Summary
Background
Vaccination remains the primary measure to prevent the spread of the SARS-CoV-2 virus, further necessitating the use of effective licensed vaccines.
Methods
From Dec 25, 2020, to July 11, 2021, we conducted a multicenter, randomised, single-blind, placebo-controlled phase 3 efficacy trial of the QazCovid-in® vaccine with a 180-day follow-up period in three clinical centres in Kazakhstan. A total of 3000 eligible participants aged 18 years or older were randomly assigned (4:1) to receive two doses of the vaccine (5 μg each, 21 days apart) or placebo administered intramuscularly. QazCovid-in® is a whole-virion formaldehyde-inactivated anti-COVID-19 vaccine, adjuvanted with aluminium hydroxide. The primary endpoint was the incidence of symptomatic cases of the SARS-CoV-2 infection confirmed by RT-PCR starting from day 14 after the first immunisation. The trial was registered with ClinicalTrials.gov NCT04691908.
Findings
The QazCovid-in® vaccine was safe over the 6-month monitoring period after two intramuscular immunisations inducing only local short-lived adverse events. The concomitant diseases of participants did not affect the vaccine safety. Out of 2400 vaccinated participants, 31 were diagnosed with COVID-19; 43 COVID-19 cases were recorded in 600 placebo participants with onset of 14 days after the first dose within the 180-day observation period. Only one severe COVID-19 case was identified in a vaccine recipient with a comorbid chronic heart failure. The protective efficacy of the QazCovid-in® vaccine reached 82·0% (95% CI 71.1–88.5) within the 180-day observation period.
Interpretation
Two immunisations with the inactivated QazCovid-in® vaccine achieved 82·0% (95% CI 71.1–88.5) protective efficacy against COVID-19 within a 180-day follow-up period.
Funding
The work was funded by the Science Committee of the Ministry of Education and Science of Kazakhstan within the framework of the Scientific and Technical Program “Development of a vaccine against coronavirus infection COVID-19”. State registration number 0.0927.
Keywords: Efficacy, COVID-19, Virus, Vaccine, Phase 3, Clinical trial
Research in context.
Evidence before this study
The PubMed, Medline, and server medRxiv search for publications on clinical trials of COVID-19 vaccines, conducted on the 1st of October 2021, showed that several inactivated whole-virion vaccines had been developed. Safety and immunogenicity were demonstrated for the BBV152 vaccine, formulated with aluminium and a Toll-like receptor 7/8 agonist and manufactured in India, within a 3-month follow-up period after the second dose. Protective efficacy was investigated in phase 3 clinical trial with a 3-month follow-up for the CoronaVac whole-virion vaccine, formulated with an aluminium adjuvant (manufactured in Turkey). Here we evaluated the protective efficacy of an inactivated vaccine within a 6-month observation period.
Added value of this study
Double immunisation with QazCovid-in® is safe and provides immunogenicity, Th1-biased cellular immunity, and the protective efficacy of 82·0% (95% CI 71.1–88.5) SARS-CoV-2 over the 6-month follow-up period starting 14 days after the first immunisation.
Implications of all the available evidence
The data obtained enable to conduct a clinical trial with the participation of pregnant women and children, as well as large-scale post-registration studies.
Alt-text: Unlabelled box
Introduction
More than 500 million confirmed cases of SARS-CoV-2 infection, which led to more than 6 million deaths worldwide, were reported by WHO in April 2022. The rapid development of effective mRNA-based and adenovirus vectored vaccines enabled vaccination of the population all over the world with more than 8·6 billion vaccine doses.1, 2, 3, 4, 5, 6, 7, 8 Several inactivated whole-virion vaccines against SARS-CoV-2 were also shown to be effective in clinical trials within a 3-month follow-up period.9,10
However, vaccination rates are insufficient for achieving global herd immunity in order to prevent the spread of SARS-CoV-2. New antigenic variants of SARS-CoV-2, namely Alpha (lineage B.1.1.7), Gamma (lineage P.1), Beta (lineage B.1.351), Delta (lineage B.1.617.2), and Omicron (lineage B.1.1.529) have emerged which carry mutations in the Spike protein leading to more efficient transmission.11, 12, 13 This has raised a concern about the efficacy of the existing vaccines.14, 15, 16, 17 The neutralisation capacity of sera from patients immunised with the BNT162b2 mRNA vaccine is shown in vitro for Alpha (lineage B.1.1.7), Beta (lineage B.1.351), and Delta (lineage B.1.617.2) mutants, and the lowest titre is detected for the most transmissible Delta variant (with a four-fold decrease).18,19 In clinical studies, the mRNA BNT162b2 and adenovirus vector ChAdOx1 vaccines are found to be highly effective in reducing the number of severe cases and deaths induced by the Alpha variant of SARS-CoV-2.20,21 The effectiveness of the mRNA-1273 vaccine against severe disease induced by Alpha mutant is shown to be 81·6%, and it reaches 95·7% against the Beta variant.22 The spreading of the Delta variant across the globe has led to its predominance and revealed reduced effectiveness of the BNT162b2, mRNA-1273, and ChAdOx1 vaccines against symptomatic infection with the SARS-CoV-2 Delta variant.23, 24, 25, 26
In Kazakhstan, the technology for production of formaldehyde-inactivated, adjuvanted with aluminium hydroxide, whole-virion vaccine QazCovid-in® against COVID-19 has been developed, and preclinical studies27 as well as phases 1/2 clinical trials of the vaccine are now completed.28 The QazCovid-in® vaccine is proven to be safe, well-tolerated, and immunogenic in two age groups, 18–49 and ≥50 years.
The QazCovid-in® vaccine has passed a temporary registration by the Ministry of Health of Kazakhstan, allowing for its industrial production and launching the preventive immunisation of the Kazakhstan population. The vaccine is also used in the Kyrgyz Republic. Here we report the results of the multicentre, randomised, single-blind, placebo-controlled, phase 3 clinical trial with a 180-day follow-up period starting from the 14th day after the first immunisation with QazCovid-in® to evaluate vaccine safety, immunogenicity, and efficacy, as well as the durability of the immune response to immunisation.
