Abstract
Background:
Bryophyllum pinnatum is a herbal medicine from Indonesia which has an anti-inflammatory effect. Adenosine monophosphate-activated protein kinase (AMPK) and inducible nitric oxide synthase (iNOS) play a function in thickening and inflammation in atherosclerosis disease.
Objective:
This research aims conducted to know the potential of Bryophyllum pinnatum as a therapy for atherosclerosis by targeting AMPK and iNOS.
Methods:
We employed a molecular docking technique to interact active compounds of Bryohyllum pinnatum with AMPK and iNOS, which were retrieved on the protein databank. Molecular docking analysis utilizing tools such as Pyrx 9.5, Pymol, and Discovery Studio, to support the interaction between the compound and protein. Molecular Dynamic (MD) simulation also performed using CABS-FLEX 2.0 server to know the stability interaction.
Results:
Bryophillin B was an active compound that possesses significant binding to AMPK and iNOS. It had same binding pocket as native ligand, and Bryophyllin B has a stronger interaction with AMPK. Based on the RMSF, the interaction biding complex Bryophyllin B with AMPK and iNOS were stable
Conclusion:
Bryophillin B was predicted to have potential therapy for atherosclerosis disease.
Keywords: Bryophyllum pinnatum, AMPK, iNOS, In Silico, Herbal medicine
1. BACKGROUND
Atherosclerosis is the main cause of vascular disease worldwide, such as ischemic stroke, peripheral arterial disease, and cardiovascular disease. Cardiovascular disease grows disproportionally in middle-low countries over the world with a mortality rate of 80%, and day by 2030 predicted 23.6 million people would die (1, 2).
Obviously, this concept of atherosclerosis over quarter-century inflammation being a primordial role in atherogenesis. Based on Virchow’s theory tells that atherosclerosis is a process of thrombus formation that action occurs in blood vessels through fatty degeneration, which directly also increases the inflammatory reaction in cells. This happens continuously, which it’s impossible to regard as simply passive processes, resulting in a transformation due to inflammation (3).
The growth of atherosclerosis is linked to the vascular inflammation that is involved by iNOS and AMPK pathways. iNOS has a role as an endogenous vasoconstrictor, formation of vascular lesion, and play infiltration of inflammatory cells (4) 28.1% in non-diabetic CAD patients and 12% in controls. The T allele frequency was higher in the nondiabetic CAD group (14%. AMPK has a major role in homeostasis involving intracellular activities, activity for reuptake of glucose, and the oxidation of fatty acid (5).
According to that pathways, Bryophyllum pinnatum leaves extract consists of the active compounds that had a role in anti-inflammatory agents (6). These compounds generate anti-inflammatory products implicated in every stage of atherosclerosis, from the initiation of atheroma to destabilization. Recently study about herbal medicine was highly concerned. The research about the effect of active compounds of Bryophyllum pinnatum on atherosclerosis is still not yet. Molecular docking study is the technique for screening the potential compounds as a drug for a disease. By molecular docking study, we screened the active compound of Bryophyllum pinnatum to be atherosclerosis therapy.
2. OBJECTIVE
The aim of the study was to investigate the potential of Bryophyllum pinnatum as a therapy for atherosclerosis by targeting AMPK and iNOS.
3. MATERIAL AND METHODS
Ligands and Protein Preparation
Bryophillum pinnatum contains the active compounds Bryophyllin A, Bryophyllin B, and Bryotoxin A, Bryotoxin B from our previous study. The chemical compounds were downloaded from PubChem database as sdf file. Besides, protein samples for AMPK and iNOS were generated using the RCSB database (https://www.rcsb.org/). Native ligands are also obtained on the web protein data bank, which is utilized as a comparison of interactions with the active chemical Bryophyllum pinnatum (7).
Anti-inflammation Bioactivity Prediction
The anti-inflammation bioactivity prediction of each compound was performed using the Way2Drug PASS Online web server (http://www.pharmaexpert.ru/passonline/) with entered SMILE code for each compound. The result was Pa (Probability Activity) and Pi (Probability inhibition). Pa must be more than 0.3, whereas Pi (Probability Inhibition) must not exceed Pa.
