Abstract
Contact dermatitis (CD), including allergic and irritant CD, are common dermatological diseases and characterized by an erythematous rash and severe itch. In this study, we investigated the function of TRPC3, a canonical TRP channel highly expressed in type 1 non-peptidergic (NP1) nociceptive primary afferents and other cell types, in a mouse CD model. Though TrpC3 null mice had little deficits in acute somatosensation, they showed significantly increased scratching with CD. In addition, TrpC3 null mice displayed no differences in mechanic and thermal hypersensitivity in an inflammatory pain model, suggesting that this channel preferentially functions to antagonize CD-induced itch. Using dorsal root ganglia (DRG) and pan-immune-specific TrpC3 conditional KO (CKO) mice, we determined that TrpC3 in DRG neurons, but not in immune cells, is required for this phenotype. Furthermore, the number of MRGPRD+ NP1 afferents in CD-affected DRGs is significantly reduced in TrpC3 mutant mice. Taken together, our results suggest that TrpC3 plays a critical role in NP1 afferents to cope with CD-induced excitotoxicity, and that degeneration of NP1 fibers may lead to an increased itch of CD. Our study identified a role of TrpC3 and NP1 afferents in CD pathology.
Keywords: Contact dermatitis, DRG, MRGPRD, non-peptidergic nociceptors, pruritus, TRPC3
Introduction
Contact dermatitis (CD) is a common group of skin diseases, which include irritant contact dermatitis (ICD) and allergic contact dermatitis (ACD) (Nosbaum et al., 2009). ICD is the clinical manifestation of a local inflammatory reaction of the skin following exposure to a physical or chemical agent (Ale and Maibach, 2014). In ACD, activation of memory T cells, by re-exposure to allergens, causes an inflammatory cascade that results in skin injury and enhanced itch (Kostner et al., 2017). Itch sensation is a hallmark and main complaint of patients with CD, which can lead to a negative cycle of scratching, skin excoriations, and even worse itch sensation. These symptoms significantly affect patients’ quality of life. At present, treatment of CD and CD-induced itch is limited due to our incomplete knowledge of the molecular mechanisms and difficulty in identifying the exact offending agent.
CD-induced itch sensation is generated by interactions of the primary sensory afferents, the immune system, and the integumentary system in the periphery. External and internal pruritogens are detected by free nerve terminals in the skin, whose cell bodies are in the dorsal root ganglion (DRG) and trigeminal ganglion. The signals are then relayed through the spinal cord to the brain to generate the perception of “itch”. The immune system plays a critical role in triggering pathological pruritus by releasing inflammatory mediators that can directly activate pruitoceptors (Storan et al., 2015). Keratinocytes, the resident epithelial cells which comprise the epidermis, promote chronic itch through both direct (barrier disruption allowing pruritogen entry) and indirect mechanisms (cytokine release) (Schwendinger-Schreck et al., 2015).
Transient receptor potential (TRP) channels in mammals comprise a superfamily of over 30 membrane-bound proteins that form tetrameric non-selective cation channels and function in a variety of sensory pathways, including itch sensation (Julius, 2013, Montell, 2011, Sun and Dong, 2016). Canonical TRP family members (TRPC) can function downstream of a G-protein coupled receptor (GPCR) or as receptors by themselves (Chen et al., 2020). TRPC channels can integrate several types of intracellular signals into changes in membrane potential and calcium entry (Chen et al., 2020). Indeed, deregulation of TRPC channels can disrupt calcium homeostasis and lead to cell damage and neuronal death (Chen et al., 2020, Jeon et al., 2021).
TRPC3 is a TRPC family member that is highly expressed in DRG neurons (Dong et al., 2017, Luo et al., 2009, Quick et al., 2012). In addition, it is also expressed in Purkinje cells, cholinergic neurons, thalamic glutamatergic neurons, and immune cells (Wenning et al., 2011, Zeisel et al., 2018). Within DRG, TrpC3 is mainly expressed in non-peptidergic (NP) nociceptors: highest in the NP1 primary afferents, marked by a GPCR MRGPRD, and followed by NP2 and NP3 primary afferents, which are marked by MRGPRA3 and a neuropeptide natriuretic polypeptide B (NPPB), respectively (Usoskin et al., 2015, Zeisel et al., 2018). NP1 is a polymodal afferent for sensing mechanical force, chemicals, and temperature, whereas NP2 and NP3 afferents are itch selective (Cranfill and Luo, 2021). Despite the high expression of TrpC3 in nonpeptidergic DRG neurons, TrpC3 null mice exhibited few deficits in a variety of behavioral tests for acute pain and itch sensation, mechanosensation, and thermosensation (Dong et al., 2017, Quick et al., 2012).
Here we examined the role of TrpC3 in a mouse CD model, which is induced by repeated application of squaric acid dibutylester (SADBE). Though this model was initially established as an ACD model (Qu et al., 2015), a later study using this model also found ICD features, including that itch could be induced in the absence of lymphocytes and that SADBE could directly activate primary sensory neurons (Feng et al., 2017). In contrast to the minor deficits in acute somatosensory assays (Dong et al., 2017, Quick et al., 2012), TrpC3 null mice displayed a significant increase in spontaneous scratching of this SADBE-CD model, suggesting that TRPC3 functions to antagonize CD-induced itch. We generated DRG neuron and immune cell specific TrpC3 CKO models and found that TrpC3 in DRG is required for this phenotype. Moreover, we performed immunohistochemistry with the DRG, skin, and spinal cord of affected and unaffected regions of control and TrpC3 null mice and revealed a significant reduction in the number of MRGPRD+ NP1 nociceptors innervating the affected region of mutant mice. Since TRPC3 is a known mediator of calcium homeostasis and plays a critical role in excitotoxicity (Alkhani et al., 2014, Jeon et al., 2021), we propose a model that TrpC3 null MRGPRD+ NP1 nociceptors are susceptible to excitotoxicity induced by this CD model and that degeneration of NP1 neurons somehow disinhibits the itch pathway, resulting in an increased amount of scratching in TrpC3 mutant mice.