Methods
Study design and participants
From Dec 25, 2020, to July 11, 2021, we conducted a randomised, single-blind, placebo-controlled phase 3 multicentre clinical trial in the International Institute of Postgraduate Education in Almaty, the 4th City Outpatient Clinic in Almaty, and Multidisciplinary City Hospital in Taraz, Kazakhstan. The study complies with the International Council for Harmonization Good Clinical Practice guidelines. The protocol (available in Supplement) is approved by the Committee for Control of Medical and Pharmaceutical Activities of the Ministry of Health of Kazakhstan (No. KZ78VMX00000211), the Central Commission on Bioethics of the Ministry of Health, and local ethical commissions of the clinical centres.
The sample size was not based on any statistical hypothesis, as the infection rate could not be prespecified, and was adjusted to study feasibility of near 3000 potential participants. Assuming the most commonly used infection rate in placebo group at 5% and the point estimate of VE at 80% (similar to another inactivated vaccine9), the designated sample size of 3000 individuals (Vaccine: Placebo = 4: 1) would ensure 54 total cases of PCR-confirmed COVID-19 by the end of the study. Using one-sample proportion test (RStudio, pwr package), the calculated number of cases would provide 98.2% power to detect the difference between the recommended null hypothesis (H0: VE ≤ 30%) and the specified alternative hypothesis (H1: VE = 80%) at the 0.05 significance level, or 81.4% power to detect the difference between the more satisfying H0 hypothesis (VE ≤ 50%) and the same H1 hypothesis.
We recruited 5476 potential participants and screened them for eligibility for the study. The main exclusion criteria comprised a history of COVID-19 diagnosis, close contacts with individuals suspected of being infected with SARS-CoV-2 or diagnosed with COVID-19 in the last 14 days, or presence of antibodies to SARS-CoV-2 in ELISA. Detailed inclusion and exclusion criteria are presented in the protocol (available in Supplement). The majority of screen faluers (1954 [35·7%] were due to the exclusion criteria, and 1518 [27·7%] had a positive test for SARS-CoV-2.
Randomisation and masking
Participants (3000) aged 18 years or older were randomly assigned (4:1) to receive the QazCovid-in® vaccine (2400 participants) or placebo (600 participants) with complete concealment. Eligible participants with a study number assigned at enrolment were vaccinated accordingly. The vaccine and placebo were assigned codes by block randomisation using a computerised randomisation schedule that was generated by an independent statistician using own SAS Programming (version 9.4). The software was built on a client-server scheme, which allowed remote viewing of randomization codes through authorization by the principal investigator, independent statistician, and sponsor's representative. We used the program MySQL version 8 as a database. GraphPad Prizm v.8.4.8 was used for data processing.
The randomization procedure was carried out by employees of the clinical base with the Principal Investigator (IK). The Principal Investigator, the sponsor's designee, and an independent statistician had the randomisation codes. Unblinding was scheduled on day 90 according to the study protocol. Premature unblinding was possible in case of a serious adverse event (AE).
All participants signed an informed consent form and underwent a screening examination. Volunteers of the study were adults 18 years of age or older, healthy or with chronic concomitant diseases listed in Table S1 (available in Supplement).
Volunteers who met all criteria were randomly assigned to the vaccine or placebo group and were immunised on study days 1 and 21. The second immunisation of 2329 participants was carried out from January 14 to February 2, 2021. We evaluated the safety, immune response, and efficacy of two doses of the QazCovid-in® vaccine (5 µg each) given intramuscularly 21 days apart compared to placebo over 180 days. The trial was registered with ClinicalTrials.gov NCT04691908.
Procedures
The QazCovid-in® vaccine was produced from the SARS-CoV-2 /human/KAZ/KZ_Almaty/2020 strain (GenBank accession number NC_045512.2 for the complete genome), similar in sequence to the SARS-CoV-2. The details of the vaccine production were described earlier.28 Follow-up visits were scheduled on days 1, 21, 42, 90 and 180.
The humoral immune response was analysed on study day 1 and day 21 (before the first and second vaccination, respectively), and on days 42, 90, and 180 by measurement of the neutralising antibody titres in a microneutralisation assay (MNA) using SARS-CoV-2/human /KAZ/KZ_Almaty/2020 strain and IgG antibodies to Spike protein of SARS-CoV-2 in ELISA. Cellular immunity was assessed by a whole-blood cytokine release assay on study day 1 (before the first vaccination), day 90, and day 180. The levels of cytokines IL-6, IFN-α, IFN-γ, TNF-α were measured in response to stimulation with two proteins (Nucleocapsid and Spike) of the SARS-CoV-2 virus.29 A detailed description of all assay methods were published earlier.28
Participants in both groups were monitored for the COVID-19 disease throughout the observation period. COVID-19 was diagnosed based on clinical data and confirmed by RT-PCR. The main clinical symptoms included fever, general weakness, malaise, sweating, myalgia and body aches, headache, sore throat, cough (rare dry with a small amount of sputum, or severe paroxysmal), tightness, burning, pain, compression in the chest, disturbance in the sensitivity of taste and smell, diarrhoea, restlessness, conjunctivitis, rash. The severity of SARS-CoV-2 infection was classified according to the WHO Clinical Progression Scale. There were no planned stopping rules for futility and no bias adjustment were planned in the protocol.
Outcomes
The primary efficacy endpoint was the incidence of symptomatic COVID-19 infection cases manifested from day 14 after the first immunisation onwards and confirmed by the detection of SARS-CoV-2 nucleic acid in RT-PCR on a clinical sample.