Ligands and Protein Interactions
Molecular interactions on molecular complexes produced by docking simulations were analyzed using PyMol 2.5 and Pyrx 9.5. By using PyMol 2.5 we sterilized protein samples before optimization by molecular docking. Protein stabilization was performed using Pymol by removing water atoms and introducing hydrogen atoms. Besides, Pyrx 9.5 program is used to lower the size of each chemical obtained, hence increasing its flexibility (8). A molecular docking simulation was used to evaluate the ability of the ligands from the plant Bryophyllum pinnatum active compounds to attach to the targeted protein domain. The goal of the molecular docking simulations was to determine the binding affinity and forms of the binding interaction. During the binding process, the binding energy created by the ligand was able to start a specific biological response at the indicated protein site. The strongest of the biological activity is the lowest binding score (9, 10).
Molecular Dynamic Simulation
We did the investigation used molecular dynamic simulations to validate the docking result. It was done using the CABS-FLEX 2.0 server (http://biocompt.chem.uw.edu.pl/CABSflex2/index) with the following parameters: Protein rigidity: 1.0, global calpha restraints weight: 1.0, global side-chain restraint weight: 1.0, number of cycles: 50, number of cycles between trajectory: 50, temperature range: 1.40, and a random number generator seed of 9056.
Table 2. Binding Affinity interaction.
| Ligand | Binding Affinity (Kcal/mol) | |
|---|---|---|
| AMPK | iNOS | |
| Bryophyllin A | -9.3 | -9.6 |
| Bryophyllin B | -9.9 | -9.9 |
| Bryotoxin A | -8.8 | -9.5 |
| Bryotoxin B | -8.7 | -9.2 |
| 6-[4-(2-piperidin-1-ylethoxy)phenyl]-3-pyridin-4-ylpyrazolo[1,5-a]pyrimidine | -9.3 | |
| 2-amino-6-(1,2-dihydroxy-propyl)-7,8-dihydro-6h-pteridin-4-one | -10.0 | |
Visualization Interaction
Molecular interactions type on molecular complexes produced by docking simulations were analyzed using the Discovery Studio software version 16. 1. 0. This allowed us to discover the chemical bonds that had been formed. In two-dimensional structures, a wide variety of chemical linkages were shown, such as hydrogen, hydrophobic, and electrostatic interaction (11, 12). PyMol structural selection and coloring software was used to represent the difficult three-dimensional structure of the mooring simulation results, which were obtained by simulation. Sticks, cartoons, ribbons, spheres, and surfaces served as the basis for the software’s interface (13).
4. RESULTS
All samples were aligned in the laboratory using the X-ray method, and then the protein had A chains with a maximum length of 400 mer, and a minimum length of 260 mer for each target protein was sequenced. AMPK structure had obtained with the code (3aqv) and iNOS (4nos). AMPK structure had a resolution of 2.08 Å, and iNOS had a resolution of 2.25 Å, as shown in Table 1. Meanwhile, in silico protein resolution refers to the clarity of the atomic distance between amino acid residues when presented in software; the greater the value, the more detailed the molecular visualization (14). AMPK is composed of a catalytic subunit alpha and two regulatory subunits, beta and gamma (15). In humans, there are two -subunit isoforms, 1 and 2, which share 90% and 61% of their amino acid sequences inside the catalytic domain and the remaining C-terminal half area, respectively. Compound C ({6-[4-(2-piperidin-1-yl-ethoxy)-phenyl]}-3-pyridin-4-yl-pyrazolo[1,5-a]pyrimidine) is a selective inhibitor of AMPK (16). While, The crystallized iNOS oxygenase domain (iNOSox) consists of the complete catalytic domain complexed with iron protoporphyrin IX (heme), BH4, and a single structural zinc atom (17).
Table 1. Target Protein Structural Information.
