Abstract
Background:
In the United States (US), urinary tract infections (UTI) lead to more than 10 million office visits each year. Temperature and season are potentially important risk factors for UTI, particularly in the context of climate change.
Methods:
We examined the relationship between ambient temperature and outpatient UTI diagnoses among patients followed from 2015 to 2017 in two California healthcare systems: Kaiser Permanente Southern California (KPSC) and Sutter Health in Northern California. We identified UTI diagnoses in adult patients using diagnostic codes and laboratory records from electronic health records. We abstracted patient age, sex, season of diagnosis, and linked community-level Index of Concentration at the Extremes (ICE-I, a measure of wealth and poverty concentration) based on residential address. Daily county-level average ambient temperature was assembled from the Parameter-elevation Regressions on Independent Slopes Model (PRISM). We implemented distributed lag nonlinear models (DLNM) to assess the association between UTI and lagged daily temperatures. Main analyses were confined to women. In secondary analyses, we stratified by season, healthcare system, and community-level ICE-I.
Results:
We observed 787,186 UTI cases (89% among women). We observed a threshold association between ambient temperature and UTI among women: an increase in daily temperature from the 5th percentile (6.0 °C) to the mean (16.2 °C) was associated with a 3.2% (95% CI: 2.4, 3.9%) increase in same-day UTI diagnosis rate, whereas an increase from the mean to 95th percentile was associated with no change in UTI risk (0.0%, 95% CI: −0.7, 0.6%). In secondary analyses, we observed the clearest monotonic increase in the rate of UTI diagnosis with higher temperatures in the fall. Associations did not differ meaningfully by healthcare system or community-level ICE-I. Results were robust to alternate model specifications.
Discussion:
Increasing temperature was related to higher rate of outpatient UTI, particularly in the shoulder seasons (spring, autumn).
Keywords: Urinary tract infections, Temperature, Climate change, Case-crossover, Electronic health records
1. Introduction
Urinary tract infections (UTI) are among the most common outpatient infections in the United States (US) (Foxman, 2010; Medina and Castillo-Pino, 2019). UTI occurs when uropathogens from fecal flora ascend the urethra to infect the bladder (Flores-Mireles et al., 2015). UTI is more common in women among whom the lifetime prevalence of UTI is 50–60% (Albert et al., 2004; Foxman et al., 2000). The clinical and economic burden associated with UTI is substantial: sequelae include frequent recurrences, pyelonephritis, bloodstream infection (BSI), and complications associated with frequent antibiotic use (Flores-Mireles et al., 2015). In the US, more than 10 million office visits, two million emergency department visits, and 100,000 hospitalizations are attributable to UTI each year (Flores-Mireles et al., 2015; Foxman, 2002; Bruxvoort et al., 2020), and UTI remains the third-leading cause of antibiotic prescriptions in adults and children (Medina and Castillo-Pino, 2019; Mazzariol et al., 2017; Hersh et al., 2011).
Established risk factors for UTI include recent sexual intercourse or a new sexual partner (Flores-Mireles et al., 2015; Scholes et al., 2000), recent personal history or family history of UTI (Flores-Mireles et al., 2015; Scholes et al., 2010), structural or functional abnormalities of the urinary tract (Hickling et al., 2017; Foxman, 2014), and diabetes (Patterson and Andriole, 1997; Stapleton, 2002). Beyond these individual-level risk factors, past research has identified increases in Google searches, medication sales, and inpatient and outpatient encounters related to UTI as well as increased frequency of culture-positive UTI during the summer months (Simmering et al., 2017; Anderson, 1983; Rosello et al., 2018; Melamed et al., 2014; Rossignol et al., 2013). Prior studies have further identified a direct link between UTI and higher ambient temperatures (Hopp et al., 2018; Malig et al., 2019; Simmering et al., 2018; Simmering et al., 2021). Several potential explanations have been put forth for the apparent seasonality of UTI, including inadvertent prophylaxis from antimicrobials prescribed in winter to treat respiratory infections (Stamm et al., 1991) or changes in sexual partners and the frequency of sexual intercourse, as supported by some evidence that sexually transmitted infections also peak during the summer months (Wright and Judson, 1978; Cornelisse et al., 2016; Shah et al., 2007; Freeman et al., 2009). It has been hypothesized previously that warmer temperatures increase UTI risk through dehydration, resulting in diminished urine production and decreased clearance of urinary pathogens (Eckford et al., 1995; Beetz, 2003). Alternatively, warmer temperatures may promote bacterial proliferation and facilitate transfer of potential uropathogens to the urethra (Anderson, 1983). A major limitation of many prior studies on season, temperature, and UTI is reliance on inpatient UTI diagnoses, which constitute the minority of cases and are often healthcare-associated.
An understanding of the relationship between temperature and outpatient UTI will be vital to both prepare for and mitigate the worst impacts of rising temperatures under climate change. The present study leveraged electronic health records (EHR) data to examine the relationship between temperature and UTI. Building upon prior research, we employed distributed lag nonlinear models (DLNM) to precisely characterize the temporal relationship between warmer temperatures and UTI. We additionally considered effect modification by season, healthcare system catchment area, and neighborhood poverty as past research indicates both season and socioeconomic position are determinants of UTI (Casey et al., 2021). We hypothesized a priori that rates of UTI would be positively associated with higher temperatures, with stronger associations during the summer and fall and in areas of concentrated poverty.
2. Methods
We conducted a case-crossover study (Maclure, 1991; Maclure and Mittleman, 2000; Armstrong et al., 2014) using EHR data between January 1, 2015 and December 31, 2017 from Kaiser Permanente Southern California (KPSC) and Sutter Health in Northern California (Bruxvoort et al., 2020; Casey et al., 2021). Case-crossover studies compare exposures that occurred prior to outcome onset with exposures that occurred in control periods for each individual. This approach eliminates confounding by time-invariant individual- and area-level confounders and is therefore well-suited to the study of short-term effects of environmental exposures (Wichmann et al., 2012; Kyobutungi et al., 2005; Buckley and Richardson, 2012).
KPSC is an integrated healthcare organization serving approximately 4.7 million individuals in nine Southern California counties who are generally representative of local populations except for modest under-representation of the highest and lowest income individuals (Koebnick et al., 2012). KPSC is a closed membership system with integration of the health plan, hospitals, and physician medical groups such that members are incentivized to receive health care within the system. Sutter Health is a mixed-payer system that delivers care to 3.5 million patients across 22 Northern California counties and is generally representative of the local population. In contrast with KPSC, Sutter Health is an open system and therefore we emulated membership by constructing a primary care cohort that included all individuals who visited a primary care clinic between 2008 and 2017. Our study included approximately 800 thousand adult KPSC members and Sutter Health primary care cohort members who received care for a UTI at an outpatient encounter (i.e., virtual and in-person outpatient clinic visits and emergency department visits) over the study period. We excluded individuals under the age of 18 at the time of UTI diagnosis, with addresses that did not geocode to county, or who lived outside their respective health care system’s catchment area (Supplemental Fig. 1).
Both KPSC and Sutter Health use Epic EHR (Epic Systems Corporation, Verona, WI, USA) systems that catalogue patient-level data including sociodemographic characteristics, diagnoses, laboratory tests, and medication orders from all care settings. The Institutional Review Boards at KPSC, Sutter Health, and Columbia University approved this study.
2.1. Urinary tract infections
We identified UTI using diagnoses, antibiotic prescription data, and positive urine culture results for E. coli, the most commonly identified organism for UTI in the general population (Gales et al., 2000; Gupta et al., 1999; Gupta et al., 1999; Hooton and Stamm, 1997; Jones et al., 1999). Diagnoses were defined using International Classification of Diseases, Ninth Revision (ICD-9) diagnostic codes 595.0, 595.9, 599.0, 590.10, 590.11 and ICD-10 codes N30.00, N30.01, N30.90, N30.91, N39.0, and N10. As previously described, positive urine cultures were defined using KPSC and Sutter Health laboratory guidelines of ≥ 1,000 colony-forming units (CFU) per mL for sterile samples and ≥ 10,000 CFU/mL for clean-catch. Thresholds for CFU were selected consistent with thresholds provided to KPSC clinicians. Therefore, we used these thresholds to define UTI instead of higher thresholds (e.g., ≥100 000 CFU/mL) to reflect real-world practice. Cultures that were identified by the laboratory as positive for contaminants were excluded (Bruxvoort et al., 2020; Armstrong et al., 2014). Patients were categorized as having UTI if they met one of two criteria: (1) UTI diagnosis with antibiotic order on the same day; or (2) positive urine culture. A complete list of eligible antibiotics is presented in Supplemental Table 1. Multiple infections in an individual patient were treated as distinct episodes. Further, because multiple health care encounters may be related to a single infection, occurrences of UTI within 30 days were defined as a single event. The first health care visit within the 30-day window was treated as the date of UTI diagnosis.
