Abstract
Background
physical functioning impairment is common among persons with cognitive impairment, but little is known about physical functioning trajectories across the US population or how trajectories may differ among persons with dementia and mild cognitive impairment (MCI).
Objective
to examine trajectories of physical functioning among persons with MCI and dementia in the USA.
Design
we used data from the National Health and Aging Trends study (NHATS) 2011–18. Physical functioning was assessed using the NHATS Expanded Short Physical Performance Battery.
Participants
the 661 individuals with MCI and 980 individuals with dementia were included in this study.
Methods
we applied group-based trajectory models to identify latent groups and estimate their trajectories. Multinomial logistic regressions were applied to examine relationships between sociodemographic and health characteristics and trajectory group memberships.
Results
both MCI- and dementia-specific trajectories differed at baseline levels and declined at varying rates across groups. Approximately, 78.43% of persons with MCI were in trajectories with a moderate rate of decline, with only 9.75% in a trajectory with good physical function and 11.82% with poor physical function without as much change over time. Among persons with dementia, approximately 81.4% experienced moderate or fast declines, and 18.52% with virtually no functional ability remained at this same low level. Worse physical functioning trajectories were found among persons who were females, Blacks, with at least four comorbidities, and among persons who had a low socioeconomic status.
Conclusions
persons with both dementia and MCI experienced steady declines in physical functioning. Socioeconomically disadvantaged groups have worse physical functioning trajectories.
Keywords: physical functioning, dementia, mild cognitive impairment, group-based trajectory models, older people
Key Points
Persons with both dementia and mild cognitive impairment (MCI) experienced steady declines in physical functioning.
Socioeconomically disadvantaged groups have worse physical functioning trajectories.
We studied a nationally representative sample of 661 individuals with MCI and 980 individuals with dementia.
Introduction
Physical functioning is an important indicator of both health and quality of life, especially for older adults [1]. Older adults with limitations in physical functioning have higher morbidity and mortality [2] and greater risks of depression [3, 4] and are less able to live independently in the community [5], with higher healthcare needs and utilisation than those without limitations in physical functioning [4]. Many persons with cognitive impairment experience declines in physical functioning [6, 7], with a risk of falling two or three times higher compared with persons without cognitive impairment [8].
Despite a wealth of literature on the interrelationships between cognitive and physical functioning and detailed explorations of whether declines between two or three time points in cognitive function precede declines in physical function and vice versa (see [2], for a review), population-based studies examining physical functioning trajectories with more than 3 years of follow-up of persons with cognitive impairment, particularly for those with mild cognitive impairment (MCI), are lacking in the USA. It is important to study physical functioning trajectories with long-term follow-up among persons with cognitive impairment because they are likely to experience functional decline that may progress over a span of 15–25 years, and the rates of change could also vary across population [9, 10]. Thus, cross-sectional studies and studies with only 1 or 2 years of follow-up cannot capture this dynamic process. Among previous cross-sectional studies, some show that cognitive functioning is a strong predictor of functional disability [11–15], whereas others only find weak or insignificant association between the two [16–18]. The discrepancies in prior studies may be clarified with a trajectory approach with long-term follow-ups. In addition, it is also important to examine population data. Compared with studies using data from a selected location or clinic [19, 20], population data are less prone to sample selection bias and survivor bias [21]. Moreover, it is crucial to examine physical functioning trajectories separately for persons with dementia and for those with MCI because they may differ in their physical abilities [22], and separately examining their physical functioning may provide novel information for researchers. However, most existing studies examining physical functioning trajectories among persons with cognitive impairment in the USA focused on persons with dementia only [23], or did not distinguish the types of cognitive impairment (i.e. dementia versus MCI) [24]. To address the above gaps in the existing literature, the objective of this study is to examine the patterns of trajectories of physical functioning over a relatively long period of time among persons with dementia and persons with MCI separately in the USA using population data.
Methods
Data and study sample
We used two longitudinal samples constructed from eight annual rounds of the National Health and Aging Trends Study (NHATS) collected in 2011–18: a sample of persons with MCI and a sample of persons with dementia. The NHATS is a nationally representative sample of Medicare beneficiaries aged 65 and above [25]. This sample is particularly apt for studying the ageing population in the USA, given 96% of all adults 65 years and older are enrolled in Medicare. In Round 1, 8,245 interviews were conducted. If a participant was unable to respond, a proxy respondent, typically a relative or a caregiver, was interviewed on their behalf. The study followed up with each participant in each round until death and had a high retention rate of over 90% at each round. In Round 5, the study included a refreshment sample of 4,182 participants to restore the Round 1 sample size by age and race [26]. The retention rate remained over 85% of all respondents after Round 5 [25].
We used the following inclusion criteria to construct our sample. First, we included individuals with either dementia or MCI in more than one rounds of data collection. Most individuals excluded here were dead or loss to follow up. Next, to model trajectories, we further restricted the sample to individuals with cognitive impairment who had at least three rounds of data for physical functioning. A flow diagram for sample construction can be found in Appendix Supplementary Figure S1. After all exclusions, we have 1,641 persons with cognitive impairment in our sample. Among them, there were 114 individuals who developed MCI first and progressed to dementia, and 20 individuals who initially developed dementia but recovered to MCI. Counting the number of years that a person had MCI and dementia diagnoses, we included persons with relatively more years of MCI diagnosis to the MCI sample and those with relatively more years of dementia diagnosis to the dementia sample. Our final samples included 661 individuals with MCI and 980 individuals with dementia.
