Abstract
Purpose
Colorectal cancer (CRC) is the third–most frequently diagnosed cancer globally. Studies have linked low serum albumin with increased risk of CRC, but the causal nature of the association remains unclear. In the present study, we explored the potential causal relationship using bidirectional Mendelian randomization (MR).
Methods
Instrumental variants for albumin were obtained from a genome-wide association study (GWAS) on 102,223 Eastern Asian participants to investigate the effect of albumin on CRC. Summary statistics of CRC were obtained from a GWAS on 7,062 CRC cases and 195,745 controls of Eastern Asian ancestry. Bidirectional MR analysis was performed using inverse variance weighting (IVW) for primary analysis, supplemented with a maximum likelihood–based method, MR-PRESSO test, leave-one-out analysis, and MR-Egger regression. Stratification analyses were further performed.
Results
We found that genetically predicted serum albumin per unit was associated with a lower risk of CRC (OR 0.75, 95% CI 0.59–0.95 with IVW). No evidence of pleiotropy was observed. Sex-stratified MR analysis showed that serum albumin was inversely associated with risk of CRC in men (OR 0.71, 95% CI 0.53–0.96), but not in women (OR 0.81, 95% CI 0.55–1.19) using IVW. Reverse MR analysis suggested a genetic predisposition toward CRC was not associated with serum albumin.
Conclusion
Our study revealed a suggestive sex disparity in the effect of albumin, which deserves further exploration of the potential biological mechanism.
Keywords: albumin, colorectal cancer, Mendelian randomization, single-nucleotide polymorphism, genome-wide association study, instrumental variables
Introduction
Colorectal cancer (CRC) is the third–most frequently diagnosed cancer and the fourth-leading cause of cancer deaths worldwide, with >1.9 million new CRC (including anus) cases and 935,000 deaths estimated to have occurred in 2020.1 Recently, increased CRC incidence has been observed in many newly developed countries in Eastern Europe, Asia, and South America, while rates have been stabilizing or decreasing in highly developed regions or countries, such as Northern Europe and Canada.2 Evidence has supported the case that modifiable risk factors are involved in CRC incidence and progression, such as obesity, tobacco smoking, alcohol consumption, and poor diet.3–8
Albumin is generally used as a biochemical marker for reflecting host nutritional status and inflammation.9 Numerous studies have suggested that albumin acts as a prognostic factor in CRC, with low levels of serum albumin predicting poor outcomes in CRC patients.10 For example, a retrospective cohort study of 1,422 CRC patients in Taiwan suggested that high serum albumin was associated with increased CRC survival (HR 1.45, 95% CI 1.09–1.92; low vs high).9 Similarly, in a retrospective cohort study of 1,465 CRC patients, serum albumin was identified as an independent prognostic factor of overall survival (HR 0.85, 95% CI 0.73–0.99; high vs low).11 Nevertheless, evidence regarding the role of albumin in the etiology of CRC is limited and unclear. Recently, a prospective cohort study of 11,320 participants in the UK Biobank suggested that higher circulating levels of albumin were associated with a reduced risk of CRC (HR 0.66, 95% CI 0.55–0.79).12 However, results from the Apolipoprotein Mortality Risk Study on 325,599 participants detected a suggestive association between albumin and CRC risk (HR 0.93, 95% CI 0.85–1.02).13 In addition, a prospective cohort study based on 256 incident CRC cases from 2,379 participants found a statistically insignificant association of serum albumin with risk of CRC (HR 0.80, 95% CI 0.52–1.22).14 The mixed findings from individual observational studies may be attributable to differences in sample size, study design, and covariates across studies. Since the presence of an ongoing inflammatory response in tumors might contribute to the progressive loss of albumin,15 reverse causality cannot be fully ruled out in observational designs.
Mendelian randomization (MR) is used to infer the causality of albumin on CRC by utilizing data from genome-wide association studies (GWASs). As genetic variants are presumed to be assigned randomly during conception, they are less susceptible to influence by confounding factors, and are hence used as proxies of serum albumin to estimate the potential causal relationship between serum albumin and CRC, thus reducing risk of confounding and reverse causation commonly found in retrospective studies. Here, we conducted a bidirectional MR analysis to explore the potential causal association between serum albumin and CRC in Asian populations. In order to explore the sex-specific effect of serum albumin on CRC risk, a sex-stratified analysis was further performed.
