cement spacer (4 g vancomycin per 40 g cement powder); intraperitoneal (IP) & Van-Cement spacer (4 g vancomycin per 40 g cement powder, and IP injection of vancomycin, 88 mg/kg, 1 ml, every 12 hours); intra-articular (IA) & Van-Cement spacer (4 g vancomycin per 40 g cement powder, and IA injection of vancomycin, 44 mg/kg, 150 µl, once per day); n = 6. The data in the figures represent the means and standard error of the means. Significance was evaluated using a Mann–Whitney U test for the comparison of bone volume. *p < 0.05, **p < 0.01 (compared with Control group), #p < 0.05, ##p < 0.01 (compared with Van-spacer group). The red arrow indicates the position of bone destruction in the distal femur or proximal tibia.
Evaluation of microbial counts
A greater quantity of MRSA was observed in the Control group by SEM, surrounded by host leucocytes, with no other microbial contamination found in any field of view (FOV). No bacteria were observed in the IA & Van-spacer group (Figure 4a). Few microorganisms remained in the Van-spacer and IP & Van-spacer groups, with fewer MRSA detected in the IP & Van-spacer group than in the Control or Van-spacer groups (Figure 4a). Significantly fewer median colonies were detected for the IP & Van-spacer group than for the Control and Van-spacer groups, whereas more colonies were detected in the IP & Van-spacer group than in the IA & Van-spacer group (no bacterial residue in any specimen; Figures 4b to 4e and Table II, p = 0.003, 0.003, 0.009, and 0.003, respectively).
Fig. 4.
Microbiological evaluation in joint tissues of rats in each treatment group on day 28. a) Microbes on the surfaces of cement spacers were observed by scanning electron microscopy, at high magnification (×5,000 and ×10,000); the red arrow indicates methicillin-resistant Staphylococcus aureus (MRSA), the blue triangle indicates leucocyte, and the yellow circle indicates erythrocyte. b) Analysis of microbial culture counts from knee joint bones (including the distal femur and proximal tibia). c) Analysis of microbial culture counts from all soft-tissues around the surgical knee joint. d) Analysis of the microbial culture counts for cement spacers. e) Analysis of microbial culture counts for the total knees. The four treatment groups were as follows: Control (antibiotic-free cement spacer); Van-Cement spacer (4 g vancomycin per 40 g cement powder); intraperitoneal (IP) & Van-Cement spacer (4 g vancomycin per 40 g cement powder, and IP injection of vancomycin, 88 mg/kg, 1 ml, every 12 hours); intra-articular (IA) & Van-Cement spacer (4 g vancomycin per 40 g cement powder, and IA injection of vancomycin, 44 mg/kg, 150 µl, once per day). n = 7. The data in the figures represent the means and standard error of the means. Significance was evaluated using a Mann–Whitney U test for the comparison of microbial counts. *p < 0.05, **p < 0.01 (compared with Control group), ##p < 0.01 (compared with Van-spacer group), △△p < 0.01 (compared with IP & Van-spacer group). MRSA was identified as spherical structures with the following features: no surface deformities and approximately 0.8 to 1.0 µm in diameter. Host leucocytes were identified as spherical objects larger than 2 µm proximal to bacteria, with or without extracellular material coverage, and not adherent to the surface of cement spacers. Erythrocytes were identified as double concave disks with an mean diameter of 7 µm.
Table II.
Mean colony-forming unit data from Figures 4b to 4e.
| Group | Animal number | Bone | Soft-tissue | Cement spacer | Total |
|---|---|---|---|---|---|
| Antibiotic-free spacer | 1 | 1.875 × 105 | 3.54 × 105 | 1.45 × 105 | 6.86 × 105 |
| 2 | 3.75 × 105 | 2.28 × 105 | 3.22 × 105 | 9.25 × 105 | |
| 3 | 3.50 × 105 | 1.84 × 105 | 6.43 × 105 | 1.17 × 106 | |
| 4 | 1.52 × 105 | 2.08 × 105 | 4.50 × 105 | 8.10 × 105 | |
| 5 | 1.80 × 105 | 6.60 × 105 | 3.54 × 105 | 1.19 × 106 | |
| 6 | 5.20 × 105 | 1.44 × 105 | 4.38 × 105 | 1.10 × 106 | |
| 7 | 9.20 × 104 | 4.96 × 105 | 2.88 × 105 | 8.76 × 105 | |
| Van-spacer | 1 | 6.55 × 104 | 2.86 × 104 | 520 | 9.46 × 104 |
| 2 | 2.84 × 104 | 4.52 × 104 | 738 | 7.43 × 104 | |
| 3 | 1.25 × 105 | 4.36 × 105 | 86 | 5.61 × 105 | |
| 4 | 4.34 × 104 | 3.70 × 104 | 475 | 8.08 × 104 | |
| 5 | 1.66 × 104 | 2.94 × 105 | 120 | 3.11 × 105 | |
| 6 | 5.35 × 104 | 8.60 × 104 | 323 | 1.39 × 105 | |
| 7 | 1.84 × 104 | 1.27 × 105 | 60 | 1.45 × 105 | |
| IP & Van-spacer | 1 | 24 | 36 | 50 | 110 |
| 2 | 37 | 240 | 26 | 303 | |
| 3 | 25 | 127 | 38 | 190 | |
| 4 | 38 | 820 | 225 | 1083 | |
| 5 | 50 | 96 | 44 | 190 | |
| 6 | 0 | 0 | 0 | 0 | |
| 7 | 132 | 54 | 128 | 314 | |
| IA & Van-spacer | 1 | 0 | 0 | 0 | 0 |
| 2 | 0 | 0 | 0 | 0 | |
| 3 | 0 | 0 | 0 | 0 | |
| 4 | 0 | 0 | 0 | 0 | |
| 5 | 0 | 0 | 0 | 0 | |
| 6 | 0 | 0 | 0 | 0 | |
| 7 | 0 | 0 | 0 | 0 |
The four treatment groups: Control (antibiotic-free cement spacer); Van-Cement spacer (4 g vancomycin per 40 g cement powder); IP & Van-Cement spacer (4 g vancomycin per 40 g cement powder, and IP injection of vancomycin, 88 mg/kg, 1 ml, every 12 hours); IA & Van-Cement spacer (4 g vancomycin per 40 g cement powder, and IA injection of vancomycin, 44 mg/kg, 150 µl, once per day); n = 7. The limit of detection of bacteria in the culture results was 0 colony-forming units, and each sample was tested in triplicate repeatedly and averaged.