2.2. Assessment of daily temperatures
We obtained data on daily mean temperature from the Parameter-elevation Regressions on Independent Slopes Model (PRISM), which gathers climate observations from a wide range of monitoring networks and applies sophisticated quality control measures to generate a nationwide temperature dataset, with full space and time coverage over our study period (Daly and Bryant, 2013). County-averaged PRISM data has been found to accurately estimate ambient temperatures at weather monitors (Spangler et al., 2019; Basu and Ostro, 2008; Weinberger et al., 2020). Consistent with prior studies, we assigned measures of daily average temperature at the county level. We used gridded daily temperature estimates at a resolution of 4 km2 to generate area-weighted daily temperatures for each county. We first intersected county shape-files with gridded daily temperature estimates to obtain the proportion overlap of the county with each 4 km2 grid cell. Then we calculated daily county temperature as the average of the intersection segments, weighted by the proportion overlap. Daily temperatures were assigned to each individual based on their county of residence for the 14-day period prior to UTI diagnosis and for the 14-day control periods. Although we anticipated that increased ambient temperatures would increase UTI risk within one to three days of exposure, we specified a 14-day exposure window to accommodate potential delays in treatment-seeking.
2.3. Covariates
EHR data provided encounter and patient-level characteristics including age at time of health care encounter, sex, race/ethnicity (non-Hispanic Asian or Pacific Islander, non-Hispanic Black, non-Hispanic white, Hispanic, and other or unknown), season (winter, Dec–Feb; spring, Mar–May; summer, June–Aug; fall, Sept–Nov), and year of encounter. We derived the Index of Concentration at the Extremes for Income (ICE-I) at the census tract level based on 2011–2015 American Community Survey data. The ICE-I captures spatial concentration of wealth and poverty simultaneously and was calculated as:
Where Ai is the count of individuals in census tract i with an income greater than or equal to the metropolitan area 80th percentile and Pi is the count of individuals in census tract i with an income less than the metropolitan area 20th percentile, and Ti is the total number of individuals in census tract i (Krieger et al., 2016). For individuals living outside metropolitan areas, we used county-level thresholds (Tran et al., 2020). An ICE-I of 1 is total concentration of wealth in that tract, and an ICE-I of −1 is total concentration of poverty. We created a categorical variable of ICE-I based on the quartile of ICE-I of the patient’s residential census tract. Relative humidity was collected from the California Irrigation Management Information System of the California Department of Water Resources (California Department of Water Resources, 2021). Relative humidity from the Monrovia monitoring station was used for the KPSC counties and the Pleasanton Station for the Sutter Health Stations.
2.4. Statistical analyses
We assessed the association between daily temperature and UTI diagnosis using a time-stratified case-crossover study, in which we matched case dates to control periods by year, month, and day of the week at the individual level, and then compared 14-day temperature exposure prior to case and control days (Armstrong et al., 2014). For example, if an individual were diagnosed on Thursday, March 26, 2015, then their control days would be the other Thursdays of March 2015; March 5, 12, and 19, and temperature exposure would be based on the individual’s county of residence. Matching on county of residence addresses potential confounders that vary across space and matching on year, month, and day of the week addresses potential confounders that vary over time (Maclure and Mittleman, 2000; Armstrong et al., 2014; Janes et al., 2005). Since exposure was measured at the county level, individuals who were diagnosed with UTI on the same day and resided in the same county had the same exposure profile and covariates; we thus pooled individuals by county and day of UTI diagnosis. Our primary analysis was restricted to women as more than 85% of UTI cases occurred among women and given that mechanism of UTI differs appreciably by sex.
Because daily temperature data are temporally autocorrelated, we used distributed lag nonlinear models (DLNM) to model the association between UTI and lagged temperature exposures. This approach addresses autocorrelation by constraining lag-specific associations to vary smoothly across lags (Gasparrini et al., 2010). An additional advantage of this approach is that DLNM can capture potentially complex nonlinear relationships, even when the shape of the exposure–response relationship varies across lags (Kim et al., 2019; Scovronick et al., 2018; Yang et al., 2012). Because of these properties, DLNMs have been implemented in past research that examines the health effects of temperatures (Kim et al., 2019; Guo et al., 2011; Rowland et al., 2020). Based on an Akaike weight of 84.4%, we constrained the exposure–response to a natural spline with three degrees of freedom (df) and we constrained the lag-response to a natural spline with three df, with knots placed along log-based intervals (Gasparrini, 2016; Wagenmakers and Farrell, 2004). (Supplemental Table 2).
2.4.1. Primary analysis
We fit conditional Poisson models in which the expected number of outcomes for matched observations are conditioned on the total number of outcomes in that matched set; the conditional Poisson yields the same result as the conditional logistic model when using area-level exposure metrics (Armstrong et al., 2014). We used a quasi-Poisson distribution to account for overdispersion and adjusted for 14-day mean relative humidity. We report effect estimates as the percent change in the daily UTI diagnosis rate calculated from (eβ ℓ −1) × 100, where βℓ is the log risk ratio for a temperature change on lag ℓ (Maclure, 1991). Because we observed an approximately threshold exposure–response relationship, for quantitative effect estimates we primarily report the association for a temperature increase from the 5th percentile (6°C) to the mean observed temperature (16.2°C), where we observe a positive association. We additionally calculated cumulative association between UTI and temperature increases over 14 consecutive days.
2.4.2. Secondary analyses
To assess whether the relationship between temperature and UTI diagnosis varies throughout the calendar year, we fit models stratified by season. We then plotted the exposure–response relationship for same-day associations, restricted to the 5th to 95th percentile of temperature observed in each season and qualitatively compared their shapes. We next examined whether the association varied across the catchment areas of the two health care systems, which also differ by geography, climate, and population demographics. We hypothesized that we would observe similar associations across catchment areas. Finally, we assessed whether individuals living in areas of concentrated poverty had a larger association than individuals in the other quartiles, by comparing census tracts in the bottom quartile of income-based ICE-I (i.e., low-ICE-I) with other quartiles. For these models we qualitatively compared the shapes of the exposure–response relationships and then compared effect estimates for temperature increase from the 5th percentile to the mean. As an exploratory secondary analysis, we restricted our analysis by catchment area and ICE-I to the fall. Finally, we separately examined the association between UTI and temperature among men.
2.4.3. Sensitivity analyses
We performed several sensitivity analyses to assess the robustness of our results to alternative model specifications. First, we present our primary analysis with the fifth percentile specified as the referent rather than the mean. Second, to evaluate the influence of relative humidity in our main analysis, we first fit a model without controlling for relative humidity and second fit a model with a DLNM for 14-day relative humidity with the same constraints as the temperature DLNM. All the above-described sensitivity analyses included the same terms for temperature as described above for our main analysis. Finally, to assess the influence of our choice of a 14-day lag, we fit a model using a 21-day lag for temperature exposure with quasi-Akaike-Information-Criteria-selected constraints (exposure: three df natural spline; lag: three df natural spline) (Rowland et al., 2020).
2.4.4. Reproducibility
All statistical analyses were conducted using R statistical software version 4.0.4 (R Foundation for Statistical Computing, Vienna, Austria). Data preparation code for the original health care data is not publicly available, otherwise the analysis code is available via GitHub (https://github.com/s-rowland/DailyTemp-UTI) with the following packages used for coding management: tictoc v 1.0.1 (Izrailev, 2016); pacman 0.5.1 (Rinker et al., 2018); data cleaning and manipulation: tidyverse 1.3.0 (Wickham et al., 2019), fst 0.9.4 (Klik, 2019); statistical analysis: MuMIn 1.43.17 (Barton and Barton, 2015); dlnm 2.4.5 (Gasparrini, 2011); gnm 1.101 (Turner and Firth, 2007); and data visualization: ggplot2 3.3.5 (Wickham et al., 2016), egg 0.4.5 (Auguie, 2019), cowplot 1.1.1 (Wilke et al., 2019), and gridExtra 2.3 (Auguie et al., 2017).
