Abstract
Obesity affects more than 650 million adults worldwide and is a major risk factor for serious comorbidities. The prevalence of obesity has tripled in the last forty years and continues to rise. Eosinophils have recently been implicated in providing a protective role against obesity. Decreasing eosinophil numbers exacerbates weight gain and contributes to glucose intolerance in high fat diet-induced obese animals, while increasing eosinophil numbers prevents high fat diet-induced adipose tissue and body weight gain. Human studies, however, do not support a protective role for eosinophils in obesity. Recent animal studies have also reported conflicting results. Considering these contradictory findings, the relationship between eosinophils and obesity may not be unidirectional. In this mini review, we summarize a recent debate regarding the role of adipose tissue eosinophils in metabolic disorders, and discuss local and systemic effects of eosinophils in obesity. Given that adipose eosinophils play a role in tissue homeostasis, more research is needed to understand the primary function of adipose tissue eosinophils in their microenvironment. Therapeutic interventions that target eosinophils in adipose tissue may have the potential to reduce inflammation and body fat, while improving metabolic dysfunction in obese patients.
Graphical Abstract
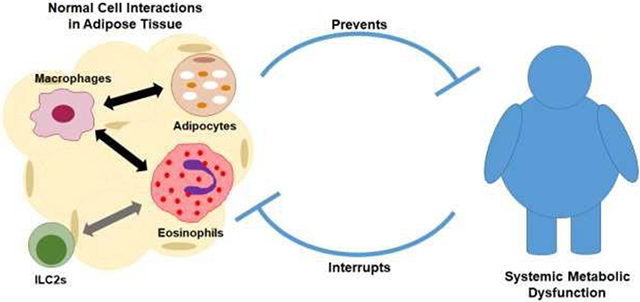
Introduction
Obesity affects more than 650 million adults worldwide, which accounts for 13% of the world’s adult population (1). The prevalence has tripled in the last forty years and continues to rise among both adults and children (2). Obesity is a major public health concern because it is an important risk factor for serious comorbidities, including asthma (3–7). While exercise and dietary changes are the cornerstones of weight loss, these interventions are not effective or achievable for many obese people. Thus, it is necessary to identify effective interventions to combat this challenging disease.
Eosinophils, known to act in allergic inflammation and in host defense against helminth infections, have recently been implicated as major players in adipose tissue homeostasis. In 2011, Wu et al. (8) identified a protective role for eosinophils in diet-induced obesity. Since then, several animal studies have confirmed that adipose eosinophils are involved in metabolic homeostasis via interactions with adipocytes and/or adipose leukocytes (9–12), suggesting a novel treatment target for obese patients. A recent study reported, however, that increasing eosinophils in obese mice to levels seen in normal lean mice failed to restore metabolic parameters (13). Another study found that eosinophil-deficient mice fed a high-fat diet instead of becoming more obese as seen in Wu’s study, these mice had reduced body fat mass, impaired enlargement of adipocytes, and decreased glucose tolerance compared to wild type mice (14). Considering these conflicting findings, the relationship between obesity and eosinophils might not be unidirectional. Discussion of different results and perspectives took place during the 2019 International Eosinophil Society (IES) meeting. Although not all data presented from this meeting is published yet, the evident presented raises important questions about the role of eosinophils in adipose tissue and metabolism. In this mini review, we summarize the recent debate over the role of adipose tissue eosinophils in metabolic disorders, and discuss local and systemic interactions between eosinophil function and obesity.
Evidence that eosinophils reduce obesity
Wu et al. were the first to report that eosinophils may protect against obesity. The authors found that when transgenic C57BL/6 mice lacking eosinophils (ΔdblGATA mice) were fed a high fat diet, they developed more body fat, impaired glucose tolerance, and decreased insulin sensitivity compared to wild type mice (8). In contrast, increasing eosinophils prevents weight gain. When wild type mice on a high fat diet were infected with helminths over 12 weeks to induce eosinophilia, mice gained less body fat, and had improved insulin sensitivity and glucose tolerance vs non-helminth infected mice. The authors suggested that eosinophils play a role in metabolic homeostasis through maintenance of adipose alternatively activated macrophages (8).