Methods
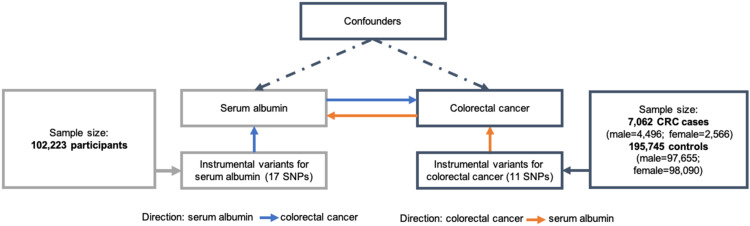
An overview of the study design is shown in Figure 1. As the current study was based on published studies and public databases, no additional ethics approval or consent to participate was required.
Figure 1.
Study flowchart.
Data Source of CRC
We obtained summary statistics on CRC from a GWAS conducted by Kazuyoshi et al.16 In this study, all cases were obtained from BioBank Japan (BBJ), one of the largest non-European BBs, while the control groups consisted of noncancer patients from BBJ and participants from population-based prospective cohorts from Tohoku University Tohoku Medical Megabank Organization, Iwate Medical University Iwate Tohoku Medical Megabank Organization, the Japan Public Health Center–based Prospective Study and the Japan Multi-institutional Collaborative Cohort Study,16 (Supplementary Table 1). Finally, 7,062 CRC cases (male:female 4,496:2,566, mean age 67.0±10.2 years) and 195,745 controls (male:female 97,655:98,090; mean age 61.6±13.9 years) were involved in the current analysis. Genotypes were imputed using 1000 Genomes Project phase III reference data,17 and a generalized linear mixed model was employed in the association analysis.18 Sex-specific GWAS summary data were also used for subsequent sex-stratified MR analysis. All participating studies obtained informed consent from the study population and gained approval from the ethics committees of the Riken Center for Integrative Medical Sciences and Institute of Medical Sciences, University of Tokyo.
Selection of Instrumental Variants Associated with Albumin
As instrumental variants (IVs) for MR analysis, we selected single-nucleotide polymorphisms (SNPs) strongly associated with serum albumin reaching a GW significance threshold of P=5×10−8 with no linkage equilibrium of r2<0.1 from a GWAS on 102,223 participants of Asian ancestry.19 Laboratory measurements of quantitative traits were retrieved from medical records by routine examination. Individuals with hepatitis B or C, cirrhosis, hepatocellular carcinoma, rheumatoid arthritis, nephrotic syndrome, or hematopoietic tumors were excluded.19 Data were further normalized by rank-based inverse normal transformation. The 1000 Genomes Project phase I was used as the reference for imputation,19 and association analysis was conducted using linear regression.20 Finally, 17 SNPs in different genomic regions were harmonized and used as IVs (Table 1). Sex-specific associations of IVs and the risk of CRC are shown in Supplementary Table 2.
Table 1.
Association of instrumental SNPs with albumin and CRC risk in Asian ancestry (sex-combined)
| SNP | Chr | Position | Albumin | CRC | |||||
|---|---|---|---|---|---|---|---|---|---|
| EA | β | SE | P | β | SE | P | |||
| rs1260326 | 2 | 27,730,940 | C | 0.059 | 0.004 | 1.23×10−40 | −0.035 | 0.017 | 0.048 |
| rs7647957 | 3 | 122,617,881 | G | 0.027 | 0.005 | 3.71×10−8 | −0.038 | 0.019 | 0.050 |
| rs4690095 | 4 | 3,421,309 | T | 0.044 | 0.004 | 1.63×10−22 | −0.028 | 0.018 | 0.113 |
| rs1449727 | 4 | 177,407,175 | T | 0.034 | 0.006 | 5.98×10−9 | 0.002 | 0.023 | 0.917 |
| rs75759936 | 4 | 79,619,199 | A | 0.057 | 0.007 | 2.10×10−17 | 0.013 | 0.023 | 0.590 |
| rs268794 | 5 | 72,236,768 | T | 0.027 | 0.005 | 1.44×10−8 | 0.000 | 0.018 | 0.978 |
| rs114584519 | 6 | 31,313,148 | T | 0.025 | 0.004 | 1.43×10−8 | −0.024 | 0.018 | 0.176 |
| rs34121855 | 7 | 73,040,814 | G | 0.044 | 0.007 | 1.65×10−9 | −0.005 | 0.029 | 0.860 |
| rs74702905 | 7 | 22,742,056 | A | 0.036 | 0.005 | 1.16×10−12 | −0.009 | 0.020 | 0.665 |
| rs111960097 | 9 | 112,230,898 | A | 0.032 | 0.005 | 1.51×10−9 | 0.019 | 0.019 | 0.323 |
