Abstract
Marine resources are today a renewable source of various compounds that are used in numerous industries. In recent years, considerable attention has been focused on diverse algae or their metabolites to develop several novel bioactive substances. Algae derivatives are defined as a food or part of food that has health benefits and prevention or treatment of disease. Algal sulfated polysaccharides have a high potential as a source of functional ingredients with a wide range of applications in the food and pharmaceutical industries. Fucoidan and carrageenan, as two main seaweed sulfated polysaccharides, possess numerous biological properties. These polysaccharides are highly valuable in food and healthy immune system diet and also can be applied in the pharmaceutical field. They have shown antiviral activity against SARS-CoV-2 causes COVID-19 infection by preventing virus entry into the cell or interfering with viral replication. Thus, they may provide some novel ingredients for the production of healthy functional foods, antiviral supplement formulations, or algal-based treatments for viral respiratory diseases, especially anti-COVID-19 and recommend solutions to this global health problem in the future. This article provides a review of recent researches on immune-boosting food ingredients, the antiviral activity of algae bioactive compounds, fucoidan, and carrageenan, in particular against SARS-CoV-2.
Keywords: Seaweed, Fucoidan, Carrageenan, Antivirus activity, Functional foods, SARS-CoV-2
Introduction
Diet and food as a source of nutrients have a crucial effect on human body function and health (Bilandžić et al. 2014). Thus, incorporating specific foods into the diet may strengthen a person’s immune response and prevent or delay chronic diseases (Kamyari et al. 2021). Since oxidative stress is involved in several diseases, the consumption of food products with antioxidant and anti-inflammatory ingredients can improve human health and the immune system. Usually, healthy foods contain omega-3 fatty acids, vitamins, minerals, and, dietary fibers (Arshad et al. 2020; Iddir et al. 2020).
Edible seaweeds have become a good source of food and alternative bioactive compounds with many industrial applications in food, cosmetics, and pharmaceuticals (Khalid et al. 2018). Algal bioactive compounds have attracted much attention in the development of functional foods and nutraceutical industries. Many reports have been published about isolated compounds from seaweeds with biological activities, demonstrating their ability to produce metabolites that can be used in functional foods, marine-based drugs and health products (Alam et al. 2021). Moreover, the industrial application of the carbohydrate isolated from macroalgae including agar and carrageenan is well known (Khalid et al. 2018). Polysaccharides are the most important compounds present in seaweeds and are well documented for their biological activities. Some seaweeds that have a large amount of polysaccharides are Ascophyllum, Porphyra, and Palmaria species. The important polysaccharides are ulvan from green seaweeds, fucoidan, alginate, and laminarin from brown seaweeds, agar, and carrageenan from red macroalgae. Two main seaweed sulfated polysaccharides, fucoidan and carrageenan possess several biological activities such as anti-cancer, anti-coagulant, antioxidant, anti-inflammation, anti-hyperglycemia, and immunoregulatory activities (Moosavi-Nasab et al. 2020; Pangestuti and Kim 2014; Wang et al. 2019). Recently, studies have been focused on novel pharmaceuticals bioactive substances from algae, in particular those with antiviral attributes which may be useful for protection against COVID-19 (Alam et al. 2021).
SARS-CoV-2, the causative agent of COVID-19 infection, is a positive-sense, single-stranded RNA (+ ssRNA) virus with 26–32 kilobase length. Coronavirus attacks the respiratory system and primarily locates at the nasal cavity and nasopharynx and according to the last studies, the disease severity is depending on high viral load and a long virus-shedding period (Bansal et al. 2020; Tsukagoshi et al. 2021). Because of the lower availability of vaccines and definitive treatment, a helpful way to strengthen the immune system against coronavirus is eating a diet high in immune-boosting nutrients that can be an active role in maintaining health and wellness (Singh et al. 2020). Indeed, foods and their ingredients can improve the body’s immune system against viruses. There are some possible bioactive compounds with the antiviral activity which can be useful against coronavirus via prevention of the viral spike (S) protein binding to host cell receptor, viral RNA replication or inhibition of protease activity and limitation of angiotensin I converting enzyme 2 (ACE2) activity (Galanakis et al. 2020). There are some important nutrients and natural ways to stimulate the immune system with foods; thus, several studies suggested the consumption of healthy foods or drinks with immune-boosting and taking essential vitamins such as vitamin A, B and C (Singh et al. 2020).
Particularly, due to virus mutation, cause the world to face "infecting outburst" crisis that virus infection worldwide spreads, improving the immune system with healthy foods is crucial. Thus, in this study, the role of fucoidan and carrageenan on immune-boosting and their antiviral activity, in particular, anti-SARS-CoV-2 activity will be reviewed which are applicable in functional foods, supplements, and pharmaceutical industries.
Algae as nutraceutical/functional foods
Bioactive compounds are the primary or secondary metabolites with pharmacological effects on metabolism and health in general (Torregrosa-Crespo et al. 2018). Seaweeds or their extracts are rich in metabolites such as proteins, vitamins, polyunsaturated fatty acids, and antioxidants that could be mined to produce several valuable ingredients for a wide range of commercial industries.
Several studies have reported that algae bioactive compounds possess biological activities. Thus, the utilization of algae for the recovery of functional substances, nutraceuticals, and pharmaceuticals for incorporation into highly value-added products and functional foods has attracted much attention (Bhattacharjee 2016; Moosavi‐Nasab et al. 2019; Rengasamy et al. 2020). The use of seaweeds as a food source has been traced back to the fourth century in many parts of Asia and their medicinal uses are even mentioned since prehistoric times. Seaweeds are increasingly used for their seaweed-derived food hydrocolloids (Ale and Meyer 2013). The most common traditional utilization of algal polysaccharides is their use as a source of the texturing ingredient in food industries. However, they have been studied as an important bioresource in the recovery of marine functional ingredients, because they exhibit several biological and physiological characteristics with health benefits (Mišurcováa et al. 2014) such as antioxidant and anti-inflammatory (Moosavi-Nasab et al. 2020), antidiabetic and anti-obesity (Oliyaei et al. 2020; Oliyaei et al. 2021), and anticancer (Liu et al. 2019), etc.
Algae as a source of anti-viral bioactive compounds
Recently, the overexploitation of the natural resource with antiviral substances has received more attention. There are numerous researches that recommended the antiviral activity of algal substances such as seaweed polysaccharides (Chi et al. 2020; Diogo et al. 2015; Elizondo-Gonzalez et al. 2012; Gomaa and Elshoubaky 2016), phlorotannins (Park et al. 2013), cyanovirin-N (Rui et al. 2008), proteins such as lectin (Hwang et al. 2020; Lee 2019; Mu et al. 2017), phycobiliproteins (Abd El Hamid et al. 2019), and cyanobacteria such as Spirulina with the potential of using for stimulating the immune system and acting as antivirus against COVID-19 by inhibition of TNF-α secretion during cytokine storm therapy (El-Sheekh and Abomohra 2020; Singh et al. 2020; Tzachor et al. 2021). There is little information about the anti-COVID 19 activity of the other algal bioactive compounds. Mostly, sulfated polysaccharides, in particular, fucoidan and carrageenan, were investigated. However, it has recently been reported that the Spirulina (Ratha et al. 2020) and Spirulina peptides (MubarakAli et al. 2021) possess the inhibitory effect on COVID-19 by binding to the spike protein. Moreover, phlorotannins are promising antiviral compounds. For instance, dieckol, isolated from Ecklonia cava, showed the SARS-CoV 3CLpro trans-/cis-cleavage inhibitory activity against in silico model (Ugur et al. 2021).
Algal sulfated polysaccharides
Seaweeds are rich in sulfated polysaccharides, which are valuable additives or ingredients because of their biological attributes. Sulfated polysaccharides are complex groups of polymers with an average molecular weight of 20,000–200,000 Da (Je et al. 2021) and are commonly found in different species of three major groups of seaweeds such as red, green, and brown algae. The chemical structure of sulfated polysaccharides is variable and dependent on seaweed species (Manlusoc et al. 2019). The main sulfated polysaccharides found in seaweeds include agar and carrageenan from red algae, fucoidan from brown algae, and ulvan from green seaweeds (Ngo and Kim 2013). The anti-viral potential of sulfated polysaccharides, in particular, fucoidan and carrageenan, has been acknowledged in the past decade. Fucoidan is a non-toxic sulfated polysaccharide and has been approved by Food and Drug Administration (FDA) as Generally Recognized as Safe (GRAS) category and can be used in food ingredients at levels up to 250 mg/day (Citkowska et al. 2019). Carrageenan also is an important water-soluble sulfated polysaccharide from red seaweeds (Rhodophyta) which can be marketed as a green product and has been used extensively in foods, cosmetics, and pharmaceuticals. Carrageenan and its derivatives have unique characteristics such as biocompatibility and biodegradability and possess reactive functional groups that make them useful in different areas of applications that are mainly related to their anionic nature (Khan et al. 2020; Pacheco-Quito et al. 2020; Pangestuti and Kim 2014).
