Abstract
Background
There has been a longstanding interest in the potential effect of vitamin D in preventing cardiac-metabolic diseases. However, there are divergent results regarding the impact of vitamin D supplementation (VDS) on managing cardiac-metabolic outcomes in the elderly population.
Material and method
We systematically searched electronic databases; Web of Science, PubMed, Scopus, EMBASE, Cochrane, and ProQuest. We included all trials that evaluated the effect of VDS on cardiac-metabolic risk factors in the elderly population, which were published until 30 September 2021. The effects of VDS on cardiac-metabolic outcomes were assessed using standardized mean difference (SMD). A random-effect model was used to pool the SMD and 95% confidence interval (CI).
Result
The literature search identified 4409 studies, of which 12 trials met inclusion criteria. Results of random effect meta-analysis indicated a significant reduction in total cholesterol (TC) (SMD: − 0.14 mg/dl; 95% CI: − 0.25, − 0.02) and triglyceride (TG) (SMD: − 0.45 mg/dl; 95% CI: − 0.86, − 0.04) with VDS compared to the placebo. The subgroup analyses revealed that the reduction of TG in patients with diabetes and vitamin D deficiency was significant. Furthermore, short-term intervention (≤ 6 months) induced a significantly lower level of TG and insulin in comparison to longer duration (> 6 months).
Conclusion
The study suggests that VDS could improve insulin concentration and dyslipidemia in the elderly population. The systematic review was registered in Alborz university of medical sciences with 2060-01-03-1397 number and the Ethics council IR.ABZUMS.REC.1397.207 number.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13098-022-00859-0.
Keywords: Vitamin D, Supplementation, Cardiac-metabolic, Elderly
Introduction
'Cardiac-metabolic' is a complex disorder characterized by dyslipidemia, hyperglycemia, visceral obesity, and hypertension [1]. The prevalence of cardiac-metabolic disorders has profoundly grown over the past decade [2, 3]. Individuals with cardiac-metabolic disorders are at a development risk for diabetes mellitus (DM) and cardiovascular disease [4]. In recent years, several studies have investigated the effect of essential nutrients on cardiac-metabolic disorders and their risk factor [5, 6]. As a global health problem, “vitamin D” deficiency is extremely prevalent in all age populations, especially in older adults (≥ 60 years old) [7]. Moreover, there has been growing data to prove the extra-skeletal role of vitamin D [8]. Evidence has been concerned with the relation between vitamin D deficiency and the development of DM, metabolic syndrome, cardiovascular disease, autoimmune diseases, and some cancers [8, 9].
A reverse association has been demonstrated between vitamin D levels and multiple pathological outcomes, including cardiovascular diseases [10, 11]. In this regard, some investigations have suggested that vitamin D supplementation (VDS) has therapeutic results on insulin resistance, serum triglycerides, and waist circumference in patients with metabolic syndrome and even in healthy adults [12–14]. In contrast, a few randomized clinical trials (RCTs) did not show any effectiveness in metabolic profile via VDS [15, 16]. In general, disagreement in the results of studies could be ascribed to differences in terms of methodology, supplementation dose, and populations' characteristics. Moreover, several meta-analyses have examined the impact of VDS on DM, dyslipidemia, body weight, and cardiovascular disease in adults and children and found conflicting results [17–24].
To the best of our knowledge, there is no comprehensive evidence outlining the effects of VDS on cardiac-metabolic outcomes in the elderly population. Given the critical assessment of the impact of vitamin D in older adults, we aimed to examine the overall effects of VDS on cardiac-metabolic outcomes in the elderly population.
Methods
Search strategy
In order to assess the relevant documents regarding the association of VDS and cardiac-metabolic outcomes in the elderly population, the biomedical electronic databases, including Scopus, Web of Science, PubMed, EMBASE, Cochrane, and also congress and conference papers as grey literature were searched for all related literature published up to 30 September 2021. Medical Subject Headings (MeSH) terms and Emtree pathways were also used in developing search terms.
The search protocol was developed based on two primary roots of "Vitamin D" and "Elderly". All related components of vitamin D including "25-hydroxyvitamin D", Dihydrotachysterol, "25(OH)D”, Calciol, Calciferol, Cholecalciferol, “25-Hydroxyergocalciferol”, “25-Hydroxycalciferol”, "Ergocalciferol", Calcitriol and elderly including "Older Adult”, Elder, or “Middle-Aged” were considered for searched queries (Additional file 1). There was no restriction for language of publication.
Inclusion and exclusion criteria
All eligible publications which considered the following criteria were included in this systematic review; (1) trials included elderly participants (participants more than 60 years were considered as elderly in the study) [25, 26], (2) trials administered vitamin D2 or vitamin D3 (cholecalciferol) as only intervention comparison placebo group, (3) trials reports cardiac-metabolic risk factors as outcomes (lipid profile, glycemic indices, anthropometric measures) (4) studies with clinical trial design, randomized or non-randomized. In addition, we included any dose, duration, and route of intervention. We exclude combination interventions, duplicate citations, narrative and systematic reviews, observational, cross-sectional, and case–control studies, book chapters, and conference papers.
Validity assessment and data extraction
After three assessment steps for titles, abstracts, and full texts, the full text of each selected record was retrieved for detailed analysis. Data were extracted using a data collection form recording citation, publication year, study year, country, type of study, population characteristics and methodological criteria (sample size, mean age, type of measure), and results and end-points. All proceedings from the comprehensive search to final data extraction were conducted by two independent expert investigators. Probable disagreements between two experts were resolved under the supervision of the primary investigator. The quality of study design, sampling strategy, and risk of bias assessments was conducted according to the Consolidated Standards of Reporting Trials (CONSORT) statement. The Kappa statistic for agreement between two research experts for quality assessment was 0.92. Baseline vitamin D deficiency was certain as serum vitamin D levels < 50 nmol/l, vitamin D insufficiency 50–75 nmol/l, and vitamin D sufficiency > 75 nmol/l [27].
Statistical analysis
For each cardiac-metabolic outcome, mean changes between baseline and follow-up for each group were compared in the meta-analysis calculation. The Chi-square based 'Q' test and 'I' square statistics were performed to examine the heterogeneity of the studies. The result of the 'Q' test was regarded to be statistically significant at P < 0.1. Standardized mean difference (SMD) was combined using a random-effect model due to heterogeneity between studies [28, 29]. SMD was calculated as [change in outcome/pooled standard deviation of outcome]. Results of the meta-analysis were presented schematically by forest plot. Effect of potential sources of heterogeneity (such as the risk of bias, duration of the intervention (≤ 6 months or > 6 months), intervention dose (< 2000 IU/d or ≥ 2000 IU/d), health condition (type of disease), and baseline vitamin D level (< 50 nmol/l or ≥ 50 nmol/l) were assessed using random-effect meta-regression analysis. Publication bias was estimated by the Egger’s and trim & fill method, which was considered statistically significant at 0.05. The analyses were conducted using STATA 11 software.
Ethical considerations
The present study was approved by the ethical committee of the Alborz University of Medical Science with IR.ABZUMS.REC.1397.207 number. Whenever we needed more information about a given study, we directly contacted the corresponding author of the cited publications.
Results
Study selection
We found 4409 records via a comprehensive search in the review. Out of this number, 2231 documents were identified as duplicates. Two reviewers exactly screened documents by title and abstract, and 2011 records were excluded from the process. Finally, from 168 full texts, 12 eligible trials [13, 30–40] were selected for the systematic review and meta-analysis (Fig. 1).