3. Results
In total, we identified 600,883 UTI cases in the KPSC population and 197,154 UTI cases in the Sutter Health population from 2015 to 2017 among individuals over 18 years old. We excluded 9,101 (1.5%) KPSC cases and 398 (0.2%) Sutter Health cases with addresses that did not geocode to a California county. We further excluded 642 (0.1%) KPSC cases and 710 (0.4%) Sutter cases outside of the healthcare catchment counties. This yielded a final sample of 591,140 UTI cases in the KPSC population and 196,046 UTI cases in the Sutter Health population (Supplemental Figure 1).
The majority of UTI cases occurred among female patients in both the KPSC population (88.1%) and in the Sutter Health population (90.8%), and median age at UTI diagnosis was older for men in both populations. Of cases occurring in female patients, within the KPSC population more UTI cases were observed among Hispanic patients (43.1%) and fewer cases were observed among white patients (38.2%) as compared with Sutter Health where 15.2% of cases occurred among Hispanic patients and more than half of cases (58.4%) occurred in white patients. More female UTI cases occurred in patients living in low ICE-I Census tracts in the KPSC population (26.9%) as compared with Sutter Health (19.5%). The distribution of UTI cases was even across seasons and year of diagnosis. (Table 1).
Table 1.
Characteristics of UTI cases in KPSC and Sutter Health, 2015 – 2017.
| KPSC | Sutter Health | |||
|---|---|---|---|---|
| Female | Male | Female | Male | |
| Total Cases | 520,653 | 70,487 | 177,934 | 18,112 |
| Age – Median (IQR) | 49 (31 – 65) | 67 (53 – 78) | 50 (32 – 69) | 68 (52 – 79) |
| Race/Ethnicity – N (%) | ||||
| Non-Hispanic Asian & PI | 43,730 (8.4) | 4791 (6.8) | 22,265 (12.5) | 1,939 (10.7) |
| Non-HIspanic Black | 41,944 (8.1) | 7546 (10.7) | 5,473 (3.1) | 787 (4.3) |
| Non-Hispanic White | 199,123 (38.2) | 32,360 (45.9) | 103,891 (58.4) | 11,561 (63.8) |
| Hispanic | 224,309 (43.1) | 24,513 (34.8) | 27,015 (15.2) | 2,062 (11.4) |
| Other | 11,547 (2.2%) | 1277 (1.8) | 19,290 (10.8) | 1,763 (9.7) |
| Season – N (%) | ||||
| Winter | 125,476 (24.1) | 17,398 (24.7) | 44,060 (24.8) | 4,656 (25.7) |
| Spring | 126,843 (24.4) | 17,531 (24.9) | 44,035 (24.7) | 4,550 (25.1) |
| Summer | 133,739 (25.7) | 17,714 (25.1) | 45,059 (25.3) | 4,413 (24.4) |
| Fall | 134,595 (25.9) | 17,844 (25.3) | 44,780 (25.2) | 4,493 (24.8) |
| Year – N (%) | ||||
| 2015 | 165,035 (31.7) | 22,350 (31.7) | 60,484 (34.0) | 5,996 (33.1) |
| 2016 | 173,609 (33.3) | 23,430 (33.2) | 59,160 (33.2) | 6,174 (34.1) |
| 2017 | 181,010 (35.0) | 24,707 (35.1) | 58,290 (32.8) | 5,942 (32.8) |
| Census Tract Poverty – N (%) | ||||
| Low ICI-I | 140,053 (26.9) | 19,055 (27.0) | 36,444 (19.5) | 3,715 (20.5) |
| High ICI-I | 380,600 (73.1) | 51,432 (73.0) | 141,490 (80.5) | 14,397 (79.5) |
UTI occurred most frequently in August and was diagnosed relatively infrequently on weekend days versus weekdays (Supplemental Figure 2). The mean overall temperature throughout the study period was 16.2°C (standard deviation; SD 6.6°C), ranging from a daily minimum of −3.1°C to a maximum temperature of 38.6°C. Average temperatures were generally higher in KPSC counties than in Sutter Health counties. Temperatures ranged from 1.2 to 38.6°C for KPSC and from −3.1 to 33.8°C for Sutter Health (Supplemental Table 3).
We observed an approximately threshold association wherein the rate of UTI diagnoses increased up to approximately 10°C but remained relatively constant thereafter. (Fig. 1) For example, an increase in daily temperature from the 5th percentile to the mean was associated with a 3.2% (95% CI: 2.4, 3.9%) increase in same-day UTI diagnosis rate, whereas an increase from the mean to 95th percentile had an association of 0.0% (95% CI: –0.7, 0.6%). This association persisted for lags between one and nine days prior to day of diagnosis, whereas associations for 10 to 13 days prior were substantially attenuated or null (Fig. 2, Supplemental Table 4). Coefficients for distributed lag terms and knots from our primary analysis which can be used to calculate any comparison of interest are presented in Supplemental Table 5.
Fig. 1.
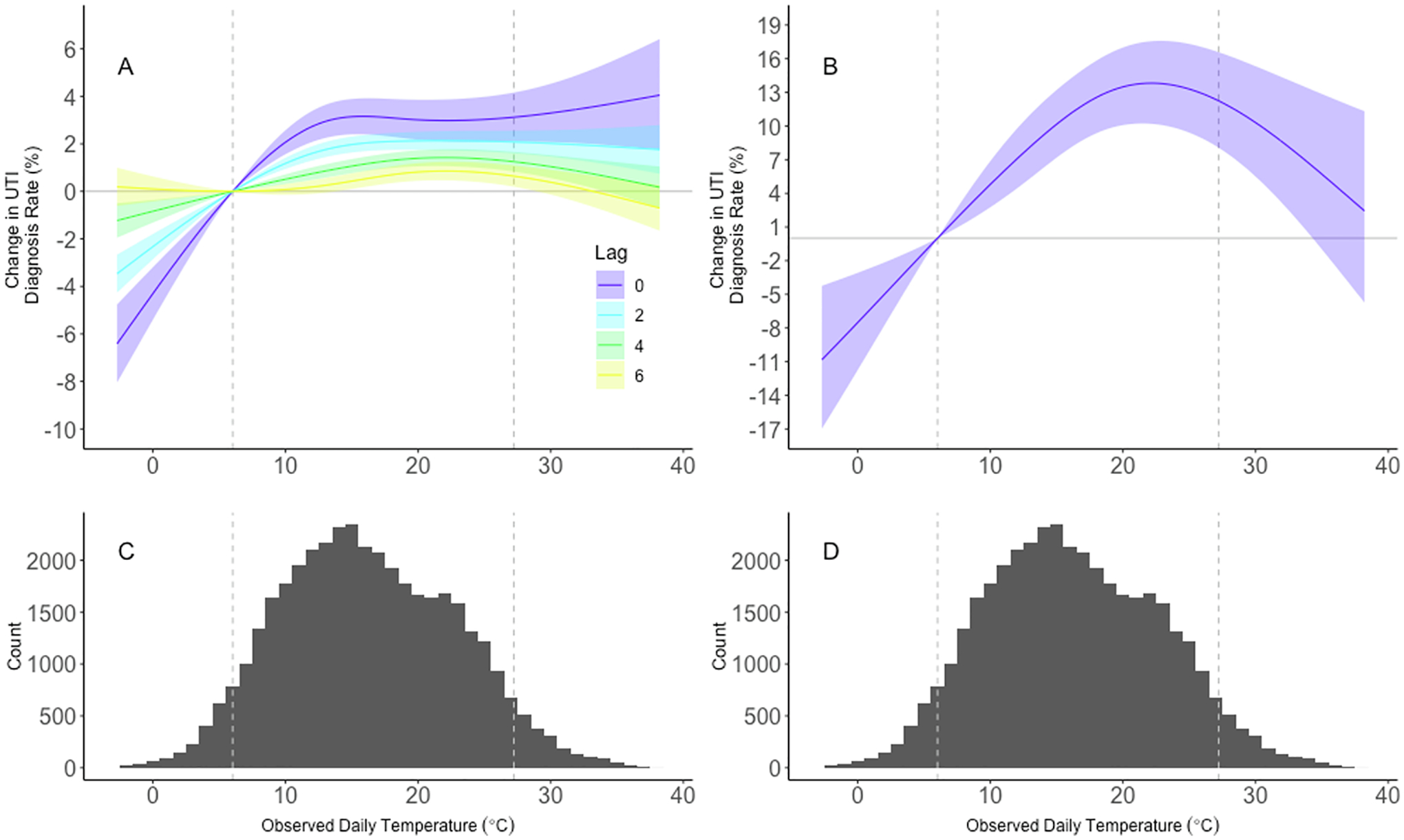
Exposure-Response relationship for selected lags. Panels A and B depict dose response curves for lag-specific and cumulative associations, respectively, between temperature and risk of UTI diagnosis relative to the 5th percentile temperature 16.2°C. We fit conditional quasi-Poisson models adjusting for 14-day relative humidity. We constrained the exposure–response to a natural spline with four degrees of freedom and we constrained the lag-response to a natural spline with three degrees of freedom. Shaded areas represent 95% confidence intervals and dashed vertical lines indicate the 5th and 95th percentiles. Panels C and D depict the distribution of observed daily mean temperature from 2015 to 2017.