MCI and dementia
NHATS participants underwent cognitive testing, including an immediate and delayed 10-word recall (memory), a clock-drawing test (executive functioning) and a series of orientation questions. MCI status was determined by a cognitive score of ≤1.5 standard deviations below the mean in one domain (executive functioning, memory and orientation) [27]. Dementia status was determined by a cognitive score in at least two cognitive domains of ≤1.5 standard deviations below the mean, a report that the individual had dementia or a proxy-reported score of at least 2 on the AD8 Dementia Screening Interview [27].
Physical functioning
Physical functioning was measured using the NHATS Expanded Short Physical Performance Battery (SPPB) [28]. This assessment-based measure is more reliable than the respondent-reported measures used in many previous studies [29–31]. The NHATS Expanded SPPB included three components: (i) balance tests, (ii) a 3 m usual walking speed to measure locomotion and (iii) rapid chair stands to measure lower body muscle function. The nested balance test of the Expanded SPPB differs from the Original SPPB version in that a more difficult balance test of standing on one leg with eyes open was added, which tests a broader range of functioning [32]. For each of the three components—balance, walking speed and chair stands—a score of 1–4 was assigned based on cut points determined from the NHATS sample distribution quartiles. A higher score indicates a better physical functioning. A score of 0 was given if the participant was deemed to have difficulties standing or walking, had major surgery in the past 3 months, did not participate in any activity due to safety reasons or attempted an activity but did not complete it. Detailed eligibility requirements for doing the SPPB are discussed in the previous literature [32]. The three components were then summed for the assessment-based score ranging from 0 to 12.
Covariates
Age, race/ethnicity, sex, socioeconomic status (SES), social support, health behaviours and health conditions have been shown to be moderators in the relationship between cognitive impairment and physical functioning [33]. Based on this evidence, we selected the following covariates available in the data: sex, age, body mass index (BMI), race/ethnicity (non-Hispanic White [hereafter ‘White’], non-Hispanic Black [hereafter ‘Black’], non-Hispanic other [hereafter ‘Other’] and Hispanic), educational attainment (less than high school, high school graduate or beyond high school), number of siblings, number of children, enrolment in Medicare Part D (drug coverage), enrolment in Medicaid and enrolment in Tricare, comorbidities (0–3 or 4+, among the following comorbidities: heart attack, heart disease, hypertension, arthritis, osteoporosis, diabetes, lung disease, stroke and cancer), married or living with a partner and smoking regularly (i.e. smoking at least one cigarette per day). A person can enrol in multiple insurance options, and therefore, we use three indicators of insurance. Because 71.82% individuals in our sample had fewer than four comorbidities, we coded this variable using three categories (0, 1–3 and 4+) following the previous studies [34]. The baseline characteristics of individuals with MCI and dementia are shown in Table 1.
Table 1.
Baseline characteristics for individuals with MCI and dementia
| N | Mean (SD)/% | Missing rate | ||||
|---|---|---|---|---|---|---|
| MCI | Dementia | MCI | Dementia | MCI | Dementia | |
| Female | 661 | 980 | 55.52% | 62.55% | 0.00% | 0.00% |
| Age | 661 | 980 | 79.50 | 81.61 | 0.00% | 0.00% |
| (7.36) | (7.54) | |||||
| Race/ethnicity | 654 | 962 | 1.06% | 1.84% | ||
| White | 350 | 553 | 53.52% | 57.48% | ||
| Black | 209 | 275 | 31.96% | 28.59% | ||
| Other | 28 | 37 | 4.28% | 3.85% | ||
| Hispanic | 67 | 97 | 10.24% | 10.08% | ||
| Education attainment | 656 | 952 | 0.76% | 2.86% | ||
| <High school | 267 | 403 | 40.70% | 42.33% | ||
| High school | 166 | 238 | 25.30% | 25.00% | ||
| >High school | 223 | 311 | 33.99% | 32.67% | ||
| # Siblings | 658 | 971 | 0.45% | 0.92% | ||
| 0 | 149 | 278 | 22.64% | 28.63% | ||
| 1–3 | 352 | 491 | 53.50% | 50.57% | ||
| 4+ | 157 | 202 | 23.86% | 20.80% | ||
| # Children | 661 | 980 | 0.00% | 0.00% | ||
| 0 | 59 | 85 | 8.93% | 8.67% | ||
| 1–3 | 364 | 517 | 55.07% | 52.76% | ||
| 4+ | 238 | 378 | 36.01% | 38.57% | ||
| Medicare drug coverage | 634 | 886 | 67.04% | 70.09% | 4.08% | 9.59% |
| Medicaid | 643 | 925 | 26.13% | 29.62% | 2.72% | 5.61% |
| Tricare | 651 | 944 | 7.22% | 3.39% | 1.51% | 3.67% |
| Comorbidity | 652 | 959 | 1.36% | 2.14% | ||
| 0 | 54 | 78 | 8.28% | 8.13% | ||
| 1–3 | 437 | 588 | 67.02% | 61.31% | ||
| 4+ | 161 | 293 | 24.69% | 30.55% | ||
| Marital status | 660 | 978 | 0.15% | 0.20% | ||
| Never married | 34 | 46 | 5.15% | 4.70% | ||
| Married/live with a partner | 268 | 369 | 40.61% | 37.73% | ||
| Separated, divorced, widowed | 358 | 563 | 54.24% | 57.57% | ||
| Smoking regularly | 519 | 753 | 49.33% | 42.10% | 21.48% | 23.16% |
Note: Mean (SD) for continuous variables and % for categorical variables.
