Abstract
Background
Penetrating keratoplasty is a corneal transplantation procedure in which a full‐thickness cornea from the host is replaced by a graft from a donor. The use of various immunosuppressants to prevent graft rejection, the most common cause of graft failure in the late postoperative period, is increasing.
Objectives
To assess the effectiveness of immunosuppressants in the prophylaxis of corneal allograft rejection after high‐ and normal‐risk keratoplasty.
Search methods
We searched CENTRAL (which contains the Cochrane Eyes and Vision Group Trials Register) (2015, Issue 4), Ovid MEDLINE, Ovid MEDLINE In‐Process and Other Non‐Indexed Citations, Ovid MEDLINE Daily, Ovid OLDMEDLINE (January 1946 to May 2015), EMBASE (January 1980 to May 2015), China National Knowledge Infrastructure (CNKI) (January 1913 to February 2015), VIP database (January 1989 to February 2015), Wanfang Data (www.wanfangdata.com) (January 1990 to February 2015), the ISRCTN registry (www.isrctn.com/editAdvancedSearch), ClinicalTrials.gov (www.clinicaltrials.gov), and the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp/search/en). We did not use any date or language restrictions in the electronic searches for trials. We last searched the English language databases on 18 May 2015 and the Chinese language databases on 20 February 2015.
Selection criteria
We included all randomised controlled trials (RCTs) assessing the use of immunosuppressants in the prevention of graft rejection, irrespective of publication language.
Data collection and analysis
We used standard procedures expected by Cochrane. The primary outcome was clear graft survival at 12 months after penetrating keratoplasty. Secondary outcomes included graft rejection, best‐corrected visual acuity, and quality of life. We defined 'high‐risk keratoplasty' as repeat keratoplasty and other indications of reduced graft survival.
Main results
We included six studies conducted in Germany (three studies), Iran, India, and China. Three studies were conducted in people undergoing high‐risk keratoplasty and investigated three different comparisons: systemic mycophenolate mofetil (MMF) versus no MMF; systemic MMF versus systemic cyclosporine A (CsA); and topical CsA versus placebo. One study compared topical tacrolimus to topical steroid in people with normal‐risk keratoplasty, and two studies compared topical CsA to placebo in people experiencing graft rejection after normal‐risk keratoplasty. Overall, we considered the trials to be at unclear or high risk of bias.
MMF may not improve clear graft survival (risk ratio (RR) 1.06, 95% confidence interval (CI) 0.84 to 1.33, 1 RCT, 87 participants, low‐quality evidence) but may reduce the risk of graft rejection (RR 0.49, 95% CI 0.22 to 1.08, 1 RCT, 87 participants, low‐quality evidence) compared to no MMF. Visual acuity was not reported.
In 1 study of 52 people comparing systemic MMF and systemic CsA, there were no graft failures in the first year of follow‐up. Data from the longest follow‐up (three years) suggest that there may be little difference in the effect of these two treatments on clear graft survival (RR 1.10, 95% CI 0.90 to 1.35, low‐quality evidence). There was low‐quality evidence of an increased risk of graft rejection with systemic MMF compared to systemic CsA, but with wide CIs compatible with increased risk with systemic CsA (RR 1.48, 95% CI 0.56 to 3.93, low‐quality evidence). Visual acuity was not reported.
One study of 84 people comparing topical CsA to placebo did not report clear graft survival at 1 year, which suggests that all grafts survived to 1 year. This study suggests that the use of topical CsA probably leads to little or no difference in graft rejection (RR 1.00, 95% CI 0.39 to 2.58, moderate‐quality evidence). At one year, the mean difference (MD) between the two groups in visual acuity was 0.07 (95% CI ‐0.01 to 0.15, moderate‐quality evidence).
Topical CsA probably does not have an effect on clear graft survival in people experiencing graft rejection after normal‐risk keratoplasty compared to placebo (RR 1.03, 95% CI 0.96 to 1.10, 2 RCTs, 283 participants, moderate‐quality evidence). There were inconsistent findings on graft rejection, with one study reporting a reduced incidence of graft rejection in the CsA group (RR 0.35, 95% CI 0.14 to 0.87, 230 participants) but the other study reporting a higher average number of episodes of graft rejection in people treated with CsA (MD 1.30, 95% CI 0.39 to 2.21, 43 participants). Overall, we judged this to be low‐quality evidence due to risk of bias and inconsistency. There was no evidence for a difference in visual acuity between the 2 groups at final follow‐up (approximately 18 months, range 2 to 33 months) (MD 0.04, 95% CI ‐0.10 to 0.18, 1 RCT, 43 participants, low‐quality evidence).
In 1 study comparing topical tacrolimus to topical steroid, the graft survived in all of the 12 treated participants and 20 control participants at 6 months. Graft rejection was rare (0 out of 12 versus 2 out of 20) (RR 0.32, 95% CI 0.02 to 6.21, low‐quality evidence). Visual acuity was not reported.
None of the studies reported on quality of life. We identified an unpublished trial of basiliximab (Simulect) (NCT00409656), probably completed in 2005.
Authors' conclusions
Current evidence on the effect of immunosuppressants in the prevention of graft failure and rejection after high‐ and normal‐risk keratoplasty is largely low quality because the number of trials was limited, and, in general, the trials were small and at risk of bias. Future trials should be large enough to detect important clinical effects, conducted with a view to minimising the risk of bias, and they should measure outcomes important to patients.
Keywords: Humans; Keratoplasty, Penetrating; Cyclosporine; Cyclosporine/therapeutic use; Graft Rejection; Graft Rejection/drug therapy; Graft Survival; Immunosuppressive Agents; Immunosuppressive Agents/therapeutic use; Mycophenolic Acid; Mycophenolic Acid/analogs & derivatives; Mycophenolic Acid/therapeutic use; Randomized Controlled Trials as Topic; Steroids; Steroids/therapeutic use; Tacrolimus; Tacrolimus/therapeutic use
Plain language summary
Immunosuppressants to prevent corneal graft rejection after penetrating keratoplasty
Background The cornea is the transparent front part of the eye that if damaged, can be replaced by a corneal transplant (keratoplasty) using healthy cornea tissue from a donor. A penetrating keratoplastyinvolves replacing all the damaged cornea. It is necessary to prevent the transplanted material (graft) from being rejected. The current strategies for preventing graft rejection are topical and oral steroids. The use of cyclosporine A (CsA), tacrolimus, mycophenolate mofetil (MMF), sirolimus, and leflunomide is increasing. However, the benefits and adverse reactions of these immunosuppressants have not yet been systematically reviewed.
Search date The evidence is up to date to May 2015.
Key findings We included six randomised controlled trials that enrolled a total of 561 people. The trials were conducted in Germany (three trials), Iran, India, and China.
In people with high‐risk keratoplasty, one study compared systemic MMF with placebo, one study compared systemic MMF with systemic CsA, and one study compared CsA eye drops versus placebo.
In people with normal‐risk keratoplasty, one study compared tacrolimus eye drops to steroid eye drops, and two studies compared CsA eye drops to placebo in people experiencing rejection after keratoplasty. All studies reported clear graft survival, incidence of graft rejection, and adverse effects.
We are uncertain as to the effects of immunosuppressants in the prevention of graft failure and rejection after high‐ and normal‐risk keratoplasty, as the number of trials is limited, and, in general, the trials are small and at risk of bias. Future trials should be large enough to detect important clinical effects, conducted with a view to minimising the risk of bias, and they should measure outcomes important to patients.
Study funding sources Three of the studies were supported by the pharmaceutical industry.
Quality of evidence We judged the quality of the evidence to be low to moderate. There was risk of bias in the included studies; the results were sometimes imprecise because of the small number of studies and small number of people enrolled in these studies; and in some analyses the results of individual trials were inconsistent.
Background
Description of the condition
Penetrating keratoplasty is a corneal transplantation procedure in which a full‐thickness cornea from the host is replaced by a graft from a donor. It has been performed in many eye diseases, including pseudophakic corneal oedema, keratoconus, aphakic corneal oedema, and stromal corneal dystrophies (Dobbins 2000; Liu 1997; Ramsay 1997). Survival of first‐time grafts is 90% at 5 years and 82% at 10 years, with reported allograft rejection rates following penetrating keratoplasty ranging from 5% to 18% (Tabbara 2007). Initial regrafts have significantly lower 5‐ and 10‐year survival rates, 53% and 41%, respectively (Thompson 2003).
The risk factors for graft failure after keratoplasty are young recipient age, the number of previous grafts, history of previous anterior segment surgery, preoperative glaucoma, quadrants of anterior synechiae, quadrants of stromal vessels, a primary diagnosis of chemical burn, and blood group ABO incompatibility. In such cases, known as high‐risk keratoplasty, the graft rejection rate may be higher than 60% (Maguire 1994).
Prevention of corneal allograft rejection
The eye has properties that permit the long‐term survival of tissue grafts that are normally rejected at extraocular sites. This ocular immune privilege was originally attributed to a putative sequestration of antigens in the eye as a result of the conspicuous absence of intraocular lymphatic drainage channels (Niederkorn 2003). However, a recent multivariate analysis suggests there is no difference between the long‐term outcomes of corneal transplantation and those of other forms of transplantation (Williams 2006). The anterior segment of the eye is still regarded as an immune‐privileged site because of the absence of vascular and lymphatic supply to the cornea. Cell‐mediated immunity in corneal allograft rejection can result from the activation of limbal Langerhans cells and from T‐cells activation by antigens released in the aqueous humor of the anterior chamber (Yamagami 2005). Nevertheless, the immunology of corneal transplantation is not fully understood (Perez 2013). Furthermore, corneal graft rejection remains the most common cause of graft failure in the late postoperative period, and prophylaxis for allograft rejection is needed (Ing 1998).
Description of the intervention
A variety of strategies to prevent corneal allograft rejection have been explored and include the use of several immunosuppressants through various delivery systems; human leukocyte antigens matching; and manipulation of antigen expression. Immunosuppressants include steroids, cyclosporine A (CsA), tacrolimus, mycophenolate mofetil (MMF), sirolimus, and leflunomide. Topical and oral steroids are currently the gold standard for routine use in the prevention of graft rejection (Hill 1991; Randleman 2006; Tabbara 2007), and the use of topical cyclosporine for routine management of high‐risk grafts is increasing (Randleman 2006).
CsA is a fungal protein that has a high degree of specificity for T‐cell lymphocytes and as a calcineurin inhibitor prevents T‐cell‐mediated immune responses. It is believed that systemic CsA significantly increases the rate of graft survival in high‐risk corneal transplantation when used prophylactically following transplantation. However, this therapy also carries significant risks, including hypertension, renal toxicity, hepatotoxicity, neurotoxicity (Hill 1989; Hill 1994), and post‐transplant lymphoproliferative disorders (Algros 2002). Although evidence on the effectiveness of topically administered CsA in the prevention of graft rejection is increasing (Belin 1990), studies have yielded inconsistent results. For example, investigators found that the use of a combination of topical CsA and steroids is better than steroids alone in preventing episodes of rejection (Cosar 2003; Inoue 2000). However, other investigators found that topical CsA did not demonstrate any significant improvement in preventing corneal graft rejection (Price 2006; Shepherd 1980).
