Dear editor,
Previously in this Journal, we described that inhibition of sorting nexin 27 (SNX27)- mediated endocytic recycling of angiotensin-converting Enzyme 2 (ACE2) by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spike (S) partially explained multiple Coronavirus Disease 19 (COVID-19) related diseases caused by ACE2 reduction.1 Together with retromer, SNX27 mediates endocytic recycling of multiple membrane proteins including glucose transporter type 1 (GLUT1) from endosomes to the plasma membrane.2 , 3 As a major glucose transporter in the blood-brain barrier, GLUT1 is the most important energy carrier of the brain. Reduction of GLUT1 protein level leads to dystonia and GLUT1 deficiency syndrome 1 (GLUT1DS1), including ataxia, sleep disturbances and muscle spasticity.4 It has been reported that the RNA level of GLUT1 was not significantly different between COVID‐19 patients and control.5 However, neuromuscular disorders, such as dystonia, ataxia, sleep disturbances and muscle spasticity are associated with COVID-19.6 To explain this phenomenon, we investigate whether the plasma membrane localization of GLUT1 controlled by endocytic recycling is disrupted by SARS-CoV-2.
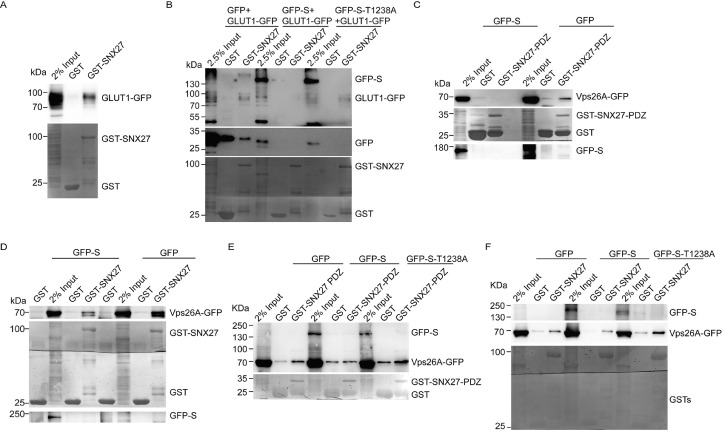
First, we accessed whether SARS-CoV-2 S abrogated the protein-protein interaction between GLUT1 and SNX27. We confirmed the interaction between GLUT1 and SNX27 by GST pulldown experiments. As shown in Fig. 1 A, bacterial expressed GST-tagged SNX27 (GST-SNX27) but not GST could pull down GFP-tagged GLUT1 (GLUT1-GFP). Our previous study found that SARS-CoV-2 S interacted with PSD95/Dlg1/ZO-1 (PDZ) domain of SNX271. Since both S and GLUT1 interacted with PDZ domain of SNX27,1 , 3 we hypothesized that SARS-CoV-2 S might compete with GLUT1 for associating with SNX27. To test this hypothesis, we performed GST pulldown experiments and found that overexpression of GFP-tagged S (GFP-S) but not GFP reduced the binding ability of GLUT1-GFP to GST-SNX27 (Fig. 1B). However, when overexpressing SARS-CoV-2 S-T1238A in which the SNX27 binding affinity was abolished, the association of GLUT1-GFP with GST-SNX27 was recovered, suggesting that SARS-CoV-2 S inhibited SNX27-GLUT1 interaction through its association with SNX27 (Fig. 1B).
Fig. 1.
Association of SNX27 with GLUT1 and Vps26A is inhibited by SARS-CoV-2 S. (A) SNX27 associates with GLUT1 in GST pulldown experiments. Lysates from 293T cells transfected with constructs expressing GFP-tagged GLUT1 were pulled down by GST–SNX27, or GST. Input represents 2% of total cell lysates. (B) SARS-CoV-2 S but not T1238A mutant of SARS-CoV-2 S suppresses the interaction between GLUT1 and SNX27 in GST pulldown experiments. Lysates from 293T cells transfected with constructs expressing GFP-tagged GLUT1 with GFP-tagged SARS-CoV-2 S, T1238A mutant of SARS-CoV-2 S, or GFP were pulled down by GST–SNX27 or GST. Input represents 2.5% of total cell lysates. (C) SARS-CoV-2 S inhibits the interaction between Vps26A-GFP and SNX27 PDZ domain in GST pulldown experiments. Lysates from 293T cells transfected with the constructs expressing GFP-tagged Vps26A with GFP-tagged SARS-CoV-2 S or GFP were pulled down by GST–SNX27-PDZ or GST. Input represents 2% of total cell lysates. (D) SARS-CoV-2 S suppresses the interaction between Vps26A and SNX27 in GST pulldown experiments. Lysates from 293T cells transfected with the constructs expressing GFP-tagged Vps26A with GFP-tagged SARS-CoV-2 S or GFP were pulled down by GST–SNX27 or GST. Input represents 2% of total cell lysates. (E) SARS-CoV-2 S but not T1238A mutant of SARS-CoV-2 S inhibits the interaction between Vps26A and SNX27 PDZ domain in GST pulldown experiments. Lysates from 293T cells transfected with the constructs expressing GFP-tagged Vps26A with GFP-tagged SARS-CoV-2 S, T1238A mutant of SARS-CoV-2 S, or GFP were pulled down by GST–SNX27-PDZ or GST. Input represents 2% of total cell lysates. (F) SARS-CoV-2 S but not T1238A mutant of SARS-CoV-2 S suppresses the interaction between Vps26A and SNX27 in GST pulldown experiments. Lysates from 293T cells transfected with the constructs expressing GFP-tagged Vps26A with GFP-tagged SARS-CoV-2 S, T1238A mutant of SARS-CoV-2 S, or GFP were pulled down by GST–SNX27 or GST. Input represents 2% of total cell lysates.
