Abstract
Context
The COVID-19 pandemic has highlighted variability in intensity of care. We aimed to characterize intensity of care among hospitalized patients with COVID-19.
Objectives
Examine the prevalence and predictors of admission code status, palliative care consultation, comfort-measures-only orders, and cardiopulmonary resuscitation (CPR) among patients hospitalized with COVID-19.
Methods
This cross-sectional study examined data from an international registry of hospitalized patients with COVID-19. A proportional odds model evaluated predictors of more aggressive code status (i.e., Full Code) vs. less (i.e., Do Not Resuscitate, DNR). Among decedents, logistic regression was used to identify predictors of palliative care consultation, comfort measures only, and CPR at time of death.
Results
We included 29,923 patients across 179 sites. Among those with admission code status documented, Full Code was selected by 90% (n = 15,273). Adjusting for site, Full Code was more likely for patients who were of Black or Asian race (ORs 1.82, 95% CIs 1.5–2.19; 1.78, 1.15–3.09 respectively, relative to White race), Hispanic ethnicity (OR 1.89, CI 1.35–2.32), and male sex (OR 1.16, CI 1.0–1.33). Of the 4951 decedents, 29% received palliative care consultation, 59% transitioned to comfort measures only, and 29% received CPR, with non-White racial and ethnic groups less likely to receive comfort measures only and more likely to receive CPR.
Conclusion
In this international cohort of patients with COVID-19, Full Code was the initial code status in the majority, and more likely among patients who were Black or Asian race, Hispanic ethnicity or male. These results provide direction for future studies to improve these disparities in care.
Key Words: Palliative care, COVID, End of life
Background
When the COVID-19 pandemic began in early 2020, healthcare systems quickly became overwhelmed with high volumes of critically ill patients, particularly older adults and those with underlying comorbidities. This surge led to a strain on available resources and unprecedented ethical questions around allocation of potentially limited resources.1 , 2 The utility of resuscitation efforts for in-hospital cardiac arrest (IHCA) specifically was scrutinized3 , 4 given the high resource utilization, concerns around exposing clinicians to COVID-19, and early studies demonstrating nearly universally poor outcomes of IHCA.5, 6, 7 As a result, both palliative care specialists and ethics teams were increasingly called upon to assist with questions related to goals of care and code status determinations for patients with COVID-19.8 , 9
With the attention paid to IHCA outcomes in the COVID-19 pandemic and the importance of code status in shaping the landscape of resuscitation efforts, we need to better understand the factors associated with a documented code status of Full Code vs. Do Not Resuscitate (DNR) for patients with COVID-19. Prior to the pandemic, patients who were older and White were more likely to have a DNR order.10 Early in the pandemic, a single center study found that patients with a DNR order were younger and had fewer comorbidities than those with a DNR order prior to the pandemic.11 In addition, palliative care consultation, which has been found to play an important role in clarifying goals of care for patients with serious illness, has been more commonly utilized during the pandemic for patients who are older and have more comorbidities.12 An association between palliative care consultation and CMO orders has been found in a similar cohort of patients with COVID-19, however separate predictors of these two outcomes have not been fully elucidated.13 A three-center study in Massachusetts found an association between non-English language preference and Full Code for patients in the ICU with COVID-19.14 To date, the factors that influence code status, as well as the factors associated with receiving cardiopulmonary resuscitation (CPR), comfort-measures only (CMO) plan of care, or a specialty palliative care consultation have not been evaluated at a population level within the context of the COVID-19 pandemic. Understanding the frequency of specific code status orders, patient characteristics associated with specific treatments near the end of life, and provision of specialty palliative care consultation may provide insights into how this care was provided during a pandemic, and inform future opportunities to improve decisions about resource allocation for intensive and palliative care. Using data from a large, multi-center registry of patients hospitalized with COVID-19, our objectives were to 1) describe the frequency and patient predictors of specific code status orders at the time of hospital admission, and 2) describe patient predictors of receiving CPR, CMO, and palliative care consultation among patients who died in the hospital of COVID-19.
Methods
Design
This study was conducted using data from the Society of Critical Care Medicine's Discovery Viral Infection and Respiratory Illness Universal Study (VIRUS) registry.15 , 16 The VIRUS: COVID-19 registry is an international database of patients from 179 sites across the six WHO regions that were hospitalized from April 2020 through March 2021 with a SARS CoV-2 infection. Academic or community-based hospitals in the US and other countries participate on a voluntary basis. Interested sites reach out to the VIRUS registry principal investigators, go through an intake process, and are invited to participate after agreeing to contribute de-identified COVID-19 hospitalized patient data. Study data were recorded and managed using the Research Electronic Data Capture system.17 Research Electronic Data Capture is a secure, web-based application designed to support data capture for research studies, providing: 1) an intuitive interface for validated data entry; 2) audit trails for tracking data manipulation and export procedures; 3) automated export procedures for seamless data downloads to common statistical packages; and 4) procedures for importing data from external sources. Data were assessed for quality every week, with weekly quality reports communicated to participating sites’ principle investigators and study coordinators. Patient data with erroneous entries were assessed, sent back and rectified. Only cleaned data were utilized for final analysis.
The data registry used for this study was determined to be exempt from Mayo Clinic's (coordinating study site) institutional review board (IRB). In addition, participating study sites obtained site-specific IRB approval and implemented data use agreements with the Mayo Clinic before data were collected. The study is registered on Clinicaltrials.gov: NCT04323787.
