Abstract
A method using calcium triflimide [Ca(NTf2)2] as a Lewis acid to activate sulfonyl fluorides toward nucleophilic addition with amines is described. The reaction converts a wide array of sterically and electronically diverse sulfonyl fluorides and amines into the corresponding sulfonamides in good yield.
Graphical Abstract

The sulfonamide structural class is prevalent within the pharmaceutical and agrochemical industries. In 2016, sulfonamides represented 15% of the top 100 most prescribed drugs, with therapeutic applications against cardiovascular, infectious, and neurological diseases.1 In the agrochemical industry, the sulfonamide motif appears in a variety of pesticides, including asulam, orzalin, fomesafen, halosafen, and sulfentrazone.2 To date, the most common methods to synthesize sulfonamides require the oxidation of sulfides or nucleophilic addition to sulfonyl chlorides. However, these approaches have several limitations. Sulfide oxidation employs the use of strong oxidants that are incompatible with many functional groups, precluding late-stage functionalization,3 and the high reactivity of sulfonyl chlorides can result in poor selectivity in the presence of competing nucleophiles, detrimental reduction of S(VI) to S(IV), as well as instability during storage.3c Therefore, developing alternative methods to generate a diverse array of sulfonamides that employ more stable reactants would benefit pharmaceutical and agrochemical syntheses (Figure 1). In addition, highlighting the value of the sulfonyl fluoride (SF) as a useful and stable functional group provides support for the development and storage of a more diverse collection of these reagents.
Figure 1.

(a) Current methods toward converting sulfonyl fluorides to sulfonamides,10 (b) proposed modes of Lewis acid activation sulfonyl fluoride for nucleophilic addition of amines, and (c) our method using Ca(NTf2)2 in tert-amyl alcohol.
In recent years, sulfonyl fluorides have gained renewed interest as alternatives to sulfonyl chlorides due to their inherent stability and chemoselective reactivity at sulfur.4 The sulfur–fluorine (S–F) bond in SO2F2 is approximately 40 kcal mol−1 stronger than the sulfur–chlorine (S–Cl) bond in SO2Cl2.5 An analogous comparison to aryl sulfonyl halides suggests that this added bond strength leads to thermodynamic stability and resistance to both reduction6 and hydrolysis.7 For the sulfonyl fluorides, a fortuitous balance between hydrolytic stability and reactivity has enabled their wide application as reactive electrophiles in chemical biology to modify specific amino acids in proteins.7c,d,8
Similarly, sulfonyl fluorides have gained importance as alternative precursors to generate sulfonamides.4,9 However, the increased stability of sulfonyl fluorides comes with the duality of reduced reactivity. Existing methods for synthesizing sulfonamides from sulfonyl fluorides typically involve using a strong base/nucleophile, a vast excess of the amine component, and/or extended periods of heating (Figure 1a).10 Additionally, sulfonamide formation is highly dependent on the electronic nature of the sulfonyl fluoride and the amine.7c Herein, we report a mild, calcium triflimide [Ca(NTf2)2] mediated activation of sulfonyl fluorides toward sulfonamide formation in tert-amyl alcohol (t-amylOH, Figure 1b,c). This method was demonstrated on a wide variety of aromatic, heteroaromatic, and alkyl sulfonamides and, to our knowledge, is the first example of a single set of reaction conditions to convert a myriad of sulfonyl fluorides and amines to sulfonamides.
The supposition that Lewis acid (LA) catalysis may facilitate nucleophilic displacement of fluoride under mild conditions guided our initial approach to this project.11,12 This could potentially occur via formation of Lewis acid/base adducts either with the sulfonyl oxygens or the fluorine atom (Figure 1b).8a,13,14 In turn, these adducts would increase the susceptibility of the sulfur atom toward nucleophilic addition. A screen of Lewis acids and solvents with benzenesulfonyl fluoride and aniline identified a promising lead: barium triflimide [Ba(NTf2)2] in t-amylOH, (Table 1, entry 2). Upon a thorough analysis of additional triflimide salts, divalent second group salts such as Ba(NTf2)2 and Ca(NTf2)2 emerged as superior Lewis acids, affording the sulfonamide product in higher yields than the monovalent cationic triflimides (Table 1, entries 1–3 and entries 4–6, respectively). Solubility of the Lewis acids showed a marked effect on the extent of reaction. In general, the triflimide salts were more soluble in the reaction than Lewis acids with other counteranions (Table 1, entries 7–10). Using the conjugate acid of the triflimide anion, HNTf2, in lieu of a Lewis acid resulted in a marginal 3% isolated yield of compound 3 (Table 1, entry 11). We also note that calcium fluoride, a potential byproduct of the reaction, does not affect sulfonamide formation (Table 1, entry 7). Attempts to reduce the molar equivalents of Lewis acid and/or amine resulted in incomplete consumption of the benzenesulfonyl fluoride.