To fulfill the endocytic recycling, PDZ domain of SNX27 associates with Vps26 (Vps26A or Vps26B), a key component of retromer.2 Because both SARS-CoV-2 S and Vps26 associated with PDZ domain of SNX27,1 , 2 we wonder whether SARS-CoV-2 S abrogates the protein-protein interaction between Vps26A and SNX27 by competing with Vps26 for associating with SNX27. To test this hypothesis, we performed GST pulldown experiments and found that overexpression of GFP-S but not GFP reduced the binding ability of GFP-tagged Vps26A (Vps26A-GFP) to PDZ domain or full length of SNX27 (Fig. 1C and D). However, when overexpressing SARS-CoV-2 S-T1238A, the association of Vps26A with PDZ domain or full length of SNX27 was reversed, suggesting that SARS-CoV-2 S inhibited SNX27-Vps26A interaction through its association with PDZ domain of SNX27 (Fig. 1E and F).
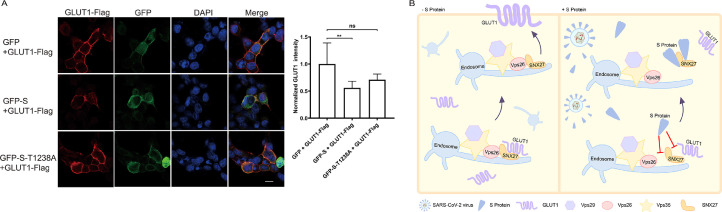
To examine whether SARS-CoV-2 S affects the surface localization of GLUT1, we performed the confocal immunofluorescent analysis experiments. Compared with GFP, GFP-S but not GSP-S-T1238A mutant reduced the surface level of Flag-tagged GLUT1-Flag (Fig. 2 A). We conclude that SARS-CoV-2 S could suppress the endocytic recycling of GLUT1 mediated by SNX27, resulting in the reduction of surface level of GLUT1.
Fig. 2.
SARS-CoV-2 S reduces surface level of GLUT1. (A) Compared with GFP, GFP-S but not GSP-S-T1238A mutant reduced the surface level of GLUT1. HeLa cells transfected with constructs expressing Flag-tagged GLUT1 with GFP-tagged SARS-CoV-2 S, T1238A mutant of SARS-CoV-2 S, or GFP were stained with HA antibody. GLUT1-HA is in red. SARS-CoV-2 S, T1238A mutant of SARS-CoV-2 S, and GFP are in green. Nucleus stained with DAPI is in Blue. Scale bar: 10 μM. Relative GLUT1 intensity was normalized by quantifying at least 20 cells through Image J. **, p value < 0.01; ns, no significance. (B) Model of how SARS-CoV-2 S inhibits GLUT1 transport mediated by SNX27. Endocytic recycling of GLUT1 is mediated by SNX27. SARS-CoV-2 S could inhibit plasma membrane targeting of GLUT1 by competing of GLUT1 and Vps26A for their association of SNX27, leading to the reduction of GLUT1.
Long COVID is characterized by multiple persisting or newly emergent symptoms following the acute infection of SARS-CoV-2.7 Our current findings expand our understanding of many neuromuscular disorders caused by GLUT1-deficiency in Long COVID patients. In no virus infection condition, SNX27 and retromer deliver GLUT1 from endosome to plasma membrane(Fig. 2B). Upon SARS-CoV-2 infection, SARS-CoV-2 S disrupts two protein-protein interactions (SNX27-Vps26A and SNX27-GLUT1) by targeting PDZ domain of SNX27(Fig. 2B). Subsequently, endocytic recycling of GLUT1 could be suppressed by SARS-CoV-2 S and surface level of GLUT1 will be decreased, leading to the deficiency of GLUT1 (Fig. 2B). Thus, many neuromuscular disorders in Long COVID patients, including dystonia, ataxia, sleep disturbances and muscle spasticity could be partially explained by the deficiency of GLUT1 caused by SARS-CoV-2 S.
