Abstract
Background:
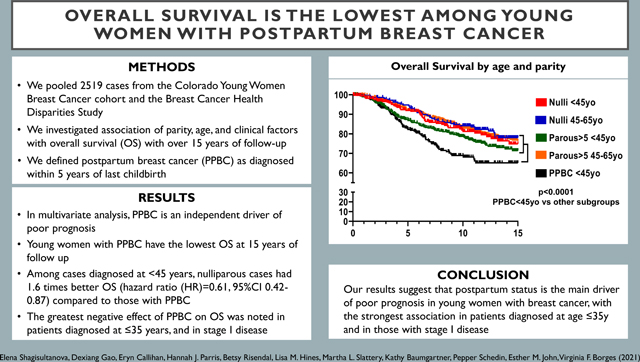
Women diagnosed with breast cancer prior to age 45 years (<45y) and within the first 5 years postpartum (postpartum breast cancer, PPBC) have the greatest risk for distal metastatic recurrence.
Methods:
Pooling data from the Colorado Young Women Breast Cancer cohort and the Breast Cancer Health Disparities Study (N=2519 cases), we examined the association of parity, age, and clinical factors with overall survival (OS) of breast cancer over 15 years of follow-up.
Results:
Women with PPBC diagnosed at <45y had the lowest OS (p<0.0001), while OS of nulliparous cases diagnosed at <45y did not differ from OS of cases diagnosed 45–65y regardless of parity status. After adjustment for study site, race/ethnicity, clinical stage, year of diagnosis, and stratification for estrogen receptor status, PPBC remained an independent factor associated with poor OS. Among cases diagnosed at <45y, nulliparous cases had 1.6 times better OS (hazard ratio (HR)=0.61, 95%CI 0.42–0.87) compared to those with PPBC, with a more pronounced survival difference among stage I breast cancers (HR=0.30, 95%CI 0.11–0.79). Among very young women diagnosed at age ≤35y, nulliparous cases had 2.3 times better OS (HR=0.44, 95%CI 0.23–0.84) compared to PPBC.
Conclusion:
Our results suggest that postpartum status is the main driver of poor prognosis in young women with breast cancer, with the strongest association in patients diagnosed at age ≤35y and in those with stage I disease.
Keywords: breast cancer, young women’s breast cancer, postpartum breast cancer, overall survival
Graphical Abstract
Introduction:
Annually in the United States, there are ~27,000 newly diagnosed cases of breast cancer in women aged less than 45 years (<45y)1. Although the definition of young women’s breast cancer (YWBC) is inconsistent, we define YWBC as breast cancer diagnosed <45y, given the increasing frequency of women having children at older ages2, 3 and the association between recent parity and increased breast cancer incidence4–6. YWBC has an increased risk of recurrence and death7, 8. Breast cancer-specific mortality is increased 1.4–2.0 times in young compared to older women9, 10. Moreover, the improvement in survival has been less in YWBC, with survival disparities between younger and older patients getting worse since 197511 and incidence of metastatic breast cancer in young women raising by 2% per year12.
Adverse outcomes in YWBC are in part due to delayed diagnosis and advanced disease presentation in the absence of screening for women <40y7. Another factor contributing to worse survival is the increased proportion of aggressive biologic subtypes in YWBC7–9, 11. Nevertheless, the association between young age at diagnosis and adverse outcomes is the strongest among women with luminal and early-stage disease9, 13, 14. A large retrospective study demonstrated that compared to patients diagnosed at age 51–60y, those diagnosed at <40y with luminal A or B tumors had significantly increased risk of breast cancer-specific death, whereas the hazard ratios (HRs) were not significant in triple negative and HER2-positive subtypes14. Multiple studies demonstrated that after adjustment for stage, tumor subtype and other prognostic tumor characteristics, young age at diagnosis is an independent risk factor for relapse and breast cancer related death7, 9, 10, 13, 14.
A substantial proportion of patients diagnosed with breast cancer at <45y are cases with postpartum breast cancer (PPBC) defined as breast cancer diagnosed within the first 5 years after childbirth. The greatest increased risk for distal recurrence is seen among women diagnosed within 5 years2, though the negative effect persists for those diagnosed up to 10 years from last childbirth2, 3. These negative outcomes are specific to PPBC2, 3, 15, 16 and not found in breast cancer diagnosed during pregnancy17, 18. Overall survival (OS) of patients diagnosed and treated during pregnancy is similar to OS of non-pregnant patients17, 18, while PPBC cases are more likely to have worse outcomes2, 3, 15, 19–21.