IA, intra-articular; IP, intraperitoneal; Van, vancomycin.
Evaluation of knee inflammation
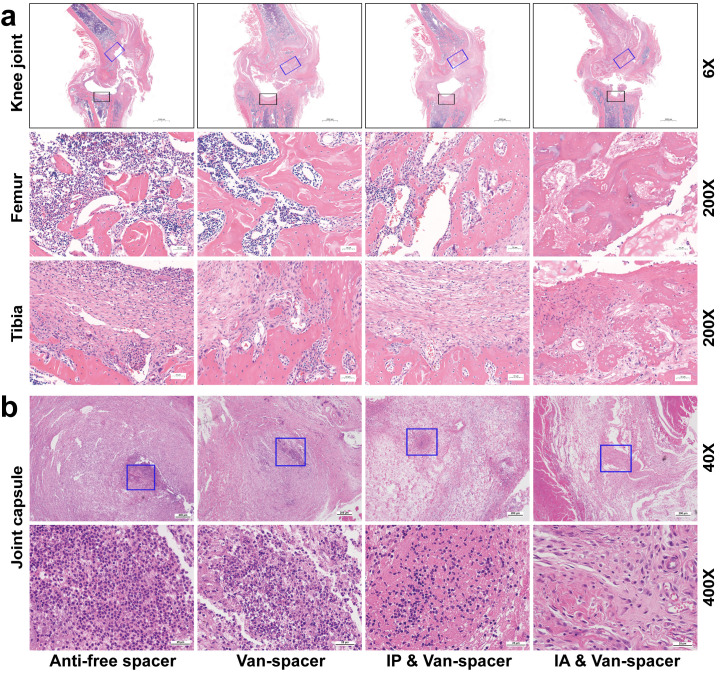
Severe osteomyelitis changes were observed in the Control and Van-spacer groups, such as intramedullary abscess, necrotic bone formation, trabecular bone defects, and inflammatory cell aggregation, whereas tissue inflammation was largely attenuated in the IP & Van-spacer and IA & Van-spacer groups, especially the IA & Van-spacer group (Figure 5a). Similar changes were also observed in the joint capsule (Figure 5b).
Fig. 5.
Histopathological assessment of the tissue surrounding the knee joint in the four treatment groups on day 28. a) Haemotoxylin and eosin (H&E) staining of knee joint (femur and tibia) at low magnification (×6) (upper) and high magnification (×200) (lower). The blue square represents the area of the distal femur osteotomy, and the black square indicates the area of the proximal tibia osteotomy. b) Representative of H&E staining of joint capsules at low magnification (×40) (upper) and high magnification (×400) (lower). The blue square shows the area where inflammatory cells were concentrated. The four treatment groups were as follows (n = 6): Control (antibiotic-free cement spacer); Van-Cement spacer (4 g vancomycin per 40 g cement powder); intraperitoneal (IP) & Van-Cement spacer (4 g vancomycin per 40 g cement powder, and IP injection of vancomycin, 88 mg/kg, 1 ml, every 12 hours); intra-articular (IA) & Van-Cement spacer (4 g vancomycin per 40 g cement powder, and IA injection of vancomycin, 44 mg/kg, 150 µl, once per day).