Fig. 1.
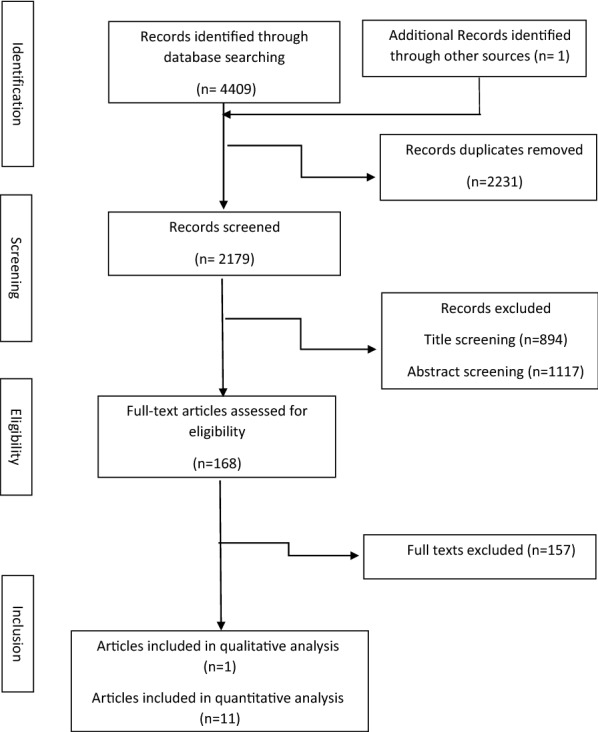
The review flowchart for selection studies
Study characteristics and qualitative synthesis
The included trial characteristics are represented in Table 1. The studies were published between 2010 and 2020. Ten of included studies have been designed as RCT [31–40] and ones as non-RCT [30], and Verrusio et al. [13] designed their study as a before-after experiment, so it was not analyzed in the meta-analysis. Six studies were conducted in the European countries [13, 30, 32, 33, 36, 38], two in Lebanon [39, 42], and four enrolled in other countries. The trials included 1328 participants; eleven studies recruited both genders, while one included only women [31]. Participants of five studies suffered from DM [30, 31, 34, 38, 40]. Three studies were conducted on apparently healthy participants [36–38]. Furthermore, other participants had a history of stroke [32], NAFLD [39], and overweight [35], and one study was conducted on postmenopausal women [33]. VDS dosage was varied between 400 IU/day to 4000 IU/day. In two studies, a single oral dose of 100,000 IU or 200,000 IU has been prescribed [30, 32]. The intervention period ranged from 2 months to one year. The included outcome variables were BMI, WC, HbA1c, insulin concentration, FBS, HOMA-IR, TG, Total cholesterol, HDL, LDL, and LDL/HDL Ratio (Table 1).
Table 1.
Characteristics of included studies
| Author, year | Country | Study population | Study design | Sex | Sample size | Dose of Vitamin D (IU) |
Mean age (SD) | Intervention duration | Baseline Vitamin D Level (nmol/L) Mean (SD) |
outcomes |
|---|---|---|---|---|---|---|---|---|---|---|
| Witham 2010 | UK | DM | RCT | M/F |
I1: 19 I2: 20 P: 22 |
Two single doses I1: 100,000 IU I2: 200,000 IU |
I1:65.3 (11.1) I2:63.3 (9.6) P: 66.7 ( 9.7) |
– |
I1:48 (21) I2:41(14) P: 45 ( 17) |
HOMA-IR HbA1c TC |
| Naharci 2012 | Turkey | IFG | Non-RCT | M/F |
I: 28 P: 23 |
Deficient: 300,000 IU/week Insufficient: 880 IU/day Normal: 400 IU/day |
I: 75.1 (7.3) P: 76.1( 5.4) |
5 months |
I: 47.5 (24.2) P: 55.8 (37.4) |
FBS HOMA-IR Insulin TC, TG, LDL, HDL LDL/HDL ratio BMI |
| Witham 2012 | UK | Patient with a history of stroke | RCT | M/F |
I: 30 P: 28 |
A single dose 100,000 IU |
I: 66.2 (13) P: 67.7 ( 6.9) |
- |
I: 38.7 (17.6) P: 38.7 (17.8) |
TC |
| Wood 2012 | UK | Postmenopausal women | RCT | F |
I1:102 I2:101 P: 102 |
I1: 400 IU/day I2: 1000 IU/day |
I1: 63.5(1.9) I2: 64.1 (2.3) P: 63.9 (2.3) |
12 months |
I1:32.7(12.9) I2:32.4(13.8) P: 36.1 (17.1) |
FBS HOMA-IR Insulin TC, TG, LDL, HDL |
| Kim 2014 | Korea | DM | RCT | M/F |
I: 11 P: 13 |
1200 IU /day |
I: 73.2 (2.0) P: 70 (1.3) |
12 weeks |
I: 26.1(4.4) P: 29.1 (6.9) |
FBS HOMA-IR Insulin TC, TG, LDL, HDL BMI |
| El-Hajj Fuleihan 2017 | Qatar | Overweight | RCT | M/F |
High dose(I): 110 Low dose(P): 112 |
High dose(I): 3750 IU/day Low dose(P): 600 IU/day |
T: 71 (4) | 12 months |
I: 52.1(17.4) P: 49.9(20.4) |
FBS HOMA-IR HbA1c, Insulin TC, TG, LDL, HDL |
| Walter Verrusio, 2017 | Italy | Healthy | Before and After | M/F | 200 | 4000 IU/day | T: 65.9 (9.8) | 6 months | – | – |
| Prusik 2018 | Poland | Healthy | RCT | M/F |
I: 48 P: 31 |
4000 IU/day | T: 68.4 ( 5.0) | 12 weeks |
I: 45.1(22.7) P: 60.4(30.2) |
TC, TG, LDL, HDL LDL/HDL ratio BMI |
| El Hajj 2019 | Lebanon | Healthy | RCT | M/F |
I: 60 P: 55 |
10,000 IU/week |
I: 73.05 (1.95) P:73.56 (2.14) |
6 months |
I: 25.2 (6.9) P:26.2 (2.14) |
BMI WC |
| Wenclewska 2019 | Poland | Healthy & DM | RCT | M/F |
I: 48 P: 44 |
2000 IU / day |
I: 63.43(1.5) P: 69.78 (2.1) |
3 months |
I: 18.2 (3.2) P:18.0(2.1) |
FBS HOMA-IR HbA1c TC, TG, LDL, HDL LDL/HDL ratio BMI |
| Hoseini 2020 | Iran | NAFLD | RCT | M/F |
I: 10 P: 10 |
50,000 IU/week |
I: 61.3 (1.4) P: 62 (1.8) |
8 weeks |
I: 22.9 (4.3) P: 24.0 (4.3) |
FBS HOMA-IR Insulin TC, TG, LDL, HDL |
| El Hajj 2020 | Lebanon | DM | RCT | M/F |
I: 45 P: 43 |
30,000 IU/week |
I: 66.9 (4.1) P: 65.7 (4.5) |
6 months |
I:14.8 (4.5) P:15.0 (4.2) |
FBS HOMA-IR HbA1c TC, TG, LDL, HDL BMI, WC |
RCT Randomized controlled trial, I Intervention, P Placebo, SD standard deviation, M male, F female, DM Diabetes mellitus, IFG Impaired fasting glucose, NAFLD Non- alcoholic fatty liver disease, IU International unit, HOMA-IR Homeostatic Model Assessment of Insulin Resistance, HbA1c Hemoglobin A1c, FBS Fasting blood sugar, TC Total cholesterol, TG Triglyceride, HDL high-density lipoprotein, LDL low-density lipoprotein, BMI Body mass index, WC Waist circumference
Quantitative synthesis
We pooled the SMD of cardiac-metabolic outcomes, which were reported through 2 or more studies, using the random-effect meta-analysis procedure owing to high heterogeneity among the included studies. The results of the (I2) test and estimated P-value are displayed in Table 2.