Using a pooled dataset of 2,519 cases, we examined the association of parity, age at diagnosis and other clinical factors with OS in women diagnosed with breast cancer at age ≤65y. Importantly, given our large sample size, we were able to stratify the case sample to examine the relationship between two additional features; a very young age at diagnosis (defined as ≤35y, an independent negative prognostic factor within YWBC9) and early disease stage at diagnosis, with parity status and OS in YWBC.
Methods:
Study sample:
We pooled data from the Colorado Young Women Breast Cancer (YWBC) Cohort, and the Breast Cancer Health Disparities Study (BCHDS) (Fig. 1). The Colorado YWBC Cohort has been previously described2, 3. Patients diagnosed at age ≥18 years were prospectively recruited from 2004–2014, or retrospectively identified using tumor registry and electronic medical record search for the presence of a breast cancer diagnosis from 1981–2003. The BCHDS22, 23 was comprised of three population-based case-control studies, two of which were included in this analysis: the 4-Corners Breast Cancer Study24 and the San Francisco Bay Area Breast Cancer Study25, with cases diagnosed at age 25–79y from 1995–2004. A total of 5,802 cases were available. This study was approved by the Institutional Review Boards of all participating institutions.
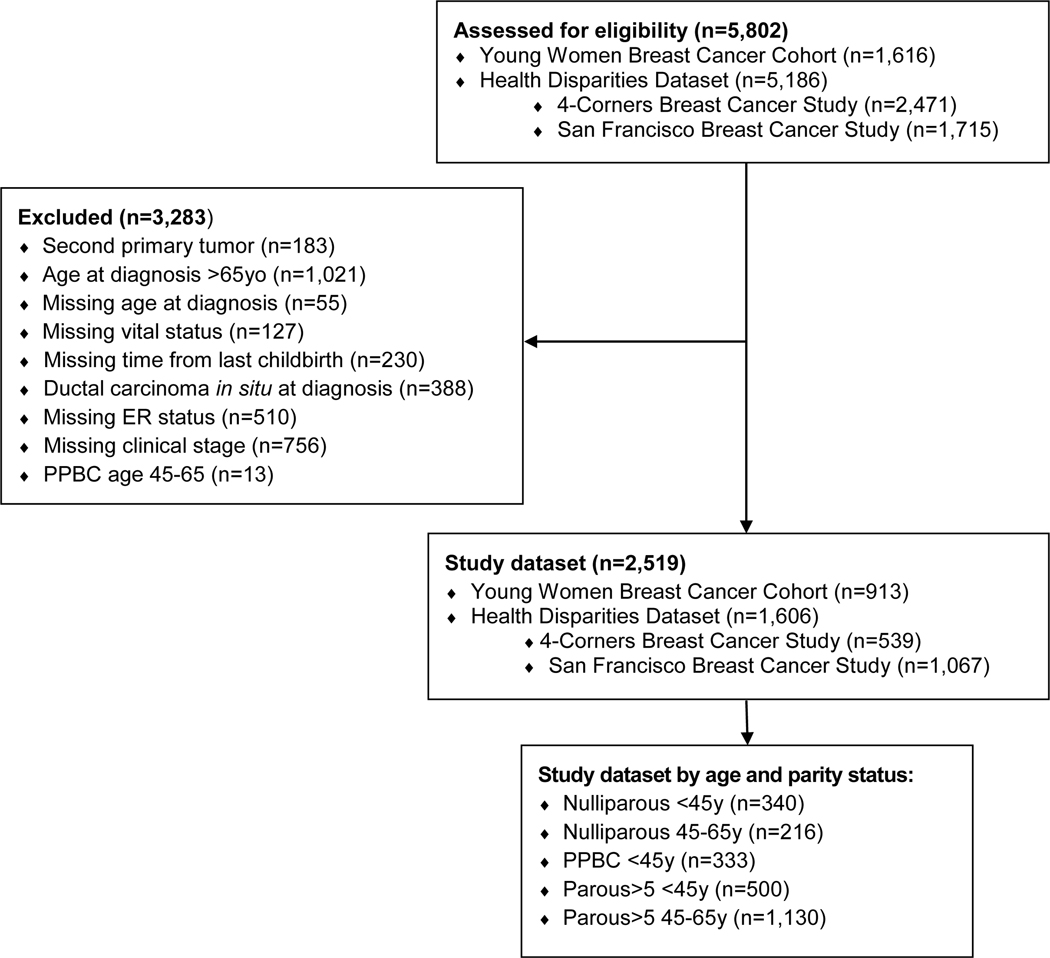
Fig. 1.