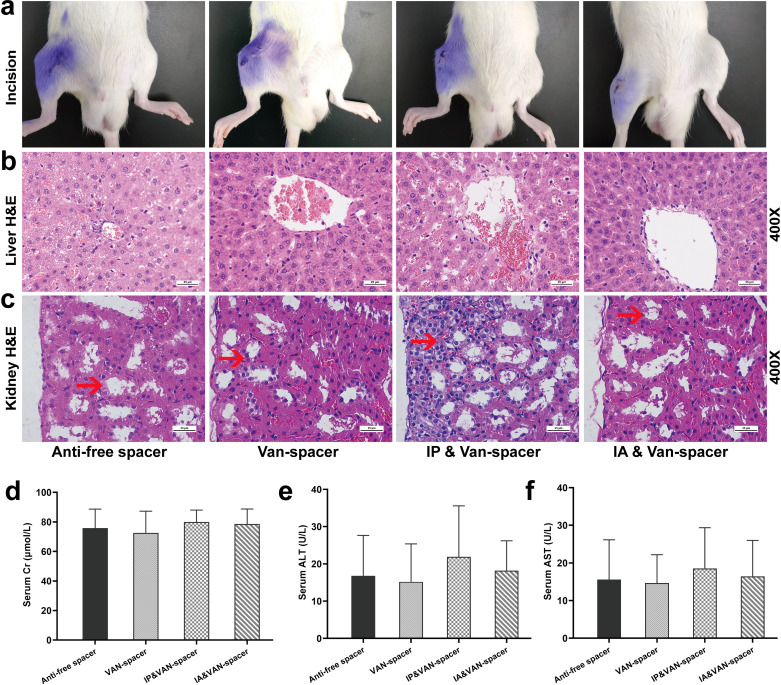
Evaluation of adverse drug effects
All the incisions were healed on day 28, without wound ruptures, exudation, or sinus tracts (Figure 6a). The serum Cr, ALT, and AST levels in each treatment group were all within the normal range, and no significant differences were observed among the four groups (Figures 6d to 6f, Cr (p = 0.924, 0.798, and 0.916, compared with Control group), ALT (p = 0.982, 0.674, and 0.993, compared with Control group), AST (p = 0.995, 0.890, and 0.997, compared with Control group)). No obvious structural changes were observed in the liver (Figure 6b), although mild changes in the renal inflammatory cell infiltration were observed in the IP & Van-spacer group compared with the IA & Van-spacer group, Van-spacer group, and Control group (Figure 6c). The serum vancomycin levels in the vancomycin treatment groups were sub-nephrotoxic (15 to 20 µg/ml) (Table III). 43-47
Fig. 6.
Evaluation of the incision healing and toxicology in the liver and kidney was carried out on day 28 in the four rat treatment groups. a) Incision healing. b) Pathological haemotoxylin and eosin (H&E) staining of liver at high magnification (×400). c) Pathological H&E staining of the kidney at high magnification ( ×400). d) Serum creatinine (Cr) levels on day 28, n = 13 per group. e) Serum alanine aminotransferase (ALT) levels on day 28, n = 13. f) Serum aspartate aminotransferase (AST) levels on day 28, n = 13. The four treatment groups were as follows: Control (antibiotic-free cement spacer); Van-Cement spacer (4 g vancomycin per 40 g cement powder); intraperitoneal (IP) & Van-Cement spacer (4 g vancomycin per 40 g cement powder, and intraperitoneal injection of vancomycin, 88 mg/kg, 1 ml, every 12 hours); intra-articular (IA) & Van-Cement spacer (4 g vancomycin per 40 g cement powder, and IA injection of vancomycin, 44 mg/kg, 150 µl, once per day). The data in the figures represent the means and standard error of the means. Significance was evaluated using two-way analysis of variance (ANOVA) for the comparison of serum ALT, AST, and Cr between the treatment groups.
Table III.
Serum levels of vancomycin pre-injection, and 0.5, two, and four hours after injection on day 28.
| Group | Pre-injection | 0.5 hrs (µg/ml) | 2 hrs (µg/ml) | 4 hrs (µg/ml) |
|---|---|---|---|---|
| Control | 0 | 0 | 0 | 0 |
| Van-Cement | * | * | * | * |
| IP & Van-Cement | * | 9.42 ± 1.38 | 3.99 ± 0.63 | * |
| IA & Van-Cement | * | 5.28 ± 0.73 | 3.30 ± 0.33 | * |
Control (antibiotic-free cement spacer); Van-Cement spacer (4 g vancomycin per 40 g cement powder); IP & Van-Cement spacer (4 g vancomycin per 40 g cement powder, and IP injection of vancomycin, 88 mg/kg, 1 ml, every 12 hours); IA & Van-Cement spacer (4 g vancomycin per 40 g cement powder, and IA injection of vancomycin, 44 mg/kg, 150 µl, once per day); n = 6.
Below the limit of detection (1 µg/ml).
IA, intra-articular; IP, intraperitoneal; Van, vancomycin.
Discussion
In the current study, we tried to extend the application of vancomycin by IA injections to treat PJI during a two-stage revision arthroplasty. Compared with routine clinical IV vancomycin administration, IA injection may be more effective after debridement or revision, allowing vancomycin to reach the joint cavity and surrounding inflamed tissues while maintaining a constant and high concentration. Thus, local vancomycin injections could maintain high drug concentrations in the infected tissues, reducing systemic adverse effects, which might represent a more effective and safe strategy for PJI control. 48 Orthopaedic surgeons have previously attempted to apply vancomycin in PJI cases caused by S. aureus or MRSA to control the infections after one-stage revision arthroplasty, either intra-articularly or systematically. The treatment duration often lasts for six weeks or more. Although promising results were obtained, the limited number of studies and the small sample sizes resulted in a low overall quality of evidence, and some data were obtained from retrospective studies or case reports. 23,25,49,50 No standard protocol or therapeutic regimen has been developed with consensus. We evaluated the local use of vancomycin for the eradication of infection after debridement and cement spacer implantation in a novel rat-based PJI model mimicking the two-stage revision process, which provided direct and reliable evidence regarding the IA application of vancomycin in the treatment of animal PJI during two-stage revision.