Fucoidan
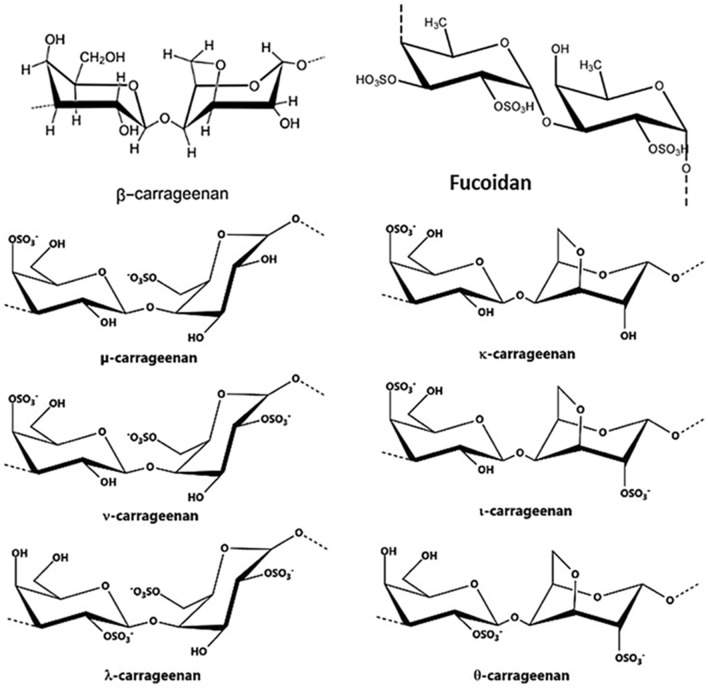
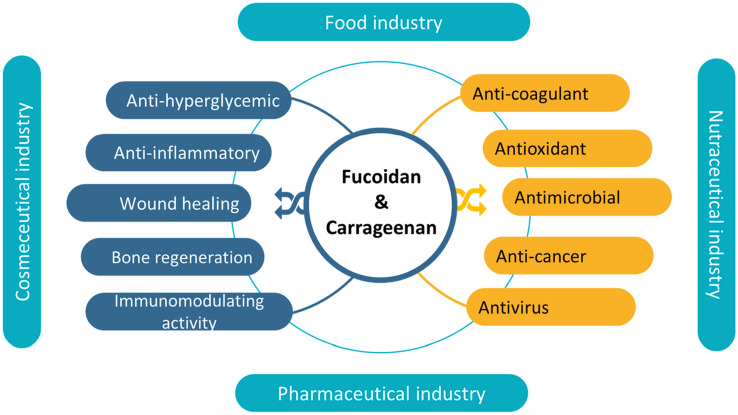
Fucoidan, as one of the most abundant seaweed sulfated polysaccharides, is localized in the cell walls and extracellular of brown seaweeds (Phaeophyceae) such as Fucus, Ascophyllum, Saccharina, and Sargassum. This polysaccharide is widely distributed in more than 265 genera and 2040 species of marine invertebrates such as sea cucumbers (Moosavi-Nasab et al. 2020; Zayed and Ulber 2020). The structure and composition of the purified fucoidan are mostly influenced by the extraction techniques used or the seaweed species. It is reported that the antiviral activity of fucoidan is mostly related to the molecular weight and sulfated groups (Wang et al. 2020; Zayed et al. 2020). From the structural point of view, fucoidan is the most abundant naturally occurring sulfated polysaccharide composed of (1–3)- and (1–4)-linked α-l-fucopyranose units and has sulfate groups at the C-2, C-4, or both positions (Fig. 1). The main repeating unit of the fucoidan chain could be altered by the insertion of different monosaccharide units such that mannose, galactose, glucose, xylose, etc. Depending on its source, fucoidan exists in different heterogeneous forms which differ in their arrangement of the sulfated group, molecular weight, and monosaccharide composition (Fernando et al. 2020; Jayawardena et al. 2019) affecting the fucoidan biological activities (Jayawardena et al. 2019). Moreover, the fucose content is dependent on the origin of algae, the time of harvest, and the species of algae (Jin et al. 2013). According to different extraction methods, the molecular weight of fucoidan products can be divided into low molecular weight (< 10 kDa), middle molecular weight (10–10,000 kDa), and high molecular weight (> 10,000 kDa) (Van Weelden et al. 2019). In addition, structure–activity relationships of bioactivities of polysaccharides are crucial because their biological activities greatly depend on their structural properties such as molecular weight and chain conformation. Thus, the modifications can exert their effect obtaining better functions with a wide range of utilization in the production of functional foods (Xu et al. 2018). Therefore, several modifications of original fucoidan such as fractionation, depolymerization and over-sulfation were evaluated through enzymatic, chemical, or physical methods (Zayed and Ulber 2020). Furthermore, seaweed species, environmental factors affecting the growth of the seaweeds and harvesting quality, processing extraction, and storage are critical for final fucoidan attributes (Flórez‐Fernández et al. 2020; Zhao et al. 2018). Usually, the bioactivities of fucoidan are related to its chemical structural make-up of monosaccharides’ composition and substituted functional groups which are differentiated by methylation analysis, Fourier-transform infrared spectroscopy, and Nuclear magnetic resonance (Fernando et al. 2020). Fucoidan has shown a broad spectrum of biological properties (Fig. 2) including antimicrobial (Poveda-Castillo et al. 2018), anticoagulant (Guan et al. 2020), antioxidant (Koh et al. 2019), anti-inflammatory (Ni et al. 2020a, b), anti-virus (Sun et al. 2018), wound healing (Park et al. 2017), bone regeneration (Kim et al. 2018), and immunomodulatory activities beneficial for the pharmaceutical, cosmeceutical, nutraceutical, and functional food industries (Fernando et al. 2020). In addition, fucoidan has been shown the anticarcinogenic properties in breast and colon cancer cells (He et al. 2019), PC-3 and U-145 prostate cancer cells (Boo et al. 2013; Choo et al. 2016), lung cancer (Wu et al. 2020). Moreover, the antiviral attributes of fucoidan (Alboofetileh et al. 2019; Krylova et al. 2020; Ponce et al. 2019) make it of great interest to isolate from various brown macroalgae.
Fig. 1.
The structure of fucoidan and carrageenans
Fig. 2.
Biological properties of fucoidan and carrageenan
Carrageenan
Carrageenan is a water-soluble sulfated polysaccharide derived from red macroalgae including Chondrus, Hypnea, Gigartina, Eucheuma, Agardhiella, Furcellaria, Iridaea, Sarconema, and Solieria and has a linear chain of partially sulfated galactans (Pacheco-Quito et al. 2020). Its molecular weight is typically between a low molecular weight of 20–40 KDa and a high molecular weight of 200–800 KDa depending on the source of algae and preparation (McKim et al. 2019). Based on carrageenan solubility in potassium chloride, it is derived into various types such as kappa (κ), iota (ι), lambda (λ), mu (μ), nu (ν), teta (θ), and beta (β) carrageenan (Fig. 1) of which kappa, iota and lambda are three main carrageenans with the wide range of application in several industries (Geonzon et al. 2020).
κ-carrageenan commercially is extracted from Kappaphycus alvarezii, whereas λ-carrageenan is derived from the genera Gigartina or Chondrus. Eucheuma denticulatum is the commercial source for ι carrageenan extraction (Khotimchenko et al. 2020). The chemical structure of carrageenan is made up of repeating units of d-galactose and 3,6-anhydro-galactose (3,6-AG) linked together. α-1,3 and β-1,4-glycosidic and carrageenan forms are different in degree and position of sulfate groups and galactose linkages (McKim et al. 2019). Different types of carrageenans usually possess a sulfated degree of about 22–35% and make them strongly anionic which influences solubility temperature and gel strength. The kappa form is characterized by a repeating unit of 4-sulfate-β-d-galactopyranosyl (1–4)-3,6-anhydro-α-d-galactose-linked (1–3) with ester sulfate and 3,6-AG value of about 25 to 30% and 28 to 35%, respectively. While iota-carrageenan contains a higher ester sulfate level (28–30%) and lower a 3,6-AG content (25−30%) compared with kappa form. Among all, lambda carrageenan contains the highest degree of ester sulfate about 32–39% and has no content of 3,6-AG (Necas and Bartosikova 2013).
Carrageenan is generally utilized as a gel-forming, thickening, stabilizing agent in foods (Pangestuti and Kim 2014), green edible films (da Rosa et al. 2020; Farhan and Hani 2020; Huang et al. 2020; Roy and Rhim 2020), and the encapsulating agent in biomedical applications (Chen et al. 2019; Yew et al. 2020). In addition, carrageenan has achieved the potential therapeutic interest in diseases due to a vast number of biological targets including antioxidant (Rafiquzzaman et al. 2016), anti-hyperglycemic (Sokolova et al. 2014), anti-tumor (Chen et al. 2018; Khotimchenko et al. 2020), anti-cancer (Liu et al. 2019), anticoagulant (Groult et al. 2019), immunomodulating properties (Cicinskas et al. 2020) and antivirus (Song et al. 2020) properties are illustrated in Fig. 2. Also, several in vitro and in vivo studies have reported that red seaweed polysaccharides can act as prebiotics and effectively regulate the composition of gut microorganisms because several microbial communities are able to digest these dietary fibers. Carrageenan derived from Kappaphycus alvarezii exhibited a positive influence on the population of beneficial bacteria such as Bifidobacterium (Qiu et al. 2022).
Antiviral mechanism of fucoidan and carrageenan
Different types of fucoidan and carrageenans extracted from brown and red algae possess substantial antiviral activity. Reports are available in either native or chemically modified forms of sulfated polysaccharides against a broad assortment of viruses including influenza A (IAV) and B (IBV), herpes simplex virus (HSV-1 and HSV-2), hepatitis C virus (HCV), hepatitis B virus (HBV), human immunodeficiency virus (HIV), and human papillomavirus (HPV). Table 1 describes the antiviral dose and properties of fucoidan. The amount of fucoidan used is varied in different studies.
Table 1.
The antiviral activity of fucoidan and carrageenan in virus infections
| Sulfated polysaccharide | Source | Dose/IC50 (µg mL−1) | Disease and Effect of treatment | Selected References |
|---|---|---|---|---|
| Fucoidan | Undaria pinnatifida | In vivo study with 5 mg day−1 twice a day for 14 days |
Anti-IAV activity Positive effect on production of antigen-specific antibody Inhibition of virus attachment and blocking virus penetration |
Hayashi et al. (2013), Richards et al. (2020), Synytsya et al. (2014) |
| Kjellmaniella crassifolia | 250 µg mL−1 | Wang et al. (2017) | ||
| Laminaria japonica | 50–500 µg mL−1 | Makarenkova et al. (2010) | ||
| Saccharina cichorioides, S. japonica | 0.001–100 µg mL−1 |
Anti-HIV activity Prevention of attachment and cell-to-cell virus spread |
Prokofjeva et al. (2013) | |
| Sargassum mcclurei, Sargassum polycystum and Turbinara ornate | IC50 value 0.33–0.7 µg mL−1 | Thuy et al. (2015) | ||
| Sargassum swartzii | 1.56 and 6.25 μg mL−1 | Dinesh et al. (2016) | ||
| Cladosiphon okamuranus Tokida | 0.83 g day−1 |
Anti-HCV activity Inhibits virus replication |
Mori et al. (2012) | |
| Scytosiphon lomentaria | IC50 value 0.76- 1.34 µg mL−1 |
Anti-HSV activity The galactofucan fractions of fucoidan showed the antiviral activity because of the low uronic acid and high sulfate esters content Inhibition of virus attachment |
Ponce et al. (2019) | |
| Sargassum henslowianum | IC50 value 0.89 and 0.82 µg mL−1 | Sun et al. (2020) | ||
| Fucus evanescens |
In vitro study with 0.25–250 µg mL−1 In vivo study with 10 mg kg−1 day−1 |
Antivirus activity against HSV, ECHO-1, and HIV-1 Inhibiting virus replication |
Krylova et al. (2020) | |
| Carrageenan | Commercial carrageenan | Iota carrageenan |
Anti- IAV Inhibition virus replication |
Leibbrandt et al. (2010) |
| Commercial carrageenan |
kappa carrageenan and sulfated derivatives In vivo study with 40 mg kg−1 d−1 |
Wang et al. (2012) | ||
| Commercial carrageenan |
Kappa, acetylated and sulfated derivatives In vivo study with 30 mg kg−1 day−1 |
Tang et al. (2013) | ||
| Commercial carrageenan |
Lambda carrageenan IC50 1–20 ng mL−1 |
Anti- HPV potential Inhibition of virus attachment and blocking virus penetration |
Rodríguez et al. (2014) | |
| Gigartina skottsbergii |
Lambda carrageenan IC50 0.52 and 10.42 for BoHv-1 and SuHV-1, respectively |
BoHV-1 and SuHV-1 Inhibition of virus attachment and blocking virus penetration |
Diogo et al. (2015) | |
| Stenogramme interrupta |
Kappa/itoa and lambda carrageenan 0.65–2.88 µg mL−1 |
Anti-HSV activity Inhibition of virus attachment and blocking virus penetration Interfere with protein binding to the heparan sulfate co-receptor in host tissues |
Cáceres et al. (2000) | |
| Gigartina skottsbergii | Lambda carrageenan 10 mg mL−1 | Carlucci et al. (2004) | ||
| Gigartina attpurpurea |
Kappa and lambda carrageenan 0.2–0.8 µg mL−1 |
Harden et al. (2009) | ||
| Solieria chordalis | Iota carrageenan 3.2–54.4 µg mL−1 | Boulho et al. (2017) | ||
| Solieria filiformis | Iota carrageenan 4.5–11.7 µg mL−1 | Ana et al. (2021) |
The scientific names of species for algae should be italic
Algae sulfated polysaccharides have shown their efficacy, particularly strongly toward pathogenic viruses in different phases of virus infection. Indeed, the special structure attributes of sulfated polysaccharides cause antiviral properties via obstruction at different phases of the life cycle of a virus. Virus entry is the initial stage of infection that is the first target of antiviral treatments. Sulfated polysaccharides can cease the virus infection by inhibiting virus adsorption and penetration to the host cell (beginning of the viral cycle). This mechanism of anti-virus activity of sulfated polysaccharides occurs by two pathways including (a) directly interacting with the positively charged regions on the surface of the viral envelope and inhibiting of the virus infection ability and killing the virus directly, (b) blocking virus interaction with the receptors via their polyanionic features and preventing to adhere thereto, thus, making them capable of deactivating viruses (Chen et al. 2020; Iravani and Varma 2021). Indeed, polysaccharides should bind to amino acids at the surface of the virus. Thus charge density and structural flexibility of polysaccharides are crucial for interaction with virus S glycoprotein (Song et al. 2020). Partly low-cost preparation and cytotoxicity, a wide range of antiviral activities, and the safety of seaweed sulfated polysaccharides make them superior as a novel drug (Dinesh et al. 2016).