Results
TrpC3 antagonizes scratching behavior in a mouse CD model
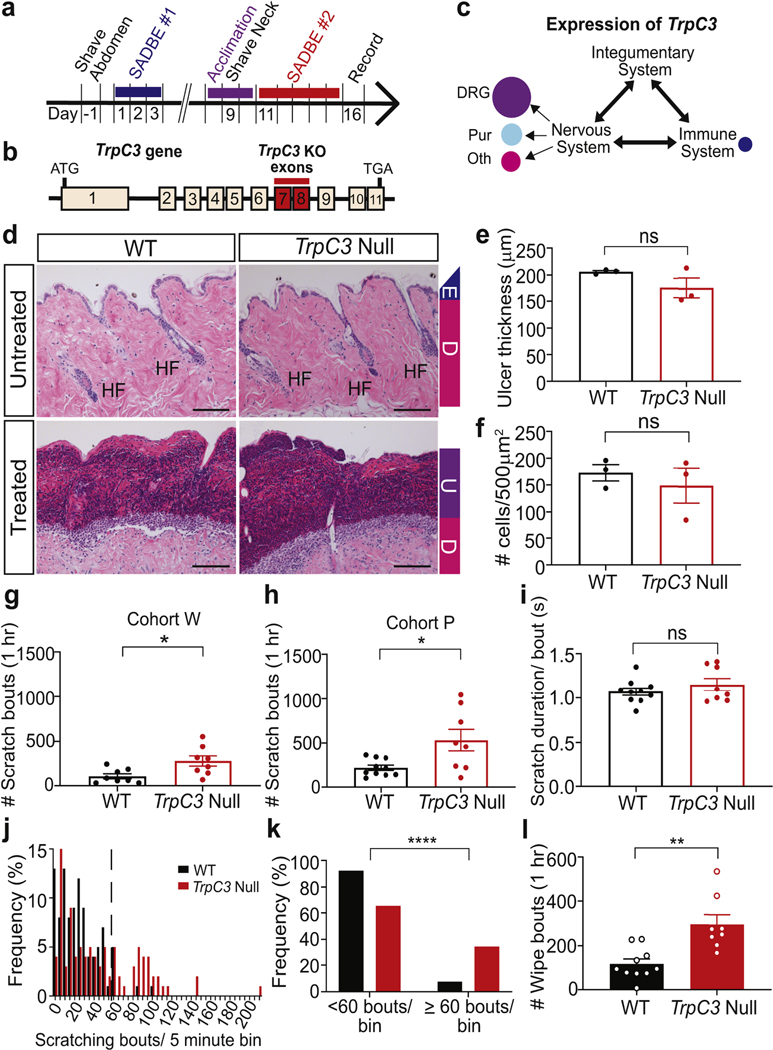
Given the high expression of Trpc3 in DRG neurons, it was surprising that almost no behavioral deficits in acute sensation were identified in TrpC3 null mice (Dong et al., 2017, Hirschler-Laszkiewicz et al., 2012). To further explore its function in somatosensation, we examined TrpC3 null mice using a CD model (Qu et al., 2014). Briefly, adult male and female TrpC3 null and wild type (WT) control mice were treated with SADBE on their abdomens and then re-challenged with SADBE at their necks after a week. Their spontaneous behavior was recorded and quantified on Day 16 (Figure 1a). As described before (Dong et al., 2017, Hirschler-Laszkiewicz et al., 2012), the TrpC3 null allele was created by excision of exons 7 and 8 (Figure 1b), which encode the pore-defining region of the channel, resulting in a transcript with premature stop codon and non-functional TRPC3. SADBE treatment induced comparable ulcerative thickness and lymphocytic infiltration in WT and null mice (Figure 1d-f), suggesting that the absence of TRPC3 did not impede the typical skin pathology associated with the CD model. Interestingly, knocking out TrpC3 resulted in a marked increase in spontaneous scratching bouts, an itch-indicating behavior, in two independent cohorts from laboratories at Washington University (Cohort W) and the University of Pennsylvania (Cohort P) (Figures 1g-h). The average scratching bout numbers in the Cohort P were higher than that in Cohort W because this cohort excluded mice that were inactive for more than 50% of the 1-hour recording time (the same criterion was used for the following experiments). We further analyzed the scratching behavior by examining the average scratch duration per bout and the number of scratching bouts in 5-minute bins. No difference in bout duration was found between genotypes (Figure 1i), but a statistically significant increase in the high scratching bout numbers/bin was seen in TrpC3 null mice (Figure 1j-k). TrpC3 null mice also displayed a marked increase in wiping behavior in this CD model (Figure 1j). In short, our results suggest the absence of functional TRPC3 may lead to an enhanced pathological itch in the SADBE-CD model.
Figure 1. TrpC3 antagonizes scratching in the SADBE-CD model.
a. Protocol and timeline of SADBE-induced CD model. b. Diagram of the TrpC3 gene, depicting excision of exons 7–8 in null mice. c. Schematic representation of relative expression levels of TrpC3 (based on mouse single neuron RNAseq data) in systems relevant to CD induced itch sensation. d. H & E staining of skin from SADBE-treated mice with dermal layers labeled: epidermis (E), dermis (D), ulcer (U). e-f. Quantification of ulcer thickness and cellular infiltration of treated neck skin (≥10 measurements/mouse, n=3). g-h. Quantification of spontaneous scratch behavior of TrpC3 null and WT mice on day 16 of the SADBE model (Cohort W, n=8 (males); Cohort P, WT n=10 (5 males, 5 females), TrpC3 null n=8 (3 males, 5 females). i. Quantification of scratch duration per bout (s). j. Frequency distribution of scratch bout numbers in 5-minute bins. k. Bar graph comparing the percentage of low (<60 bouts) and high (≥60 bouts) scratching bout bins. l. Quantification of wiping bouts. Scale bars = 50 μm. Student’s two-tailed t test (e-i, l). Chi-squared test (k). ns, not significant. Asterisks indicate statistical significance. *p<0.05, **p<0.01; error bar, SEM.
Loss of TRPC3 does not alter mechanical allodynia, thermal hyperalgesia, or gait.
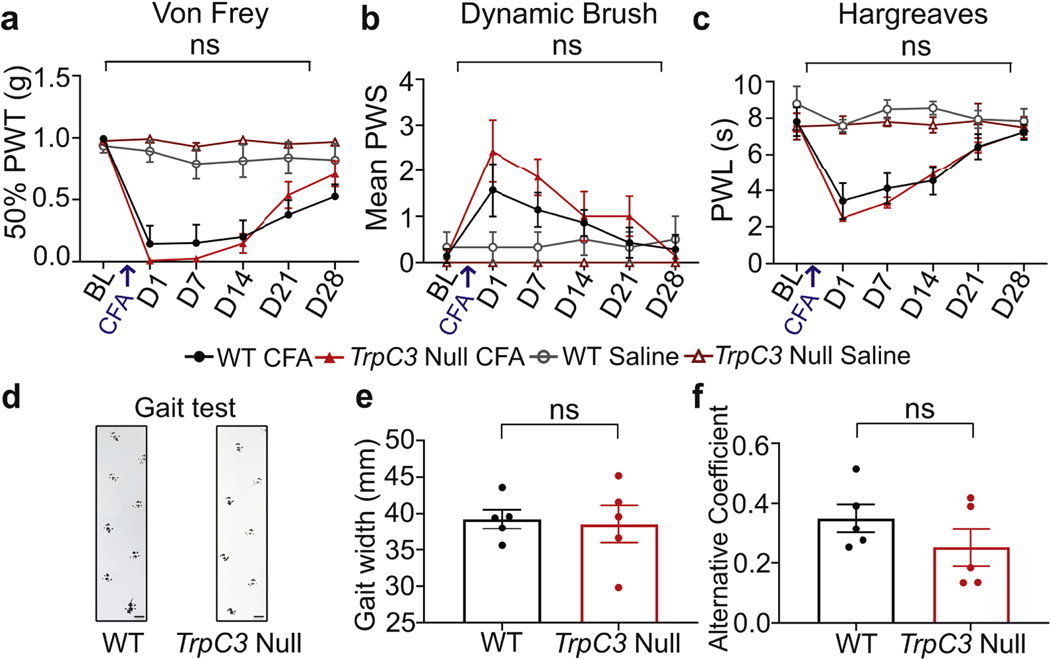
To determine whether TrpC3 is also required in chronic inflammatory pain sensation, Complete Freund’s Adjuvant (CFA) was injected in the hindpaw to induce inflammatory pain. Dynamic and static mechanical allodynia, as well as thermal sensitivity, were tested before and after CFA treatment. No significant differences were found for any of these behavioral assays (Figure 2a-c), suggesting that TrpC3 is dispensable for modulating pain sensation in this inflammatory pain model. Of note, a TrpC3 gain-of-function point mutation mouse line, Moonwalker (Mwk) mice, caused Purkinje cell degeneration and cerebellar ataxia (Becker et al., 2009). To test whether TrpC3 null mice had the same deficit, we performed a footprint assay, measured their gait width, and calculated the alternative coefficient. We found no statistically significant differences in gait width and alternative coefficient (Figure 2d-f). These results are consistent with our previous finding that TrpC3 null mice displayed normal motor coordination on the rotarod assay (Dong et al., 2017). Taken together, these findings highlight the preferential requirement of TrpC3 in modulating CD-induced scratching behavior.