The in vitro elution data showed that vancomycin release from the cement primarily occurred within the first seven days (92.86% of cumulative release), especially on the first day. The cumulative release of vancomycin from the cement did not exceed 4% of the total vancomycin added to the cement throughout the 28-day period. These in vitro elution results of vancomycin cement were consistent with previous studies. 19-22 The PJI rat model indicated that implantation of vancomycin-loaded cement spacers alone following debridement was unable to eliminate bacteria, either on the spacer or from joint tissues during a two-week treatment course; systemic vancomycin combined with a vancomycin-spacer was also insufficient. However, a vancomycin spacer combined with IA vancomycin was effective for eliminating the bacteria both on the spacer and in joint tissues after a two-week treatment course in the current rat model. General status, weightbearing activity, serum α1-AGP levels, and histopathology also indicated that the combination of a vancomycin spacer and IA vancomycin injection resulted in better infection control outcomes than the vancomycin spacer alone, or the vancomycin spacer with systematic vancomycin. However, no statistical differences in the radiological evaluation were observed between the IA & Van-spacer group and IP & Van-spacer group. The possible reason was that weightbearing activity of the rats in the IA & Van-spacer group was accelerated after bacterial eradication, which resulted in the accelerated wear of knee bone and cement spacer. Moreover, the sample size used in the CT analysis was probably too small, which might bias our results. Thus, an IA vancomycin injection combined with a vancomycin-loaded cement spacer might be the ideal option for eliminating PJI caused by MRSA.
In this rat model study, no notable local or systemic adverse reactions were observed following IA vancomycin injection, such as sinus tract, poor incision healing, drug-induced osteolysis, nephrotoxicity, hepatotoxicity, body weight reductions, or decreased activity. However, some mild pathological changes in the kidney were observed in the IP & Van-spacer group, which were considered evidence of nephrotoxicity caused by systemic vancomycin, despite serum vancomycin levels in the current study being lower than the reported concentration necessary to induce nephrotoxicity (15 to 20 µg/ml). 43-47 This phenomenon was consistent with the previous experimental studies in rats describing the renal toxicity of vancomycin. 51-54 Clinical cases of nephrotoxicity following the use of conventional vancomycin doses have also been reported, with an incidence ranging from 5% to 22%. 55-57 Therefore, the use of IA vancomycin in our current study appears safer for rats than systemic vancomycin.
However, this study still has some limitations. First, it is a rat-based study, which cannot accurately represent human PJI and treatment conditions, as the absorption, distribution, metabolism, and excretion of drugs in rats differs from those in humans, 58 as well as the biodynamics and biomechanical characteristics of the knee. Second, although the available literature suggests that different debridement methods can influence PJI prognosis, 59,60 and some novel biomaterials can enhance the elution of vancomycin without attenuating the strength of cement, 61 our study aimed to compare the efficacy of different vancomycin administration approaches based on uniform debridement and vancomycin-loaded acrylic cement spacer implantation procedures. Third, our study compared the efficacy between vancomycin application approaches without the use of adjuvant oral antibiotics, such as rifampicin, which might affect the ability of the vancomycin spacer and/or systemic vancomycin treatment to eliminate the infection. Further investigations remain necessary to investigate the efficacy of additional oral antibiotics. Fourth, because the synovial fluid volume in the rat knee cavity was too small for sampling, we were unable to determine the concentrations and pharmacodynamics of vancomycin in the synovial fluid to test this hypothesis. Experiments based on larger animals or even humans are necessary. Fifth, we did not detect serum CRP or interleukin-6 levels, which are elevated during early-stage infection in humans. Instead, α1-AGP was used as the characteristic serum biomarker for acute infection in rats, based on evidence that acute inflammation induces acute-phase protein synthesis, such as α1-AGP. 62-66 Sixth, SEM could not be used to quantify bacteria accurately on the cement in our study because the surface of the cement spacers was rough. Moreover, microorganisms under the fibrous or scar tissue could not be visualized by SEM, making it inferior to cement sonication plus microbial culture counts in determining the number of adherent bacteria. Last, our current experiment and available clinical studies have not examined secondary infections associated with IA vancomycin injections, but potential adverse effects may occur due to the invasive administration approach.
In conclusion, in the current TKA rat model of PJI, IA vancomycin injection was effective and safe for controlling MRSA infection, and was able to eradicate the infection during a 14-day treatment course after debridement and vancomycin spacer implantation. More clinical trials and follow-up studies remain necessary to further evaluate the safety and efficacy of IA vancomycin injection in PJI patients.
Author contributions
J. Wei: Methodology, Investigation, Formal analysis, Writing – original draft.
K. Tong: Methodology, Investigation, Formal analysis.
H. Wang: Methodology, Formal analysis, Writing – review & editing.
Y. Wen: Methodology, Formal analysis, Writing – review & editing.
L. Chen: Methodology, Formal analysis, Writing – review & editing.
Funding statement
This work was supported by grants from the National Natural Science Foundation of China (No. 81603214, 81972036) and Translational Medicine and Interdisciplinary Research Joint Fund project (No. ZXJC202005). Each author certifies that there are no other funding or commercial associations (consultancies, stock ownership, equity interest, patent/licensing arrangements, etc.) that might pose a conflict of interest in connection with the submitted article related to the author or any immediate family members.
Data sharing
All relevant data analyzed during the current study are included within the paper.
