Abstract
Objectives
Recently, the Omicron strain of SARS-CoV-2 has spread and replaced the previously dominant Delta strain. Several Omicron sublineages (BA.1, BA.1.1, and BA.2) have been identified, with in vitro and preclinical reports showing that the pathogenicity and therapeutic efficacy differs between BA.1 and BA.2. We sought to develop a TaqMan assay to identify these subvariants.
Methods
A TaqMan assay was constructed for rapid identification and genotyping of Omicron sublineages with 171 samples. We analyzed three characteristic mutations of the spike gene, Δ69–70, G339D, and Q493R, by TaqMan assay. The accuracy of the TaqMan assay was examined by comparing its results with the results of whole genome sequencing (WGS) analysis.
Results
A total of 171 SARS-CoV-2 positive samples were analyzed by WGS and TaqMan assay. The 127 samples determined as BA.1/BA.1.1 by WGS were all positive for Δ69–70, G339D and Q493R by TaqMan assay. A total of 42 samples, determined as BA.2 by WGS, were negative for Δ69–70 but positive for G339D and Q493R by TaqMan. Two samples with G339N were determined to be inconclusive by the TaqMan method. Except for these two samples, the concordance rate between WGS and the TaqMan assay was 100% (169/169).
Conclusion
TaqMan assays targeting characteristic mutations are useful for identification and discrimination of Omicron sublineages.
Keywords: SARS-CoV-2, VOC, Omicron, BA.1, BA.2
Introduction
SARS-CoV-2 has mutated continuously, which affected the transmissibility of the virus and the efficacy of antiviral drugs and vaccines. To control the emergence of new strains, it is important to continuously monitor viral evolution through genomic epidemiologic analysis (Munnink et al., 2021).
Recently, a new strain, designated as B.1.1.529, was detected and isolated in Gauteng Province, South Africa (World Health Organization, 2021). The World Health Organization designated the B.1.1.529 lineage as a variant of concern (VOC) on November 26, 2021 (World Health Organization, 2021), naming it Omicron, in line with the naming convention adopted for previous VOCs using the Greek alphabet, and it has since spread to a number of countries to become a globally dominant strain (Desingu and Nagarajan, 2022). Omicron contains more than 30 nonsynonymous mutations in the spike protein; therefore, Omicron viruses may reduce the efficacy of antibody therapy and evade vaccine-induced immunity.
Omicron viruses are classified into several sublineages, including BA.1, BA.1.1, BA.2, and BA.3, which have been observed worldwide. In the early period after Omicron emergence, BA.1 was the dominant sublineage; however, in Denmark, the United Kingdom, India, the Philippines, and South Africa, BA.2 became dominant (Hodcroft, 2021; Fonager et al., 2022; Yamasoba et al., 2022), with BA.2 reported to be more transmissible than BA.1 (Ito et al., 2022).
Omicron contains numerous mutations compared with other VOCs (Gangavarapu, et al. 2022; Saxena et al., 2022; Wang and Cheng, 2022). A total of 33 mutations have been identified in the spike protein in BA.1, 34 in BA.1.1, and 29 in BA.2. BA.1 and BA.1.1 share 33 mutations, but BA.1.1 has an additional mutation in R346K compared with BA.1. Compared with BA.1 and BA.1.1, the spike protein mutations in BA.2 are very different. Unique mutations in BA.1/BA.1.1 are A67V, Δ69–70, T95I, Δ143–145, N211I, Δ212, S371L, G466S, G496S, T547K, N856K, and L981F. Conversely, unique mutations in BA.2 are T19I, L24S, Δ25–27, V213G, S371F, T376A, D405N, and R408S.
In this study, we present a TaqMan assay to discriminate between BA.1/BA.1.1 and BA.2. This method is feasible for any laboratory equipped to perform quantitative real-time polymerase chain reaction (PCR).
Methods
Samples
The study includes patients who are SARS-CoV-2 positive (n = 294) who were collected from all areas of Yamanashi Prefecture in Japan and tested positive at our hospital. The sample collection period was from January 12 to March 10, 2022. During this period, 294 positive samples were subjected to whole genome analysis (Hirotsu et al., 2022), and 171 of them were randomly selected to evaluate the performance of the TaqMan assay.