The protective role of eosinophils in obesity is supported by two additional studies reporting that eosinophils were reduced in high fat diet-induced obese mice. The decrease in eosinophils was associated with increased weight gain and glucose intolerance (9, 10). Conversely, increasing eosinophils appears to decrease body fat. In other studies, both chronic helminth infection and soluble helminth egg antigens were used to increase adipose tissue eosinophils in high fat diet-induced obese mice. Treated mice had less weight gain, less fat mass gain, and decreased adipocyte size as well as improved peripheral glucose uptake and increased white adipose tissue insulin sensitivity (11, 15). In another study, honeybee pollen extract was used to increase eosinophils in mesenteric and epididymal adipose tissue of ob/ob mice, which develop obesity and insulin resistance due to a mutation in the leptin gene. The increased eosinophils restored glucose tolerance and insulin sensitivity in pollen treated mice(12). Similarly, our group recently found that increasing circulating eosinophils through overexpression of IL-5 prevented high fat diet-induced weight and fat gain (16). All of these data suggest a role for eosinophils in maintaining metabolic homeostasis.
All of the studies discussed above used whole-body knockout mice or mice with elevated blood eosinophils. Eosinophil character and function, however, is tissue specific (17). Ting et al. built on this concept by using an adipose-specific transgenic mouse model, as reported at the 2019 International Eosinophil Society (IES) meeting (18). Mice were generated that overexpress human eotaxin2, an eosinophil specific chemokine, under the control of a fat specific promoter. This genetic manipulation selectively increases eosinophil migration into adipose tissue. When fed a high fat diet, these eotaxin2 mice gained less weight and less adipose tissue and showed increased glucose tolerance compared to wild type mice on a high fat diet. Furthermore, their adipocytes were smaller, and the expression of genes involved in fat oxidation were upregulated compared to wild type mice (18). This study demonstrated that tissue-specific eosinophils promote metabolic homeostasis.
A potential mechanism for the protective effects of eosinophils was suggested by the work of Quinlan et al. Mice with a deletion in the gene encoding the transcription factor Kruppel-like Factor 3 (KLF3), were described as having three times the number of eosinophils in their adipose tissue compared to wild type mice. They were additionally protected from diet-induced obesity. Mice lacking KLF3 had increased capacity for thermogenesis (19) and their eosinophils expressed genes for proteins known to enhance thermogenesis (20). These data suggest that eosinophil mediated thermogenesis is a potential protective mechanism against diet-induced obesity.
Evidence that eosinophils do not reduce obesity
In contrast to mice, human studies have not supported a protective role for eosinophils in obesity. Eosinophilia coexists with obesity, as several small epidemiological studies have demonstrated a positive correlation between blood eosinophil counts and body mass index (BMI) or metabolic syndrome (21–23). Sunadome et al. found that blood eosinophils are correlated with BMI up to a plateau of 40 kg/m2 (23). Likewise, Moussa et al. showed that elevated eosinophils in subcutaneous adipose tissue were associated with metabolic syndrome (24). Kuruvilla et al. found in a small study, over six months, that anti-IL-5 therapy was associated with a mild but significant decrease in BMI in patients with severe asthma (25). However, this particular study was not randomized or placebo controlled, and lacked other measurements related to obesity including body fat and glucose tolerance (25). Similar results were reported by Klion et al during the 2019 International Eosinophil Society (IES) meeting. Two years of treatment with Benralizumab (anti-IL-5 receptor antibody) resulted in decreased body weight along with completely depleted peripheral blood eosinophils (26). Thus, data on a role for eosinophils, in metabolic diseases in humans with asthma disagrees with the initial studies in obese, non-asthmatic mice.
Recent data from animal studies have also challenged the hypothesis that eosinophils prevent obesity. Because elevated eosinophils are associated with reduced body weight and improved metabolic parameters, Bolus et al. artificially restored eosinophils to normal physiological levels (not to above normal levels) in obese C57BL/6 mice, via administration of recombinant IL-5. Intraperitoneal administration of IL-5 restored eosinophils in adipose tissue to normal, but did not prevent obesity or obesity-related metabolic dysfunction (13). Furthermore, acute treatment with helminth antigen, increased eosinophils, but did not protect against diet-induced obesity (27). BALB/c mice also gained weight on a high fat diet and developed metabolic dysfunction. Eosinophil depletion using ΔdblGATA BALB/c mice did not worsen obesity (17). Separate from obesity, eosinophil depletion did worsen glucose tolerance, suggesting a protective role of eosinophils in metabolic dysfunction (14). This paper suggests there may be an unexplained disconnect between eosinophils, obesity and metabolic dysfunction.