Secondary efficacy endpoints were the rate of hospitalisation and death, level of neutralising antibodies against SARS-CoV-2, rate of the antibody seroconversion, the longevity of antibody response, and changes in the level of antigen-specific cellular immunity. The safety outcomes included the incidence of all local reactions observed in participants within 2 h after the first and second doses of the vaccine, as well as the incidence of solicited adverse reactions within 7 days after the first or second vaccine administration, and the incidence of unsolicited adverse events AEs from the first vaccination up to day 180 of the study. During their daily visits to the study centres within 7 days after the first and second vaccination, volunteers were monitored for local and systemic reactions which included temperature, blood pressure, pulse, and other symptoms. From day 8 to day 20 and from day 28 to day 41, volunteers recorded all symptoms in self-observation Diaries 1 and 2, respectively. From day 43 to day 180 of the study, the participants reported all local and systemic reactions to the investigator by phone. All AEs were graded as mild, moderate, or severe and were assigned as vaccine-related or non-related according to the Protocol (Appendix 1). Monthly calls were made by investigators to register AEs and weekly calls to evaluate the effectiveness of the QazCovid-in® vaccine. If AEs (local and systemic reactions) and signs of ARVI were detected, the participant informed the investigator by the phone.
Vaccine efficacy
The vaccine efficacy was calculated for the intention-to-treat population using the following formula: VE (%)=(1 – RR) × 100, where the RR is as follows: RR= a/b* c/d, where a - is the number of vaccinated participants with COVID-19, b - is the total number of vaccinated participants, c - is the number of placebo participants with COVID-19, and d - is the total number of placebo participants.30 The 95% confidence intervals (95%) CI were calculated using the Koopman asymptotic score confidence interval for the ratio of proportions as implemented in the GraphPad Prizm v8.4.8 software.30
Statistical analysis
The sample size was not based on any statistical hypothesis and was adjusted to study feasibility. Qualitative characteristics of a study group are presented as percentages; quantitative parameters are presented as median with an interquartile range (IQR) and a min-max range. The safety analysis set included all randomised participants who received at least one dose of the study vaccine or placebo. Descriptive summary data were provided as numbers and per group percentages and included the participants who reported at least one solicited local reaction or systemic AE, any unsolicited AEs, serious AEs, or AEs of special interest after the first or second dose. The attributable risk was calculated for particular groups and presented with 95% CI calculated by the Newcombe-Wilson method with continuity correction. Immunogenicity analysis included the participants who received at least one dose of the study vaccine or placebo and provided blood samples according to their allocated vaccination schedule. Immunogenicity metrics were presented as geometric mean titres (GMTs) with 95% CIs. A seroconversion rate was assessed as the percentage of participants with a fourfold or greater increase in antibody titre compared to day 1 and was presented with 95% Wilson CI. Post hoc statistical analyses with appropriate correction for multiple comparisons were performed for cytokine data on day 90 and day 180 in comparison to day 1 in each group; or between the vaccine group and the placebo group on indicated days. Analyses were performed independently for each cytokine data set. In case when immunogenicity data were missing for several participants, the data omission procedure was run, and the data for these participants were excluded from the analysis. Statistical analysis was performed using GraphPad Prism 8 (version 8.4.3) software by an independent external statistician.
Role of the funding source
The sponsor had no role in the design of the study, as well as in the data collection, its analyses and interpretation, in the writing of the manuscript, and in the decision to publish the results. All authors confirm that they had full access to all the data in the study and accept responsibility to submit for publication.
Results
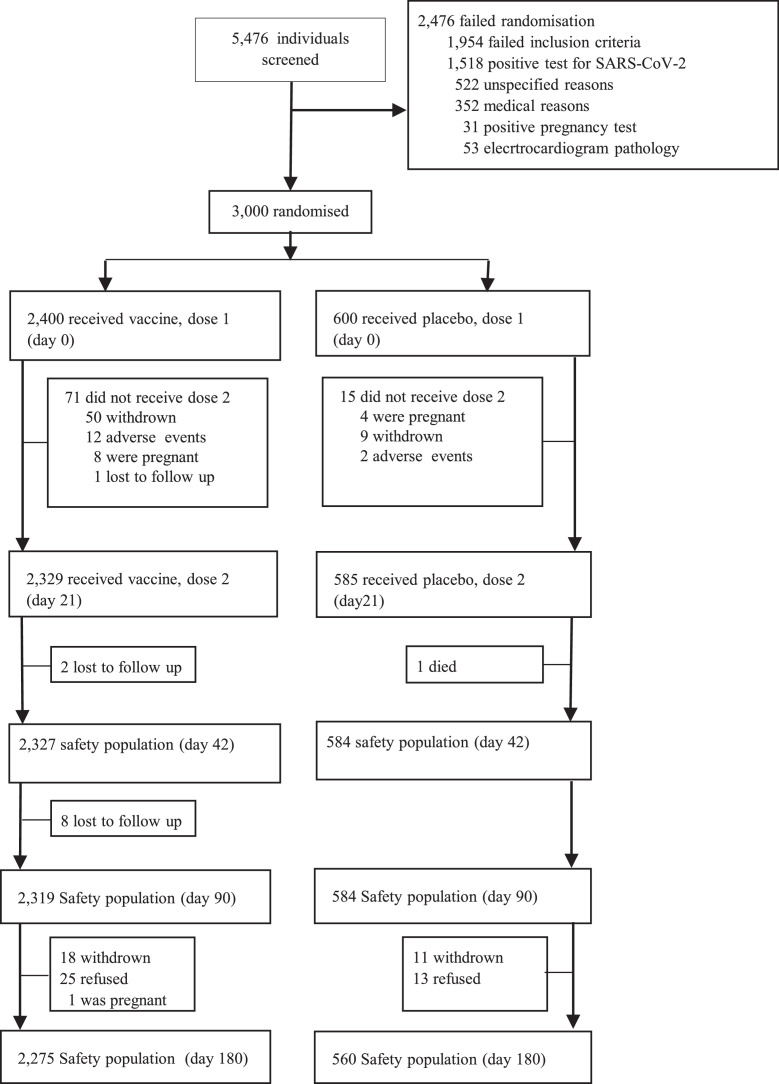
From Dec 25, 2020, until Jan 13, 2021, 5476 potential participants aged 18 or older were screened to participate in the phase 3 study. The majority of screen failures (2476 [55·21%]) did not pass the inclusion criteria. Participants meeting the inclusion criteria (3000 participants in 3 centres) were randomised to receive the QazCovid-in® vaccine (2400 participants) or placebo (600 participants) on days 1 and 21 and completed all scheduled visits to the centres, except 165 participants who dropped out of the study for various reasons. The distribution of study participants is shown in Figure 1.