SARS-CoV-2 diagnostic testing
Nasopharyngeal swab samples were collected using cotton swabs and placed in viral transport media (Copan Diagnostics, Murrieta, CA, USA). Multiple molecular diagnostic testing platforms, including SARS-CoV-2 quantitative reverse transcriptase-PCR in accordance with the protocol developed by the National Institute of Infectious Diseases in Japan (Shirato et al., 2020), FilmArray Respiratory Panel 2.1 with the FilmArray Torch system (bioMérieux, Marcy-l'Etoile, France) (Hirotsu et al., 2020a), Xpert Xpress SARS-CoV-2 test using Cepheid GeneXpert (Cepheid, Sunnyvale, CA, USA) (Hirotsu et al., 2022), and Lumipulse antigen test with the LUMIPULSE G600II system (Fujirebio, Inc., Tokyo, Japan) were used to identify positive samples for this study (Hirotsu et al., 2021; Hirotsu et al., 2020b).
Whole genome sequencing (WGS)
WGS analysis was performed as previously described (Hirotsu and Omata, 2021a; Hirotsu and Omata, 2021b; Hirotsu and Omata, 2021c). In brief, SARS-CoV-2 genomic RNA was reverse transcribed into cDNA and amplified by using the Ion AmpliSeq SARS-CoV-2 Insight Research Assay (Thermo Fisher Scientific, Waltham, MA, USA) on the Ion Torrent Genexus System. Sequencing reads and quality were processed using Genexus software with SARS-CoV-2 plugins. The sequencing reads were mapped and aligned using the torrent mapping alignment program. Assembly was performed with the Iterative Refinement Meta-Assembler (Shepard et al., 2016).
To determine the viral clade and lineage classifications, the consensus FASTA files were downloaded and processed through Nextstrain (Hadfield et al., 2018) and Phylogenetic Assignment of Named Global Outbreak Lineages (PANGOLIN) (Rambaut et al., 2020). The FASTA files were deposited in the Global Initiative on Sharing Avian Influenza Data (GISAID) EpiCoV database (Shu and McCauley, 2017). All GISAID Accession IDs are noted in Table S1.
TaqMan assay
To distinguish BA.1/BA.1.1 from BA.2, we constructed a TaqMan assay system to analyze the spike protein mutations that characterize the Omicron sublineages. Thus, we used a predesigned TaqMan SARS-CoV-2 Mutation Panel (Thermo Fisher Scientific) to distinguish Δ69–70, G339D, and Q493R. The TaqMan probe detected both wild-type and variant sequences of SARS-CoV-2, with the TaqMan minor groove binder probe for the wild-type allele labeled with VIC dye and the variant allele with FAM dye fluorescence. TaqPath 1-Step RT-qPCR Master Mix CG was used as master mix and the qPCR was performed on the Step-One Plus Real-Time PCR System (Thermo Fisher Scientific). The allelic discrimination software (Thermo Fisher Scientific) was used to analyze data and identify variant and wild-type alleles. The amplification curves of each data set were visually confirmed.
According to the GISAID database, as of March 11, 2022, the frequency of mutations targeted in this study detected in each sublineage were as follows. Δ69-70 in 96.1%, 95.7%, and 0.1% of BA.1, BA.1.1, and BA.2, respectively; G339D in 87.9%, 99.3%, and 96.3% of BA.1, BA.1.1, and BA.2, respectively; Q493R in 88.3%, 91.2%, and 92% of BA.1, BA.1.1, and BA.2, respectively (Gangavarapu, et al. 2022). The spike Δ69–70 mutation was also used to distinguish sublineages because it was detected in BA.1/BA.1.1 but not in BA.2 (Supplemental Figure 1a). Spike G339D and Q493R mutations are detected in BA.1/BA.1.1 and BA.2 but not in other VOCs (Supplemental Figure 1b).