| rs76024129 | 12 | 13,274,823 | T | 0.043 | 0.008 | 1.14×10−8 | 0.008 | 0.029 | 0.792 |
| rs6119 | 14 | 95,054,012 | G | 0.039 | 0.005 | 6.52×10−14 | 0.013 | 0.020 | 0.536 |
| rs79755028 | 15 | 75,060,413 | A | 0.039 | 0.007 | 4.90×10−9 | −0.050 | 0.026 | 0.054 |
| rs34562254 | 17 | 16,842,991 | A | 0.031 | 0.005 | 2.25×10−11 | −0.002 | 0.018 | 0.919 |
| rs7212936 | 17 | 1,646,651 | C | 0.032 | 0.005 | 1.66×10−11 | −0.022 | 0.019 | 0.248 |
| rs1688043 | 19 | 35,553,341 | T | 0.08 | 0.009 | 3.73×10−20 | −0.015 | 0.034 | 0.652 |
| rs34010237 | 19 | 50,012,574 | A | 0.089 | 0.007 | 2.41×10−40 | −0.022 | 0.027 | 0.410 |
Abbreviations: CRC, colorectal cancer; Chr, chromosome; EA, effect allele; SNP, single-nucleotide polymorphism.
Reverse MR Analysis on the Association Between CRC and Albumin
IVs for reverse MR analysis were selected from the CRC GWAS,19 which identified 13 independent SNPs at the threshold of genome-wide association (r2<0.1, P=5×10−8; Supplementary Table 3). GWAS summary-level statistics of albumin were also used to assess the potential reverse association of genetic predisposition of CRC with serum albumin.16
Statistical Analysis
We performed MR analysis via inverse variance weighting (IVW) as primary analysis and applied maximum likelihood–based and leave-one-out analyses as sensitivity analyses. In addition, MR pleiotropy residual sum and outlier (MR-PRESSO) testing and MR-Egger regression were used to test for potential outliers and pleiotropy across variants. Specifically, IVW requires that either all variants be valid instruments or that there is balanced horizontal pleiotropy that is independent of IV strength across all SNPs.21 IVW estimates the causal association of risk factors with outcome by combining the Wald ratio, which is the weighted average of ratio estimates of the effect of exposure on outcome for each genetic variant in an approach analogous to meta-analysis.22 Heterogeneity across each variant was evaluated with Cochran’s Q test. If no substantial heterogeneity was observed, the fixed-effect model was employed. Otherwise the random-effect model was used.23
The maximum likelihood–based method provides estimated causal association by assuming that there is a linear relationship between exposure and outcome and normal bivariate distribution for the genetic association estimates.22 Leave-one-out analysis was further applied to test the influence of individual SNPs on the overall effect of serum albumin on the risk of CRC by excluding each SNP sequentially and then performing IVW in turn. The MR-PRESSO test was used to detect outliers with potential horizontal pleiotropy and provide corrected causal estimates of exposure on outcome after outlier removal.24 MR-Egger regression analysis was used to detect the potential directional pleiotropic effect, with a P value for the intercept term <0.05 suggesting directional pleiotropy of the SNPs.25
The strength of IVs was measured by the Fstatistic using the equation 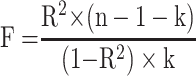 , with n, k, and R2 indicating sample size, number of IVs used, and genetic variance explained by the IVs, respectively.26 A low F-value indicates less bias in genetic variants, with <10 suggesting the possibility of IVs being subjected to weak instrumental variable bias. Power to estimate causal effects of exposure on outcome was calculated for α=0.05.27 ORs with their 95% CIs in the current MR analysis were reported to increase by 1 g/dL in serum albumin. All P values were two-sided, and associations between exposure and outcome were considered statistically significant at P<0.05. MR analyses were conducted using R version 4.0.2 with the Mendelian Randomization28 and MR-PRESSO24 R packages.