Statistical analyses
We applied group-based trajectory modelling (GBTM) to identify distinct trajectories for physical functioning among respondents. The GBTM assumes multiple unobserved subgroups with distinct trajectories within a population [35]. Intuitively, it uses statistical methods to determine the probability of an individual belonging to a given trajectory group. In contrast to assigning groups a priori to the data based on a certain variable (e.g. sex, race, etc.), GBTM permits clustering of individuals with similar trajectories. The use of statistical method allows the evaluation of individual changes to determine meaningful changes over time versus random variation. We used the first observation (in the first or the fifth Rounds) in our data as the baseline, with years since the first observation as the time variable [36].
Following previous studies [37] and based on the baseline distribution of physical functioning shown in Supplementary Figure S2, we modelled physical functioning using a zero-inflated Poisson distribution. Specifically, the distribution for individuals with dementia suggests that zero-inflated Poisson should be used instead of Poisson. In addition, both distributions are slightly over-dispersed, and zero-inflated Poisson typically fits better than standard Poisson when there is overdispersion [38]. We chose to use zero-inflated Poisson instead of zero-inflated negative binomial because incorporating the latter into GBTM is technically challenging, and the two models typically give consistent estimates despite different standard errors [38]. For each sample, we first fitted models with varying numbers of latent groups and included linear, quadratic and cubic terms to select the best functional form. We selected the best fitted model based on the Bayesian information criterion as well as diagnosis statistics such as the average posterior probability (greater than 0.7) and the odds of correction classification (greater than 5) [39]. Our model selection and diagnosis results are shown in Appendix Supplementary Tables S1 and S2, respectively. Each participant was assigned to the latent group with the highest posterior probability. Trajectories for all groups were plotted with 95% confidence intervals (CIs). As a conservative test, if the CIs for two trajectories do not overlap, they are considered statistically different. All analyses were performed using the ‘traj’ package in Stata 15 [40].
After identifying distinct trajectory groups, we applied multinomial logistic regression models to predict latent group memberships using various individual SES, demographic and physiological characteristics. To impute a small number of missing values in these characteristics, we performed multiple imputation by chained equations, with all the covariates mentioned above together with trajectory group memberships as predictors [41]. The regression coefficients from 10 imputed data sets were then combined based on the Rubin’s rule [42]. The analyses were conducted using the Stata packages ‘ice’ and ‘mim’. Results without multiple imputations are provided in Appendix Supplementary Tables S3 and S4, and they are substantively consistent with our main results.
Results
Physical functioning trajectories for persons with MCI
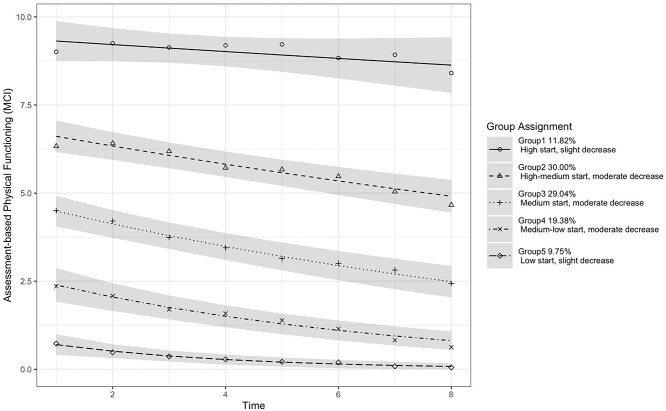
We identified five latent groups for persons with MCI. Figure 1 shows the estimated physical functioning trajectories. The estimated trajectory parameters are shown in Appendix Supplementary Table S5, and the characteristics of individuals in each latent group are shown in Appendix Supplementary Table S6. The five groups started at different baselines and declined at various rates over time. The 11.82% of individuals were in Group 1 (‘high start, slight decrease’) with the highest baseline value close to a score of 9 of 12, 30% were in Group 2 (‘high–medium start, moderate decrease’) with a baseline value of 6.3, 29.04% were in Group 3 (‘medium start, moderate decrease’) with a baseline value of 4.5, 19.38% were in Group 4 (‘medium–low start, moderate decrease’) with a baseline value of 2.4 and 9.75% were in Group 5 (‘low start, slight decrease’) with the lowest baseline value of 0.7. Groups 1 (‘high start, slight decrease’) and 5 (‘low start, slight decrease’) experienced slight decreases in physical functioning over time (the speed of decline was −0.1 for Group 1 and −0.08 for Group 5), and the other three groups experienced moderate decreases over time (the speed of decline was −0.24, −0.28 and −0.22 for Groups 2–4, respectively).
Figure 1.
Physical functioning trajectories for persons with MCI, with 95% CI.