Table 2.
Meta-analysis of the effect of vitamin D supplementation on cardiac-metabolic outcomes in elderly population
| Variables | Number of study | Pooled SMD ( 95% CI) | Model | Heterogeneity assessment | ||
|---|---|---|---|---|---|---|
| I2 | Q test | P-value | ||||
| HbA1c | 4 | 0.06(− 0.11, 0.24) | Random | 0.0 | 0.57 | 0.96 |
| FBS (mg/dl) | 7 | 0.01(− 0.2, 0.21) | Random | 52.5 | 14.75 | 0.04 |
| HOMA-IR | 8 | 0.0(− 0.14, 0.13) | Random | 6 | 9.57 | 0.38 |
| Insulin (μU/ml) | 5 | − 0.11(− 0.34, 0.14) | Random | 54.4 | 10.97 | 0.05 |
| TG (mg/dl) | 8 | − 0.45(− 0.86, − 0.04) | Random | 88.5 | 69.46 | 0.0 |
| TC (mg/dl) | 10 | − 0.14(− 0.25, − 0.02) | Random | 0.0 | 10.49 | 0.48 |
| HDL (mg/dl) | 8 | 0.34(− 0.07, 0.74) | Random | 88.6 | 69.91 | 0.0 |
| LDL (mg/dl) | 8 | − 0.17(− 0.36, 0.02) | Random | 47.9 | 15.37 | 0.05 |
| LDL/HDL Ratio | 3 | − 0.08(− 0.35, 0.19) | Random | 0.0 | 0.14 | 0.93 |
| BMI (kg/m2) | 6 | 0.18(− 0.3,0.67) | Random | 83.7 | 30.72 | 0.0 |
| WC(cm) | 2 | − 0.24(− 0.98, 0.24) | Random | 85.3 | 6.80 | 0.0 |
The effect on lipid profile
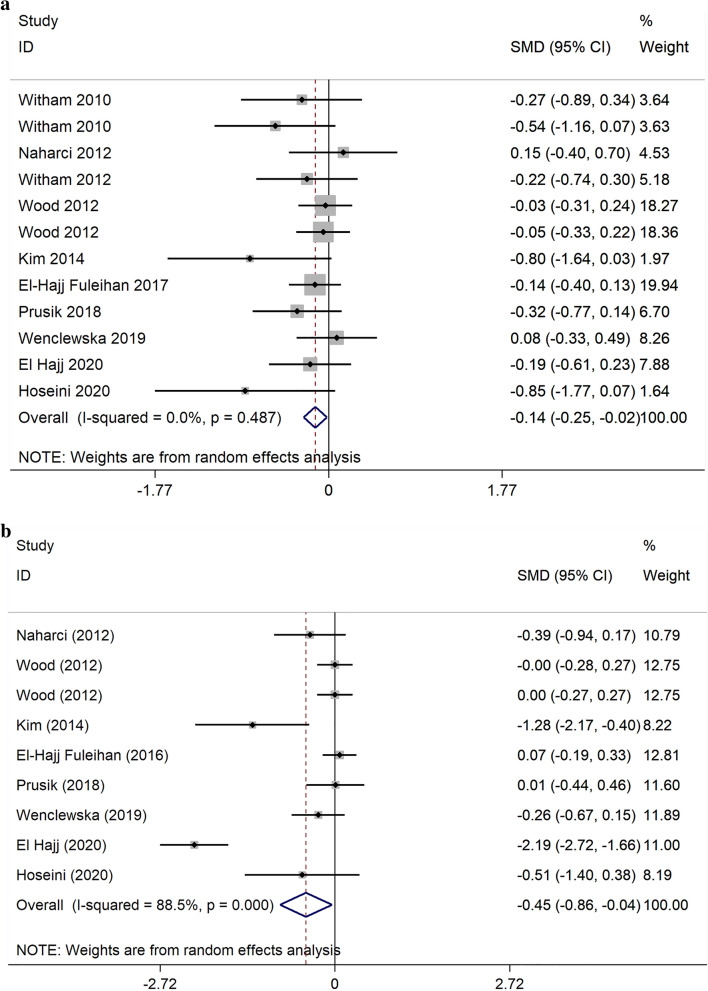
Ten trials [30–36, 38–40] reported the VDS effects on TC. Pooling the SMD revealed significant reduction in TC following intervention compared to placebo (SMD: − 0.14 mg/dl; 95% CI: − 0.25, − 0.02), without any significant heterogeneity (I2 = 0; P = 0.48) (Fig. 2). Also, changes in TG was assessed in 8 studies. Compared with placebo, the difference in TG decrease was significant, (SMD: − 0.45 mg/dl; 95%CI: − 0.86, − 0.04). The heterogeneity of the analysis was high between studies (I2 = 88.5%; P = 0.00) (Fig. 2). The pooled SMD for LDL (SMD: − 0.45 mg/dl; 95%CI: − 0.88, − 0.04), HDL (SMD: 0.34 mg/dl; 95%CI: − 0.07, 0.74) and LDL/HDL Ratio (SMD: − 0.08 mg/dl; 95%CI: − 0.35, 0.19) showed a non-significant change in the VDS group in comparison with the placebo (Additional file 2.
Fig. 2.
Forest plot of the effect of vitamin D supplementation on a total cholesterol, b triglyceride
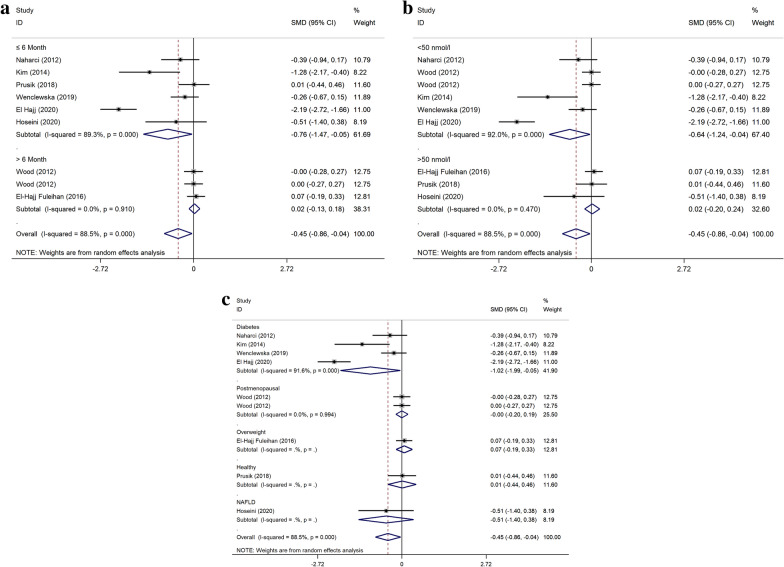
The subgroup analysis finding as regards the risk of bias of selected articles, baseline vitamin D, diseases, dose, and duration of intervention revealed a significant effect of VDS in the reduction of TG concentrations in the population with vitamin D deficiency, DM, and study duration ≤ 6 months subgroup (Fig. 3). The subgroup analysis did not statistically meaningful effect concerning other lipid profile outcomes (data was not shown).
