Abstract
Background:
Ocular herpes simplex is usually caused by herpes simplex virus type 1 (HSV-1) and less commonly by the type 2 virus (HSV-2). Ocular manifestations of HSV include blepharitis, conjunctivitis, lacrimal system obstruction, corneal involvement, and uveitis. Corneal involvement is one of the causes of loss of vision and can be epithelial herpetic keratitis or stromal herpetic keratitis.
Objective:
A significant population has a colonization of herpes viruses. Under certain circumstances, these viruses can reactivate with a significant ocular morbidity. Globally, COVID-19 vaccines are recommended; however, the vaccine safety data are limited.
Case report:
Herein, we reported a case of herpetic keratitis reactivation that occurred 2 days after receiving SARS-CoV-2 mRNA vaccine. The patient is a 50-year-old man who underwent penetrating keratoplasty (PKP) in 2020 for corneal opacity caused by a previous herpes simplex keratitis in 2013. Herpetic keratitis was treated successfully with topical antiviral acyclovir along with topical moxifloxacin and artificial tears. After treatment, prophylactic oral acyclovir was started.
Conclusion:
Both ophthalmologist and patients should be aware of this phenomenon. Long-term prophylactic antiviral treatment may be recommended for those patients.
Keywords: Penetrating keratoplasty, herpes simplex keratitis, cornea, COVID-19, vaccination
1. BACKGROUND
Ocular herpes simplex is usually caused by herpes simplex virus type 1 (HSV-1) and less commonly by the type 2 virus (HSV-2). Ocular manifestations of HSV include blepharitis, conjunctivitis, lacrimal system obstruction, corneal involvement, and uveitis. Corneal involvement is one of the causes of loss of vision and can be epithelial herpetic keratitis or stromal herpetic keratitis.
The latter is thought to result from a combination of viral infection and compromised immune mechanisms (1). One of the important factors of recurrence is the previous history of stromal keratitis. Age, sex, ethnicity, and previous history of non-ocular HSV disease were not associated with an increased risk of recurrence (1). Other virus in this family is human herpesvirus 3 (VZV) which causes herpes zoster ophthalmicus with variable manifestations of skin rash, conjunctivitis, keratitis and uveitis (2).
In general, different types of vaccines (such as influenza and hepatitis B) have been associated with ocular symptoms. The severity ranged from mild symptoms (conjunctivitis) to severe and organ-threatening sequela such as uveitis, acute idiopathic maculopathy, Vogt-Koyanagi-Harada disease, and multiple evanescent white dot syndrome (3, 4). With the occurrence of SARS-CoV-2 virus and the development of different types of vaccines against it, many reports were published regarding the ocular manifestations of COVID-19, as well as ocular adverse effects of COVID-19 vaccinations (3, 5). The reported ocular sequela of COVID-19 vaccinations include facial nerve palsy, abducens nerve palsy, uveitis, central serous retinopathy, ophthalmic vein thrombosis, multiple evanescent white dot syndrome, Vogt-Koyanagi-Harada disease reactivation, acute corneal graft rejection, and new-onset Graves’ Disease (3, 5). One of the reported events post COVID-19 vaccination is the reactivation of HSV keratitis in patients who have a previous history of HSV keratitis (6, 7).
2. OBJECTIVE
We report the first case of HSV keratitis reactivation on a corneal graft for a patient who underwent a penetrating keratoplasty (PKP) for a previous HSV keratitis after 2 days of receiving SARS-CoV-2 mRNA vaccination (Pfizer/BioNTech vaccine).
3. CASE REPORT
A 50-year-old man, who underwent left penetrating keratoplasty (PKP) in February 2020, presented on 19th of December 2021 to our services complaining of left eye redness, tearing and pain in the left eye 2 days after receiving the first dose of SARS-CoV-2 mRNA vaccination (Pfizer/BioNTech vaccine) on the 12th from the same month. The patient underwent PKP and anti-vascular endothelial growth factor (anti-VEGF) injection as a result of left corneal opacity and vascularization which developed from a previous herpetic keratitis in 2013. During the postoperative follow up period, the patient received two additional subconjunctival anti-VEGF injections and a corneal topography-guided stitches removal was performed. He achieved a best corrected visual acuity (BCVA) of 6/15 (compared to 6/120 before the PKP). A prophylactic dose of 400 mg oral acyclovir and topical prednisolone acetate were maintained during the postoperative period until 7 months before receiving the vaccine where the patient lost follow up and stopped the prophylactic acyclovir by his own. The patient is known to have hypertension and ischemic heart disease.