, with n, k, and R2 indicating sample size, number of IVs used, and genetic variance explained by the IVs, respectively.26 A low F-value indicates less bias in genetic variants, with <10 suggesting the possibility of IVs being subjected to weak instrumental variable bias. Power to estimate causal effects of exposure on outcome was calculated for α=0.05.27 ORs with their 95% CIs in the current MR analysis were reported to increase by 1 g/dL in serum albumin. All P values were two-sided, and associations between exposure and outcome were considered statistically significant at P<0.05. MR analyses were conducted using R version 4.0.2 with the Mendelian Randomization28 and MR-PRESSO24 R packages.
Results
Sex-Combined MR Analysis of Association Between Albumin and CRC
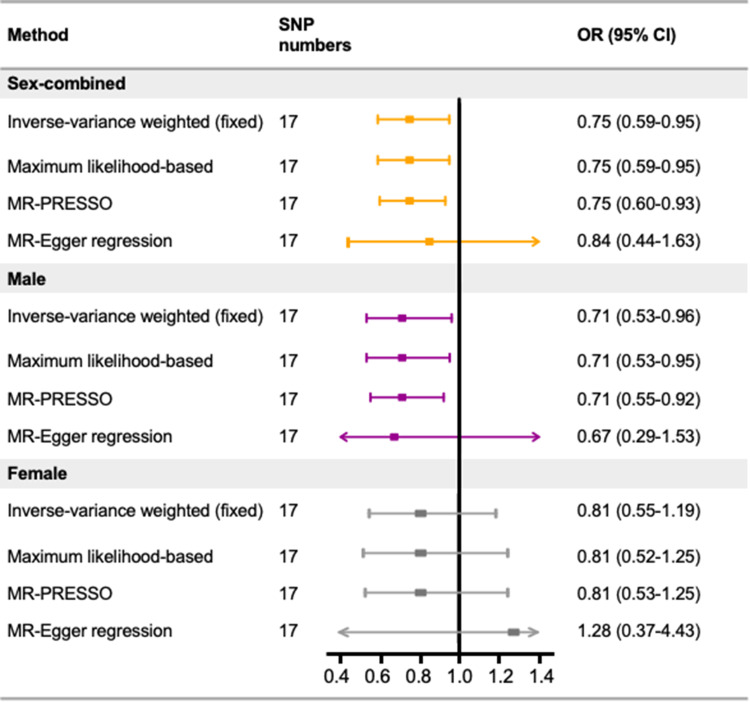
In the current MR study, the 17 SNPs included as IVs explained a total of 1.08% of the proportion of variance, and the F statistics showed no evidence of potential weak instrumental bias, with a range of 28.89–217.56 (Supplementary Table 1). We found that genetically predicted serum albumin per unit was negatively associated with the risk of CRC (OR 0.75, 95% CI 0.59–0.95; Figure 2). Statistical power was calculated to be 69.83% to detect an effect size of 0.75 at a significance level of 0.05. Results from the maximum likelihood–based method produced similar results (OR 0.75, 95% CI 0.59–0.95). No evidence of potential outliers was detected by MR-PRESSO (OR 0.75, 95% CI 0.60–0.93). The results of Cochran’s Q test showed that the IVs were homogeneous. No evidence of potential directional pleiotropic effect was observed on MR-Egger regression. In the leave-one-out analysis, we sequentially removed each SNP in turn to assess the robustness of results, and found that no single SNP exerted influence on overall MR estimates (Supplementary Figure 1).
Figure 2.
Estimated causal effects of genetically predicted serum albumin on the risk of colorectal cancer.
Abbreviations: MR, Mendelian randomization; MR-PRESSO, MR pleiotropy residual sum and outlier; SNP, single-nucleotide polymorphism.
Sex-Stratified MR Analysis of Association Between Albumin and CRC
Using the sex-stratified GWAS summary data of CRC, an inverse association was also observed in the male population (OR 0.71, 95% CI 0.53–0.96 by IVW), while the association was not statistically significant in the female population (OR 0.81, 95% CI 0.55–1.19 by IVW; Figure 2).