Results
Construction of a TaqMan assay to distinguish Omicron sublineages
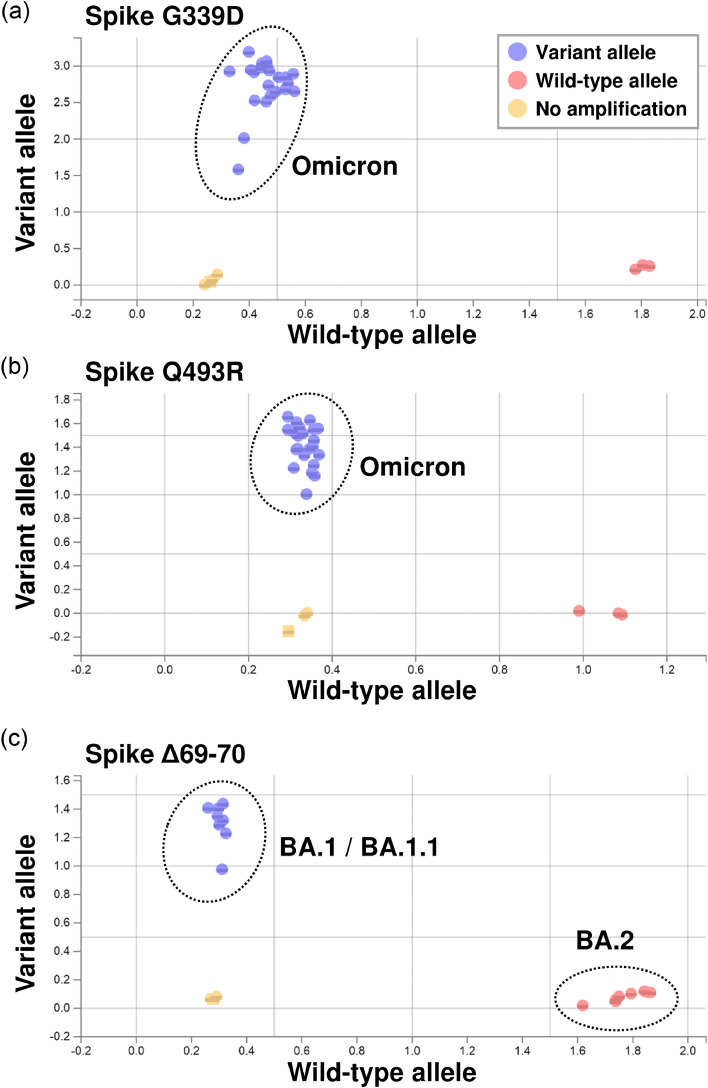
When we analyzed using nucleic acids extracted from nasopharyngeal swabs, G339D and Q493R mutations were specifically detected in Omicron-infected patients (Figure 1 a and 1b). In addition, the Δ69–70 mutation was distinct between BA1/BA.1.1 and BA.2 (Figure 1c). These results showed that TaqMan assays could detect Omicron strains and distinguish between Omicron sublineages.
Figure 1.
Genotyping of Omicron sublineages by TaqMan assay. (a-c) Samples were analyzed with a TaqMan assay that detects mutations in Omicron spike proteins. Spike protein mutations G339D (a), Q493R (b), and Δ69–70 (c) were targeted. G339D and Q493R indicated Omicron (including BA.1/BA.1.1 and BA.2), while Δ69–70 was used to distinguish BA.1/BA.1.1 from BA.2. Blue circles indicate variant alleles (FAM dye) and red circles indicate wild-type alleles (VIC dye). The results were plotted on a scatterplot of wild-type alleles (x axis) versus variant alleles (y axis) using the allelic discrimination software.
Comparison of TaqMan assay and WGS data
To examine whether the TaqMan assay could accurately distinguish Omicron sublineages, we compared WGS data and TaqMan assay results using 171 SARS-CoV-2-positive samples (Table 1 and Table S1). WGS analysis determined 127 samples to be BA.1/BA.1.1 and 44 samples to be BA.2 (Table 1). In these samples, TaqMan assay analysis showed that all BA.1/BA.1.1 samples were positive for Δ69–70, G339D, and Q493R, whereas BA.2 samples were negative for Δ69–70 and positive for G339D and Q493R (Table 1). Two samples were determined to be inconclusive because of equivocal results in the G339D assay. Excluding these two samples, the TaqMan assay data were consistent with WGS data, demonstrating 100% (169/169) agreement. Therefore, the TaqMan assay represents a useful technique for distinguishing Omicron sublineages.