Table 1.
Lewis Acid Optimizationa

| ||
|---|---|---|
| entry | Lewis acid | yield (%) |
| 1 | Ca(NTf2)2 | 88 |
| 2 | Ba(NTf2)2 | 80 |
| 3 | Mg(NTf2)2 | 38 |
| 4 | LiNTf2 | 9 |
| 5 | AgNTf2 | N/Rb |
| 6 | KNTf2 | <2 |
| 7 | CaF2 | N/Rb |
| 8 | Ca(OTf)2 | 4 |
| 9 | La(OTf)3·xH2O | 30 |
| 10 | LiCl | 2 |
| 11 | HNTf2 | 3 |
| 12 | none | N/Rb |
Reaction conditions: PhSO2F (1 equiv), aniline (2 equiv), Lewis acid (1 equiv), t-amylOH (0.32 M), 60 °C, 24 h.
N/R denotes no reaction.
Calcium triflimide was selected for further examination of solvent effects due to its improved organic solubility and reduced cost vs barium triflimide. The reaction could be run in water, although a reduction in yield was observed, and the solubility of the starting materials was generally poor (Table 2, entry 2). Alcoholic solvents afforded the desired sulfonamide as well, with sterically congested alcohols providing higher conversion to sulfonamide (Table 2, entries 3–5). Switching to polar aprotic solvents such as MeCN and DMF (Table 2, entries 6 and 7), the conversion dropped significantly, while a nonpolar solvent such as toluene produced moderate yields of the sulfonamide (Table 2, entry 8). Reports in the literature suggest using hexafluoroisopropanol (HFIP) as a solvent or NBu4PF6 as an additive could improve the activation of Ca(NTf2)2.12a,15 However, Ca(NTf2)2 showed minimal solubility in HFIP, and only 9% sulfonamide product 3 was isolated (Table 2, entry 9). Additionally, the NBu4PF6/Ca(NTf2)2 combination also resulted in considerably decreased yields of 3 (see the Supporting Information (SI)). Overall, t-amylOH and t-BuOH provided the highest yields with t-amylOH emerging as the solvent of choice due to convenience of use and environmental factors.16
Table 2.
Solvent Optimizationa

| ||
|---|---|---|
| entry | solvent | yield (%) |
| 1 | t-amylOH | 88 |
| 2 | H2O | 63 |
| 3 | MeOH | 40 |
| 4 | i-PrOH | 71 |
| 5 | t-BuOH | 87 |
| 6 | MeCN | 42 |
| 7 | DMF | 13 |
| 8 | toluene | 51 |
| 9 | HFIP | 9b |
Reaction conditions: PhSO2F (1 equiv), aniline (2 equiv), Ca(NTf2)2 (1 equiv), solvent (0.20 M), 60 °C, 24 h. Isolated yields are averages of two independent trials.
Ca(NTf2)2 showed minimal solubility in HFIP. Isolated yield is reported for one trial.
With favorable conditions in hand, we next focused our attention on exploring the versatility of the method using sterically and electronically diverse aromatic and aliphatic amines as nucleophiles (Scheme 1). When aromatic and heteroaromatic amines were reacted with benzenesulfonyl fluoride, in the presence of Ca(NTf2)2, sulfonamide formation was achieved in good to excellent yield. It is noteworthy that in the absence of Lewis acid these reactions proceeded with little to no sulfonamide formation, highlighting the crucial role Ca(NTf2)2 plays in activating the sulfonyl fluorides toward nucleophilic addition. We generally used two molar equivalents of the amine component for these reactions but were pleased to show that when only 1 equiv of aniline was used in combination with triethylamine sulfonamide 3 was isolated in 71% yield (Scheme 1).17
Scheme 1.

