Summary
In this issue of Cell Chemical Biology, Douglas et al. (2022) describe a potent, specific, and cell-permeable furin inhibitor that interacts with a cryptic binding site to rescue hallmarks of cystic fibrosis in human ex vivo models. BOS-318 holds promise for development of therapeutics targeting an array of furin-dependent pathologies.
Cystic Fibrosis (CF) is a progressive genetic disease that causes severe damage to the respiratory and digestive systems, resulting in lifelong health problems and early death, typically from end-stage lung disease. Mucociliary clearance (MCC) is critical for maintenance of healthy airways and is aided by a thin layer of fluid known as airway surface liquid (ASL). The overall volume of ASL is essential for this process and depends on a balance of Na+/Cl− ions, which are regulated by the activities of cystic fibrosis transmembrane conductance regulator (CFTR) and epithelial sodium channel (ENaC). Inactivating mutations in CFTR disrupt this ion balance, leading to dehydration of airways and progression of hallmark CF symptoms. Furin is a protease abundantly present in the airways that activate ENaC, resulting in additional ion imbalance and exacerbation of CF symptoms. Due to this key role in pathogenesis, furin has emerged as an attractive target for therapeutic intervention in CF (Couture et al., 2015).
Furin is the prototypical member of the preprotein convertase family of subtilisin-like proteases that generate bioactive proteins and peptides by cleaving precursor proteins at polybasic motifs (Dahms et al., 2014). Peptide and peptidomimetic inhibitors have been developed that bind to furin with picomolar to low nanomolar affinity by exploiting the properties of the polybasic cleavage site (Figure 1) (Thomas et al., 2022). While extremely effective at inhibiting furin activity in vitro, these molecules had limited success in cellular and in vivo contexts due to relatively low cell permeability, poor efficacy, and poor target selectivity.
Figure 1. BOS-318 binds a cryptic pocket proximal to the furin active site.
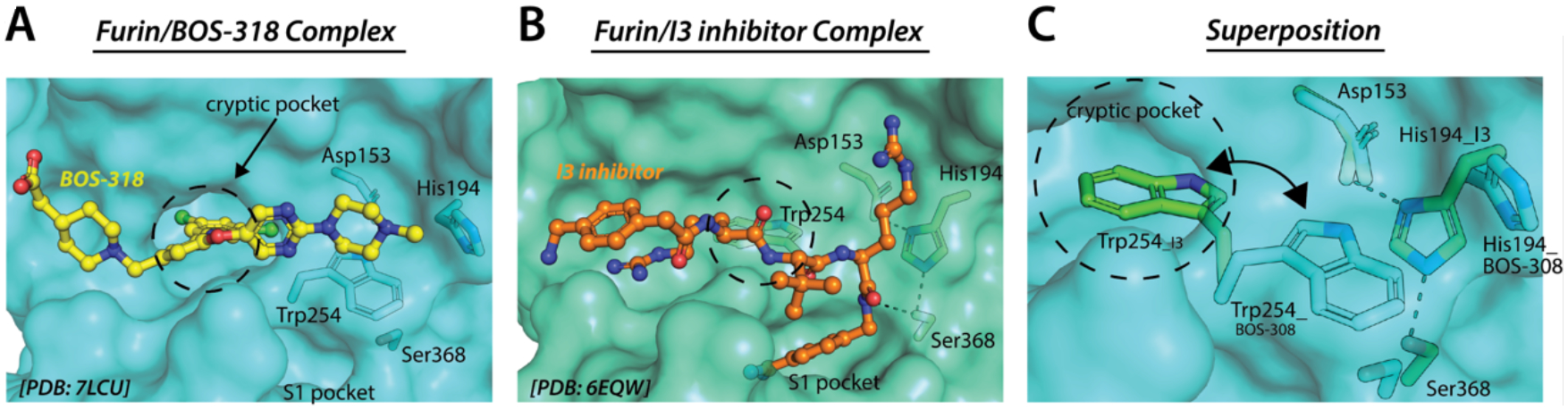
(A and B) Furin/BOS-318 complex (PDB:7LCU and Furin/13 complex (PDB:6EQW). Inhibitors are shown as ball and stick and furin is shown as semitransparent cyan (BOS-318) and green (13) surface representations with selected residues and structural features labeled. Note that Asp153, Hisl 94, and Ser368 constitute the catalytic triad of furin. (C) Furin from the BOS-318 and 13 complexes were superimposed and selected amino acids and structural elements are labeled. Furin from the BOS-318 complex is shown as a semitransparent cyan surface while the furin surface of the 13 complex is omitted for clarity.