Figure 2. Loss of TRPC3 does not alter mechanical allodynia, thermal hyperalgesia, or gait.
Behavioral assays with WT and TrpC3 null mice after CFA (n=7/genotype; 2 males, 5 females) or saline (n=6/genotype; 1 male, 5 females) injection. a. 50% Paw withdrawal threshold (PWT) in response to von Frey filaments. b. Paw withdrawal score (PWS) in response to dynamic brush stimulation. c. Paw withdrawal latency (PWL) in Hargreaves test. d. Representative footprints of TrpC3 null and WT mice in gait assay. e-f. Quantification of gait width and the alternative coefficient (indicating step alternation uniformity) (n=5/genotype, females). Scale bar = 100 μm. Two-way ANOVA (a-c); Student’s one-tail t test (e-f); error bar, SEM.
TrpC3 in DRG neurons is required to antagonize CD-induced scratching
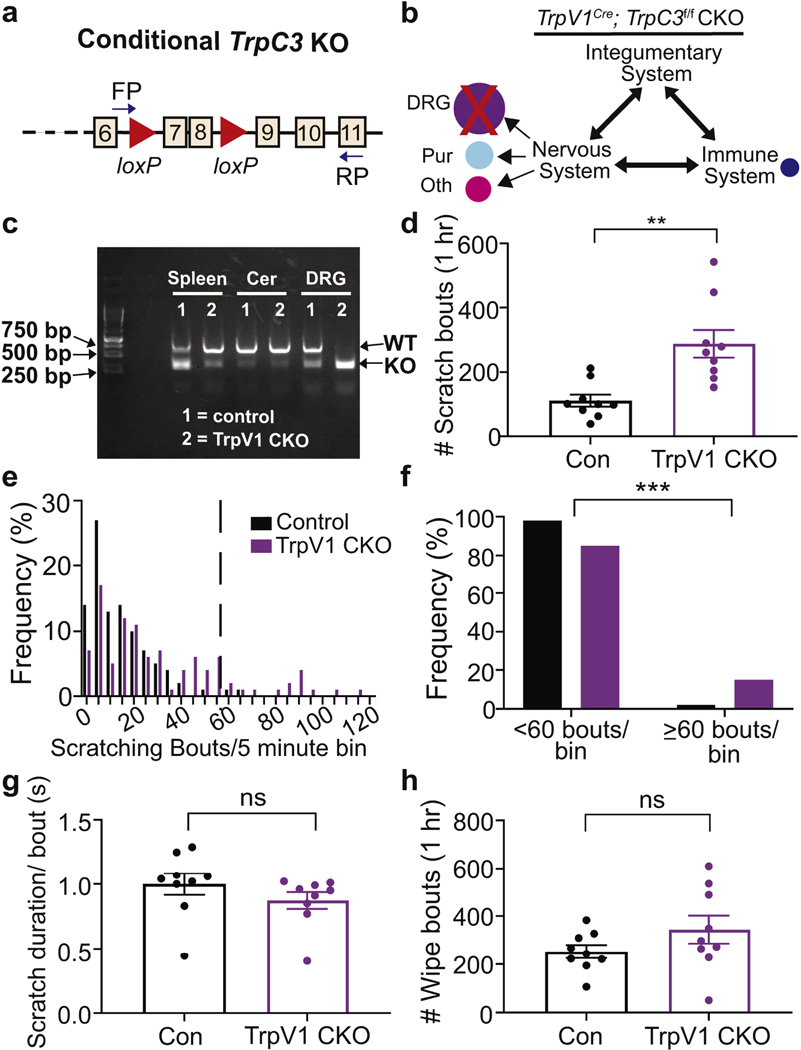
Based on mouse skin single cell RNAseq data (Joost et al., 2020) and our own RT-PCR result, TrpC3 is barely detected in the mouse skin. Thus, we focused on determining TRPC3 function in neuronal and immune cells in modulating CD-induced scratching. We generated TrpC3 conditional knockout (CKO) mice by crossing a genetic allele, in which exons 7 and 8 were floxed by loxP sites (Hirschler-Laszkiewicz et al., 2012), with different Cre lines (Figure 3a). Given the high expression level of TrpC3 in DRG neurons, we generated a CKO mouse line that specifically knocked out TrpC3 expression in DRG nociceptors, including all NP afferents, using the TrpV1Cre mouse line (Figure 3b-c)(Cavanaugh et al., 2011). We then induced CD in control (TrpC3f/f) and TrpV1-CKO (TrpV1Cre;TrpC3f/f) mice. Interestingly, TrpV1-CKO mice showed a significant increase in scratching bouts when compared to control mice (Figure 3d). Similar to the null mice, the TrpV1-CKO mice scratched with a greater frequency than the control mice (Figure 3e-f) but displayed no difference in the average scratch bout duration (Figure 3g). The number of wiping bouts was not significantly changed in TrpV1-CKO mice (Figure 3h). These results indicate that TrpC3 in DRG neurons is required for antagonizing CD-induced scratching.
Figure 3. TrpC3 is required in DRG neurons to antagonize CD-induced scratching.
a. Diagram of floxed TrpC3 gene illustrating the loxP sites surrounding exons 7 and 8 of TrpC3 gene and the location of the forward (FP) and reverse RT-PCR primers (RP). b. Schematic representation of knocking out TrpC3 expression in DRG neurons. c. RT-PCR performed on RNA isolated from floxed control and TrpV1 CKO DRG, cerebellum, and spleen tissue. d. Quantification of scratch behavior of TrpV1 CKO (n =9; 4 males, 5 females) and control mice on Day 16 (n =9; 5 males, 4 females). e. Frequency distribution of bout numbers in 5-minute bins. f. Bar graph comparing the total percentage of low (<60 bouts) and high (≥60 bouts) scratching bout bins. g. Quantification of scratch duration per bout (s). h. Quantification of wiping bouts. Student’s two-tailed t test (b, e, f); Chi-squared test (d). Asterisks indicate statistical significance. **p<0.01; error bar, SEM.