Consort diagram, selection of patients for survival analysis
We limited the study sample to cases diagnosed at ≤65y to minimize the risk of non-breast cancer related deaths. We defined YWBC as diagnoses at <45y2, 26, and breast cancer in very young women as diagnoses at ≤35y9. We distinguished cases with a breast cancer diagnosis within 5 years of last childbirth (PPBC)3, or more than 5 years since last childbirth (Parous>5). Nulliparous cases had no reported childbirth prior to breast cancer diagnosis. We categorized cases into 5 subgroups according to parity and age at diagnosis: Nulliparous <45y, PPBC <45y, Parous>5 <45y, Nulliparous 45–65y, and Parous>5 45–65y. We did not include PPBC cases diagnosed at age 45–65y, as the number of cases was too small for analysis.
Of 5,802 cases, 3,283 cases were excluded from the study because of a history of a second primary tumor, in situ diagnosis, diagnosed at >65y, or missing data on vital status, age at diagnosis, ER status, clinical stage, or time since last childbirth (Fig. 1). Additionally, we excluded 13 PPBC 45–65y cases. The final study dataset included 2,519 cases.
Definitions for clinical and pathologic parameters:
Clinical stage was defined according to American Joint Committee of Cancer Staging Manual (7th edition). Cancer biologic subtypes were defined as follows: Luminal A subtype: estrogen receptor positive (ER+) and progesterone receptor positive (PR+), Human epidermal receptor-2 negative (HER2-); Luminal B subtype: ER+ and HER2+ with any PR status, or ER+, PR- and HER2-; HER2+ subtype: ER-, PR-, and HER2+; Triple negative (TNBC) subtype: ER-, PR-, and HER2-. For analyses, cases were categorized as ER+ or ER-, given missing HER2 status for many cases. Body mass index (BMI) was calculated as weight (kilograms) divided by height squared (meters). BMI was missing for 903 cases.
Study endpoint:
Primary outcome was OS, defined as the percentage of cases alive at 5, 10 and 15 years after breast cancer diagnosis. OS was collected for the Colorado YWBC cohort through tumor registry data and medical record review. For the BCHDS, OS was obtained via linkage with the cancer registries.
Statistical approach:
Data fields within BCHDS were harmonized22 and then the two studies were harmonized to assure common definitions. The main variable requiring integration was the parity data, where the time (in years) between last childbirth and date of diagnosis were uniformly identified for all parous cases. Variables of interest were merged in SAS 9.4 (SAS institute) and stored in RedCap. Statistical analysis was performed in PRISM 7.0 (GraphPad), or SAS 9.4. P-values <0.05 were considered statistically significant, except in Table 1 where p<0.0025 was considered significant after correction for multiple comparisons. Chi-square, or Fisher’s exact test were used to evaluate differences in categorical variables, one-way ANOVA was used for continuous variables. We estimated OS probabilities by the Kaplan-Meier method and compared survival among subgroups by log rank test. Multivariable Cox proportional hazard models were applied to estimate hazard ratios (HRs) and 95% confidence intervals (CIs) after adjustment for study site, race/ethnicity, clinical stage, and diagnosis year categorized as <1999 (bone marrow transplant era), 1999–2003 (treatment dominated by chemotherapy), and 2004–2007 (adjuvant HER2-targeted agents became available) and 2008–2014 (current treatment based on biologic subtype and genomic data). ER status was treated as the stratum variable assuming that the baseline hazard function differed between ER+ and ER- disease. We performed an exploratory analysis to look at frequency of TNBC and HER2+ cases among YWBC, as we have not found an increased frequency of the TNBC and HER2+ in PPBC previously2,3, though that is often assumed the reason for poorer prognosis in PPBC. Cases were censored at date of last follow-up, or if they were alive past 15 years from the cancer diagnosis.
Table 1.