In this issue of Cell Chemical Biology, Douglas et al. (2022) describe the development of BOS-318, a small molecule that represents a significant breakthrough in overcoming barriers to development of furin inhibitors with therapeutic value. BOS-318 was discovered through extensive medicinal chemistry optimization of a screening hit identified using DNA-encoded library technology (Axten et al., 2019). BOS-318 harbors a novel core motif ((3,5-dichlorophenyl)pyridine) that is much less charged compared to existing inhibitors that exploit the polybasic furin consensus motif. The result is an inhibitor that is readily cell-permeable, displays exceptional selectivity for furin, and is highly potent in curing CF symptoms in a human ex vivo model (Douglas et al., 2022). BOS-318 significantly reduces ENaC-mediated Na+ absorption and protects against neutrophil-elastase-mediated activation of ENaC in fully differentiated CF primary human bronchial cells. BOS-318 also increases ASL height and MCC clearance rates and exhibits cytoprotection against furin-activated P. aeruginosa exotoxin A-induced airway epithelial cell toxicity, which is one of the central features of CF lung disease (Douglas et al., 2022). These results altogether make BOS-318 a promising molecule for development of an efficacious therapeutic for treating CF, though critical next steps will be to assess the effectiveness and safety of BOS-318 in animal models and beyond.
While the major focus of the Douglas et al., (2022) study was on the role of furin in the pathogenesis of CF (Douglas et al., 2022), the diverse array of furin substrates make it an attractive target for therapeutic intervention in a number of other diseases such as hypertension, cancer, infectious, respiratory and neurodegenerative disorders (Couture et al., 2015). With respect to furin’s role in infectious diseases, acquisition of polybasic sites on surface glycoproteins enhances the virulence of many viruses including highly pathogenic influenza viruses (Izaguirre, 2019). Of note, the SARS-CoV-2 spike protein has acquired a polybasic site which when cleaved, promotes the infectivity and cell-cell spread of the virus. A number of studies have suggested that the major protease responsible for cleaving S protein is furin. Further demonstrating effectiveness, BOS-318 and related compounds were recently reported to reduce virus production in SARS-CoV-2-infected cells (Essalmani et al., 2022). Taken together, it is clear that furin inhibition holds promise in therapeutic development for a diverse array of human pathologies and the unique features of BOS-318 and related small molecules will place it at the forefront of these efforts.
To understand the mechanism of action, the authors determined a crystal structure of BOS-318 in complex with furin (Douglas et al., 2022). This structure displayed an unexpected binding mode that is different from that observed in any previous furin-inhibitor/substrate bound structures (Figure 1A, B). BOS-318 binding to furin is accompanied by a reorganization of the substrate binding site unraveling a new cryptic hydrophobic binding pocket into which the dichlorophenyl moiety of BOS-318 inserts and engages in a unique network of interactions (Figure 1C). Selectivity of BOS-318 for furin is enhanced by relatively low conservation of the cryptic binding site in related proteases and because it fails to engage the highly conserved regions of furin such as the P1 pocket and catalytic triad (Douglas et al., 2022). The network of contacts between the dichlorophenyl moiety of BOS-318 and its cryptic binding pocket allow for high potency in the absence of electrostatic interactions that occur in inhibitors that exploit the polybasic consensus sequence of furin. Notably, BOS-318 exhibits a dual mechanism of inhibition by both obstructing the substrate binding region of furin and perturbing the architecture of the catalytic triad due to steric effects (Figure 1A, B). Of note, shortly after publication of the Douglas et. al. (2022) study, Dahms et al. reported crystal structures and kinetic studies of a series of BOS-318-like furin inhibitors utilizing the (3,5-dichlorophenyl)pyridine core motif and observed similar mode of binding (Dahms et al., 2022).