Fig. 3.
Forest plot of the effect of vitamin D supplementation on triglyceride (mg/dL) stratified by intervention duration (a) stratified by baseline vitamin D levels (b) stratified by disease subtype (c)
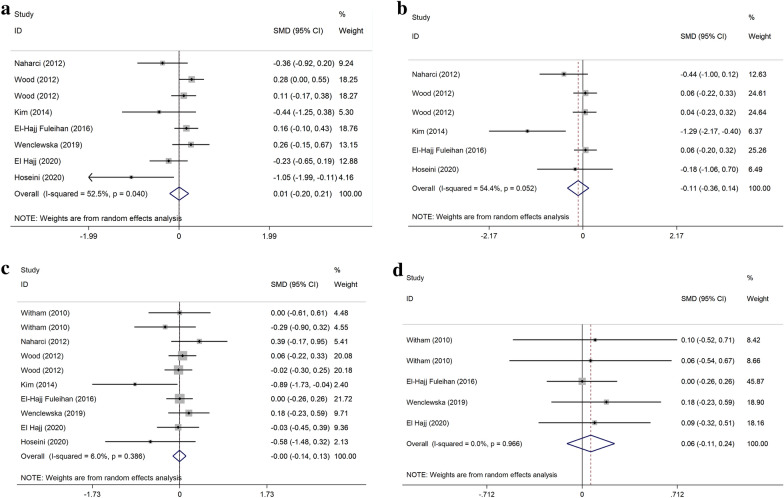
The effect on glycemic indices
The pooled SMD of five studies [31, 33–35, 39] indicated a decreased in insulin concentration in vitamin D group, however it was not statistically significant (SMD = − 0.11 μU/ml; 95% CI: − 0.34, 0.14), with moderate heterogeneity (I2 = 54.4% P = 0.05). Additionally, Pooled the effects of VDS on FBS, HOMA-IR, and HbA1c compared to the placebo was (SMD: 0.01 mg/dl; 95% CI: − 0.2, 0.21), (SMD: 0.0;95% CI: − 0.14, 0.13), and (SMD:0.06;95% CI: − 0.11, 0.24) respectively (Fig. 4). The results of subgroup analysis were demonstrated a significant effect in insulin concentration in study duration ≤ 6 months (SMD: − 0.6 μU/ml; 95% CI: − 1.18, − 0.02), (I2 = 42.0% P = 0.17) (Additional file 4). However, FBS levels in study duration > 6 months significantly increased (SMD: 0.18 mg/dl; 95% CI: 0.02, 0.34), (I2 = 0.0% P = 0.67) (Additional file 3).
Fig. 4.
Forest plot of the effect of vitamin D supplementation on fasting blood sager (a), insulin concentration (b), Homeostatic Model Assessment of Insulin Resistance (c), Hemoglobin A1c (d)
The effect on anthropometric measures
Six studies measured the effect of VDS on BMI [30, 34, 36–38, 40]. Overall, the meta-analysis revealed no difference in BMI between the study groups (SMD: 0.18 kg/m2; 95% CI: − 0.30, 0.67); results showed high heterogeneity (I2 = 83.7% P = 0.00) (Additional file 4). Furthermore, VDS did not change WC compared with placebo (SMD: − 0.24 Cm; 95% CI: − 0.98, 0.49), (I2 = 85.3% P = 0.00) (Additional file 4). The subgroup analyzed based on the risk of bias, diseases, and vitamin D dose didn't show any notable changes in the overall SMD of BMI (P > 0.05) (data was not shown).
Risk of bias assessment
The risk of bias assessments of the selected RCTs is shown in Table 3. Two studies had CONSORT score equal to 33, which is interpreted as high quality. Six trials were labeled as medium quality according to CONSORT scores (range: 25–30). The score of the three studies was lower than 25 and was classified as low quality. Random allocation and concealment mechanisms and blinding details were the main sources of risk of bias in the included trials.
Table 3.
Risk of bias assessment of included studies according to the CONSORT Checklist
| Item | Cynthia El Hajj, 2019 |
Cynthia El Hajj, 2020 |
Ghada El-Hajj Fuleihan, 2017 | Zahra Hoseini, 2020 | Ilkin Naharci, 2012 | Kim, Hyoung-Jun, 2014 | Krzysztof Prusik, 2018 |
Sylwia Wenclewska, 2019 | M. D. Witham, 2010 |
M.D. Witham, 2012 |
Adrian D. Wood, 2012 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1a | Yes | Yes | Yes | Yes | No | No | Yes | Yes | Yes | Yes | Yes |
| 1b | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 2a | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes |
| 2b | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | No |
| 3a | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 3b | No | No | No | No | No | No | No | No | No | No | No |
| 4a | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 4b | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 5 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 6a | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 6b | No | No | No | No | No | No | No | No | Yes | No | No |
| 7a | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 7b | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 8a | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes |
| 8b | No | No | No | No | No | Yes | Yes | Yes | No | Yes | Yes |
| 9 | Yes | Yes | No | No | No | Yes | Yes | Yes | Yes | Yes | Yes |
| 10 | Yes | Yes | No | No | No | Yes | No | No | No | Yes | Yes |
| 11a | Yes | Yes | No | No | No | No | No | No | No | Yes | Yes |
| 11b | No | No | No | No | No | No | No | No | No | Yes | Yes |
| 12a | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 12b | Yes | No | No | No | No | No | No | Yes | No | Yes | Yes |
| 13a | Yes | Yes | Yes | Yes | No | No | No | No | Yes | Yes | Yes |
| 13b | Yes | Yes | Yes | Yes | No | No | No | No | Yes | Yes | Yes |
| 14a | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 14b | No | No | No | No | No | No | No | No | No | No | Yes |
| 15 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 16 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 17a | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 17b | Yes | Yes | No | Yes | No | No | No | Yes | No | Yes | Yes |
| 18 | No | No | Yes | No | Yes | No | No | No | Yes | Yes | No |
| 19 | No | No | Yes | No | Yes | Yes | Yes | Yes | Yes | No | Yes |
| 20 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 21 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 22 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 23 | Yes | Yes | Yes | Yes | No | No | Yes | Yes | Yes | Yes | Yes |
| 24 | Yes | Yes | No | Yes | No | No | No | No | Yes | Yes | Yes |
| 25 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Total | 30 | 29 | 26 | 23 | 21 | 23 | 25 | 26 | 29 | 33 | 33 |
Publication bias and sensitivity analysis
Publication bias was performed by Egger's test. According of Egger’s test results the publication bias was seen by VDS on TC (coefficient = − 1.69, standard error = 0.59, P = 0.018, 95% CI = − 3.02, − 0.36) and FBS (coefficient = − 3.02, standard error = 0.77, P = 0.008, 95% CI = − 4.91, − 1.14). Because of the evidence of publication bias, "trim-and-fill" analysis was conducted to estimate potentially missing trials. The result of "trim-and-fill" presented no missing trials, and the publication bias has an ignorable effect on pooled SMD of TG and FBS. There was no evidence of publication bias on the impact of vitamin D with other studies' outcomes (Egger's P > 0.05). The results of the sensitivity analyses, based on the one-out remove method, showed that there was no substantial difference in the effect sizes of any of the pooled results (Data was not shown).