Numerous studies recommend fucoidan as an alternative form of treatment or prophylaxis in cases of viral infection (Table 1). Different source of fucoidan extract from Undaria pinnatifida (Hayashi et al. 2013; Richards et al. 2020; Synytsya et al. 2014), Kjellmaniella crassifolia (Wang et al. 2017), Laminaria japonica (Makarenkova et al. 2010) has been found to be quite active against IAV via inhibiting virus binding to host cell and replication. Furthermore, inactivation of HIV and prevention of cell-to-cell virus spread by two high molecular weight fucoidans isolated from Saccharina cichorioides (α-l-fucan) and S. japonica (galactofucan) have been found. These two fractions were the most effective inhibitors at a concentration of 0.001–100 µg mL−1 and did not exert cytotoxicity at concentrations up to 100 µg mL−1 (Prokofjeva et al. 2013). A similar anti-HIV activity was observed by fucoidan extracted from Sargassum mcclurei, Sargassum polycystum, and Turbinara ornate with a mean IC50 value of 0.33–0.7 µg mL–1 (Thuy et al. 2015) and two fucoidan fractions FF1 (45 kDa) and FF2 (30 kDa) isolated from Sargassum swartzii with no toxicity up to 1000 µg mL−1 (Dinesh et al. 2016). It has been proposed that the fucoidan derived from Kjellmaniella crassifolia inactivates virus particles via binding to neuraminidase and block the release of viral particles. The viral neuraminidase protein is responsible for IAV entry into the host cells and the release process of the virus from the cells. Thus, the inhibition of the cellular EGFR pathway and neuraminidase is a useful therapeutic pathway for IAV disease. Fucoidan inhibits the EGFR pathway via interfering with the activation of EGFR, PKCalpha, NF-kappaB, and Akt (Wang et al. 2017). Furthermore, fucoidan derived from Cladosiphon okamuranus Tokida has a positive effect on HCV treatment at 0.83 g day−1 and interference virus replication. Direct antiviral activity of fucoidan is related to its interaction with the virus envelope glycoprotein. Moreover, fucoidan indirectly inhibits anti-HCV activity by reducing the RNA replication and serum α-interferon (IFNα levels in FLR3-1 replicon cells and serum alanine aminotransferase levels (Mori et al. 2012). Li et al. (2017) found that fucoidan from Fucus vesiculosus has an HBV replication suppressive effect by limiting the HBsAg and ABeAg expression and secretion. Moreover, fucoidan has a positive effect on inhibition of HBV DNA replicative intermediates in a dose-dependent manner. Indeed, fucoidan exhibits the anti-HBV activity and virus replication via activation of MEK-ERK pathway and treatment with 100 μg/ml of fucoidan enhanced the level of phosphorylated ERK in hepatocytes. Fucoidan also promotes the type I interferon response by activation of interferon regulatory factor 3 (IRF3) and IRF7.
A recent study revealed that fucoidan extracted from Scytosiphon lomentaria showed anti-HSV-1 and anti-HSV-2 with no cytotoxicity up to 1000 µg mL−1 (CC50 > 1000 µg mL−1). Indeed, the galactofucan fractions of fucoidan offer antiviral activities against the herpes simplex virus. Galactofucan exhibits antiherpetic property with high selectively against HSV and its activity is due to the low uronic acid and high sulfate ester content of galactofucan (Ponce et al. 2019). Given the lack of antiviral activity of uronic acid, fractions with higher content of sulfate groups exhibit stronger anti-HSV activity (Sun et al. 2020). In parallel to this investigation, two fractions of fucoidan SHAP-1and SHAP-2 isolated from Sargassum henslowianum have shown inhibition toward the HSV-1 on Vero- cells with the IC50 value 0.89 and 0.82 µg mL−1, respectively. They claimed that the SHAP-1 and SHAP-2 interfere at the first phase of infection and destructive virus adsorption to the host cell surface. Indeed, sulfate ester content and galactofucan fractions are key factors in the virucidal activity of fucoidan. In addition, depending on the number and position of the sulfate groups, sulfated polysaccharides exhibit different levels of antiviral activity. The backbone of fucoidan consists of α-(1 → 3)-linked L-Fucp with sulfate groups on the C-2 and C-4 positions that are responsible for the high anti-HSV activity of SHAP-1 and SHAP-2 (Sun et al. 2020). Recently, native and enzymatic modified fucoidans from Fucus evanescens were evaluated for antiviral activity against HSV-1, HSV-2, enterovirus (ECHO-1), and HIV-1 in Vero and human MT-4 cell lines. The in vitro assay revealed the beneficial effects of both types of fucoidan against HSV-1, HSV-2, ECHO-1, and HIV-1 by inhibiting virus replication in different phases of pretreatment of cells, pretreatment of the virus, simultaneous treatment, and treatment of infected cells. These studies suggested that the main target for antiviral action of fucoidan is virus adsorption. Treatment with fucoidans causes increase in the resistance to virus infection (preventive effect), directly affects virus particles (virucidal effect), and inhibits the early stage of virus replication (virus-inhibiting effect). Indeed, the primary mechanism of antivirus properties of fucoidan is due to the interaction of sulfated groups of fucoidan with positively charged virus capsid protein and hinders the attachment of the virus to the host cell, consequently suppressing the entry process of the virus. However, both types of fucoidans exhibited potential antiviral activity by downregulation replication of the DNA-containing HSV-1 and HSV-2 (Krylova et al. 2020). Thus, the higher degree of sulfation exhibits more interaction points for inactivating viruses.
Carrageenan has also been used to treat various viral diseases and several studies have described the in vitro or in vivo antivirus activity of carrageenans (Table 1). The anti-viral activity of carrageenan is attributed to several factors including the sulfate group distribution in the repeating galactose units, molecular weight, and the interaction with other biomolecules such as proteins and creating complexes with new colloidal entities which causes the inhibition or promotion of carrageenan bioactivity. It has been reported that the low molecular weight of carrageenans has a higher solubility and can easily penetrate cell membranes and, therefore, are more effective in antiviral activity. Sugar composition with the lowest molecular weight may have a synergist influence on the antiviral activity of carrageenan. Furthermore, carrageenan has an influence on different phases of viral infection processes based on its structural diversity and complexity (Ana et al. 2021). Leibbrandt et al. (2010) revealed that the viral infection by influenza A PR8/34 H1N1 virus in a mouse model was reduced upon exposure to treatment with iota-carrageenan nasal spray. Similarly, Wang et al. (2012) confirmed the antiviral activity of kappa carrageenan against IAV with an inhibition rate of 45–47% at a dose of 40 mg kg−1 day−1which has a similar concentration of Ribavirin (39.9% inhibition rate). They confirmed carrageenan oligosaccharide with 1–3 kDa molecular weight and sulfated content of 0.8–1.0 mol mole−1 possesses appropriate inhibition of IAV replication in vitro and in vivo. In parallel with this observation, the low molecular weight of kappa carrageenans (3, 5 and 10 kDa) and its acetylated and sulfated derivatives (acetylated degree of 1.0 and sulfation degree of 2.4) showed an inhibitory effect against influenza virus A/FM1/47 (H1N1). As the in vivo study using FM 1-induced pulmonary oedema model exhibited that acetylation significantly enhanced their inhibition activity against influenza virus and both types of kappa carrageenan showed higher antiviral activity compared with Rabivirin at the dose of 30 mg kg−1 daily. Considering that the low molecular weight of carrageenans has a better water solubility, they can be a promising substance against the influenza virus (Tang et al. 2013). The inhibitory effect of κ-carrageenan against H1N1 viruses mainly is in inhibiting the HA binding to Madin-Daby canine kidney cells (MDCK), internalization, downregulation of mRNA, and protein expression without affecting adsorption. The low molecular weight kappa-carrageenan prevents the mRNA transcription via interfering with the polymerase activity while the high molecular weight κ-carrageenan directly blocks the virus attachment to the host cell. However, the antiviral activity of carrageenan is influenced by the sulfation level, molecular weight, the serotype of the virus, and the host cells (Shao et al. 2015). Lambda carrageenan had also been reported for its potent anti- HPV potential in vitro with IC50 1–20 ng mL−1 and in vivo study (Rodríguez et al. 2014). Similarly, Diogo et al. (2015) reported the potent antiviral efficacy of lambda carrageenan isolated from Gigartina skottsbergii against laboratory strains bovine herpesvirus type 1 (BoHV-1) and suid herpesvirus type 1 (SuHV-1). Moreover, the carrageenan derivatives were found to have better antivirus activity.