Fig. 2.
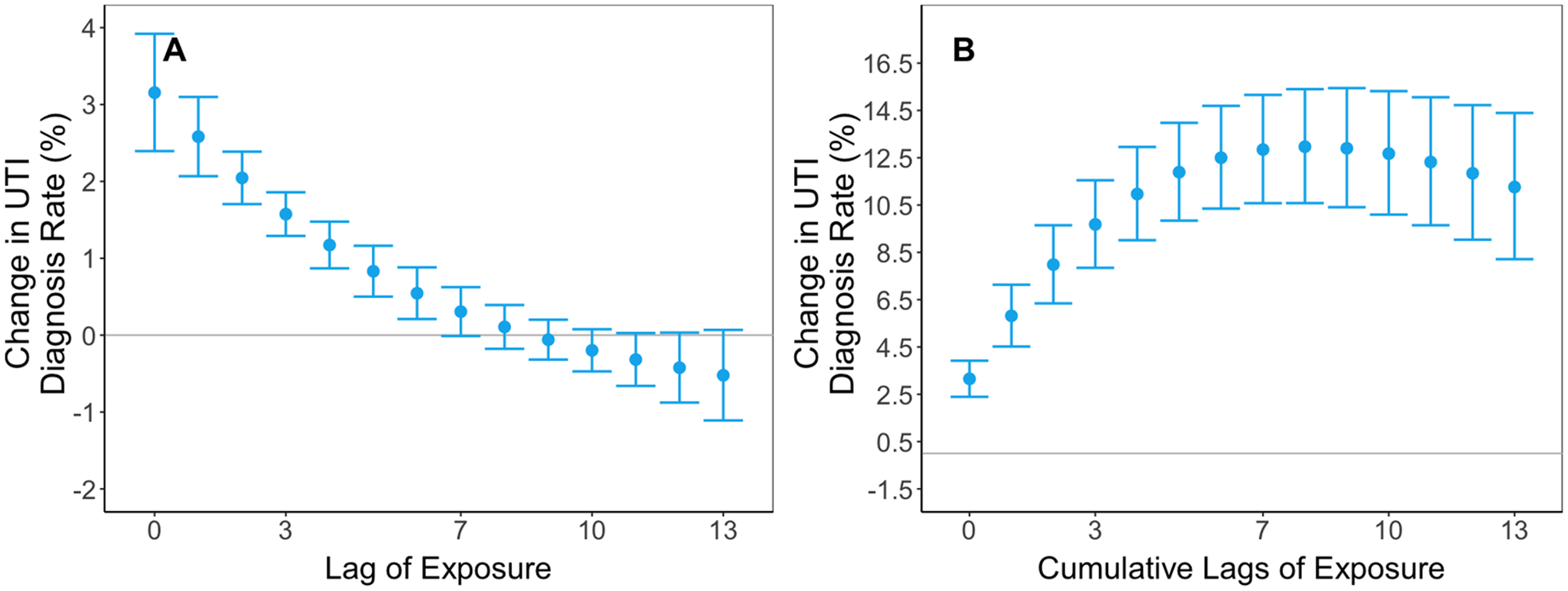
Lag-response effect estimates. Panel A illustrates the percent change in daily UTI diagnosis rate for an increase in temperature from the 5th percentile to the mean for each lag. We fit conditional quasi-Poisson models adjusting for 14-day relative humidity. We constrained the exposure–response to a natural spline with four degrees of freedom and we constrained the lag-response to a natural spline with three degrees of freedom. Panel B illustrates the cumulative association for the same temperature change. Error bars represent 95% confidence intervals.
3.1. Secondary analyses
We observed mostly positive associations between temperature and UTI across temperatures in the fall and spring with inverse associations in the winter. Higher temperatures were not clearly associated with increased rates of UTI diagnosis during the summer. We observed the clearest monotonic increase in the rate of UTI diagnosis with higher temperatures in the fall (Fig. 3, Supplemental Fig. 3). By catchment area, we observed a monotonic increase in UTI diagnosis rate for the KPSC catchment with decreased UTI diagnosis rate for temperatures below the mean and increased rates for temperatures above the mean. For the Sutter Health catchment area, the relationship was similar at lower temperature, but temperatures above the 85th percentile were associated with lower rates of UTI diagnosis (Fig. 4). Results were similar by census tract-level concentration of poverty, with slightly stronger cumulative associations for an increase from 5th percentile to mean seen in low ICE-I communities. (Fig. 5). Patterns of effect modification by catchment area and ICE-I were consistent when restricted our analysis to UTI cases that occurred during the fall. (Supplemental Figs. 4, 5) Among men, we generally observed an attenuated and less precise exposure–response. (Supplemental Figure 6).
Fig. 3.
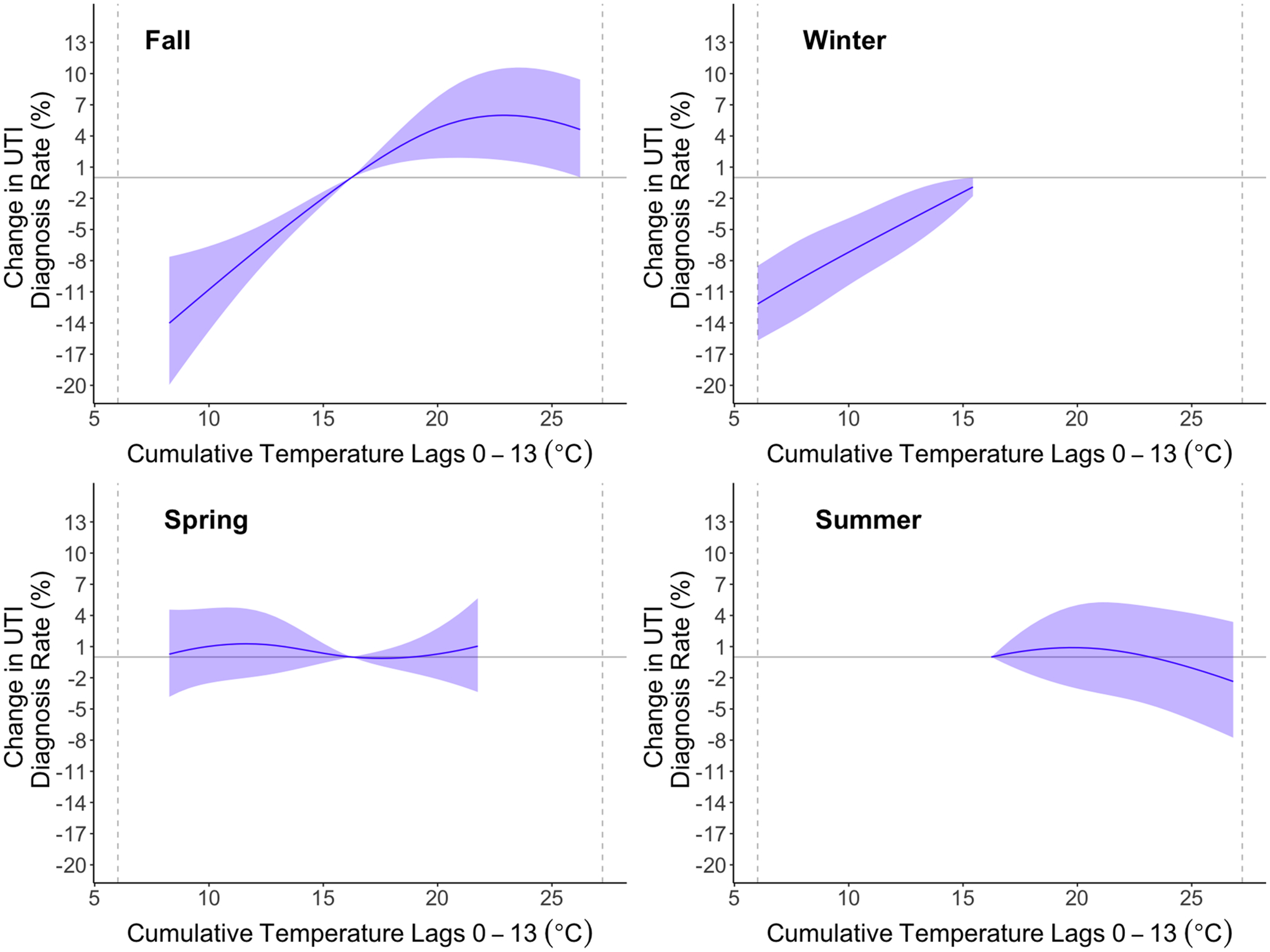
Season-stratified exposure–response relationship for 14-day cumulative association between temperature and UTI risk relative to the mean (16.4°C). We fit conditional quasi-Poisson models adjusting for 14-day relative humidity. We constrained the exposure–response to a natural spline with four degrees of freedom and we constrained the lag-response to a natural spline with three degrees of freedom. For each season, we restricted the temperature range to the 5th to 95th percentile of observed temperature for that season. Shaded areas represent 95% confidence intervals.