Reverse MR Analysis of Association Between CRC and Albumin
Of the 13 instrumental SNPs for CRC, eleven were available in the summary data of albumin for reverse MR analysis (Supplementary Table 3).The results showed that there was no evidence of potential reverse association between genetic predisposition to CRC and serum albumin (OR 1.00, 95% CI 0.98–1.03 by IVW) (Supplementary Table 4).
Discussion
In the present study, we found high circulating levels of albumin were associated with a decreased risk of CRC by utilizing the largest-scale GWAS summary data of CRC among Asian populations. In the sex-stratified analysis, the inverse association was found in men, but not women. Conversely, genetic predisposition to CRC risk did not affect serum albumin. Consistently with our results, a recent prospective population-based study from China suggested that prediagnostic serum albumin was inversely associated with CRC risk in 82,061 participants (HR 0.43, 95% CI 0.26–0.72).29 In addition, evidence from two population-based cohorts in Estonia and Finland of 17,345 participants detected a strong inverse association between albumin and risk of CRC (HR 0.70, 95% CI 0.65–0.76).30 Ko et al also assessed the association between serum albumin and risk of developing colon cancer in 118 cases and 118 controls matched to each case patient by age, sex, and month of blood donation, with mean levels of albumin in cases slightly lower than their counterpart controls (P=0.01).31 Moreover, colon cancer risk was found to increase when comparing the highest quartiles to the lowest quartiles of serum albumin (Ptrend=0.02).31 Such studies provide evidence supporting findings from the present MR study suggesting that high albumin levels have a protective effect on risk of CRC.
Albumin is synthesized in the liver, and its level depends on adequate dietary protein intake and proper absorption, with low intake of specific amino acids possibly reducing serum levels of albumin.32 Albumin is commonly regarded as an indicator of nutritional state, liver function, biosynthesis, detoxification capability, and inflammation.33 The potential mechanism to explain the protective role of albumin on CRC risk may be related to peroxidation processes, systemic inflammatory responses, and tumor volume.34 As an antioxidant, albumin is able to reduce reoxygenation injury.35 Also, it has several immunomodulatory effects and anti-inflammatory effects by binding bacterial products, modulating functions of antigen-presenting cells, and producing cytokines.36 Therefore, the dysregulated immunoresponse and oxidative damage caused by aberrant albumin levels could possibly initiate the occurrence of CRC.37,38
Of note, our study sheds new light on the very limited evidence for sex-specific effects of albumin on risk of CRC by showing a stronger protective effect for men than women. Evidence from observational studies suggested that there might be a sex-different effect of low serum albumin on worse outcome. Grimm et al39 reported that low albumin concentrations were associated with significantly higher HRs for all-cause mortality in men than women in a large cohort of 285,930 patients from the General Hospital Vienna between 1992 and 2002. However, in the current study, we detected no statistically significant association of albumin with CRC risk in the female population. In the GWAS of CRC, female cases consisted of only 36.34% of the total number of cases, which may yield relatively low power to detect a significant association in women.
The study has several strengths. First, this is the only MR study exploring the association between albumin and CRC risk in Asian populations to date. In the past few decades, the incidence and mortality of CRC has increased worldwide, especially in Asian countries,40 so ongoing exploration of the etiology of CRC is much needed. Our study suggested serum albumin as a potential protective factor in the incidence of CRC, suggesting the value of serum albumin as a predictor of CRC incidence in the general population. Second, we performed both sex-combined and sex-stratified MR analyses on the association of albumin with the risk of CRC, and found that the sex-specific effect of albumin on the risk of CRC was more apparent in men than women.
Nevertheless, some limitations are worth considering. First, although the results from MR-Egger regression suggested no evidence of pleiotropy across IVs, we cannot totally rule out the pleiotropic effect across the variants, given that pleiotropy is widespread in the genome and completely valid IVs are almost impossible to be satisfied in real MR practice. Second, due to the limited publicly available data sets for CRC, we could not investigate the association of albumin with subtypes of CRC, eg, colon cancer and rectal cancer. Third, the potential linear or nonlinear relationship between albumin and CRC could not be fully assessed, since the current study was based on summary-level data rather than individual-level statistics. Fourth, our study was based on the GWAS of Asian populations, and thus it may be difficult to extrapolate to other ethnicities. Lastly, the variance explained by the IVs of CRC iwa 0.28%, which may have led to the low power in the reverse MR study. As such, we cannot exclude the possibility of a potential reverse association between serum albumin and the risk of CRC. In future, GWASs of CRC in Asians with larger samples are warranted to reveal more significant loci with higher heritability.