Physical functioning trajectories for persons with dementia
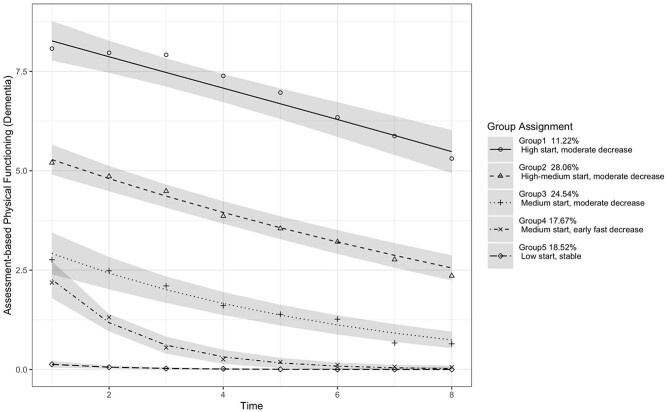
We also identified five latent groups for persons with dementia. Figure 2 shows the estimated physical functioning trajectories. The estimated trajectory parameters are shown in Appendix Supplementary Table S7, and the characteristics of individuals in each latent group are shown in Appendix Supplementary Table S8. Overall, compared with the MCI trajectories, the five groups with dementia started with lower baselines and declined at faster rates. The 11.22% individuals were in Group 1 (‘high start, moderate decrease’), 28.06% in Group 2 (‘high–medium start, moderate decrease’), 24.54% in Group 3 (‘medium start, moderate decrease’), 17.67% in Group 4 (‘medium start, early fast decrease’) and 18.52% in Group 5 (‘low start, stable’). Groups 1 (‘high start, moderate decrease’), 2 (‘high–medium start, moderate decrease’) and 3 (‘medium start, moderate decrease’) started with baseline values of 8.1, 5.2 and 2.8, respectively, and deteriorated at similarly moderate rates (the speed of decline was −0.4, −0.39 and −0.31, respectively) across time. Group 4 (‘medium start, early fast decrease’) started at a score of 2.2 and declined quickly in the first 4 years (the speed of decline was −0.64), but the deterioration slowed down later and eventually levelled off. Group 5 (‘low start, stable’) started at almost no physical abilities and stayed stable over time (the speed of decline was −0.02).
Figure 2.
Physical functioning trajectories for persons with dementia, with 95% CI.
Multinomial logistics regression results
In both samples for dementia and MCI, we selected Group 1 as the reference category because it had the highest baseline values and relatively little changes over time compared with other groups. Relative risk ratios from the multinomial logistic regression for persons with MCI are shown in Table 2. Individuals who were Black or had at least four comorbidities were less likely to be in Group 1 (‘high start, slight decrease’), whereas individuals with beyond high school education were more likely to be in Group 1 (‘high start, slight decrease’). In addition, compared with being in Group 1 (‘high start, slight decrease’), females were more likely to be in Groups 4 (‘medium–low start, moderate decrease’) and 5 (‘low start, slight decrease’).
Table 2.
Risk factors of physical functioning trajectories for individuals with MCI (relative risk ratios and 95% confidence interval)
| Group 2 High–medium start, moderate decrease |
Group 3 Medium start, moderate decrease |
Group 4 Medium–low start, moderate decrease |
Group 5 Low start, slight decrease |
|
|---|---|---|---|---|
| Female | 1.45 | 1.82 | 2.94** | 5.04** |
| (0.71, 2.96) | (0.86, 3.86) | (1.30, 6.63) | (1.94, 13.09) | |
| Age | 0.56 | 0.89 | 0.32 | 0.73 |
| (0.15, 2.08) | (0.23, 3.51) | (0.08, 1.28) | (0.16, 3.31) | |
| Age squared | 1.00 | 1.00 | 1.01 | 1.00 |
| (1.00, 1.01) | (0.99, 1.01) | (1.00, 1.02) | (0.99, 1.01) | |
| Race/ethnicity (Ref. White) | ||||
| Black | 2.77* | 2.45* | 3.47** | 4.29** |
| (1.21, 6.35) | (1.03, 5.85) | (1.37, 8.79) | (1.50, 12.25) | |
| Other | 0.70 | 0.30 | 0.56 | 0.43 |
| (0.21, 2.35) | (0.07, 1.31) | (0.12, 2.63) | (0.06, 2.94) | |
| Hispanic | 2.23 | 2.24 | 1.40 | 1.56 |
| (0.71, 7.06) | (0.67, 7.46) | (0.35, 5.55) | (0.33, 7.42) | |
| Educational attainment (Ref. < High school) | ||||
| High school | 1.19 | 0.84 | 0.69 | 0.78 |
| (0.48, 2.93) | (0.33, 2.16) | (0.25, 1.85) | (0.26, 2.38) | |
| >High school | 0.42* | 0.42* | 0.22** | 0.45 |
| (0.18, 0.94) | (0.18, 0.98) | (0.09, 0.55) | (0.16, 1.27) | |
| # Siblings (Ref. 0) | ||||
| 1–3 | 0.79 | 0.83 | 0.85 | 0.59 |
| (0.31, 1.99) | (0.32, 2.16) | (0.31, 2.33) | (0.20, 1.74) | |
| 4+ | 0.67 | 0.48 | 0.54 | 0.39 |
| (0.24, 1.84) | (0.16, 1.40) | (0.17, 1.70) | (0.11, 1.35) | |
| # Children (Ref. 0) | ||||
| 1–3 | 1.11 | 0.97 | 0.96 | 1.32 |
| (0.37, 3.34) | (0.30, 3.12) | (0.27, 3.39) | (0.31, 5.55) | |
| 4+ | 1.18 | 1.02 | 0.80 | 1.55 |
| (0.35, 3.95) | (0.29, 3.62) | (0.20, 3.16) | (0.33, 7.22) | |
| Medicare drug coverage | 0.68 | 0.73 | 0.53 | 0.63 |
| (0.34, 1.37) | (0.35, 1.52) | (0.24, 1.16) | (0.25, 1.61) | |
| Medicaid | 0.69 | 1.16 | 0.87 | 1.97 |
| (0.32, 1.53) | (0.51, 2.67) | (0.35, 2.15) | (0.73, 5.35) | |
| Tricare | 0.57 | 0.46 | 0.80 | 0.51 |
| (0.20, 1.63) | (0.14, 1.47) | (0.24, 2.73) | (0.10, 2.45) | |
| Comorbidity (Ref. 0) | ||||
| 1–3 | 1.35 | 2.26 | 1.99 | 2.20 |
| (0.54, 3.37) | (0.78, 6.53) | (0.58, 6.79) | (0.40, 11.95) | |
| 4+ | 2.10 | 6.84** | 10.87** | 11.67* |
| (0.64, 6.94) | (1.86, 25.15) | (2.59, 45.61) | (1.81, 75.12) | |
| Marital status (Ref. Never married) | ||||
| Married/live with a partner | 1.23 | 0.87 | 1.03 | 0.42 |
| (0.26, 5.69) | (0.18, 4.26) | (0.18, 5.77) | (0.07, 2.49) | |