Tacrolimus has been shown to be effective in preventing corneal allograft rejection (Reinhard 2005; Sloper 2001), causing a lower incidence of side effects related to toxicity or over immunosuppression at a much lower dosage than CsA (Reis 1998b). Systematic adverse effects such as hypertension and renal toxicity may be encountered with oral tacrolimus (Sloper 2001). MMF is thought to be a safe and effective immunosuppressive agent following renal transplantation due to less nephrotoxicity (Guerra 2007; Land 2005), but is teratogenic and is unsafe for use in pregnant women (Jackson 2009; Klieger‐Grossmann 2010). MMF has been shown to be as effective as CsA in preventing acute rejection following high‐risk corneal transplantation (Reinhard 2005; Reis 1999), but inferior to systemic tacrolimus in preventing graft rejection (Reis 1998a). Sirolimus is a bacterial macrolide with both antifungal and immunosuppressive properties. It is commonly used in conjunction with CsA or tacrolimus after solid‐organ transplantation. Similar to MMF, sirolimus is fetotoxic, although not teratogenic, and should be used with caution in corneal transplantation in pregnant women (Guerra 2007).
How the intervention might work
Immunosuppressants prevent corneal graft rejection by inhibiting the immunity of the host. Different drugs have different targets. The mechanism of CsA prophylaxis of corneal graft rejection is mainly by selectively inhibiting cellular immunity, which primarily inhibits the proliferation and action of T‐cells (Utine 2010). MMF prevents the replication of T‐ and B‐lymphocytes by inhibiting the de novo pathway of purine synthesis (Siconolfi 1996). Tacrolimus, a calcineurin inhibitor, is a macrolide antibiotic with potent immunosuppressive activity (Pillans 2006). Steroids have an antiproliferative function (Taylor 2005).
Why it is important to do this review
Immunosuppressants are widely used for the prophylaxis of corneal graft rejection after high‐ and normal‐risk keratoplasty. However, the benefits and adverse reactions from their use have not yet been systematically reviewed.
Objectives
Our primary objective was to assess the effectiveness of immunosuppressants in the prophylaxis of corneal allograft rejection after high‐ and normal‐risk keratoplasty.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) only.
Types of participants
We included people undergoing high‐ and normal‐risk keratoplasty and evaluated them as two separate groups. We defined the term 'high‐risk keratoplasty' as repeat keratoplasty, graft position close to the limbus, presence of three or four quadrants with deep vascularisation, transplantation of a highly immunogenic graft (for example central limbo‐keratoplasty), severe atopic dermatitis, and steroid‐response glaucoma.
Types of interventions
We included trials in which systemic or topical immunosuppressants such as CsA, tacrolimus, sirolimus, and MMF were compared to placebo, corticosteroids, or other immunosuppressants.
Types of outcome measures
Primary outcomes
Clear graft survival 12 months after penetrating keratoplasty.
Secondary outcomes
Graft rejection 12 months after penetrating keratoplasty. We defined rejection as any immune reaction requiring a change in therapy.
Best‐corrected visual acuity.
Quality of life measured using a validated questionnaire.
Cost‐effectiveness. This includes the cost of the drugs and other palliative medications, the need for bed rest or hospitalisation versus outpatient care, and the length of hospital stay.
-
Adverse effects
Epithelial keratitis
High intraocular pressure as defined by study investigators
Major calcineurin‐inhibitor toxicity (e.g. new‐onset diabetes or renal failure)
Minor calcineurin‐inhibitor toxicity (e.g. tremor, gingivitis, or hirsutism)
Dose reductions due to adverse events
Withdrawals and dropouts due to adverse events
We measured most outcomes during a 1‐year, 2‐year, 5‐year, and 10‐year follow‐up where possible. For those studies where the aforementioned follow‐up was not available even after correspondence with the principal investigator, we included the nearest time point available in the general and subgroup analyses.
Search methods for identification of studies
Electronic searches
We searched CENTRAL (which contains the Cochrane Eyes and Vision Group Trials Register) (2015, Issue 4), Ovid MEDLINE, Ovid MEDLINE In‐Process and Other Non‐Indexed Citations, Ovid MEDLINE Daily, Ovid OLDMEDLINE (January 1946 to May 2015), EMBASE (January 1980 to May 2015), China National Knowledge Infrastructure (CNKI) (January 1913 to February 2015), VIP database (January 1989 to February 2015), Wanfang Data (www.wanfangdata.com) (January 1990 to February 2015), the ISRCTN registry (www.isrctn.com/editAdvancedSearch), ClinicalTrials.gov (www.clinicaltrials.gov), and the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp/search/en). We did not use any date or language restrictions in the electronic searches for trials. We last searched the English language databases on 18 May 2015 and the Chinese language databases on 20 February 2015.
See: Appendices for details of search strategies for CENTRAL (Appendix 1), MEDLINE (Appendix 2), EMBASE (Appendix 3), China National Knowledge Infrastructure (CNKI) (Appendix 4), VIP database (Appendix 5), Wanfang Data (Appendix 6), ISRCTN (Appendix 7), ClinicalTrials.gov (Appendix 8), and the ICTRP (Appendix 9).
Searching other resources
We searched reference lists of identified trial reports to find additional trials. We also searched the Social Science Citation Index to find studies that had cited the identified trials.
Data collection and analysis
Selection of studies
Two review authors (MA, TXW) scanned the titles, abstracts, and keywords of every record retrieved to find studies that met our inclusion criteria. We retrieved full‐text copies of the studies for further assessment if the information given suggested that the studies:
included participants after penetrating keratoplasty;
compared immunosuppressants such as CsA, tacrolimus, and MMF with corticosteroids only;
assessed one or more relevant clinical outcome measures;
used random allocation for the comparison groups.
After reviewing the full text, we included only those studies that fulfilled the inclusion criteria. We excluded studies if they used a false randomisation procedure or included participants complicated with other diseases. We listed excluded studies in the Characteristics of excluded studies section with reasons for exclusion.
Any disagreements were resolved through discussion or, if required, through consultation with a third review author.
Data extraction and management
Two review authors (MA, TXW) independently extracted data concerning details of study population, intervention used, and outcomes using a data extraction form and then entered into RevMan 2014. The form included the following items.
General information: setting, country, year of publication, sponsor
Trial characteristics: design, duration of follow‐up, method of randomisation, allocation concealment, masking (blinding) (participants, people administering treatment, outcome assessors)
Intervention(s): intervention(s) (dose, route, timing), comparison intervention(s) (dose, route, timing), co‐medication(s) (dose, route, timing)
Participants: exclusion criteria, total number and number in comparison groups, age (adults), baseline characteristics, diagnostic criteria, similarity of groups at baseline (including any comorbidity), assessment of compliance, withdrawals/losses to follow‐up (reasons/description), subgroups
Outcomes: outcomes specified in this review, any other outcomes assessed, other events, length of follow‐up, quality of reporting of outcomes
Results: for outcomes (including a measure of variation) and times of assessment
MA and TXW independently abstracted original reports of trial results. We contacted authors of the primary studies for further information. There were no disagreements in this step.
For binary outcomes, we extracted the number of events and total number in each group. For continuous outcomes, we extracted the mean, standard deviation, and sample size of each group.
Assessment of risk of bias in included studies
We assessed the quality of reporting for each trial based largely on the criteria specified by Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). See Appendix 10 for further details.
We also planned to explore the influence of individual quality criteria in a sensitivity analysis.
Measures of treatment effect
For dichotomous data, we used risk ratios and 95% confidence intervals. For continuous data, we used mean difference and 95% confidence intervals to express the effects if we could extract the data as mean and standard deviation.
Unit of analysis issues
Participants were randomly allocated to treatment in all studies. In two studies it was stated that one eye per person was included (Javadi 2010; Sinha 2010); in the other studies this was not clear.
Dealing with missing data
We assessed and reported the presence or absence or an intention‐to‐treat (ITT) analysis in the following way (ITT analysis refers to the analysis of outcomes based on the treatment arm to which participants were randomly allocated, rather than the treatment they actually received):
Yes: Specifically reported by authors that ITT analysis was undertaken and confirmed on study assessment.
Yes: Not specifically reported, but confirmed on study assessment.
No: Not reported and lack of ITT analysis confirmed on study assessment (participants who were randomised were not included in the analysis because they did not receive the study intervention, they withdrew from the study, or were not included because of protocol violation).
No: Stated but not confirmed on study assessment.
Unclear: Not reported and not clear from study assessment.
Assessment of heterogeneity
We examined heterogeneity by the Chi2 test, and the significance was set at P greater than 0.1; I2 is used to estimate total variation across studies that is due to heterogeneity using percentages. I2 less than 40% is considered as not having important heterogeneity, 30% to 60% as moderate heterogeneity, 50% to 90% as substantial heterogeneity, and 75% to 100% as considerable heterogeneity as outlined in Chaper 9 of the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2011). If there was evidence of heterogeneity, we planned to explore it and perform subgroup analysis to determine the possible reason. We performed sensitivity analysis to explore whether or not the heterogeneity was due to low‐quality trials. If so, we excluded the lowest quality trials.
Assessment of reporting biases
We did not assess potential publication bias using a funnel plot as planned as we included only six studies. See Chapter 10 of the Cochrane Handbook for Systematic Reviews of Interventions (Sterne 2011).
Data synthesis
We performed statistical analyses according to the statistical guidelines in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
When data were reported in various forms that could not easily be converted into a standard measure, we summarised the data in a narrative format, and analysed different comparisons separately.
We included data in a meta‐analysis if they were of sufficient quality and sufficiently similar. We used a fixed‐effect model because less than three trials were included in each analysis.
Subgroup analysis and investigation of heterogeneity
We did not perform subgroup analyses in this review. In future updates we plan to conduct subgroup analysis with the following:
Normal‐ versus high‐risk keratoplasty
Different dosage of immunosuppressants
Sensitivity analysis
We were unable to conduct our planned sensitivity analysis due to only one or two studies belonging to each subgroup. If more trials are included in future updates of this review, we plan to conduct sensitivity analysis to assess how robust the review results are to key decisions and assumptions made during the review. We will repeat analysis of data with the following adjustments.
Exclusion of studies with high risk of bias; studies with low risk of bias were defined as having adequate allocation concealment and a 'reasonably expected loss to follow‐up' classified as less than 20%, given the stated importance of attrition as a quality measure (Tierney 2005).
Exclusion of unpublished studies.
Comparing the difference between the combined analysis results from the random‐effects model and the fixed‐effect model.
Summary of findings table
In a modification to our published protocol, we planned to prepare a 'Summary of findings' table presenting relative and absolute risks for the outcomes listed below. However, as the data were limited for each comparison, we did not include such a table in the current version of the review.
We graded the overall quality of the evidence for each outcome using the GRADE classification (www.gradeworkinggroup.org/).
In future updates, we will include the following outcomes in the 'Summary of findings' table.
Clear graft survival
Graft rejection
Best‐corrected visual acuity
Quality of life
Adverse effects
Follow‐up: 12 months after penetrating keratoplasty
Results
Description of studies
Results of the search
The electronic searches yielded a total of 6660 records. The Trials Search Co‐ordinator ran the electronic searches and identified 577 records, and we ran searches on the VIP and China National Knowledge Infrastructure Chinese databases and identified 6023 records. See Figure 1 for details of the screening process of the search results. We obtained full‐text records of 20 reports for further investigation. We included 9 reports of 6 studies and excluded 10 reports of 9 studies. See Characteristics of included studies; Characteristics of excluded studies. We have also included one study in Ongoing studies that is completed but the results have not yet been published (NCT00409656). We tried but failed to make contact the investigators of this study to obtain the data; we will add this study to the review if the data becomes available.
1.