Figure 1.
Trial profile for the phase 3 study.
The demographic characteristics of the participants enrolled in the study are shown in Table 1 and S3. The study involved 1482 (49·4%) females and 1518 males (50·6%). The median age in the vaccine group was 35 years (IQR 26, 45), and 34 years (IQR 26, 46) in the placebo group. The mean height and weight of the volunteers were comparable between the groups.
Table 1.
Demographic characteristics of study participants, ITT population.
| Vaccine group (n = 2400) | Placebo group (n = 600) | |
|---|---|---|
|
Sex Female, (%) Male, (%) |
1195 (49·79) 1205 (50·21) |
287 (47·83) 313 (52·17) |
|
Ethnicity White, (%) Asian, (%) |
132 (5·50) 2268 (94·50) |
46 (7·67) 554 (92·33) |
|
Age, years Median (IQR) 18-55, (%) >55, (%) |
35·0 (26 0, 45 0) 2225 (92 7) 175 (7 3) |
34·0 (26 0, 46 0) 554 (92 3) 46 (7 7) |
|
Body-mass index* Median (IQR) ˂25, (%) 25–30, (%) ≥30.0:obese |
24 3 (21 7, 27 8) 1342 (55 9) 688 (28 7) 370 (15 4) |
24 9 (21 5, 28 1) 347 (57 8) 167 (27 8) 86 (14 4) |
Data are presented as numbers (%).
The body-mass index is the weight in kilograms divided by the square of the height in meters.
The safety subset included the participants who received at least one dose of the study vaccine or placebo. Within 2 h after immunisation, only local reactions, with pain at the injection site as the most common one, were recorded: 13·04% after the first dose, and 4·59% after the second dose (Figure S1). The complete list of AEs recorded within 2 h after each immunisation is presented in Table 2. No immediate serious AEs within the first 2 h after both immunisations were reported.
Table 2.
Any foreseen AEs within 2 h after the first or the second vaccination, ITT population.
| Local and systemic AEs | AEs after the first Vaccination |
AEs after the second Vaccination |
||
|---|---|---|---|---|
| Vaccine (n = 2400) (%) | Placebo (n = 600) (%) | Vaccine (n = 2329) (%) | Placebo (n = 585) (%) | |
| Any foreseen local and systemic AEs | 1168 (48·67) | 31 (5 17) | 278 (11 94) | 21 (3 59) |
| Local reactions | 1502 (62 58) | 31 (5 17) | 350 (15 03) | 25 (4 27) |
| Soreness at the injection site | 1094 (45 58) | 15 (2 5) | 214 (9 19) | 9 (1 54) |
| Pain | 313 (13 04) | 10 (1 67) | 107 (4 59) | 7 (1 19) |
| Swelling | 44 (1 83) | 4 (0 67) | 7 (0 30) | 4 (0 68) |
| Hyperaemia | 37 (1 54) | 0 (0 0) | 22 (0 94) | 5 (0 85) |
| Systemic reactions | 26 (1 08) | 0 (0 0) | 3 (0 16) | 2 (0 34) |
| Fever | 3 (0 125) | 0 (0 0) | 1 (0 04) | 0 (0 0) |
| Headache | 14 (0 58) | 0 (0 0) | 1 (0 04) | 1 (0 17) |
| Cough | 0 (0 0) | 0 (0 0) | 1 (004) | 1 (0 17) |
| Mild (Grade 1) | 0 (0 0) | 0 (0 0) | 0 (0 0) | 0 (0 0) |
| Moderate (Grade 2) | 0 (0 0) | 0 (0 0) | 0 (0 0) | 0 (0 0) |
| Any serious AE | 0 (0 0) | 0 (0 0) | 0 (0 0) | 0 (0 0) |
Data are presented as n (%).
Within the first 7 days, the most often local reactions included pain at the injection site (19·37% after the first, 6·14% after the second dose), hyperaemia (5·04% after the first, 2·96% after the second dose), and swelling (3·08% after the first dose, 2·02% after the second one) (Figure S1, Table S2). Local reactions after the second dose of the vaccine were recorded less frequently. All local AEs within the first 7 days after both vaccinations were mild (Grade 1) and short-lived.
The most often systemic reaction in the vaccine group within the first 7 days was headache (1·83% after the first and 2·0% after the second dose) (Figure S2, Table S2). All noted AEs in the vaccine group were mild (Grade 1). Within this period, we recorded more systemic reactions after the second immunisation in the placebo group compared to the vaccine group. These reactions included headache (2·39%), weakness (3·08%), sore throat (1·19%), fever (1·37%), cough (1·02%), and rheum (1·02%), and were related to COVID-19 diagnosed in 10 volunteers of the placebo group within this observation period.
During the entire study period, only mild AEs (Grade 1) and no severe AEs associated with vaccination were revealed, as well as no cases of allergisation and thrombi formation were recorded. One suicide death unrelated to the vaccination was recorded in the placebo group. Overall, the QazCovid-in® vaccine was well-tolerated and safe within the 180-day observation period after two intramuscular immunisations in volunteers aged 18 years or older. The concomitant diseases of participants did not affect the safety parameters of the vaccine.
Two immunisations with the QazCovid-in® vaccine induced a fourfold increase in MNA titres in 99% (95% CI 99, 100) of participants with a GMT of 109 (105, 113) on day 42. Antibodies were still present on days 90 and 180, although their titres decreased to 47·3 (95% CI 45·8, 48·9) and 9·1 (95% CI 8·4, 9·8) respectively (Table 3). The increase in cellular immunity in response to stimulation with Nucleocapsid and Spike proteins of SARS-CoV-2 was observed as statistically significant elevation of IFN-α, IFN-γ, IL-6, and TNF-α cytokine levels (p < 0.0001) in the vaccine group on study days 90 and 180 compared to day 1 (Figure S3), as measured by a whole-blood cytokine release assay. These data indicate that the QazCovid-in® vaccine, administered intramuscularly twice with an interval of 21 days, stimulated both humoral and cellular immune response against SARS-CoV-2 which persisted for at least 180 days.