Conclusion
In closing, in this study we demonstrated that in using an MR method, there was a causal protective effect of serum albumin and risk of CRC. This effect was statistically significant in the general population, but when stratified based on sex, the effect remained in men, but was attenuated in women. Further studies are warranted to explore possible biological mechanistic pathways behind the effect of serum albumin on the incidence of CRC.
Acknowledgments
We sincerely appreciate the BioBank Japan and related institutions for providing the GWAS summary data of colorectal cancer and serum albumin.
Funding Statement
This work was supported by grants from the National Natural Science Foundation of China (81803326, 82103936), Natural Science Foundation of Zhejiang Province (LQ20H260008, LQ21H260001), Talent Project of the Zhejiang Association for Science and Technology (2018YCGC003), Medical Health Science and Technology Project of Zhejiang Provincial Health Commission (2020KY195), and Zhejiang Chinese Medical University Foundation (2020ZG01, 2020ZG16).
Abbreviations
BBJ, BioBank Japan; CRC, colorectal cancer; GWAS, genome-wide association study; MR, Mendelian randomization; IVs, instrumental variants; IVW, inverse variance weighting; MR-PRESSO, MR pleiotropy residual sum and outlier; RR, relative risk; SNP, single-nucleotide polymorphism.
Novelty and Impact
This is the first bidirectional MR study to explore the association between serum albumin and risk of CRC. Genetically predicted high circulating albumin levels were associated with a reduced risk of CRC in an Asian population. Sex-stratified MR analysis suggested that the inverse association was more pronounced in men, which deserves further studies to clarify the potential mechanism.
Data Sharing
The data sets supporting the conclusions of this article are available from the BioBank Japan website: http://jenger.riken.jp/en.
Ethics Approval and Informed Consent
All participating studies involved in the GWASs obtained informed consent from the study population and gained approval from the ethics committees of the Riken Center for Integrative Medical Sciences and the Institute of Medical Sciences, University of Tokyo. As we utilized publicly available data sets to perform MR, no additional ethics approval was required. An ethics-approval waiver was consented to by the ethics committee of Zhejiang Chinese Medical University.
Author Contributions
All authors made a significant contribution to the work reported, whether is in conception, study design, execution, acquisition of data, analysis and interpretation, or all these areas, took part in drafting, revising, or critically reviewing the article, gave final approval to the version to be published, have agreed on the journal to which the article has been submitted, and agree to be accountable for all aspects of the work.
Disclosure
The authors have no relevant financial or nonfinancial interests to disclose.
References
- 1.Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660 [DOI] [PubMed] [Google Scholar]
- 2.Arnold M, Abnet CC, Neale RE, et al. Global Burden of 5 major types of gastrointestinal cancer. Gastroenterology. 2020;159(1):335–349 e15. doi: 10.1053/j.gastro.2020.02.068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Botteri E, Iodice S, Bagnardi V, Raimondi S, Lowenfels AB, Maisonneuve P. Smoking and colorectal cancer: a meta-analysis. JAMA. 2008;300(23):2765–2778. doi: 10.1001/jama.2008.839 [DOI] [PubMed] [Google Scholar]
- 4.Fedirko V, Tramacere I, Bagnardi V, et al. Alcohol drinking and colorectal cancer risk: an overall and dose-response meta-analysis of published studies. Ann Oncol. 2011;22(9):1958–1972. doi: 10.1093/annonc/mdq653 [DOI] [PubMed] [Google Scholar]
- 5.Islami F, Goding Sauer A, Miller KD, et al. Proportion and number of cancer cases and deaths attributable to potentially modifiable risk factors in the United States. CA Cancer J Clin. 2018;68(1):31–54. doi: 10.3322/caac.21440 [DOI] [PubMed] [Google Scholar]
- 6.Park Y, Hunter DJ, Spiegelman D, et al. Dietary fiber intake and risk of colorectal cancer: a pooled analysis of prospective cohort studies. JAMA. 2005;294(22):2849–2857. doi: 10.1001/jama.294.22.2849 [DOI] [PubMed] [Google Scholar]