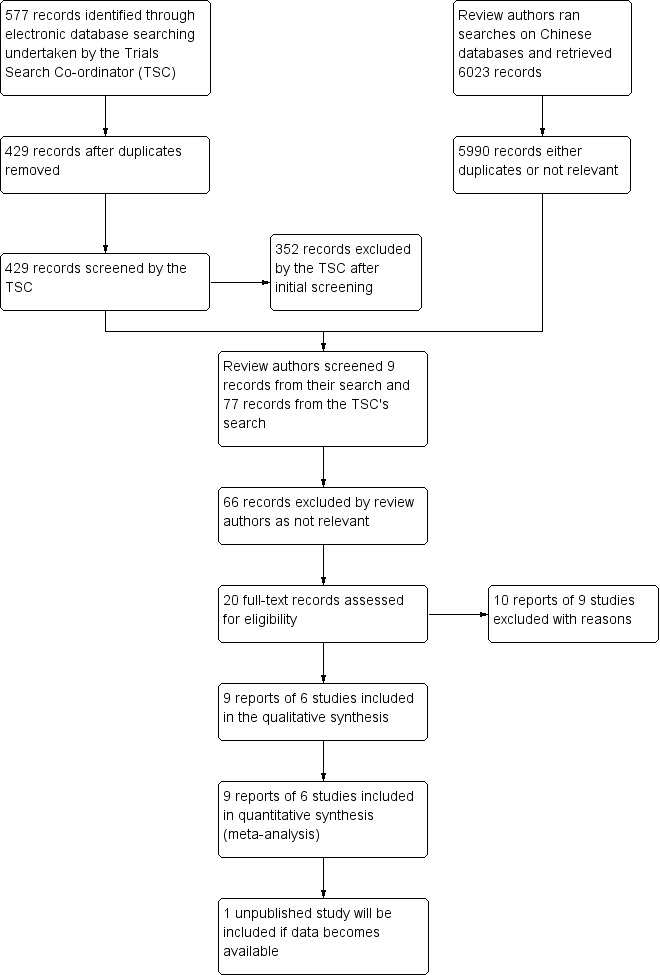
Study flow diagram
Included studies
Six studies met our inclusion criteria (Birnbaum 2009; Javadi 2010; Reinhard 2001; Reinhard 2005; Sinha 2010; Zhang 2009). See Table 1 and Characteristics of included studies for further information.
1. Characteristics of included studies.
| Study | Country | Type of keratoplasty | Number of participants (eyes) | Intervention | Comparator | Additional treatment to all participants |
| Birnbaum2009 | Germany | High risk | 98 enrolled, 87 per‐protocol | MMF (systemic) 1 g b.i.d. for 6 months | No MMF | Systemic fluocortolone 1 mg/kg bodyweight/day tapered within 3 weeks postoperatively; prednisolone acetate 1% eye drops 5 times/day, tapered over 5 months. |
| Javadi 2010 | Iran | Participants enrolled at first episode of graft rejection | 43 (43) | CsA (topical) 2% prepared in olive oil q.i.d. for 6 months | Placebo (olive oil) q.i.d. for 6 months | Based on the severity of graft rejection reaction, 0.1% topical betamethasone every 1 h during waking hours with its ophthalmic ointment during sleep, alone (in the presence of subepthelial infiltration or some scattered keratic precipitates) or in combination with 1 mg/kg oral prednisolone for 2 weeks (in the presence of rejection lines or graft oedema overlying the keratic precipitates). The topical corticosteroid was gradually tapered off over 2 weeks after resolution of the rejection episode, which was defined as complete clearance of keratic precipitates or anterior chamber reaction, or both. |
| Reinhard 2001 | Germany | High risk | 56 | CsA (systemic) target blood levels 120 ng/ml to 150 ng/ml | MMF (systemic) 1 g b.i.d. | Systemic fluocortolone 1 mg/kg bodyweight/day tapered within 3 weeks postoperatively; prednisolone acetate 1% eye drops 5 times/day, tapered over 5 months. |
| Reinhard 2005 | Germany | Normal risk | 40 enrolled; in FK 506 group, 8 with premature withdrawal of the drug | tacrolimus (topical) 0.06% t.i.d. for 6 months | Prednisolone acetate (topical) 1% 5 drops/day tapered within 6 months | Systemic fluocortolone 1 mg/kg bodyweight/day tapered within 3 weeks postoperatively; acetazolamide 500 mg/day for 5 days postop. |
| Sinha 2010 | India | High risk | 84 (84) | CsA (topical) 2% prepared in 1.4% polyvinyl alcohol q.i.d. for 1 year | Polyvinyl alcohol 1.4% | Topical prednisolone acetate 1% eye drops 2 hourly during waking hours for the initial 2 weeks followed by every 6 hours for 1 month and 4 times a day for 1 year. Topical 0.3% ofloxacin hydrochloride eye drops 4 times a day for the initial 1 month. |
| Zhang 2009 | China | Participants with graft rejection | 240 participants enrolled; 119 in the CsA group and 111 in the placebo group per‐protocol | CsA (topical) 1% prepared in ? q.i.d. for 6 months | Placebo (?) | 0.1% dexamethasone |
MMF: mycophenolate mofetil; CsA: cyclosporine A
b.i.d.: twice daily; t.i.d.: three times daily; q.i.d.: four times daily
Study design
All studies used a parallel‐group design. The unit of allocation for treatment was individual participants, although it was not always clear how many eyes were included. Birnbaum 2009, Javadi 2010, Reinhard 2001, and Sinha 2010 were single‐centre studies; Birnbaum 2009 and Zhang 2009 were multicentre studies; for Reinhard 2005 this was unclear.
Participants
Three studies were conducted in Germany (Birnbaum 2009; Reinhard 2001; Reinhard 2005), one study in Iran (Javadi 2010), one study in India (Sinha 2010), and one study in China (Zhang 2009).
Three studies enrolled a total of 238 participants undergoing high‐risk keratoplasty (Birnbaum 2009; Reinhard 2001; Sinha 2010).
One study enrolled 40 participants undergoing normal‐risk keratoplasty (Reinhard 2005).
Two studies enrolled a total of 283 participants experiencing graft rejection after keratoplasty (Javadi 2010; Zhang 2009).
Interventions
Birnbaum 2009 compared MMF versus no systemic immunosuppression (in midterm). All participants in both groups received systemic and topical corticosteroids: fluocortolone at 1 mg/kg body weight per day, tapered over three weeks, and prednisolone acetate 1% eye drops five times a day, tapered over five months.
Javadi 2010 compared 2% topical CsA versus placebo. Participants were randomly given 2% topical CsA or placebo four times a day for six months in addition to corticosteroid treatment. Based on the severity of graft rejection reaction, corticosteroid treatment consisted of 0.1% topical betamethasone every one hour during waking hours with its ophthalmic ointment during sleep, alone or in combination with 1 mg/kg oral prednisolone for two weeks. The topical corticosteroid was gradually tapered off over two weeks after resolution of the rejection episode, which was defined as complete clearance of keratic precipitates or anterior chamber reaction, or both. Graft rejection episodes recurring after the termination of CsA were treated with corticosteroids as usual.
Reinhard 2001 compared systemic MMF versus systemic CsA. All participants except those with steroid‐induced glaucoma received corticosteroids systemically (1 mg/kg body weight fluocortolone, tapered within three weeks postoperatively) and topically (five drops prednisolone acetate 1% daily after epithelial consolidation, tapered within five months).
Reinhard 2005 compared topical tacrolimus versus topical steroids. Twenty participants were treated with tacrolimus 0.06% three times topically per day for six months postoperatively (tacrolimus group). An additional 20 participants received 5 drops of prednisolone acetate 1% tapered within six months (control group). All participants received 1 mg/kg body weight per day of systemic fluocortolone, tapered within three weeks postoperatively.
Sinha 2010 compared 2% topical CsA (in vehicle polyvinyl alcohol) in the experimental group versus 1.4% polyvinyl alcohol drops in control group. Both groups also received corticosteroid eye drops after surgery.
Zhang 2009 compared 1% CsA eye drops versus control. Both groups also received 1% dexamethasone eye drops.
Outcome measures
All studies reported clear graft survival, immune reactions causing rejection, and side effects. No study reported best‐corrected visual acuity, quality of life, or cost‐effectiveness.
Only three studies reported details of the postoperative visits. One study scheduled these visits after 1, 3, 6, 9, 12, 24, and 36 months (Reinhard 2001). In another study they were daily in the first week and after 2, 3, 6, 9, 12, 18, and 24 months (Birnbaum 2009). Reinhard 2005 did not describe the visit time in detail and only stated follow‐up time of tacrolimus group and steroid group as 5.8 to 14.8 and 9.1 to 13.3 months, respectively.
Excluded studies
We excluded nine studies because although the authors used the term "randomly allocated patients" in their text, they were not real RCTs (He 1999; Liu 2007; Lu 2009; Reinhard 1999; Wu 2001; Xi 2003; Ye 2004; Zhao 2005; Zhou 2008). We telephoned the authors of these articles for clarification on this point. One study included participants with different conditions and was therefore excluded (Reinhard 1999). See Characteristics of excluded studies for further information.
Risk of bias in included studies
The risk of bias is described below and summarised in Figure 2 and Figure 3.
2.
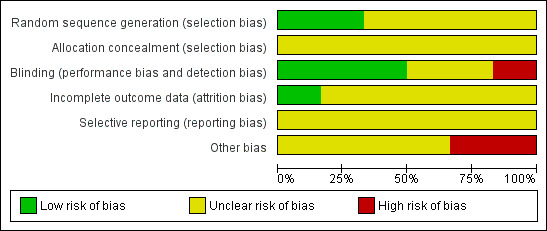
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
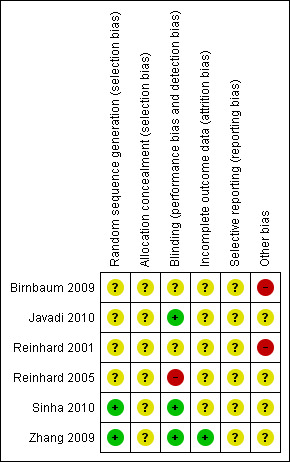
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Random sequence generation
All included trials stated that they "randomly allocated the participants", but only two studies described adequate measures for generating the allocation sequence. In Zhang 2009 they used a random numbers table and in Sinha 2010, they used computer‐generated random numbers with a block randomisation strategy.
Allocation concealment
None of the studies reported on the use of allocation concealment.
Blinding
Blinding (masking) was performed in three studies (Javadi 2010; Sinha 2010; Zhang 2009).
In Javadi 2010, the participants and the ophthalmologist who analysed the results were unaware of the type of drops.
In Sinha 2010, to eliminate assessment bias, the ocular pharmacologist was masked to the outcome variables. Also, the investigator measuring the outcome variables was masked to the nature of the drug therapy.
In Zhang 2009, dedicated personnel were responsible for maintaining masking and keeping the allocation table hidden, thus both participants and outcome assessor were masked.
The remaining three studies did not provide any information about masking (Birnbaum 2009; Reinhard 2001; Reinhard 2005).
Incomplete outcome data
Four studies had participants who did not complete the study.
In Birnbaum 2009, 11 participants withdrew from the study: 7 in the MMF group and 4 in the control group. Of the 11 participants, 9 withdrew due to protocol deviation, 2 due to side effects from the MMF.
In Javadi 2010, one participant was excluded because of intolerance to the medication given.
In Reinhard 2001, premature withdrawal from immunosuppressive prophylaxis occurred in two participants in the CsA group (one case of severe gingival hyperplasia, one of hepatotoxicity), and in two participants in the MMF group (one diagnosis of Hodgkin's lymphoma one month after keratoplasty, one case of dermatological problems in a participant with severe atopic dermatitis).
In Reinhard 2005, premature withdrawal from the drug occurred in eight participants in the tacrolimus group due to local side effects, and all the participants completed the study in accordance with the protocol.