Some investigations reported anti-HSV-1 activity of kappa/iota and lambda-carrageenan from Stenogramme interrupta (Phyllophoraceae) (Cáceres et al. 2000), anti-HSV-2 activity of lambda carrageenans from Gigartina skottsbergii (Carlucci et al. 2004), and Gigartina attpurpurea (Harden et al. 2009) by the interaction between virus-sulfated polysaccharides and interfering with the attachment of virions to host cells. Cell-to-cell fusion and transmission of HSV-1 infection were also blocked by iota-carrageenan from Solieria chordalis at 3.2–54.4 µg mL−1 concentrations without any cytotoxicity (Boulho et al. 2017). Recently, carrageenan-rich enzymatic extracts from Solieria filiformis were screened for antiviral activity against HSV-1 and exhibited significant anti-HSV-1 activity at the effective concentration (EC50) of 4.5 µg mL−1 without toxic effects on cells in in vitro study. These mechanisms of anti-viral activity of carrageenans are mainly related to negatively charged groups of structure that are linked to the positive charges of virus envelop and inhibit the virus attachment to the cell surface and penetration (Ana et al. 2021). Carrageenans inhibit virus infection at different steps of the viral life, from inhibition of initial viral adsorption and entry to the host cell to blocking viral replication. Moreover, carrageenans can suppress the DNA replication of the enveloped virus (HSV-1) significantly rather than non-enveloped RNA virus (ECHO-1). According to the chemical structure of carrageenan, conformation, 3,6anhydrogalactose, and sulfate group content show different antiviral activity. For instance, carrageenan structure in the configuration of a chaotic coil has more flexibility and provides better attachment to certain viral envelope glycoproteins, which are necessary for the binding the virus to the host cell (Krylova et al. 2022).
Treatment and or prevention of SARS-CoV-2
In the past years, researchers have developed vaccines and medicine such as remdesivir, favipiravir, simeprevir, various monoclonal antibodies, paxlovid, and molnupiravir to prevent the infection or propagation of SARS-CoV-2 (Zhang et al. 2022). Moreover, in vitro anti-SARS-CoV-2 activity of ivermectin, an antiparasitic agent, is reported (Caly et al. 2020). Also, the clinical trials of anti-hepatitis C virus (HCV) activity of Sofosbuvir showed that Sofosbuvir was effective against patients infected with SARS-CoV-2 via RdRp Inhibiting mechanism (Sayad et al,. 2020). Despite that the treatment is crucial, especially in the first days, the development of novel drugs for the prophylactic approach is necessary (Zhang et al. 2022). Although the efficacy of these medicines is confirmed, their adverse effects remain unclear. Therefore, promising antiviral bioactive compounds from natural sources need to be developed as safe agents.
Several studies reported that the diverse structure of sulfated polysaccharides has a crucial role in boosting the host antiviral response by interfering with virus attachment, adsorption, and its replication process (Hans et al. 2021). Carrageenans (kappa-, iota- and lambda- carrageenan) are commercially available in the market and used in food products and pharmaceuticals (Pacheco-Quito et al. 2020). Moreover, fucoidan derived from Undaria pinnatifida is a commercially available dietary supplement (Richards et al. 2020). Recently, some studies have developed novel nasal sprays formulated with carrageenan, and their clinical investigations suggested their effective antiviral activity in particular against COVID-19. According to the clinical trial studies, lambda-carrageenan nasal spray can decrease the risk of SARS-CoV-2 infection (Moakes et al. 2021).
Anti-SARS-CoV-2 activity
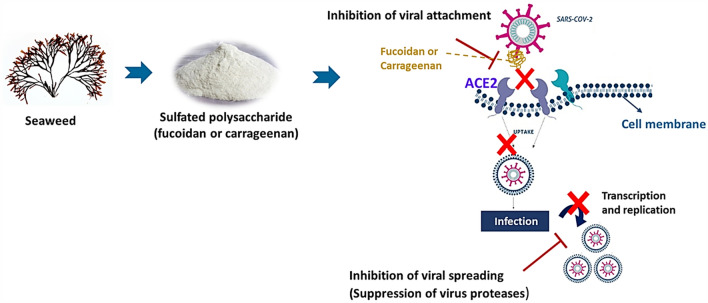
Two main mechanisms of antiviral action of natural compounds include inhibition of interfering virus entry and replication (Fig. 3). First, S glycoprotein is the main antigen on the surface of coronavirus and involves virus attachment and mediating the membrane and membrane fusion to enter a cell. Thus, the antiviral compounds should inhibit the S protein attachment to the host receptor in a dose-dependent manner. Second, the virus proteases such as 3CLpro and PLpro, RdRP, or RNA replicas participate in virus transcription and replication. Therefore, the inhibition of CoV-2 protein such as N and M protein can suppress and block the virus replication (Xian et al. 2020).
Fig. 3.
Antiviral activity of seaweed polysaccharides
SARS-CoV-2 like other viruses circulating in wildlife for human infection initially should encounter humans and a cellular receptor allowing the virus to bind. Recently, it was shown that the SARS-CoV-2 spike protein, binds to the human ACE2 (Bian and Li 2020). The ACE2 is an integral membrane glycoprotein that is known for the highest expression in most tissues such as the kidneys, endothelium, lungs, and heart. According to the structural database of ACE2, besides inhibiting SARS-CoV-2, the inhibition of the ACE2 protein is absolutely necessary to reduce the operability of the host receptor of SARS-CoV-2. If the ACE2 protein is inhibited, it suggests that coronavirus is prevented and treated (Ni et al. 2020a, b).
Some reports have been conducted to investigate the inhibitory activity of fucoidan against HSV1 (Wozniak et al. 2015), IAV (Synytsya et al. 2014), HPIV1 (Sun et al. 2018) and HIV (Dinesh et al. 2016). It is supposed that the sulfate groups in fucoidan structure play a key role in the protective effect against SARS-CoV-2 and these fucoidan-structure polyanions act as receptors for viruses, subsequently, limiting the connection of the positive part of virus capsid to the host cell receptor, thus inhibiting virus entry and cease the cycle of infection as shown in Table 2 (Dimitrova-Shumkovska et al. 2020).
Table 2.
Anti-SARS-CoV-2 activity of fucoidan and carrageenan
| Sulfated polysaccharide | Cell line | Dose (µg mL−1) | Mechanism/Results | References |
|---|---|---|---|---|
| Fucoidan fractions (RPI-27 and RPI-28) | Vero-CCL81 | EC50 values of RPI-27 (8.3 µg mL−1) | Inhibiting of viral attachment via binding to the S-protein of SARSE-CoV-2 | Kwon et al. (2020) |
| Extracted from Saccharina japonica | RPI-28 (1.2 µM), Remdesivir (11.4 µM) | Fucoidan was more effective than remdesivir | ||
| Commercial fucoidan, iota carrageenan and sea cucumber sulfated polysaccharide (SCSP) | Vero E6 cells | Fucoidan concentration ≥ 15.6 µg mL−1 |
Inhibition of virus attachment via binding to S protein of virus Virus aggregation by iota-carrageenan |
Song et al. (2020) |
| Iota-carrageenan concentration ≥ 125 µg mL−1 |
SCSP had highest sulfation degree and inhibitory effect Fucoidan was stronger than iota-carrageenan |
|||
| Commercial iota-, kappa- and lambda- carrageenan | SARS-CoV-2 Spike Pseudotyped Lentivirus | Iota carrageenan (10 and 100 µg mL−1) | Inhibiting of virus entry and replication | Morokutti-Kurz et al. (2020) |
| Kappa- and lambda-carrageenan (100 µg mL−1) | Iota carrageenan was more effective than others | |||
| Nasal spray formulated with iota carrageenan and xylitol | Vero E6 cell culture | Iota carrageenan (at least concentration 6 µg mL−1) xylitol (50 mg mL−1) |
Inhibiting of virus entry and replication Combination of carrageenan and xylitol was more effective |
Bansal et al. (2020) |
| Nasal spray formulated with lambda-carrageenan |
Madin–Darby canine kidney (MDCK) cells BALB/c mice |
EC50 value of 0.9 µg mL−1 |
Blocking of viral attachment to host cell receptors Prevention of virus entry and production |
Jang et al. (2021) |
| Nose and mouth sprays formulated with iota- and kappa- carrageenan | TMPRSS2-Vero E6 cells |
Iota carrageenan (1.2 mg mL−1) Kappa–carrageenan (0.4 mg mL−1) |
Decreased viral attachment and entry into target cells because the sulfated polysaccharide mimics cellular heparin sulfates or aggregates viral particles | Schütz et al. (2021) |
| Nasal spray formulated with iota-carrageenan and sodium chloride | Calu-3, a human respiratory model cell line | Iota carrageenan (1.7 mg mL−1) and sodium chloride (9 mg mL−1) | Inhibiting of viral entry and production | Varese et al. (2021) |
Kwon et al. (2020) explained the potential of fucoidan fractions to inhibit SARS-CoV-2 attachment and entry. SARS-CoV-2 entry is initiated by the binding of viral S-protein to cell surface receptors. Their results indicated that RPI-27 (MW ≃ 100 kDa) and RPI-28 (MW ≃ 12 kDa), two complex sulfated polysaccharides (fucoidans), were derived from Saccharina japonica functions against SARS-CoV-2. Indeed, RPI-27 with EC50 values of 8.3 ± 4.6 µg/m was more effective than the antiviral drug remdesivir at 11.4 µM in Vero-CCL81 cells. Vero-CCL81 cell was capable to express ACE-2. RPI-28 caused about 75% inhibition of SARS-CoV-2 particle attachment. Indeed, RPI-27 and RPI-28 provide multiple binding groups with virus envelop, thus these two fractions showed a stronger protective effect compared with other polysaccharides tested. Highly branched fucoidan fractions (RPI-27 and RPI-28) contained several binding ligands in chemical structure. However, RPI-27 had higher antiviral activity rather than RPI-28 because RPI-27 possessed higher molecular weight and exhibited more multiple interaction points with the S-protein of the virus (Kwon et al. 2020). According to Song et al. (2020), in an in vitro model of SARS-CoV-2 infection of Vero E6 cells, fucoidan and iota carrageenan demonstrated inhibitory activity at concentrations of ≥ 15.6 µg mL−1 and ≥ 125 µg mL−1, respectively. Moreover, the inhibitory property could be related to the sulfate content and among these two sulfated polysaccharides, fucoidan with a higher sulfate group (22.8%) was stronger than iota-carrageenan (10.4%). However, gel-forming iota-carrageenan caused virus aggregation.