Fig. 4.
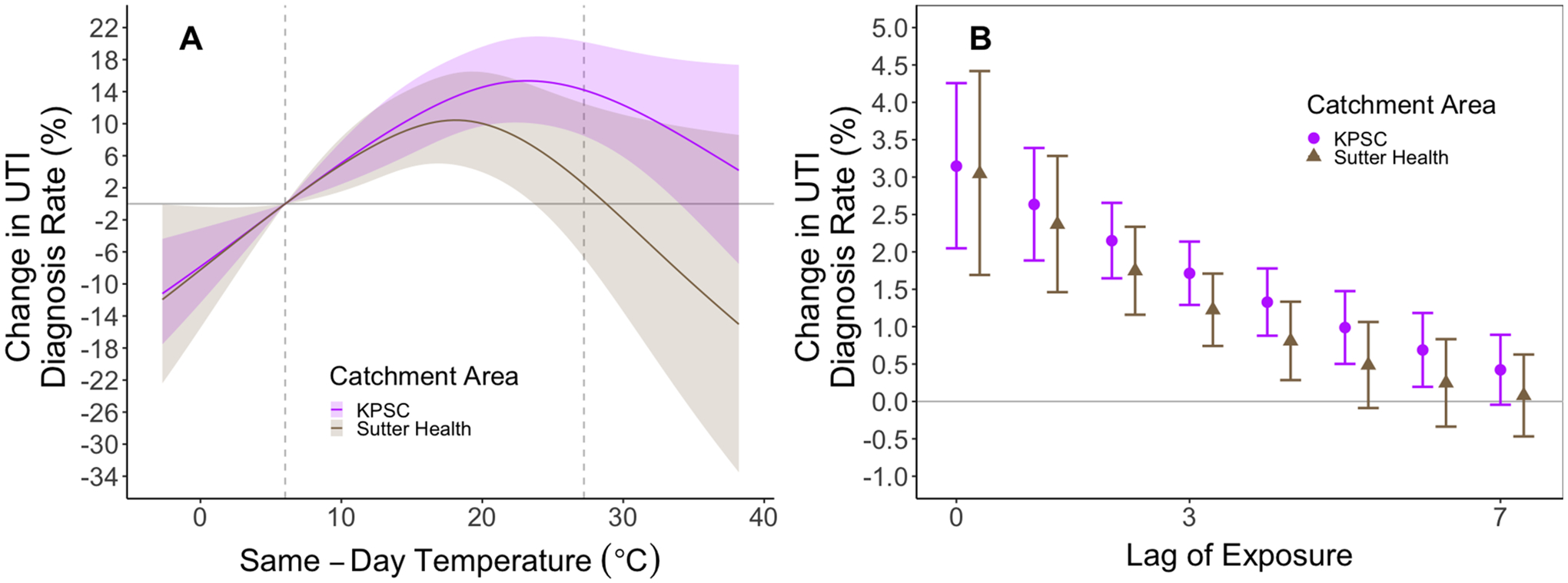
Stratified analysis of exposure–response relationship for UTI diagnosis for KPSC and Sutter Health. Panel A depicts the exposure–response relationship for 14-day cumulative temperatures separately for each catchment area and Panel B depicts the associations for same-day through seven-day lags. We constrained the exposure–response to a natural spline with four degrees of freedom and we constrained the lag-response to a natural spline with three degrees of freedom. Shaded areas and error bars represent 95% confidence intervals.
Fig. 5.
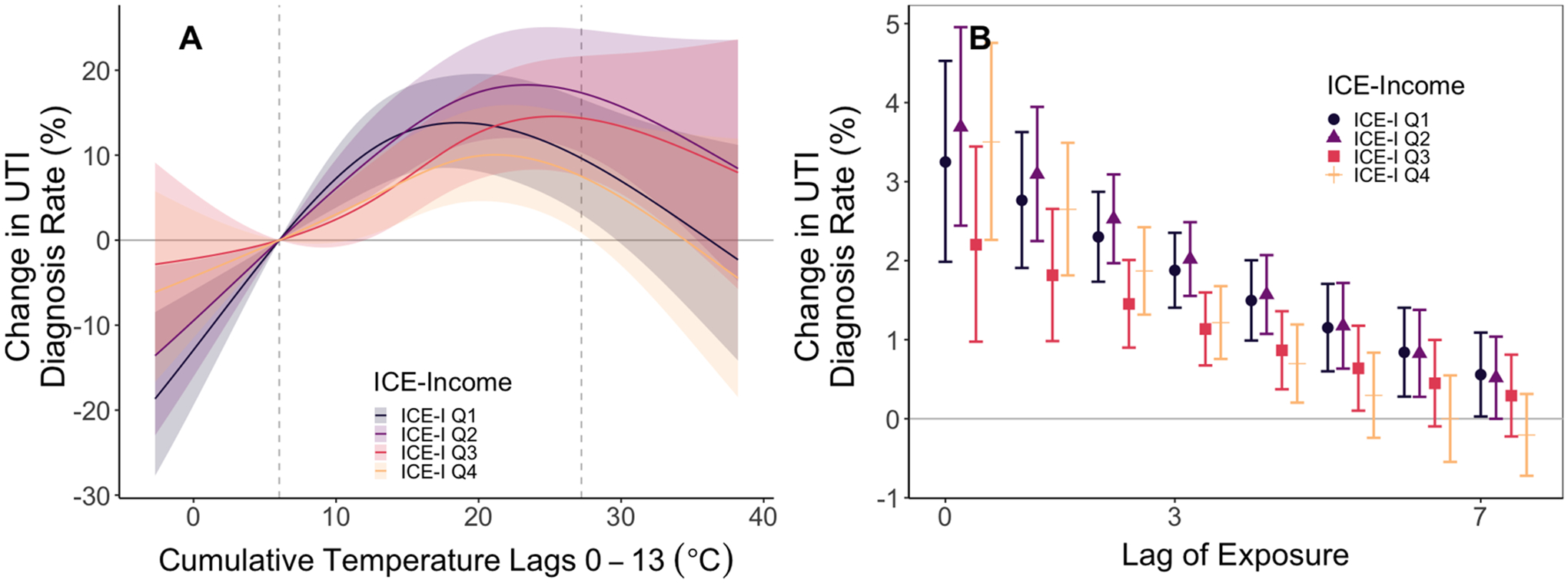
Stratified analysis of exposure–response relationship for UTI diagnosis for low-ICE-I and high-ICE-I Census tracts. Panel A depicts the exposure–response relationship for 14-day cumulative temperatures separately for high- and low-poverty Census tracts. Panel B depicts associations for the same-day through seven-day lags for the exposure–response relationship. We constrained the exposure–response to a natural spline with four degrees of freedom and we constrained the lag-response to a natural spline with three degrees of freedom. Shaded areas and error bars represent 95% confidence intervals.
3.2. Sensitivity analyses
Results with the fifth percentile specified as the referent showed a similar dose–response, but associations stronger in magnitude than our primary analysis in which the mean temperature was specified as the referent. Findings were robust to alternative model specifications with no adjustment for relative humidity, and with DLNM for relative humidity incorporated (Supplemental Figure 7). Associations were slightly greater in magnitude for the 21-day lag model, with protective associations for lags 14 through 20 (Supplemental Figure 8).