Characteristics of breast cancer patients, by age at diagnosis and parity status
| Clinico-pathologic characteristics | Nulliparous <45y | PPBCa <45y | Parous>5 <45y | pb <45y | Nulliparous 45–65y | Parous>5 45–65y | pc 45–65y | pd | |
|---|---|---|---|---|---|---|---|---|---|
| N | 340 | 333 | 500 | 216 | 1130 | ||||
|
| |||||||||
| Mean age at diagnosis | 37.0±5.1 | 36.1±4.9 | 40.4±3.2 | <0.001 | 53.3±6.5 | 54.3±6.0 | 0.034 | <0.001 | |
|
| |||||||||
| Mean BMIe (kg/m2) | 25.2±6.4 | 25.7±5.2 | 27.6±6.6 | 0.001 | 27.9±7.0 | 29.1±6.3 | 0.047 | <0.001 | |
|
| |||||||||
| Study site n (%) | YWBCf Cohort | 260 (76.5) | 249 (74.8) | 279 (55.8) | 43 (19.9) | 82 (7.3) | |||
| 4-Corners Study | 24 (7.0) | 30 (9.0) | 69 (13.8) | 43 (19.9) | 373 (33.0) | ||||
| SFBCSg | 56 (16.5) | 54 (16.2) | 152 (30.4) | 130 (60.2) | 675 (59.7) | ||||
|
| |||||||||
| Race / ethnicity n (%) | Black | 7 (2.1) | 12 (3.6) | 15 (3.0) | <0.001 | 0 (0.0) | 4 (0.3) | <0.001 | <0.001 |
| Hispanic | 56 (16.5) | 68 (20.4) | 175 (35.0) | 75 (34.7) | 591 (52.3) | ||||
| Non-Hispanic White | 242 (71.2) | 224 (67.3) | 284 (56.8) | 138 (63.9) | 530 (46.9) | ||||
| Other | 11 (3.2) | 8 (2.4) | 7 (1.4) | 1 (0.5) | 2 (0.2) | ||||
| Unknown | 24 (7.0) | 21 (6.3) | 19 (3.8) | 2 (0.9) | 3 (0.3) | ||||
|
| |||||||||
| Cancer Histology n (%) | Ductal | 283 (83.2) | 282 (84.7) | 407 (81.4) | 0.089 | 166 (76.9) | 859 (76.0) | 0.499 | 0.003 |
| Ductal + lobular | 22 (6.5) | 13 (3.9) | 20 (4.0) | 11 (5.1) | 84 (7.4) | ||||
| Lobular | 10 (2.9) | 12 (3.6) | 33 (6.6) | 19 (8.8) | 82 (7.3) | ||||
| Other | 17 (5.0) | 21 (6.3) | 27 (5.4) | 12 (5.5) | 76 (6.7) | ||||
| Unknown | 8 (2.4) | 5 (1.5) | 13 (2.6) | 8 (3.7) | 29 (2.6) | ||||
|
| |||||||||
| Cancer subtype n (%) | Luminal A | 77 (22.6) | 65 (19.5) | 84 (16.8) | <0.001 | 14 (6.5) | 19 (1.7) | <0.001 | <0.001 |
| Luminal B | 101 (29.7) | 93 (28.0) | 101 (20.2) | 14 (6.5) | 19 (1.7) | ||||
| Luminal, HER2h unknown | 66 (19.4) | 66 (19.8) | 158 (31.6) | 145 (67.1) | 797 (70.5) | ||||
| HER2 positive | 16 (4.7) | 26 (7.8) | 26 (5.2) | 2 (0.9) | 7 (0.6) | ||||
| Triple negative HRi negative | 42 (12.4) | 49 (14.7) | 47 (9.4) | 4 (1.8) | 16 (1.4) | ||||
| HER2 unknown | 38 (11.2) | 34 (10.2) | 84 (16.8) | 31 (14.4) | 251 (22.2) | ||||
|
| |||||||||
| Stage n (%) | 1 | 124 (36.5) | 88 (26.4) | 176 (35.2) | 0.100 | 112 (51.9) | 537 (47.5) | 0.605 | <0.001 |
| 2 | 155 (45.6) | 166 (49.8) | 225 (45.0) | 87 (40.3) | 482 (42.7) | ||||
| 3 | 45 (13.2) | 60 (18.0) | 77 (15.4) | 13 (6.0) | 90 (8.0) | ||||
| 4 | 16 (4.7) | 19 (5.8) | 22 (4.4) | 4 (1.8) | 21 (1.8) | ||||
|
| |||||||||
| Grade n (%) | Grade I | 41 (12.1) | 23 (6.9) | 62 (12.4) | 0.018 | 56 (25.9) | 201 (17.8) | 0.052 | <0.001 |
| Grade II | 113 (33.2) | 105 (31.5) | 182 (36.4) | 79 (36.6) | 463 (41.0) | ||||