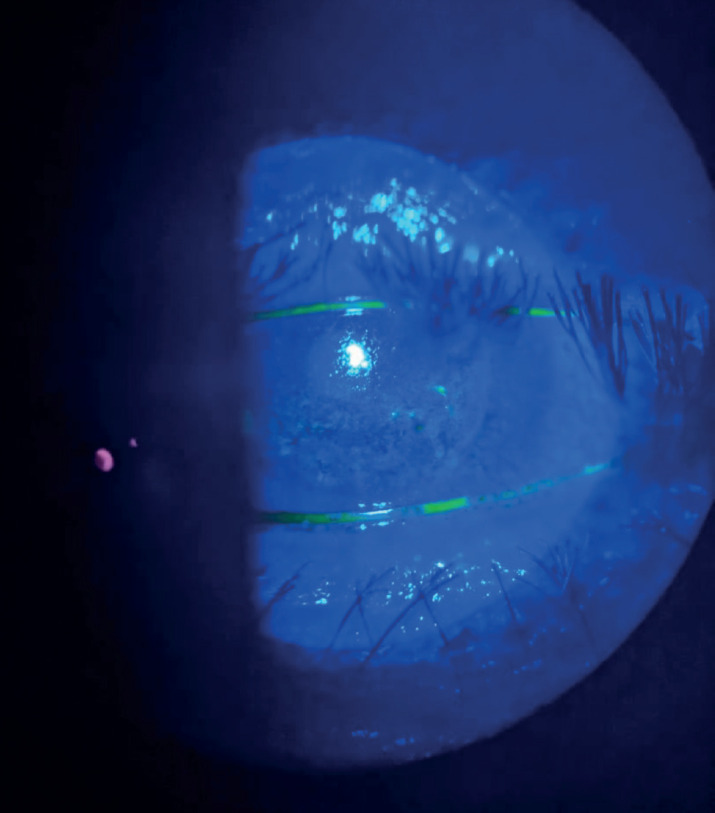
On exam, the patient achieved a BCVA of 6/60, anterior segment exam revealed a reduction in the corneal sensation along with multiple dendritic ulcers scattered all over the graft with ciliary injection and mild anterior chamber cells (Figure 1). The dilated fundus exam was normal. The intraocular pressure was 15 mmHg. Topical acyclovir eye ointment 5 times a day, topical moxifloxacin 4 times a day and excessive lubricant eye drops were commenced. With close monitoring and regular follow up visits, the dendritic ulcers were resolved after 2 weeks of treatment and the graft was clear (except for punctate epithelial erosions) with a visual acuity of 6/30 which was explained by the presence of cataract (Figure 2). Acyclovir ointment was stopped, topical fluorometholone twice a day was started, and 400 mg oral acyclovir was commenced. The patient was encouraged to receive the second dose of the vaccine.
Figure 1. Slit-lamp photo showing multiple dendritic ulcers at presentation.
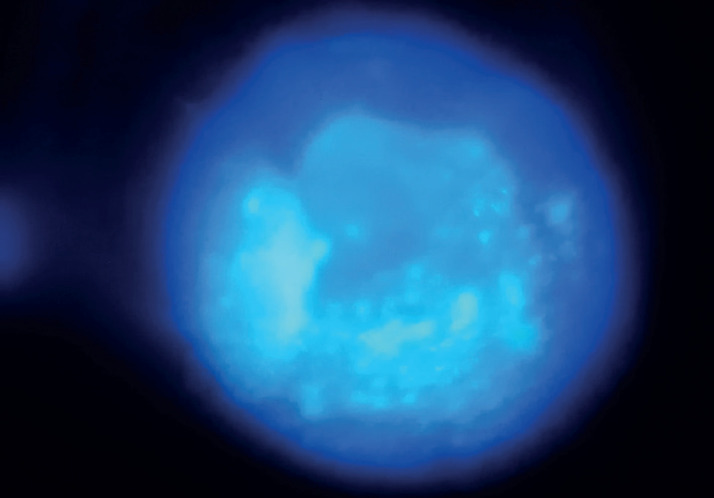
Figure 2. After 2 weeks of the treatment, dendritic ulcers were resolved with punctate epithelial erosions.
4. DISCUSSION
To the best of our knowledge, this is the first report of HSV recurrence after SARS-CoV-2 mRNA vaccination (Pfizer/BioNTech vaccine) on a corneal graft for a patient who underwent PKP for a corneal scar that resulted from a previous HSV keratitis. Li et al reported 2 cases of HSV keratitis reactivation preceded by COVID-19 vaccination (7). In one case, the reactivation was developed on a corneal graft of a 60-year-old lady 2 days after receiving inactivated COVID-19 vaccines (Sinovac) (7). She underwent PKP as a result of HSV keratitis with residual scar (7). In the other case, VZV was detected 2 days following the inactivated COVID-19 vaccines (Sinovac) (7). Richardson-May et al reported a case of 82-year-old male presented with a recurrence of herpetic keratitis 1 day following COVID-19 vaccination (AstraZeneca) (6). Herbort Jr and Papasavvas reported a case of 53-year-old man with reactivation of herpetic infection 5 days after the second dose of Moderna vaccine (8). He was treated in the past for right herpes kerato-uveitis that had been inactive for 18 months without treatment (8). Alkhalifaha et al reported 2 cases of HSV reactivation following the SARS-CoV-2 mRNA (Pfizer/BioNTech) vaccine; one of the cases 4 days after the vaccine and the other 4 weeks after the vaccine (9).