Table 3.
Immunogenicity of the QazCovid-in® vaccine in phase 3 study measured by MNA and ELISA, PP population.
| Days after the first immunisation | Vaccine GMT % seroconversion |
Placebo GMT % seroconversion |
Vaccine/Placebo GMT ratio (95% CI) % seroconversion ratio (95% CI) |
|||
|---|---|---|---|---|---|---|
| MNA | ELISA | MNA | ELISA | MNA | ELISA | |
| Day 1 | 1·0 n/a * |
1·0 n/a |
1·0 n/a |
1·0 n/a |
1·0 (1·0, 1·0) n/a |
1·0 (1·0, 1·0) n/a |
| Day 21 | 17·1 99 |
169·7 99 |
1·4 11 |
1·8 16 |
12·6 (11·6, 13·8) 9·0 (7·2, 11·4) |
97·0 (83·0, 113·3) 6·1 (5·1, 7·4) |
| Day 42 | 109 99 |
711·3 100 |
2·3 24 |
3·1 26 |
47·6 (43·3, 52·3) 4·1 (3·5, 4·7) |
228·9 (200·7, 261·0) 3·8 (3·3, 4·3) |
| Day 90 | 47·3 99 |
48·3 99 |
2·8 29 |
3·8 30 |
16·7 (15·1, 18·3) 3·4 (3·0, 3·9) |
91·4 (79·6, 105·0) 3·3 (2·9, 3·7) |
| Day 180 | 9·1 64 |
32.8 76 |
4·3 37 |
8·6 40 |
2·1 (1·8, 2·5) 1·7 (1·5, 1·9) |
3·8 (3·0, 4·9) 1·9 (1·7, 2·1) |
n/a – not applicable.
95% CI for GMT ratio was calculated based on the 95% CI for difference between means of logarithmic values with assumption that the variances in the populations are similar.
95% CI for %seroconversion ratios was calculated using the Koopman asymptotic score confidence interval for the ratio of proportions as implemented in the GraphPad Prizm v8.4.8.
The preventive efficacy of the QazCovid-in® vaccine was studied through epidemiological surveillance of the study participants. Both the vaccine (2275 participants) and placebo (560 participants) groups were monitored for COVID-19 throughout 180 days of the observation period. The preventive efficacy of the QazCovid-in® vaccine was assessed as the number of PCR-confirmed COVID-19 cases of any severity determined in the vaccine group compared to the placebo from day 14 to day 180 of the study.30
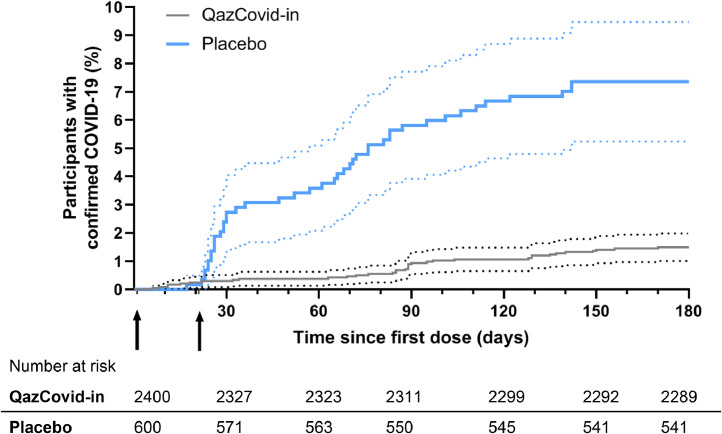
Within 14 days after the first vaccination, 4 (0·17%) vaccine recipients and 0 (0%) placebo recipients were diagnosed with mild COVID-19. Figure 2, S4 illustrate the percentage of confirmed COVID-19 infection cases diagnosed after the first dose of the vaccine or placebo and time dependence of hazard functions for both groups. A marked difference in the number of cases between vaccine and placebo recipients is observed immediately after the second vaccination, which indicates the early protection provided by the vaccine.
Figure 2.
Kaplan–Meier cumulative incidence curves for the symptomatic, PCR-confirmed COVID-19 in participants who received at least one dose of vaccine or placebo.
Cumulative incidence was calculated using the Kaplan–Meier method as implemented in GraphPad Prism 8.4.8. Dotted lines represent the 95% confidence intervals. Arrows indicate the first and second immunisations. Numbers of individuals at risk for the specified time points are shown below the graph.
From day 14 to day 180, COVID-19 was confirmed in 31 (1·36%) vaccine recipients, with 30 (5·35%) mild cases and 1 (0·005%) severe case. In the placebo group, 43 (7·16%) cases of mild COVID-19 were confirmed. The preventive efficacy of vaccination was calculated as the ratio of confirmed COVID-19 cases of any severity with onset on day 14 after the first vaccination or later in the vaccine group to placebo. As shown in Table 4, the QazCovid-in® vaccine efficacy amounted to 82·0% (95% CI, 71·1–88·5). Among participants diagnosed with COVID-19, two participants had underlying medical conditions: one participant with HCV from the placebo group had a mild COVID-19, and severe disease was recorded in a vaccinated participant with chronic heart failure. The latter was the only severe COVID-19 case recorded in the study.
Table 4.