There were no exclusions in Sinha 2010.
Zhang 2009 used ITT analysis and reported 1 case where treatment was not completed in the CsA group (1 out of 120) and 9 cases in the control group (9 out of 120). The other studies did not report the use of ITT analysis.
Selective reporting
The outcomes in the methods section of the study reports were reported in detail, but we were unable to check the protocols of the included studies. We therefore judged the risk of reporting bias as unclear.
Other potential sources of bias
A potential conflict of interest may be present in Sinha 2010 as the trialists themselves prepared the 2% CsA drops in 1.4% polyvinyl alcohol. We found no other potential sources of bias in the other studies.
Effects of interventions
We considered adverse effects separately at the end of this section.
Systemic MMF versus no MMF in people undergoing high‐risk keratoplasty
One study compared MMF and no MMF in 98 participants (87 followed up) undergoing high‐risk keratoplasty with an average follow‐up of 34.9 ± 16.3 months (Birnbaum 2009) (Table 2).
2. Systemic MMF versus no MMF in people undergoing high‐risk keratoplasty.
| Outcome | Systemic MMF | No MMF | RR (95% CI) |
| Clear graft survival at 3 years | 40/50 (80.0%) | 28/37 (75.7%) | 1.06 (0.84 to 1.33) |
| Graft rejection (immune reactions) at 3 years | 8/50 (16.0%) | 12/37 (32.4%) | 0.49 (0.22 to 1.08) |
Data from Birnbaum 2009
MMF: mycophenolate mofetil RR: risk ratio CI: confidence interval
Results of this study suggest that MMF may not improve clear graft survival (risk ratio (RR) 1.06, 95% confidence interval (CI) 0.84 to 1.33), but may reduce the risk of graft rejection (immune reactions) (RR 0.49, 95% CI 0.22 to 1.08).
We judged this to be low‐quality evidence, downgrading for risk of bias and imprecision.
Visual acuity, quality of life, and costs were not reported.
Systemic MMF versus systemic CsA in people undergoing high‐risk keratoplasty
One study compared systemic MMF and systemic CsA in 52 participants with high‐risk keratoplasty with three years follow‐up (Reinhard 2001) (Table 3).
3. Systemic MMF versus systemic CsA in people undergoing high‐risk keratoplasty.
| Outcome | Systemic MMF | Systemic CsA | RR (95% CI) |
| Clear graft survival at 3 years | 25/27 (92.6%) | 21/25 (84.0%) | 1.10 (0.90 to 1.35) |
| Graft rejection (immune reactions) at 3 years | 8/27 (29.6%) | 5/25 (20.0%) | 1.48 (0.56 to 3.93) |
Data from Reinhard 2001
MMF: mycophenolate mofetil CsA: cyclosporine A RR: risk ratio CI: confidence interval
There were no graft failures in the first year of follow‐up. Data from the longest follow‐up (three years) suggests that there may be little difference in the effect of these two treatments on clear graft survival (RR 1.10, 95% CI 0.90 to 1.35). There was low‐quality evidence of an increased risk of graft rejection with systemic MMF compared to systemic CsA, but with wide CIs compatible with increased risk with systemic CsA (RR 1.48, 95% CI 0.56 to 3.93, low‐quality evidence).
We judged this to be low‐quality evidence, downgrading for risk of bias and imprecision.
Visual acuity, quality of life, and costs were not reported.
Topical CsA versus placebo in people undergoing high‐risk keratoplasty
One study compared topical CsA to placebo in 84 participants undergoing high‐risk keratoplasty (Sinha 2010) (Table 4, Table 5).
4. Topical CsA versus placebo in people undergoing high‐risk keratoplasty.
| Outcome | Topical CsA | Placebo | RR (95% CI) |
| Graft rejection (immune reactions) at 1 year | 7/39 (17.9%) | 7/39 (17.9%) | 1.00 (0.39 to 2.58) |
Data from Sinha 2010
CsA: cyclosporine A RR: risk ratio CI: confidence interval
5. Topical CsA versus placebo in people undergoing high‐risk keratoplasty (visual acuity).
| Outcome | Topical CsA | Placebo | MD (95% CI) | ||
| mean (SD) | N | mean (SD) | N | ||
| Mean best‐corrected visual acuity at 1 year | 0.31 (0.18) | 39 | 0.24 (0.17) | 39 | 0.07 (‐0.01 to 0.15) |
Data from Sinha 2010
CsA: cyclosporine A SD: standard deviation MD: mean difference CI: confidence interval
The study did not report clear graft survival at one year, which suggests that all grafts survived to one year. Results of this study suggest that topical CsA probably leads to little or no difference in graft rejection (RR 1.00, 95% CI 0.39 to 2.58, moderate‐quality evidence).
At one year, the mean difference (MD) between the two groups in visual acuity was 0.07 (95% CI ‐0.01 to 0.15).
We judged this to be moderate‐quality evidence, downgrading for imprecision.
Quality of life and costs were not reported.
Topical CsA versus placebo in people experiencing graft rejection after normal‐risk keratoplasty
Two studies compared topical CsA versus placebo in participants experiencing graft rejection after normal‐risk keratoplasty (Javadi 2010;Zhang 2009). Average follow‐up was 16 months in Javadi 2010 and 6 months in Zhang 2009.
Topical CsA probably does not have an effect on clear graft survival (RR 1.03, 95% CI 0.96 to 1.10; participants = 283; studies = 2; I2 = 34%) (Analysis 1.1). We judged this to be moderate‐quality evidence, downgrading for risk of bias.
1.1. Analysis.
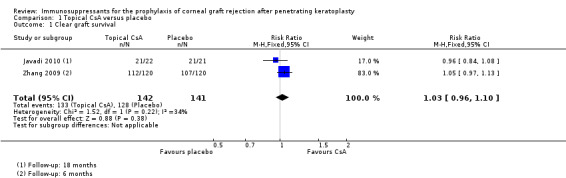
Comparison 1 Topical CsA versus placebo, Outcome 1 Clear graft survival.
Evidence from Zhang 2009 suggests that topical CsA probably reduces the incidence of graft rejection (RR 0.35, 95% CI 0.14 to 0.87) (Table 6). Javadi 2010 did not report the incidence of graft rejection but reported that participants treated with CsA on average had 2.7 (standard deviation (SD) 1.8) episodes of graft rejection compared to 1.4 (SD 1.2) episodes in the placebo group (MD 1.30, 95% CI 0.39 to 2.21). Overall, we judged the evidence to be low quality, downgrading for risk of bias and inconsistency between the two studies.
6. Topical CsA versus placebo in people with graft rejection after normal‐risk keratoplasty.
| Outcome | Topical CsA | Placebo | RR (95% CI) |
| Graft rejection (immune reactions) at 6 months | 7/119 (5.9%) | 17/111 (15.3%) | 0.35 (0.14 to 0.87) |
Data from Zhang 2009
CsA: cyclosporine A RR: risk ratio CI: confidence interval
Javadi 2010 reported visual acuity at final follow‐up, which was approximately 18 months (range 2 to 33 months follow‐up) (Table 7). The MD in visual acuity between the two groups was 0.04 (95% CI ‐0.10 to 0.18).
7. Topical CsA versus placebo in people with graft rejection after normal‐risk keratoplasty (visual acuity).
| Outcome | Topical CsA | Placebo | MD (95% CI) | ||
| mean (SD) | N | mean (SD) | N | ||
| Mean best‐corrected visual acuity at 18 months | 0.23 (0.31) | 22 | 0.19 (0.12) | 21 | 0.04 (‐0.10 to 0.18) |
Data from Javadi 2010
CsA: cyclosporine A SD: standard deviation MD: mean difference CI: confidence interval
Neither study reported quality of life or costs.
Topical tacrolimus versus topical steroid in people undergoing normal‐risk keratoplasty
One study compared topical tacrolimus with topical steroid in 32 normal‐risk keratoplasty participants and reported at 6 months follow‐up (Reinhard 2005) (Table 8).
8. Topical tacrolimus versus topical steroid in people undering normal‐risk keratoplasty.
| Outcome | Topical tacrolimus | Topical steroid | RR (95% CI) |
| Clear graft survival at 6 months | 12/12 (100%) | 20/20 (100%) | 1.00 (0.88 to 1.14) |
| Graft rejection (immune reactions) at 6 months | 0/12 (0%) | 2/20 (10%) | 0.32 (0.02 to 6.21) |
Data from Reinhard 2005
RR: risk ratio CI: confidence interval
The graft survived in all of the 12 treated participants and 20 control participants. The immune reactions were also very rare: 0 out of 12 versus 2 out of 20 in the two groups, respectively (RR 0.32, 95% CI 0.02 to 6.21).
We judged this to be low‐quality evidence, downgrading for risk of bias and imprecision.
Visual acuity, quality of life, and costs were not reported.
Adverse effects
Systemic MMF
We saw a range of adverse effects with MMF, which we have summarised in Table 9.
9. MMF: adverse effects.
| Category | Study | Details of adverse event |
MMF Number of events/Number followed up |
Control* Number of events/Number followed up |
| Epithelial keratitis | Birnbaum 2009 | Not reported | ||
| Reinhard 2001 | Not reported | |||
| High intraocular pressure | Birnbaum 2009 | Not reported | ||
| Reinhard 2001 | Glaucoma | 1/29 | 2/27 | |
| Major calcineurin‐inhibitor toxicity | Birnbaum 2009 | Hyperglycaemia | 7/57 | 4/41 |
| Reinhard 2001 | Hepatotoxicity | 2/29 | 3/27 | |
| Minor calcineurin‐inhibitor toxicity | Birnbaum 2009 | |||
| Reinhard 2001 | Gingival problems | 0 | 3/41 | |
| Dose reductions due to adverse events | Birnbaum 2009 | Due to raised liver enzymes | 2/57 | 0 |
| Reinhard 2001 | Not reported | |||
| Withdrawals and dropouts due to adverse events | Birnbaum 2009 | Gastrointestinal disturbances (1) Asthma, pruritus, and fatigue (1) |
2/57 | 0 |
| Reinhard 2001 | 2/29 | 2/27 | ||
Birnbaum 2009 control group received systemic and topical corticosteroids same as MMF group.
Reinhard 2001 control group received systemic cyclosporine A.
MMF: mycophenolate mofetil
Other adverse effects reported in MMF group in Birnbaum 2009: Hyperlipidaemia (11), Infections (8), Tachycardia (4), Weight loss (4), Fatigue (4), Weight gain (3), Insomnia (3), Headache (3), Malignancies (2), Myalgia (1), Renal colic (1), Myocardial infarction (1), Erythema (1), Deterioration of atopic eczema (1), Muscular cramps (1), Paresthesia (1), Ostealgia (1), Agranulocytosis (1), Anaemia (1).
Topical CsA
Sinha 2010 reported that “Overall, topical cyclosporine A 2% was well tolerated by all the patients included in this study. There were no reports of ocular irritation. There was no epithelial toxicity in the form of corneal erosions or superficial punctuate keratopathy in any of the patients”.
Javadi 2010 reported "No significant complications occurred during the study. As mentioned, only one case from group 1 [CsA group] was excluded due to medication intolerance”.
Zhang 2009 reported that the incidence of adverse events was similar in the topical CsA (1 case) and placebo groups.
Systemic CsA
We have summarised adverse effects seen with systemic CsA in Table 10.