Outcomes and Predictors of Interest
The main outcomes of interest were the first code status documented after admission for all patients, and processes of care for those who died. Code status on admission was categorized as Full Code, DNR with endotracheal intubation as an option, or DNR with Do Not Intubate (DNR/DNI). This was treated as an ordinal variable reflecting treatment plans from the most to least aggressive care. The processes of care for deceased patients that were evaluated were: 1) palliative care consultation; 2) an order for CMO; and 3) the receipt of CPR at the time of death. The predictors of interest included patient age, sex, race, ethnicity, admission location (intensive care unit [ICU], or acute care), study site, and number of comorbidities (0, 1, 2, or 3 or more; listed in Table 1 ). ICU admission was included as a predictor rather than an outcome because it is a surrogate for disease severity, which could in turn affect the care processes of interest. In the VIRUS registry race was categorized as White Caucasian, Black or African American, Asian American, American Indian or Alaskan Native, Hawaiian or Pacific Islander, East Asian, South Asian, West Asian, Southeast Asian, Mixed, or Other. Due to small sample sizes in some of these categories, we combined East Asian, South Asian, West Asian, and Southeast Asian into one category (Asian Native), and American Indian or Alaskan Native, Hawaiian or Pacific Islander, Mixed, and Other into one category (Other).
Table 1.
Comorbidities Documented and Included in Analyses
| Coronary artery disease |
| Hypertension |
| Cardiac arrhythmias |
| Congestive heart failure |
| Valvular heart disease |
| Chronic pulmonary disease (not asthma) |
| Asthma (physician diagnosed) |
| Pulmonary circulation disorder |
| Chronic kidney disease |
| Chronic dialysis |
| Diabetes |
| Hypothyroidism |
| Liver disease |
| Hepatitis B |
| Hepatitis C |
| Peptic ulcer disease excluding bleeding |
| Solid tumor without metastasis |
| Hematologic malignancy |
| Metastatic cancer |
| History of solid organ or bone marrow transplant |
| HIV/AIDS or other immunosuppression |
| Stroke or other neurological disorders |
| Paralysis |
| Rheumatoid arthritis/collagen vascular disease |
| Blood loss anemia |
| Iron deficiency anemia |
| Coagulopathy |
| Malnutrition |
| Obesity |
| Substance use disorder |
| Depression |
| Psychosis |
| Dementia |
| Obstructive sleep apnea |
| Venous thromboembolic disease |
| Dyslipidemia/hyperlipidemia |
| Other comorbidity not otherwise specified |
Statistical Analysis
To determine the association between predictors and the three different code status orders on admission, a proportional odds mixed model with random intercepts for different study sites was used. Standard error estimates and 95% confidence intervals (CIs) of the effect sizes were obtained via bootstrapping methods.
A proportional odds model can be viewed as an extension of logistic models. For an ordinal response with more than two levels (such as code status in our analysis), assigning numeric values to the levels and analyzing them as a continuous variable may not be justifiable. On the other hand, dichotomizing the ordinal response and conducting a binary logistic regression relies on the choice of cut point in such dichotomization, which is often arbitrary. In a proportional odds model, the effect size for each predictor is taken to be constant (known as the proportional odds assumption) across all possible combinations of the response variables, which are A) Full Code and DNR/endotracheal intubation as an option vs. DNR/DNI; and B) Full Code vs. DNR/endotracheal intubation as an option and DNR/DNI in our setting. This model takes into account the ordinality of the three outcome categories from least aggressive to most aggressive care, without making the (somewhat arbitrary) decisions to convert the response to be numerical or binary. These effect sizes are interpreted as the odds ratio of opting for more aggressive IHCA care for each covariate.
Including study site into the analysis as a covariate could lead to inaccurate estimates, because the large number of sites will lead to a high degree of freedom in the model. Therefore, we chose to account for the variability between study sites using a mixed model. Specifically, we included random intercepts by site into our proportional odds model, which specified site-specific intercepts in the model, representing a site-specific effect on the baseline odds of opting for more aggressive code status. These site-specific intercepts were assumed to be random, following a normal distribution. In a proportional odds model with random intercepts, the interpretation of covariate effects is restricted to comparison of patients within the same site. Though the interpretation is site-specific (i.e., conditioning on the same random effect), the estimation for fixed, covariate effects is based on observations across all sites.
Missing data were addressed using inverse probability weighting (IPW).18 By removing observations with missing values, biases could be introduced since the remaining complete observations may not be representative of the whole population. Incorporating IPW in our analyses put more weight on the less represented observations (those predicted as more likely to be missing) to account for this. Missing data were modeled using logistic regression with age, race, death, and admission location as predictors, and the inverse of the predicted probabilities of nonmissing were used as weights in our proportional odds mixed model. An IPW procedure relies on the assumption of missing at random, which means that the missing data only depend on the fully observed variables, but not the ones with missing values.
To determine the association between each covariate and process of care, we fit logistic models with random intercepts for each of three processes of care (palliative care consultation, CMO orders, or receipt of CPR) as a binary outcome. Similar to the mixed model for code status, these models specified site-specific effects on each of the three treatments. These models were adjusted for patient age, sex, race, ethnicity, admission location, and number of comorbidities.