- 7.Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371(9612):569–578. doi: 10.1016/s0140-6736(08)60269-x [DOI] [PubMed] [Google Scholar]
- 8.Yao Y, Suo T, Andersson R, et al. Dietary fibre for the prevention of recurrent colorectal adenomas and carcinomas. Cochrane Database Syst Rev. 2017;1(1):Cd003430. doi: 10.1002/14651858.CD003430.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sun LC, Chu KS, Cheng SC, et al. Preoperative serum carcinoembryonic antigen, albumin and age are supplementary to UICC staging systems in predicting survival for colorectal cancer patients undergoing surgical treatment. BMC Cancer. 2009;9:288. doi: 10.1186/1471-2407-9-288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dixon MR, Haukoos JS, Udani SM, et al. Carcinoembryonic antigen and albumin predict survival in patients with advanced colon and rectal cancer. Arch Surg. 2003;138(9):962–966. doi: 10.1001/archsurg.138.9.962 [DOI] [PubMed] [Google Scholar]
- 11.González-Trejo S, Carrillo JF, Carmona-Herrera DD, et al. Baseline serum albumin and other common clinical markers are prognostic factors in colorectal carcinoma: a retrospective cohort study. Medicine. 2017;96(15):e6610. doi: 10.1097/md.0000000000006610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.He MM, Fang Z, Hang D, et al. Circulating liver function markers and colorectal cancer risk: a prospective cohort study in the UK Biobank. Int J Cancer. 2021;148(8):1867–1878. doi: 10.1002/ijc.33351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ghuman S, Van Hemelrijck M, Garmo H, et al. Serum inflammatory markers and colorectal cancer risk and survival. Br J Cancer. 2017;116(10):1358–1365. doi: 10.1038/bjc.2017.96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuhn T, Sookthai D, Graf ME, et al. Albumin, bilirubin, uric acid and cancer risk: results from a prospective population-based study. Br J Cancer. 2017;117(10):1572–1579. doi: 10.1038/bjc.2017.313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McMillan DC, Watson WS, O’Gorman P, Preston T, Scott HR, McArdle CS. Albumin concentrations are primarily determined by the body cell mass and the systemic inflammatory response in cancer patients with weight loss. Nutr Cancer. 2001;39(2):210–213. doi: 10.1207/S15327914nc392_8 [DOI] [PubMed] [Google Scholar]
- 16.Ishigaki K, Akiyama M, Kanai M, et al. Large-scale genome-wide association study in a Japanese population identifies novel susceptibility loci across different diseases. Nat Genet. 2020;52(7):669–679. doi: 10.1038/s41588-020-0640-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Auton A, Brooks LD, Durbin RM; 1000 Genomes Project Consortium. A global reference for human genetic variation. Nature. 2015;526(7571):68–74. doi: 10.1038/nature15393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou W, Nielsen JB, Fritsche LG, et al. Efficiently controlling for case-control imbalance and sample relatedness in large-scale genetic association studies. Nat Genet. 2018;50(9):1335–1341. doi: 10.1038/s41588-018-0184-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kanai M, Akiyama M, Takahashi A, et al. Genetic analysis of quantitative traits in the Japanese population links cell types to complex human diseases. Nat Genet. 2018;50(3):390–400. doi: 10.1038/s41588-018-0047-6 [DOI] [PubMed] [Google Scholar]
- 20.Li Y, Willer CJ, Ding J, Scheet P, Abecasis GR. MaCH: using sequence and genotype data to estimate haplotypes and unobserved genotypes. Genet Epidemiol. 2010;34(8):816–834. doi: 10.1002/gepi.20533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hartwig FP, Davey Smith G, Bowden J. Robust inference in summary data Mendelian randomization via the zero modal pleiotropy assumption. Int J Epidemiol. 2017;46(6):1985–1998. doi: 10.1093/ije/dyx102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Burgess S, Butterworth A, Thompson SG. Mendelian randomization analysis with multiple genetic variants using summarized data. Genet Epidemiol. 2013;37(7):658–665. doi: 10.1002/gepi.21758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hemani G, Bowden J, Davey Smith G. Evaluating the potential role of pleiotropy in Mendelian randomization studies. Hum Mol Genet. 