TrpC3 in immune cells is not required to modulate CD-induced scratching
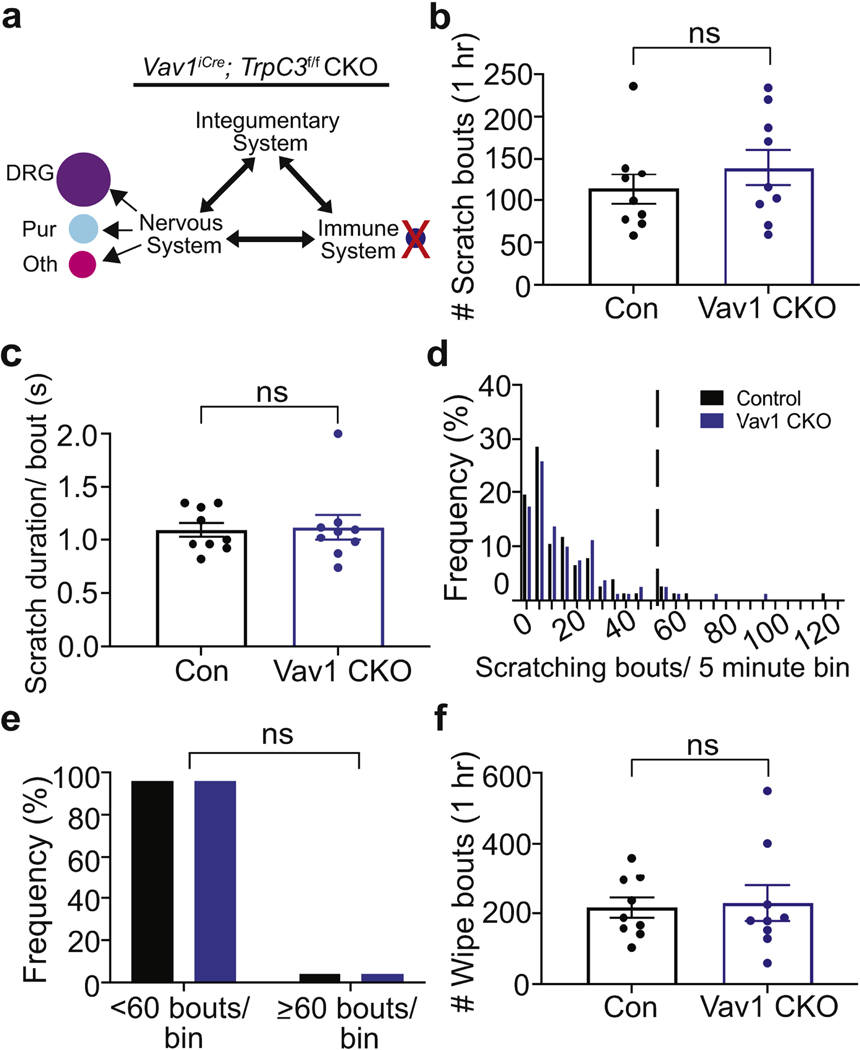
To determine TRPC3 function in the immune system, we knocked out TrpC3 expression in pan-immune cells utilizing the Vav1iCre line (Figure 4a) (Yang et al., 2008) and induced CD in control (TrpC3f/f) and Vav1-CKO (Vav1iCre;TrpC3f/f) mice. No differences in spontaneous scratching behavior (including bout number, duration per bout, and frequency) or wiping were found between the genotypes (Figure 4b-d). These findings are consistent with the histological result: similar amount of inflammatory cell infiltration in affected skin of TrpC3 null and WT mice (Figure 1d-f). Together, our data suggest that TrpC3 in the immune cells is not required to modulate scratching and/or skin inflammation in SADBE-induced CD (Figure 4b-f).
Figure 4. TrpC3 in immune cells is not required to modulate CD-induced scratching.
a. Schematic illustration showing CKO of TrpC3 from immune cells. b. Quantification of scratch bouts of Vav1-CKO and control mice on Day 16 (n =9; 4 males, 5 females/genotype). c. Quantification of scratch duration per bout (s). d. Frequency distribution of bout numbers in a 5-minute bins. e. Bar graph comparing the percentage of low (<60 bouts) and high (≥60 bouts) scratching bout bins. f. Quantification of wiping bouts. Student’s two-tailed t test (c, d, g); Chi-squared test (f); error bar, SEM.
Degeneration of MRGPRD+ neurons in the CD-affected region of TrpC3 null mice
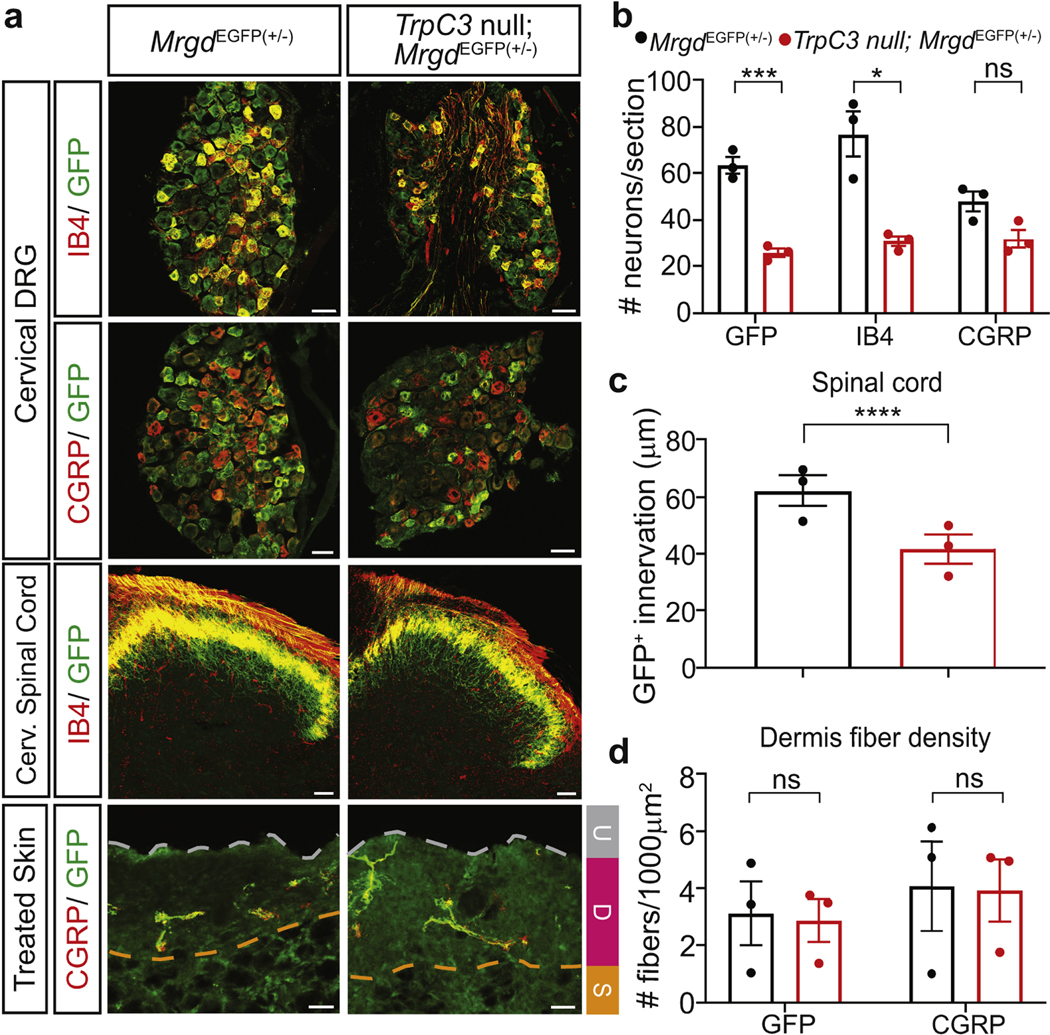
To reveal potential mechanisms underlying the increased CD-induced itch sensation of TrpC3 mutant mice, we performed immunohistochemistry with the DRG, skin, and spinal cord of the affected cervical region of control and TrpC3 null mice. Since TrpC3 shows a highly overlapped expression with Mrgprd (Dong et al., 2017), we utilized the MrgprdEGFP(+/−) allele (Zylka et al., 2005) to visualize the NP1 afferents. We found a significant reduction in IB4+ (marker of NP1 neurons) and GFP+ DRG neurons and a reduction of GFP+ fibers innervating the lamina II of the spinal cord (Figure 5a-c). In contrast, the number and central terminals of peptidergic (CGRP+) neurons, which normally had low expression level of TrpC3, did not differ between genotypes (Figure 5a-b). Though CGRP+ and GFP+ fibers normally innervate the epidermis layer of the skin (Zylka et al., 2005), both types of intraepidermal free nerve terminals were lost in the SADBE treated neck skin, and only few dermis innervating fibers remained. No significant difference was found in the number of these remaining dermis nerve fibers (Figure 5d). Together, these results indicate that TrpC3 null MRGPRD+ NP1 neurons innervating the CD-affected skin region degenerate and die.