This efficient blockage of the SARS-COV-2 spike protein binding to ACE-2 receptor by carrageenan was further confirmed. Morokutti-Kurz, Graf, Grassauer, and Prieschl-Grassauer (2020) described the in vitro anti-SARS-CoV-2 activities of carrageenan. They suggested that the iota-carrageenan is a safe compound for the treatment of coronavirus infection because their study revealed the iota-carrageenan interference in SARS-CoV-2 Spike Pseudotyped Lentivirus (SSPL) entry with an IC50 value of 2.6 µg mL−1. Moreover, the same result was obtained against various Rhino- and Coronaviruses. Iota-carrageenan not only exhibited the inhibitory activity against SSPL at 10 µg mL−1, but also was active at 100 µg mL−1 concentration. While kappa-carrageenan and lambda-carrageenan were only active at 100 µg mL−1. Nevertheless, iota-carrageenan had stronger antiviral activity rather than the others (Morokutti-Kurz et al. 2020). Mechanistically, a binding competition between anionic groups of sulfated polysaccharides and cationic regions of virus envelope glycoprotein was proposed as a potential mode of action for SARS-CoV-2 inhibition by carrageenan (Jang et al. 2021).
In addition, the algal-derived nasal spray can be a promising self-administered antiviral spray. In vitro study against in Vero cell culture showed the effective reduction in SARS-Cov-2 infection by iota carrageenan at least concentration of 6 µg mL−1, while antiviral activity could promote by the combination of 5% m V−1 xylitol (Bansal et al. 2020). Similarly, Jang et al. (2021) reported potent antiviral activity, the efficacy of lambda-carrageenan against laboratory strains of IAV and IBV viruses, and primary isolation of SARS-CoV-2 with an EC50 value of 0.3–1.4 µg mL−1 and 0.9 µg mL−1, respectively. Regarding its cytotoxicity, the CC50 concentration for lambda- carrageenan was safe up to 300 μg mL−1. According to the analysis, lambda-carrageenan treatment induced gene expression reduction of viral proteins and prevention of virus production in Vero cell culture. Interestingly, lambda-carrageenan is composed of (1,3)-linked α-d-galactose-2-sulfated and (1,4)-linked β-d-galactose-2,6,-disulfat units. Lambda-carrageenan is more soluble in cold water compare with kappa- and iota-carrageenan due to its higher sulfate content (32–39% of ester sulfate degree). Thus lambda-carrageenan is a promising antiviral seaweed polysaccharide applicable in nasal spray formulation (Jang et al. 2021).
In order to harness the SARS-Cov-2 inactivating power of algal sulfated polysaccharides, new nasal and oral sprays were formulated by carrageenan. Hui (2020) suggested the povidone-iodine and carrageenan-containing sprays as a promising candidate for chemoprophylaxis and suppression of the coronavirus outbreak. In parallel with this observation, Schütz et al. (2021) applied two types of nose and mouth sprays formulated by iota- and kappa- carrageenan with 1.2 mg mL−1 and 0.4 mg mL−1 concentration, respectively. Both types of sprays exhibited the anti-COVID-19 activity because of their polyanionic structure properties. Therefore, using carrageenan-based sprays could be useful to eradicate the COVID-19 pandemic.
Similarly, the in vitro respiratory epithelium model treated with nasal spray formulated with iota-carrageenan (1.7 mg mL−1) and sodium chloride showed adequate inhibition against SARS-CoV-2 (Varese et al. 2021).
Clinical trial
There are a few clinical trials about the anti-SARS-CoV-2 activity of carrageenan and fucoidan, while these two sulfated polysaccharides are suitable for anti-COVID-19 researches. The clinical trials of a nasal spray containing carrageenan showed the practical therapeutic against COVID-19. Héctor et al. (2020) showed a decreased coronavirus spread in humans after carrageenan nasal spray administration. A randomized controlled clinical trial was conducted in 2020 in 229 healthy personals (females and males < 40 and > 40–55 years) with no COVID-19 symptoms; some groups consumed one carrageenan spray (containing 0.17 g of carrageenan) into a nostril and the other groups consumed four sprays into the oral cavity after utilization of one drop of ivermectin (0.6 mg mL−1) five times a day for 14 days. This study showed an appropriate dosage of ivermectin and carrageenan and improvement in protection in the subjects treated with ivermectin and carrageenan combination, suggesting the importance of consuming carrageenan spray with such an antiviral drug by coronavirus patients. Furthermore, Figueroa et al. (2021) found that iota-carrageenan spray possesses an inhibitory effect against SARS-CoV-2. Clinically, 394 hospital personnel were classified into two treatment groups who administrated iota-carrageenan (1.7 g L−1) and placebo four times daily for 21 days. The lower infection rate in the carrageenan treatment group (1%) compared with placebo ones (5%) explained that iota-carrageenan was active against COVID-19. This valuable information proved the potential of carrageenan against COVID-19 and nasal sprays containing carrageenan are approved and available in some countries.
Despite carrageenan being approved by FDA and recognized as safe food additive, some investigations revealed that carrageenan has an adverse effect on the immune system and blood coagulation because of its sulfate groups. In addition, it is reported that exposure of human intestinal epithelial cells to the carrageenan (1–10 mg/l) for 1–8 days caused cell death in both primary cells and a cell line (Liu et al. 2015). Furthermore, a considerable amount of research has been conducted on different biological activities and therapeutic potential of fucoidan for the treatment of disease. However, it is difficult to absolutely confirm the beneficial effects of fucoidan because of the different findings in various studies. Therefore, more investigations are needed to evaluate the side effects of fucoidan on human health in various study models.
Conclusion
It is noteworthy that several recent works have stated that numerous poly sulfated compounds are considered for their inhibitory potential for the virus attachment and replication. In the past decade, sulfated polysaccharides were mainly derived from seaweeds because of their antiviral properties. Several studies confirmed that various fucoidan and carrageenan preparations have been shown to exhibit a wide spectrum of antivirus activity and propose a potential approach to the use of natural seaweed metabolites to tackle the current pandemic SARS-CoV-2. Moreover, seaweed-derived functional foods can provide health benefits by reducing the risk of diseases and enhancing the immune body system, thus improving the quality of life. Thus, promoting the utilization of a healthy diet containing seaweeds or the production of functional foods will be suggested. Considering the role of structural properties of fucoidan and carrageenan, they are highly valuable in face masks and other medical and hygiene materials. Therefore, more attention should be paid to the scale-up of production and application of fucoidan as a novel algal-based medicine.
Acknowledgements
The work was financially supported by Shiraz University of Medical Sciences under project number 23643 and Shiraz University (Grant number: 96GCU5M1984).
Author contributions
NO: investigation and writing original draft; MM-N: supervision and editing; SMM: funding acquisition and project administration.
Declarations
Conflict of interest
The authors declare no conflict of interest.
Contributor Information
Marzieh Moosavi-Nasab, Email: marzieh.moosavi-nasab@mail.mcgill.ca.
Seyed Mohammad Mazloomi, Email: smmazloomi@gmail.com.
References
- Abd El Hamid MI, Abd El Fatah WM, El Morsi AA, Draz MS, Kallakuri S, Bungau S, Hafez E. Anti-HIV/HCV activity of cyanobacterial phycobiliproteins by a new standardized method using bacteriophage surrogates. Rev Chim. 2019;70:3115–3122. [Google Scholar]
- Alam M, Parra-Saldivar R, Bilal M, Afroze CA, Ahmed M, Iqbal H, Xu J. Algae-derived bioactive molecules for the potential treatment of SARS-CoV-2. Molecules. 2021;26(8):2134. doi: 10.3390/molecules26082134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alboofetileh M, Rezaei M, Tabarsa M, Rittà M, Donalisio M, Mariatti F, Cravotto G. Effect of different non-conventional extraction methods on the antibacterial and antiviral activity of fucoidans extracted from Nizamuddinia zanardinii. Int J Biol Macromol. 2019;124:131–137. doi: 10.1016/j.ijbiomac.2018.11.201. [DOI] [PubMed] [Google Scholar]
- Ale MT, Meyer AS. Fucoidans from brown seaweeds: an update on structures, extraction techniques and use of enzymes as tools for structural elucidation. RSC Adv. 2013;3(22):8131–8141. [Google Scholar]
- Ana P, Nathalie B, Gilles B, Daniel R, Tomás M-S, Yolanda F-P. Anti-Herpes simplex virus (HSV-1) activity and antioxidant capacity of carrageenan-rich enzymatic extracts from Solieria filiformis (Gigartinales, Rhodophyta) Int J Biol Macromol. 2021;168:322–330. doi: 10.1016/j.ijbiomac.2020.12.064. [DOI] [PubMed] [Google Scholar]
- Arshad MS, Khan U, Sadiq A, Khalid W, Hussain M, Yasmeen A, Rehana H. Coronavirus disease (COVID-19) and immunity booster green foods: a mini review. Food Sci Nutr. 2020;8(8):3971–3976. doi: 10.1002/fsn3.1719. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Bansal S, Jonsson CB, Taylor SL, Figueroa JM, Dugour AV, Palacios C, Vega JC. Iota-carrageenan and Xylitol inhibit SARS-CoV-2 in cell culture. PloS one. 2020;16(11):e0259943. doi: 10.1371/journal.pone.0259943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharjee M. Pharmaceutically valuable bioactive compounds of algae. Asian J Pharm Clin Res. 2016;9:43–47. [Google Scholar]
- Bian J, Li Z. Angiotensin-converting enzyme 2 (ACE2): SARS-CoV-2 receptor and RAS modulator. Acta Pharm Sin B. 2020;11(1):1–12. doi: 10.1016/j.apsb.2020.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilandžić N, Sedak M, Đokić M, Varenina I, Kolanović BS, Božić Đ, Šimić B. Determination of zinc concentrations in foods of animal origin, fish and shellfish from Croatia and assessment of their contribution to dietary intake. J Food Compos Anal. 2014;35(2):61–66. [Google Scholar]