Protective efficacy of the QazCovid-in® vaccine against SARS-CoV-2 infection, ITT population.
| Efficacy end point | Vaccine | Placebo |
|---|---|---|
| Number and severity of SARS-CoV-2 cases recorded on day 90 after the first vaccination | ||
| No of cases <14 days, Mild, n |
4 | 0 |
| No of cases >14 days, Mild, n Moderate, n Severe, n Sum |
13 4 0 17 |
34 0 0 34 |
| Hospitalisation, deaths and other secondary outcomes | 4 | 1 |
| Vaccine efficacy (95%CI) |
87·5% (77·9–92·9) |
|
| Number and severity of SARS-CoV-2 cases recorded on day 180 after the first vaccination | ||
| No of cases < 14 | 4 | 0 |
| No of cases > 14 days, Mild, n Moderate, n Severe, n Sum |
23 7 1 31 |
42 1 0 43 |
| Vaccine efficacy (95%CI) |
82·0% (71·1–88·5) |
|
| Hospitalisation, deaths and other secondary outcomes | 2 | 1 |
The 95% confidence interval for vaccine efficacy was calculated using the Koopman asymptotic score confidence interval for the ratio of proportions as implemented in the GraphPad Prizm v8.4.8.
Analysis of sera samples obtained from the placebo group participants revealed a fourfold or more increase in neutralising antibody titres to SARS-CoV-2 in 307 (52·5%) out of 585 participants tested on any scheduled study day (day 21, 42, 90, or 180) in comparison to day 1 (Table 3). From the total 307 cases, 43 people had symptoms of COVID-19 confirmed by RT-PCR, while 264 participants (45%) were likely to have asymptomatic infection. In the vaccine group, a fourfold or more increase in neutralising antibody titers was observed in 248 (10·7%) of 2,319 participants from day 42 to day 180 of the study, which includes symptomatic and supposed asymptomatic cases. We also observed a statistically significant increase (p < 0.0001) in the levels of IFN-α, IFN-γ, IL-6, and TNF-α cytokines on days 90 and 180 compared to the placebo group (Figure S3). These data suggest a nearly fivefold reduction in the overall COVID-19 infection rate (symptomatic and asymptomatic) in the vaccine group in comparison to the placebo, which is comparable with the estimated vaccine efficacy.
Over the period from December 2020 to July 2021, 45 virus samples were isolated from the participants with confirmed COVID-19 and sequenced. The results of sequencing showed that alongside the 19 original SARS-CoV-2 viruses (5 in the vaccine and 14 in the placebo recipients), 16 isolates were similar to the Alpha (lineage B.1.1.7) mutant variant (9 in the vaccine group and 7 in placebo recipients), and 10 – to the Delta (lineage B.617.2) variant of SARS-CoV-2 (7 from the vaccine group and 3 from placebo recipients). The results indicate that at least three variants of SARS-CoV-2 circulated in Kazakhstan during the clinical trial period.
Discussion
This phase 3 clinical trial of the inactivated whole-virion QazCovid-in® vaccine indicated that its double intramuscular administration was well-tolerated and safe for 180 days. No serious vaccine-related AEs were reported during the study. All local AEs recorded in the vaccine group were classified as foreseeable, typical for injectable inactivated vaccines. All local reactions detected within 7 days after the vaccine administration were mild, lasted from 1 to 3 days, and required no treatment. One systemic AE of mild severity (37·1 °C body temperature on the 1st day after vaccination) was revealed. No acute allergic reactions, blood clots, or autoimmune conditions were observed in the QazCovid-in® vaccine recipients. Vaccination with QazCovid-in® was also safe for patients with underlying medical conditions who were at high risk of severe COVID-19 and death.
The inactivated whole-virion QazCovid-in® vaccine ensured the formation of humoral immunity after two immunisations, with GMTs of neutralising antibodies reaching 109 (95% CI 105, 113) on study day 42 and gradually decreasing to 47·3 (95% CI 45·8, 48·9) and 9·1 (95% CI 8·4, 9·8) in 3 and 6 months, respectively. As we have shown in the phase 1 study using the IFN-γ /IL-4 cytokine ratio, the QazCovid-in® vaccine induces the Th1-biased cellular immune response 28 which can contribute to the formation of functional memory T cells. The protective efficacy of the QazCovid-in® vaccine amounts to 82·0% (95% CI 71·1–88·5) within 180 days starting 14 days after the first vaccination, which suggests early protection. This efficacy is comparable to that of CoronaVac inactivated whole-virion vaccine produced in Turkey (83·5%) and observed during a follow-up period of 43 days.9
The correlates of protection for vaccines against SARS-CoV-2 are not defined yet, although the positive correlation between the titres of neutralising antibodies with protection from SARS-CoV-2 infection is recognised.31, 32, 33 Studies of the immune response in participants infected with SARS-CoV-2 have shown that neutralising antibody titres wane within 50 days after mild disease, and they can be detected over 6–8 months in case of moderate and severe disease, but also wane with the time.33, 34, 35, 36 A deeper analysis of patients recovered from COVID-19 have revealed that memory B and T cells persist in patients with moderate disease and potent virus-specific immunity is formed which can be protective in case of a repeated encounter with SARS-CoV-2.37, 38, 39 These data suggest that potent humoral immunity induced by the QazCovid-in® vaccine in combination with increased cellular immunity lasting up to 6 months is capable of providing protective immune memory response against SARS-CoV-2.
The regular monitoring of viruses circulated from March to July 2021 in Kazakhstan revealed that the Alpha (lineage B.1.1.7) variant of SARS-CoV-2 circulated in March along with the original SARS-CoV-2 virus, and later they were substituted by the Delta (lineage B.1.617.2.1.2) variant. The results of sequencing of viruses isolated from the study participants diagnosed with COVID-19 within the period from December 2020 to July 2021 showed that, alongside the original SARS-CoV-2 virus, Alpha (lineage B.1.1.7) and Delta (lineage B.1.617.2) variants induced the disease in vaccine recipients with a similar frequency. The study was completed before the Omicron (lineage B.1.1.529) strain reached Kazakhstan. Therefore, no data about the QazCovid-in® vaccine efficacy against Omicron strain are available.These data indicate that the QazCovid-in® vaccine promotes the protection not only against the SARS-CoV-2 strain used for vaccine preparation but also against more transmissible Alpha and Delta variants within 6 months after the first vaccination with an efficacy of 82·0% (71·1–88·5). The broad immunity could be explained by the formation of B- and T-cell immune response by QazCovid-in® vaccine.