| Grade III | 161 (47.4) | 171 (51.4) | 208 (41.6) | 62 (28.7) | 351 (31.0) | ||||
| Unknown | 25 (7.3) | 34 (10.2) | 48 (9.6) | 19 (8.8) | 115 (10.2) | ||||
|
| |||||||||
| ERj n (%) | Positive | 243 (71.5) | 224 (67.3) | 342 (68.4) | 0.468 | 179 (82.9) | 856 (75.8) | 0.029 | <0.001 |
| Negative | 97 (28.5) | 109 (32.7) | 158 (31.6) | 37 (17.1) | 274 (24.2) | ||||
|
| |||||||||
| PRk n (%) | Positive | 222 (65.3) | 215 (64.6) | 308 (61.6) | 0.592 | 151 (69.9) | 741 (65.6) | 0.290 | 0.338 |
| Negative | 115 (33.8) | 114 (34.2) | 183 (36.6) | 63 (29.2) | 372 (32.9) | ||||
| Unknown | 3 (0.9) | 4 (1.2) | 9 (1.8) | 2 (0.9) | 17 (1.5) | ||||
|
| |||||||||
| Menopausal status n (%) | Premenopausal | 319 (93.8) | 322 (96.7) | 449 (89.8) | 0.003 | 88 (40.7) | 347 (30.7) | 0.006 | <0.001 |
| Postmenopausal | 13 (3.8) | 7 (2.1) | 35 (7.0) | 115 (53.2) | 698 (61.8) | ||||
| Unknown | 8 (2.4) | 4 (1.2) | 16 (3.2) | 13 (6.0) | 85 (7.5) | ||||
PPBC – post-partum breast cancer;
p-value for comparison of Nulliparous <45y, Parous>5 <45y, and PPBC <45y;
p-value for comparisson of Nulliparous 45–65y and Parous>5 45–65y;
p-value for comparison of 5 subgroups; p-values were calculated not including unknown / missing variables; adjusting for multiple comparisons, p<0.0025 was considered statistically significant;
BMI – body mass index, calculated as weight (kg) divided by height squared (m);
YWBC – young women breast cancer;
SFBCS – San Francisco Bay Area Breast Cancer Study;
HER2 – human epidermal growth factor receptor 2;
HR – hormonal receptors,
ER – estrogen receptor;
PR – progesterone receptor.
Results:
Characteristics of study sample
We compared the clinical and pathologic parameters among the subgroups classified by parity and age at diagnosis (Table 1). Cancer histology, tumor stage, grade, ER and PR status did not differ within the subgroups of cases diagnosed at <45y, and cases 45–65y. There were significant differences between cases diagnosed at <45y and those diagnosed at 45–65y for BMI, race/ethnicity, cancer biologic subtype, tumor stage, grade, ER status and menopausal status (p<0.001). Older patients had higher BMI and younger patients had more advanced stage, higher grade, and a greater proportion of ER negative cases. Overall, ~70% of women diagnosed with breast cancer <45y were parous, and ~30% were diagnosed within 5 years of their most recent childbirth. There was a strong correlation between menopausal status and age (Pearson’s r=0.94, p=0.002). Multivariable regression models were adjusted for study site, race/ethnicity, tumor stage, and ER status. In an exploratory sub-set analysis, among cases with complete biologic subtype information [n=849], the proportion of TNBC and HER2+ cases were similar for parity-based subgroups within each age category (Fisher exact test p<0.05).