Reconciling opposing data
There is an active debate over the role of eosinophils in diet-induced obesity in the scientific literature. In all experimental protocols, feeding mice a high fat diet-induced obesity (8, 11, 13, 14, 16, 18–20, 27) and decreased eosinophils in adipose tissue. Increasing IL-5 using transgenic animals, globally increased eosinophils above normal in all tissues and decreased obesity and prevented metabolic dysfunction. In contrast, administering IL-5 i.p., restored eosinophils in abdominal adipose tissue to normal physiologic levels but did not prevent obesity. Similarly, increasing eosinophils using helminth infection increases eosinophils globally, and above normal. However, one paper using this method over 12 weeks, demonstrated a protective effect of eosinophils, while the other, testing over 10 days, did not (11, 27). These data suggest that either eosinophil activity or duration of activity, not simply eosinophil number, protects against metabolic dysfunction and obesity. An additional complication is that different strains of mice, respond to high fat diet differently. Both C57BL/6 and BALB/c develop obesity, but there are strain-dependent variations in the immunometabolic response to a high fat diet (28) that may alter the role of eosinophils in metabolic regulation of obesity. This may explain why depleting eosinophils in BALB/c ΔdblGATA mice did not worsen obesity (14), while it did in the C57BL/6 ΔdblGATA mice (8). Of note, all current transgenic animal models of eosinophilic disease have elevated or diminished eosinophils from pre-birth, making it difficult to distinguish the role of eosinophils on obesity from the effects of genetic manipulation on metabolic homeostasis (29). The development and use of inducible eosinophil knockout mice may help answer these questions.
In humans, reducing or depleting eosinophils in asthma reduced body weight in thin and overweight asthma subjects (25, 26). Although these studies were not designed to test the role of eosinophils, this data does not support a protective role for eosinophils in obesity in humans. However, severe asthmatic patients are often on corticosteroids and their asthma may limit their ability to exercise, both of which may lead to weight gain, independent of eosinophils. The decrease in body weight on anti-IL-5 or anti-IL-5 receptor treatment may, therefore, be due to a reduction in steroid use or an increase in physical activity. Additionally, eosinophils were only measured in blood, not in body fat (25, 26). This is important because, animal studies have demonstrated that adipose eosinophils, rather than circulating eosinophils, promote leanness (9). Thus, future studies should investigate the effects of adipose tissue eosinophils, in addition to circulating eosinophils, on weight gain and metabolic dysfunction in obesity.
The systemic and local effects of adipose eosinophils in obesity
Eosinophils are normally present in adipose tissue, adjacent to adipocytes and other adipose resident leukocytes. These eosinophils are the primary source of IL-4 in adipose tissue (8). Additionally, it has been suggested that eosinophils may mediate glucose homeostasis and energy expenditure (8–10, 14).
Adipose tissue can be divided into two types, visceral and subcutaneous; visceral tissue is the more metabolically active of the two. Eosinophils are reduced in visceral tissue of high fat diet-induced obese animals compared to lean animals (8–10, 27). Acute high fat diet-induced a rapid decline in chemotactic signals for eosinophils, a rapid decline in eosinophils in visceral adipose tissue, followed later by a decline in eosinophils in subcutaneous adipose tissue (27). Notably, Bolus et al. showed that the decrease in adipose tissue eosinophils with obesity can be reversed after dietary intervention and weight loss (30).
The systemic effects of eosinophils on glucose homeostasis and energy expenditure may be mediated by local cell-cell interactions (Figure 1). Eosinophils are capable of influencing adipocytes, which are normally critical regulators of energy balance and nutrient homeostasis. White adipose tissue (WAT) and brown adipose tissue (BAT) are comprised of white fat cells and brown fat cells, respectively. White fat cells serve as the principal site for energy storage and release, producing and secreting numerous cytokines and hormones (31). Brown fat cells (also called thermogenic adipocytes) are the other major class of adipocytes, catabolizing stored lipids and producing heat. Brown fat cells are characterized by their multinodular lipid droplet appearance, high mitochondrial content, and expression of uncoupling protein 1 (Ucp1) (32, 33). Under physiologic conditions, adipose tissue eosinophils can induce white adipocytes to become thermogenic adipocytes, a process known as browning or beiging, indirectly through macrophages (34). These “beige” adipocytes, resulting from eosinophil signaling, increase energy expenditure and thermogenesis, thereby reducing adiposity (Figure 1).
Figure 1. Systemic effects of eosinophils on obesity may be mediated by local cell-cell interactions.