Acknowledgements
We thank Mrs Qi Yin from the microbiology laboratory of Zhongnan Hospital of Wuhan University of China for MRSA ATCC-43300, and Dr. Yihang Wang for examination of the surfaces of cement spacers by scanning electron microscopy.
Ethical review statement
All rat studies have been performed in accordance with the ethical standards in the 1964 Declaration of Helsinki. The protocol of all animal experiments was approved by the Committee on the Ethics of Animal Experiments of the School of Medicine, Wuhan University (No. AF339). All animal experimental procedures were performed following the Guidelines for the Care and Use of Laboratory Animals of the Chinese Animal Welfare Committee.
Open access funding
The authors confirm that the open access fee for this study was self-funded.
Supplementary material
Figure displaying total knee arthroplasty procedure, establishment of the periprosthetic joint infection model, debridement, and cement spacer implantation in rats. An ARRIVE checklist is also included to show that the ARRIVE guidelines were adhered to in this study.
© 2022 Author(s) et al.This is an open-access article distributed under the terms of the Creative Commons Attribution Non-Commercial No Derivatives (CC BY-NC-ND 4.0) licence, which permits the copying and redistribution of the work only, and provided the original author and source are credited. See https://creativecommons.org/licenses/by-nc-nd/4.0/
Contributor Information
Jian Wei, Email: wei19828@163.com.
Kai Tong, Email: ttyjgtk@163.com.
Hui Wang, Email: wh20210130@163.com.
Yinxian Wen, Email: wenyinxian@whu.edu.cn.
Liaobin Chen, Email: lbchen@whu.edu.cn.
References
- 1. Ahmed SS, Haddad FS. Prosthetic joint infection. Bone Joint Res. 2019;8(11):570–572. 10.1302/2046-3758.812.BJR-2019-0340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lenguerrand E, Whitehouse MR, Beswick AD, Jones SA, Porter ML, Blom AW. Revision for prosthetic joint infection following hip arthroplasty: Evidence from the National Joint Registry. Bone Joint Res. 2017;6(6):391–398. 10.1302/2046-3758.66.BJR-2017-0003.R1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Argenson JN, Arndt M, Babis G, et al. . Hip and knee section, treatment, debridement and retention of implant: proceedings of International Consensus on Orthopedic Infections. J Arthroplasty. 2019;34(2S):S399–S419. 10.1016/j.arth.2018.09.025 [DOI] [PubMed] [Google Scholar]
- 4. Dosler S, Karaaslan E. Inhibition and destruction of Pseudomonas aeruginosa biofilms by antibiotics and antimicrobial peptides. Peptides. 2014;62:32–37. 10.1016/j.peptides.2014.09.021 [DOI] [PubMed] [Google Scholar]
- 5. Lechner S, Lewis K, Bertram R. Staphylococcus aureus persisters tolerant to bactericidal antibiotics. J Mol Microbiol Biotechnol. 2012;22(4):235–244. 10.1159/000342449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Roy ME, Peppers MP, Whiteside LA, Lazear RM. Vancomycin concentration in synovial fluid: direct injection into the knee vs. intravenous infusion. J Arthroplasty. 2014;29(3):564–568. 10.1016/j.arth.2013.08.017 [DOI] [PubMed] [Google Scholar]
- 7. Byren I, Bejon P, Atkins BL, et al. . One hundred and twelve infected arthroplasties treated with “DAIR” (debridement, antibiotics and implant retention): antibiotic duration and outcome. J Antimicrob Chemother. 2009;63(6):1264–1271. 10.1093/jac/dkp107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bernard L, Legout L, Zürcher-Pfund L, et al. . Six weeks of antibiotic treatment is sufficient following surgery for septic arthroplasty. J Infect. 2010;61(2):125–132. 10.1016/j.jinf.2010.05.005 [DOI] [PubMed] [Google Scholar]
- 9. Tam VH, Louie A, Fritsche TR, et al. . Impact of drug-exposure intensity and duration of therapy on the emergence of Staphylococcus aureus resistance to a quinolone antimicrobial. J Infect Dis. 2007;195(12):1818–1827. 10.1086/518003 [DOI] [PubMed] [Google Scholar]
- 10. Smirnova MV, Vostrov SN, Strukova EV, et al. . The impact of duration of antibiotic exposure on bacterial resistance predictions using in vitro dynamic models. J Antimicrob Chemother. 2009;64(4):815–820. 10.1093/jac/dkp287 [DOI] [PubMed] [Google Scholar]
- 11. Costelloe C, Metcalfe C, Lovering A, Mant D, Hay AD. Effect of antibiotic prescribing in primary care on antimicrobial resistance in individual patients: systematic review and meta-analysis. BMJ. 2010;340:c2096. 10.1136/bmj.c2096 [DOI] [PubMed] [Google Scholar]
- 12. Kurtz SM, Lau EC, Son MS, Chang ET, Zimmerli W, Parvizi J. Are we winning or losing the battle with periprosthetic joint infection: trends in periprosthetic joint infection and mortality risk for the Medicare population. J Arthroplasty. 2018;33(10):3238–3245. 10.1016/j.arth.2018.05.042 [DOI] [PubMed] [Google Scholar]