Discussion
The present meta-analysis finding revealed that VDS compared with placebo improved TC and TG management in the elderly. In contrast, we observed no significant beneficial effects of VDS in overall or subgroup analyses on LDL, HDL, FBS, HbA1c, HOMA-IR, BMI, and WC. Nevertheless, characteristics of the selected trials such as baseline vitamin D, duration of supplementation, and health condition of participants partly influenced the impacts that emerged in some overall findings.
Given the existence of much evidence about the effectiveness of vitamin D in glucose metabolism, some meta-analyses similar to our overall pooled results found that VDS may not improve glycemic indices[23, 41]. While, others [22, 42, 43] proposed that vitamin D could be a useful adjuvant therapy in controlling glycemic parameters in patients with DM or impaired glucose tolerance because it reduces insulin resistance and improves the function of beta cells [44]. Most systematic reviews have evaluated the effects of vitamin D on glycemic parameters in diabetics or adults with impaired glucose tolerance. Our selection criteria were different from the meta-analysis, and we included the elderly population (more than 60 years) with any type of disease. Despite that, the subgroup analysis showed a decrease in glycemic parameters (i.e., insulin concentration, FBS, and HOMA-IR) in DM subgroups; but, because of the few eligible trials, it is not statistically significant.
Further, in terms of the intervention duration, present findings demonstrated that intervention duration ≤ six months significantly improved insulin concentration; however, long-term duration (> 6 months) was associated with increased FBS levels. In line with our findings, some meta-analyses displayed improvement in glycemic control in short-term interventions [22, 41, 45]. Contrary to the results, some studies found that longer intervention durations were associated with more decrease in glycemic parameters [20, 46, 47]. It seems that the effect of the duration of VDS on glycemic control is controversial. This ambiguity may be attributed to the wide diversity between the study's population, health condition, dose, frequency, and baseline vitamin D level.
Concerning lipid profile, our findings agree with the results of most previous meta-analyses revealed that vitamin D could be a valuable treatment to decrease TC and TG. In addition, a significant reduction was shown in TG in the short-term intervention (≤ 6 months) studies, patients with DM and low levels of baseline vitamin D. Dibaba et al. (2019), in a comprehensive meta-analysis found administration of vitamin D diminish TC, TG, and LDL cholesterol, while the improvements were more noticeable in patients with vitamin D deficiency [18]. Another meta-analysis reported that treatment with vitamin D has a beneficial impact on TC; lowering LDL in diabetics and patients with insufficient vitamin D and shorter duration was significant. However, there was no positive effect on TG and HDL cholesterol [48]. In Mirhosseini et al. meta-analysis vitamin D administration improved cardiac-metabolic parameters such as lipid profile. Also, the improvement effect of vitamin D was noticeable in trials with less than six months of intervention and patients with baseline serum vitamin D levels < 50 nmol/l [22]. Wang et al. provided that vitamin D changed LDL cholesterol but not TG, TC, and HDL cholesterol. As well as, the effect on LDL-C was more evident in the short-term intervention duration [49]. However, a recent meta-analysis of 4 RCTs that evaluate the impact of vitamin D on lipid profile in adults with metabolic syndrome has shown no significant effect on lipid profile following supplementation [17]. In the present study, probably due to insufficient studies, no statistically meaningful change was seen in LDL and HDL cholesterol.
It is considered that an inverse association was seen between serum vitamin D levels and serum lipid profile [10, 50]. Several possible reasons may explain the role of vitamin D in modifying lipid profiles. Vitamin D might increase lipoprotein lipase activity and mRNA in adipocytes and lower serum TG levels [51, 52]. Also, it decreases the absorption of fatty acid via improving calcium absorption, and the formation of insoluble calcium-fatty acid complexes in the bowel leading to a diminution cholesterol concentration [53].
There were masses of evidence outlining the emphasis of vitamin D on human health and diseases [54, 55]. Many studies have revealed that impaired vitamin D levels may result in multiple pathological outcomes, including obesity, hypertension, cardiovascular diseases, and DM [56, 57]. Studies have demonstrated that older papulation are highly at risk of severe vitamin D deficiency [58]. The effectiveness of vitamin D in preventing chronic diseases, including metabolic syndrome and cardiovascular diseases, has received extra attention in recent years [54, 59]. Several studies suggested that vitamin D deficiency or insufficiency increases the risk of all-cause mortality in elderly participants, even before the onset of DM and cardiovascular diseases [60]. The result of a meta-analysis demonstrated that serum vitamin D levels of 75 to 87.5 nmol/L (or 30 to 35 ng/mL) are associated with the lowest mortality rate [61]. Taking into expanding the elderly population in the world, the importance of chronic disease and its complications in the population increases. Therefore, as a cheap and safe treatment, vitamin D might prevent morbidities and fracture events in the elderly [62]. As mentioned above, in our study, vitamin D administration has a therapeutic effect on some cardiac-metabolic parameters, though no beneficial effect was observed on BMI or WC. The inverse relationship between BMI and vitamin D status has been provided in numerous pieces of evidence [13, 63]. However, the vitamin D effects on anthropometric measures are controversial. A recent meta-analysis indicates no significant impact on the BMI and WC of healthy adults [64]. With regard to that, a few RCTs investigated vitamin D impacts on anthropometric measures in the elderly.
This is the first systematic review examining the VDS effects on cardiac-metabolic outcomes in the elderly population (≥ 60 years) to the best of our knowledge. We conducted a comprehensive literature search that included all possible related eligible studies. Also, Subgroup and sensitivity analysis was executed to mitigate the study's heterogeneity. Finally, there was no evidence of significant publication bias in most of the study's outcomes, except in TC and FBS. After using Trim and fill method, the overall effect size of vitamin D on TG remained unchanged. The study has some limitations; First, we found only 11 eligible studies in this meta-analysis, clearly underlining the lack of adequate investigation of the elderly population in this issue. Second, even after performance subgroup analysis, we could not diminish potential heterogeneity in some outcomes such as TG. The third limitation is that data on WC and BMI was available in few studies, thus precluding evaluation of the role of vitamin D on anthropometric measures.
Conclusion
This study suggests that VDS may improve TC, TG, and insulin concentration in the elderly, especially in short-term intervention duration and in patients with vitamin D deficiency and diabetes. Although the number of studies on this issue is minimal, nonetheless, the finding of this study will enhance our understanding of the importance of vitamin D in the elderly population as well as potentially provide the best evidence-based practical interventions to improve vitamin D-related cardiac-metabolic diseases in the elderly people. Due to, the high heterogeneity between included studies the study results must be interpreted with caution, and large and well-designed RCTs are necessary to validate the review findings.
Supplementary Information
Additional file 2. Forest plot of the effect of vitamin D supplementation on high-density lipoprotein; HDL (a) low-density lipoprotein; LDL (b) LDL/HDL ratio (c)
Additional file3. Forest plot of the effect of vitamin D supplementation on insulin concentration stratified by intervention duration (a) Forest plot of the effect of vitamin D supplementation on fasting blood sager stratified by intervention duration (b).
Additional file 4. Forest plot of the effect of vitamin D supplementation on body mass index (BMI) (a) Waist circumference (b).
Acknowledgements
This study was funded by Alborz University of Medical Sciences. The authors appreciate Emam Ali hospital's Clinical Research Development Unit of Alborz University of Medical Sciences for their comprehensive cooperation.