Results
Code Status on Admission
Data were extracted for 29,923 patients representing 179 sites in the registry as of April 2021. There were 129 sites from the US, eight non-US sites in the Region of the Americas, 14 sites in the European Region, 12 sites in the South-East Asian Region, 9 sites in the Eastern Mediterranean/African Region, and 7 sites in the Western Pacific Region. Participating countries are listed in Supplemental Table 1. Patients had a median age of 60.7 years (standard deviation [SD]: 17.5 years), 45% were female, 23% were black, 19% were Hispanic/Latino, and 45% had three or more comorbidities (Table 2 ). Code status on admission was not documented for 12,981 patients (40%), with clusters of missing code status data by site (22 sites did not document code status, and another 30 had >50% of patients without admission code status documented). Distribution of missing code status data does not appear to be related the prevalence of code status orders, and is detailed in Supplemental Figure 1A. Patients with code status documented, compared to those without, and had a higher proportion of ICU admissions (42% vs. 33%) and a higher proportion of individuals with 3 or more comorbidities (55% vs. 45%). Of the 16,942 patients for whom code status was documented on admission, 90% had an order for Full Code, 2% for DNR with endotracheal intubation as an option, and 7% for DNR/DNI (Table 3 ). A majority of subjects in each racial and ethnic category had an order for Full Code (Table 4 ). The remaining <1% were documented to have an order for DNI with resuscitation as an option, however these were excluded from the analyses due to the low number and limited clinical significance.
Table 2.
Baseline Characteristics for All Patients and Those With Code Status Documented
| All Patients | Code Status Present | |
|---|---|---|
| Characteristic | N = 29,923 | N = 16,942 |
| Patient age, yrs, mean (SD) | 60.7 (17.5) | 61.4 (17.2) |
| Female, n (%) | 13,344 (45) | 7437 (44) |
| Race, n (%) | ||
| White | 16,829 (56) | 9118 (54) |
| Black/African American | 6939 (23) | 4299 (25) |
| Asian American | 479 (2) | 343 (2) |
| Asian Native | 2212 (7) | 843 (5) |
| Other | 2972 (10) | 2131 (13) |
| Hispanic/Latino, n (%) | 5770 (19) | 4130 (24) |
| ICU admission | 9919 (33) | 7165 (42) |
| Comorbidities, n (%) | ||
| 0 | 3487 (12) | 1865 (11) |
| 1 | 4888 (16) | 2587 (15) |
| 2 | 4792 (16) | 2755 (16) |
| 3+ | 13,442 (45) | 9341 (55) |
Missing data:
Patient age: 16 for all patients, 8 for code status presents; Sex: 272 for all patients, 9 for code status present; Race: 492 for all patients, 9 for code status present; Ethnicity: 7435 for all patients, 2142 for code status present; Comorbidities: 3314 for all patients, 394 for code status present.
Table 3.
Overall Code Status Orders on Admission (n = 16,942)
| Code Status Order | Number of Patients (%) |
|---|---|
| Full Code | 15,273 (90) |
| DNR, Intubation okay | 411 (2) |
| DNR/DNI | 1206 (7) |
| Resuscitate, DNI | 52 (<1) |
Abbreviations: DNAR, do not attempt resuscitation; DNI, do not intubate.
Table 4.
Code Status by Covariate
| Race | ||||||
|---|---|---|---|---|---|---|
| White (N = 16,829) | Asian American (N = 479) | Black or African American (N = 6939) | Other (N = 2972) | Asian Other (N = 2212) | Missing (N = 492) | |
| Code status | ||||||
| Full Code | 8029 (48) | 313 (65) | 3999 (58) | 2031 (68) | 714 (32) | 187 (38) |
| DNR/Intubation OK | 219 (1) | 3 (1) | 80 (1) | 32 (1) | 70 (3) | 7 (1) |
| DNR/DNI | 838 (5) | 27 (6) | 214 (3) | 68 (2) | 45 (2) | 14 (3) |
| Missing | 7711 (46) | 136 (28) | 2640 (38) | 841 (28) | 1369 (62) | 284 (58) |
| Ethnicity | ||||||
|---|---|---|---|---|---|---|
| Hispanic (N = 5770) | Non-Hispanic (N = 16,718) | Missing (N = 7435) | ||||
| Code status | ||||||
| Full code | 3914 (68) | 9374 (56) | 1985 (27) | |||
| DNR/Intubation OK | 44 (1) | 326 (2) | 41 (1) | |||
| DNR/DNI | 166 (3) | 939 (6) | 101 (1) | |||
| Missing | 1640 (28) | 6048 (36) | 5293 (71) |
| Sex | ||||||
|---|---|---|---|---|---|---|
| Female (N = 13,344) | Male (N = 16,307) | Non binary (N = 14) | Missing (N = 258) | |||
| Code status | ||||||