- Boo H-J, Hong J-Y, Kim S-C, Kang J-I, Kim M-K, Kim E-J, Kwon J-M. The anticancer effect of fucoidan in PC-3 prostate cancer cells. Mar Drugs. 2013;11(8):2982–2999. doi: 10.3390/md11082982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulho R, Marty C, Freile-Pelegrín Y, Robledo D, Bourgougnon N, Bedoux G. Antiherpetic (HSV-1) activity of carrageenans from the red seaweed Solieria chordalis (Rhodophyta, Gigartinales) extracted by microwave-assisted extraction (MAE) J Appl Phycol. 2017;29(5):2219–2228. [Google Scholar]
- Cáceres PJ, Carlucci MAJ, Damonte EB, Matsuhiro B, Zúñiga EA. Carrageenans from chilean samples of Stenogramme interrupta (Phyllophoraceae): structural analysis and biological activity. Phytochemistry. 2000;53(1):81–86. doi: 10.1016/s0031-9422(99)00461-6. [DOI] [PubMed] [Google Scholar]
- Caly L, Druce JD, Catton MG, Jans DA, Wagstaff KM. The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro. Antivir Res. 2020;178:104787. doi: 10.1016/j.antiviral.2020.104787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlucci M, Scolaro L, Noseda M, Cerezo A, Damonte E. Protective effect of a natural carrageenan on genital herpes simplex virus infection in mice. Antivir Res. 2004;64(2):137–141. doi: 10.1016/j.antiviral.2004.07.001. [DOI] [PubMed] [Google Scholar]
- Chen X, Zhao X, Gao Y, Yin J, Bai M, Wang F. Green synthesis of gold nanoparticles using carrageenan oligosaccharide and their in vitro antitumor activity. Mar Drugs. 2018;16(8):277. doi: 10.3390/md16080277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Han W, Zhao X, Tang W, Wang F. Epirubicin-loaded marine carrageenan oligosaccharide capped gold nanoparticle system for pH-triggered anticancer drug release. Sci Rep. 2019;9(1):1–10. doi: 10.1038/s41598-019-43106-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen RR, Li YJ, Chen JJ, Lu CL. A review for natural polysaccharides with anti-pulmonary fibrosis properties, which may benefit to patients infected by 2019-nCoV. Carbohydr Polym. 2020;247:116740. doi: 10.1016/j.carbpol.2020.116740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi Y, Zhang M, Wang X, Fu X, Guan H, Wang P. Ulvan lyase assisted structural characterization of ulvan from Ulva pertusa and its antiviral activity against vesicular stomatitis virus. Int J Biol Macromol. 2020;157:75–82. doi: 10.1016/j.ijbiomac.2020.04.187. [DOI] [PubMed] [Google Scholar]
- Choo G-S, Lee H-N, Shin S-A, Kim H-J, Jung J-Y. Anticancer effect of fucoidan on DU-145 prostate cancer cells through inhibition of PI3K/Akt and MAPK pathway expression. Mar Drugs. 2016;14(7):126. doi: 10.3390/md14070126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicinskas E, Kalitnik AA, Karetin YA, Ram MSGM, Achary A, Kravchenko AO. Immunomodulating properties of Carrageenan from Tichocarpus crinitus. Inflammation. 2020;43(4):1387–1396. doi: 10.1007/s10753-020-01216-x. [DOI] [PubMed] [Google Scholar]
- Citkowska A, Szekalska M, Winnicka K. Possibilities of fucoidan utilization in the development of pharmaceutical dosage forms. Mar Drugs. 2019;17(8):458. doi: 10.3390/md17080458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Rosa GS, Vanga SK, Gariepy Y, Raghavan V. Development of biodegradable films with improved antioxidant properties based on the addition of carrageenan containing olive leaf extract for food packaging applications. J Polym Environ. 2020;28(1):123–130. [Google Scholar]
- Dimitrova-Shumkovska J, Krstanoski L, Veenman L. Potential beneficial actions of fucoidan in brain and liver injury, disease, and intoxication—Potential implication of sirtuins. Mar Drugs. 2020;18(5):242. doi: 10.3390/md18050242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinesh S, Menon T, Hanna LE, Suresh V, Sathuvan M, Manikannan M. In vitro anti-HIV-1 activity of fucoidan from Sargassum swartzii. Int J Biol Macromol. 2016;82:83–88. doi: 10.1016/j.ijbiomac.2015.09.078. [DOI] [PubMed] [Google Scholar]
- Diogo JV, Novo SG, González MJ, Ciancia M, Bratanich AC. Antiviral activity of lambda-carrageenan prepared from red seaweed (Gigartina skottsbergii) against BoHV-1 and SuHV-1. Res Vet Sci. 2015;98:142–144. doi: 10.1016/j.rvsc.2014.11.010. [DOI] [PubMed] [Google Scholar]
- Elizondo-Gonzalez R, Cruz-Suarez LE, Ricque-Marie D, Mendoza-Gamboa E, Rodriguez-Padilla C, Trejo-Avila LM. In vitro characterization of the antiviral activity of fucoidan from Cladosiphon okamuranus against Newcastle Disease Virus. Virology Journal. 2012;9(1):1–9. doi: 10.1186/1743-422X-9-307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Sheekh M, Abomohra AE-F. The therapeutic potential of spirulina to combat COVID-19 infection. Egypt J Bot. 2020;60(3):605–609. [Google Scholar]
- Farhan A, Hani NM. Active edible films based on semi-refined κ-carrageenan: Antioxidant and color properties and application in chicken breast packaging. Food Packag Shelf Life. 2020;24:100476. [Google Scholar]
- Fernando IPS, Sanjeewa KKA, Lee HG, Kim H-S, Vaas APJP, De Silva HIC, Lee J-S. Fucoidan purified from Sargassum polycystum induces apoptosis through mitochondria-mediated pathway in HL-60 and MCF-7 cells. Mar Drugs. 2020;18(4):196. doi: 10.3390/md18040196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueroa JM, Lombardo M, Dogliotti A, Flynn L, Giugliano RP, Simonelli G, Marcote M. Efficacy of a nasal spray containing Iota-Carrageenan in the prophylaxis of COVID-19 in hospital personnel dedicated to patients care with COVID-19 disease. A pragmatic multicenter, randomized, double-blind, placebo-controlled trial (CARR-COV-02) medRxiv. 2021 doi: 10.1101/2021.04.13.21255409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flórez-Fernández N, Balboa EM, Domínguez H. Extraction and purification of fucoidan from marine sources. Encycl Mar Biotechnol. 2020;2:1093–1125. [Google Scholar]
- Galanakis CM, Aldawoud T, Rizou M, Rowan NJ, Ibrahim SA. Food ingredients and active compounds against the Coronavirus disease (COVID-19) pandemic: a comprehensive review. Foods. 2020;9(11):1701. doi: 10.3390/foods9111701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geonzon LC, Descallar FBA, Du L, Bacabac RG, Matsukawa S. Gelation mechanism and network structure in gels of carrageenans and their mixtures viewed at different length scales—a review. Food Hydrocolloids. 2020;108:106039. [Google Scholar]
- Gomaa HH, Elshoubaky GA. Antiviral activity of sulfated polysaccharides carrageenan from some marine seaweeds. Int J Curr Pharm Rev Res. 2016;7(1):34–42. [Google Scholar]
- Groult H, Cousin R, Chot-Plassot C, Maura M, Bridiau N, Piot J-M, Fruitier-Arnaudin I. λ-Carrageenan oligosaccharides of distinct anti-heparanase and anticoagulant activities inhibit MDA-MB-231 breast cancer cell migration. Mar Drugs. 2019;17(3):140. doi: 10.3390/md17030140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan Z, Xu T, Xu Y, Shi L, Wang T. In Vitro anti-coagulant effects of Fucoidan sulfate grafted on TiO2 nano-film. J Nanosci Nanotechnol. 2020;20(12):7753–7760. doi: 10.1166/jnn.2020.18604. [DOI] [PubMed] [Google Scholar]
- Hans N, Malik A, Naik S. Antiviral activity of sulfated polysaccharides from marine algae and its application in combating COVID-19: mini review. Bioresource Technol Rep. 2021;13:100623. doi: 10.1016/j.biteb.2020.100623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harden EA, Falshaw R, Carnachan SM, Kern ER, Prichard MN. Virucidal activity of polysaccharide extracts from four algal species against herpes simplex virus. Antivir Res. 2009;83(3):282–289. doi: 10.1016/j.antiviral.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi K, Lee J-B, Nakano T, Hayashi T. Anti-influenza A virus characteristics of a fucoidan from sporophyll of Undaria pinnatifida in mice with normal and compromised immunity. Microbes Infect. 2013;15(4):302–309. doi: 10.1016/j.micinf.2012.12.004. [DOI] [PubMed] [Google Scholar]
- He X, Xue M, Jiang S, Li W, Yu J, Xiang S. Fucoidan promotes apoptosis and inhibits emt of breast cancer cells. Biol Pharm Bull. 2019;42(3):442–447. doi: 10.1248/bpb.b18-00777. [DOI] [PubMed] [Google Scholar]
- Héctor C, Roberto H, Psaltis A, Veronica C. Study of the efficacy and safety of topical ivermectin+ iota-carrageenan in the prophylaxis against COVID-19 in health personnel. J Biomed Res Clin Investig. 2020;2(1):1007. [Google Scholar]
- Huang X, Luo X, Liu L, Dong K, Yang R, Lin C, Huang Q. Formation mechanism of egg white protein/κ-Carrageenan composite film and its application to oil packaging. Food Hydrocolloids. 2020;105:105780. [Google Scholar]
- Hui K. Povidone-iodine and carrageenan are candidates for SARS-CoV-2 infection control. Hong Kong Med J Xianggang Yi Xue Za Zhi. 2020;26(5):464. doi: 10.12809/hkmj208889. [DOI] [PubMed] [Google Scholar]
- Hwang H-J, Han J-W, Jeon H, Cho K, Kim J-H, Lee D-S, Han JW. Characterization of a novel mannose-binding lectin with antiviral activities from red alga. Grateloupia Chiangii Biomol. 2020;10(2):333. doi: 10.3390/biom10020333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iddir M, Brito A, Dingeo G, Fernandez Del Campo SS, Samouda H, La Frano MR, Bohn T. Strengthening the immune system and reducing inflammation and oxidative stress through diet and nutrition: considerations during the COVID-19 crisis. Nutrients. 2020;12(6):1562. doi: 10.3390/nu12061562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iravani S, Varma RS. Important roles of oligo-and polysaccharides against SARS-CoV-2: recent advances. Appl Sci. 2021;11(8):3512. [Google Scholar]
- Jang Y, Shin H, Lee MK, Kwon OS, Shin JS, Kim Y-I, Kim M. Antiviral activity of lambda-carrageenan against influenza viruses and severe acute respiratory syndrome coronavirus 2. Sci Rep. 2021;11(1):1–12. doi: 10.1038/s41598-020-80896-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayawardena TU, Fernando IS, Lee WW, Sanjeewa KA, Kim H-S, Lee D-S, Jeon Y-J. Isolation and purification of fucoidan fraction in Turbinaria ornata from the Maldives; inflammation inhibitory potential under LPS stimulated conditions in in-vitro and in-vivo models. Int J Biol Macromol. 2019;131:614–623. doi: 10.1016/j.ijbiomac.2019.03.105. [DOI] [PubMed] [Google Scholar]