Abbreviations
- BMI
Body mass index
- CI
Confidence interval
- CONSORT
Consolidated Standards of Reporting Trials
- DM
Diabetes mellitus
- FBS
Fasting blood sugar
- HbA1c
Hemoglobin A1c
- HDL
High-density lipoprotein
- HOMA-IR
Homeostatic Model Assessment of Insulin Resistance
- LDL
Low-density lipoprotein
- MESH
Medical Subject Headings
- RCT
Randomized clinical trial
- SMD
Standardized mean difference
- TC
Total cholesterol
- TG
Triglyceride
- VDS
Vitamin D supplementation
- WC
Waist circumference
Author contributions
The authors confirm contribution to the paper as follows: study conception and design: MQ and MZ; contributed to the acquisition of the data: SHD, GA, YM; participated in its design, coordination, and statistical analysis: MKH, KP, and AM; performed the extra analyses; MKH, EKN, and drafted the manuscript: MKH, HE. All authors read and approved the final manuscript.
Funding
None.
Availability of data and materials
The datasets generated or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
The authors give their consent for the publication of details within the text (tables, figures, and supplementary files) to be published in the Journal.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Mostafa Qorbani and Maryam Zarei are Equally contributed as first authors
Contributor Information
Mostafa Qorbani, Email: mqorbani1379@yahoo.com.
Yousef Moradi, Email: Yousefmoradi211@yahoo.com.
Geeta Appannah, Email: geeta@upm.edu.my.
Shirin Djalainia, Email: shdjalalinia@gmail.com.
Kumars Pourrostami, Email: dr.porrostami1975@gmail.com.
Armita Mahdavi-Gorabi, Email: armitamahdavi61@gmail.com.
Ebrahim Khalil Naderali, Email: naderae@hope.ac.uk.
Maryam Khazdouz, Email: Maryam.khazdouz@hotmail.com.
References
- 1.Detection NCEPEPo, Adults ToHBCi. Third report of the National Cholesterol Education Program (NCEP) Expert Panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III): The Program; 2002. 10.1007/s40200-020-00673-3 [PubMed]
- 2.Mohammadi Y, Parsaeian M, Farzadfar F, Kasaeian A, Mehdipour P, Sheidaei A, et al. Levels and trends of child and adult mortality rates in the Islamic Republic of Iran, 1990–2013; protocol of the NASBOD study. Arch Iran Med. 2014;17(3):176–181. [PubMed] [Google Scholar]
- 3.Djalalinia S, Khosravi M, Hasani M, Moghaddam SS, Atoofi MK, Mahdavi-Gorabi A, et al. Effects of selenium supplementation on cardiometabolic risk factors, inflammatory, and antioxidant markers: a systematic review and meta-analysis protocol. Int J Prev Med. 2019 doi: 10.4103/ijpvm.IJPVM_509_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fisher M. Cardiometabolic disease: the new challenge? Wiley Online Library; 2006. [Google Scholar]
- 5.Gorabi AM, Hasani M, Djalalinia S, Zarei M, Ejtahed H, Abdar ME, et al. Effect of selenium supplementation on glycemic indices: a meta-analysis of randomized controlled trials. J Diabetes Metab Disord. 2019;18(2):349–362. doi: 10.1007/s40200-019-00419-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hasani M, Mansour A, Asayesh H, Djalalinia S, Gorabi AM, Ochi F, et al. Effect of glutamine supplementation on cardiometabolic risk factors and inflammatory markers: a systematic review and meta-analysis. BMC Cardiovasc Disord. 2021;21(1):1–21. doi: 10.1186/s12872-021-01986-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Holick M, Matsuoka L, Wortsman J. Age, vitamin D, and solar ultraviolet. Lancet (British edition) 1989;2(8671):1104–1105. doi: 10.1016/S0140-6736(89)91124-0. [DOI] [PubMed] [Google Scholar]
- 8.Bouillon R. Extra-skeletal effects of vitamin D. Vitam D Clin Med. 2018;50:72–88. doi: 10.1159/000486072. [DOI] [PubMed] [Google Scholar]
- 9.Pittas AG, Lau J, Hu FB, Dawson-Hughes B. The role of vitamin D and calcium in type 2 diabetes. A systematic review and meta-analysis. J Clin Endocrinol Metab. 2007;92(6):2017–2029. doi: 10.1210/jc.2007-0298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hyppönen E, Boucher BJ, Berry DJ, Power C. 25-hydroxyvitamin D, IGF-1, and metabolic syndrome at 45 years of age: a cross-sectional study in the 1958 British Birth Cohort. Diabetes. 2008;57(2):298–305. doi: 10.2337/db07-1122. [DOI] [PubMed] [Google Scholar]
- 11.Barbalho SM, Tofano RJ, de Campos AL, Rodrigues AS, Quesada K, Bechara MD, et al. Association between vitamin D status and metabolic syndrome risk factors. Diabetes Metab Syndr. 2018;12(4):501–507. doi: 10.1016/j.dsx.2018.03.011. [DOI] [PubMed] [Google Scholar]
- 12.Roosta S, Kharadmand M, Teymoori F, Birjandi M, Adine A, Falahi E. Effect of vitamin D supplementation on anthropometric indices among overweight and obese women: a double blind randomized controlled clinical trial. Diabetes Metab Syndr. 2018;12(4):537–541. doi: 10.1016/j.dsx.2018.03.022. [DOI] [PubMed] [Google Scholar]
- 13.Verrusio W, Andreozzi P, Renzi A, Musumeci M, Gueli N, Cacciafesta M. Association between serum vitamin D and metabolic syndrome in middle-aged and older adults and role of supplementation therapy with vitamin D. Annali dell'Istituto superiore di sanita. 2017;53(1):54–59. doi: 10.4415/ANN_17_01_11. [DOI] [PubMed] [Google Scholar]
- 14.Salekzamani S, Bavil AS, Mehralizadeh H, Jafarabadi MA, Ghezel A, Gargari BP. The effects of vitamin D supplementation on proatherogenic inflammatory markers and carotid intima media thickness in subjects with metabolic syndrome: a randomized double-blind placebo-controlled clinical trial. Endocrine. 2017;57(1):51–59. doi: 10.1007/s12020-017-1317-2. [DOI] [PubMed] [Google Scholar]
- 15.Kampmann U, Mosekilde L, Juhl C, Moller N, Christensen B, Rejnmark L, et al. Effects of 12 weeks high dose vitamin D3 treatment on insulin sensitivity, beta cell function, and metabolic markers in patients with type 2 diabetes and vitamin D insufficiency–a double-blind, randomized, placebo-controlled trial. Metabo Clin Exp. 2014;63(9):1115–1124. doi: 10.1016/j.metabol.2014.06.008. [DOI] [PubMed] [Google Scholar]
- 16.Angellotti E, D'Alessio D, Dawson-Hughes B, Chu Y, Nelson J, Hu P, et al. Effect of vitamin D supplementation on cardiovascular risk in type 2 diabetes. Clin Nutr (Edinburgh, Scotland) 2019;38(5):2449–2453. doi: 10.1016/j.clnu.2018.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.AlAnouti F, Abboud M, Papandreou D, Mahboub N, Haidar S, Rizk R. Effects of vitamin d supplementation on lipid profile in adults with the metabolic syndrome: a systematic review and meta-analysis of randomized controlled trials. Nutrients. 2020;12(11):3352. doi: 10.3390/nu12113352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dibaba DT. Effect of vitamin D supplementation on serum lipid profiles: a systematic review and meta-analysis. Nutr Rev. 2019;77(12):890–902. doi: 10.1093/nutrit/nuz037. [DOI] [PubMed] [Google Scholar]