4. Discussion
In this case-crossover study, we examined the relationship between ambient temperature and outpatient UTI diagnoses in two California health care systems from 2015 to 2017. Using DNLMs to account for temporal autocorrelation in daily temperatures and flexibly model potentially complex non-linear relationships, we observed increased frequency of UTI diagnosis among women associated with higher temperatures in the spring and fall. Our findings were robust to alternative model specifications and the inclusion of men who comprised ~ 11% of outpatient UTI diagnoses in the combined study population.
Our findings are generally consistent with prior research that examines temperature and UTI. In a case-control study using data from the National Inpatient Sample from 1998 to 2011, Simmering et al. found that the odds of hospitalization with a primary diagnosis of UTI were increased during months with higher ambient temperatures in a dose-dependent manner (Simmering et al., 2018). Among 23.7 million Medicare enrollees, Hopp et al. found that risk of UTI hospitalization was increased during heatwaves–defined as at least two consecutive days exceeding the 99th percentile of daily temperatures for county of residence–as compared with non-heatwave periods from 1999 to 2010 (Hopp et al., 2018). Malig et al. estimated the probability of hospital admission for UTI increased by a cumulative 7.3% over a 14-day period for every 10°F (i.e., 5.5°C) increase in daily temperature with DLNMs applied to statewide California data, 1999–2009 (Malig et al., 2019). Over a 14-day period, we observed approximately 12% cumulative increase in UTI diagnosis rate for a shift from 6.0 to 16.2°C.
In analysis of all hospital admissions in Adelaide, South Australia from 2003 to 2014, Borg et al. found that a 1°C increase in daily minimum temperature was associated with increased rates of daily emergency department admissions for urinary tract infection ands and lower urinary tract infections (Borg et al., 2017). Using data from Beijing, Chu et al. used time-series analyses from 2013 to 2018 and found the strongest association between increased temperature and hospital admissions for urinary system disease occurred on lag day zero, with elevated risk through lag day two (Chu et al., 2021). We observed elevated risk beginning on lag day zero and extending through lag day seven, with minimal risk associated with lags after day nine. Future studies are needed to further refine the critical period of temperature exposure in relation to UTI risk, particularly when more accurate measures of symptom onset (rather than treatment-seeking) are available.
Finally, in the only prior study to assess the relationship between temperature and outpatient UTI using national claims data, Simmering et al. found an association between increases in week-prior temperature and UTI among women based on 15 million unique outpatient encounters from 2001 to 2015 (Simmering et al., 2021). Our analysis offers a substantial extension of these prior works, considering UTI in the outpatient setting, where the majority of infections are diagnosed and treated, using DLMNs as a modeling strategy, and relying on electronic health record data that provided both diagnosis codes and laboratory data from which to identify UTI cases. Only one prior study employed DLNMs to account for temporal autocorrelation of daily temperature data and capture potential non-linearities in the exposure–response relationship.
In our primary analysis, we observed a threshold phenomenon wherein the frequency of UTI diagnosis increased monotonically up until 10°C but remained relatively constant thereafter. Our findings suggest that in California changes from mildly cool to mildly warm temperatures may confer the greatest increase in UTI risk, rather than extreme heat. These findings are particularly relevant as the magnitude of average daily temperatures are projected to increase as the global climate changes. It has been previously hypothesized that higher temperatures may increase UTI risk through dehydration – even subclinically – leading to decreased urinary frequency and reduced clearance of potential pathogens from the urethral meatus (Eckford et al., 1995; Beetz, 2003; Nygaard and Linder, 1997). Our findings suggest nuance in this mechanism, as one would expect dehydration – and therefore rates of UTI diagnosis – to increase in a monotonic fashion with rising temperature. One possible explanation for the threshold phenomenon we observe is behavior change wherein individuals protect themselves against higher temperatures, for example by spending time in air-conditioned indoor spaces, that mitigate exposure to higher temperatures.
Past research has also focused on seasonality with increased rates of UTI often, though not always (Vorland et al., 1985; Stansfeld, 1966), observed during the fall and summer when temperatures are generally warmer (Falagas et al., 2009; Yolbas et al., 2013; Anderson, 1983; Rosello et al., 2018; Melamed et al., 2014; Rossignol et al., 2013). We therefore considered effect modification by season in our analysis. Whereas we observed a slightly decreased frequency of UTI diagnosis at higher temperatures relative to the mean during the summer, there was a clear monotonic increase in the frequency of UTI diagnosis during the fall. Again, behavior change could explain this relationship. Individuals may take protective measures during the summer, but not during the fall or spring when warmer temperatures may be unexpected.
Finally, we considered effect modification by catchment area and by ICE-I, a measure of concentrated neighborhood poverty and wealth. By catchment area, we observed decreased rates of UTI diagnoses for the highest temperatures in the Sutter Health catchment, which has a more temperate climate relative to the KPSC catchment. Although we caution that the divergence in dose–response curves by catchment was only observed for the highest temperatures where estimates were relatively imprecise due to data sparsity. We observed no clear evidence of a difference in response between ambient temperature and UTI by concentration of poverty (low ICE-I) community status. This finding is consistent with prior studies using these data that showed no clear relationship between low individual- or community-level socioeconomic status and overall UTI diagnosis rate (Casey et al., 2021).
4.1. Limitations
While the electronic health records data employed in this analysis provided detailed, longitudinal health records for a large population of individuals in Northern and Southern California, they represent only a subset of individuals residing in these areas. While the Sutter Health population is generally representative of the underlying population in their region, individuals in with the highest and lowest incomes are under-represented in the KPSC patient population. This limits generalizability of our results to the region in generally, and certainly to other parts of the country where local climates, behaviors, and healthcare utilization may differ. Another limitation of EHR data is that we are only able to identify UTIs among individuals who sought care. This means that the least severe cases may not appear in our dataset. We suspect it is unlikely that care-seeking for UTI differs systematically by ambient temperatures.
We used daily average county-level temperature estimates, which may have resulted in exposure misclassification due to error in the exposure model, spatial variability within county, and participant housing quality and behaviors. Our seasonal results suggest a role of behavioral adaptation to hot temperatures. It is possible that other temperature metrics not included in our analysis, for example maximum daily temperature, nighttime average temperature, or temperature variability, matter more for UTI diagnosis rates (Li et al., 2017). We used ICE-I to stratify infections based on neighborhood poverty, however the ICE-I variable was constructed with data collected from 2011 to 2015. Little overlap between these dates and the study period may introduce misclassification, as neighborhoods can change quickly. We have no reason to suspect this misclassification would be systematic with respect to UTI and temperature. Finally, our case-crossover study design was able to handle time-invariant confounding and analyses that additionally controlled for relative humidity did not change effect estimates. The DLNM also assumes that the effect of temperature on rates of UTI diagnosis would vary smoothly over the days; we selected the degree of smoothness based on quasi-Akaike Information Criteria, a metric that balances goodness-of-fit with penalties for model complexity.
5. Conclusion
In this time-stratified case-crossover analysis, we examined the relationship between ambient temperature and the rate of outpatient UTI diagnosis using EHR data from two California healthcare systems. We observed a threshold phenomenon wherein frequency of UTI diagnosis increased with higher temperatures and then plateaued at approximately 10°C. Our results further suggest the dose–response differs by season with a clear monotonic increase in frequency of UTI diagnoses observed in the fall and an increase at temperatures above the mean during the spring. Overall, our findings underscore the importance of modeling approaches that can capture potential non-linearities and seasonal variation in the exposure–response relationship. Further, as climate change intensifies, public health and healthcare professionals should pay attention to the joint implications of temperature and season in future study and treatment of outpatient UTI.
Supplementary Material
Acknowledgments
This work was supported by the National Institute of Allergy and Infectious Diseases at the National Institutes of Health (grant number R01AI130066). Dr Parks was supported by the National Institute of Environmental Health Sciences (grant number K99ES033742) and the Earth Institute postdoctoral research fellowship at Columbia University.
Footnotes
CRediT authorship contribution statement
Holly Elser: Methodology, Writing – original draft. Sebastian T. Rowland: Formal analysis, Methodology, Visualization, Writing – original draft. Sara Y. Tartof: Conceptualization, Funding acquisition, Resources, Supervision, Writing – review & editing. Robbie M. Parks: Data curation, Writing – review & editing. Katia Bruxvoort: Data curation, Writing – review & editing. Rachel Morello-Frosch: Writing – review & editing. Sarah C. Robinson: Data curation, Project administration, Resources, Writing – review & editing. Alice R. Pressman: Data curation, Project administration, Resources, Writing – review & editing. Rong X. Wei: Data curation, Writing – review & editing. Joan A. Casey: Conceptualization, Methodology, Resources, Supervision, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Data Sharing
Original, diagnosis-level data tied to individuals, locations, and time are considered personally identifiable health information. These data cannot be shared owing to risks of breaching patient confidentiality. Study data are available for researchers who meet the criteria for access to confidential data upon reasonable request.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.envint.2022.107303.