| Full Code | 6624 (50) | 8643 (53) | 5 (36) | 1 (0) | ||
| DNR/Intubation OK | 172 (1) | 236 (1) | 3 (21) | 0 (0) | ||
| DNR/DNI | 623 (5) | 583 (4) | 0 (0) | 0 (0) | ||
| Missing | 5907 (44) | 6811 (42) | 6 (43) | 257 (100) |
| ICU | ||||||
|---|---|---|---|---|---|---|
| 0 (N = 20,004) | 1 (N = 9919) | |||||
| Code status | ||||||
| Full code | 8680 (43) | 6593 (66) | ||||
| DNR/Intubation OK | 156 (1) | 255 (3) | ||||
| DNR/DNI | 914 (5) | 292 (3) | ||||
| Missing | 10,227 (51) | 2754 (28) |
| Comorbidities | ||||||
|---|---|---|---|---|---|---|
| 0 (N = 3487) | 1 (N = 4888) | 2 (N = 4792) | 3+ (N = 13,442) | Missing (N = 3314) | ||
| Code status | ||||||
| Full code | 1787 (51) | 2427 (50) | 2526 (53) | 8165 (61) | 368 (11) | |
| DNR/Intubation OK | 40 (1) | 46 (1) | 61 (1) | 259 (2) | 5 (0) | |
| DNR/DNI | 35 (1) | 101 (2) | 163 (3) | 886 (7) | 21 (1) | |
| Missing | 1622 (47) | 2301 (47) | 2037 (43) | 4101 (31) | 2920 (88) |
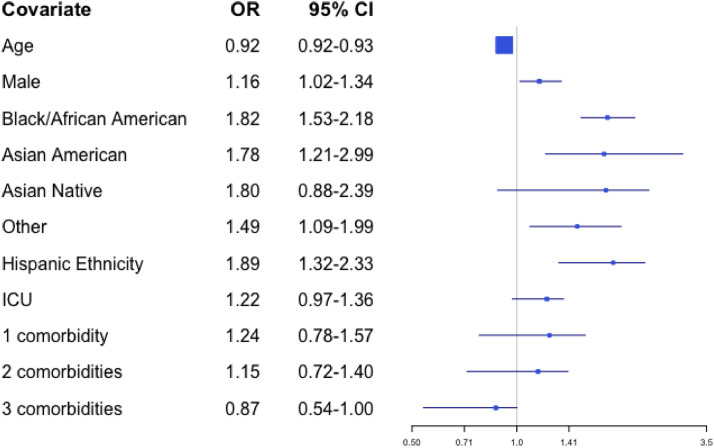
In our proportional odds model, people of Black or African American race, Asian American race, Hispanic ethnicity, and male sex had higher odds of having a more aggressive code status. The regression parameters between these two logistic models were similar for each covariate (Supplemental Table 2), except for the ICU covariate, which had a larger effect size in model A (Full Code and DNR/Intubation vs. DNR/DNI) relative to model B (Full Code vs. DNR/Intubation and DNR/DNI). After controlling for random effects by site, Black or African American patients and Asian American patients had higher odds of a more aggressive code status (OR 1.82; 95% CI 1.53–2.18 and 1.78, 95% CI 1.21–2.99, respectively), compared to White patients. For patients of Hispanic ethnicity, the odds ratio for having a more aggressive code status was 1.89 (95% CI 1.32–2.33) compared to non-Hispanic patients, and for patients who were male the odds ratio for having a more aggressive code status was 1.16 (95% CI 1.02–1.34) compared to female patients. Conversely, a more aggressive code status became less likely with increasing age (OR 0.92, 95% CI 0.92–0.93 for patients one year older). All odds ratios were based on comparisons between patients at the same site. These results are presented in Fig. 1 .
Fig. 1.
Odds from multivariate analyses of selecting a more aggressive code status order by patient characteristic.
To better understanding how missing data for code status may have affected results, we also performed a sensitivity analysis in which missing code status was imputed as Full Code. This imputation was derived from clinical practice, in which patients are presumed to be Full Code in the absence of indication otherwise. The odds ratios from the sensitivity analysis were consistent with those from the main analysis for all covariates except Hispanic ethnicity (Supplemental Table 3). For patients of Hispanic ethnicity, the odds ratio of having a more aggressive code status decreased from 1.89 (95% CI 1.32–2.33) to 1.19 (0.99–1.42) in the sensitivity analysis.
Treatment for Deceased Patients
Among the 4951 patients who died during their hospital stay, processes of care (palliative care consultation, order for CMO, or receipt of CPR at time of death) were documented for 2967 patients (60%). Data were missing for the other 1984 patients. Palliative care was consulted for 867 patients (29%), an order for CMO was in place for 1740 patients (59%), and CPR was performed at the time of death for 686 patients (29%; Table 5 ).
Table 5.
Processes of Care for Deceased Patients (n = 2967)
| Outcome | Number of Patients (%) |
|---|---|
| Palliative care consultation | 867 (29) |
| CMO | 1740 (59) |
| CPR | 868 (29) |
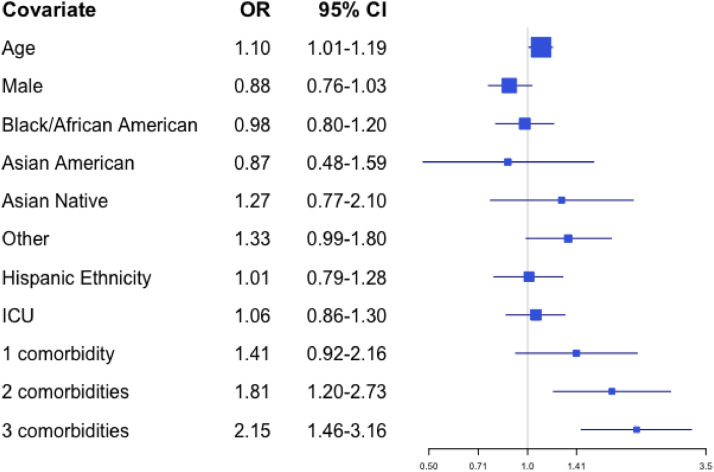
The multivariable logistic regression model that included all potential predictors as covariates found that decedents were more likely to have a palliative care consultation if they were older (OR 1.10 for a difference of one year, 95% CI 1.01–1.19) or had two comorbidities or three or more comorbidities (OR 1.81, 95% CI 1.20–2.73, and OR 2.15, 95% CI 1.46–3.16, respectively relative to no comorbidities). These results are presented in Fig. 2.