| Separated, divorced, widowed | 1.62 | 1.36 | 1.40 | 0.57 |
| (0.34, 7.66) | (0.28, 6.71) | (0.25, 7.84) | (0.10, 3.24) | |
| Smoking regularly | 1.53 | 1.35 | 1.90 | 1.85 |
| (0.76, 3.07) | (0.64, 2.87) | (0.85, 4.24) | (0.76, 4.49) | |
Note: *P < 0.05; **P < 0.01; ***P < 0.001. N = 661. Reference group is Group 1 (High start, slight decrease).
The results for persons with dementia are shown in Table 3. Persons who were female or had at least four comorbidities were less likely to be in Group 1 (‘high start, moderate decrease’), whereas persons who had beyond high school education or siblings were more likely to be in Group 1 (‘high start, moderate decrease’). In addition, compared with being in Group 1 (‘high start, moderate decrease’), individuals who smoked regularly were more likely to be in Group 2 (‘high-medium start, moderate decrease’); Individuals who had less than high school education or without Medicare drug coverage were more likely to be in Group 3 (‘medium start, moderate decrease’); Those who were Black, had comorbidities, received Medicaid but without Medicare drug coverage were more likely to be in Group 5 (‘low start, stable’).
Table 3.
Risk factors of physical functioning trajectories for individuals with dementia (relative risk ratios and 95% confidence interval)
| Group 2 High–medium start, moderate decrease |
Group 3 Medium start, moderate decrease |
Group 4 Medium start, early fast decrease |
Group 5 Low start, stable |
|
|---|---|---|---|---|
| Female | 1.95* | 2.47** | 2.10* | 4.20*** |
| (1.10, 3.46) | (1.34, 4.54) | (1.10, 4.01) | (2.17, 8.13) | |
| Age | 0.53 | 0.57 | 0.38* | 0.35* |
| (0.22, 1.25) | (0.23, 1.39) | (0.15, 0.93) | (0.14, 0.85) | |
| Age squared | 1.00 | 1.00 | 1.01* | 1.01** |
| (1.00, 1.01) | (1.00, 1.01) | (1.00, 1.01) | (1.00, 1.01) | |
| Race/ethnicity (Ref. White) | ||||
| Black | 1.06 | 1.08 | 1.59 | 2.36* |
| (0.54, 2.09) | (0.53, 2.21) | (0.76, 3.32) | (1.14, 4.92) | |
| Other | 0.53 | 0.55 | 0.80 | 0.62 |
| (0.14, 1.98) | (0.14, 2.18) | (0.19, 3.39) | (0.14, 2.67) | |
| Hispanic | 0.70 | 0.92 | 1.18 | 0.91 |
| (0.27, 1.86) | (0.34, 2.51) | (0.41, 3.36) | (0.30, 2.71) | |
| Educational attainment (Ref. < High school) | ||||
| High school | 0.48 | 0.40* | 0.51 | 0.61 |
| (0.23, 1.03) | (0.18, 0.89) | (0.23, 1.17) | (0.26, 1.41) | |
| >High school | 0.23*** | 0.26*** | 0.26** | 0.39* |
| (0.12, 0.47) | (0.13, 0.55) | (0.12, 0.57) | (0.18, 0.83) | |
| # Siblings (Ref. 0) | ||||
| 1–3 | 0.43* | 0.40* | 0.44* | 0.40* |
| (0.21, 0.86) | (0.19, 0.83) | (0.21, 0.94) | (0.19, 0.86) | |
| 4+ | 0.36* (0.15, 0.84) | 0.33* (0.14, 0.81) | 0.39* (0.16, 0.98) | 0.34* (0.14, 0.87) |
| # Children (Ref. 0) | ||||
| 1–3 | 1.08 | 0.85 | 0.87 | 0.63 |
| (0.36, 3.24) | (0.27, 2.62) | (0.27, 2.77) | (0.20, 2.03) | |
| 4+ | 1.03 | 0.71 | 0.56 | 0.70 |
| (0.33, 3.17) | (0.22, 2.26) | (0.17, 1.86) | (0.21, 2.33) | |
| Medicare drug coverage | 0.62 | 0.47* | 0.63 | 0.44* |
| (0.34, 1.13) | (0.25, 0.88) | (0.33, 1.22) | (0.23, 0.85) | |
| Medicaid | 1.52 | 1.68 | 1.58 | 2.85** |
| (0.72, 3.20) | (0.78, 3.63) | (0.70, 3.57) | (1.30, 6.28) | |
| Tricare | 2.51 | 0.71 | 2.12 | 2.54 |
| (0.64, 9.80) | (0.13, 3.92) | (0.45, 9.87) | (0.52, 12.42) | |
| Comorbidity (Ref. 0) | ||||
| 1–3 | 1.44 | 2.38 | 1.34 | 5.15** |
| (0.66, 3.14) | (0.97, 5.84) | (0.56, 3.22) | (1.67, 15.88) | |
| 4+ | 2.62* | 7.64*** | 4.36** | 15.72*** |
| (1.02, 6.71) | (2.70, 21.65) | (1.56, 12.22) | (4.50, 54.89) | |
| Marital status (Ref. Never married) | ||||
| Married/live with a partner | 0.70 | 0.40 | 0.37 | 0.43 |
| (0.14, 3.48) | (0.08, 2.05) | (0.07, 1.91) | (0.08, 2.19) | |
| Separated, divorced, widowed | 1.09 | 0.68 | 0.46 | 0.48 |
| (0.22, 5.41) | (0.13, 3.39) | (0.09, 2.33) | (0.09, 2.42) | |
| Smoking regularly | 1.82* | 1.50 | 1.32 | 1.14 |
| (1.01, 3.29) | (0.80, 2.84) | (0.66, 2.62) | (0.57, 2.27) | |
Note: *P < 0.05; **P < 0.01; ***P < 0.001. N = 980. Reference group is Group 1 (High start, moderate decrease).
Discussion
In this study, we performed GBTM on assessment-based physical functioning for persons with MCI and those with dementia using population data in the USA. For both persons with dementia and MCI, the results showed five distinct latent trajectory groups. Among persons with MCI, about 78.43% belonged to groups with steady, moderate declines, distinguished by their baseline function. An additional 11.82% had good physical functioning without much decline, and the remainder had the poorest physical functioning without much decline. Among persons with dementia, about 63.82% belonged to groups with steady, moderate declines, distinguished by their baseline physical functioning. A fourth group (17.67%) with impaired function at baseline had a steeper rate of decline over the first 4 years, and a fifth group (18.52%) with virtually no functional ability remained at this same low level. In total, approximately 81.4% of persons with dementia experienced moderate or fast declines. Several populations tended to have worse baseline physical functioning among persons with MCI (Groups 4–5), including females, Blacks and those with at least four comorbidities. Among persons with dementia, several populations were more likely to be in the trajectory groups with low or medium baseline values (Groups 3–5), including females, Blacks, those with relatively low SES and those had at least four comorbidities.