Substrate Scope Reacting Aryl, Heteroaryl, and Aliphatic Amines with PhSO2F*
*Reaction conditions: PhSO2F (1 equiv), amine (2 equiv), Ca(NTf2)2 (1 equiv), t-amylOH (0.20 M), 60 °C, 24 h. Isolated yields for Ca(NTf2)2 reactions are averages of two independent trials. Reactions without Ca were conducted as a single reaction. Mass balance is unreacted starting material. aPhSO2F (1 equiv), aniline (1 equiv), Ca(NTf2)2 (1 equiv), Et3N (1 equiv), t-amylOH (0.20 M), 60 °C, 24 h. bN/R denotes no reaction. cTrace product was detected by LC/MS; however, it could not be isolated. dYield of sulfonamide 14 on 6.2 mmol scale.
Interestingly, 4-aminophenol reacts with sulfonyl fluoride 1 to form the sulfonamide 7 as the major product. This preference for sulfonamide formation is opposite to the observed reactivity in the presence of Cs2CO3, which instead provides the corresponding sulfonic ester.18 Analogues of compound 10 with variations on the arylsulfonyl were valuable leads in a drug discovery project within Pfizer.19 Under our reaction conditions, we obtained compound 10 in 63% yield from benzenesulfonyl fluoride, highlighting the value of our chemistry on a pharmaceutically relevant template. Additionally, both primary and secondary aliphatic amines reacted with sulfonyl fluoride 1 to generate their corresponding sulfonamides (11–14) in good yield, with the synthesis of compound 14 exemplified on gram scale. Initial attempts to react phenylsulfonyl fluoride with weakly nucleophilic anilines containing electron withdrawing groups at the 4-position (e.g., –SCF3 and –CF3), using our general procedure, did not result in detectable sulfonamide product.
We selected 1-(5-(trifluoromethyl)-2-pyridinyl)piperazine (15) as the nucleophilic amine to assess the versatility of the method with various sulfonyl fluorides. Toward this end, we successfully synthesized an array of sulfonamides using aryl- and heteroarylsulfonyl fluorides (Scheme 2). Additionally, alkylsulfonyl fluorides provided the desired sulfonamides (24 and 25) under identical reaction conditions. Interestingly, while Ca(NTf2)2 was required for reactivity of electron-rich arylsulfonyl fluorides, reactions of electron-deficient sulfonyl fluorides with amine 15 proceeded in the absence of any Lewis acid (see 21–23, Scheme 2). This is presumably due to the increased electrophilicity of the sulfonyl fluoride resulting from the electron-withdrawing groups in the aryl ring.7c
Scheme 2.

Substrate Scope Reacting Aryl- and Alkylsulfonyl Fluorides with Amine 15*
*Reaction conditions: RSO2F (1 equiv), 1-(5-trifluoromethyl)-2-pyrdinyl piperazine, 18 (2 equiv), Ca(NTf2)2 (1 equiv), t-amylOH (0.20 M), 60 °C, 24 h. Isolated yields for Ca(NTf2)2 reactions are averages of two independent trials. Reactions without Ca where conducted as a single reaction. aN/R denotes no reaction.
We next wanted to explore a less nucleophilic amine in combination with the electron-deficient sulfonyl fluorides to determine if Ca(NTf2)2 would be required in those cases. Accordingly, 4-cyanobenzenesulfonyl fluoride was reacted with aniline in the presence and absence of Ca(NTf2)2 in t-amyl alcohol at 60 °C and analyzed by LC/MS (see the SI). In the absence of Ca(NTf2)2 no sulfonamide formation was detected after 24 h. However, in the presence of Ca(NTf2)2, the reaction was nearly complete after 1 h, affording sulfonamide 26 in 85% yield (Scheme 3 and SI). We observed similar reactivity with the heteroarylsulfonyl fluorides, as demonstrated in the synthesis of sulfonamides 27 and 28 (Scheme 3). Although Ca(NTf2)2 is not required for reaction between an electron-deficient sulfonyl fluoride and a highly nucleophilic amine, for all other combinations of sulfonyl fluorides and amines that we explored, Ca(NTf2)2 activation to generate sulfonamides in high yield has been demonstrated to be essential.
Scheme 3.