2018;27(R2):R195–R208. doi: 10.1093/hmg/ddy163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Verbanck M, Chen CY, Neale B, Do R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat Genet. 2018;50(5):693–698. doi: 10.1038/s41588-018-0099-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bowden J, Del Greco MF, Minelli C, Davey Smith G, Sheehan NA, Thompson JR. Assessing the suitability of summary data for two-sample Mendelian randomization analyses using MR-Egger regression: the role of the I2 statistic. Int J Epidemiol. 2016;45(6):1961–1974. doi: 10.1093/ije/dyw220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yarmolinsky J, Bonilla C, Haycock PC, et al. Circulating selenium and prostate cancer risk: a Mendelian Randomization analysis. J Natl Cancer Inst. 2018;110(9):1035–1038. doi: 10.1093/jnci/djy081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Burgess S. Sample size and power calculations in Mendelian randomization with a single instrumental variable and a binary outcome. Int J Epidemiol. 2014;43(3):922–929. doi: 10.1093/ije/dyu005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yavorska OO, Burgess S. MendelianRandomization: an R package for performing Mendelian randomization analyses using summarized data. Int J Epidemiol. 2017;46(6):1734–1739. doi: 10.1093/ije/dyx034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang Z, Zheng Y, Wu Z, et al. Association between pre-diagnostic serum albumin and cancer risk: results from a prospective population-based study. Cancer Med. 2021;10(12):4054–4065. doi: 10.1002/cam4.3937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fischer K, Kettunen J, Wurtz P, et al. Biomarker profiling by nuclear magnetic resonance spectroscopy for the prediction of all-cause mortality: an observational study of 17,345 persons. PLoS Med. 2014;11(2):e1001606. doi: 10.1371/journal.pmed.1001606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ko WF, Helzlsouer KJ, Comstock GW. Serum albumin, bilirubin, and uric acid and the anatomic site-specific incidence of colon cancer. J Natl Cancer Inst. 1994;86(24):1874–1875. doi: 10.1093/jnci/86.24.1874 [DOI] [PubMed] [Google Scholar]
- 32.Phillips A, Shaper AG, Whincup PH. Association between serum albumin and mortality from cardiovascular disease, cancer, and other causes. Lancet. 1989;2(8677):1434–1436. doi: 10.1016/s0140-6736(89)92042-4 [DOI] [PubMed] [Google Scholar]
- 33.Newsome PN, Cramb R, Davison SM, et al. Guidelines on the management of abnormal liver blood tests. Gut. 2018;67(1):6–19. doi: 10.1136/gutjnl-2017-314924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Al-Shaiba R, McMillan DC, Angerson WJ, Leen E, McArdle CS, Horgan P. The relationship between hypoalbuminaemia, tumour volume and the systemic inflammatory response in patients with colorectal liver metastases. Br J Cancer. 2004;91(2):205–207. doi: 10.1038/sj.bjc.6601886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Halliwell B. Albumin–an important extracellular antioxidant? Biochem Pharmacol. 1988;37(4):569–571. doi: 10.1016/0006-2952(88)90126-8 [DOI] [PubMed] [Google Scholar]
- 36.Ferrer R, Mateu X, Maseda E, et al. Non-oncotic properties of albumin. A multidisciplinary vision about the implications for critically ill patients. Expert Rev Clin Pharmacol. 2018;11(2):125–137. doi: 10.1080/17512433.2018.1412827 [DOI] [PubMed] [Google Scholar]
- 37.Janney A, Powrie F, Mann EH. Host-microbiota maladaptation in colorectal cancer. Nature. 2020;585(7826):509–517. doi: 10.1038/s41586-020-2729-3 [DOI] [PubMed] [Google Scholar]
- 38.Li SKH, Martin A. Mismatch repair and colon cancer: mechanisms and therapies explored. Trends Mol Med. 2016;22(4):274–289. doi: 10.1016/j.molmed.2016.02.003 [DOI] [PubMed] [Google Scholar]
- 39.Grimm G, Haslacher H, Kampitsch T, et al. Sex differences in the association between albumin and all-cause and vascular mortality. Eur J Clin Invest. 2009;39(10):860–865. doi: 10.1111/j.1365-2362.2009.02189.x [DOI] [PubMed] [Google Scholar]
- 40.Arnold M, Sierra MS, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global patterns and trends in colorectal cancer incidence and mortality. Gut. 2017;66(4):683–691. doi: 10.1136/gutjnl-2015-310912 [DOI] [PubMed] [Google Scholar]