Abstract
Objectives
: Viral respiratory infections are common in children, and usually associated with non-specific symptoms. Respiratory panel-based testing was implemented during the COVID-19 pandemic, for the rapid differentiation between SARS-CoV-2 and other viral infections, in children attending the emergency department (ED) of the teaching hospital of Lille, northern France, between February 2021 and January 2022.
Methods
: Samples were collected using nasopharyngeal swabs. Syndromic respiratory testing was performed with two rapid multiplex molecular assays: the BioFire® Respiratory Panel 2.1 - plus (RP2.1 plus) or the QIAstat-Dx Respiratory SARS-CoV-2 Panel. SARS-CoV-2 variant was screened using mutation-specific PCR-based assays and genome sequencing.
Results
: A total of 3517 children were included in the study. SARS-CoV-2 was detected in samples from 265 children (7.5%). SARS-CoV-2 infected patients were younger than those without SARS-CoV-2 infection (median age: 6 versus 12 months, p < 0.0001). The majority of infections (61.5%) were associated with the Omicron variant. The median weekly SARS-CoV-2 positivity rate ranged from 1.76% during the Alpha variant wave to 24.5% with the emergence of the Omicron variant. Most children (70.2%) were treated as outpatients, and seventeen patients were admitted to the intensive care unit. Other respiratory viruses were more frequently detected in SARS-CoV-2 negative children than in positive ones (82.1% versus 37.4%, p < 0.0001). Human rhinovirus/enterovirus and respiratory syncytial virus were the most prevalent in both groups.
Conclusions
: We observed a low prevalence of SARS-CoV-2 infection in children attending pediatric ED, despite the significant increase due to Delta and Omicron variants, and an important circulation of other respiratory viruses. Severe disease was overall rare in children.
Keywords: Syndromic testing, SARS-CoV-2, Respiratory viruses, Children, Emergency department
1. Introduction
Viruses are the leading cause of acute respiratory tract infections in children [1]. Respiratory viruses are diverse, with variable circulation patterns, even if most common viral respiratory infections tend to follow seasonal patterns with a peak incidence during winter in temperate regions [2]. Reinfections are possible and co-infections have been frequently reported in symptomatic young children [3].
The emergence and the rapid spread of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) have modified the existing overall picture. The burden of SARS-CoV-2 infection in children, although remaining low as compared to adults [4], has increased with the emergence of more transmissible variants such as the Delta or Omicron variants [5]. During the SARS-CoV-2 pandemic, the non-pharmaceutical interventions implemented to reduce the spreading of the virus, have also impacted the dynamics of other seasonal diseases that commonly affect children, most notably on respiratory syncytial virus (RSV) and influenza [6]. However, the magnitude, timing, and duration of this impact varied among viruses. Indeed, several respiratory viruses continued to circulate in the midst of the pandemic and could even rapidly return to prepandemic levels when COVID-19 mitigation practices become less stringent [7].
Respiratory viral infections lead to variable symptoms and disease severity in children, contributing to substantial morbidity, and cannot often be differentiated clinically. Therefore, testing for other pathogens, beyond SARS-CoV-2, is useful for clinical management.
Multiplex molecular assays have significantly improved the diagnosis of infectious diseases, and especially viral infections because they allow simultaneous detection of multiple pathogens in the same analysis. Moreover, some of these technologies with short turnaround time and minimal technical expertise allow rapid screening of pathogens associated with a specific infectious syndrome. This so-called ‘syndromic approach’ can provide a rapid result, with the promise of timely clinical decisions such as hospital admission, isolation, and antimicrobial treatment or lack thereof [8,9].
We here describe data provided by the implementation of respiratory panel-based testing for the rapid differentiation between SARS-CoV-2 and other viral infections, in children attending the emergency department (ED) of the teaching hospital of Lille, northern France between February 2021 and January 2022.
2. Materials and methods
2.1. Patients and samples
This is a retrospective monocentric study conducted at the Lille University Hospital (CHU Lille), France. Children aged ≤ 15 years, attending the pediatric ED with suspicion of respiratory infection i.e. children presenting with fever and/or respiratory symptoms and/or digestive symptoms on admission [10], and who underwent a syndromic testing between February 15th, 2021 and January 30th, 2022, were included.