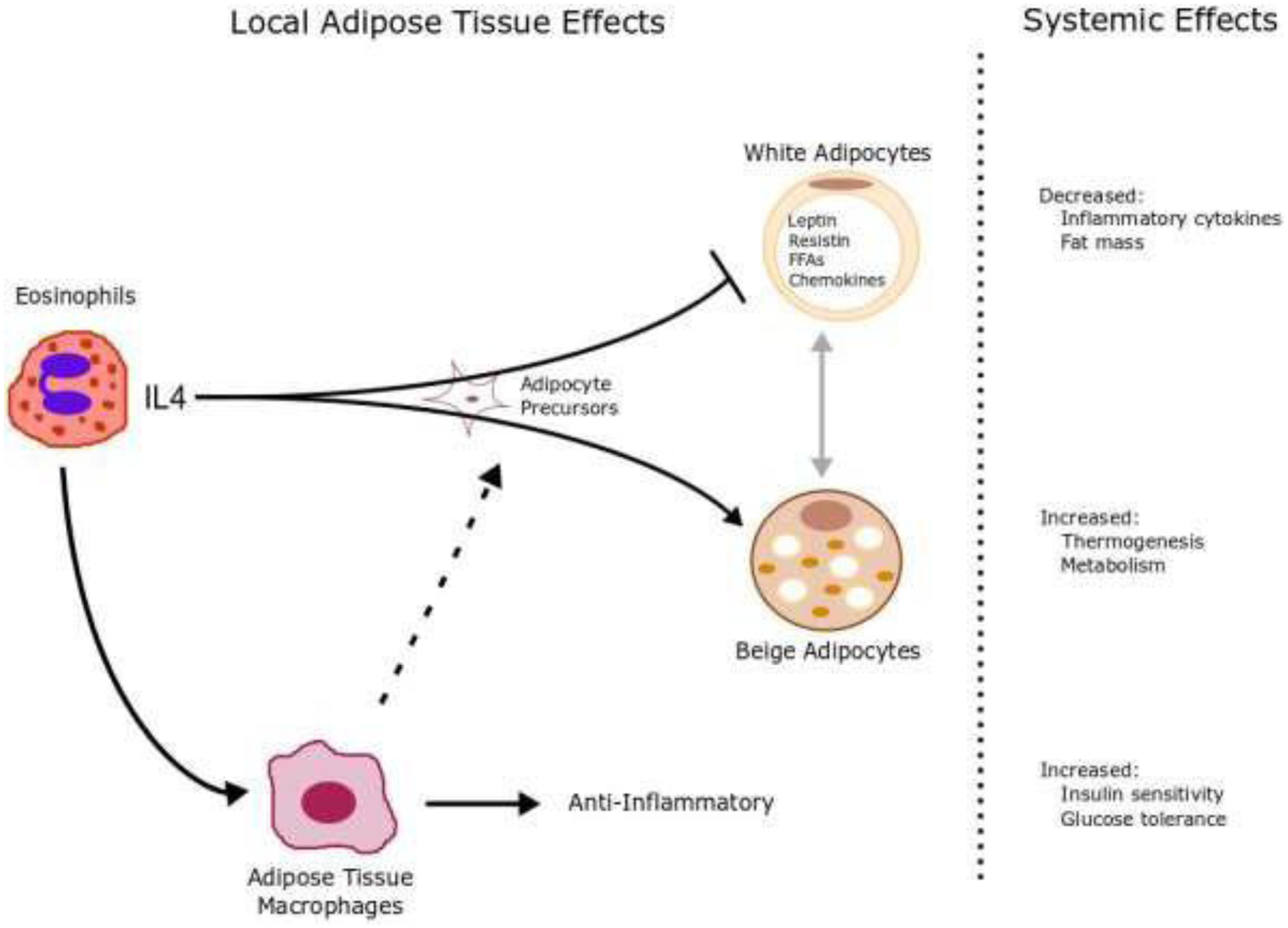
Eosinophils are a major source of locally produced IL-4 in white adipose tissue. Eosinophils induce adipocyte precursor cells to develop into thermogenic, UCP1-expressing “beige” adipocytes. Systemically, beige adipocytes increase thermogenesis and metabolism. Eosinophils simultaneously inhibit expansion of white adipocytes, which consequently decreases inflammatory cytokines and fat mass. The expression of IL-4 also causes alternative activation of adipose tissue macrophages promoting anti-inflammatory Th2 cytokines and insulin sensitivity and inhibits classical activation of macrophages and inflammatory Th1 cytokines and insulin resistance.
Eosinophils interact with many leukocytes in adipose tissue. These interactions determine their function. Cells such as group 2 innate lymphoid cells (ILC2s) communicate with eosinophils and are important in maintaining metabolic homeostasis. IL-33 signaling drives ILC2 cells to secrete IL-5 and IL-13, which promote the accumulation of eosinophils and macrophages in adipose tissue. (35, 36) Moreover, eosinophils are the major source of IL-4 in adipose tissue. Eosinophils use IL-4 to communicate with macrophages, (37) which triggers expression of uncoupling protein 1 in white adipose tissue, inducing beiging, and increasing thermogenesis (Figure 1). This is potentially a mechanism of eosinophil induced protection against obesity. Macrophages are another important resident cell type in visceral adipose tissue. Activated macrophages in obese adipose tissue are derived from multiple, distinct cell obese-associated populations recently identified via transcriptome and functional analyses (38, 39). In obese adipose tissue, a distinct class of metabolically activated macrophages contribute to metabolic dysfunction and insulin resistance (38, 40, 41). Other subsets of adipose tissue macrophages, including those that express the lipid receptor Trem2, have been identified as playing a protective role during obesity and regulation of adipocytes (42). Overall, these cell-cell interactions determine the role adipose tissue eosinophils have in maintaining metabolic homeostasis.