- Je J-G, Lee H-G, Fernando KH, Jeon Y-J, Ryu B. Purification and structural characterization of sulfated polysaccharides derived from brown algae, Sargassum binderi: inhibitory mechanism of iNOS and COX-2 pathway interaction. Antioxidants. 2021;10(6):822. doi: 10.3390/antiox10060822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin W, Zhang Q, Wang J, Zhang W. A comparative study of the anticoagulant activities of eleven fucoidans. Carbohyd Polym. 2013;91(1):1–6. doi: 10.1016/j.carbpol.2012.07.067. [DOI] [PubMed] [Google Scholar]
- Kamyari N, Soltanian AR, Mahjub H, Moghimbeigi A. Diet, nutrition, obesity, and their implications for COVID-19 mortality: Development of a marginalized two-part model for semicontinuous data. JMIR Public Health Surveill. 2021;7(1):e22717. doi: 10.2196/22717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalid S, Abbas M, Saeed F, Bader-Ul-Ain H, Suleria HAR. Therapeutic potential of seaweed bioactive compounds. IntechOpen; 2018. [Google Scholar]
- Khan MUA, Raza MA, Mehboob H, Kadir MRA, Abd Razak SI, Shah SA, Amin R. Development and in vitro evaluation of κ-carrageenan based polymeric hybrid nanocomposite scaffolds for bone tissue engineering. RSC Adv. 2020;10(66):40529–40542. doi: 10.1039/d0ra07446b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khotimchenko M, Tiasto V, Kalitnik A, Begun M, Khotimchenko R, Leonteva E, Khotimchenko Y. Antitumor potential of carrageenans from marine red algae. Carbohydr Polym. 2020;246:116568. doi: 10.1016/j.carbpol.2020.116568. [DOI] [PubMed] [Google Scholar]
- Kim BS, Yang SS, You HK, Shin HI, Lee J. Fucoidan-induced osteogenic differentiation promotes angiogenesis by inducing vascular endothelial growth factor secretion and accelerates bone repair. J Tissue Eng Regen Med. 2018;12(3):e1311–e1324. doi: 10.1002/term.2509. [DOI] [PubMed] [Google Scholar]
- Koh HSA, Lu J, Zhou W. Structure characterization and antioxidant activity of fucoidan isolated from Undaria pinnatifida grown in New Zealand. Carbohyd Polym. 2019;212:178–185. doi: 10.1016/j.carbpol.2019.02.040. [DOI] [PubMed] [Google Scholar]
- Krylova NV, Ermakova SP, Lavrov VF, Leneva IA, Kompanets GG, Iunikhina OV, Silchenko AS. The comparative analysis of antiviral activity of native and modified fucoidans from brown algae Fucus evanescens in vitro and in vivo. Mar Drugs. 2020;18(4):224. doi: 10.3390/md18040224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krylova NV, Kravchenko AO, Iunikhina OV, Pott AB, Likhatskaya GN, Volod’koYermak AVIM. Influence of the structural features of carrageenans from red algae of the Far Eastern Seas on their antiviral properties. Mar Drugs. 2022;20(1):60. doi: 10.3390/md20010060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon PS, Oh H, Kwon S-J, Jin W, Zhang F, Fraser K, Dordick JS. Sulfated polysaccharides effectively inhibit SARS-CoV-2 in vitro. Cell Discov. 2020;6(1):1–4. doi: 10.1038/s41421-020-00192-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C. Griffithsin, a highly potent broad-spectrum antiviral lectin from red algae: from discovery to clinical application. Mar Drugs. 2019;17(10):567. doi: 10.3390/md17100567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibbrandt A, Meier C, König-Schuster M, Weinmüllner R, Kalthoff D, Pflugfelder B, Fazekas T. Iota-carrageenan is a potent inhibitor of influenza A virus infection. PLoS ONE. 2010;5(12):e14320. doi: 10.1371/journal.pone.0014320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Li J, Tang Y, Lin L, Xie Z, Zhou J, Chen Z. Fucoidan from Fucus vesiculosus suppresses hepatitis B virus replication by enhancing extracellular signal-regulated Kinase activation. Virol J. 2017;14(1):1–8. doi: 10.1186/s12985-017-0848-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Zhan X, Wan J, Wang Y, Wang C. Review for carrageenan-based pharmaceutical biomaterials: favourable physical features versus adverse biological effects. Carbohyd Polym. 2015;121:27–36. doi: 10.1016/j.carbpol.2014.11.063. [DOI] [PubMed] [Google Scholar]
- Liu Z, Gao T, Yang Y, Meng F, Zhan F, Jiang Q, Sun X. Anti-cancer activity of porphyran and carrageenan from red seaweeds. Molecules. 2019;24(23):4286. doi: 10.3390/molecules24234286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makarenkova I, Deriabin P, L'vovZviagintsevaBesednova DTN. Antiviral activity of sulfated polysaccharide from the brown algae Laminaria japonica against avian influenza A (H5N1) virus infection in the cultured cells. Vopr Virusol. 2010;55(1):41–45. [PubMed] [Google Scholar]
- Manlusoc JKT, Hsieh C-L, Hsieh C-Y, Salac ESN, Lee Y-T, Tsai P-W. Pharmacologic application potentials of sulfated polysaccharide from marine algae. Polymers. 2019;11(7):1163. doi: 10.3390/polym11071163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKim JM, Willoughby JA, Sr, Blakemore WR, Weiner ML. Clarifying the confusion between poligeenan, degraded carrageenan, and carrageenan: a review of the chemistry, nomenclature, and in vivo toxicology by the oral route. Crit Rev Food Sci Nutr. 2019;59(19):3054–3073. doi: 10.1080/10408398.2018.1481822. [DOI] [PubMed] [Google Scholar]
- Mišurcováa L, Orsavováb J, Ambrožováa JV. Algal polysaccharides and health polysaccharides: bioactivity and biotechnology. Springer International Publishing Switzerland; 2014. [Google Scholar]
- Moakes RJ, Davies SP, Stamataki Z, Grover LM. Formulation of a composite nasal spray enabling enhanced surface coverage and prophylaxis of SARS-COV-2. Adv Mater. 2021;33(26):2008304. doi: 10.1002/adma.202008304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moosavi‐Nasab M, Mirzapour‐Kouhdasht A, Oliyaei N (2019) Application of essential oils for shelf‐life extension of seafood products. Essent Oils‐oils of Nat
- Moosavi-Nasab M, Oliyaei N, Eun J-B, Mirzapour-Kouhdasht A. Innovation in the seafood sector through the valorization of by-products innovation in the food sector through the valorization of food and agro-food by-products. IntechOpen; 2020. [Google Scholar]
- Mori N, Nakasone K, Tomimori K, Ishikawa C. Beneficial effects of fucoidan in patients with chronic hepatitis C virus infection. World J Gastroenterol: WJG. 2012;18(18):2225. doi: 10.3748/wjg.v18.i18.2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morokutti-Kurz M, Graf P, Grassauer A, Prieschl-Grassauer E. SARS-CoV-2 in-vitro neutralization assay reveals inhibition of virus entry by iota-carrageenan. BioRxiv. 2020 doi: 10.1101/2020.07.28.224733. [DOI] [Google Scholar]
- Mu J, Hirayama M, Sato Y, Morimoto K, Hori K. A novel high-mannose specific lectin from the green alga Halimeda renschii exhibits a potent anti-influenza virus activity through high-affinity binding to the viral hemagglutinin. Mar Drugs. 2017;15(8):255. doi: 10.3390/md15080255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MubarakAli D, MohamedSaalis J, Sathya R, Irfan N, Kim J-W. An evidence of microalgal peptides to target spike protein of COVID-19: in silico approach. Microb Pathog. 2021;160:105189. doi: 10.1016/j.micpath.2021.105189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Necas J, Bartosikova L. Carrageenan: a review. Veterinarni Medicina. 2013;58(4):187–205. [Google Scholar]
- Ngo D-H, Kim S-K. Sulfated polysaccharides as bioactive agents from marine algae. Int J Biol Macromol. 2013;62:70–75. doi: 10.1016/j.ijbiomac.2013.08.036. [DOI] [PubMed] [Google Scholar]
- Ni L, Wang L, Fu X, Duan D, Jeon Y-J, Xu J, Gao X. In vitro and in vivo anti-inflammatory activities of a fucose-rich fucoidan isolated from Saccharina japonica. Int J Biol Macromol. 2020;156:717–729. doi: 10.1016/j.ijbiomac.2020.04.012. [DOI] [PubMed] [Google Scholar]
- Ni W, Yang X, Yang D, Bao J, Li R, Xiao Y, Yang D. Role of angiotensin-converting enzyme 2 (ACE2) in COVID-19. Crit Care. 2020;24(1):1–10. doi: 10.1186/s13054-020-03120-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliyaei N, Moosavi-Nasab M, Tamaddon AM, Fazaeli M. Encapsulation of fucoxanthin in binary matrices of porous starch and halloysite. Food Hydrocolloids. 2020;100:105458. [Google Scholar]
- Oliyaei N, Moosavi-Nasab M, Tamaddon AM, Tanideh N. Antidiabetic effect of fucoxanthin extracted from Sargassum angustifolium on streptozotocin-nicotinamide-induced type 2 diabetic mice. Food Sci Nutr. 2021;9(7):3521–3529. doi: 10.1002/fsn3.2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacheco-Quito E-M, Ruiz-Caro R, Veiga M-D. Carrageenan: drug delivery systems and other biomedical applications. Mar Drugs. 2020;18(11):583. doi: 10.3390/md18110583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pangestuti R, Kim S-K. Biological activities of carrageenan. Adv Food Nutr Res. 2014;72:113–124. doi: 10.1016/B978-0-12-800269-8.00007-5. [DOI] [PubMed] [Google Scholar]
- Park J-Y, Kim JH, Kwon JM, Kwon H-J, Jeong HJ, Kim YM, Ryu YB. Dieckol, a SARS-CoV 3CLpro inhibitor, isolated from the edible brown algae Ecklonia cava. Bioorg Med Chem. 2013;21(13):3730. doi: 10.1016/j.bmc.2013.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J-H, Choi S-H, Park S-J, Lee YJ, Park JH, Song PH, Song C-H. Promoting wound healing using low molecular weight fucoidan in a full-thickness dermal excision rat model. Mar Drugs. 2017;15(4):112. doi: 10.3390/md15040112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponce NM, Flores ML, Pujol CA, Becerra MB, Navarro DA, Córdoba O, Stortz CA. Fucoidans from the phaeophyta Scytosiphon lomentaria: chemical analysis and antiviral activity of the galactofucan component. Carbohyd Res. 2019;478:18–24. doi: 10.1016/j.carres.2019.04.004. [DOI] [PubMed] [Google Scholar]
- Poveda-Castillo GDC, Rodrigo D, Martínez A, Pina-Pérez MC. Bioactivity of fucoidan as an antimicrobial agent in a new functional beverage. Beverages. 2018;4(3):64. [Google Scholar]