Nasal or naso-pharyngeal specimens were collected using flocked swabs eluted in 3 mL of viral transport medium (Yocon, Beijing, China).
This study was based on medical and laboratory records, in strict compliance with the French reference methodology MR-004 established by French National Commission on Informatics and Liberties (CNIL), and approved by the Institutional data protection authority of CHU Lille under the number DEC21–364.
2.2. Laboratory methods
Upon admission, samples were heat-inactivated (incubation at 56 °C for 30 min) before analysis. Two assays were used for rapid respiratory-panel testing depending on reagents availability: (i) the BioFire® Respiratory Panel 2.1 - plus (RP2.1 plus) – by bioMérieux® on the FILMARRAY Multiplex Real-Time PCR System (bioMérieux®, Lyon, France), and (ii) the QIAstat-Dx Respiratory SARS-CoV-2 Panel – by Qiagen® on the QIAstat-Dx Analyzer (Qiagen®, Les Ulis, France). A brief description of the two methods is provided in Table 1 .
Table 1.
Diagnostic assays.
| Characteristics | BioFire ® Respiratory Panel 2.1 - plus | QIAstat-Dx Respiratory SARS-CoV-2 Panel |
|---|---|---|
| Pathogens detected | - Viruses: Adenovirus, Coronaviruses 229E, HKU1, NL63, and OC43, SARS-CoV-2, Human Metapneumovirus A/B, Influenza A (with differentiation of Influenza A/H1, A/H3, A/H1–2009), Influenza B, Parainfluenza virus 1,2 3 and 4, MERS-CoV, Rhinovirus/Enterovirus, Respiratory Syncytial Virus A/B, SARS-CoV-2 - Bacteria: Bordetella pertussis, Bordetella parapertussis, Chlamydia pneumoniae, Mycoplasma pneumoniae |
- Viruses: Adenovirus, Bocavirus, Coronaviruses 229E, HKU1, NL63, and OC43, SARS-CoV-2, Human Metapneumovirus A/B, Influenza A (with differentiation of Influenza A/H1, A/H3, A/H1–2009), Influenza B, Parainfluenza virus 1,2 3 and 4, Rhinovirus/Enterovirus, Respiratory Syncytial Virus A/B, SARS-CoV-2 - Bacteria: Bordetella pertussis, Mycoplasma pneumoniae, Legionella pneumophila |
| Turnaround time | 45 min | 60 min |
| Sample input volume | 300 µL | 300 µL |
| Report of Ct value | No (melting curves) | Yes |
SARS-CoV-2 variant screening was routinely performed using mutation-specific PCR-based assays, including VirSNiP SARS B117 (Tib MolBiol, Roche Diagnostics, Meylan, France), EurobioPlex SARS-CoV-2 SNPs (Eurobio Scientific, Les Ulis, France). Inconclusive results were confirmed using viral whole genome sequencing for samples with optimal viral load (Ct value ≤ 28). Briefly, total RNA extraction was performed with the MGIEasy Nucleic Acid Extraction Kit on the MGISP-960 instrument (BGI®, Shenzen, China). The libraries were prepared using Illumina® COVIDSeq protocol, and paired-end sequencing with 150 bp read length was carried out on NextSeq 550 platform. Data were processed using DRAGEN COVIDSeq Test Pipeline 1.0.0 (Illumina®, Evry, France).
2.3. Statistical analysis
GraphPad Prism software was used for statistical analyses. Data were presented as median and interquartile range (IQR), or as percentage. Fisher's exact test was used to compare categorical variables. Mann–Whitney U and Kruskal-Wallis tests were used to compare quantitative variables when appropriate. A two-sided p-value < 0.05 was considered statistically significant.
3. Results
A total of 3517 children admitted at the pediatric (ED during the study period were included. Children were mainly male (56.1%), and the median age was 12 months. Children aged less than 5 years represented the majority (92.8%) of the population. The age distribution is shown in Table 2 .
Table 2.