| Name | PDB ID | Visualization Method | Resolution | Atom count | Weight (kDa) | Chain | Sequence Length |
|---|---|---|---|---|---|---|---|
| AMPK | 3AQV | X-ray diffraction | 2.08 Å | 2301 | 31.97 | 1 | 276 |
| iNOS | 4NOS | X-ray diffraction | 2.25 Å | 15317 | 201.82 | 1 | 427 |
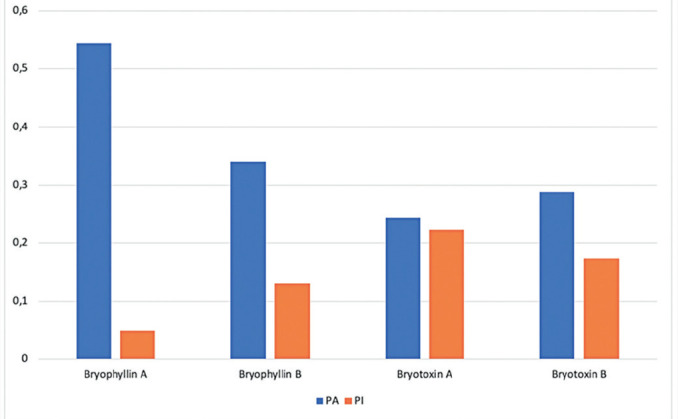
Analyzed results from the probability effect of Bryophyllum pinnatum as an anti-inflammatory agent shown in Figure 1. Bryophyllin A had the highest inflammatory effect, following with Bryophyllin B. The higher probability value shown, the greater possibility effect given (18). If Pa >0.7, the compound predicted has the same activity in the experiment. If 0.5<Pa>0.7, the drug is more likely to display the activity in an experiment, but the probability is lower. If Pa is less than 0.5, it is improbable that the drug will display has the same activity as the experiment (19). However, true experimental is needed to know the anti-inflammatory activity of each compound.
Figure 1. Anti-Inflammatory Properties active compounds of Bryophyllum pinnatum.
Bond strength was predicted using a grid box with the center coordinates and dimensions shown below. The coordinates were chosen based on the location of a novel ligand-binding pocket identified in the literature research. Coordinates and dimensions for AMPK (X: -4.767 Y: 47.767 Z: 8.615; X: 23 Y: 23, Z: 23), and iNOS (X: 0.694 Y: 96.553 Z: 19.798; X: 23, Y:23, Z:23)
5. DISCUSSION
In this study, Bryophyllin B formed the strongest interaction with AMPK and iNOS than other compounds. Bryophyllin B had a -9.9 Kcal/mol binding affinity with AMPK and iNOS. Bryophyllin B had a stronger binding affinity than native ligand, but not stronger than iNOS native ligand. This binding inhibited AMPK activity, which is involved in the invasion and accumulation of white blood cells (WBC), hence inhibiting arterial wall thickening and vascular remodeling. Besides, Bryophyllin B bonds with iNOS, which will impede vasoconstrictor activity, vascular lesions development, and inflammatory cell infiltration. The same binding to the control ligand will cause the same biological impact, so the form of contact link between the molecules created is very important to decide the resulting action potential (20, 21).
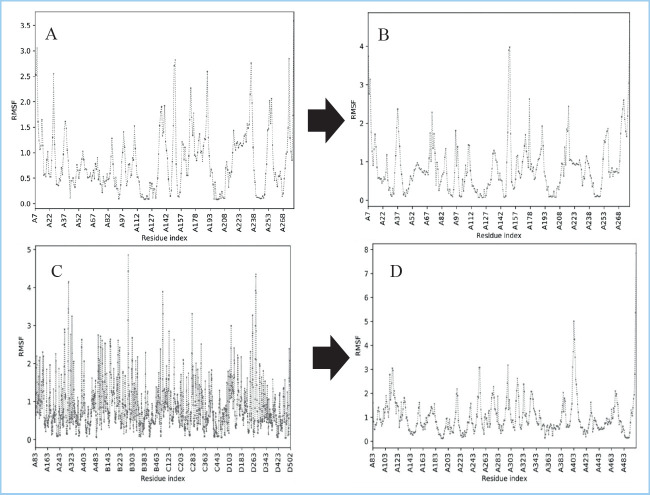
Comparing protein dynamics requires selecting one or more features that define protein dynamics and comparing their dissimilarity. Several characteristics and dissimilarity measurements have been described to capture protein dynamics conservation. The atomic root mean square fluctuations are the most basic (RMSF) (22). The results of the molecular dynamic analysis revealed a substantial change in RMSF in the absence of Bryophyllin B binding to AMPK and iNOS. The interaction between Bryophyllin B and AMPK exhibited an RMSF of 0-2 before binding (Figure 2A), but increased to 2-4 after binding (Figure 2B). This indicates that Bryophyllin B binding to the target protein domain is still stable and has biological activity. The interaction between Bryophyllin B and iNOS is more stable than the interaction of AMPK (Figure 2C, 2D). RMSF is caused by atoms interacting with peptide and protein residues at a specified distance. When the resultant distance is smaller than 4, the interaction complex is considered to be stable (23).