Figure 5. Degeneration of MRGPRD+ neurons in the CD affected region of TrpC3 null mice.
a. Immunostaining of adult MrgprdEGFP(+/−) and TrpC3 null; MrgprdEGFP(+/−) mouse cervical DRG, spinal cord, and treated neck skin sections using IB4 and antibodies against CGRP and GFP. Skin layers: ulcer (U); dermis (D); subcutaneous layer (S). b. Quantification of marker positive DRG neuron numbers per section. c. Quantification of the innervation thickness of MRGPRD+ central terminals. d. Quantification of dermal innervation of MRGPRD+ fibers in 1000μm2 area (≥4 sections/mouse; n=3). Scale bars = 50 μm. Student’s two-tailed t test. Asterisks indicate statistical significance. *p<0.05, ***p<0.001, ****p<0.0001; error bar, SEM.
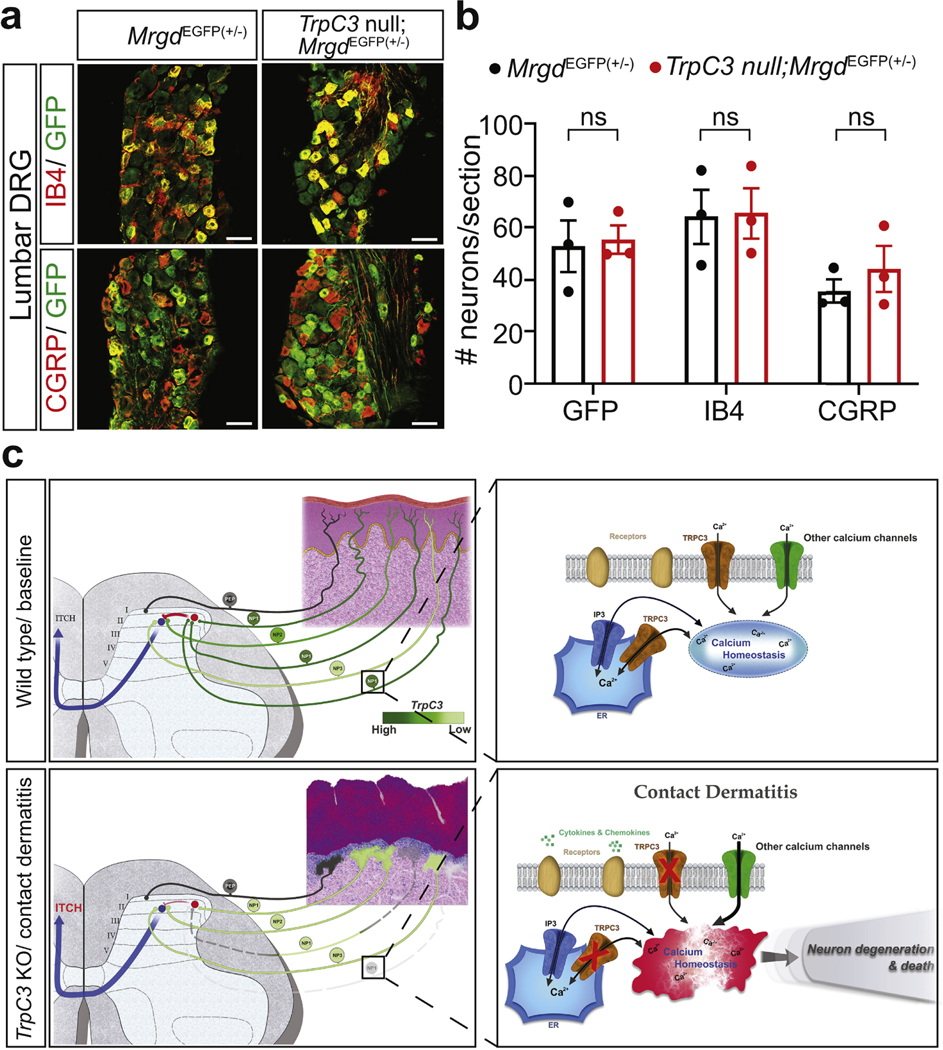
Since our previous data demonstrated a normal number of MRGPRD+ DRG neurons and spinal cord and skin innervation in untreated juvenile TrpC3 null mice (Dong et al., 2017), this result suggests either an age-dependent increase in cell death or an increased cell death caused by the SADBE-CD model. To differentiate the two possibilities, we conducted immunohistochemistry on lumbar DRGs that innervate the untreated mouse skin (Figure 6a). No differences in MRGPRD+ neurons, nonpeptidergic (IB4), or peptidergic (CGRP) neurons were found (Figure 6b). This result suggests that the decrease in cervical MRGPRD+ (NP1) neurons of TrpC3 null mice is due to an increased cell death caused by the SADBE-CD model.
Figure 6. MRGPRD+ neurons are not affected in the control region of TrpC3 null mice.
a. Immunostaining of adult MrgprdEGFP(+/−) and TrpC3 null; MrgprdEGFP(+/−) mouse lumbar DRG, sections using IB4 and antibodies against CGRP and GFP. b. Quantification of marker positive DRG neuron numbers per section (≥4 sections/mouse, n=3). c. Schematic of the hypothetical model of how TRPC3 functions in MRGPRD+ neurons to antagonize CD-induced itch. Scale bars = 50 μm. Student’s two-tailed t test.; error bar, SEM.
Discussion
In this study, we identified a function of TRPC3 in somatosensation: antagonizing pathological scratching of the SADBE-CD model, and determined that TrpC3 in DRG neurons, but not in immune cells, is required for this function. Interestingly, in the affected region of TrpC3 null mice, there is a marked reduction in the number of MRGPRD+ DRG neurons, where TrpC3 is normally expressed at the highest level. Based on these results, we propose a hypothetical model that TrpC3 is highly expressed in NP1 neurons, which normally antagonize the skin inflammation (Zhang et al., 2021) and itch pathway mediated by NP2 and NP3 neurons (Figure 6c). Upon CD induction, NP1 neurons encounter excitotxity and degenerate in the absence of TrpC3, which leads to the disinhibition of the itch pathway and results in increased itch behaviors/sensation (Figure 6c).