Demographics of patients.
| Characteristics | All patients (n = 3517) | SARS-CoV-2 positive patients (n = 265) | SARS-CoV-2 negative patients (n = 3252) |
|---|---|---|---|
| Sex ratio,% of male | 56.1 | 53.2 | 56.3 |
| Median age (IQR), months | 12 (4 – 26) | 6 (2 – 20.5) | 12 (5 – 27) |
| Age distribution,% | |||
| ▒< 1month | 4.6 | 5.3 | 4.5 |
| ▒1 – 23 months | 66.4 | 73.6 | 65.8 |
| ▒24 - 59 months | 21.8 | 12.1 | 22.6 |
| ▒60 – 119 months | 4.9 | 4.9 | 4.9 |
| ▒≥ 120 months | 2.3 | 4.1 | 2.2 |
3.1. SARS-CoV-2 infection
SARS-CoV-2 was detected in samples from 265 children (7.5%). SARS-CoV-2 infected patients were younger than those without SARS-CoV-2 infection (median age: 6 months versus 12 months, p < 0.0001) (see Table 2). Most patients (234/265, 88.3%) presented with acute upper respiratory tract infections. Fourteen patients (5.3%) did not present respiratory symptoms on admission. Ten (3.8%) and seven (2.6%) patients were diagnosed on admission with moderate and severe pneumonia respectively.
The majority of children (70.2%) were treated as outpatients. The hospitalization occurred for 94.1% (16/17) of children with lower respiratory tract infections and for 25% (63/248) of other children. A total of 62 children (23.4%) were hospitalized in general pediatric wards, including six hospitalizations (2.3%) not related to COVID-19. Seventeen admissions (6.4%) to the intensive care unit (ICU) were recorded (Table 3 ). Overall, they were either very young needing a respiratory or enteral support, or presenting a viral coinfection and/or an underlying condition. One death was recorded in a 2-month-old boy who presented with severe pneumonia on admission, and who rapidly developed an acute respiratory distress syndrome. A bacterial pulmonary co-infection with E coli K1 was also evidenced. The death occurred two days post-admission.
Table 3.
Description of SARS-CoV-2 positive children admitted to ICU.
| Patient number | Age | Underlying condition | Clinical features | Outcome |
|---|---|---|---|---|
| 1 | 22 days | None | Acute upper respiratory tract infection. Non-invasive respiratory support needed. No viral coinfection | Alive |
| 2 | 23 days | None | Acute upper respiratory tract infection. Non-invasive respiratory support and enteral nutrition support needed. No viral coinfection. | Alive |
| 3 | 1 month | None | Acute upper respiratory tract infection. Enteral nutrition support needed. No viral coinfection. | Alive |
| 4 | 1 month | None | Severe acute bronchiolitis. Non-invasive respiratory support needed. Coinfection with influenzavirus | Alive |
| 5 | 1 month | None | Gastrointestinal symptoms. Enteral nutrition support needed. No viral coinfection | Alive |
| 6 | 1 month | Preterm birth | Acute upper respiratory tract infection. Non-invasive respiratory support needed. Coinfection with Parainfluenzavirus 3 | Alive |
| 7 | 1 month | None | Severe acute bronchiolitis. Non-invasive respiratory support needed. Coinfection with RSV | Alive |
| 8 | 1 month | None | Severe acute bronchiolitis. Non-invasive respiratory support needed. Coinfection with RSV | Alive |
| 9 | 1 month | None | Severe acute bronchiolitis. Non-invasive respiratory support needed. Coinfection with RSV | Alive |