Lastly, from a broader perspective, targeting of cryptic binding sites, such as the BOS-318 binding site of furin, holds particular promise when the protein of interest lacks obvious druggable pockets based on existing structural data, or if the functional site of the protein cannot be targeted with sufficient specificity. Targeting the cryptic site allows for development of compounds with superior drug-like properties. Together with structures of other proteins harboring cryptic sites exploited by inhibitor molecules including Bcl-xL and Bcl-2, β-lactamase, and SUMO E1 activating enzyme (Lv et al., 2018), lessons learned from BOS-318 (Douglas et al., 2022) and related molecules (Dahms et al., 2022) will guide the development of methods for identifying and rationally targeting cryptic binding that may open up new avenues for therapeutic development moving forward.
ACKNOWLEDGEMENTS
This work was supported by the NIH R01 GM115668, R01 GM128731, and CPRIT RR200030 (S.K.O.)
Footnotes
DECLARATION OF INTERESTS
The authors declare no competing interests.
REFERENCES
- Axten JM, Cheung M, Memartino MP, Guan HA, Hu Y, Miller AB, Qin D, Wu C, Zhang Z, Lin X Furin Inhibitors. (2019) WO 2019/215341
- Couture F, Kwiatkowska A, Dory YL, and Day R (2015). Therapeutic uses of furin and its inhibitors: a patent review. Expert Opin Ther Pat 25, 379–396. 10.1517/13543776.2014.1000303. [DOI] [PubMed] [Google Scholar]
- Dahms SO, Hardes K, Becker GL, Steinmetzer T, Brandstetter H, and Than ME (2014). X-ray structures of human furin in complex with competitive inhibitors. ACS chemical biology 9, 1113–1118. 10.1021/cb500087x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahms SO, Schnapp G, Winter M, Büttner FH, Schlepütz M, Gnamm C, Pautsch A, and Brandstetter H (2022). Dichlorophenylpyridine-Based Molecules Inhibit Furin through an Induced-Fit Mechanism. ACS chemical biology 17, 816–821. 10.1021/acschembio.2c00103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas LE, Reihill JA, Ho MW, Axten JM, Campobasso N, Schneck JL, Rendina AR, Wilcoxen KM, and Martin SL (2022). A highly selective, cell-permeable furin inhibitor BOS-318 rescues key features of cystic fibrosis airway disease. Cell Chemical Biology. [DOI] [PubMed] [Google Scholar]
- Essalmani R, Jain J, Susan-Resiga D, Andreo U, Evagelidis A, Derbali RM, Huynh DN, Dallaire F, Laporte M, Delpal A, et al. (2022). Distinctive Roles of Furin and TMPRSS2 in SARS-CoV-2 Infectivity. J Virol 96, e0012822. 10.1128/jvi.00128-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izaguirre G (2019). The Proteolytic Regulation of Virus Cell Entry by Furin and Other Proprotein Convertases. Viruses 11, 837. 10.3390/v11090837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv Z, Yuan L, Atkison JH, Williams KM, Vega R, Sessions EH, Divlianska DB, Davies C, Chen Y, and Olsen SK (2018). Molecular mechanism of a covalent allosteric inhibitor of SUMO E1 activating enzyme. Nature communications 9, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas G, Couture F, and Kwiatkowska A (2022). The Path to Therapeutic Furin Inhibitors: From Yeast Pheromones to SARS-CoV-2. International Journal of Molecular Sciences 23, 3435. [DOI] [PMC free article] [PubMed] [Google Scholar]