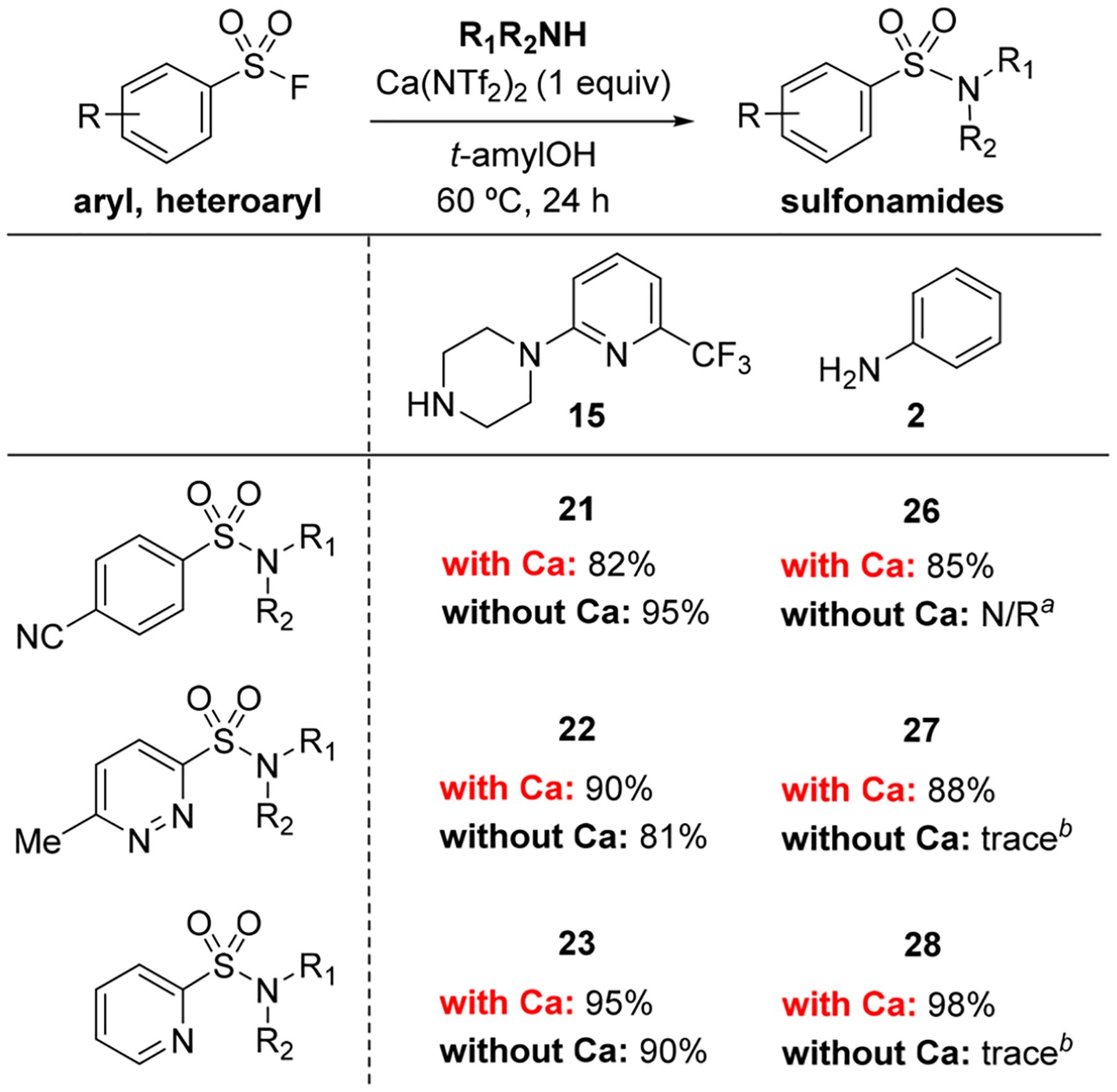
Comparing the Reactivity of Electron-Deficient Sulfonyl Fluorides with Amines 2 and 15*
*Reaction conditions: RSO2F (1 equiv), amine, 15 or 2 (2 equiv), Ca(NTf2)2 (1 equiv), t-amylOH (0.20 M), 60 °C, 24 h. Isolated yields for Ca(NTf2)2 reactions are averages of independent trials. Reactions without Ca weere conducted as a single reaction. aN/R denotes no reaction. bTrace product was detected by LC/MS; however, it could not be isolated.
We wanted to show the complementarity of this method and the stability of the sulfonyl fluoride toward traditional sulfonamide synthesis from sulfonyl chlorides. Therefore, we subjected piperazine 29 to a sequential Boc deprotection, sulfonamidation with benzenesulfonyl chloride. The reactivity of the sulfonyl fluoride was then unveiled for reaction with 3-aminopyridine via the action of Ca(NTf2)2 to afford compound 31 in high yield (Scheme 4). Notably, sulfonyl fluoride 30 withstood the Boc-deprotection under acidic conditions, and the first sulfonamidation in the presence of triethylamine proceeded without detectable self-condensation. The synthesis of compound 31 demonstrates how synthetic strategies can exploit the robust nature of sulfonyl fluorides and then unlock the latent reactivity using calcium triflimide.