References
- Foxman B, 2010. The epidemiology of urinary tract infection. Nature Reviews Urology. 7 (12), 653–660. [DOI] [PubMed] [Google Scholar]
- Medina M, Castillo-Pino E, 2019. An introduction to the epidemiology and burden of urinary tract infections. Therapeutic advances in urology. 11, 1756287219832172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores-Mireles AL, Walker JN, Caparon M, Hultgren SJ, 2015. Urinary tract infections: epidemiology, mechanisms of infection and treatment options. Nature reviews microbiology. 13 (5), 269–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert X, Huertas I, Pereiro I, Sanfélix J, Gosalbes V, Perrotta C, 2004. Antibiotics for preventing recurrent urinary tract infection in non-pregnant women. Cochrane Database of Systematic Reviews. 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foxman B, Barlow R, D’Arcy H, Gillespie B, Sobel JD, 2000. Urinary tract infection: self-reported incidence and associated costs. Annals of epidemiology. 10 (8), 509–515. [DOI] [PubMed] [Google Scholar]
- Foxman B, 2002. Epidemiology of urinary tract infections: incidence, morbidity, and economic costs. The American journal of medicine. 113 (1), 5–13. [DOI] [PubMed] [Google Scholar]
- Bruxvoort KJ, Bider-Canfield Z, Casey JA, et al. , 2020. Outpatient urinary tract infections in an era of virtual healthcare: trends from 2008 to 2017. Clinical Infectious Diseases. 71 (1), 100–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzariol A, Bazaj A, Cornaglia G, 2017. Multi-drug-resistant Gram-negative bacteria causing urinary tract infections: a review. Journal of Chemotherapy. 29 (sup1), 2–9. [DOI] [PubMed] [Google Scholar]
- Hersh AL, Shapiro DJ, Pavia AT, Shah SS, 2011. Antibiotic prescribing in ambulatory pediatrics in the United States. Pediatrics. 128 (6), 1053–1061. [DOI] [PubMed] [Google Scholar]
- Scholes D, Hooton TM, Roberts PL, Stapleton AE, Gupta K, Stamm WE, 2000. Risk factors for recurrent urinary tract infection in young women. The Journal of infectious diseases. 182 (4), 1177–1182. [DOI] [PubMed] [Google Scholar]
- Scholes D, Hawn TR, Roberts PL, et al. , 2010. Family history and risk of recurrent cystitis and pyelonephritis in women. The Journal of urology. 184 (2), 564–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickling DR, Sun TT, Wu XR, 2017. Anatomy and physiology of the urinary tract: relation to host defense and microbial infection. Molecular Pathogenesis and Clinical Management, Urinary Tract Infections, pp. 1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foxman B, 2014. Urinary tract infection syndromes: occurrence, recurrence, bacteriology, risk factors, and disease burden. Infectious Disease Clinics. 28 (1), 1–13. [DOI] [PubMed] [Google Scholar]
- Patterson JE, Andriole VT, 1997. Bacterial urinary tract infections in diabetes. Infectious Disease Clinics. 11 (3), 735–750. [DOI] [PubMed] [Google Scholar]
- Stapleton A, 2002. Urinary tract infections in patients with diabetes. The American journal of medicine. 113 (1), 80–84. [DOI] [PubMed] [Google Scholar]
- Simmering JE, Tang F, Cavanaugh JE, Polgreen LA, Polgreen PM. The increase in hospitalizations for urinary tract infections and the associated costs in the United States, 1998–2011. Paper presented at: Open forum infectious diseases, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson JE, 1983. Seasonality of symptomatic bacterial urinary infections in women. Journal of Epidemiology & Community Health. 37 (4), 286–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosello A, Pouwels K, De Cellès MD, et al. , 2018. Seasonality of urinary tract infections in the United Kingdom in different age groups: longitudinal analysis of The Health Improvement Network (THIN). Epidemiology & Infection. 146 (1), 37–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melamed RD, Khiabanian H, Rabadan R, 2014. Data-driven discovery of seasonally linked diseases from an Electronic Health Records system. BMC bioinformatics. 15 (6), 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossignol L, Pelat C, Lambert B, Flahault A, Chartier-Kastler E, Hanslik T, 2013. A method to assess seasonality of urinary tract infections based on medication sales and google trends. PloS one. 8 (10), e76020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopp S, Dominici F, Bobb JF, 2018. Medical diagnoses of heat wave-related hospital admissions in older adults. Preventive medicine. 110, 81–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malig BJ, Guirguis K, Gershunov A, Basu R, 2019. Associations between ambient temperature and hepatobiliary and renal hospitalizations in California, 1999 to 2009. Environmental research. 177, 108566. [DOI] [PubMed] [Google Scholar]
- Simmering J, Cavanaugh J, Polgreen L, Polgreen P, 2018. Warmer weather as a risk factor for hospitalisations due to urinary tract infections. Epidemiology & Infection. 146 (3), 386–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmering JE, Polgreen LA, Cavanaugh JE, Erickson BA, Suneja M, Polgreen PM, 2021. Warmer weather and the risk of urinary tract infections in women. The Journal of Urology. 205 (2), 500–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamm WE, McKevitt M, Roberts PL, White NJ, 1991. Natural history of recurrent urinary tract infections in women. Reviews of infectious diseases. 13 (1), 77–84. [DOI] [PubMed] [Google Scholar]
- Wright RA, Judson FN, 1978. Relative and seasonal incidences of the sexually transmitted diseases. A two-year statistical review. Sexually Transmitted Infections. 54 (6), 433–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelisse VJ, Chow EP, Chen MY, Bradshaw CS, Fairley CK, 2016. Summer heat: a cross-sectional analysis of seasonal differences in sexual behaviour and sexually transmissible diseases in Melbourne. Australia. Sexually transmitted infections. 92 (4), 286–291. [DOI] [PubMed] [Google Scholar]
- Shah AP, Smolensky MH, Burau KD, Cech IM, Lai D, 2007. Recent change in the annual pattern of sexually transmitted diseases in the United States. Chronobiology international. 24 (5), 947–960. [DOI] [PubMed] [Google Scholar]
- Freeman J, Anderson D, Sexton D, 2009. Seasonal peaks in Escherichia coli infections: possible explanations and implications. Clinical Microbiology and Infection. 15 (10), 951–953. [DOI] [PubMed] [Google Scholar]
- Eckford S, Keane D, Lamond E, Jackson S, Abrams P, 1995. Hydration monitoring in the prevention of recurrent idiopathic urinary tract infections in pre-menopausal women. British journal of urology. 76 (1), 90–93. [DOI] [PubMed] [Google Scholar]
- Beetz R, 2003. Mild dehydration: a risk factor of urinary tract infection? European journal of clinical nutrition. 57 (2), S52–S58. [DOI] [PubMed] [Google Scholar]
- Casey JA, Rudolph KE, Robinson SC, et al. , 2021. Sociodemographic inequalities in urinary tract infection in two large California health systems. Open Forum Infectious Diseases, Paper presented at. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maclure M, 1991. The case-crossover design: a method for studying transient effects on the risk of acute events. American journal of epidemiology. 133 (2), 144–153. [DOI] [PubMed] [Google Scholar]
- Maclure M, Mittleman MA, 2000. Should we use a case-crossover design? Annual review of public health. 21 (1), 193–221. [DOI] [PubMed] [Google Scholar]
- Armstrong BG, Gasparrini A, Tobias A, 2014. Conditional Poisson models: a flexible alternative to conditional logistic case cross-over analysis. BMC medical research methodology. 14 (1), 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wichmann J, Ketzel M, Ellermann T, Loft S, 2012. Apparent temperature and acute myocardial infarction hospital admissions in Copenhagen, Denmark: a case-crossover study. Environmental Health. 11 (1), 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyobutungi C, Grau A, Stieglbauer G, Becher H, 2005. Absolute temperature, temperature changes and stroke risk: a case-crossover study. European journal of epidemiology. 20 (8), 693–698. [DOI] [PubMed] [Google Scholar]