- 13. de Beaubien B, Belden K, Bell K, et al. . Hip and knee section, treatment, antimicrobials: proceedings of International Consensus on Orthopedic Infections. J Arthroplasty. 2019;34(2S):S477–S482. 10.1016/j.arth.2018.09.033 [DOI] [PubMed] [Google Scholar]
- 14. Klinder A, Zaatreh S, Ellenrieder M, et al. . Antibiotics release from cement spacers used for two-stage treatment of implant-associated infections after total joint arthroplasty. J Biomed Mater Res B Appl Biomater. 2019;107(5):1587–1597. 10.1002/jbm.b.34251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kurd MF, Ghanem E, Steinbrecher J, Parvizi J. Two-stage exchange knee arthroplasty: does resistance of the infecting organism influence the outcome? Clin Orthop Relat Res. 2010;468(8):2060–2066. 10.1007/s11999-010-1296-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Drexler M, Dwyer T, Kuzyk PRT, et al. . The results of two-stage revision TKA using Ceftazidime-Vancomycin-impregnated cement articulating spacers in Tsukayama Type II periprosthetic joint infections. Knee Surg Sports Traumatol Arthrosc. 2016;24(10):3122–3130. 10.1007/s00167-015-3753-y [DOI] [PubMed] [Google Scholar]
- 17. Rava A, Bruzzone M, Cottino U, Enrietti E, Rossi R. Hip spacers in two-stage revision for periprosthetic joint infection: a review of literature. Joints. 2019;7(2):56–63. 10.1055/s-0039-1697608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Warth LC, Hadley CJ, Grossman EL. Two-stage treatment for total knee arthroplasty infection utilizing an articulating prefabricated antibiotic spacer. J Arthroplasty. 2020;35(3S):S57–S62. 10.1016/j.arth.2019.10.049 [DOI] [PubMed] [Google Scholar]
- 19. Hsu Y-H, Hu C-C, Hsieh P-H, Shih H-N, Ueng SWN, Chang Y. Vancomycin and ceftazidime in bone cement as a potentially effective treatment for knee periprosthetic joint infection. J Bone Joint Surg Am. 2017;99-A(3):223–231. 10.2106/JBJS.16.00290 [DOI] [PubMed] [Google Scholar]
- 20. Boelch SP, Rueckl K, Fuchs C, et al. . Comparison of Elution Characteristics and Compressive Strength of Biantibiotic-Loaded PMMA Bone Cement for Spacers: Copal® Spacem with Gentamicin and Vancomycin versus Palacos® R+G with Vancomycin. Biomed Res Int. 2018;2018:4323518. 10.1155/2018/4323518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Duffy RK, Shafritz AB. Bone cement. J Hand Surg Am. 2011;36(6):1086–1088. 10.1016/j.jhsa.2011.01.041 [DOI] [PubMed] [Google Scholar]
- 22. Lee S-H, Tai C-L, Chen S-Y, Chang C-H, Chang Y-H, Hsieh P-H. Elution and mechanical strength of vancomycin-loaded bone cement: in vitro study of the influence of brand combination. PLoS One. 2016;11(11):e0166545. 10.1371/journal.pone.0166545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Whiteside LA, Peppers M, Nayfeh TA, Roy ME. Methicillin-resistant Staphylococcus aureus in TKA treated with revision and direct intra-articular antibiotic infusion. Clin Orthop Relat Res. 2011;469(1):26–33. 10.1007/s11999-010-1313-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Whiteside LA, Roy ME, Nayfeh TA. Intra-articular infusion: a direct approach to treatment of infected total knee arthroplasty. Bone Joint J. 2016;98-B(1 Suppl A):31–36. 10.1302/0301-620X.98B.36276 [DOI] [PubMed] [Google Scholar]
- 25. Whiteside LA, Roy ME. One-stage revision with catheter infusion of intra-articular antibiotics successfully treats infected THA. Clin Orthop Relat Res. 2017;475(2):419–429. 10.1007/s11999-016-4977-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rybak MJ, Lomaestro BM, Rotschafer JC, et al. . Vancomycin therapeutic guidelines: a summary of consensus recommendations from the Infectious Diseases Society of America, the American Society of Health-System Pharmacists, and the Society of Infectious Diseases Pharmacists. Clin Infect Dis. 2009;49(3):325–327. 10.1086/600877 [DOI] [PubMed] [Google Scholar]
- 27. O’Donnell JN, Rhodes NJ, Miglis CM, et al. . Dose, duration, and animal sex predict vancomycin-associated acute kidney injury in preclinical studies. Int J Antimicrob Agents. 2018;51(2):239–243. 10.1016/j.ijantimicag.2017.08.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Avedissian SN, Pais GM, O’Donnell JN, et al. . Twenty-four hour pharmacokinetic relationships for intravenous vancomycin and novel urinary biomarkers of acute kidney injury in a rat model. J Antimicrob Chemother. 2019;74(8):2326–2334. 10.1093/jac/dkz167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wei J, Wen Y, Tong K, Wang H, Chen L. Local application of vancomycin in one-stage revision of prosthetic joint infection caused by Methicillin-Resistant Staphylococcus aureus. Antimicrob Agents Chemother. 2021;65(9):e0030321. 10.1128/AAC.00303-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Slane J, Gietman B, Squire M. Antibiotic elution from acrylic bone cement loaded with high doses of tobramycin and vancomycin. J Orthop Res. 2018;36(4):1078–1085. 10.1002/jor.23722 [DOI] [PubMed] [Google Scholar]