- Prokofjeva MM, Imbs TI, Shevchenko NM, Spirin PV, Horn S, Fehse B, Prassolov VS. Fucoidans as potential inhibitors of HIV-1. Mar Drugs. 2013;11(8):3000–3014. doi: 10.3390/md11083000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu SM, Aweya JJ, Liu X, Liu Y, Tang S, Zhang W, Cheong KL. Bioactive polysaccharides from red seaweed as potent food supplements: a systematic review of their extraction, purification, and biological activities. Carbohydr Polym. 2022;275:118696. doi: 10.1016/j.carbpol.2021.118696. [DOI] [PubMed] [Google Scholar]
- Rafiquzzaman S, Ahmed R, Lee JM, Noh G, Jo G-A, Kong I-S. Improved methods for isolation of carrageenan from Hypnea musciformis and its antioxidant activity. J Appl Phycol. 2016;28(2):1265–1274. [Google Scholar]
- Ratha SK, Renuka N, Rawat I, Bux F. Prospective options of algae-derived nutraceuticals as supplements to combat COVID-19 and human coronavirus diseases. Nutrition. 2020;83:111089. doi: 10.1016/j.nut.2020.111089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rengasamy KR, Mahomoodally MF, Aumeeruddy MZ, Zengin G, Xiao J, Kim DH. Bioactive compounds in seaweeds: an overview of their biological properties and safety. Food Chem Toxicol. 2020;135:111013. doi: 10.1016/j.fct.2019.111013. [DOI] [PubMed] [Google Scholar]
- Richards C, Williams NA, Fitton JH, Stringer DN, Karpiniec SS, Park AY. Oral fucoidan attenuates lung pathology and clinical signs in a severe influenza a mouse model. Mar Drugs. 2020;18(5):246. doi: 10.3390/md18050246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez A, Kleinbeck K, Mizenina O, Kizima L, Levendosky K, Jean-Pierre N, Teleshova N. In vitro and in vivo evaluation of two carrageenan-based formulations to prevent HPV acquisition. Antivir Res. 2014;108:88–93. doi: 10.1016/j.antiviral.2014.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy S, Rhim J-W. Preparation of gelatin/carrageenan-based color-indicator film integrated with shikonin and propolis for smart food packaging applications. ACS Appl Bio Mater. 2020;4(1):770–779. [Google Scholar]
- Rui L, Yu H, Yan B-m (2008) Antiviral activities of the recombinant cyanovirin-N against CVB_3 in vitro. J Med Postgrad 12
- Sayad B, Sobhani M, Khodarahmi R. Sofosbuvir as repurposed antiviral drug against COVID-19: why were we convinced to evaluate the drug in a registered/approved clinical trial? Arch Med Res. 2020;51(6):577–581. doi: 10.1016/j.arcmed.2020.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schütz D, Conzelmann C, Fois G, Groß R, Weil T, Wettstein L, Frick M. Carrageenan containing over-the-counter nasal and oral sprays inhibit SARS-CoV-2 infection of airway epithelial cultures. Am J Physiol-Lung Cell Mol Physiol. 2021;320:750–756. doi: 10.1152/ajplung.00552.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao Q, Guo Q, Xu WP, Li Z, Zhao TT. Specific inhibitory effect of κ-carrageenan polysaccharide on swine pandemic 2009 H1N1 influenza virus. PLoS ONE. 2015;10(5):e0126577. doi: 10.1371/journal.pone.0126577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh S, Dwivedi V, Sanyal D, Dasgupta S. Therapeutic and nutritional potential of Spirulina in combating COVID-19 infection. AIJR Preprints. 2020;49(1):2–8. [Google Scholar]
- Sokolova E, Bogdanovich L, Ivanova T, Byankina A, Kryzhanovskiy S, Yermak I. Effect of carrageenan food supplement on patients with cardiovascular disease results in normalization of lipid profile and moderate modulation of immunity system markers. PharmaNutrition. 2014;2(2):33–37. [Google Scholar]
- Song S, Peng H, Wang Q, Liu Z, Dong X, Wen C, Zhu B. Inhibitory activities of marine sulfated polysaccharides against SARS-CoV-2. Food Funct. 2020;11(9):7415–7420. doi: 10.1039/d0fo02017f. [DOI] [PubMed] [Google Scholar]
- Sun T, Zhang X, Miao Y, Zhou Y, Shi J, Yan M, Chen A. Studies on antiviral and immuno-regulation activity of low molecular weight fucoidan from Laminaria japonica. J Ocean Univ China. 2018;17(3):705–711. [Google Scholar]
- Sun Q-L, Li Y, Ni L-Q, Li Y-X, Cui Y-S, Jiang S-L, Dong C-X. Structural characterization and antiviral activity of two fucoidans from the brown algae Sargassum henslowianum. Carbohydr Polym. 2020;229:115487. doi: 10.1016/j.carbpol.2019.115487. [DOI] [PubMed] [Google Scholar]
- Synytsya A, Bleha R, Synytsya A, Pohl R, Hayashi K, Yoshinaga K, Hayashi T. Mekabu fucoidan: structural complexity and defensive effects against avian influenza A viruses. Carbohyd Polym. 2014;111:633–644. doi: 10.1016/j.carbpol.2014.05.032. [DOI] [PubMed] [Google Scholar]
- Tang F, Chen F, Li F. Preparation and potential in vivo anti-influenza virus activity of low molecular-weight κ-carrageenans and their derivatives. J Appl Polym Sci. 2013;127(3):2110–2115. [Google Scholar]
- Thuy TTT, Ly BM, Van TTT, Van Quang N, Tu HC, Zheng Y, Ai U. Anti-HIV activity of fucoidans from three brown seaweed species. Carbohyd Polym. 2015;115:122–128. doi: 10.1016/j.carbpol.2014.08.068. [DOI] [PubMed] [Google Scholar]
- Torregrosa-Crespo J, Montero Z, Fuentes JL, Reig García-Galbis M, Garbayo I, Vílchez C, Martínez-Espinosa RM. Exploring the valuable carotenoids for the large-scale production by marine microorganisms. Mar Drugs. 2018;16(6):203. doi: 10.3390/md16060203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukagoshi H, Shinoda D, Saito M, Okayama K, Sada M, Kimura H, Saruki N. Relationships between viral load and the clinical course of COVID-19. Viruses. 2021;13(2):304. doi: 10.3390/v13020304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzachor A, Rozen O, Khatib S, Jensen S, Avni D. Photosynthetically controlled spirulina, but not solar spirulina, inhibits TNF-α secretion: potential implications for COVID-19-related cytokine storm therapy. Mar Biotechnol. 2021;23(1):149–155. doi: 10.1007/s10126-021-10020-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ugur S, Karaaslan MG, Aktas OC, Khan H. Investigation of promising antiviral candidate molecules based on algal phlorotannin for the prevention of COVID-19 pandemic by in silico studies. Biochemistry. 2021;7(1):3. [Google Scholar]
- Van Weelden G, Bobiński M, Okła K, Van Weelden WJ, Romano A, Pijnenborg J. Fucoidan structure and activity in relation to anti-cancer mechanisms. Mar Drugs. 2019;17(1):32. doi: 10.3390/md17010032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varese A, Ceballos A, Palacios CA, Figueroa JM, Dugour AV. Iota-carrageenan prevents the replication of SARS-CoV-2 on an in vitro respiratory epithelium model. Biorxiv. 2021 doi: 10.1101/2021.04.27.441512. [DOI] [Google Scholar]
- Wang W, Zhang P, Yu G-L, Li C-X, Hao C, Qi X, Guan H-S. Preparation and anti-influenza A virus activity of κ-carrageenan oligosaccharide and its sulphated derivatives. Food Chem. 2012;133(3):880–888. [Google Scholar]
- Wang W, Wu J, Zhang X, Hao C, Zhao X, Jiao G, Yu G. Inhibition of influenza A virus infection by fucoidan targeting viral neuraminidase and cellular EGFR pathway. Sci Rep. 2017;7(1):1–14. doi: 10.1038/srep40760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Xing M, Cao Q, Ji A, Liang H, Song S. Biological activities of fucoidan and the factors mediating its therapeutic effects: A review of recent studies. Mar Drugs. 2019;17(3):183. doi: 10.3390/md17030183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S-H, Huang C-Y, Chen C-Y, Chang C-C, Huang C-Y, Dong C-D, Chang J-S. Structure and biological activity analysis of fucoidan isolated from Sargassum siliquosum. ACS Omega. 2020;5(50):32447–32455. doi: 10.1021/acsomega.0c04591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wozniak M, Bell T, Dénes Á, Falshaw R, Itzhaki R. Anti-HSV1 activity of brown algal polysaccharides and possible relevance to the treatment of Alzheimer's disease. Int J Biol Macromol. 2015;74:530–540. doi: 10.1016/j.ijbiomac.2015.01.003. [DOI] [PubMed] [Google Scholar]
- Wu T-C, Hong Y-H, Tsai Y-H, Hsieh S-L, Huang R-H, Kuo C-H, Huang C-Y. Degradation of Sargassum crassifolium fucoidan by ascorbic acid and hydrogen peroxide, and compositional, structural, and in vitro anti-lung cancer analyses of the degradation products. Mar Drugs. 2020;18(6):334. doi: 10.3390/md18060334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xian Y, Zhang J, Bian Z, Zhou H, Zhang Z, Lin Z, Xu H. Bioactive natural compounds against human coronaviruses: a review and perspective. Acta Pharm Sin B. 2020;10(7):1163–1174. doi: 10.1016/j.apsb.2020.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Chang Y, Xue C, Wang J, Shen J. Gastric protective activities of sea cucumber fucoidans with different molecular weight and chain conformations: a structure–activity relationship investigation. J Agric Food Chem. 2018;66(32):8615–8622. doi: 10.1021/acs.jafc.8b01497. [DOI] [PubMed] [Google Scholar]
- Yew YP, Shameli K, Mohamad SE, Lee KX, Teow S-Y. Green synthesized montmorillonite/carrageenan/Fe3O4 nanocomposites for pH-responsive release of protocatechuic acid and its anticancer activity. Int J Mol Sci. 2020;21(14):4851. doi: 10.3390/ijms21144851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zayed A, Ulber R. Fucoidans: downstream processes and recent applications. Mar Drugs. 2020;18(3):170. doi: 10.3390/md18030170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zayed A, El-Aasr M, Ibrahim A-RS, Ulber R. Fucoidan characterization: determination of purity and physicochemical and chemical properties. Mar Drugs. 2020;18(11):571. doi: 10.3390/md18110571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, He P, Rodrigues AL, Jeske W, Tandon R, Bates JT, Linhardt RJ. Potential anti-SARS-CoV-2 activity of pentosan polysulfate and mucopolysaccharide polysulfate. Pharmaceuticals. 2022;15(2):258. doi: 10.3390/ph15020258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao D, Xu J, Xu X. Bioactivity of fucoidan extracted from Laminaria japonica using a novel procedure with high yield. Food Chem. 2018;245:911–918. doi: 10.1016/j.foodchem.2017.11.083. [DOI] [PubMed] [Google Scholar]