Approximately 52% - 84% of adults have latent colonization to HSV-1 within the trigeminal ganglia. Under certain conditions, the viral will replicate and this can lead to recurrent clinical disease (6). Latency is an active and complex immune mechanism where CD8-positive T cells play an important role. A possible mechanism that can explain the reactivation of HSV keratitis following vaccination is that mRNA vaccines T dysregulate latency mechanisms in the sensory nerve ganglions (8). A further proposed mechanism includes auto-immunological reaction triggered by the vaccine, with possible reduction in neurotrophin allowing HSV replication (10). The role of the disruption of humoral immunity was also reported (10).
Since HSV-1 and VZV infections can cause substantial ocular morbidity, prompt antiviral management should be started. Many efficient antiviral agents are available and the management of herpetic viruses ocular complications are successfully treated in most cases (8). However, each new episode can affect eye structures. Furthermore, some complications such as acute retinal necrosis are much more dangerous and their management is difficult. Therefore, in patients with a previous history of herpetic infections, preventive antiviral therapy may be recommended. This is more important in patients with a corneal graft as the graft is more prone for rejection after attacks of herpetic infections.
5. CONCLUSION
This article reported the first case of HSV keratitis in a corneal graft following SARS-CoV-2 mRNA vaccination. It highlights the importance of considering prophylactic antiviral treatment for those patients.
Consent for publication:
Written informed consent was obtained from the patient for publication of this Case report and any accompanying images. A copy of the written consent is available for review by the Editor of this journal.
Availability data and materials:
Data sharing does not apply to this article as no datasets were generated or analyzed during the current study.
Author's contribution:
All authors were involved in all steps of preparation this case report. Final proofreading was made by the first author.
Competing interests:
The authors declare that they have no competing interests.
Financial support and sponsorship:
No funding.
REFERENCES
- 1.Barker NH. Ocular herpes simplex. BMJ Clin Evid. 2008;2008:0707. [PMC free article] [PubMed] [Google Scholar]
- 2.Naumann G, Gass JD, Font RL. Histopathology of herpes zoster ophthalmicus. Am J Ophthalmol. 1968;65:533–541. doi: 10.1016/0002-9394(68)93869-5. [DOI] [PubMed] [Google Scholar]
- 3.Ng XL, Betzler BK, Testi I, et al. Ocular Adverse Events After COVID-19 Vaccination. Ocul Immunol Inflamm. 2021:1–9. doi: 10.1080/09273948.2021.1976221. [published online ahead of print, 2021 Sep 24] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Biancardi AL, Moraes HV Jr. Anterior and intermediate uveitis following yellow fever vaccination with fractional dose: case reports. Ocul Immunol Inflamm. 2019;27(4):521–523. doi: 10.1080/09273948.2018.1510529. [DOI] [PubMed] [Google Scholar]
- 5.Phylactou M, Li JO, Larkin DFP. Characteristics of endothelial corneal transplant rejection following immunisation with SARS-CoV-2 messenger RNA vaccine. Br J Ophthalmol. 2021 Jul;105(7):893–896. doi: 10.1136/bjophthalmol-2021-319338. [DOI] [PubMed] [Google Scholar]
- 6.Richardson-May J, Rothwell A, Rashid M. Reactivation of herpes simplex keratitis following vaccination for COVID-19. BMJ Case Rep. 2021;14:e245792. doi: 10.1136/bcr-2021-245792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li S, Jia X, Yu F, Wang Q, Zhang T, Yuan J. Herpetic Keratitis Preceded by COVID-19 Vaccination. Vaccines. 2021;9(12):1394. doi: 10.3390/vaccines9121394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Herbort CP Jr, Papasavvas I. Effect of SARS-CoV-2 mRNA vaccination on ocular herpes simplex and varicella-zoster virus reactivation: should preventive antiviral treatment be given in known herpes patients. J Ophthalmic Inflamm Infect. 2021;11(1):33. doi: 10.1186/s12348-021-00262-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alkhalifah MI, Alsobki HE, Alwael HM, Al Fawaz AM, Al-Mezaine HS: Herpes simplex virus keratitis reactivation after SARS-CoV-2 BNT162b2 mRNA vaccination: a report of two cases. Ocul Immunol Inflamm. 2021:1–3. doi: 10.1080/09273948.2021.1986548. [DOI] [PubMed] [Google Scholar]
- 10.Hassman LM, DiLoreto DA. Immunologic factors may play a role in herpes simplex virus 1 reactivation in the brain and retina after influenza vaccination. IDCases. 2016;6:47–51. doi: 10.1016/j.idcr.2016.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]