Scheme 4.

Sulfonyl Fluoride as a Latent Electrophile
In conclusion, we have developed a method that employs calcium triflimide activation of aryl- and alkylsulfonyl fluorides to synthesize sulfonamides. This method can be used to couple a wide array of sterically and electronically diverse sulfonyl fluorides and amines to produce sulfonamides in good to excellent yields. Preliminary results suggest that divalent cations and the triflimide anion are essential for efficient conversion, with future studies focusing on developing a better understanding of the reaction mechanism. With the contribution of this new method, we envision that the sulfonyl fluorides will emerge as the preferred sulfonyl halide for a myriad of sulfonylation reactions.
Supplementary Material
ACKNOWLEDGMENTS
N.D.B. thanks Pomona College for start-up funds. Additionally, acknowledgment is made to the Donors of the American Chemical Society Petroleum Research Fund for support (544180-UNI). S.M.E., R.W.F., S.C.K., M.R., and C.P.W. thank the Pomona SURF program and the Pomona Department of Chemistry for their funding. C.P.W. and N.D.B. thank the ACS Scholars Program. C.P.W., N.D.B. and C.W.A. thank the Beckman Scholars Program. The authors also thank Mona Shahgholi and Nassem Torian (Caltech) for the HRMS analyses, Ivan Samardjiev (Pfizer) for X-ray crystallography, and Patrick Mullins (Pfizer) and Christopher Helal (Pfizer) for critical review of the manuscript.
Footnotes
The authors declare no competing financial interest.
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.orglett.8b01520.
Experimental methods and NMR spectra (PDF)
Accession Codes
CCDC 1842164–1842166 contain the supplementary crystallographic data for this paper. These data can be obtained free of charge via www.ccdc.cam.ac.uk/data_request/cif, or by emailing data_request@ccdc.cam.ac.uk, or by contacting The Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, UK; fax: +44 1223 336033.
REFERENCES
- (1).McGrath NA; Brichacek M; Njardarson JT J. Chem. Educ 2010, 87, 1348. [Google Scholar]
- (2).Devendar P; Yang G-F Top. Curr. Chem 2017, 375, 1. [DOI] [PubMed] [Google Scholar]
- (3).(a) Wright SW; Hallstrom KN J. Org. Chem 2006, 71, 1080. [DOI] [PubMed] [Google Scholar]; (b) Bahrami K; Khodaei MM; Abbasi J Tetrahedron 2012, 68, 5095. [Google Scholar]; (c) Goldberg FW; Kettle JG; Kogej T; Perry MWD; Tomkinson NP Drug Discovery Today 2015, 20, 11. [DOI] [PubMed] [Google Scholar]
- (4).(a) Dong J; Krasnova L; Finn MG; Sharpless KB Angew. Chem., Int. Ed 2014, 53, 9430 and references cited therein. [DOI] [PubMed] [Google Scholar]; (b) Bogolubsky AV; Moroz YS; Mykhailiuk PK; Pipko SE; Konovets AI; Sadkova IV; Tolmachev A ACS Comb. Sci 2014, 16, 192. [DOI] [PubMed] [Google Scholar]
- (5).(a) Kiang T; Zare RNJ Chem. Soc., Chem. Commun 1980, 24, 1228. [Google Scholar]; (b) Wray KL; Feldman EV J. Chem. Phys 1971, 54, 3445. [Google Scholar]
- (6).(a) Bertrand MP Org. Prep. Proced. Int 1994, 26, 257. [Google Scholar]; (b) Chatgilialoglu C Sulfonyl Radicals. In Sulphones and Sulfoxides; Patai S, Rappoport Z, Stirling C, Eds.; John Wiley & Sons, Ltd: Chichester, 1988; pp 1089–1113. [Google Scholar]