- 31. Choi S, Moon SM, Park S-J, et al. . Antagonistic effect of colistin on vancomycin activity against Methicillin-Resistant Staphylococcus aureus in in vitro and in vivo studies. Antimicrob Agents Chemother. 2020;64(4):e01925-19. 10.1128/AAC.01925-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Carli AV, Bhimani S, Yang X, et al. . Quantification of peri-implant bacterial load and in vivo biofilm formation in an innovative, clinically representative mouse model of periprosthetic joint infection. J Bone Joint Surg Am. 2017;99-A(6):e25. 10.2106/JBJS.16.00815 [DOI] [PubMed] [Google Scholar]
- 33. Canham PB, Potter RF, Woo D. Geometric accommodation between the dimensions of erythrocytes and the calibre of heart and muscle capillaries in the rat. J Physiol. 1984;347:697–712. 10.1113/jphysiol.1984.sp015091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Thompson JM, Saini V, Ashbaugh AG, et al. . Oral-only linezolid-rifampin is highly effective compared with other antibiotics for periprosthetic joint infection: study of a mouse model. J Bone Joint Surg Am. 2017;99-A(8):656–665. 10.2106/JBJS.16.01002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zebala LP, Chuntarapas T, Kelly MP, Talcott M, Greco S, Riew KD. Intrawound vancomycin powder eradicates surgical wound contamination: an in vivo rabbit study. J Bone Joint Surg Am. 2014;96-A(1):46–51. 10.2106/JBJS.L.01257 [DOI] [PubMed] [Google Scholar]
- 36. Singh R, Ray P, Das A, Sharma M. Role of persisters and small-colony variants in antibiotic resistance of planktonic and biofilm-associated Staphylococcus aureus: an in vitro study. J Med Microbiol. 2009;58(Pt 8):1067–1073. 10.1099/jmm.0.009720-0 [DOI] [PubMed] [Google Scholar]
- 37. Lovati AB, Bottagisio M, Maraldi S, et al. . Vitamin E phosphate coating stimulates bone deposition in implant-related infections in a rat model. Clin Orthop Relat Res. 2018;476(6):1324–1338. 10.1097/01.blo.0000534692.41467.02 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Morris JL, Letson HL, Grant A, Wilkinson M, Hazratwala K, McEwen P. Experimental model of peri-prosthetic infection of the knee caused by Staphylococcus aureus using biomaterials representative of modern TKA. Biol Open. 2019;8(9):bio045203. 10.1242/bio.045203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Alt V, Lips KS, Henkenbehrens C, et al. . A new animal model for implant-related infected non-unions after intramedullary fixation of the tibia in rats with fluorescent in situ hybridization of bacteria in bone infection. Bone. 2011;48(5):1146–1153. 10.1016/j.bone.2011.01.018 [DOI] [PubMed] [Google Scholar]
- 40. Lovati AB, Romanò CL, Bottagisio M, et al. . Modeling Staphylococcus epidermidis-Induced Non-Unions: Subclinical and Clinical Evidence in Rats. PLoS One. 2016;11(1):e0147447. 10.1371/journal.pone.0147447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bostian PA, Karnes JM, Cui S, et al. . Novel rat tail discitis model using bioluminescent Staphylococcus aureus. J Orthop Res. 2017;35(9):2075–2081. 10.1002/jor.23497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Edelstein AI, Weiner JA, Cook RW, et al. . Intra-articular vancomycin powder eliminates Methicillin-resistant S. aureus in a rat model of a contaminated intra-articular implant. J Bone Joint Surg Am. 2017;99-A(3):232–238. 10.2106/JBJS.16.00127 [DOI] [PubMed] [Google Scholar]
- 43. Lodise TP, Patel N, Lomaestro BM, Rodvold KA, Drusano GL. Relationship between initial vancomycin concentration-time profile and nephrotoxicity among hospitalized patients. Clin Infect Dis. 2009;49(4):507–514. 10.1086/600884 [DOI] [PubMed] [Google Scholar]
- 44. Jeffres MN, Isakow W, Doherty JA, Micek ST, Kollef MH. A retrospective analysis of possible renal toxicity associated with vancomycin in patients with health care-associated methicillin-resistant Staphylococcus aureus pneumonia. Clin Ther. 2007;29(6):1107–1115. 10.1016/j.clinthera.2007.06.014 [DOI] [PubMed] [Google Scholar]
- 45. Cano EL, Haque NZ, Welch VL, et al. . Incidence of nephrotoxicity and association with vancomycin use in intensive care unit patients with pneumonia: retrospective analysis of the IMPACT-HAP Database. Clin Ther. 2012;34(1):149–157. 10.1016/j.clinthera.2011.12.013 [DOI] [PubMed] [Google Scholar]