Fig. 2.
Odds from multivariate analyses of receiving a palliative care consultation by patient characteristic.
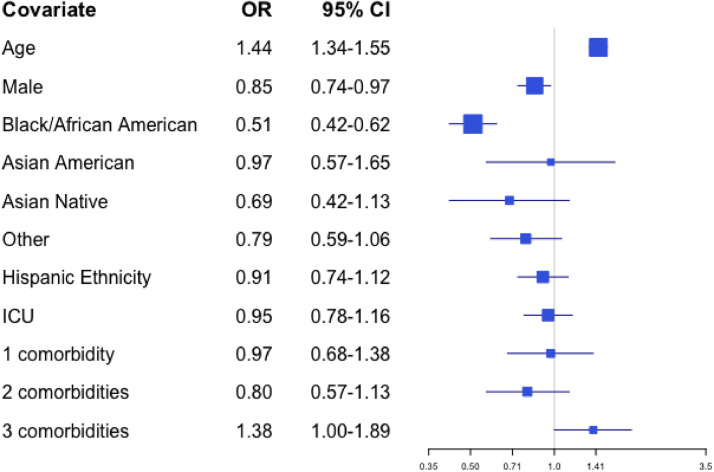
A similar model evaluating orders for CMO as the outcome suggests that patients were more likely to have CMO if they were older (OR 1.44 for a difference of one year, 95% CI 1.34–1.55) or had three or more comorbidities (OR 1.38 relative to no comorbidities, 95% CI 1.00–1.89), and less likely if they were Black or African American race (OR 0.51 relative to White race, 95% CI 0.42–0.62) or male (OR 0.85 relative to female, 95% CI 0.74–0.97). These results are presented in Fig. 3.
Fig. 3.
Odds from multivariate analyses of receiving CMO by patient characteristic.
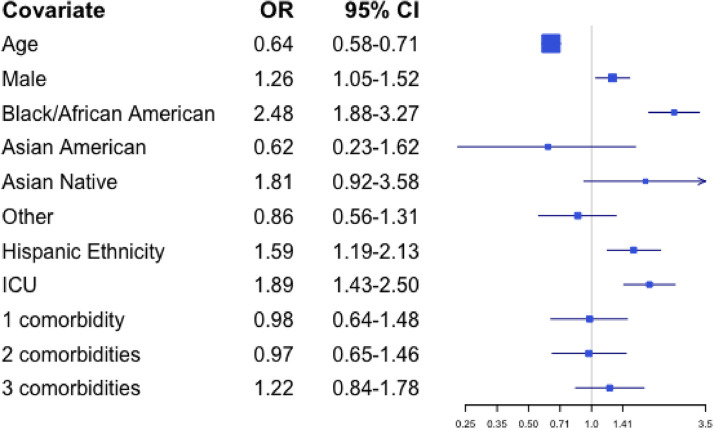
Finally, receipt of CPR at the time of death appeared less likely for patients who were older (OR 0.64 for patients older by one year, 95% CI 0.58–0.71). Patients were more likely to receive CPR at time of death if they were Black or African American (OR 2.48 relative to White, 95% CI 1.88–3.27), of Hispanic ethnicity (OR 1.59 relative to non-Hispanic, 95% CI 1.19–2.13), or in the ICU (OR 1.89 relative to acute care, 95% CI 1.43–2.50). These results are presented in Fig. 4.
Fig. 4.
Odds from multivariate analyses of receiving CPR by patient characteristic.
In sensitivity analyses, we ran the logistic regression models for treatments for deceased patients without weighting missing values, and found similar results (Supplement Table 4).
Discussion
Results from this international cohort of hospitalized patients with COVID-19 suggest that the overwhelming majority had an order for Full Code on admission, and that predictors of more aggressive code status preferences included Black or African American race, Asian American race, Hispanic ethnicity, and male sex. The 90% prevalence of Full Code orders on admission in this cohort appears lower than the prevalence of Full Code orders prior to the pandemic, based on studies that found this prevalence to be in the 94–97% range for patients admitted to the ICU.19 , 20 While direct comparisons between these numbers are limited by the different times and conditions of the studies, our findings suggest that fewer patients with COVID-19 had orders for Full Code. This may be related to increased parentalism in the setting of the pandemic,21 stemming from the documented poor outcomes for patients who underwent CPR, as well as concerns around limited resources and exposure to medical staff.5, 6, 7 It also may be true that the pandemic has encouraged more informed decision-making by both patients and families regarding end-of-life preferences, and that this additional consideration has led to fewer orders for Full Code.