- Buckley JP, Richardson DB, 2012. Seasonal modification of the association between temperature and adult emergency department visits for asthma: a case-crossover study. Environmental Health. 11 (1), 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koebnick C, Langer-Gould AM, Gould MK, et al. , 2012. Sociodemographic characteristics of members of a large, integrated health care system: comparison with US Census Bureau data. The Permanente Journal. 16 (3), 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gales AC, Jones RN, Gordon KA, et al. , 2000. Activity and spectrum of 22 antimicrobial agents tested against urinary tract infection pathogens in hospitalized patients in Latin America: report from the second year of the SENTRY antimicrobial surveillance program (1998). Journal of Antimicrobial Chemotherapy. 45 (3), 295–303. [DOI] [PubMed] [Google Scholar]
- Gupta K, Hooton T, Wobbe C, Stamm W, 1999. The prevalence of antimicrobial resistance among uropathogens causing acute uncomplicated cystitis in young women. International journal of antimicrobial agents. 11 (3–4), 305–308. [DOI] [PubMed] [Google Scholar]
- Gupta K, Scholes D, Stamm WE, 1999. Increasing prevalence of antimicrobial resistance among uropathogens causing acute uncomplicated cystitis in women. Jama. 281 (8), 736–738. [DOI] [PubMed] [Google Scholar]
- Hooton TM, Stamm WE, 1997. Diagnosis and treatment of uncomplicated urinary tract infection. Infectious Disease Clinics. 11 (3), 551–581. [DOI] [PubMed] [Google Scholar]
- Jones RN, Kugler KC, Pfaller MA, Winokur PL, 1999. Characteristics of pathogens causing urinary tract infections in hospitals in North America: results from the SENTRY Antimicrobial Surveillance Program, 1997. Diagnostic microbiology and infectious disease. 35 (1), 55–63. [DOI] [PubMed] [Google Scholar]
- Daly C, Bryant K, 2013. The PRISM climate and weather system—an introduction. PRISM climate group, Corvallis, OR. [Google Scholar]
- Spangler KR, Weinberger KR, Wellenius GA, 2019. Suitability of gridded climate datasets for use in environmental epidemiology. Journal of exposure science & environmental epidemiology. 29 (6), 777–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu R, Ostro BD, 2008. A multicounty analysis identifying the populations vulnerable to mortality associated with high ambient temperature in California. American journal of epidemiology. 168 (6), 632–637. [DOI] [PubMed] [Google Scholar]
- Weinberger KR, Harris D, Spangler KR, Zanobetti A, Wellenius GA, 2020. Estimating the number of excess deaths attributable to heat in 297 United States counties. Environmental Epidemiology (Philadelphia, Pa) 4 (3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieger N, Waterman PD, Spasojevic J, Li W, Maduro G, Van Wye G, 2016. Public health monitoring of privilege and deprivation with the index of concentration at the extremes. American journal of public health. 106 (2), 256–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran KV, Casey JA, Cushing LJ, Morello-Frosch R, 2020. Residential proximity to oil and gas development and birth outcomes in California: a retrospective cohort study of 2006–2015 births. Environmental health perspectives. 128 (6), 067001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- California Department of Water Resources. California Irrigation Management Information System https://cimis.water.ca.gov/. Published 2021. Accessed December 25, 2021.
- Janes H, Sheppard L, Lumley T, 2005. Case-crossover analyses of air pollution exposure data: referent selection strategies and their implications for bias. Epidemiology. 717–726. [DOI] [PubMed] [Google Scholar]
- Gasparrini A, Armstrong B, Kenward MG, 2010. Distributed lag non-linear models. Statistics in medicine. 29 (21), 2224–2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y, Kim H, Gasparrini A, et al. , 2019. Suicide and ambient temperature: a multi-country multi-city study. Environmental health perspectives. 127 (11), 117007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scovronick N, Sera F, Acquaotta F, et al. , 2018. The association between ambient temperature and mortality in South Africa: A time-series analysis. Environmental research. 161, 229–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Ou C-Q, Ding Y, Zhou Y-X, Chen P-Y, 2012. Daily temperature and mortality: a study of distributed lag non-linear effect and effect modification in Guangzhou. Environmental Health. 11 (1), 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y, Barnett AG, Pan X, Yu W, Tong S, 2011. The impact of temperature on mortality in Tianjin, China: a case-crossover design with a distributed lag nonlinear model. Environmental health perspectives. 119 (12), 1719–1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowland ST, Boehme AK, Rush J, Just AC, Kioumourtzoglou M-A, 2020. Can ultra short-term changes in ambient temperature trigger myocardial infarction? Environment International. 143, 105910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasparrini A, 2016. Modelling lagged associations in environmental time series data: a simulation study. Epidemiology (Cambridge, Mass). 27 (6), 835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagenmakers E-J, Farrell S, 2004. AIC model selection using Akaike weights. Psychonomic bulletin & review. 11 (1), 192–196. [DOI] [PubMed] [Google Scholar]
- Izrailev S Functions for timing R scripts, as well as implementations of Stack and List structures., 1.0 Package ‘tictoc’Version 1.0 1–8 (2016). In.
- Rinker T, Kurkiewicz D. pacman: Package management for R. Buffalo, New York: Retrieved from http://github.com/trinker/pacman [Google Scholar]. 2018.
- Wickham H, Averick M, Bryan J, et al. , 2019. Welcome to the Tidyverse. Journal of open source software. 4 (43), 1686. [Google Scholar]
- Klik M fst: Lightning Fast Serialization of Data Frames for R. R package version 0.9. 0. In: 2019.
- Barton K, Barton MK, 2015. Package ‘mumin’. Version. 1 (18), 439. [Google Scholar]
- Gasparrini A, 2011. Distributed lag linear and non-linear models in R: the package dlnm. Journal of statistical software. 43 (8), 1. [PMC free article] [PubMed] [Google Scholar]
- Turner H, Firth D, 2007. gnm: A package for generalized nonlinear models. New Functions for Multivariate Analysis. 7, 8. [Google Scholar]
- Wickham H, Chang W, Wickham MH, 2016. Package ‘ggplot2’. Create Elegant Data Visualisations Using the Grammar of Graphics Version. 2 (1), 1–189. [Google Scholar]
- Auguie B egg: Extensions for ‘ggplot2’: Custom Geom, Custom Themes, Plot Alignment, Labelled Panels, Symmetric Scales, and Fixed Panel Size. R package version 04. 2019; 5. [Google Scholar]
- Wilke CO, Wickham H, Wilke MCO. Package ‘cowplot’. Streamlined Plot Theme and Plot Annotations for ‘ggplot2. 2019.
- Auguie B, Antonov A, Auguie MB, 2017. Package ‘gridExtra’. Miscellaneous Functions for “Grid” Graphics.
- Borg M, Bi P, Nitschke M, Williams S, McDonald S, 2017. The impact of daily temperature on renal disease incidence: an ecological study. Environmental Health. 16 (1), 1–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu L, Du H, Li T, et al. , 2021. Short-term associations between particulate matter air pollution and hospital admissions through the emergency room for urinary system disease in Beijing, China: A time-series study. Environmental Pollution. 289, 117858. [DOI] [PubMed] [Google Scholar]
- Nygaard I, Linder M, 1997. Thirst at work—an occupational hazard? International Urogynecology Journal. 8 (6), 340–343. [DOI] [PubMed] [Google Scholar]
- Vorland L, Carlson K, Aalen O, 1985. Antibiotic resistance and small R plasmids among Escherichia coli isolates from outpatient urinary tract infections in northern Norway. Antimicrobial agents and chemotherapy. 27 (1), 107–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stansfeld J, 1966. Clinical observations relating to incidence and aetiology of urinary-tract infections in children. British Medical Journal. 1 (5488), 631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falagas M, Peppas G, Matthaiou D, Karageorgopoulos D, Karalis N, Theocharis G, 2009. Effect of meteorological variables on the incidence of lower urinary tract infections. European journal of clinical microbiology & infectious diseases. 28 (6), 709–712. [DOI] [PubMed] [Google Scholar]
- Yolbas I, Tekin R, Kelekci S, et al. , 2013. Community-acquired urinary tract infections in children: pathogens, antibiotic susceptibility and seasonal changes. Eur Rev Med Pharmacol Sci. 17 (7), 971–976. [PubMed] [Google Scholar]
- Li J, Xu X, Yang J, et al. , 2017. Ambient high temperature and mortality in Jinan, China: A study of heat thresholds and vulnerable populations. Environmental research. 156, 657–664. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