10. Systemic MMF versus systemic CsA: adverse effects.
| Study ID and comparisons | Side effects | Number of events/number followed up in the experimental group | Number of events/number followed up in the control group | OR (95% CI) |
|
Reinhard 2001 Systemic MMF versus systemic CsA |
Hepatotoxicity | 2/29 | 3/27 | 0.6 (0.10 to 3.72) |
| Arterial hypertension | 0/29 | 3/27 | 0.12 (0.01 to 1.17) | |
| Gingiva problems | 0/29 | 3/27 | 0.12 (0.01 to 1.17) | |
| Neurovegetative disorders | 0/29 | 3/27 | 0.12 (0.01 to 1.17) | |
| Hodgkin's lymphoma | 0/29 | 1/27 | 0.13 (0.00 to 6.35) | |
| Recurrence of acoustic neurinoma | 1/29 | 0/27 | 6.90 (0.14 to 348.44) | |
| Exacerbation of atopic dermatitis | 1/29 | 0/27 | 6.90 (0.14 to 348.44) |
MMF: mycophenolate mofetil CsA: cyclosporine A OR: odds ratio CI: confidence interval
Tacrolimus
Eight participants withdrew due to local side effects in the intervention group. Reinhard 2005 reported adverse events such as superficial punctate keratitis (8 out of 20 versus 8 out of 20), injection of the conjunctiva (6 out of 20 versus 2 out of 20), burning sensation (6 out of 20 versus 0 out of 20), superficial opacification (2 out of 20 versus 1 out of 20), and erosion (1 out of 20 versus 0 out of 20), in the experimental and control groups, respectively. There were no significant differences between the two arms except a burning sensation (see Table 11).
11. Tacrolimus: adverse effects.
| Study ID and comparisons | Side effects | Number of events/number followed up in the experimental group | Number of events/number followed up in the control group | OR (95% CI) |
|
Reinhard 2005 Topical tacrolimus versus topical steroid |
Superficial punctate | 8/20 | 8/20 | 1.00 (0.29 to 3.49) |
| Injection of the conjunctiva | 6/20 | 2/20 | 3.38 (0.73 to 15.62) | |
| Burning sensation | 6/20 | 0/20 | 9.92 (1.79 to 55.04) | |
| Superficial opacification | 2/20 | 1/20 | 2.02 (0.20 to 20.62) | |
| Erosion | 1/20 | 0/20 | 7.39 (0.15 to 372.38) |
OR: odds ratio CI: confidence interval
Discussion
Summary of main results
We included six studies conducted in Germany, Iran, India, and China. Three studies were conducted in people undergoing high‐risk keratoplasty and investigated three different comparisons: systemic mycophenolate mofetil (MMF) versus no MMF; systemic MMF versus systemic cyclosporine A (CsA); and topical CsA versus placebo. One study compared topical tacrolimus to topical steroid in people with normal‐risk keratoplasty, and two studies compared topical CsA to placebo in people experiencing graft rejection after normal‐risk keratoplasty. All participants in these trials received additional steroid treatment.
There was uncertainty as to the effect of systemic MMF, systemic CsA, or topical CsA on both clear graft survival and graft rejection in people with high‐risk keratoplasty.
Topical CsA probably does not have an effect on clear graft survival in people experiencing graft rejection after normal‐risk keratoplasty compared to placebo. There were inconsistent findings on graft rejection.
There was uncertainty as to the relative effects of topical tacrolimus to topical steroid.
Adverse effects were common with systemic MMF, but less common with topical treatments CsA and tacrolimus.
Overall completeness and applicability of evidence
The included studies have addressed the main objectives of the review including graft survival and incidence of graft rejection at 12 months. However, the question about other outcomes including best‐corrected visual acuity, quality of life, and cost‐effectiveness analysis have not been answered.
The evidence from this review came from both normal‐ and high‐risk keratoplasty populations, and therefore can be applied to both of these populations.
Quality of the evidence
Overall, we rated the quality of evidence from the included studies from low to moderate due to methodological limitations in the included trials and imprecision in the summary estimates. For some analyses, results of individual trials were inconsistent. Publication bias was also a possibility, but we were unable to assess this due to the low number of trials for each comparison.
Two trials failed to report the information on interventions adequately (Reinhard 2001; Reinhard 2005). Dosage and duration of therapy should be considered when evaluating the effectiveness of immunosuppressants, but we could not do this due to the small number of included trials.
Potential biases in the review process
As the review included fewer than 10 trials, we were unable to investigate publication bias using a funnel plot. We searched English and Chinese databases only, but not other language databases.
Authors' conclusions
Implications for practice.
Currently there is insufficient evidence to ascertain which immunosuppressant is better for penetrating keratoplasty. There was uncertainty as to the effects of systemic MMF, systemic CsA, or topical CsA on both clear graft survival and graft rejection in people with high‐risk keratoplasty. Topical CsA probably does not have an effect on clear graft survival in people experiencing graft rejection after normal‐risk keratoplasty compared to placebo. There were inconsistent findings on graft rejection. There was uncertainty as to the relative effects of topical tacrolimus to topical steroid. Adverse effects were common with systemic MMF, but less common with topical treatments CsA and tacrolimus.
Implications for research.
Large, randomised, placebo‐controlled, double‐masked trials using polyvinyl alcohol as the vehicle of topical CsA should be conducted by a third party who has no conflict of interest to evaluate the effectiveness of immunosuppressant and adverse events that may occur. For all immunosuppressants, future studies should take into account the following factors: the study should be powered to detect important clincial differences, and methods (randomisation procedure, allocation concealment, masking of participants and outcome assessors) should be performed and reported in detail. Other factors impacting study quality include baseline of participants and the manufacturer, composition, dosage, and course of treatment of the drugs. The method of outcomes detection should be designed, performed, and reported carefully; ITT analysis should be applied to the outcomes when there are missing participants due to drop‐out or loss to follow‐up. The outcomes best‐corrected visual acuity, quality of life, and cost‐effectiveness analysis should be assessed. Follow‐up should be at least one year.
Acknowledgements
We thank Anupa Shah, Richard Wormald, and Iris Gordon from the Cochrane Eyes and Vision Group for help with writing this protocol and Jod Mehta, Catey Bunce, Keryn Williams, and Doug Coster for their comments on the protocol and/or review. We also thank Drs. Wei Xin, Chen Zhiyu, and Deng Yinping for their previous hard work in developing the protocol.
Appendices
Appendix 1. CENTRAL search strategy
#1 MeSH descriptor Corneal Transplantation #2 cornea* near/3 transplant* #3 cornea* near/3 graft* #4 MeSH descriptor Keratoplasty, Penetrating #5 keratoplast* #6 (#1 OR #2 OR #3 OR #4 OR #5) #7 MeSH descriptor Immunosuppressive Agents #8 immunosuppress* #9 MeSH descriptor Cyclosporine #10 cyclosporin* #11 MeSH descriptor Tacrolimus #12 tacrolimus$ or FK506* #13 MeSH descriptor Mycophenolic Acid #14 mycophenolate* near/2 mofetil* #15 MMF* #16 MeSH descriptor Sirolimus #17 sirolimus or rapam* #18 (#7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17) #19 (#6 AND #18)
Appendix 2. MEDLINE (OvidSP) search strategy
1. randomized controlled trial.pt. 2. (randomized or randomised).ab,ti. 3. placebo.ab,ti. 4. dt.fs. 5. randomly.ab,ti. 6. trial.ab,ti. 7. groups.ab,ti. 8. or/1‐7 9. exp animals/ 10. exp humans/ 11. 9 not (9 and 10) 12. 8 not 11 13. exp corneal transplantation/ 14. (cornea$ adj3 transplant$).tw. 15. (cornea$ adj3 graft$).tw. 16. keratoplast$.tw. 17. or/13‐16 18. exp immunosuppressive agents/ 19. immunosuppress$.tw. 20. cyclosporin$.tw. 21. (tacrolimus$ or FK506$).tw. 22. mycophenolic acid/ 23. ((mycophenolate$ adj2 mofetil$) or MMF$).tw. 24. (sirolimus$ or rapam$).tw. 25. or/18‐24 26. 17 and 25 27. 12 and 26
The search filter for trials at the beginning of the MEDLINE strategy is from the published paper by Glanville et al (Glanville 2006).
Appendix 3. EMBASE (OvidSP) search strategy
1. exp randomized controlled trial/ 2. exp randomization/ 3. exp double blind procedure/ 4. exp single blind procedure/ 5. random$.tw. 6. or/1‐5 7. (animal or animal experiment).sh. 8. human.sh. 9. 7 and 8 10. 7 not 9 11. 6 not 10 12. exp clinical trial/ 13. (clin$ adj3 trial$).tw. 14. ((singl$ or doubl$ or trebl$ or tripl$) adj3 (blind$ or mask$)).tw. 15. exp placebo/ 16. placebo$.tw. 17. random$.tw. 18. exp experimental design/ 19. exp crossover procedure/ 20. exp control group/ 21. exp latin square design/ 22. or/12‐21 23. 22 not 10 24. 23 not 11 25. exp comparative study/ 26. exp evaluation/ 27. exp prospective study/ 28. (control$ or prospectiv$ or volunteer$).tw. 29. or/25‐28 30. 29 not 10 31. 30 not (11 or 23) 32. 11 or 24 or 31 33. exp cornea transplantation/ 34. (cornea$ adj3 transplant$).tw. 35. (cornea$ adj3 graft$).tw. 36. exp keratoplasty, penetrating/ 37. keratoplast$.tw. 38. or/33‐37 39. exp immunosuppressive agent/ 40. immunosuppress$.tw. 41. exp cyclosporine A/ 42. cyclosporin$.tw. 43. exp tacrolimus/ 44. (tacrolimus$ or FK506$).tw. 45. exp Mycophenolic Acid 2 Morpholinoethyl Ester/ 46. ((mycophenolate$ adj2 mofetil$) or MMF$).tw. 47. exp sirolimus/ 48. (sirolimus$ or rapam$).tw. 49. or/39‐48 50. 38 and 49 51. 32 and 50
Appendix 4. CNKI search strategy
1. 角膜移植 ti.
2. 角膜移植 ti. AND 免疫抑制剂 ti.
3. 角膜移植 ti. AND 他克莫司 ti.
4. 角膜移植 ti. AND 环孢霉素A ti.
5. 角膜移植 ti. AND 雷帕霉素 ti.
6. 角膜移植 ti. AND 环磷酰胺 ti.
7. 角膜移植 ti. AND 苯丁酸氮芥 ti.
8. 角膜移植 ti. AND 丝裂霉素C ti.
9. 角膜移植 ti. AND 糖皮质激素 ti.
10. 角膜移植 ti. AND 5‐氟脲嘧啶 ti.
11. 角膜移植 ti. AND 6‐巯基嘌呤 ti.
12. 角膜移植 ti. AND 6‐巯基鸟嘌呤 ti.
13. 角膜移植 ti. AND 8‐重氮鸟嘌呤 ti.
14. 角膜移植 ti. AND 5‐溴去氧脲核甙 ti.
15. 角膜移植 ti. AND 氨甲喋呤 ti.
16. 角膜移植 ti. AND FK506 ti.
17. 角膜移植 ti. AND 放线菌素D ti.
18. 角膜移植 ti. AND 正定霉素 ti.
19. 角膜移植 ti. AND 氯霉素 ti.
20. 角膜移植 ti. AND 长春新碱 ti.
21. 角膜移植 ti. AND 无环鸟苷 ti.
22. 角膜移植 ti. AND (随机 ti. OR 随机 ab. OR 随机 tx) NOT (鼠 OR 兔 OR 干细胞移植)
Appendix 5. VIP search strategy
1. 角膜移植 ti.
2. 角膜移植 ti. AND 免疫抑制剂 ti.