The role of eosinophils in obese patients with asthma
Eosinophils are a hallmark of allergic asthma. However, the role of eosinophils in obesity-related asthma is unclear. Some obese patients with asthma have severe asthma symptoms with low sputum eosinophils while others have high eosinophils (43, 44). Thus, the obese asthma phenotype can be divided into at least two classes: Th2 high obese patients, characterized by pre-existing allergic asthma, and high eosinophils that is additionally complicated by obesity vs Th2 low obese patients with asthma who develop asthma symptoms as a consequence of obesity and who have normal eosinophils. Farahi et al. reported that more eosinophils were recruited to the lungs of obese patients with asthma compared with non-obese patients with asthma (45). Furthermore, eosinophils isolated from asthmatic obese patients had increased chemotaxis and adhesion activity after being stimulated by eotaxin or platelet activating factor, compared to eosinophils isolated from asthmatic non-obese patients or non-asthmatic non-obese individuals (46). In animals, airway hyperresponsiveness induced by antigen challenge is greater in obese than in non-obese mice (47, 48). These data, combined with studies in humans that eosinophil recruitment to lungs is greater with obesity, suggest that obesity can promote eosinophil recruitment to the lung, and once recruited, eosinophils from obese patients may be more active.
The role of eosinophils in obesity induced asthma may be complex. It is well known that eosinophils are a source of pro-inflammatory cytokines and preformed proteins including TNF- α (49) and eosinophil major basic protein (50) which have profound effects on neuronal M2 muscarinic receptors that normally function to inhibit acetylcholine release from airway parasympathetic nerves (51–55). Loss of M2 function, either through down regulation (53, 54) or acute blockade with a selective antagonist (50, 55) increases acetylcholine release and causes airway hyperresponsiveness (51, 53, 54). Therefore, increased eosinophil recruitment to the lungs, as seen with obesity (45), may complicate pre-existing allergic asthma by enhancing eosinophil recruitment to airways and nerves. For Th2 low obese asthmatic patients, obesity may cause airway hyperresponsiveness through an eosinophil-independent mechanism (2, 56, 57). Weight loss has been shown to be an effective treatment for Th2 low obese asthmatic patients (58). Therapeutic targets will need to consider both the subtype of asthma, and also that eosinophils exist in multiple locations including adipose tissue.
Summary, Considerations, and Future Directions
Obesity, which is characterized by poor metabolic control, insulin resistance, inflammation, and impaired immune function, is an important risk factor for many comorbidities, including asthma. Eosinophils have traditionally been associated with chronic inflammatory diseases including in the lung, skin and gut, but their role in obesity has been underappreciated. Recent studies of adipose tissue show that eosinophils play a key role in metabolic homeostasis. Data from animal studies show that adipose tissue eosinophils are inversely correlated with body weight and body fat. Eosinophil presence and activation state within adipose tissue is related to prevention of obesity, but the exact relationship is unclear. Future studies should focus on cell-cell interactions between eosinophils and adipocytes and other adipose tissue resident leukocytes to identify the role of adipose eosinophils in obesity and asthma, to identify therapeutic strategies that may be relevant in either asthma, or obesity, or obesity-related asthma.
Table 1.
Comparing obesity and metabolic dysfunction in altered models of eosinophils
| Strain | Animal model | Eosinophils* | Obesity | Metabolic dysfunction | Ref |
|---|---|---|---|---|---|
| BALB/c | ΔdblGATA | Depleted | Prevents | Worsens | 17 |
| C57BL/6 | ΔdblGATA | Depleted | Worsens | Worsens | 11 |
| Helminth (acute) | Increased above normal | No change | No change | 30 | |
| Helminth (chronic) | Increased above normal | Prevents | Prevents | 14 | |
| Helminth antigen | Increased above normal | Prevents | Prevents | 18 | |
| Honeybee extract | Increased above normal | Prevents | Prevents | 15 | |
| IL-5 transgenic | Increased above normal | Prevents | Prevents | 19 | |
| IL-5 i.p. | Normal | No change | No change | 16 | |
| Eotaxin2 transgenic | Increased adipose eosinophils above normal | Prevents | Prevents | 12 |
Eosinophils are all circulating except in Eoxtaxin2 transgenic as noted.