Our findings have several important implications. First, the participants in our study came from the NHATS, which is nationally representative, and therefore our estimates can be arguably extrapolated to the US population of older adults with cognitive impairment as a whole. Specifically, the trajectories estimated in this study can give us a clear picture of the different types of physical functioning trajectories as well as their relative prevalence among persons with cognitive impairment in the USA. On a population level, the huge majority of persons with both MCI and dementia experienced physical functioning declines. Clinical interventions are needed to examine whether how this decline is preventable or reversible for each of the trajectory groups. In addition, these trajectories imply increased burdens over time on the healthcare system and caregivers of taking care of individuals who have both physical and cognitive impairments. The worsening physical impairment over time may also impose additional barriers to the receipt of healthcare among these individuals.
Second, we find that persons with MCI typically have steady functional declines, which adds to the debate on the degree of associated functional decline in MCI by providing novel information on the extent of functional change. Future research should aim to determine the causes of the physical functioning decline: perhaps this reflects these persons’ worsening cognitive function or their increased number of comorbidities compared with the general population. Further research should examine the potential for early interventions to ameliorate decline before it is too late for these persons.
Finally, our findings highlight the disparities in physical functioning trajectories among persons with cognitive impairment across sex, racial/ethnic and SES groups. Hale et al. found that for the most advantaged groups including Whites and the highly educated, cognitive impairment occurs later and is compressed towards the end of life [43]. We additionally discover that being female, Black, or having comorbidities, dementia or low SES are associated with lower baselines and more rapid declines in physical functioning. Future efforts in advancing health equity should address multiple dimensions of disadvantages that are associated with troubling physical functioning trajectories.
There are several limitations of this study. First, unhealthy individuals were less likely to be included in our sample due to survival bias. Specifically, those who had worse health including physical functioning might be more likely to die over time, and therefore, death can be a competing mechanism. However, our goal is not to examine any treatment effects, but rather to describe physical functioning trajectories among persons with cognitive impairment who were alive. Although those who died during the study period may have had even more rapidly declining physical functioning trajectories than observed in our figures, from a policy perspective, understanding and improving the physical functioning among people who are still alive is most relevant. Relatedly, there could be potential sample selection issues due to our sample selection criteria. To better understand any potential sample selection issues, we conducted two sets of formal comparisons. First, we compared the samples before and after excluding individuals with less than two rounds of cognitive impairment (see Appendix Supplementary Table S9). Results suggest that the individuals excluded in this step were likely to be a mix of high- and low-SES groups. Second, we compared the samples before and after excluding individuals with at least three rounds of data for physical functioning samples (see Appendix Supplementary Table S10). Results suggest that individuals excluded in this step were more likely to have fewer children or do not have Medicare drug coverage. Second, although we used a variety of individual characteristics to predict trajectory group memberships, we may still have missed some important characteristics, such as living arrangements. Finally, due to page limit, we only examined persons with MCI and those with dementia separately. There is heterogeneity within each of these two groups. Future studies are needed to further examine subgroups within persons with MCI or dementia.