- (7).(a) Baker BR; Lourens GJ J. Med. Chem 1967, 10, 1113. [DOI] [PubMed] [Google Scholar]; (b) Ciuffarin E; Senatore L; Isola M J. Chem. Soc., Perkin Trans 2 1972, 4, 468.5066070 [Google Scholar]; (c) Mukherjee H; Debreczeni J; Breed J; Tentarelli S; Aquila B; Dowling JE; Whitty A; Grimster NP Org. Biomol. Chem 2017, 15, 9685. [DOI] [PubMed] [Google Scholar]; (d) Huang JL; Nagy A; Ivleva VB; Blackstock D; Arnold F; Cai CX Anal. Chem 2018, 90, 4293. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Kice JL; Lunney EA J. Org. Chem 1975, 40, 2125. [Google Scholar]
- (8).(a) Narayanan A; Jones LH Chem. Sci 2015, 6, 2650 and references therein. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Zhao Q; Ouyang X; Wan X; Gajiwala KS; Kath JC; Jones LH; Burlingame AL; Taunton JJ Am. Chem. Soc 2017, 139, 680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).(a) Zelli R; Tommasone S; Dumy P; Marra A; Dondoni A Eur. J. Org. Chem 2016, 2016, 5102. [Google Scholar]; (b) Toulgoat F; Langlois BR; Médebielle M; Sanchez J-Y J. Org. Chem 2008, 73, 5613. [DOI] [PubMed] [Google Scholar]
- (10).(a) Empfield JR; Mayhugh D; Ohnmacht CJ; Frank CA; Grant T; Li J Bioorg. Med. Chem. Lett 1997, 7, 775. [Google Scholar]; (b) Bebernitz GR; Aicher TD; Stanton JL; Gao J; Shetty SS; Knorr DC; Strohschein RJ; Tan J; Brand LJ; Liu C; Wang WH; Vinluan CC; Kaplan EL; Dragland CJ; DelGrande D; Islam A; Lozito RJ; Liu X; Maniara WM; Mann WR J. Med. Chem 2000, 43, 2248. [DOI] [PubMed] [Google Scholar]; (c) Berg S; Bergh M; Hellberg S; Högdin K; Lo-Alfredsson Y; Söderman P; von Berg S; Weigelt T; Ormö M; Xue Y; Tucker J; Neelissen J; Jerning E; Nilsson Y; Bhat RJ Med. Chem 2012, 55, 9107. [DOI] [PubMed] [Google Scholar]; (d) Harris PA; Cheung M; Hunter RN; Brown ML; Veal JM; Nolte RT; Wang L; Liu W; Crosby RM; Johnson JH; Epperly AH; Kumar R; Luttrell DK; Stafford JA J. Med. Chem 2005, 48, 1610. [DOI] [PubMed] [Google Scholar]
- (11).For an example of base-catalyzed activation of sulfonyl fluorides, see:; Gembus V; Marsais F; Levacher V Synlett 2008, 2008, 1463. [Google Scholar]
- (12).For representation of Lewis acid complexation with sulfonyl compounds, see:; (a) Qi C; Hasenmaile F; Gandon V; Lebœuf D ACS Catal. 2018, 8, 1734. [Google Scholar]; (b) Peyronneau M; Roques N; Mazieres S; Le Roux C Synlett 2003, 5, 0631. [Google Scholar]
- (13).Begouin J-M; Niggemann M Chem. - Eur. J 2013, 19, 8030. [DOI] [PubMed] [Google Scholar]
- (14).There is also potential for interaction of the Lewis acid with the amine nucleophile. For an example of Ca(NTf2)2 participating in coordination of sulfonyl oxygens and NH bonds with the assistance of an alcoholic solvent, see:; Lebœuf D; Gandon V Synthesis 2017, 49, 1500. [Google Scholar]
- (15).Lebœuf D; Gandon V Synthesis 2017, 49, 1500 and references cited therein. [Google Scholar]
- (16).Ramgren SD; Hie L; Ye Y; Garg NK Org. Lett 2013, 15, 3950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17). Additional stoichiometry/base combinations were explored; see the Supporting Information for selected examples.
- (18).Tribby AL; Rodríguez I; Shariffudin S; Ball ND J. Org. Chem 2017, 82, 2294. [DOI] [PubMed] [Google Scholar]
- (19).Chappie TA; Henderson JL; Young JM; Wager TT; Kormos BL; Patel NC; Sciabola S; Tuttle JB; Verhoest PR; Tucker JW (Pfizer, Inc., USA: ). Preparation of 6,7,8,9-tetrahydro-5H-pyrido[2,3-d]azepine compounds as selective dopa-mine D3 ligands. PCT Int. Appl WO 2017122116A1 20170720, 2017. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