Predictors of code status preference appeared similar in the setting of COVID-19 compared to before the pandemic, with individuals who were male, Black or African American, or of Hispanic ethnicity, more likely than their White, non-Hispanic counterparts to have orders for Full Code.10 , 22 Similarly, in this cohort individuals who were Black or African American race or of Hispanic ethnicity appeared more likely to die undergoing CPR and less likely to die in the setting of CMO compared to White, non-Hispanic patients. In studies in the U. S. prior to the pandemic, non-White race has been found to be associated with more aggressive end of life care.23 , 24 These similarities suggest that the factors driving racial differences in preferences and treatment prior to the pandemic persist during the pandemic.
Differences between race and ethnicity in care preferences (within the same study site) have previously been attributed in part to lack of trust,25, 26, 27 stemming from centuries of racism, compromised access to care for racial and ethnic minority groups,28 and overt disparities in health outcomes.29, 30, 31 It is not surprising that after the repeated failures of sociopolitical and medical systems, particularly in the United States, to provide equitable healthcare, individuals from these groups may feel compelled to advocate for more aggressive end-of-life treatment. This may be particularly relevant during the COVID-19 pandemic, where racial disparities in outcomes for patients with COVID-19 and concerns about potential constraints on resources may perpetuate existing mistrust of the healthcare system.32 Prepandemic research has indicated that racial disparities in code status preference may be reduced by a counselor-based intervention,33 , 34 suggesting that palliative and supportive care services may have a particularly important role during a pandemic in addressing some of the observed disparities by building rapport and assisting patients and families with informed decision-making.
In our cohort, specialty palliative care consultation was received by 29% of the patients who died from COVID-19. Prior research suggests the percentage of ICU patients receiving a specialty palliative care consultation before death prior to the pandemic was around 36%.35 , 36 Although comparisons are confounded by different timing, methodology, and settings of these studies, some differences are mitigated by the fact that the majority of decedents in our study (85%) were also located in the ICU at time of death. These results imply that the prevalence of palliative care consultation prior to death may have been slightly lower during the pandemic than prior to the pandemic, raising concerns that an overburdened healthcare system may have been unable to meet the demand for palliative care services. Given the established and important role that specialty palliative care plays in clarifying values, goals and preferences for end-of-life care for seriously ill patients,37, 38, 39 coupled with high mortality rates from COVID-19 in older patients with chronic conditions, and the documented poor outcomes for patients with IHCA early in the pandemic, these findings highlight the need for hospitals to develop the resources and bandwidth to adjust palliative care service capacity during a pandemic. This reinforces the important role of primary palliative care, an adaptation that has received substantial attention during the pandemic because of these concerns around overwhelmed specialty palliative care services.40 , 41
Strengths of this study include the number of participants and sites contributing data to the VIRUS registry, making it one of the largest available COVID-19 datasets. To our knowledge, this is the first study that uses such a large and diverse patient population to examine predictors of code status and end-of-life care during the COVID-19 pandemic. The study's results should also be considered in the context of its limitations. First, common to large registry-based studies, there was a high level of missing data which could bias the results. We mitigated this by using inverse probability weighting in the analyses. Second, in this registry-based study we were able to identify disparities but not determine drivers of the disparities. The cross-sectional nature also prohibited analysis of how palliative care consultation may influence changes in code status throughout a hospital admission. Without multiple data points through a single hospitalization or across the pandemic, we were unable to determine trends in code status over time. Third, the registry is comprised of sites that volunteer to participate, which may predispose the sample to selection bias. Fourth, the absence of a control group of patients without COVID-19 may limit interpretations of how COVID-19 may have changed end-of-life care processes. Fifth, assigning subjects to a limited number of racial categories inevitably fails to capture the nuances of racial identification and lived experience, particularly given potential variability in racial definitions across countries. While this is an imperfect measure, we chose to include race as a covariate given previously described racial disparities in end-of-life care.42, 43, 44 Lastly, the predictors and covariates that we were able to examine were constrained to those available in the registry, and unmeasured confounding may persist. For example we did not account for specific co-morbidities due to unavailability of disease severity, which could potentially confound results.
In conclusion, in a large international cohort of hospitalized patients with COVID-19, orders for Full Code were substantially more common than DNR or DNI orders on admission, with patients more likely to receive an aggressive plan of care if they were younger, of non-White race or ethnicity, or had fewer comorbidities. Among patients who died, those in non-White racial groups were also less likely to die with a CMO order and more likely to die in the setting of CPR. Future research should be directed at understanding how a pandemic setting may exacerbate existing racial disparities in end-of-life care, and how to efficiently use palliative care resources to maximize informed decision-making prior to death.
Disclosures and Acknowledgments
Financial Support: This project was supported by the National Institutes of Health (Grants T32HL125195 and K23HL144830), and by NIH/NCRR/NCATS CTSA Grant Number UL1 TR002377. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH. The registry is funded in part by the Gordon and Betty Moore Foundation, and Janssen Research & Development, LLC. They had no influence on analysis, interpretation and reporting of pooled data.
There are no notable conflicts of interest to disclose. All author funding disclosures have been reported in the attached ICMJE forms.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.jpainsymman.2022.06.014.