Acknowledgments:
Thank you to Dr. David Jacoby and Dr. Brenda Marsh for proofreading this article.
GRANTS:
R01HL131525, R01 HL113023, R01 AR061567, R01 HL124165
Footnotes
Conflict of Interest Disclosure: All authors have no conflict of interest.
References
- 1.Organization WH. Obesity and Overweight. Accessed Dec 22, 2019.
- 2.Carpaij OA, and van den Berge M. The asthma-obesity relationship: underlying mechanisms and treatment implications. Curr Opin Pulm Med. 2018;24(1):42–9. [DOI] [PubMed] [Google Scholar]
- 3.Mosen DM, Schatz M, Magid DJ, and Camargo CA Jr., The relationship between obesity and asthma severity and control in adults. J Allergy Clin Immunol. 2008;122(3):507–11 e6. [DOI] [PubMed] [Google Scholar]
- 4.Ford ES. The epidemiology of obesity and asthma. J Allergy Clin Immunol. 2005;115(5):897–909; quiz 10. [DOI] [PubMed] [Google Scholar]
- 5.Lessard A, Turcotte H, Cormier Y, and Boulet LP. Obesity and asthma: a specific phenotype? Chest. 2008;134(2):317–23. [DOI] [PubMed] [Google Scholar]
- 6.Akerman MJ, Calacanis CM, and Madsen MK. Relationship between asthma severity and obesity. J Asthma. 2004;41(5):521–6. [DOI] [PubMed] [Google Scholar]
- 7.Taylor B, Mannino D, Brown C, Crocker D, Twum-Baah N, and Holguin F. Body mass index and asthma severity in the National Asthma Survey. Thorax. 2008;63(1):14–20. [DOI] [PubMed] [Google Scholar]
- 8.Wu D, Molofsky AB, Liang HE, Ricardo-Gonzalez RR, Jouihan HA, Bando JK, et al. Eosinophils sustain adipose alternatively activated macrophages associated with glucose homeostasis. Science. 2011;332(6026):243–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li T, Lesuer W, Singh A, Hernandez JD, Zhang X, Jelinek D, et al. 2006-P: The Role of Adipose Tissue-Resident Eosinophils in Adipocyte Metabolism and Whole-Body Energy Homeostasis. Diabetes. 2019;68(Supplement 1):2006-P. [Google Scholar]
- 10.Hams E, Locksley RM, McKenzie ANJ, and Fallon PG. Cutting Edge: IL-25 Elicits Innate Lymphoid Type 2 and Type II NKT Cells That Regulate Obesity in Mice. The Journal of Immunology. 2013;191(11):5349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hussaarts L, García-Tardón N, van Beek L, Heemskerk MM, Haeberlein S, van der Zon GC, et al. Chronic helminth infection and helminth-derived egg antigens promote adipose tissue M2 macrophages and improve insulin sensitivity in obese mice. The FASEB Journal. 2015;29(7):3027–39. [DOI] [PubMed] [Google Scholar]
- 12.Kitamura H, Naoe Y, Kimura S, Miyamoto T, Okamoto S, Toda C, et al. Beneficial effects of Brazilian propolis on type 2 diabetes in ob/ob mice: Possible involvement of immune cells in mesenteric adipose tissue. Adipocyte. 2013;2(4):227–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bolus WR, Peterson KR, Hubler MJ, Kennedy AJ, Gruen ML, and Hasty AH. Elevating adipose eosinophils in obese mice to physiologically normal levels does not rescue metabolic impairments. Mol Metab. 2018;8:86–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee EH, Itan M, Jang J, Gu HJ, Rozenberg P, Mingler MK, et al. Eosinophils support adipocyte maturation and promote glucose tolerance in obesity. Sci Rep. 2018;8(1):9894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hams E, Bermingham R, Wurlod FA, Hogan AE, O’Shea D, Preston RJ, et al. The helminth T2 RNase ω1 promotes metabolic homeostasis in an IL-33– and group 2 innate lymphoid cell–dependent mechanism. The FASEB Journal. 2016;30(2):824–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Calco GN, Proskocil BJ, Jacoby DB, Fryer AD, and Nie Z International Eosinophil Society Meeting. Portland, OR; 2019. [Google Scholar]
- 17.Weller PF, and Spencer LA. Functions of tissue-resident eosinophils. Nature reviews Immunology. 2017;17(12):746–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li T, Hernandez J, LeSuer W, Tianna S, Zhang X, Jacobsen E, De Filippis E. International Eosinophil Society Symposium. 2019.