| 10 | 1 month | None | Gastrointestinal symptoms. Enteral nutrition support needed. No viral coinfection | Alive |
| 11 | 2 months | None | Severe pneumonia with evolution to acute respiratory distress syndrome. No viral coinfection. Bacterial pulmonary co-infection with E. coli K1 | Death |
| 12 | 2 months | None | Severe acute bronchiolitis. Non-invasive respiratory support needed. Coinfection with RSV | Alive |
| 13 | 2 months | None | Severe pneumonia following aspiration from dysphagia. Non-invasive respiratory support needed. No viral coinfection. | Alive |
| 14 | 4 months | polycystic kidney disease | Moderate pneumonia. Non-invasive respiratory support needed. Corticosteroid therapy. No viral coinfection. | Alive |
| 15 | 3.2 years | Asthma | Severe pneumonia. Coinfection with RSV and HRV/EV | Alive |
| 16 | 3.8 years | sickle cell disease | Acute chest syndrome and acute hemolytic anemia. No viral coinfection | Alive |
| 17 | 8.4 years | Pansinusitis complicated by cerebral empyema | Postoperative ICU admission. Not related to COVID-19 | Alive |
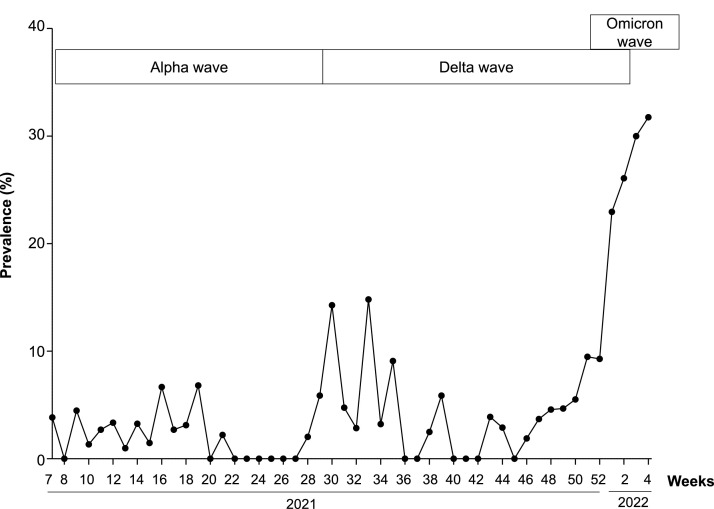
The distribution of cases over time is shown in Fig. 1 , and the number of tests and positive results per week is detailed in table S1 (supplementary material).
Fig. 1.
Distribution over time of SARS-CoV-2 infections.
SARS-CoV-2 variant information was available for 208 patients (78.5%). Alpha, Delta and Omicron variants were detected in 19 (9.1%), 60 (28.8%) and 128 (61.5%) samples respectively. B.1.640 lineage was found in one sample.
Alpha variant has been detected in children until week 28 (2021). The Delta variant was detected from week 29 (2021) to week 3 (2022), and the Omicron variant was detected since week 51 (2021). The median weekly SARS-CoV-2 positivity rate (ratio between cases and total number of tested children) was 1.76% and 3.5% during the Alpha wave, and the “Delta only” circulation period respectively. This rate increased to 24.5% with the emergence of Omicron variant (p = 0.0002). The median age was 7, 4 and 5 months in the Alpha, Delta and Omicron cases, respectively (p = 0.12). The hospitalization rate during the Alpha wave (5/19, 26.3%) was similar to that observed in Delta cases (22/60, 36.7%, p = 0.58) and in Omicron cases (31/128, 24.2%, p = 0.78).
3.2. Other respiratory viruses
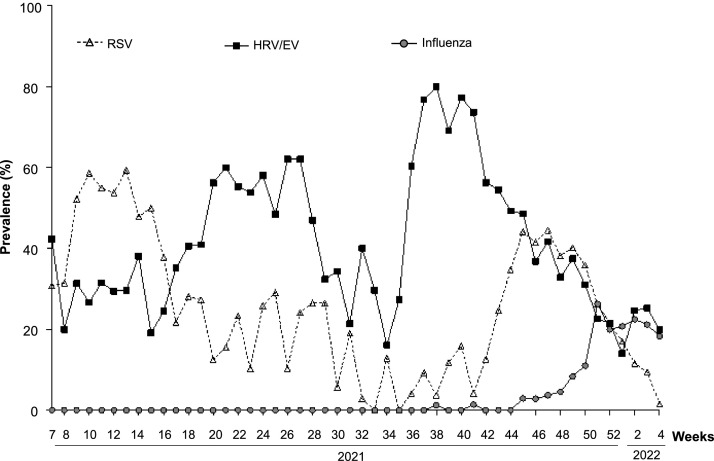
At least one other respiratory virus was detected in 2768 children (78.7%). The two most common viruses were human rhinovirus/enterovirus (HRV/EV) and respiratory syncytial virus (RSV) detected in 39.5% and 29.1% of the study population, respectively. The weekly distribution of these viruses is shown in Fig. 2 . The median weekly proportion of HRV/EV positive samples was 37.7%, with prevalence ranging from 14% to 80% during the study period (Fig. 2). The highest prevalences were observed during weeks 18 to 28 (May to July), and weeks 36–45 (September to November). RSV circulation was detected throughout the study period, with a median weekly proportion of positive samples at 24.4% (Fig. 2). A first peak was observed from February to April (weeks 7–16). A second important circulation period was observed from week 44 (November) to week 1 (January 2022).