Figure 2. Root Mean Square Fluctuation (RMSF) Bryophyllin B interaction with AMPK without Bryophyllin B (A), Bryophyllin B-AMPK (B), iNOS without Bryophyllin B (C), and iNOS-AMPK (D).
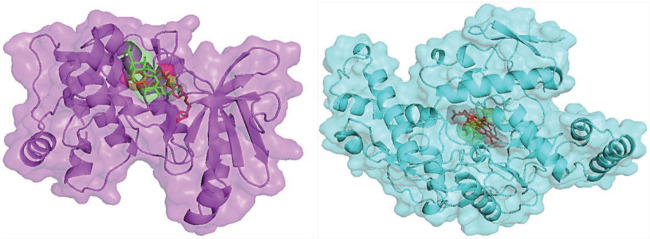
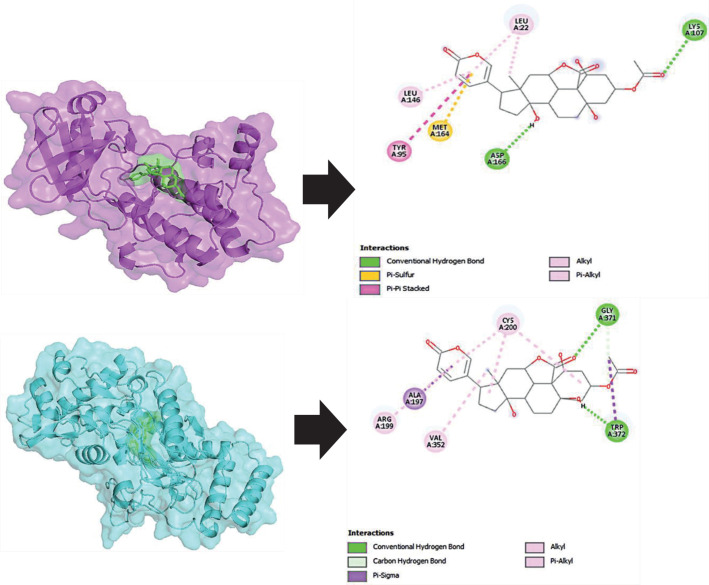
As seen in Figure 3, Bryophyllin B was attached to the same binding pocket site as the control, implying that it will have the same effect. The images of the ligand-protein molecular interaction in Figures 4 illustrate the binding mechanism of Bryophyllin B at the active site of AMPK and iNOS, respectively. Bryophyllin B has 2 hydrogen bonds and 4 hydrophobic bonds. Hydrogen interactions were Asp166 and Lys107. Besides, Bryophyllin B and iNOS interaction has 2 hydrogen bonds and 5 hydrophobic bonds. Bryophyllin B formed molecular interaction action at Gly 371, Trp372, Cys 200, Ala197, Val352, and Arg 199. The data above demonstrate that the Bryophyllin B compound in Bryophyllum Pinnatum had the potential to inhibit AMPK and iNOS. In our previously study, Bryophyllum pinnatum had anti-flamatory in Systemic Lupus Erythematosus (SLE) mice model (24). AMPK has been examined utilizing numerous substances such as Bicalin, Curcumin and Gingerol (5). However, compared with our findings, Bryophyllin B has more negative bond energy, meaning that it has a stronger bond than previous research. While, iNOS over expression in atheroslcerosis can be upregulated in macrophage tissues in response to inflammatory signals. Its promote pro-inflammatory activity on cell (25, 26). So, inhibition of iNOS is key role for the target therapy for atherosclerosis disease progression. However, additional study is required to assess the usefulness and toxicity of these compounds in the therapy of atherosclerosis.
Figure 3. Bryophyllin B interaction with AMPK (A) and iNOS (B) in the same binding pocket. Bryphyllin B (Green), Control (Red), AMPK (Magenta), iNOS (Cyan).
Figure 4. Binding interaction between Bryophyllin B with AMPK (Upper) and iNOS (Lower). Bryophyllin(Green), AMPK (Magenta), iNOS (Cyan).
6. CONCLUSION
In Sum, Bryophyllin B is a chemical found in Bryophyllum pinnatum that heve potential as therapy for atherosclerosis by suppressing AMPK and iNOS activity. Additional study is required to evaluate the effectiveness and toxicity of Bryiphyllin B as an atherosclerosis disease therapy.
Author’s contribution:
All authors were involved in all steps of preparationthis article. Final proofreading was made by the first author.
Conflict of Interest:
There are no conflicts of interest.