- 19.Knights AJ, Vohralik EJ, Houweling PJ, Hoehn KL, Crossley M, Quinlan KG. International Eosinophil Society Symposium. Portland, OR; 2019. [Google Scholar]
- 20.Vohralik EJ, Knights AJ, Houweling PJ, Hoehn KL, Crossley M, Quinlan KG. International Eosinophil Society Symposium. Portland, OR; 2019. [Google Scholar]
- 21.Shim WS, Kim HJ, Kang ES, Ahn CW, Lim SK, Lee HC, et al. The association of total and differential white blood cell count with metabolic syndrome in type 2 diabetic patients. Diabetes Res Clin Pract. 2006;73(3):284–91. [DOI] [PubMed] [Google Scholar]
- 22.Babio N, Ibarrola-Jurado N, Bullo M, Martinez-Gonzalez MA, Warnberg J, Salaverria I, et al. White blood cell counts as risk markers of developing metabolic syndrome and its components in the PREDIMED study. PLoS One. 2013;8(3):e58354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sunadome H, Matsumoto H, Izuhara Y, Nagasaki T, Kanemitsu Y, Ishiyama Y, et al. Correlation between eosinophil count, its genetic background and body mass index: The Nagahama Study. Allergol Int. 2019. [DOI] [PubMed] [Google Scholar]
- 24.Moussa K, Gurung P, Adams-Huet B, Devaraj S, and Jialal I. Increased eosinophils in adipose tissue of metabolic syndrome. Journal of Diabetes and its Complications. 2019. [DOI] [PubMed] [Google Scholar]
- 25.Kuruvilla M, Patrawala M, Levy JM, Shih J, and Lee FE. Association of antieosinophil therapy with decreased body mass index in patients with severe asthma: A preliminary retrospective analysis. Ann Allergy Asthma Immunol. 2019;122(6):649–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klion A International Eosinophil Society Meeting. Portland, OR; 2019. [Google Scholar]
- 27.van den Berg SM, van Dam AD, Kusters PJH, Beckers L, den Toom M, van der Velden S, et al. Helminth antigens counteract a rapid high-fat diet-induced decrease in adipose tissue eosinophils. J Mol Endocrinol. 2017;59(3):245–55. [DOI] [PubMed] [Google Scholar]
- 28.Jovicic N, Jeftic I, Jovanovic I, Radosavljevic G, Arsenijevic N, Lukic ML, et al. Differential Immunometabolic Phenotype in Th1 and Th2 Dominant Mouse Strains in Response to High-Fat 347 Feeding. PLoS One. 2015;10(7):e0134089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bolus WR, and Hasty AH. Contributions of Innate Type 2 Inflammation to Adipose Function. Journal of lipid research. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bolus WR, Kennedy AJ, and Hasty AH. Obesity-induced reduction of adipose eosinophils is reversed with low-calorie dietary intervention. Physiol Rep. 2018;6(22):e13919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rosen ED, and Spiegelman BM. What we talk about when we talk about fat. Cell. 2014;156(1–2):20–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cohen P, and Spiegelman BM. Brown and Beige Fat: Molecular Parts of a Thermogenic Machine. Diabetes. 2015;64(7):2346–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Harms M, and Seale P. Brown and beige fat: development, function and therapeutic potential. Nat Med. 2013;19(10):1252–63. [DOI] [PubMed] [Google Scholar]
- 34.Brestoff JR, Kim BS, Saenz SA, Stine RR, Monticelli LA, Sonnenberg GF, et al. Group 2 innate lymphoid cells promote beiging of white adipose tissue and limit obesity. Nature. 2015;519(7542):242–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Molofsky AB, Nussbaum JC, Liang H-E, Van Dyken SJ, Cheng LE, Mohapatra A, et al. Innate lymphoid type 2 cells sustain visceral adipose tissue eosinophils and alternatively activated macrophages. The Journal of experimental medicine. 2013;210(3):535–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nussbaum JC, Van Dyken SJ, von Moltke J, Cheng LE, Mohapatra A, Molofsky AB, et al. Type 2 innate lymphoid cells control eosinophil homeostasis. Nature. 2013;502(7470):245–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rao RR, Long JZ, White JP, Svensson KJ, Lou J, Lokurkar I, et al. Meteorin-like is a hormone that regulates immune-adipose interactions to increase beige fat thermogenesis. Cell. 2014;157(6):1279–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hill DA, Lim H-W, Kim YH, Ho WY, Foong YH, Nelson VL, et al. Distinct macrophage populations direct inflammatory versus physiological changes in adipose tissue. Proceedings of the National Academy of Sciences. 2018;115(22):E5096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Silva HM, Báfica A, Rodrigues-Luiz GF, Chi J, Santos PdEA, Reis BS, et al. Vasculature-associated fat macrophages readily adapt to inflammatory and metabolic challenges. The Journal of experimental medicine. 