Appendix. Supplementary materials
References
- 1.White DB, Lo B. A framework for rationing ventilators and critical care beds during the COVID-19 Pandemic. JAMA. 2020;323:1773–1774. doi: 10.1001/jama.2020.5046. [DOI] [PubMed] [Google Scholar]
- 2.Hsu A, Weber W, Heins A, Josephson E, Kornberg R, Diaz R. A proposal for selective resuscitation of adult cardiac arrest patients in a pandemic [published online ahead of print, 2020 Jun 11] J Am Coll Emerg Physicians Open. 2020;1:408–415. doi: 10.1002/emp2.12096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chan PS, Berg RA, Nadkarni VM. Code blue during the COVID-19 Pandemic. Circ Cardiovasc Qual Outcomes. 2020;13 doi: 10.1161/CIRCOUTCOMES.120.006779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kopar PK, Brown DE, Turnbull IR. Ethics of codes and codes of ethics: when is it ethical to provide cardiopulmonary resuscitation during the COVID-19 Pandemic? Ann Surg. 2020;272:930–934. doi: 10.1097/SLA.0000000000004318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shah P, Smith HABJ, Olarewaju A, et al. MD9 is cardiopulmonary resuscitation futile in coronavirus disease 2019 patients experiencing in-hospital cardiac arrest? Crit Care Med. 2021;49(2):201–208. doi: 10.1097/CCM.0000000000004736. [DOI] [PubMed] [Google Scholar]
- 6.Thapa SB, Kakar TS, Mayer C, Khanal D. Clinical outcomes of in-hospital cardiac arrest in COVID-19. JAMA Intern Med. 2021;180(2):279–281. doi: 10.1001/jamainternmed.2020.4796. Published online September 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hayek SS, Brenner SK, Azam TU, et al. In-hospital cardiac arrest in critically ill patients with COVID-19: multicenter cohort study. BMJ. 2020;371:m3513. doi: 10.1136/bmj.m3513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moriyama D, Scherer JS, Sullivan R, Lowy J, Berger JT. The impact of COVID-19 surge on clinical palliative care: a descriptive study from a New York hospital system. J Pain Symptom Manage. 2021;61:e1–e5. doi: 10.1016/j.jpainsymman.2020.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huberman BJ, Mukherjee D, Gabbay E, et al. Phases of a pandemic surge: the experience of an ethics service in New York City during COVID-19. J Clin Ethics. 2020;31:219–227. [PubMed] [Google Scholar]
- 10.Johnson RW, Newby LK, Granger CB, et al. Differences in level of care at the end of life according to race. Am J Crit Care. 2010;19:335–344. doi: 10.4037/ajcc2010161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coleman JJ, Botkai A, Marson EJ, et al. Bringing into focus treatment limitation and DNACPR decisions: How COVID-19 has changed practice. Resuscitation. 2020;155:172–179. doi: 10.1016/j.resuscitation.2020.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Obata R, Maeda T, Rizk D, Kuno T. Palliative care team involvement in patients with COVID-19 in New York City. Am J Hosp Palliat Care. 2020;37:869–872. doi: 10.1177/1049909120940986. Epub 2020 Jul 8. PMID: 32638632. [DOI] [PubMed] [Google Scholar]
- 13.Cheruku SR, Barina A, Kershaw CD, et al. Palliative care consultation and end-of-life outcomes in hospitalized COVID-19 patients. Resuscitation. 2022;170:230–237. doi: 10.1016/j.resuscitation.2021.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moin EE, Okin D, Jesudasen SJ, et al. Code status orders in patients admitted to the intensive care unit with COVID-19: a retrospective cohort study. Resusc Plus. 2022;10 doi: 10.1016/j.resplu.2022.100219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Walkey AJ, Kumar VK, Harhay MO, et al. The Viral Infection and Respiratory Illness Universal Study (VIRUS): an International Registry of Coronavirus 2019-related critical illness. Crit Care Explor. 2020;2:e0113. doi: 10.1097/CCE.0000000000000113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Walkey AJ, Sheldrick RC, Kashyap R, et al. Guiding principles for the conduct of observational critical care research for coronavirus disease 2019 pandemics and beyond: the society of critical care medicine discovery viral infection and respiratory illness universal study registry. Crit Care Med. 2020;48:e1038–e1044. doi: 10.1097/CCM.0000000000004572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap) - a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mansournia MA, Altman DG. Inverse probability weighting. BMJ. 2016;352:i189. doi: 10.1136/bmj.i189. Published 2016 Jan 15. [DOI] [PubMed] [Google Scholar]
- 19.Higgins TL, Teres D, Copes WS, Nathanson BH, Stark M, Kramer AA. Assessing contemporary intensive care unit outcome: an updated Mortality Probability Admission Model (MPM0-III) Crit Care Med. 2007;35:827–835. doi: 10.1097/01.CCM.0000257337.63529.9F. [DOI] [PubMed] [Google Scholar]
- 20.Creutzfeldt CJ, Wunsch H, Curtis JR, Hua M. Prevalence and outcomes of patients meeting palliative care consultation triggers in neurological intensive care units. Neurocrit Care. 2015;23:14–21. doi: 10.1007/s12028-015-0143-8. [DOI] [PubMed] [Google Scholar]
- 21.Fins JJ. Resuscitating patient rights during the pandemic: COVID-19 and the risk of resurgent paternalism. Camb Q Healthc Ethics. 2021;30:215–221. doi: 10.1017/S0963180120000535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ankuda CK, Fonger E, O'Neil T. Electing full code in hospice: patient characteristics and live discharge rates. J Palliat Med. 2018;21:297–301. doi: 10.1089/jpm.2017.0276. [DOI] [PubMed] [Google Scholar]