Fig. 2.
Distribution over time of Respiratory syncytial virus, rhinovirus/enterovirus and influenzavirus infections.
Respiratory viruses were detected in 99 out of 265 (37.4%) SARS-CoV-2 positive patients and in 2669 out 3252 (82.1%) of SARS-CoV-2 negative children, showing a more important circulation of other respiratory viruses in SARS-CoV-2 negative children (p < 0.0001).
The repartition of detected viruses in COVID-19 and non-COVID-19 patients is presented in Table 4 . Of note, the circulation of influenza virus weakly started in weeks 38–40, and peaked from week 51. Beyond viruses, the panel testing allowed the detection of Bordetella pertussis in 2 children.
Table 4.
Other respiratory viruses.
| Respiratory viruses | COVID-19 children (n = 265) | Non-COVID-19 children (n = 3252) | p value |
|---|---|---|---|
| Enterovirus/human rhinovirus | 43 (16.2%) | 1283 (39.5%) | P < 0.0001 |
| Respiratory syncytial virus | 21 (7.9%) | 947 (29.1%) | P < 0.0001 |
| Adenovirus | 16 (6%) | 373 (11.5%) | P < 0.0001 |
| Human parainfluenza virus 1–4 | 10 (3.8%) | 362 (11.1%) | P < 0.0001 |
| Human metapneumovirus | 4 (1.5%) | 188 (5.8%) | p = 0.002 |
| Coronaviruses (OC43, HKU1, 229E, NL63) | 18 (6.8%) | 345 (10.6%) | p = 0.06 |
| Influenzavirus | 8 (3%) | 216 (6.6%) | p = 0.02 |
| Bocavirus | 11 (4.2%) | 151 (4.6%) | p = 0.9 |
4. Discussion
The measures adopted to contain the COVID-19 pandemic in our hospital included a systematic screening of SARS-CoV-2 infection in children attending pediatric emergency department, especially those with respiratory symptoms. Since these symptoms are usually non-specific, testing for other viral pathogens seemed reasonable, and a respiratory syndromic testing approach was implemented.
The overall prevalence of SARS-CoV-2 infection during the study period was 7.5%. However the prevalence before the emergence of the Omicron variant ranged from 1.76 to 3.5%, and was similar to that previously reported [11]. SARS-CoV-2 infected children were younger than those without SARS-CoV-2 infection. This observation is probably related to milder SARS-CoV-2 symptoms in older children, and therefore fewer ED visits. No difference regarding the age was observed between children infected with the different variants. In addition, the hospitalization rates were similar between all groups.
The emergence of more transmissible variants of concern was associated with an increase of infection rates in unvaccinated populations, including children. However, previous data with the Alpha and Delta variants showed that hospitalization rates remained stable in children, suggesting that these variants do not lead to more severe disease in children [12]. More interestingly, a recent analysis (preprint paper) of the Omicron wave suggested even fewer hospitalizations and less severe outcomes in children under 5 years, as compared to the Delta wave [13].
Findings in this study suggest that severe disease is overall rare in children because the few patients admitted to ICU were fragile or presenting a viral coinfection and/or an underlying disease. Nevertheless, COVID-19 can lead to death in children [14].
The multiplex testing approach also gave us the opportunity to investigate the circulation of the other respiratory viruses. We found that more than a third of COVID-19 patients had a coinfection with another virus. However, the detection rate of other respiratory viruses was significantly higher in the non-COVID-19 patients than in SARS-CoV-2 infected children.
The HRV/EV complex was the most detected in samples, with high detection rates maintained all over the year. Rhinoviruses and other respiratory enteroviruses (belong to the genus Enterovirus) are usually grouped and targeted together in commercial respiratory assays.
Rhinovirus detection is very common in children and is usually asymptomatic or associated with mild symptoms; however acute and severe symptoms (wheezing and asthma exacerbations) can be observed in younger children [15], [16], [17].
Our data are in agreement with previous studies that reported an unchanged rhinovirus circulation during the COVID-19 pandemic (data of year 2020), with no impact of social restrictions [18], [19], [20], [21].