Financial support and sponsorship:
Nil.
REFERENCES
- 1.Sakakura K, Nakano M, Otsuka F, Ladich E, Kolodgie FD, Virmani R. Corrigendum to ‘Pathophysiology of Atherosclerosis Plaque Progression’ [Heart, Lung and Circulation (2013) 399–411] Heart Lung Circ. 2014 Apr;23(4):387. doi: 10.1016/j.hlc.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 2.Herrington W, Lacey B, Sherliker P, Armitage J, Lewington S. Epidemiology of Atherosclerosis and the Potential to Reduce the Global Burden of Atherothrombotic Disease. Circ Res. 2016 Feb 19;118(4):535–546. doi: 10.1161/CIRCRESAHA.115.307611. [DOI] [PubMed] [Google Scholar]
- 3.Libby P. Inflammation in Atherosclerosis. Arterioscler Thromb Vasc Biol. 2012 Sep;32(9):2045–2051. doi: 10.1161/ATVBAHA.108.179705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saini V, Bhatnagar MK, Bhattacharjee J. Endothelial nitric oxide synthase Glu298Asp (G894T) gene polymorphism in coronary artery disease patients with type 2 diabetes mellitus. Diabetes Metab Syndr Clin Res Rev. 2012 Apr;6(2):106–109. doi: 10.1016/j.dsx.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 5.Zuo H, Li Y, Hao P, Liu J-H. Drug design targeting the AMPK signalling pathway with herbal medicines for atherosclerosis therapy. [2021 Dec 17];LaboratoriumsMedizin [Internet] 2017 Apr 25;41(2) Available from: https://www.degruyter.com/document/doi/10.1515/labmed-2016-0086/html. [Google Scholar]
- 6.Andrade AWL, Guerra GCB, de Souza Araújo DF, de Araújo Júnior RF, de Araújo AA, de Carvalho TG, et al. Anti-Inflammatory and Chemopreventive Effects of Bryophyllum pinnatum (Lamarck) Leaf Extract in Experimental Colitis Models in Rodents. Front Pharmacol. 2020 Jul 29;11:998. doi: 10.3389/fphar.2020.00998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nurdiana , Dantara TWI, Syaban MFR, Mustafa SA, Ikhsani H, Syafitri FE, et al. Effect of Bryophyllum pinnatum Leaves Ethanol Extract in TNF-α and TGF-β as Candidate Therapy of SLE in Pristane-Induced SLE BALB/c Mice Model. Res J Pharm Technol. 2021 Feb 16;14(2):1069–1072. [Google Scholar]
- 8.Syaban MFR, Erwan NE, Syamsuddin MRR, Zahra FA, Sabila FL. Molecular Docking Approach of Viscosin as Antibacterial for Methicillin-resistant Staphylococcus Aureus Via β-Lactamase Inhibitor Mechanism. Clin Res J Intern Med. 2021 Nov 8;2(2):187–192. [Google Scholar]
- 9.Yueniwati Y, Syaban MFR, Faratisha IFD, Yunita KC, Putra GFA, Kurniawan DB, et al. Molecular Docking Approach of Natural Compound from Herbal Medicine in Java against Severe Acute Respiratory Syndrome Coronavirus-2 Receptor. 2021;6 [Google Scholar]
- 10.Nugraha RYB, Faratisha IFD, Mardhiyyah K, Ariel DG, Putri FF, Nafisatuzzamrudah , et al. Antimalarial Properties of Isoquinoline Derivative from Streptomyces hygroscopicus subsp. Hygroscopicus: An In Silico Approach. BioMed Res Int. 2020 Jan 9;2020:1–15. doi: 10.1155/2020/6135696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yueniwati Y, Syaban MFR, Erwan NE, Putra GFA, Krisnayana AD. Molecular Docking Analysis of Ficus religiosa Active Compound with Anti-Inflammatory Activity by Targeting Tumour Necrosis Factor Alpha and Vascular Endothelial Growth Factor Receptor in Diabetic Wound Healing. Open Access Maced J Med Sci. 2021 Nov 16;9(A):1031–1036. [Google Scholar]
- 12.Nugraha A, Rahmadhani D, Puspitaningrum M, Rizqianti Y, Kharisma V, Ernawati D. Molecular docking of anthocyanins and ternatin in Clitoria ternatea as coronavirus disease oral manifestation therapy. J Adv Pharm Technol Res. 2021;12(4):362. doi: 10.4103/japtr.japtr_126_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kharisma VD, Widyananda MH, Ansori ANM, Nege AS, Naw SW, Nugraha AP. Tea catechin as antiviral agent via apoptosis agonist and triple inhibitor mechanism against HIV-1 infection: A bioinformatics approach. J Pharm Pharmacogn Res. 2021;9(4):435–445. [Google Scholar]