- 23.Brown CE, Engelberg RA, Sharma R, et al. Race/ethnicity, socioeconomic status, and healthcare intensity at the end of life. J Palliat Med. 2018;21:1308–1316. doi: 10.1089/jpm.2018.0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Muni S, Engelberg RA, Treece PD, Dotolo D, Curtis JR. The influence of race/ethnicity and socioeconomic status on end-of-life care in the ICU. Chest. 2011;139:1025–1033. doi: 10.1378/chest.10-3011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Halbert CH, Armstrong K, Gandy OH, Jr, Shaker L. Racial differences in trust in health care providers. Arch Intern Med. 2006;166:896–901. doi: 10.1001/archinte.166.8.896. [DOI] [PubMed] [Google Scholar]
- 26.Armstrong K, McMurphy S, Dean LT, et al. Differences in the patterns of health care system distrust between blacks and whites. J Gen Intern Med. 2008;23:827–833. doi: 10.1007/s11606-008-0561-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tong E, McGraw SA, Dobihal E, Baggish R, Cherlin E, Bradley EH. What is a good death? Minority and non-minority perspectives. J Palliat Care. 2003;19:168–175. [PubMed] [Google Scholar]
- 28.Manuel JI. Racial/ethnic and gender disparities in health care use and access. Health Serv Res. 2018;53:1407–1429. doi: 10.1111/1475-6773.12705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Murphy SL, Jiaquan X, Kochanek KD. Deaths: final data for 2010. Natl Vital Stat Rep. 2013;61(14):1–118. [PubMed] [Google Scholar]
- 30.Williams DR. Miles to go before we sleep: racial inequities in health. J Health Soc Behav. 2012;53:279–295. doi: 10.1177/0022146512455804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brandon DT, Isaac LA, LaVeist TA. The legacy of Tuskegee and trust in medical care: is Tuskegee responsible for race differences in mistrust of medical care? J Natl Med Assoc. 2005;97:951–956. [PMC free article] [PubMed] [Google Scholar]
- 32.Warren RC, Forrow L, Hodge DA, Sr, Truog RD. Trustworthiness before trust - COVID-19 vaccine trials and the Black Community. N Engl J Med. 2020;383:e121. doi: 10.1056/NEJMp2030033. [DOI] [PubMed] [Google Scholar]
- 33.Benton K, Stephens J, Vogel R, et al. The influence of race on end-of-life choices following a counselor-based palliative consultation. Am J Hosp Palliat Care. 2015;32:84–89. doi: 10.1177/1049909113506782. [DOI] [PubMed] [Google Scholar]
- 34.Mayeda DP, Ward KT. Methods for overcoming barriers in palliative care for ethnic/racial minorities: a systematic review. Palliat Support Care. 2019;17:697–706. doi: 10.1017/S1478951519000403. [DOI] [PubMed] [Google Scholar]
- 35.Lee JD, Jennerich AL, Engelberg RA, Downey L, Curtis JR, Khandelwal N. Type of intensive care unit matters: variations in palliative care for critically Ill patients with chronic, life-limiting illness. J Palliat Med. 2021;24:857–864. doi: 10.1089/jpm.2020.0412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Evans BA, Turner MC, Gloria JN, Pickett LC, Galanos AN. Palliative care consultation is underutilized in critically ill general surgery patients. Am J Hosp Palliat Care. 2020;37:149–153. doi: 10.1177/1049909119864025. [DOI] [PubMed] [Google Scholar]
- 37.Ma J, Chi S, Buettner B, et al. Early palliative care consultation in the medical ICU: a cluster randomized crossover trial. Crit Care Med. 2019;47:1707–1715. doi: 10.1097/CCM.0000000000004016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Diop MS, Rudolph JL, Zimmerman KM, Richter MA, Skarf LM. Palliative care interventions for patients with heart failure: a systematic review and meta-analysis. J Palliat Med. 2017;20:84–92. doi: 10.1089/jpm.2016.0330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Scott M, Shaver N, Lapenskie J, et al. Does inpatient palliative care consultation impact outcomes following hospital discharge? A narrative systematic review. Palliat Med. 2020;34:5–15. doi: 10.1177/0269216319870649. [DOI] [PubMed] [Google Scholar]
- 40.Ferguson L, Barham D. Palliative care pandemic pack: a specialist palliative care service response to planning the COVID-19 Pandemic. J Pain Symptom Manage. 2020;60:e18–e20. doi: 10.1016/j.jpainsymman.2020.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mitchell S, Maynard V, Lyons V, Jones N, Gardiner C. The role and response of primary healthcare services in the delivery of palliative care in epidemics and pandemics: a rapid review to inform practice and service delivery during the COVID-19 pandemic. Palliat Med. 2020;34:1182–1192. doi: 10.1177/0269216320947623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brown CE, Engelberg RA, Sharma R, et al. Race/ethnicity, socioeconomic status, and healthcare intensity at the end of life. J Palliat Med. 2018;21:1308–1316. doi: 10.1089/jpm.2018.0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sharma RK, Cameron KA, Chmiel JS, et al. Racial/ethnic differences in inpatient palliative care consultation for patients with advanced cancer. J Clin Oncol. 2015;33:3802–3808. doi: 10.1200/JCO.2015.61.6458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hanchate A, Kronman AC, Young-Xu Y, Ash AS, Emanuel E. Racial and ethnic differences in end-of-life costs: why do minorities cost more than whites? Arch Intern Med. 2009;169:493–501. doi: 10.1001/archinternmed.2008.616. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.