Table 1.
Comparison of results between WGS and TaqMan assay.
| WGS | TaqMan assay |
|||
|---|---|---|---|---|
| Lineage | n | Spike Δ69-70 | Spike G339D | Spike Q493R |
| Pos / Neg / Inc | Pos / Neg / Inc | Pos / Neg / Inc | ||
| BA.1/BA.1.1 | 127 | 127 / 0/ 0 | 127 / 0 / 0 | 127 / 0/ 0 |
| BA.2 | 44 | 0 / 44/ 0 | 42/ 0 / 2a | 44 / 0/ 0 |
Inc, inconclusive; Neg, negative; Pos, positive; WGS, whole genome sequencing.
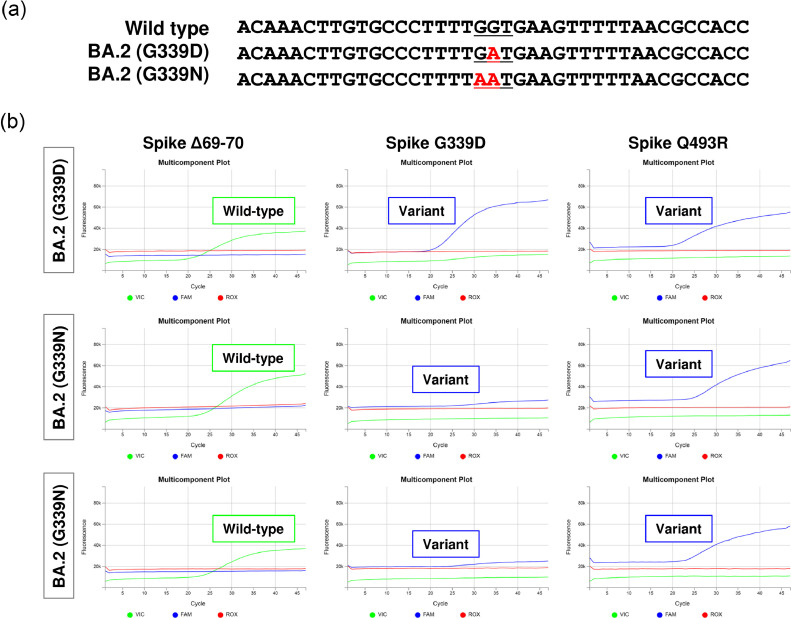
Two samples with spike G339N were included, and a TaqMan probe targeting spike G339D showed only low amplification in Figure 2. These samples were determined to be inconclusive.
Mutations in TaqMan assay target sites
In the two samples determined to be inconclusive aforementioned, WGS analysis results revealed these samples had the spike G339N mutation (position 22577–22578: c.1015_1016delGGGinsAA) (Accession ID: EPI_ISL_11018144 and EPI_ISL_11018145) (Figure 2 a). Analysis with PANGOLIN classified these samples as BA.2. The mutation that occurred at codon 339 overlapped with the target site of the TaqMan probe. Therefore, we examined whether the TaqMan probe targeting G339D could efficiently amplify the target in the samples with G339N. In both BA.2 G339D and BA.2 G339N samples, sufficient amplification signals were obtained for Δ69–70 and Q493R. However, compared with BA.2 G339D, BA.2 G339N showed a lower amplification efficiency of the variant allele-specific signal (blue line in Figure 2b). In conclusion, the TaqMan assay used in this study specifically detected characteristic mutations related to Omicron sublineages in clinical samples.
Figure 2.
Analysis of samples with mutations in the TaqMan probe site. Two samples were classified by WGS analysis as BA.2 containing a G339N mutation. (a) The mutation site occurred at codon 339, from wild-type (GGT, glycine [G]) to the variant form (GAT, aspartic acid [D] or AAT, asparagine [N]). (b) Most BA.2 lineage viruses carry the G339D mutation, but two samples showed a G339N mutation by WGS. Compared with BA.2 G339D, the fluorescent signal (blue line) was lower in the BA.2 G339N samples.