The mode of transmission of rhinoviruses or other enteroviruses can explain this observation. These non-enveloped viruses are usually transmitted by direct contact, and can survive on fingers with possible self‐inoculation of the nose or conjunctiva following inadvertent contamination of the fingers [22].
Regarding RSV circulation in 2021, this study clearly confirms the unusual delay of the 2020/2021 winter outbreak previously reported in France [23], with a peak around weeks 10–13. This observation is probably related to an increase in social restrictions with a second lockdown between end October and mid-December 2020, even if less strict than the first one. Our data also suggest that the third lockdown that started in Week 13 at the peak of 2020/2021 epidemic might contribute to the following decrease in the number of cases. We also observed the return to prepandemic patterns regarding the circulation of RSV and influenzavirus during 2021/2022 winter.
The main strength of our study is the high number of samples tested, using a multiplex approach yielding a result within 4 h after sampling and available 24 h/24 h. The study is limited by the lack of comparison with data from previous years and the absence of impact evaluation of such testing.
In conclusion, the implementation of the syndromic testing approach aimed to timely differentiate between SARS-CoV-2 and other viral infections in order to improve patient management in a context of reduced hospital beds. Overall, we observed a low prevalence of SARS-CoV-2 infection in children attending pediatric ED, despite the significant increase due to Delta and Omicron variants, and an important circulation of other respiratory viruses.
The identification of a viral etiology can reduce unnecessary investigations and promote antibiotic stewardship. However, this diagnostic approach is expensive, and its utility and contributions need to be further evaluated [24].
Funding
This work was supported by the University Hospital of Lille.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.jcv.2022.105221.
Appendix. Supplementary materials
References
- 1.Lozano R., Naghavi M., Foreman K., Lim S., Shibuya K., Aboyans V., et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the global burden of disease study 2010. Lancet. 2012;380:2095–2128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Price R.H.M., Graham C., Ramalingam S. Association between viral seasonality and meteorological factors. Sci. Rep. 2019;9:929. doi: 10.1038/s41598-018-37481-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mandelia Y., Procop G.W., Richter S.S., Worley S., Liu W., Esper F. Dynamics and predisposition of respiratory viral co-infections in children and adults. Clin. Microbiol. Infect. 2021;27 doi: 10.1016/j.cmi.2020.05.042. 631.e1-631.e6. [DOI] [PubMed] [Google Scholar]
- 4.Hoang A., Chorath K., Moreira A., Evans M., Burmeister-Morton F., Burmeister F., et al. COVID-19 in 7780 pediatric patients: a systematic review. EClinicalMedicine. 2020;24 doi: 10.1016/j.eclinm.2020.100433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marks K.J., Whitaker M., Anglin O., Milucky J., Patel K., Pham H., et al. Hospitalizations of children and adolescents with laboratory-confirmed COVID-19 - COVID-NET, 14 States, July 2021-January 2022. MMWR Morb. Mortal Wkly Rep. 2022;71:271–278. doi: 10.15585/mmwr.mm7107e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Williams T.C., Sinha I., Barr I.G., Zambon M. Transmission of paediatric respiratory syncytial virus and influenza in the wake of the COVID-19 pandemic. Euro. Surveill. 2021;26 doi: 10.2807/1560-7917.ES.2021.26.29.2100186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Olsen S.J., Azziz-Baumgartner E., Budd A.P., Brammer L., Sullivan S., Pineda R.F., et al. Decreased influenza activity during the COVID-19 pandemic - United States, Australia, Chile, and South Africa, 2020. MMWR Morb. Mortal Wkly Rep. 2020;69:1305–1309. doi: 10.15585/mmwr.mm6937a6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ramanan P., Bryson A.L., Binnicker M.J., Pritt B.S., Patel R. Syndromic panel-based testing in clinical microbiology. Clin. Microbiol. Rev. 2018;31:e00024. doi: 10.1128/CMR.00024-17. -17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zanella M.-.C., Meylan P., Kaiser L. Syndromic panels or “panel syndrome”? A perspective through the lens of respiratory tract infections. Clin. Microbiol. Infect. 2020;26:665–668. doi: 10.1016/j.cmi.2019.12.018. [DOI] [PubMed] [Google Scholar]
- 10.Shen K., Yang Y., Wang T., Zhao D., Jiang Y., Jin R., et al. Diagnosis, treatment, and prevention of 2019 novel coronavirus infection in children: experts’ consensus statement. World J. Pediatr. 2020;16:223–231. doi: 10.1007/s12519-020-00343-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meyer M., Holfter A., Ruebsteck E., Gruell H., Dewald F., Koerner R.W., et al. The alpha variant (B.1.1.7) of SARS-CoV-2 in children: first experience from 3544 nucleic acid amplification tests in a cohort of children in Germany. Viruses. 2021;13:1600. doi: 10.3390/v13081600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ladhani S.N., sKIDs Investigation Team Children and COVID-19 in schools. Science. 2021;374:680–682. doi: 10.1126/science.abj2042. [DOI] [PubMed] [Google Scholar]
- 13.Wang L., Berger N.A., Kaelber D.C., Davis P.B., Volkow N.D., Xu R. COVID infection severity in children under 5 years old before and after Omicron emergence in the US. MedRxiv 2022:2022.01.12.22269179. https://doi.org/10.1101/2022.01.12.22269179.