A small sample size (2400 participants in the vaccine group and 600 in placebo) is the main limitation of this phase 3 study performed with the QazCovid-in® vaccine as compared to phase 3 studies of other vaccines against SARS-CoV-2. However, 94·5% (2835 participants) from 3000 randomised participants completed the whole 180-day trial including the estimation of safety, immunogenicity, and efficacy parameters. Young population with median age 35 years and predominance of the Asian participants in the groups (2268 from 2400 of vaccinated participants) is the second limitation of the study.
Contributors
The study was carried out at the clinical base of the International Institute of Postgraduate Education in Almaty, the 4th City Outpatient Clinic in Almaty, and the Multidisciplinary City Hospital in Taraz Ministry of Health of Kazakhstan by a contract organisation centre for Clinical Medicine and Research. The director Ilyas Kulmagambetov is the Principal Investigator who organised and conducted the studies and made the final decision to publish the results. The corresponding author confirms that he had full access to all study data and had final responsibility for the decision to submit the paper for publication. GS, MSt, MSe, ZhB, and BKh coordinated the studies, contributed to the data analysis and interpretation, and edited the report. KZ, LK, MO, YA, KS, MK, OCh, BM, AN, AN, KZh, NA, SN, AK, ZE, and BKh contributed to the vaccine development, implementation of the studies, and data collection. TD, LK, MO, YA, KS, GS, MK, and BKh were responsible for the laboratory research. GS and MSe verified the underlying data. All authors confirm that they had full access to all the data in the study and accept responsibility to submit for publication. All authors critically reviewed the manuscript and approved the final version.
Data sharing statement
The Individual details of a participant are available upon request to BKh. Once the request is approved, the data can be transferred via a secure online platform.
Declaration of interests
All authors declare no competing interests.
Acknowledgements
We would like to thank the study participants, the staff of the Research Centres in Almaty and Taraz, as well as members of the test management groups, and the leadership of the Ministry of Education and Science of Kazakhstan. The work was funded by the Science Committee of the Ministry of Education and Science of Kazakhstan within the framework of the Scientific and Technical Program “Development of a vaccine against coronavirus infection COVID-19”. State registration number 0.0927.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.eclinm.2022.101526.
Appendix. Supplementary materials
References
- 1.Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384(5):403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xia S, Zhang Y, Wang Y, et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBIBP-CorV: a randomised, double-blind, placebo-controlled, phase 1/2 trial. Lancet Infect Dis. 2021;21(1):39–51. doi: 10.1016/S1473-3099(20)30831-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Logunov DY, Dolzhikova IV, Shcheblyakov DV, et al. Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: an interim analysis of a randomised controlled phase 3 trial in Russia. Lancet. 2021;397(10275):671–681. doi: 10.1016/S0140-6736(21)00234-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Voysey M, Clemens SAC, Madhi SA, et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397(10269):99–111. doi: 10.1016/S0140-6736(20)32661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang Y, Zeng G, Pan H, et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18-59 years: a randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect Dis. 2021;21(2):181–192. doi: 10.1016/S1473-3099(20)30843-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roest S, Hoek RAS, Manintveld OC. BNT162b2 mRNA Covid-19 vaccine in a nationwide mass vaccination setting. N Engl J Med. 2021;384(20):1968–1970. doi: 10.1056/NEJMc2104281. [DOI] [PubMed] [Google Scholar]
- 8.Lopez Bernal J, Andrews N, Gower C, et al. Effectiveness of the Pfizer-BioNTech and Oxford-AstraZeneca vaccines on covid-19 related symptoms, hospital admissions, and mortality in older adults in England: test negative case-control study. BMJ. 2021;373:n1088. doi: 10.1136/bmj.n1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tanriover MD, Doganay HL, Akova M, et al. Efficacy and safety of an inactivated whole-virion SARS-CoV-2 vaccine (CoronaVac): interim results of a double-blind, randomised, placebo-controlled, phase 3 trial in Turkey. Lancet. 2021;398(10296):213–222. doi: 10.1016/S0140-6736(21)01429-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ella R, Reddy S, Jogdand H, et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBV152: interim results from a double-blind, randomised, multicentre, phase 2 trial, and 3-month follow-up of a double-blind, randomised phase 1 trial. Lancet Infect Dis. 2021;21(7):950–961. doi: 10.1016/S1473-3099(21)00070-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hasan MS, Islam MT, Alam A, et al. Initial reports of the SARS-CoV-2 Delta variant (B.1.617.2 lineage) in Bangladeshi patients: risks of cross-border transmission from India. Health Sci Rep. 2021;4(3):e366. doi: 10.1002/hsr2.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tegally H, Wilkinson E, Lessells RJ, et al. Sixteen novel lineages of SARS-CoV-2 in South Africa. Nat Med. 2021;27(3):440–446. doi: 10.1038/s41591-021-01255-3. [DOI] [PubMed] [Google Scholar]