- 14.Prahasanti C, Nugraha AP, Putri TPS, Ramadhani NF, Narmada IB. A bioinformatic approach of hydroxyapatite and polymethylmethacrylate composite exploration as dental implant biomaterial. 10 [Google Scholar]
- 15.Handa N, Takagi T, Saijo S, Kishishita S, Takaya D, Toyama M, et al. Structural basis for compound C inhibition of the human AMP-activated protein kinase α2 subunit kinase domain. Acta Crystallogr D Biol Crystallogr. 2011 May 1;67(5):480–487. doi: 10.1107/S0907444911010201. [DOI] [PubMed] [Google Scholar]
- 16.Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, et al. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest. 2001 Oct 15;108(8):1167–1174. doi: 10.1172/JCI13505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fischmann TO, Hruza A, Niu XD, Fossetta JD, Lunn CA, Dolphin E, et al. Structural characterization of nitric oxide synthase isoforms reveals striking active-site conservation. Nat Struct Biol. 1999;6(3):10. doi: 10.1038/6675. [DOI] [PubMed] [Google Scholar]
- 18.Lagunin A, Filimonov D, Poroikov V. Multi-Targeted Natural Products Evaluation Based on Biological Activity Prediction with PASS. Curr Pharm Des. 2010 May 1;16(15):1703–1717. doi: 10.2174/138161210791164063. [DOI] [PubMed] [Google Scholar]
- 19.Lagunin A, Stepanchikova A, Filimonov D, Poroikov V. PASS: prediction of activity spectra for biologically active substances. Bioinformatics. 2000 Aug 1;16(8):747–748. doi: 10.1093/bioinformatics/16.8.747. [DOI] [PubMed] [Google Scholar]
- 20.Syaban MFR, Rachman HA, Arrahman AD, Hudayana N, Purna J, Pratama FA. Allium sativum as Antimalaria Agent via Falciapin Protease-2 Inhibitor Mechanism: Molecular Docking Perspective. 2021;02(1):6. [Google Scholar]
- 21.Dhea Kharisma V, Nur Muhammad Ansor rif, Hermawan Widyananda M, Lusia Utami S, PateraNugraha A. Molecural Simulation: The Potency of Conserved Region on E6 HPV-16 as a Binding Target of Black Tea Compounds Against Cervical Cancer. 9 [Google Scholar]
- 22.Fuglebakk E, Echave J, Reuter N. Measuring and comparing structural fluctuation patterns in large protein datasets. Bioinformatics. 2012 Oct 1;28(19):2431–2440. doi: 10.1093/bioinformatics/bts445. [DOI] [PubMed] [Google Scholar]
- 23.Rantam FA, Kharisma VD, Sumartono C, Nugraha J, Wijaya AY, Susilowati H, et al. Molecular docking and dynamic simulation of conserved B cell epitope of SARS-CoV-2 glycoprotein Indonesian isolates: an immunoinformatic approach. F1000Research. 2021 Aug 16;10 doi: 10.12688/f1000research.54258.1. Chem Inf Sci-813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nurdiana , Dantara TWI, Syaban MFR, Mustafa SA, Ikhsani H, Syafitri FE, et al. Efficacy and side effects studies of Bryophyllum pinnatum leaves ethanol extract in pristane-induced SLE BALB/c mice model. AIP Conf Proc. 2019 Jun 4;2108(1):020016. [Google Scholar]
- 25.Aktan F. iNOS-mediated nitric oxide production and its regulation. Life Sci. 2004 Jun 25;75(6):639–653. doi: 10.1016/j.lfs.2003.10.042. [DOI] [PubMed] [Google Scholar]
- 26.Zhong HJ, Liu LJ, Chong CM, Lu L, Wang M, Chan DSH, et al. Discovery of a Natural Product-Like iNOS Inhibitor by Molecular Docking with Potential Neuroprotective Effects In Vivo. PLOS ONE. 2014 Apr 1;9(4):e92905. doi: 10.1371/journal.pone.0092905. [DOI] [PMC free article] [PubMed] [Google Scholar]