2019;216(4):786–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bigornia SJ, Farb MG, Mott MM, Hess DT, Carmine B, Fiscale A, et al. Relation of depot-specific adipose inflammation to insulin resistance in human obesity. Nutrition & diabetes. 2012;2:e30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Coats BR, Schoenfelt KQ, Barbosa-Lorenzi VC, Peris E, Cui C, Hoffman A, et al. Metabolically Activated Adipose Tissue Macrophages Perform Detrimental and Beneficial Functions during Diet-Induced Obesity. Cell reports. 2017;20(13):3149–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jaitin DA, Adlung L, Thaiss CA, Weiner A, Li B, Descamps H, et al. Lipid-Associated Macrophages Control Metabolic Homeostasis in a Trem2-Dependent Manner. Cell. 2019;178(3):686–98.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Desai D, Newby C, Symon FA, Haldar P, Shah S, Gupta S, et al. Elevated sputum interleukin-5 and submucosal eosinophilia in obese individuals with severe asthma. Am J Respir Crit Care Med. 2013;188(6):657–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Haldar P, Pavord ID, Shaw DE, Berry MA, Thomas M, Brightling CE, et al. Cluster analysis and clinical asthma phenotypes. Am J Respir Crit Care Med. 2008;178(3):218–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Farahi N, Loutsios C, Tregay N, Wright AKA, Berair R, Lok LSC, et al. In vivo imaging reveals increased eosinophil uptake in the lungs of obese asthmatic patients. J Allergy Clin Immunol. 2018;142(5):1659–62.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Grotta MB, Squebola-Cola DM, Toro AA, Ribeiro MA, Mazon SB, Ribeiro JD, et al. Obesity increases eosinophil activity in asthmatic children and adolescents. BMC Pulm Med. 2013;13:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Calixto MC, Lintomen L, Andre DM, Leiria LO, Ferreira D, Lellis-Santos C, et al. Metformin attenuates the exacerbation of the allergic eosinophilic inflammation in high fat-diet-induced obesity in mice. PLoS One. 2013;8(10):e76786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Calixto MC, Lintomen L, Schenka A, Saad MJ, Zanesco A, and Antunes E. Obesity enhances eosinophilic inflammation in a murine model of allergic asthma. Br J Pharmacol. 2010;159(3):617–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Costa JJ, Matossian K, Resnick MB, Beil WJ, Wong DT, Gordon JR, et al. Human eosinophils can express the cytokines tumor necrosis factor-alpha and macrophage inflammatory protein-1 alpha. J Clin Invest. 1993;91(6):2673–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jacoby DB, Gleich GJ, and Fryer AD. Human eosinophil major basic protein is an endogenous allosteric antagonist at the inhibitory muscarinic M2 receptor. J Clin Invest. 1993;91(4):1314–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fryer AD, and Maclagan J. Muscarinic inhibitory receptors in pulmonary parasympathetic nerves in the guinea-pig. Br J Pharmacol. 1984;83(4):973–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fryer AD, and Wills-Karp M. Dysfunction of M2-muscarinic receptors in pulmonary parasympathetic nerves after antigen challenge. J Appl Physiol. 1991;71(6):2255–61. [DOI] [PubMed] [Google Scholar]
- 53.Nie Z, Jacoby DB, and Fryer AD. Etanercept Prevents Airway Hyperresponsiveness by Protecting Neuronal M2 Muscarinic Receptors in Antigen Challenged Guinea Pigs British Journal of Pharmacology. 2009;156(1):201–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nie Z, Scott GD, Weis PD, Itakura A, Fryer AD, and Jacoby DB. Br J Pharmacol. 2011. [DOI] [PMC free article] [PubMed]
- 55.Yost BL, Gleich GJ, and Fryer AD. Ozone-induced hyperresponsiveness and blockade of M2 muscarinic receptors by eosinophil major basic protein. J Appl Physiol. 1999;87(4):1272–8. [DOI] [PubMed] [Google Scholar]
- 56.Nie Z, Jacoby DB, and Fryer AD. Hyperinsulinemia potentiates airway responsiveness to parasympathetic nerve stimulation in obese rats. Am J Respir Cell Mol Biol. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rasmussen F, and Hancox RJ. Mechanisms of obesity in asthma. Curr Opin Allergy Clin Immunol. 2014;14(1):35–43. [DOI] [PubMed] [Google Scholar]
- 58.van Huisstede A, Rudolphus A, Castro Cabezas M, Biter LU, van de Geijn GJ, Taube C, et al. Effect of bariatric surgery on asthma control, lung function and bronchial and systemic inflammation in morbidly obese subjects with asthma. Thorax. 2015;70(7):659–67. [DOI] [PubMed] [Google Scholar]