- 13.Davies NG, Abbott S, Barnard RC, et al. Estimated transmissibility and impact of SARS-CoV-2 lineage B.1.1.7 in England. Science. 2021;372(6538) doi: 10.1126/science.abg3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Plante JA, Liu Y, Liu J, et al. Spike mutation D614G alters SARS-CoV-2 fitness. Nature. 2021;592(7852):116–121. doi: 10.1038/s41586-020-2895-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Korber B, Fischer WM, Gnanakaran S, et al. Tracking changes in SARS-CoV-2 spike: evidence that D614G increases infectivity of the COVID-19 virus. Cell. 2020;182(4) doi: 10.1016/j.cell.2020.06.043. 812-27 e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yurkovetskiy L, Wang X, Pascal KE, et al. Structural and functional analysis of the D614G SARS-CoV-2 spike protein variant. Cell. 2020;183(3):739–751.e8. doi: 10.1016/j.cell.2020.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hou YJ, Chiba S, Halfmann P, et al. SARS-CoV-2 D614G variant exhibits efficient replication ex vivo and transmission in vivo. Science. 2020;370(6523):1464–1468. doi: 10.1126/science.abe8499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu J, Liu Y, Xia H, et al. BNT162b2-elicited neutralization of B.1.617 and other SARS-CoV-2 variants. Nature. 2021;596(7871):273–275. doi: 10.1038/s41586-021-03693-y. [DOI] [PubMed] [Google Scholar]
- 19.Liu Y, Liu J, Xia H, et al. Neutralizing activity of BNT162b2-elicited serum. N Engl J Med. 2021;384(15):1466–1468. doi: 10.1056/NEJMc2102017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sheikh A, McMenamin J, Taylor B, Robertson C, Public Health Scotland and the EAVE II Collaborators SARS-CoV-2 Delta VOC in Scotland: demographics, risk of hospital admission, and vaccine effectiveness. Lancet. 2021;397(10293):2461–2462. doi: 10.1016/S0140-6736(21)01358-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abu-Raddad LJ, Chemaitelly H, Butt AA. National study group for C-V. Effectiveness of the BNT162b2 Covid-19 vaccine against the B.1.1.7 and B.1.351 variants. N Engl J Med. 2021;385(2):187–189. doi: 10.1056/NEJMc2104974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chemaitelly H, Yassine HM, Benslimane FM, et al. mRNA-1273 COVID-19 vaccine effectiveness against the B.1.1.7 and B.1.351 variants and severe COVID-19 disease in Qatar. Nat Med. 2021;27(9):1614–1621. doi: 10.1038/s41591-021-01446-y. [DOI] [PubMed] [Google Scholar]
- 23.Chemaitelly H, Tang P, Hasan MR, et al. Waning of BNT162b2 vaccine protection against SARS-CoV-2 infection in Qatar. N Engl J Med. 2021;385(24):e83. doi: 10.1056/NEJMoa2114114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lopez Bernal J, Andrews N, Gower C, et al. Effectiveness of Covid-19 vaccines against the B.1.617.2 (Delta) variant. N Engl J Med. 2021;385(7):585–594. doi: 10.1056/NEJMoa2108891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tartof SY, Slezak JM, Fischer H, et al. Effectiveness of mRNA BNT162b2 COVID-19 vaccine up to 6 months in a large integrated health system in the USA: a retrospective cohort study. Lancet. 2021;398(10309):1407–1416. doi: 10.1016/S0140-6736(21)02183-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tang P., Hasan M.R., Chemaitelly H., et al. BNT162b2 and mRNA-1273 COVID-19 vaccine effectiveness against the SARS-CoV-2 Delta variant in Qatar. Nat Med. 2021;27:2136–2143. doi: 10.1038/s41591-021-01583-4. [DOI] [PubMed] [Google Scholar]
- 27.Zhugunissov K, Zakarya K, Khairullin B, et al. Development of the inactivated QazCovid-in vaccine: protective efficacy of the vaccine in Syrian hamsters. Front Microbiol. 2021;12 doi: 10.3389/fmicb.2021.720437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zakarya K, Kutumbetov L, Orynbayev M, et al. Safety and immunogenicity of a QazCovid-in® inactivated whole-virion vaccine against COVID-19 in healthy adults: a single-centre, randomised, single-blind, placebo-controlled phase 1 and an open-label phase 2 clinical trials with a 6 months follow-up in Kazakhstan. EClinicalMedicine. 2021;39 doi: 10.1016/j.eclinm.2021.101078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Petrone L, Petruccioli E, Vanini V, et al. A whole blood test to measure SARS-CoV-2-specific response in COVID-19 patients. Clin Microbiol Infect. 2021;27(2) doi: 10.1016/j.cmi.2020.09.051. 286 e7- e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fagerland MW, Lydersen S, Laake P. Recommended confidence intervals for two independent binomial proportions. Stat Methods Med Res. 2015;24(2):224–254. doi: 10.1177/0962280211415469. [DOI] [PubMed] [Google Scholar]
- 31.Addetia A, Crawford KHD, Dingens A, et al. Neutralizing antibodies correlate with protection from SARS-CoV-2 in humans during a fishery vessel outbreak with a high attack rate. J Clin Microbiol. 2020;58(11):e02107–e02120. doi: 10.1128/JCM.02107-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cromer D, Juno JA, Khoury D, et al. Prospects for durable immune control of SARS-CoV-2 and prevention of reinfection. Nat Rev Immunol. 2021;21(6):395–404. doi: 10.1038/s41577-021-00550-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Khoury DS, Cromer D, Reynaldi A, et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med. 2021;27(7):1205–1211. doi: 10.1038/s41591-021-01377-8. [DOI] [PubMed] [Google Scholar]
- 34.Seow J, Graham C, Merrick B, et al. Longitudinal observation and decline of neutralizing antibody responses in the three months following SARS-CoV-2 infection in humans. Nat Microbiol. 2020;5(12):1598–1607. doi: 10.1038/s41564-020-00813-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ibarrondo FJ, Fulcher JA, Goodman-Meza D, et al. Rapid decay of anti-SARS-CoV-2 antibodies in persons with mild Covid-19. N Engl J Med. 2020;383(11):1085–1087. doi: 10.1056/NEJMc2025179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wajnberg A, Amanat F, Firpo A, et al. Robust neutralizing antibodies to SARS-CoV-2 infection persist for months. Science. 2020;370(6521):1227–1230. doi: 10.1126/science.abd7728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sherina N, Piralla A, Du L, et al. Persistence of SARS-CoV-2-specific B and T cell responses in convalescent COVID-19 patients 6-8 months after the infection. Med (N Y) 2021;2(3) doi: 10.1016/j.medj.2021.02.001. 281-95 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rodda LB, Netland J, Shehata L, et al. Functional SARS-CoV-2-specific immune memory persists after mild COVID-19. Cell. 2021;184(1) doi: 10.1016/j.cell.2020.11.029. 169-83 e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Byazrova M, Yusubalieva G, Spiridonova A, et al. Pattern of circulating SARS-CoV-2-specific antibody-secreting and memory B-cell generation in patients with acute COVID-19. Clin Transl Immunol. 2021;10(2):e1245. doi: 10.1002/cti2.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.