Spike-based SARS-CoV-2 vaccines including BNT162b2 from BioNTech and mRNA-1273 from Moderna have been administrated globally. Previously, we discussed that side effects of S-based SARS-CoV-2 vaccine, such as myocarditis and pericarditis, may due to the reduction of ACE2 recycling by S.1 Cervical dystonia and ataxia were occasionally reported after spike-based SARS-CoV-2 mRNA vaccination.8, 9, 10 Those adverse events might due to GLUT1-dificiency by SARS-CoV-2 S because we uncovered the role for SARS-CoV-2 S in suppressing endocytic recycling of GLUT1 by targeting SNX27. Taken together, to generate a better mRNA vaccine against SARS-CoV-2 with less adverse events, T1238A mutant of SARS-CoV-2 S with less inhibition of SNX27- mediated endocytic recycling could be a promising candidate.
In summary, we reveal the mechanism how SARS-CoV-2 S reduces the surface level of GLUT1. SARS-CoV-2 S abrogates two protein-protein interactions including SNX27-Vps26A and SNX27-GLUT1. Consequently, S suppresses endocytic recycling of GLUT1 mediated by SNX27 and causes GLUT1 deficiency. GLUT1 deficiency by SARS-CoV-2 may partially explain some neuromuscular disorders of COVID-19 related syndromes, such as seizures, abnormal movements, and cognitive disorders. Our study provides new ideas for understanding COVID-19 related syndromes induced by GLUT1 reduction, which could help the treatment of some Long COVID diseases.
Declaration of Competing Interest
The authors declare that there are no conflicts of interest.
Acknowledgements
This work was supported by grants from National Natural Science Foundation of China [82072270 and 81871663], and Academic promotion programme of Shandong First Medical University [2019LJ001].
References
- 1.Ren Y., Lv L., Li P., Zhang L. Inhibition of endocytic recycling of ACE2 by SARS-CoV-2 S protein partially explains multiple COVID-19 related diseases caused by ACE2 reduction. J Infect. 2022;85:e21–e23. doi: 10.1016/j.jinf.2022.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lucas M., Gershlick D.C., Vidaurrazaga A., Rojas A.L., Bonifacino J.S., Hierro A. Structural mechanism for cargo recognition by the retromer complex. Cell. 2016;167:1623–1635. doi: 10.1016/j.cell.2016.10.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Steinberg F., Gallon M., Winfield M., Thomas E.C., Bell A.J., Heesom K.J., Tavaré J.M., Cullen P.J. A global analysis of SNX27-retromer assembly and cargo specificity reveals a function in glucose and metal ion transport. Nat Cell Biol. 2013;15:461–471. doi: 10.1038/ncb2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koch H., Weber Y.G. The glucose transporter type 1 (Glut1) syndromes. Epilepsy Behav. 2019;91:90–93. doi: 10.1016/j.yebeh.2018.06.010. [DOI] [PubMed] [Google Scholar]
- 5.Mustroph J., Hupf J., Hanses F., Evert K., Baier M.J., Evert M., Meindl C., Wagner S., Hubauer U., Pietrzyk G., Leininger S., Staudner S., Vogel M., Wallner S., Zimmermann M., Sossalla S., Maier L.S., Jungbauer C. Decreased GLUT1/NHE1 RNA expression in whole blood predicts disease severity in patients with COVID-19. ESC Heart Fail. 2021;8:309–316. doi: 10.1002/ehf2.13063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shimohata T. Neuro-COVID-19. Clin Exp Neuroimmunol. 2021 doi: 10.1111/cen3.12676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alwan N.A. The road to addressing Long Covid. Science. 2021;373:491–493. doi: 10.1126/science.abg7113. [DOI] [PubMed] [Google Scholar]
- 8.Algahtani H.A., Shirah B.H., Alwafi E. Acute cervical dystonia following the BNT162b2 mRNA COVID-19 vaccine. Clin Neurol Neurosurg. 2022;218 doi: 10.1016/j.clineuro.2022.107304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Katada E., Toyoda T., Yamada G., Morishima A., Matsukawa N. A case of chronic inflammatory demyelinating polyneuropathy following COVID-19 vaccine. Neurol Clin Neurosci. 2022 doi: 10.1111/ncn3.12604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Christensen S.K., Ballegaard M., Boesen M.S. [Guillian Barré syndromeafter mRNA-1273 vaccination against COVID-19] Ugeskr Laeger. 2021:183. [PubMed] [Google Scholar]