TRPC3 is a non-selective cation channel which mediates calcium homeostasis and sensitization of primary nociceptors through its ability to engage in both receptor-operated calcium influx and in store-operated calcium entry (Alkhani et al., 2014). In addition, TRPC3 can be coupled to metabotropic glutamate receptors (Hartmann et al., 2008) and is required for IgG immune complex-induced excitation of DRG neurons (Qu et al., 2012). Disruptions in TrpC3 expression or TRPC3 activity can lead to dysfunction of calcium homeostatis and cell death. A gain-of-function mutation of TRPC3 in Mwk mice led to increased intracellular Ca2+ concentration, which resulted in purkinje cell death and ataxia (Becker et al., 2009). An additional mouse model of spinocerebellar ataxia found that TrpC3 was downregulated before the onset of neural degeneration (Lin et al., 2000). MRGPRD+ (NP1) afferents innervating SADBE treated skin become hyperexcitable and display spontaneous firing (Qu et al., 2014). Since NP1 afferents have the highest level of TrpC3 expression, 2–5 fold higher than its expression in NP2 and NP3 afferents (Zeisel et al., 2018), knocking out TrpC3 should disrupt calcium homeostasis in NP1 afferents to the greatest extent. We speculate that a combination of increased calcium entry and a deficit of TRPC3 renders NP1 neurons susceptible to chronic excitotoxicity induced by SADBE-CD (and ultimately cell death) (Figure 6c).
MRGPRD is expressed in ∼20% of mouse DRG neurons whose afferents form the densest innervation of the epidermis, specifically in the stratum granulosum (Zylka et al., 2005). MRGPRD+ (NP1) neurons are polymodal and respond to noxious heat, mechanical pain, inflammatory pain, and pruitogens such as β-alanine (Abdus-Saboor et al., 2019, Cavanaugh et al., 2009, Liu et al., 2012, Shinohara et al., 2004). Unlike the NP1 subpopulation, NP2 (which express Mrgpra3 and Mrgprc11) and NP3 (which express somatostatin, the interleukin-31 receptor A (IL31r), and Nppb) neurons predominantly mediate histaminergic and non-histaminergic itch (Han et al., 2013, Liu et al., 2009, Mishra and Hoon, 2013). Some recent studies further highlighted the critical role of NP2+ and NP3+ afferents in mediating dermatitis-induced itch (Solinski et al., 2019, Wang et al., 2021, Zhu et al., 2017) . Though it is generally believed that the “pain” pathway antagonizes the “itch” pathway (Lagerström et al., 2010, Liu et al., 2010), whether the NP1 pathway could inhibit the NP2/NP3 itch pathway has not been directly tested and established. Our study revealed that TrpC3 mutant mice had increased scratching but decreased number of NP1 neurons when challenged with the SADBE-CD model. A possible model to explain these data is that the NP1 pathway normally inhibits the NP2/NP3 itch pathway, so NP1 afferent degeneration leads to disinhibition of the itch pathway, resulting in an increased scratching phenotype. Future experiments will be needed to test and establish this model, including the direct manipulation of NP1 neuronal activities while stimulating NP2 and/or NP3 afferents. A recent study revealed NP1 fibers function in inhibiting mast cell activities and skin inflammation (Zhang et al., 2021), which is also consistent with our model. In addition to the degeneration of MRGPRD+ afferents, other cellular and molecular mechanisms could also contribute to this phenotype and warrant future investigation.
In summary, our study discovered a previously unreported function of TRPC3 in modulating itch sensation of a mouse CD model. The molecular and cellular mechanisms we identified here will help to understand the full pathology of CD and develop additional treatment strategies.
Materials and Methods
Mice
Three to four-month-old male and female mice were used for all behavioral and histology experiments. TrpC3 null mice and floxed TrpC3 mice were described as previously (Dong et al., 2017, Hirschler-Laszkiewicz et al., 2012). MrgdEGFP mice were obtained from Xinzhong Dong’s lab (Zylka et al., 2005). C57BL/6J (000664), Trpv1Cre (017769), and Vav1iCre(008610) mice were purchased from Jackson Laboratories (Cavanaugh et al., 2011, Yang et al., 2008). All experiments were conducted in accordance with the National Institute of Health guidelines and with approval from the Animal Care and Use Committee of University of Pennsylvania and Washington University School of Medicine. Mice were housed in a 12-hour light/dark cycle with food and water ad libitum.
Behavior
Contact dermatitis model
A murine model of contact dermatitis was produced with repeated topical application of 25 μl of squaric acid dibutyl ester (SADBE, 1% in acetone, Sigma, 339792) on shaved skin. The protocol timeline was adapted from previously described procedures as summarized in Figure 1 (Qu et al., 2014, Scott et al., 2002). On day 16, spontaneous behavior was recorded for 1 hour (23 hours after the treatment), and scored for scratching behavior, wiping behavior, and inactive periods by researchers blinded to mouse genotype. Scratching bouts were defined as an uninterrupted movement of the hindpaw directed at the treated site and ended when paws were placed on the floor, licked, or paused in the air for more than 1 second. A wipe was defined as a downward movement of the forepaw to the head/neck. Scratch frequency distribution graphs were made by dividing the 1-hour video into 5-minute bins, quantifying the number of bouts that occurred in each interval, and sorting the interval into bins based on bout number. Then the percentage of low (< 60 bouts/bin) and high (≥ 60 bouts/bin) scratch bins were calculated and compared. Experiments on two cohorts of mice were independently conducted at the Washington University of St. Louis (Cohort W) and the University of Pennsylvania (Cohort P).
Inflammatory pain behaviors
Either saline or 10 μl intraplantar injection of CFA (Sigma, St. Louis; 1:1 dilution with saline, final concentration 5μg/10 μl) was injected into the plantar skin of mice. Static and dynamic mechanical sensitivity using von Frey hair and paintbrush respectively, and radiant heat response latencies using Hargreaves apparatus prior to CFA injection and again on days 1, 7, 14, 21, and 28 post-CFA were measured (Cheng et al., 2017).
Gait
Footprint analysis of TrpC3 null and WT naïve mice was performed with ink and analyzed for gait with and alternative coefficient as previously published (Becker et al., 2009). Gait width was measured as the average lateral distance between right and left step pairs. The alternative coefficient was calculated by determining the mean of the absolute value of 0.5 minus the ratio of right-left step distance to right-right step distance for every right-left step pair.
Hematoxylin and eosin staining, Imaging, and Quantification
On day 17, treated and untreated neck skin was collected and fixed overnight in 4% buffered paraformaldehyde at 4°C (Thermo Scientific, AAJ19943K2), serially dehydrated using ethanol and xylene. Tissue was paraffin embedded, sliced (5 µm), and collected on positively charged slides and dried overnight at room temperature. Slides were stained with Hematoxylin (Leica, 3801540) and Eosin (Leica, 3801600) using an automated stainer (Leica, Autostainer XL). Images were collected at 20X magnification utilizing brightfield microscopy. Cellular infiltration and ulcer thickness were analyzed in Fiji.
RT-PCR.
≥4-month-old mice were deeply anesthetized with CO2 and DRGs, cerebellum, and spleen tissue were dissected under RNase free conditions. Tissue was mechanically homogenized, RNA was isolated using the RNeasy Micro Kit (Qiagen 74004), and cDNA was synthesized with oligo-dT primers using the SuperScript First-Strand Synthesis system (Invitrogen 18080051). RT-PCR was performed on cDNA with primers for TrpC3 (forward primer CCTGGCTTTCATGATTGGCATGTTC for exon 6 and reverse primer CACTCACATCTCAGCACACTGGGG for exon 11).