Despite these limitations, the findings of this study have important implications for future research and policymaking. The sheer number of individuals with cognitive impairment who were likely to experience physical impairment highlights the burden of their caregivers and potential barriers to receiving healthcare. Both clinical interventions to slow down the declines in physical functioning and social policies to help their caregivers and to make it easier for them to receive healthcare are needed. Future interventions to prevent and slow down physical functioning declines among persons with cognitive impairment should start as early as in the MCI stage. Finally, our study revealed worse physical functioning trajectories in women, Blacks and persons with low SES. Policymakers should be aware of these disparities and design effective policies targeting these groups.
Supplementary Material
Contributor Information
Emma Zang, Department of Sociology, Yale University, New Haven, CT 06520, USA; Department of Biostatistics, Yale University, New Haven, CT 06520, USA.
Yu Shi, Department of Biostatistics, Yale University, New Haven, CT 06520, USA.
Xueqing Wang, Office of Population Research, Princeton University, Princeton, NJ 08540, USA; School of Public and International Affairs, Princeton University, Princeton, NJ 08540, USA.
Bei Wu, Rory Meyers College of Nursing, New York University, New York, NY 10010, USA.
Terri R Fried, Veterans Affairs Connecticut Healthcare System, West Haven, CT 06516, USA; Department of Medicine, Yale School of Medicine, New Haven, CT 06520, USA.
Declaration of Conflicts of Interest
None.
Declaration of Sources of Funding
This study was supported by the National Institute on Aging under grant numbers R21AG074238-01, 1R56AG067619-01 and P30AG021342.
References
- 1. Guralnik JM, Fried LP, Salive ME. Disability as a public health outcome in the aging population. Annu Rev Public Health 1996; 17: 25–46. [DOI] [PubMed] [Google Scholar]
- 2. Bruce ML. Depression and disability in late life: directions for future research. Am J Geriatr Psychiatry Off J Am Assoc Geriatr Psychiatry 2001; 9: 102–12. [PubMed] [Google Scholar]
- 3. Pearson JL, Teri L, Reifler BV et al. Functional status and cognitive impairment in Alzheimer’s patients with and without depression. J Am Geriatr Soc 1989; 37: 1117–21. [DOI] [PubMed] [Google Scholar]
- 4. Millar RJ. Neighborhood cohesion, disorder, and physical function in older adults: an examination of racial/ethnic differences. J Aging Health 2020; 32: 1133–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fried LP, Guralnik JM. Disability in older adults: evidence regarding significance, etiology, and risk. J Am Geriatr Soc 1997; 45: 92–100. [DOI] [PubMed] [Google Scholar]
- 6. Shaw FE. Falls in cognitive impairment and dementia. Clin Geriatr Med 2002; 18: 159–73. [DOI] [PubMed] [Google Scholar]
- 7. Allan LM, Ballard CG, Rowan EN et al., eds. Incidence and prediction of falls in dementia: a prospective study in older people. Baune B (ed.). PLoS One 2009; 4: e5521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Härlein J, Dassen T, Halfens RJG et al. Fall risk factors in older people with dementia or cognitive impairment: a systematic review. J Adv Nurs 2009; 65: 922–33. [DOI] [PubMed] [Google Scholar]
- 9. Melis RJF, Haaksma ML, Muniz-Terrera G. Understanding and predicting the longitudinal course of dementia. Curr Opin Psychiatry 2019; 32: 123–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jutkowitz E, MacLehose RF, Gaugler JE et al. Risk factors associated with cognitive, functional, and behavioral trajectories of newly diagnosed dementia patients. J Gerontol A Biol Sci Med Sci 2017; 72: 251–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Scherr PA, Albert MS, Funkenstein HH et al. Correlates of cognitive function in an elderly community population. Am J Epidemiol 1988; 128: 1084–101. [DOI] [PubMed] [Google Scholar]
- 12. Kay DWK, Holding TA, Jones B et al. Dependency in old age: a comparison of mental and physical factors. Int J Geriatr Psychiatry 1991; 6: 833–44. [Google Scholar]
- 13. Barberger-gateau P, Chaslerie A, Dartigues JF et al. Health measures correlates in a French elderly community population: the Paquid study. J Gerontol 1992; 47: S88–97. [DOI] [PubMed] [Google Scholar]
- 14. Atkinson HH, Rapp SR, Williamson JD et al. The relationship between cognitive function and physical performance in older women: results from the women’s health initiative memory study. J Gerontol A Biol Sci Med Sci 2010; 65A: 300–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mielke MM, Roberts RO, Savica R et al. Assessing the temporal relationship between cognition and gait: slow gait predicts cognitive decline in the Mayo Clinic study of aging. J Gerontol A Biol Sci Med Sci 2013; 68: 929–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Winograd CH. Mental status tests and the capacity for self-care. J Am Geriatr Soc 1984; 32: 49–55. [DOI] [PubMed] [Google Scholar]