3. 角膜移植 ti. AND 他克莫司 ti.
4. 角膜移植 ti. AND 环孢霉素A ti.
5. 角膜移植 ti. AND 雷帕霉素 ti.
6. 角膜移植 ti. AND 丝裂霉素 ti.
7. 角膜移植 ti. AND 糖皮质激素 ti.
Appendix 6. Wanfang Data search strategy
1. 角膜移植 tx.
2. 角膜移植 tx. AND 免疫抑制剂 tx.
3. 角膜移植 tx. AND 他克莫司 tx.
4. 角膜移植 tx. AND 环孢霉素A tx.
5. 角膜移植 tx. AND 雷帕霉素 tx.
6. 角膜移植 tx. AND 丝裂霉素 tx.
7. 角膜移植 tx. AND 糖皮质激素 tx.
Appendix 7. ISRCTN search strategy
"(Cornea transplant) AND immunosuppressant"
Appendix 8. ClinicalTrials.gov search strategy
(Cornea transplant OR graft) AND immunosuppressant
Appendix 9. ICTRP search strategy
cornea transplant
Appendix 10. Parameters used to assess risk of bias in included studies
Sequence generation (checking for possible selection bias)
Methods used to generate the allocation sequence were categorised as:
adequate (any truly random process, e.g. random number table; computer random number generator);
inadequate (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number); or
unclear.
Allocation concealment (checking for possible selection bias)
Methods used to conceal the procedure of allocation were categorised as:
adequate (e.g. telephone or central randomisation; consecutively numbered, sealed, opaque envelopes);
inadequate (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
unclear.
Blinding (masking) (checking for possible performance bias)
Masking was categorised as:
adequate, inadequate, or unclear for participants;
adequate, inadequate, or unclear for personnel;
adequate, inadequate, or unclear for outcome assessors.
Incomplete outcome data (checking for possible attrition bias through withdrawals, dropouts, protocol deviations)
We stated whether attrition and exclusions were reported; the numbers included in the analysis at each stage (compared with the total randomised participants); reasons for attrition or exclusion where reported; and whether missing data were balanced across groups or were related to outcomes.
The risk of incomplete outcome data was categorised as:
low risk: trials where few dropouts/losses to follow‐up are noted and an intention‐to‐treat analysis is possible;
high risk: the rate of exclusion was at least 20%, or wide differences in exclusions between groups
unclear.
Selective reporting bias
The risk of selective reporting bias was categorised as:
low risk: where it is clear that all of the study's prespecified outcomes and all expected outcomes of interest to the review have been reported;
high risk: where not all of the study's prespecified outcomes have been reported; one or more reported primary outcomes were not prespecified; outcomes of interest are reported incompletely and so cannot be used; study fails to include results of a key outcome that would be expected to have been reported;
unclear.
Other sources of bias
We assessed whether each study was free of other problems that could put it at risk of bias:
low risk: free of any other possible source of bias;
high risk: which may result in bias e.g. conflict of interest;
unclear.
Data and analyses
Comparison 1. Topical CsA versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Clear graft survival | 2 | 283 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.96, 1.10] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Birnbaum 2009.
| Methods | Multicentre (9 centres) Parallel‐group RCT, unclear how many eyes included |
|
| Participants | Country: Germany Number of participants enrolled: 98 Number of participants per‐protocol: 87 Average age (range): 59 years (not reported) Sex: 47% women Inclusion criteria: High‐risk keratoplasty, which was defined as "repeat keratoplasty, graft position close to the limbus, presence of three or four quadrants with deep vascularisation, transplantation of a highly immunogenic graft (eg, central limbokeratoplasty), severe atopic dermatitis, and steroid‐response glaucoma". Exclusion criteria:
|
|
| Interventions | Intervention:
Comparator:
Participants received MMF at a fixed dosage (2 x 1000 mg daily) for 6 months postoperatively. Thereafter, they took 2 x 500 mg MMF daily for 2 weeks. Participants in both groups received systemic and topical corticosteroids: fluocortolone at 1 mg/kg body weight per day, tapered over 3 weeks, and prednisolone acetate 1% eye drops at 5 times a day, tapered over 5 months. |
|
| Outcomes |
|
|
| Method and times of measuring the outcomes | Postoperative examination was performed after 6 weeks, 3, 6, 9, 12, 18, and 24 months, and then yearly, including visual acuity, slit‐lamp examination, specular microscopy of the graft endothelium, determination of intraocular pressure, and fundus examination. Adverse events and possible systemic side effects were monitored (in cooperation with each participant's general practitioner) by means of a standard list of questions. The mean follow‐up time was 34.9 ± 16.3 (mean ± SD) months. All participants were monitored for efficacy postoperatively. A scoring system was not used. If opacities could not be detected in any of the corneal layers regarding the central 3 mm, the grafts were considered clear. |
|
| Funding source and statement of interest | Roche Pharma AG, Grenzach‐Wyhlen, Germany, for financial support of the study. There was no declaration of interest in the article. | |
| Notes | Date study conducted: June 2000 to August 2006 Trial registration number: NCT00411515 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation was performed by drawing a lot. |
| Allocation concealment (selection bias) | Unclear risk | Not used. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not used. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 11 participants withdrew from the study: 7 in the MMF group and 4 in the control group. Of the 11 participants, 9 withdrew due to protocol deviation, 2 due to side effects from the MMF. |
| Selective reporting (reporting bias) | Unclear risk | Not mentioned. |
| Other bias | High risk | "Study recruitment was stopped prematurely due to a statistically significant result from the interim evaluation once 2/3 of the patients had been recruited." Page 2066 |
Javadi 2010.
| Methods | Single‐centre Parallel‐group RCT, 1 eye per person included |
|
| Participants | Country: Iran Number of participants: 43 Average age (range): 34 years (17 to 59) Sex: 49% women Inclusion criteria: People who had at least 1 episode of graft rejection after penetrating keratoplasty. Graft rejection reaction was defined as "the presence of subepithelial infiltration, the presence of keratic precipitates with or without anterior chamber reaction, or graft oedema in a previously clear graft with or without keratic precipitates or anterior chamber reaction. In the case of graft oedema without keratic precipitates or anterior chamber reaction, oedema reversal after taking corticosteroids differentiated graft rejection from endothelial decompensation". Exclusion criteria:
|
|
| Interventions | Intervention:
Comparator:
Upon graft rejection, the participants were given CsA or placebo 4 times a day for 6 months in addition to corticosteroid treatment. Based on the severity of graft rejection reaction, corticosteroid treatment consisted of 0.1% topical betamethasone every 1 hour during waking hours with its ophthalmic ointment during sleep, alone or in combination with 1 mg/kg oral prednisolone for 2 weeks. The topical corticosteroid was gradually tapered off over 2 weeks after resolution of the rejection episode, which was defined as complete clearance of keratic precipitates or anterior chamber reaction, or both. Graft rejection episodes recurring after the termination of CsA were treated with corticosteroids as usual. |
|
| Outcomes |
|
|
| Method and times of measuring the outcomes | The participants were followed up every week until complete resolution of the graft rejection episode and then every month for 3 months, every 2 months for 1 year, and every 6 months onwards. In the case of visual acuity reduction or eye redness, participants had access to the ophthalmologist; participants were examined between the follow‐up examinations as necessary. | |
| Funding source and statement of interest | Funded by Sina Daru Pharmacy Co, Tehran, Iran. Competing interests: Author Ahmad Karbasian is the executive manager of Sina Daru Pharmacy Co, from which 2% topical CsA and placebo were procurred. The other authors had no financial or propriety interest in any of the materials used in this study. |
|
| Notes | Date study conducted: not reported Trial registration number: NCT01028443 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomised, double‐blind clinical trial" was mentioned, but lack of detailed information. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not mentioned. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Both the participants and the ophthalmologist were masked. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Only one participant was excluded, because of intolerance to the medication given. |
| Selective reporting (reporting bias) | Unclear risk | Not mentioned. |
| Other bias | Unclear risk | Not mentioned. |
Reinhard 2001.
| Methods | Single‐centre Parallel‐group RCT, unclear how many eyes included | |
| Participants | Country: Germany Number of participants: 56 Average age (range): 55 years (18 to 87) Sex: 48% women Inclusion criteria: High‐risk keratoplasty, which was defined by "the presence of deep vascularization in three or four quadrants, a history of previous keratoplasty, position of the graft close to the limbus, transplantation of a highly immunogenic graft (limbokeratoplasty), severe atopic dermatitis or steroid response glaucoma". Exclusion criteria:
|
|
| Interventions | Intervention:
Comparator:
MMF was administered in a daily dose of 2 × 1 g; the CsA dose was adjusted according to blood trough levels, with a target of 120 to 150 ng/ml (monoclonal TDx, Abbott Exsym). After 6 months, immunosuppressive medication was tapered within 2 weeks. Additionally, all participants except those with steroid‐induced glaucoma received corticosteroids systemically (1 mg/kg body weight fluocortolone, tapered within 3 weeks postoperatively) and topically (5 drops prednisolone acetate 1% daily after epithelial consolidation, tapered within 5 months). |
|
| Outcomes |
|
|
| Method and times of measuring the outcomes | Postoperative visits were performed after 1, 3, 6, 9, 12, 24, and 36 months. Immune reactions were diagnosed by endothelial precipitates adhering to graft endothelium with (severe endothelial immune reactions) or without (mild endothelial immune reactions) stromal oedema or by the presence of non‐infectious stromal infiltration (stromal immune reactions). Endothelial cell loss was assessed only in participants with at least 3 postoperative endothelial cell density values. Participants with endothelial immune reactions were excluded from this calculation. The individual mean loss of endothelial cells per day and per square millimetre was derived from the postoperatively acquired endothelial values of each participant individually. This was done by calculating the slope of the regression line for each scatter plot of endothelial cell density values plotted against time. |
|
| Funding source and statement of interest | No information on funding source and declaration of interest. | |
| Notes | Date study conducted: not reported Trial registration number: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "The 56 patients were randomised to receive CsA (27 patients) or MMF (29 patients)", but no detailed information about randomisation procedure. |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not used. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Premature withdrawal from immunosuppressive prophylaxis occurred in 2 participants in the CsA group (1 case of severe gingival hyperplasia, 1 of hepatotoxicity), and in 2 participants in the MMF group (1 diagnosis of Hodgkin's lymphoma 1 month after keratoplasty, 1 case of dermatological problems in a participant with severe atopic dermatitis). |
| Selective reporting (reporting bias) | Unclear risk | Not mentioned. |
| Other bias | High risk | "Study recruitment was stopped prematurely due to a statistically significant result from the scheduled interim evaluation". Page 2064 |
Reinhard 2005.
| Methods | Multicentre/single‐centre: not reported Parallel‐group RCT, unclear how many eyes included |
|
| Participants | Country: Germany Number of participants: 40 Average age (range): 58 years in tacrolimus group and 70 years in steroid group (range not reported) Sex: 48% women Normal‐risk keratoplasty |
|
| Interventions | Intervention:
Comparator:
Participants were treated with topical tacrolimus 0.06% 3 times per day for 6 months postoperatively or 5 drops of prednisolone acetate 1% tapered within 6 months. All participants received 1 mg/kg bodyweight/day of systemic fluocortolone tapered within 3 weeks postoperatively. |
|
| Outcomes |
|
|
| Method and times of measuring the outcomes | Controls of the graft at the slit‐lamp were scheduled 7 weeks, 4, 12, and 18 months postoperatively and thereafter annually. Endothelial immune reactions were diagnosed via endothelial precipitates and stromal oedema, stromal immune reactions via subepithelial infiltrates. Postoperative visits: trial authors did not describe the visit time in detail and only stated that follow‐up times of tacrolimus group and steroid group were 5.8 to 14.8 and 9.1 to 13.3, respectively. | |
| Funding source and statement of interest | Funding source was not mentioned and no declaration of interest. | |
| Notes | Date study conducted: not reported Trial registration number: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "A randomised clinical pilot study" was mentioned in the article title, but there was no information about the randomisation procedure in the text. |
| Allocation concealment (selection bias) | Unclear risk | Not performed. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Not performed. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Premature withdrawal of the drug occurred in 8 participants due to local side effects in the tacrolimus group, and all the participants completed the study in accordance with the protocol. |
| Selective reporting (reporting bias) | Unclear risk | No information. |
| Other bias | Unclear risk | No information. |
Sinha 2010.