Immunostaining
Mice were anesthetized with ketamine and xylazine and transcardially perfused with 4% PFA (in PBS) on day 16. Immunostaining was performed according to previously described protocol (Dong et al., 2017, Olson et al., 2017)). Cervical (C2-C5) and lumbar (L2-L5) DRG and spinal cord (SC) were dissected and post-fixed for 2–4 hours in 4% PFA at 4°C. Neck skin was dissected and post-fixed in 4% PFA O/N. All tissues were cryoprotected in 30% sucrose in PBS O/N at 4°C and embedded in OCT. DRG sections were sliced at 20μm, collected on Superfrost Plus slides, and dried O/N at room temperature. Neck skin and SC were sliced at 20 μm and 30 μm, respectively, and processed as floating sections. Primary antibodies used include chicken anti-GFP (1:2,000 (skin), 1:1,000 (SC, DRG); Aves, GFP-1020), rabbit anti-GFP (1:1,000, Invitrogen, A-11122), rabbit anti-CGRP (1:1,000; Immunostar, 24112), Guinea pig anti-VGLUT1 (1:1,000, Millipore, AB5905), and Alexa 594 conjugated IB4 (1:500; Life Sciences, I21413). Anti-GFP antibodies were used to label the expression of GFP in MrgprdEGFP mice. Secondary antibodies (all 1:500) were Alexa 488 conjugated goat anti-chicken antibody (Invitrogen, A-11039), Alexa 594 conjugated goat anti-rabbit antibody (Invitrogen, A21207), and Alexa 647 conjugated goat anti-guinea pig antibody (Jackson Immunoresearch, 106-605-003). Images were collected using a Leica SP5 confocal microscope.
Data Analysis
All data, except for frequency graphs and figure 2 graphs, are presented as mean ± standard error of mean (SEM) and analyzed with using two-tailed t-tests. Frequency graphs are presented as the mean percentage of low and high scratch bins and analyzed with Chi-squared tests. Figure 2 graphs were analyzed using two-way ANOVA. Statistical analysis was performed using GraphPad Prism. Differences were considered significant if p < 0.05.
Data Availability Statement
Datasets related to this article can be found at https://data.mendeley.com/datasets/hbrhfhfjj7/draft?a=c422df58-45aa-44a6-a1b7-ccccc6182b6a, hosted at Mendeley Data (Beattie, Katherine (2021), “TRPC3 Antagonizes Pruritus in A Mouse Contact Dermatitis Model”, Mendeley Data, V1, doi: 10.17632/hbrhfhfjj7.1).
Acknowledgements
We thank members of Katherine’s thesis committee for their advice, the Luo lab members for their help and support, and Dr. Phillip Scott and his lab members for immunology expertise. This work was supported by the National Institute of Health through grants from the NIAMS (F31 AR075436-01 to KB) and the NINDS (grant R01 NS083702 and R01NS094224 to WL). Histology research was supported by the Penn Skin Biology and Diseases Resource-based Center, funded by P30-AR069589.
Abbreviations:
- ACD
Allergic contact dermatitis
- CD
contact dermatitis
- CFA
Complete Freund’s Adjuvant
- CKO
conditional knock out
- DRG
dorsal root ganglion
- ICD
Irritant contact dermatitis
- MRGPRs
Mas-related G-protein-coupled receptors
- NP
non-peptidergic
- SADBE
squaric acid dibutylester
- SC
spinal cord
- TRPC
transient receptor potential channel subfamily C
Footnotes
Conflict of Interest
The authors state no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdus-Saboor I, Fried NT, Lay M, Burdge J, Swanson K, Fischer R, et al. Development of a Mouse Pain Scale Using Sub-second Behavioral Mapping and Statistical Modeling. Cell Rep 2019;28(6):1623–34.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ale IS, Maibach HI. Irritant contact dermatitis. Reviews on environmental health 2014;29(3):195–206. [DOI] [PubMed] [Google Scholar]
- Alkhani H, Ase AR, Grant R, O’Donnell D, Groschner K, Séguéla P. Contribution of TRPC3 to store-operated calcium entry and inflammatory transductions in primary nociceptors. Molecular Pain 2014;10:43-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker EBE, Oliver PL, Glitsch MD, Banks GT, Achilli F, Hardy A, et al. A point mutation in TRPC3 causes abnormal Purkinje cell development and cerebellar ataxia in moonwalker mice. Proceedings of the National Academy of Sciences 2009;106(16):6706–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanaugh DJ, Chesler AT, Jackson AC, Sigal YM, Yamanaka H, Grant R, et al. Trpv1 Reporter Mice Reveal Highly Restricted Brain Distribution and Functional Expression in Arteriolar Smooth Muscle Cells. The Journal of Neuroscience 2011;31(13):5067–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanaugh DJ, Lee H, Lo L, Shields SD, Zylka MJ, Basbaum AI, et al. Distinct subsets of unmyelinated primary sensory fibers mediate behavioral responses to noxious thermal and mechanical stimuli. Proc Natl Acad Sci U S A 2009;106(22):9075–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Sooch G, Demaree IS, White FA, Obukhov AG. Transient Receptor Potential Canonical (TRPC) Channels: Then and Now. Cells 2020;9(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng L, Duan B, Huang T, Zhang Y, Chen Y, Britz O, et al. Identification of spinal circuits involved in touch-evoked dynamic mechanical pain. Nat Neurosci 2017;20(6):804–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cranfill SL, Luo W. The development of somatosensory neurons: Insights into pain and itch. Current topics in developmental biology 2021;142:443–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong P, Guo C, Huang S, Ma M, Liu Q, Luo W. TRPC3 Is Dispensable for β-Alanine Triggered Acute Itch. Scientific Reports 2017;7(1):13869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng J, Yang P, Mack MR, Dryn D, Luo J, Gong X, et al. Sensory TRP channels contribute differentially to skin inflammation and persistent itch. Nature Communications 2017;8(1):980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han L, Ma C, Liu Q, Weng H-J, Cui Y, Tang Z, et al. A subpopulation of nociceptors specifically linked to itch. Nature Neuroscience 2013;16(2):174–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann J, Dragicevic E, Adelsberger H, Henning HA, Sumser M, Abramowitz J, et al. TRPC3 Channels Are Required for Synaptic Transmission and Motor Coordination. Neuron 2008;59(3):392–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschler-Laszkiewicz I, Zhang W, Keefer K, Conrad K, Tong Q, Chen S-j, et al. Trpc2 depletion protects red blood cells from oxidative stress-induced hemolysis. Experimental Hematology 2012;40(1):71–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon J, Bu F, Sun G, Tian J-B, Ting S-M, Li J, et al. Contribution of TRPC Channels in Neuronal Excitotoxicity Associated With Neurodegenerative Disease and Ischemic Stroke. Frontiers in Cell and Developmental Biology 2021;8(1755). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joost S, Annusver K, Jacob T, Sun X, Dalessandri T, Sivan U, et al. The Molecular Anatomy of Mouse Skin during Hair Growth and Rest. Cell stem cell 2020;26(3):441–57.e7. [DOI] [PubMed] [Google Scholar]