- 46. Bosso JA, Nappi J, Rudisill C, et al. . Relationship between vancomycin trough concentrations and nephrotoxicity: a prospective multicenter trial. Antimicrob Agents Chemother. 2011;55(12):5475–5479. 10.1128/AAC.00168-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Carreno JJ, Jaworski A, Kenney RM, Davis SL. Comparative incidence of nephrotoxicity by age group among adult patients receiving vancomycin. Infect Dis Ther. 2013;2(2):201–208. 10.1007/s40121-013-0022-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Xu H, Yang J, Xie J, et al. . Efficacy and safety of intrawound vancomycin in primary hip and knee arthroplasty. Bone Joint Res. 2020;9(11):778–788. 10.1302/2046-3758.911.BJR-2020-0190.R2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Whiteside LA, Nayfeh TA, LaZear R, Roy ME. Reinfected revised TKA resolves with an aggressive protocol and antibiotic infusion. Clin Orthop Relat Res. 2012;470(1):236–243. 10.1007/s11999-011-2087-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ji B, Li G, Zhang X, Wang Y, Mu W, Cao L. Effective treatment of single-stage revision using intra-articular antibiotic infusion for culture-negative prosthetic joint infection. Bone Joint J. 2020;102-B(3):336–344. 10.1302/0301-620X.102B3.BJJ-2019-0820.R1 [DOI] [PubMed] [Google Scholar]
- 51. Fuchs TC, Frick K, Emde B, Czasch S, von Landenberg F, Hewitt P. Evaluation of novel acute urinary rat kidney toxicity biomarker for subacute toxicity studies in preclinical trials. Toxicol Pathol. 2012;40(7):1031–1048. 10.1177/0192623312444618 [DOI] [PubMed] [Google Scholar]
- 52. Vaidya VS, Ozer JS, Dieterle F, et al. . Kidney injury molecule-1 outperforms traditional biomarkers of kidney injury in preclinical biomarker qualification studies. Nat Biotechnol. 2010;28(5):478–485. 10.1038/nbt.1623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Sabler IM, Berkovitch M, Sandbank J, et al. . Exposure to hyperbaric oxygen intensified vancomycin-induced nephrotoxicity in rats. PLoS One. 2016;11(4):e0152554. 10.1371/journal.pone.0152554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Pais GM, Liu J, Avedissian SN, et al. . Lack of synergistic nephrotoxicity between vancomycin and piperacillin/tazobactam in a rat model and a confirmatory cellular model. J Antimicrob Chemother. 2020;75(5):1228–1236. 10.1093/jac/dkz563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Farber BF, Moellering RC. Retrospective study of the toxicity of preparations of vancomycin from 1974 to 1981. Antimicrob Agents Chemother. 1983;23(1):138–141. 10.1128/AAC.23.1.138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Lodise TP, Lomaestro B, Graves J, Drusano GL. Larger vancomycin doses (at least four grams per day) are associated with an increased incidence of nephrotoxicity. Antimicrob Agents Chemother. 2008;52(4):1330–1336. 10.1128/AAC.01602-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Cosgrove SE, Vigliani GA, Fowler VG, et al. . Initial low-dose gentamicin for Staphylococcus aureus bacteremia and endocarditis is nephrotoxic. Clin Infect Dis. 2009;48(6):713–721. 10.1086/597031 [DOI] [PubMed] [Google Scholar]
- 58. Reagan-Shaw S, Nihal M, Ahmad N. Dose translation from animal to human studies revisited. FASEB J. 2008;22(3):659–661. 10.1096/fj.07-9574LSF [DOI] [PubMed] [Google Scholar]
- 59. Tsang S-T, Morgan-Jones R, Simpson A. Debridement for prosthetic joint infections: future therapies. Bone Joint Res. 2020;9(6):311–313. 10.1302/2046-3758.96.BJR-2020-0238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Deng Z, Liu F, Li C. Therapeutic effect of ethylenediaminetetraacetic acid irrigation solution against wound infection with drug-resistant bacteria in a rat model: an animal study. Bone Joint Res. 2019;8(5):189–198. 10.1302/2046-3758.85.BJR-2018-0280.R3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Funk GA, Menuey EM, Ensminger WP, Kilway KV, McIff TE. Elution of rifampin and vancomycin from a weight-bearing silorane-based bone cement. Bone Joint Res. 2021;10(4):277–284. 10.1302/2046-3758.104.BJR-2020-0430.R1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Cray C, Zaias J, Altman NH. Acute phase response in animals: a review. Comp Med. 2009;59(6):517–526. [PMC free article] [PubMed] [Google Scholar]
- 63. Mestriner FLAC, Spiller F, Laure HJ, et al. . Acute-phase protein alpha-1-acid glycoprotein mediates neutrophil migration failure in sepsis by a nitric oxide-dependent mechanism. Proc Natl Acad Sci U S A. 2007;104(49):19595–19600. 10.1073/pnas.0709681104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Matsumoto K, Nishi K, Kikuchi M, et al. . Alpha1-acid glycoprotein suppresses rat acute inflammatory paw edema through the inhibition of neutrophils activation and prostaglandin E2 generation. Biol Pharm Bull. 2007;30(7):1226–1230. 10.1248/bpb.30.1226 [DOI] [PubMed] [Google Scholar]
- 65. Søe NH, Jensen NV, Nürnberg BM, et al. . A novel knee prosthesis model of implant-related osteomyelitis in rats. Acta Orthop. 2013;84(1):92–97. 10.3109/17453674.2013.773121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Zarrabian S, Attaix D, Marcy J, et al. . Effects of alimentary whole proteins versus their small peptide hydrolysates on liver and skeletal muscle during the acute inflammation phase in the rat. Clin Nutr. 1998;17(4):169–176. 10.1016/s0261-5614(98)80053-8 [DOI] [PubMed] [Google Scholar]