- 19.Hauger H, Laursen RP, Ritz C, Mølgaard C, Lind MV, Damsgaard CT. Effects of vitamin D supplementation on cardiometabolic outcomes in children and adolescents: a systematic review and meta-analysis of randomized controlled trials. Eur J Nutr. 2020;59(3):873–884. doi: 10.1007/s00394-019-02150-x. [DOI] [PubMed] [Google Scholar]
- 20.Lee CJ, Iyer G, Liu Y, Kalyani RR, Ligon CB, Varma S, et al. The effect of vitamin D supplementation on glucose metabolism in type 2 diabetes mellitus: a systematic review and meta-analysis of intervention studies. J Diabetes Complicat. 2017;31(7):1115–1126. doi: 10.1016/j.jdiacomp.2017.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li X, Liu Y, Zheng Y, Wang P, Zhang Y. The effect of vitamin D supplementation on glycemic control in type 2 diabetes patients: a systematic review and meta-analysis. Nutrients. 2018;10(3):375. doi: 10.3390/nu10030375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mirhosseini N, Rainsbury J, Kimball SM. Vitamin D supplementation, serum 25 (OH) D concentrations and cardiovascular disease risk factors: a systematic review and meta-analysis. Front Cardiovasc Med. 2018;5:87. doi: 10.3389/fcvm.2018.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.George P, Pearson E, Witham M. Effect of vitamin D supplementation on glycaemic control and insulin resistance: a systematic review and meta-analysis. Diabet Med. 2012;29(8):e142–e150. doi: 10.1111/j.1464-5491.2012.03672.x. [DOI] [PubMed] [Google Scholar]
- 24.Seida JC, Mitri J, Colmers IN, Majumdar SR, Davidson MB, Edwards AL, et al. Effect of vitamin D3 supplementation on improving glucose homeostasis and preventing diabetes: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2014;99(10):3551–3560. doi: 10.1210/jc.2014-2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.De Andrade FB, de França Caldas JA, Kitoko PM. Relationship between oral health, nutrient intake and nutritional status in a sample of Brazilian elderly people. Gerodontology. 2009;26(1):40–45. doi: 10.1111/j.1741-2358.2008.00220.x. [DOI] [PubMed] [Google Scholar]
- 26.Forman DE, Berman AD, McCabe CH, Baim DS, Wei JY. PTCA in the elderly: the “young-old” versus the “old-old”. J Am Geriatr Soc. 1992;40(1):19–22. doi: 10.1111/j.1532-5415.1992.tb01823.x. [DOI] [PubMed] [Google Scholar]
- 27.Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96(7):1911–1930. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- 28.Whitehead A, Whitehead J. A general parametric approach to the meta-analysis of randomized clinical trials. Stat Med. 1991;10(11):1665–1677. doi: 10.1002/sim.4780101105. [DOI] [PubMed] [Google Scholar]
- 29.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Witham M, Dove F, Dryburgh M, Sugden J, Morris A, Struthers A. The effect of different doses of vitamin D3 on markers of vascular health in patients with type 2 diabetes: a randomised controlled trial. Diabetologia. 2010;53(10):2112–2119. doi: 10.1007/s00125-010-1838-1. [DOI] [PubMed] [Google Scholar]
- 31.Naharci I, Bozoglu E, Kocak N, Doganci S, Doruk H, Serdar M. Effect of vitamin D on insulin sensitivity in elderly patients with impaired fasting glucose. Geriatr Gerontol Int. 2012;12(3):454–460. doi: 10.1111/j.1447-0594.2011.00791.x. [DOI] [PubMed] [Google Scholar]
- 32.Witham M, Dove F, Sugden J, Doney A, Struthers A. The effect of vitamin D replacement on mrkers of vascular health in stroke patients–a randomised controlled trial. Nutr Metab Cardiovasc Dis. 2012;22(10):864–870. doi: 10.1016/j.numecd.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 33.Wood AD, Secombes KR, Thies F, Aucott L, Black AJ, Mavroeidi A, et al. Vitamin D3 supplementation has no effect on conventional cardiovascular risk factors: a parallel-group, double-blind, placebo-controlled RCT. J Clin Endocrinol Metab. 2012;97(10):3557–3568. doi: 10.1210/jc.2012-2126. [DOI] [PubMed] [Google Scholar]
- 34.Kim H-J, Kang C-K, Park H, Lee M-G. Effects of vitamin D supplementation and circuit training on indices of obesity and insulin resistance in T2D and vitamin D deficient elderly women. J Exerc Nutr Biochem. 2014;18(3):249. doi: 10.5717/jenb.2014.18.3.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.El-Hajj Fuleihan G, Baddoura R, Habib RH, Halaby G, Arabi A, Rahme M, et al. Effect of vitamin D replacement on indexes of insulin resistance in overweight elderly individuals: a randomized controlled trial. Am J Clin Nutr. 2016;104(2):315–323. doi: 10.3945/ajcn.116.132589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Prusik K, Kortas J, Prusik K, Mieszkowski J, Jaworska J, Skrobot W, et al. Nordic walking training causes a decrease in blood cholesterol in elderly women supplemented with Vitamin D. Front Endocrinol. 2018;9:42. doi: 10.3389/fendo.2018.00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.El Hajj C, Fares S, Chardigny JM, Boirie Y, Walrand S. Vitamin D supplementation and muscle strength in pre-sarcopenic elderly Lebanese people: a randomized controlled trial. Arch Osteoporos. 2019 doi: 10.1007/s11657-018-0553-2. [DOI] [PubMed] [Google Scholar]
- 38.Wenclewska S, Szymczak-Pajor I, Drzewoski J, Bunk M, Śliwińska A. Vitamin D supplementation reduces both oxidative DNA damage and insulin resistance in the elderly with metabolic disorders. Int J Mol Sci. 2019;20(12):2891. doi: 10.3390/ijms20122891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hoseini Z, Behpour N, Hoseini R. Co-treatment with vitamin D supplementation and aerobic training in elderly women with Vit D deficiency and NAFLD: A single-blind controlled trial. Hepat Monthly. 2020 doi: 10.5812/hepatmon.96437. [DOI] [Google Scholar]
- 40.El Hajj C, Walrand S, Helou M, Yammine K. Effect of vitamin D supplementation on inflammatory markers in non-obese lebanese patients with type 2 diabetes: a randomized controlled trial. Nutrients. 2020;12(7):1–12. doi: 10.3390/nu12072033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Krul-Poel YH, Ter Wee MM, Lips P, Simsek S. Management of endocrine disease: the effect of vitamin D supplementation on glycaemic control in patients with type 2 diabetes mellitus: a systematic review and meta-analysis. Eur J Endocrinol. 2017;176(1):R1–R14. doi: 10.1530/EJE-16-0391. [DOI] [PubMed] [Google Scholar]
- 42.Mohamad MI, El-Sherbeny EE, Bekhet MM. The effect of vitamin D supplementation on glycemic control and lipid profile in patients with type 2 diabetes mellitus. J Am Coll Nutr. 2016;35(5):399–404. doi: 10.1080/07315724.2015.1026427. [DOI] [PubMed] [Google Scholar]
- 43.Ostadmohammadi V, Milajerdi A, Ghayour-Mobarhan M, Ferns G, Taghizadeh M, Badehnoosh B, et al. The effects of vitamin D supplementation on glycemic control, lipid profiles and C-reactive protein among patients with cardiovascular disease: a systematic review and meta-analysis of randomized controlled trials. Curr Pharm Des. 2019;25(2):201–210. doi: 10.2174/1381612825666190308152943. [DOI] [PubMed] [Google Scholar]