- Julius D TRP channels and pain. Annual review of cell and developmental biology 2013;29:355–84. [DOI] [PubMed] [Google Scholar]
- Kostner L, Anzengruber F, Guillod C, Recher M, Schmid-Grendelmeier P, Navarini AA. Allergic Contact Dermatitis. Immunology and Allergy Clinics of North America 2017;37(1):141–52. [DOI] [PubMed] [Google Scholar]
- Lagerström MC, Rogoz K, Abrahamsen B, Persson E, Reinius B, Nordenankar K, et al. VGLUT2-dependent sensory neurons in the TRPV1 population regulate pain and itch. Neuron 2010;68(3):529–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin X, Antalffy B, Kang D, Orr HT, Zoghbi HY Polyglutamine expansion down-regulates specific neuronal genes before pathologic changes in SCA1. Nature Neuroscience 2000;3(2):157–63. [DOI] [PubMed] [Google Scholar]
- Liu Q, Sikand P, Ma C, Tang Z, Han L, Li Z, et al. Mechanisms of itch evoked by β-alanine. The Journal of neuroscience : the official journal of the Society for Neuroscience 2012;32(42):14532–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Tang Z, Surdenikova L, Kim S, Patel KN, Kim A, et al. Sensory Neuron-Specific GPCR Mrgprs Are Itch Receptors Mediating Chloroquine-Induced Pruritus. Cell 2009;139(7):1353–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Abdel Samad O, Zhang L, Duan B, Tong Q, Lopes C, et al. VGLUT2-dependent glutamate release from nociceptors is required to sense pain and suppress itch. Neuron 2010;68(3):543–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo W, Enomoto H, Rice FL, Milbrandt J, Ginty DD. Molecular Identification of Rapidly Adapting Mechanoreceptors and Their Developmental Dependence on Ret Signaling. Neuron 2009;64(6):841–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra SK, Hoon MA. The Cells and Circuitry for Itch Responses in Mice. Science 2013;340(6135):968–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montell C The history of TRP channels, a commentary and reflection. Pflügers Archiv - European Journal of Physiology 2011;461(5):499–506. [DOI] [PubMed] [Google Scholar]
- Nosbaum A, Vocanson M, Rozieres A, Hennino A, Nicolas JF. Allergic and irritant contact dermatitis. European journal of dermatology : EJD 2009;19(4):325–32. [DOI] [PubMed] [Google Scholar]
- Olson W, Abdus-Saboor I, Cui L, Burdge J, Raabe T, Ma M, et al. Sparse genetic tracing reveals regionally specific functional organization of mammalian nociceptors. Elife 2017;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu L, Fan N, Ma C, Wang T, Han L, Fu K, et al. Enhanced excitability of MRGPRA3- and MRGPRD-positive nociceptors in a model of inflammatory itch and pain. Brain 2014;137(4):1039–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu L, Fu K, Yang J, Shimada SG, LaMotte RH. CXCR3 chemokine receptor signaling mediates itch in experimental allergic contact dermatitis. PAIN 2015;156(9):1737–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu L, Li Y, Pan X, Zhang P, LaMotte RH, Ma C. Transient receptor potential canonical 3 (TRPC3) is required for IgG immune complex-induced excitation of the rat dorsal root ganglion neurons. J Neurosci 2012;32(28):9554–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quick K, Zhao J, Eijkelkamp N, Linley JE, Rugiero F, Cox JJ, et al. TRPC3 and TRPC6 are essential for normal mechanotransduction in subsets of sensory neurons and cochlear hair cells. Open Biol 2012;2(5):120068-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwendinger-Schreck J, Wilson SR, Bautista DM. Interactions Between Keratinocytes and Somatosensory Neurons in Itch. In: Cowan A, Yosipovitch G, editors. Pharmacology of Itch. Berlin, Heidelberg: Springer Berlin Heidelberg; 2015. p. 177–90. [DOI] [PubMed] [Google Scholar]
- Scott AE, Kashon ML, Yucesoy B, Luster MI, Tinkle SS. Insights into the Quantitative Relationship between Sensitization and Challenge for Allergic Contact Dermatitis Reactions. Toxicology and Applied Pharmacology 2002;183(1):66–70. [DOI] [PubMed] [Google Scholar]
- Shinohara T, Harada M, Ogi K, Maruyama M, Fujii R, Tanaka H, et al. Identification of a G Protein-coupled Receptor Specifically Responsive to β-Alanine. Journal of Biological Chemistry 2004;279(22):23559–64. [DOI] [PubMed] [Google Scholar]
- Solinski HJ, Kriegbaum MC, Tseng PY, Earnest TW, Gu X, Barik A, et al. Nppb Neurons Are Sensors of Mast Cell-Induced Itch. Cell Rep 2019;26(13):3561–73.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storan ER, O’Gorman SM, McDonald ID, Steinhoff M. Role of Cytokines and Chemokines in Itch. In: Cowan A, Yosipovitch G, editors. Pharmacology of Itch. Berlin, Heidelberg: Springer Berlin Heidelberg; 2015. p. 163–76. [DOI] [PubMed] [Google Scholar]
- Sun S, Dong X. Trp channels and itch. Seminars in immunopathology 2016;38(3):293–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usoskin D, Furlan A, Islam S, Abdo H, Lönnerberg P, Lou D, et al. Unbiased classification of sensory neuron types by large-scale single-cell RNA sequencing. Nature Neuroscience 2015;18(1):145–53. [DOI] [PubMed] [Google Scholar]
- Wang F, Trier AM, Li F, Kim S, Chen Z, Chai JN, et al. A basophil-neuronal axis promotes itch. Cell 2021;184(2):422–40.e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenning AS, Neblung K, Strauß B, Wolfs M-J, Sappok A, Hoth M, et al. TRP expression pattern and the functional importance of TRPC3 in primary human T-cells. Biochimica et Biophysica Acta (BBA) - Molecular Cell Research 2011;1813(3):412–23. [DOI] [PubMed] [Google Scholar]
- Yang J, Hills D, Taylor E, Pfeffer K, Ure J, Medvinsky A. Transgenic tools for analysis of the haematopoietic system: Knock-in CD45 reporter and deletor mice. Journal of Immunological Methods 2008;337(2):81–7. [DOI] [PubMed] [Google Scholar]
- Zeisel A, Hochgerner H, Lönnerberg P, Johnsson A, Memic F, van der Zwan J, et al. Molecular Architecture of the Mouse Nervous System. Cell 2018;174(4):999–1014.e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Edwards TN, Chaudhri VK, Wu J, Cohen JA, Hirai T, et al. Nonpeptidergic neurons suppress mast cells via glutamate to maintain skin homeostasis. Cell 2021;184(8):215166.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Hanson CE, Liu Q, Han L. Mrgprs activation is required for chronic itch conditions in mice. Itch (Phila) 2017;2(3):e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zylka MJ, Rice FL, Anderson DJ. Topographically Distinct Epidermal Nociceptive Circuits Revealed by Axonal Tracers Targeted to Mrgprd. Neuron 2005;45(1):17–25. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Datasets related to this article can be found at https://data.mendeley.com/datasets/hbrhfhfjj7/draft?a=c422df58-45aa-44a6-a1b7-ccccc6182b6a, hosted at Mendeley Data (Beattie, Katherine (2021), “TRPC3 Antagonizes Pruritus in A Mouse Contact Dermatitis Model”, Mendeley Data, V1, doi: 10.17632/hbrhfhfjj7.1).