- 14.Goldman D.L., Aldrich M.L., Hagmann S.H.F., Bamford A., Camacho-Gonzalez A., Lapadula G., et al. Compassionate Use of Remdesivir in children with severe COVID-19. Pediatrics. 2021;147 doi: 10.1542/peds.2020-047803. [DOI] [PubMed] [Google Scholar]
- 15.Erkkola R., Turunen R., Räisänen K., Waris M., Vuorinen T., Laine M., et al. Rhinovirus C is associated with severe wheezing and febrile respiratory illness in young children. Pediatr. Infect. Dis. J. 2020;39:283–286. doi: 10.1097/INF.0000000000002570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lejeune S., Pichavant M., Engelmann I., Béghin L., Drumez E., Le Rouzic O., et al. Severe preschool asthmatics have altered cytokine and anti-viral responses during exacerbation. Pediatr Allergy Immunol. 2020;31:651–661. doi: 10.1111/pai.13268. [DOI] [PubMed] [Google Scholar]
- 17.Ruohola A., Waris M., Allander T., Ziegler T., Heikkinen T., Ruuskanen O. Viral etiology of common cold in children, Finland. Emerg. Infect. Dis. 2009;15:344–346. doi: 10.3201/eid1502.081468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuitunen I., Artama M., Haapanen M., Renko M. Rhinovirus spread in children during the COVID-19 pandemic despite social restrictions-A nationwide register study in Finland. J. Med. Virol. 2021;93:6063–6067. doi: 10.1002/jmv.27180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Park S., Michelow I.C., Choe Y.J. Shifting patterns of respiratory virus activity following social distancing measures for coronavirus disease 2019 in South Korea. J. Infect. Dis. 2021;224:1900–1906. doi: 10.1093/infdis/jiab231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rodgers L., Sheppard M., Smith A., Dietz S., Jayanthi P., Yuan Y., et al. Changes in seasonal respiratory illnesses in the United States during the coronavirus disease 2019 (COVID-19) pandemic. Clin. Infect. Dis. 2021;73:S110–S117. doi: 10.1093/cid/ciab311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Takashita E., Kawakami C., Momoki T., Saikusa M., Shimizu K., Ozawa H., et al. Increased risk of rhinovirus infection in children during the coronavirus disease-19 pandemic. Influenza Other Respir. Viruses. 2021;15:488–494. doi: 10.1111/irv.12854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.L'Huillier A.G., Tapparel C., Turin L., Boquete-Suter P., Thomas Y., Kaiser L. Survival of rhinoviruses on human fingers. Clin. Microbiol. Infect. 2015;21:381–385. doi: 10.1016/j.cmi.2014.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Delestrain C., Danis K., Hau I., Behillil S., Billard M.-.N., Krajten L., et al. Impact of COVID-19 social distancing on viral infection in France: a delayed outbreak of RSV. Pediatr. Pulmonol. 2021;56:3669–3673. doi: 10.1002/ppul.25644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Diallo D., Hochart A., Lagree M., Dervaux B., Martinot A., Dubos F. Impact of the Sofia® Influenza A+B FIA rapid diagnostic test in a pediatric emergency department. Arch. Pediatr. 2019;26:6–11. doi: 10.1016/j.arcped.2018.10.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.