- 44.Mitri J, Dawson-Hughes B, Hu FB, Pittas AG. Effects of vitamin D and calcium supplementation on pancreatic β cell function, insulin sensitivity, and glycemia in adults at high risk of diabetes: the Calcium and Vitamin D for Diabetes Mellitus (CaDDM) randomized controlled trial. Am J Clin Nutr. 2011;94(2):486–494. doi: 10.3945/ajcn.111.011684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Haroon NN, Anton A, John J, Mittal M. Effect of vitamin D supplementation on glycemic control in patients with type 2 diabetes: a systematic review of interventional studies. J Diabetes Metab Disord. 2015;14(1):1–11. doi: 10.1186/s40200-015-0130-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mirhosseini N, Vatanparast H, Mazidi M, Kimball SM. The effect of improved serum 25-hydroxyvitamin D status on glycemic control in diabetic patients: a meta-analysis. J Clin Endocrinol Metab. 2017;102(9):3097–3110. doi: 10.1210/jc.2017-01024. [DOI] [PubMed] [Google Scholar]
- 47.Wu C, Qiu S, Zhu X, Li L. Vitamin D supplementation and glycemic control in type 2 diabetes patients: a systematic review and meta-analysis. Metab Clin Exp. 2017;73:67–76. doi: 10.1016/j.metabol.2017.05.006. [DOI] [PubMed] [Google Scholar]
- 48.Jafari T, Fallah AA, Barani A. Effects of vitamin D on serum lipid profile in patients with type 2 diabetes: a meta-analysis of randomized controlled trials. Clin Nutr. 2016;35(6):1259–1268. doi: 10.1016/j.clnu.2016.03.001. [DOI] [PubMed] [Google Scholar]
- 49.Wang H, Xia N, Yang Y, Peng D-Q. Influence of vitamin D supplementation on plasma lipid profiles: a meta-analysis of randomized controlled trials. Lipids Health Dis. 2012;11(1):1–9. doi: 10.1186/1476-511X-11-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Scragg R, Sowers M, Bell C. Serum 25-hydroxyvitamin D, diabetes, and ethnicity in the Third National Health and Nutrition Examination Survey. Diabetes Care. 2004;27(12):2813–2818. doi: 10.2337/diacare.27.12.2813. [DOI] [PubMed] [Google Scholar]
- 51.Wang J-H, Keisala T, Solakivi T, Minasyan A, Kalueff AV, Tuohimaa P. Serum cholesterol and expression of ApoAI, LXRβ and SREBP2 in vitamin D receptor knock-out mice. J Steroid Biochem Mol Biol. 2009;113(3–5):222–226. doi: 10.2337/diacare.27.12.2813. [DOI] [PubMed] [Google Scholar]
- 52.Querfeld U, Hoffmann MM, Klaus G, Eifinger F, Ackerschott M, Michalk D, et al. Antagonistic effects of vitamin D and parathyroid hormone on lipoprotein lipase in cultured adipocytes. J Am Soc Nephrol. 1999;10(10):2158–2164. doi: 10.1681/ASN.V10102158. [DOI] [PubMed] [Google Scholar]
- 53.Christensen R, Lorenzen JK, Svith CR, Bartels E, Melanson E, Saris W, et al. Effect of calcium from dairy and dietary supplements on faecal fat excretion: a meta-analysis of randomized controlled trials. Obes Rev. 2009;10(4):475–486. doi: 10.1111/j.1467-789X.2009.00599.x. [DOI] [PubMed] [Google Scholar]
- 54.Ramly M, Ming MF, Chinna K, Suboh S, Pendek R. Effect of vitamin D supplementation on cardiometabolic risks and health-related quality of life among urban premenopausal women in a tropical country–a randomized controlled trial. PLoS ONE. 2014;9(10):e110476. doi: 10.1371/journal.pone.0110476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang TJ, Pencina MJ, Booth SL, Jacques PF, Ingelsson E, Lanier K, et al. Vitamin D deficiency and risk of cardiovascular disease. Circulation. 2008;117(4):503–511. doi: 10.1161/CIRCULATIONAHA.107.706127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Parker J, Hashmi O, Dutton D, Mavrodaris A, Stranges S, Kandala N-B, et al. Levels of vitamin D and cardiometabolic disorders: systematic review and meta-analysis. Maturitas. 2010;65(3):225–236. doi: 10.1016/j.maturitas.2009.12.013. [DOI] [PubMed] [Google Scholar]
- 57.Chen C, Chen Y, Weng P, Xia F, Li Q, Zhai H, et al. Association of 25-hydroxyvitamin D with cardiometabolic risk factors and metabolic syndrome: a mendelian randomization study. Nutr J. 2019;18(1):61. doi: 10.1186/s12937-019-0494-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sato Y, Asoh T, Oizumi K. Retraction notice to" High prevalence of vitamin D deficiency and reduced bone mass in elderly women with Alzheimer’s disease". Bone. 2019;23(6 December 1998):555–557. doi: 10.1016/j.bone.2019.05.019. [DOI] [PubMed] [Google Scholar]
- 59.Pilz S, Verheyen N, Grübler MR, Tomaschitz A, März W. Vitamin D and cardiovascular disease prevention. Nat Rev Cardiol. 2016;13(7):404–417. doi: 10.1038/nrcardio.2016.73. [DOI] [PubMed] [Google Scholar]
- 60.Fan H, Yu W, Cao H, Li J, Liu B, Wang J, et al. Meta-analysis of circulating 25-hydroxyvitamin D levels and risk of cardiovascular and all-cause mortality in elderly population. Int J Cardiol. 2014;176(3):1025–1029. doi: 10.1016/j.ijcard.2014.07.074. [DOI] [PubMed] [Google Scholar]
- 61.Schöttker B, Ball D, Gellert C, Brenner H. Serum 25-hydroxyvitamin D levels and overall mortality. A systematic review and meta-analysis of prospective cohort studies. Ageing Res Rev. 2013;12(2):708–718. doi: 10.1016/j.arr.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 62.Rolland Y, de Souto BP, Van Kan GA, Annweiler C, Beauchet O, Bischoff-Ferrari H, et al. Vitamin D supplementation in older adults: searching for specific guidelines in nursing homes. J Nutr Health Aging. 2013;17(4):402–412. doi: 10.1007/s12603-013-0007-x. [DOI] [PubMed] [Google Scholar]
- 63.Gangloff A, Bergeron J, Lemieux I, Després J-P. Changes in circulating vitamin D levels with loss of adipose tissue. Curr Opin Clin Nutr Metab Care. 2016;19(6):464–470. doi: 10.1097/MCO.0000000000000315. [DOI] [PubMed] [Google Scholar]
- 64.Duan L, Han L, Liu Q, Zhao Y, Wang L, Wang Y. Effects of vitamin D supplementation on general and central obesity: results from 20 randomized controlled trials involving apparently healthy populations. Ann Nutr Metab. 2020;76(3):153–164. doi: 10.1159/000507418. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 2. Forest plot of the effect of vitamin D supplementation on high-density lipoprotein; HDL (a) low-density lipoprotein; LDL (b) LDL/HDL ratio (c)
Additional file3. Forest plot of the effect of vitamin D supplementation on insulin concentration stratified by intervention duration (a) Forest plot of the effect of vitamin D supplementation on fasting blood sager stratified by intervention duration (b).
Additional file 4. Forest plot of the effect of vitamin D supplementation on body mass index (BMI) (a) Waist circumference (b).
Data Availability Statement
The datasets generated or analyzed during the current study are available from the corresponding author on reasonable request.