| Methods | Single‐centre Parallel‐group RCT, 1 eye per person included |
|
| Participants | Country: India Number of participants: 84 Average age (range): 46 years (21 to 87) Sex: 44% women High‐risk keratoplasty |
|
| Interventions | Intervention:
Comparator:
Additional treatment given to all participants: systemic fluocortolone 1 mg/kg bodyweight/ day tapered within 3 weeks postoperatively; acetazolamide 500 mg/day for 5 days postoperatively, |
|
| Outcomes |
|
|
| Method and times of measuring the outcomes | Subsequent follow‐up was done at 1, 3, 6 months, and 1 year postoperatively. At each follow‐up, participants were evaluated on parameters of best‐corrected visual acuity, slit‐lamp biomicroscopy for corneal and anterior segment evaluation, anterior chamber reaction (flare and cells), corneal thickness, and intraocular pressure. Rejection was defined as the occurrence of 1 of the following:
|
|
| Funding source and statement of interest | The study was funded by a financial grant from the Indian Council of Medical Research, New Delhi, India. The authors reported that they had no financial, proprietary or competing interests. |
|
| Notes | Date study conducted: January 2002 to December 2004 Trial registration number: ISRCTN52781697 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A computer‐generated sequence, block randomisation strategy was used. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The ocular pharmacologist was masked to the outcome variables, and the results assessor was masked. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No exclusion. |
| Selective reporting (reporting bias) | Unclear risk | No information. |
| Other bias | Unclear risk | No information. |
Zhang 2009.
| Methods | Multicentre (4 centres) Parallel‐group RCT, unclear how many eyes included |
|
| Participants | Country: China Number of participants: 240 Average age (range): 18 to 65 years Sex: F/M: 36/84 and 27/93, respectively High‐risk keratoplasty |
|
| Interventions | Intervention:
Comparator:
CsA given as eye drops 4 ˜ 6 times a day, 2 drops a time, total 6 months. In both groups, 1% dexamethasone eye drops 4 times a day, 1 ˜ 2 drops a time. 2 weeks later 3 times a day, 30 days later 2 times a day, 2 months later 1 time a day. |
|
| Outcomes | Primary outcomes:
Secondary outcomes:
|
|
| Method and times of measuring the outcomes | Follow‐up over 180 days. | |
| Funding source and statement of interest | The funding source was not mentioned, but two of the authors came from the Huabei Pharmaceutical Group New Drug Development LTD., where the experimental drug is made. The declaration of interest was not provided. |
|
| Notes | Date study conducted: not reported. We telephoned the first author, Professor Zhang, and known that the study was conducted between July, 2003 to August, 2004. Trial registration number: not registered |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table was mentioned, but procedure not described in detail. |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Placebo used and adequately masked. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Treatment not completed in 1 case in the CsA group (1/120) and 9 cases in the control group (9/120). |
| Selective reporting (reporting bias) | Unclear risk | No information. |
| Other bias | Unclear risk | No information. |
CsA: cyclosporine A MMF: mycophenolate mofetil RCT: randomised controlled trial SD: standard deviation
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| He 1999 | Claimed to be a RCT, but was identified in a telephone interview with the original author as a non‐RCT. |
| Liu 2007 | Claimed to be a RCT, but was identified in a telephone interview with the original author as a non‐RCT. |
| Lu 2009 | This study, which compared local and systemic administration of glucocorticoids, was not a RCT. |
| NCT00553735 | Trial assessed treatment of dry eye syndrome in people who had recently received a bone marrow transplant. |
| Reinhard 1999 | Participants with different conditions. |
| Wu 2001 | Claimed to be a RCT, but was identified in a telephone interview with the original author as a non‐RCT. |
| Xi 2003 | Claimed to be a RCT, but was identified in a telephone interview with the original author as a non‐RCT. |
| Ye 2004 | Claimed to be a RCT, but was identified in a telephone interview with the original author as a non‐RCT. |
| Zhao 2005 | Claimed to be a RCT, but was identified in a telephone interview with the original author as a non‐RCT. |
| Zhou 2008 | A quasi‐RCT, allocated participants by the number of birth date. |
RCT: randomised controlled trial
Characteristics of ongoing studies [ordered by study ID]
NCT00409656.
| Trial name or title | Prospective, randomised trial of basiliximab (Simulect) in the prophylaxis of high‐risk keratoplasty patients |
| Methods | Allocation: randomised Control: active control Endpoint classification: efficacy study Intervention model: parallel assignment Masking: open label Primary purpose: prevention |
| Participants | Inclusion criteria: high‐risk keratoplasty Exclusion criteria: normal‐risk keratoplasty Age minimum: N/A Age maximum: N/A Gender: both |
| Interventions | Basiliximab |
| Outcomes | Primary outcome: graft rejection Secondary outcome: clear graft survival |
| Starting date | December 2003 |
| Contact information | Thomas Reinhard, MD, Prof. (http://clinicaltrials.gov/show/NCT00409656) |
| Notes |
Differences between protocol and review
We have added methods for preparing a 'Summary of findings' table and GRADE assessment. This was not in the original protocol, which was published in 2008.
Contributions of authors
Conceiving the review: MA, XW Designing the review: TXW Co‐ordinating the review: XYC, TXW Designing electronic search strategies: TXW, Cochrane Eyes and Vision Group editorial team Undertaking searches: MA, ZYC Screening search results: MA, TXW Appraising quality of papers: MA, TXW Writing to authors of papers for additional information: TXW Data collection for the review: MA, TXW Data management for the review: MA Entering data into RevMan: MA, JE
Analysis of data: MA Interpretation of data: MA, XYC Providing a methodological perspective: TXW, JE
Providing a clinical perspective: XYC Providing a policy perspective: TXW Providing a consumer perspective: TXW Writing the review: MA, TXW, JE
Providing general advice on the review: XYC, TXW, JE
Performing previous work that was the foundation of the current study: TXW Guarantor for the review: MA, XYC, TXW
Sources of support
Internal sources
Chinese Cochrane Centre, West China Hospital of Sichuan University, China.
External sources
Chinese Medical Board of New York (CMB), USA.
Declarations of interest
None known.
New
References
References to studies included in this review
Birnbaum 2009 {published data only}
- Birnbaum F, Mayweg S, Reis A, Bohringer D, Seitz B, Engelmann K, et al. Mycophenolate mofetil (MMF) following penetrating high risk keratoplasty: long‐term results of a prospective, randomised, multicentre study. Eye 2009;23(11):2063‐70. [DOI] [PubMed] [Google Scholar]
- Reinhard T, Mayweg S, Sokolovska Y, Seitz B, Mittelviefhaus H, Engelmann K, et al. Systemic mycophenolate mofetil avoids immune reactions in penetrating high‐risk keratoplasty: preliminary results of an ongoing prospectively randomized multicentre study. Transplant International 2005;18(6):703‐8. [DOI] [PubMed] [Google Scholar]
Javadi 2010 {published data only}
- Javadi MA, Feizi S, Karbasian A, Rastegarpour A. Efficacy of topical ciclosporin A for treatment and prevention of graft rejection in corneal grafts with previous rejection episodes. British Journal of Ophthalmology 2010;94(11):1464‐7. [DOI] [PubMed] [Google Scholar]
- NCT01028443. Efficacy of topical cyclosporine A for treatment and prevention of graft rejection in corneal grafts with previous rejection episodes. clinicaltrials.gov/ct2/show/record/NCT01028443 (accessed 22 December 2014).
Reinhard 2001 {published data only}
- Reinhard T, Reis A, Bohringer D, Malinowski M, Voiculescu A, Heering P, et al. Systemic mycophenolate mofetil in comparison with systemic cyclosporin A in high‐risk keratoplasty patients: 3 years' results of a randomised prospective clinical trial. Graefe's Archive for Clinical and Experimental Ophthalmology 2001;239(5):367‐72. [DOI] [PubMed] [Google Scholar]
- Reinhard T, Reis A, Kutkuhn B, Voiculescu A, Sundmacher R. Mycophenolate mofetil after penetrating high risk keratoplasty. A pilot study [Mycophenolatmofetil nach perforierender hochrisiko‐keratoplastik. Eine pilot study]. Klinische Monatsblatter fur Augenheilkunde 1999;215(3):201‐2. [DOI] [PubMed] [Google Scholar]
Reinhard 2005 {published data only}
- Reinhard T, Mayweg S, Reis A, Sundmacher R. Topical FK506 as immunoprophylaxis after allogeneic penetrating normal‐risk keratoplasty: a randomised clinical pilot study. Transplant International 2005;18(2):193‐7. [DOI] [PubMed] [Google Scholar]
Sinha 2010 {published data only}
- Sinha R, Jhanji V, Verma K, Sharma N, Biswas NR, Vajpayee RB. Efficacy of topical cyclosporine A 2% in prevention of graft rejection in high‐risk keratoplasty: a randomised controlled trial. Graefe's Archive for Clinical and Experimental Ophthalmology 2010;248(8):1167‐72. [DOI] [PubMed] [Google Scholar]
Zhang 2009 {published data only}
- Zhang YQ, Chen JQ, Zhou LH, Shi WY, Wang LY, Wang YQ, et al. The randomized, double blindness, multi‐center clinical trail for CsA on rejection following PRK. Chinese Ophthalmic Research 2009;27(5):407‐11. [Google Scholar]
References to studies excluded from this review
He 1999 {published data only}
- He Y, Sun BJ, Li YC, Wang YQ, Zhao DQ, Li JC, et al. Suppression of corneal allograft rejection by topical cyclosporine A in high‐risk keratoplasty. China Academic Journal Electronic Publishing House 1999;21(5):420‐2. [Google Scholar]
Liu 2007 {published data only}
- Liu YM, Chen JQ. Prevention and treatment of topical FK506 for allograft rejection after therapeutic total keratoplasty. Chinese Journal of Practice Ophthalmology 2007;25(4):360‐5. [Google Scholar]
Lu 2009 {published data only}
- Lu J, Zhang BJ, Wang LN, Wang D, Hao JL. Comparative research of local versus systematic use glucocorticoids for rejection after keratoplasty. Chinese Laboratory Diagnosticology 2009;13(12):1781‐2. [Google Scholar]
NCT00553735 {published data only}
- NCT00553735. The prophylactic use of topical cyclosporine A 0.05% (Restasis) to prevent onset and progression of graft‐versus‐host disease‐related dry eye. clinicaltrials.gov/ct2/show/record/NCT00553735 (accessed 22 December 2014).