- 17. Reed BR, Jagust WJ, Seab JP. Mental status as a predictor of daily function in progressive dementia. Gerontologist 1989; 29: 804–7. [DOI] [PubMed] [Google Scholar]
- 18. Blaum CS, Ofstedal MB, Liang J. Low cognitive performance, comorbid disease, and task-specific disability: findings from a nationally representative survey. J Gerontol A Biol Sci Med Sci 2002; 57: M523–31. [DOI] [PubMed] [Google Scholar]
- 19. Rooth V, van Oostrom SH, Deeg DJH et al. Common trajectories of physical functioning in the Doetinchem Cohort Study. Age Ageing 2016; 45: 382–8. [DOI] [PubMed] [Google Scholar]
- 20. Gandotra S, Lovato J, Case D et al. Physical function trajectories in survivors of acute respiratory failure. Ann Am Thorac Soc 2019; 16: 471–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Clouston SAP, Brewster P, Kuh D et al. The dynamic relationship between physical function and cognition in longitudinal aging cohorts. Epidemiol Rev 2013; 35: 33–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jia R, Liang J, Xu Y et al. Effects of physical activity and exercise on the cognitive function of patients with Alzheimer disease: a meta-analysis. BMC Geriatr 2019; 19: 181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tolea MI, Morris JC, Galvin JE. Trajectory of mobility decline by type of dementia. Alzheimer Dis Assoc Disord 2016; 30: 60–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Law C-K, Lam FM, Chung RC et al. Physical exercise attenuates cognitive decline and reduces behavioural problems in people with mild cognitive impairment and dementia: a systematic review. J Physiother 2020; 66: 9–18. [DOI] [PubMed] [Google Scholar]
- 25. Freedman VA, Kasper JD. Cohort profile: the National Health and Aging Trends Study (NHATS). Int J Epidemiol 2019; 48: 1044–1045g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kasper JD, Freedman VA. National Health and Aging Trends Study User Guide: Rounds 1–9 Final Release. Baltimore: Johns Hopkins University School of Public Health, 2020. Available at www.NHATS.org. [Google Scholar]
- 27. Kasper JD, Freedman VA, Spillman B. Classification of persons by dementia status in the National Health and Aging Trends Study. Technical Paper #5. Baltimore: Johns Hopkins University School of Public Health. Available at www.NHATS.org. [Google Scholar]
- 28. Guralnik JM, Simonsick EM, Ferrucci L et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol 1994; 49: M85–94. [DOI] [PubMed] [Google Scholar]
- 29. Dey AK, Alyass A, Muir RT et al. Validity of self-report of cardiovascular risk factors in a population at high risk for stroke. J Stroke Cerebrovasc Dis 2015; 24: 2860–5. [DOI] [PubMed] [Google Scholar]
- 30. Daniels T, Goodacre L, Sutton C et al. Accurate assessment of adherence. Chest 2011; 140: 425–32. [DOI] [PubMed] [Google Scholar]
- 31. Babiarczyk B, Sternal D. Accuracy of self-reported and measured anthropometric data in the inpatient population: anthropometric data in inpatients. Int J Nurs Pract 2015; 21: 813–9. [DOI] [PubMed] [Google Scholar]
- 32. Kasper JD, Chan KS, Freedman VA. Measuring physical capacity: an assessment of a composite measure using self-report and performance-based items. J Aging Health 2017; 29: 289–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Livingston G, Huntley J, Sommerlad A et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet 2020; 396: 413–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Keeney T, Fox AB, Jette DU et al. Functional trajectories of persons with cardiovascular disease in late life: functional trajectories in CVD. J Am Geriatr Soc 2019; 67: 37–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Nagin DS, Odgers CL. Group-based trajectory modeling in clinical research. Annu Rev Clin Psychol 2010; 6: 109–38. [DOI] [PubMed] [Google Scholar]
- 36. Roe CM, Xiong C, Grant E et al. Education and reported onset of symptoms among individuals with Alzheimer disease. Arch Neurol 2008; 65: 108–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ayyagari P, Ullrich F, Malmstrom TK et al., eds. Self-rated health trajectories in the African American health cohort. Bayer A (ed.). PLoS One 2012; 7: e53278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yang Z, Hardin JW, Addy CL. Testing overdispersion in the zero-inflated Poisson model. J Stat Plan Inference 2009; 139: 3340–53. [Google Scholar]
- 39. Nagin DS. Group-Based Modeling of Development. Cambridge, MA: Harvard University Press, 2005. [Google Scholar]
- 40. Jones BL, Nagin DS. A note on a Stata plugin for estimating group-based trajectory models. Sociol Methods Res 2013; 42: 608–13. [Google Scholar]
- 41. Royston P, White I. Multiple imputation by chained equations (MICE): implementation in Stata. J Stat Softw 2011; 45: 1–20. [Google Scholar]
- 42. Rubin DB. Multiple Imputation for Nonresponse in Surveys. New York: Wiley, 1987. [Google Scholar]
- 43. Hale JM, Schneider DC, Mehta NK et al. Cognitive impairment in the U.S.: lifetime risk, age at onset, and years impaired. SSM Popul Health 2020; 11: 100577. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.