Discussion
In this study, we present data regarding the use of a TaqMan assay to distinguish between Omicron strains and their sublineages. WGS analysis is the most standard method to determine these sublineages. However, WGS analysis of all specimens is restricted by limited resources and is difficult to apply under conditions of rapid spread of infection. In this regard, we have established a TaqMan assay that more conveniently and rapidly identifies Omicron strains and distinguishes the sublineages. BA.1/BA.1.1 and BA.2 are reported to differ in transmissibility and treatment response and so, the World Health Organization recommends monitoring BA.2 as a separate sublineage (World Health Organization, 2022). Therefore, assay systems that distinguish these subtypes will be important in determining preventive measures, infection control, and treatment strategies.
Each viral lineage has its own characteristic mutations. By targeting these mutations, it is possible to distinguish mutant strains and sublineages. On the basis of the accumulating WGS data during surveillance, we consider the TaqMan assay to be a suitable approach to detect characteristic mutations that occur frequently among viral lineages. In this study, we subjected SARS-CoV-2-positive samples collected from all areas of Yamanashi Prefecture in Japan between January 12, 2022 and March 10, 2022. To evaluate the performance of the TaqMan assay, 171 randomly selected samples of the 294 samples that completed the WGS analysis were also analyzed by the TaqMan assay in this study. The main prevalent mutant strain during this period was the Omicron strain (Hirotsu et al., 2022), which was in the process of replacing BA.1/BA.1.1 with BA.2. The TaqMan assay and WGA results were consistent in almost all samples, thus TaqMan assay results reflected the viral genome epidemiology of the region at the time.
Of the total 171 tested samples, two samples harbored the D339N mutation, which was revealed by WGS. These two samples were determined to be negative when the results of a TaqMan probe targeting D339D were analyzed using automated assay software. However, a weak but low signal amplification was observed by visual confirmation; therefore, these samples were considered as inconclusive in this study for convenience. On the basis of these results, it should be noted that if a mutation occurs in the TaqMan probe sequence, it will not be amplified efficiently due to insufficient annealing. Mutations in primer sequences can also cause similar problems. Therefore, visual examinations of the results may help to avoid missing subtle changes caused by mutations around the primers and TaqMan probe.
To determine each lineage, it is efficient to know which mutant strain is prevalent at a given time and analyze the characteristic mutations of that strain. In this analysis, spike Δ69-70 was used to distinguish between BA.1/BA.1.1 and BA.2. There are other mutations that are also useful for distinguishing Omicron strains (Erster et al., 2022; Lee et al., 2022). For example, Q493R is a useful site to distinguish BA.1, BA.2, and BA.3 from BA.4 and BA.5. Q493R is found in BA.1, BA.2, and BA.3 but wild-type in BA.4 and BA.5 (Gangavarapu, et al. 2022). On the other hand, BA.4 and BA.5 have spike L452R and F486V mutations, which can be analyzed to distinguish them from BA.1, BA.2, and BA.3. The TaqMan method could be adapted to identify any such newly emerging mutant strains. Analysis of multiple characteristic mutations is expected to improve the accuracy of classification of virus lineage.
The TaqMan assay has a shorter turnaround time and lower cost than WGS. TaqMan assays require 2-3 hours until results are available. However, WGS generally requires 1-2 days due to the labor-intensive procedures, such as reverse transcription reactions from RNA to cDNA, library preparation, purification, and library quantification. In addition, facilities and laboratories with limited analytical equipment and resources cannot perform WGS analysis. Alternatively, TaqMan assays are useful to distinguish VOCs and their sublineages quickly and easily in any laboratory with qualitative PCR facilities. In addition, the TaqMan assay costs only a few thousand Japanese yen (JPY) per specimen, whereas WGS costs tens of thousands of JPY. Therefore, the TaqMan assay is considered useful for rapid screening of other samples.
There are, however, several limitations. TaqMan assays do not always accurately determine the sublineages in some situations. WGS analysis may be necessary when the characteristic mutations of newly emerging strains or sublineages remain under investigation. It should also be noted that if mutations occur at the TaqMan probe or primer locations, the PCR amplification efficiency will be reduced and the signal will be attenuated, which may make interpretation difficult (Figure 2b).