Reinhard 1999 {published data only}
- Reinhard T, Moller M, Sundmacher R. Penetrating keratoplasty in patients with atopic dermatitis with and without systemic cyclosporin A. Cornea 1999;18(6):645‐51. [DOI] [PubMed] [Google Scholar]
Wu 2001 {published data only}
- Wu GJ, Ding W, Yang FY, Feng JY. Administration of immunosuppressive drug and corticosteroid after penetrating keratoplasty in fungal corneal ulcer. Chinese Ophthalmic Research 2001;19(1):64‐6. [Google Scholar]
Xi 2003 {published data only}
- Xi XH, Qin B, Jiang DY. Cyclosporin A combined with dexamethasone in preventing and treating immune rejection after penetrating keratoplasty. Bulletin of Hunan Medical University 2003;28(6):627‐30. [PubMed] [Google Scholar]
- Xi XH, Qin B, Jiang DY, Tang LS, Cao NY. Effects of topical cyclosporin A and cyclosporin A combined dethmathason for long term on immune rejection of high risk corneal transplantation. Chinese Journal of Modern Medicine 2004;14(6):30‐3. [Google Scholar]
Ye 2004 {published data only}
- Ye CT, Lin YS, Tang XL, Diao HT. Suppression of corneal allograft rejection by topical FK506 and cyclosporine A in penetrating keratoplasty. Journal of Guangdong Pharmaceutical College 2004;14(6):43‐4. [Google Scholar]
Zhao 2005 {published data only}
- Zhao J, Xie LX, Shi WH, Li SW, Li W. Suppression of corneal allograft rejection by systemic cyclosporine A in high‐risk keratoplasty. Chinese Journal of Ophthalmology 2005;14(3):147‐50. [Google Scholar]
Zhou 2008 {published data only}
- Zhou Q, Bi YL, Wang HM, Wu SY, Cui HP. The effects of topical corticosteroid treatment on the chronic corneal allograft dysfunction after penetrating keratoplasty. Journal of Tongji University (Medical Science) 2008;29(5):115‐8. [Google Scholar]
References to ongoing studies
NCT00409656 {unpublished data only}
- NCT00409656. Prospective, randomized trial of basiliximab (Simulect) in the prophylaxis of high‐risk keratoplasty patients. clinicaltrials.gov/ct2/show/record/NCT00409656 (accessed 23 February 2015).
Additional references
Algros 2002
- Algros MP, Angonin R, Delbosc B, Cahn JY, Kantelip B. Danger of systemic cyclosporine for corneal graft. Cornea 2002;21(6):613‐4. [DOI] [PubMed] [Google Scholar]
Belin 1990
- Belin MW, Bouchard CS, Phillips TM. Update on topical cyclosporin A. Background, immunology, and pharmacology. Cornea 1990;9(3):184‐95. [PubMed] [Google Scholar]
Cosar 2003
- Cosar CB, Laibson PR, Cohen EJ, Rapuano CJ. Topical cyclosporine in pediatric keratoplasty. Eye and Contact Lens 2003;29(2):103‐7. [DOI] [PubMed] [Google Scholar]
Deeks 2011
- Deeks JJ, Higgins JPT, Altman DG (editors). Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Dobbins 2000
- Dobbins KR, Price FW Jr, Whitson WE. Trends in the indications for penetrating keratoplasty in the midwestern United States. Cornea 2000;19(6):813‐6. [DOI] [PubMed] [Google Scholar]
Glanville 2006
- Glanville JM, Lefebvre C, Miles JN, Camosso‐Stefinovic J. How to identify randomized controlled trials in MEDLINE: ten years on. Journal of the Medical Library Association 2006;94(2):130‐6. [PMC free article] [PubMed] [Google Scholar]
Guerra 2007
- Guerra G, Srinivas TR, Meier‐Kriesche HU. Calcineurin inhibitor‐free immunosuppression in kidney transplantation. Transplant International 2007;20(10):813‐27. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hill 1989
- Hill JC. The use of cyclosporine in high‐risk keratoplasty. American Journal of Ophthalmology 1989;107(5):506‐10. [DOI] [PubMed] [Google Scholar]
Hill 1991
- Hill JC, Maske R, Watson P. Corticosteroids in corneal graft rejection. Oral versus single pulse therapy. Ophthalmology 1991;98(3):329‐33. [DOI] [PubMed] [Google Scholar]
Hill 1994
- Hill JC. Systemic cyclosporine in high‐risk keratoplasty. Short versus long‐term therapy. Ophthalmology 1994;101(1):128‐33. [DOI] [PubMed] [Google Scholar]
Ing 1998
- Ing JJ, Ing HH, Nelson LR, Hodge DO, Bourne WM. Ten‐year postoperative results of penetrating keratoplasty. Ophthalmology 1998;105(10):1855‐65. [DOI] [PubMed] [Google Scholar]
Inoue 2000
- Inoue K, Amano S, Kimura C, Sato T, Fujita N, Kagaya F, et al. Long‐term effects of topical cyclosporine A treatment after penetrating keratoplasty. Japanese Journal of Ophthalmology 2000;44(3):302‐5. [DOI] [PubMed] [Google Scholar]
Jackson 2009
- Jackson P, Paquette L, Watiker V, Randolph L, Ramanathan R, Seri I. Intrauterine exposure to mycophenolate mofetil and multiple congenital anomalies in a newborn: possible teratogenic effect. American Journal of Medical Genetics Part A 2009;149(A)(6):1231‐6. [DOI] [PubMed] [Google Scholar]
Klieger‐Grossmann 2010
- Klieger‐Grossmann C, Chitayat D, Lavign S, Kao K, Garcia‐Bournissen F, Quinn D, et al. Prenatal exposure to mycophenolate mofetil: an updated estimate. Journal of Obstetrics and Gynaecology of Canadian 2010;32(8):794‐7. [DOI] [PubMed] [Google Scholar]
Land 2005
- Land W, Vincenti F. Toxicity‐sparing protocols using mycophenolate mofetil in renal transplantation. Transplantation 2005;80(2 Suppl):S221‐34. [DOI] [PubMed] [Google Scholar]
Liu 1997
- Liu E, Slomovic AR. Indications for penetrating keratoplasty in Canada, 1986‐1995. Cornea 1997;16(4):414‐9. [PubMed] [Google Scholar]
Maguire 1994
- Maguire MG, Stark WJ, Gottsch JD, Stulting RD, Sugar A, Fink NE, et al. Risk factors for corneal graft failure and rejection in the collaborative corneal transplantation studies. Collaborative Corneal Transplantation Studies Research Group. Ophthalomology 1994;101(9):1536‐47. [DOI] [PubMed] [Google Scholar]
Niederkorn 2003
- Niederkorn JY. Mechanisms of immune privilege in the eye and hair follicle. Journal of Investigative Dermatology. Symposium Proceedings 2003;8(2):168‐72. [DOI] [PubMed] [Google Scholar]
Perez 2013
- Perez VL, Saeed AM, Tan Y, Urbieta M, Cruz‐Guilloty F. The eye: A window to the soul of the immune system. Journal of Autoimmunity 2013;45:7‐14. [DOI] [PubMed] [Google Scholar]
Pillans 2006
- Pillans P. Immunosuppressants ‐ mechanisms of action and monitoring. Australian Prescriber 2006;29(4):99‐101. [Google Scholar]
Price 2006
- Price MO, Price FW. Efficacy of topical cyclosporine 0.05% for prevention of cornea transplant rejection episodes. Ophthalmology 2006;113(10):1785‐90. [DOI] [PubMed] [Google Scholar]
Ramsay 1997
- Ramsay AS, Lee WR, Mohammed A. Changing indications for penetrating keratoplasty in the west of Scotland from 1970 to 1995. Eye 1997;11(Pt 3):357‐60. [DOI] [PubMed] [Google Scholar]
Randleman 2006
- Randleman JB, Stulting RD. Prevention and treatment of corneal graft rejection: current practice patterns (2004). Cornea 2006;25(3):286‐90. [DOI] [PubMed] [Google Scholar]
Reis 1998a
- Reis A, Reinhard T, Sundmacher R, Braunstein S, Godehardt E. Mycophenolate mofetil and FK506: two novel immunosuppressants in murine corneal transplantation. Transplantation Proceedings 1998;30(8):4344‐7. [DOI] [PubMed] [Google Scholar]
Reis 1998b
- Reis A, Reinhard T, Sundmacher R, Braunstein S, Godehardt E. Comparative investigation of FK506 and cyclosporin A in murine corneal transplantation. Graefe's Archive for Clinical and Experimental Ophthalmology 1998;236(10):785‐9. [DOI] [PubMed] [Google Scholar]
Reis 1999
- Reis A, Reinhard T, Voiculescu A, Kutkuhn B, Godehardt E, Spelsberg H, et al. Mycophenolate mofetil versus cyclosporin A in high risk keratoplasty patients: a prospectively randomised clinical trial. British Journal of Ophthalmology 1999;83(11):1268‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Shepherd 1980
- Shepherd WF, Coster DJ, Fook TC, Rice NS, Jones BR. Effect of cyclosporin A on the survival of corneal grafts in rabbits. British Journal of Ophthalmology 1980;64(3):148‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
Siconolfi 1996
- Siconolfi L. Mycophenolate mofetil (CellCept): immunosuppression on the cutting edge. AACN Clinical Issues 1996;7(3):390‐402. [DOI] [PubMed] [Google Scholar]
Sloper 2001
- Sloper CM, Powell RJ, Dua HS. Tacrolimus (FK506) in the management of high‐risk corneal and limbal grafts. Ophthalmology 2001;108(10):1838‐44. [DOI] [PubMed] [Google Scholar]
Sterne 2011
- Sterne JAC, Egger M, Moher D (editors). Chapter 10: Addressing reporting biases. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Tabbara 2007
- Tabbara KF. Pharmacologic strategies in the prevention and treatment of corneal transplant rejection. International Ophthalmology 2007;28(3):223‐32. [DOI] [PubMed] [Google Scholar]
Taylor 2005
- Taylor AL, Watson CJ, Bradley JA. Immunosuppressive agents in solid organ transplantation: Mechanisms of action and therapeutic efficacy. Critical Reviews in Oncology/Hematology 2005;56(1):23‐46. [DOI] [PubMed] [Google Scholar]
Thompson 2003
- Thompson RW, Price MO, Bowers PJ, Price FW. Long‐term graft survival after penetrating keratoplasty. Ophthalmology 2003;110(7):1396‐402. [DOI] [PubMed] [Google Scholar]
Tierney 2005
- Tierney JF, Stewart LA. Investigating patient exclusion bias in meta‐analysis. International Journal of Epidemiology 2005;34(1):79‐87. [DOI] [PubMed] [Google Scholar]
Utine 2010
- Utine CA, Stern M, Akpek EK. Clinical review: topical ophthalmic use of cyclosporin A. Ocular Immunology and Inflammation 2010;18(5):352‐61. [DOI] [PubMed] [Google Scholar]
Williams 2006
- Williams KA, Esterman AJ, Bartlett C, Holland H, Hornsby NB, Coster DJ. How effective is penetrating corneal transplantation? Factors influencing long‐term outcome in multivariate analysis. Transplantation 2006;81(6):896‐901. [DOI] [PubMed] [Google Scholar]
Yamagami 2005
- Yamagami S, Hamrah P, Zhang Q, Liu Y, Huq S, Dana MR. Early ocular chemokine gene expression and leukocyte infiltration after high‐risk corneal transplantation. Molecular Vision 2005;11:632‐40. [PubMed] [Google Scholar]
References to other published versions of this review
Wei 2009
- Wei X, Chen Z, Wu T, Yinping D. Immunosuppressants for the prophylaxis of corneal graft rejection after penetrating keratoplasty. Cochrane Database of Systematic Reviews 2009, Issue 1. [DOI: 10.1002/14651858.CD007603] [DOI] [PMC free article] [PubMed] [Google Scholar]