Our study showed that the TaqMan assay can be used to specifically detect Omicron viruses and classify subvariants. By applying this method to SARS-CoV-2-positive specimens, it is possible to analyze multiple specimens rapidly. Although accumulated data are still needed (Iketani et al., 2022; Takashita et al., 2022; Yamasoba et al., 2022; Zhou et al., 2022), BA.1 and BA.2 show different susceptibilities to antibody and antiviral therapy (Takashita et al., 2022). Rapid classification of Omicron sublineages may be clinically critical in providing appropriate treatment to patients with COVID-19.
Acknowledgments
Data availability
The sequences of SARS-CoV-2 genomes are available on GISAID (www.gisaid.org). We have provided the accession numbers in the Supplementary Table 1. Source data are provided with this report.
Acknowledgments
We thank all medical and ancillary hospital staff for their support. We thank Dr. Gillian Campbell from Edanz (https://www.jp.edanz.com/ac) for editing a draft of this manuscript.
Conflict of interest
The authors have no competing interests to declare.
Funding source
This study was supported by a Grant-in-Aid for the Genome Research Project from Yamanashi Prefecture (to M.O. and Y.H.), the Japan Society for the Promotion of Science (JSPS) KAKENHI Early-Career Scientists JP18K16292 (to Y.H.), a Grant-in-Aid for Scientific Research (B) 20H03668 (to Y.H.), a Research Grant for Young Scholars (to Y.H.), the YASUDA Medical Foundation (to Y.H.), the Uehara Memorial Foundation (to Y.H.), and Medical Research Grants from the Takeda Science Foundation (to Y.H.).
Ethical Approval statement
The institutional review board of the Clinical Research and Genome Research Committee at Yamanashi Central Hospital approved this study and the use of an opt-out consent method (Approval No. C2019-30). The requirement for written informed consent was waived owing to the observational nature of this study and the urgent need to collect COVID-19 data.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ijid.2022.06.039.
Appendix. Supplementary materials
References
- Desingu PA, Nagarajan K. Omicron BA.2 lineage spreads in clusters and is concentrated in Denmark. J Med Virol. 2022;94:2360–2364. doi: 10.1002/jmv.27659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erster O, et al. Novel RT-qPCR assays enable rapid detection and differentiation between SARS-CoV-2 Omicron (BA.1) and BA.2 variants. medRxiv. 2022 [Google Scholar]
- Fonager J, et al. Molecular epidemiology of the SARS-CoV-2 variant Omicron BA.2 sub-lineage in Denmark, 29 November 2021 to 2. Eurosurveillance 2022. 2022;27 doi: 10.2807/1560-7917.ES.2022.27.10.2200181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangavarapu K, et al. Outbreak.info genomic reports: scalable and dynamic surveillance of SARS-CoV-2 variants and mutations. medRxiv. 2022 doi: 10.1101/2022.01.27.22269965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadfield J, et al. Nextstrain: real-time tracking of pathogen evolution. Bioinformatics. 2018;34:4121–4123. doi: 10.1093/bioinformatics/bty407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirotsu Y, Omata M. SARS-CoV-2 B.1.1.7 lineage rapidly spreads and replaces R.1 lineage in Japan: serial and stationary observation in a community. Infect Genet Evol. 2021;95 doi: 10.1016/j.meegid.2021.105088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirotsu Y, Omata M. Discovery of a SARS-CoV-2 variant from the P.1 lineage harboring K417T/E484K/N501Y mutations in Kofu. Japan. J Infect. 2021;82:276–316. doi: 10.1016/j.jinf.2021.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirotsu Y, Omata M. Detection of R.1 lineage severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) with spike protein W152L/E484K/G769V mutations in Japan. PLOS Pathog. 2021;17 doi: 10.1371/journal.ppat.1009619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirotsu Y, et al. Comparison of automated SARS-CoV-2 antigen test for COVID-19 infection with quantitative RT-PCR using 313 nasopharyngeal swabs including from 7 serially followed patients. Int J Infect Dis. 2020;99:397–402. doi: 10.1016/j.ijid.2020.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirotsu Y, et al. Analysis of Covid-19 and non-Covid-19 viruses, including influenza viruses, to determine the influence of intensive preventive measures in Japan. J Clin Virol. 2020;129 doi: 10.1016/j.jcv.2020.104543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirotsu Y, et al. Prospective study of 1,308 nasopharyngeal swabs from 1,033 patients using the LUMIPULSE SARS-CoV-2 antigen test: comparison with RT-qPCR. Int J Infect Dis. 2021;105:7–14. doi: 10.1016/j.ijid.2021.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirotsu Y, et al. Direct comparison of Xpert Xpress, FilmArray Respiratory Panel, Lumipulse antigen test, and RT-qPCR in 165 nasopharyngeal swabs. BMC Infect Dis. 2022;22:221. doi: 10.1186/s12879-022-07185-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirotsu Y, et al. SARS-CoV-2 Omicron sublineage BA.2 replaces BA.1.1: genomic surveillance in Japan from September 2021 to March 2022. J Infect. 2022;85(2):174–211. doi: 10.1016/j.jinf.2022.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodcroft EB. CoVariants: SARS-CoV-2 mutations and variants of interest, 2021.
- Iketani S, et al. Antibody evasion properties of SARS-CoV-2 Omicron sublineages. Nature. 2022;604:553–556. doi: 10.1038/s41586-022-04594-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito K, Piantham C, Nishiura H. Relative instantaneous reproduction number of Omicron SARS-CoV-2 variant with respect to the Delta variant in Denmark. J Med Virol. 2022;94:2265–2268. doi: 10.1002/jmv.27560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee WL, et al. Quantitative detection of SARS-CoV-2 Omicron BA.1 and BA.2 variants in wastewater through allele-specific RT-qPCR. medRxiv. 2022 [Google Scholar]
- Munnink OBB, et al. The next phase of SARS-CoV-2 surveillance: real-time molecular epidemiology. Nat Med. 2021;27:1518–1524. doi: 10.1038/s41591-021-01472-w. [DOI] [PubMed] [Google Scholar]
- Rambaut A, et al. A dynamic nomenclature proposal for SARS-CoV-2 lineages to assist genomic epidemiology. Nat Microbiol. 2020;5:1403–1407. doi: 10.1038/s41564-020-0770-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxena SK, et al. Characterization of the novel SARS-CoV-2 Omicron (B.1.1.529) variant of concern and its global perspective. J Med Virol. 2022;94:1738–1744. doi: 10.1002/jmv.27524. [DOI] [PubMed] [Google Scholar]
- Shepard SS, et al. Viral deep sequencing needs an adaptive approach: IRMA, the iterative refinement meta-assembler. BMC Genomics. 2016;17:708. doi: 10.1186/s12864-016-3030-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirato K, et al. Development of genetic diagnostic methods for novel coronavirus 2019 (nCoV-2019) in Japan. Jpn J Infect Dis. 2020;73:304–307. doi: 10.7883/yoken.JJID.2020.061. [DOI] [PubMed] [Google Scholar]
- Shu Y, McCauley J. GISAID: global initiative on sharing all influenza data - from vision to reality. Euro Surveill. 2017;22:30494. doi: 10.2807/1560-7917.ES.2017.22.13.30494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takashita E, et al. Efficacy of antiviral agents against the SARS-CoV-2 omicron subvariant BA.2. N Engl J Med. 2022;386:1475–1477. doi: 10.1056/NEJMc2201933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Cheng G. Sequence analysis of the emerging SARS-CoV-2 variant Omicron in South Africa. J Med Virol. 2022;94:1728–1733. doi: 10.1002/jmv.27516. [DOI] [PubMed] [Google Scholar]
- World Health Organization . 2021. Classification of omicron (B.1.1.529): SARS-CoV-2 variant of concern. [Google Scholar]
- World Health Organization . 2022. Statement on Omicron sublineage BA.2. [Google Scholar]
- Yamasoba D, et al. Virological characteristics of the SARS-CoV-2 Omicron BA.2 spike. Cell. 2022;185:2103–2115. doi: 10.1016/j.cell.2022.04.035. e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H, et al. Neutralization of SARS-CoV-2 omicron BA.2 by Therapeutic Monoclonal Antibodies. bioRxiv. 2022 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The sequences of SARS-CoV-2 genomes are available on GISAID (www.gisaid.org). We have provided the accession numbers in the Supplementary Table 1. Source data are provided with this report.