Overall survival
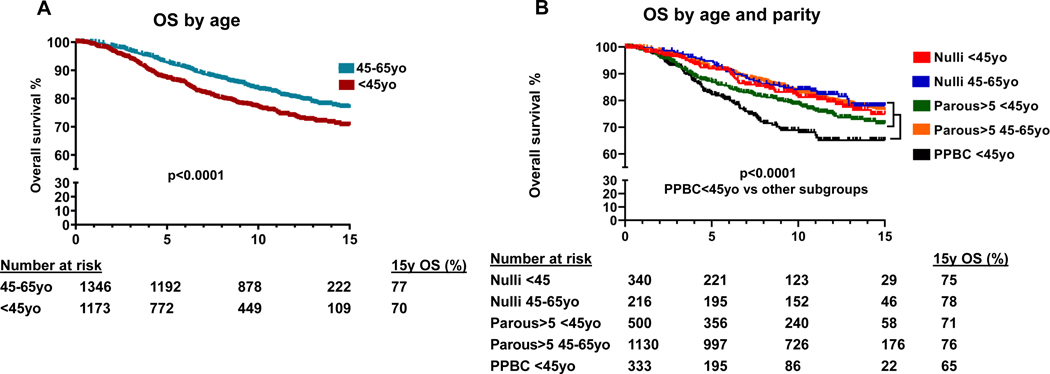
Comparing OS among the five subgroups (Fig. 2, Table S1), women diagnosed at <45y had worse survival than those diagnosed at 45–65y (p<0.001; Fig. 2A), and in women <45y survival differed by parity status. PPBC cases diagnosed at <45y had the lowest OS compared to all other subgroups (p<0.0001; Fig. 2B), whereas survival of Nulliparous <45y cases and Parous>5 <45y cases did not differ compared to all cases 45–65y. At 15 years of follow-up, survival probability was 65% for PPBC <45y cases, compared to 75% and 71% for Nulliparous <45y cases and Parous>5 <45y cases, respectively, and 76–78% for cases 45–65y regardless of parity status. In multivariable models (Table 2), compared to PPBC <45y cases, all other subgroups had better survival. OS was highest for Nulliparous <45y compared to PPBC <45y cases (HR=0.61, 95%CI 0.42–0.87). Additional analysis showed that negative effect of post-partum status on OS persisted up to 10 years after the last childbirth, while survival of Nulliparous <45y remained significantly better (Table S2). Furthermore, removal of the menopausal patients from the study groups did not alter the outcomes in multivariate analysis with significant OS difference between PPBC and Nulliparous <45y (Table S3).
Fig. 2.
Overall survival (OS) in years (N=2,519) grouped by age (A) and by age at diagnosis and parity status (B); number of cases at risk is indicated; Nulli – Nulliparous cases; 15y OS – overall survival 15 years post diagnosis
Table 2.
Multivariable adjusted overall survival hazard ratios among women with breast cancer, by age at diagnosis and parity status
| Group | Subgroup by age/parity | N | Deaths (%) | HRa | 95%CIb |
|---|---|---|---|---|---|
| All cases (N=2,519) | PPBC <45y | 333 | 78 (23.4) | Refc | |
| Nulliparous <45y | 340 | 50 (14.7) | 0.61* | 0.42–0.87 | |
| Parous>5 <45y | 500 | 105 (21.0) | 0.78 | 0.57–1.04 | |
| Nulliparous 45–65y | 216 | 40 (18.5) | 0.71 | 0.48–1.06 | |
| Parous>5 45–65y | 1130 | 213 (18.9) | 0.67* | 0.50–0.89 | |
|
| |||||
| <45y (N=1,173) | PPBC ≤35y | 128 | 33 (25.8) | Ref | |
| Nulliparous ≤35y | 123 | 13 (10.6) | 0.44* | 0.23–0.84 | |
| Parous>5 ≤35y | 45 | 10 (22.2) | 0.62 | 0.31–1.24 | |
| PPBC 36–44y | 205 | 45 (22.0) | 0.79 | 0.48–1.28 | |
| Nulliparous 36–44y | 217 | 37 (17.0) | 0.55* | 0.33–0.91 | |
| Parous>5 36–44 | 455 | 97 (21.3) | 0.68 | 0.44–1.05 | |
|
| |||||
| Stage I (N=1,037) | PPBC <45y | 88 | 13 (14.8) | Ref | |
| Nulliparous <45y | 124 | 6 (4.8) | 0.30* | 0.11–0.79 | |
| Parous>5 <45y | 176 | 15 (8.5) | 0.46* | 0.22–0.99 | |
| Parous>5 45–65y | 537 | 59 (10.9) | 0.56 | 0.28–1.10 | |
| Nulliparous 45–65y | 112 | 14 (12.5) | 0.61 | 0.28–1.36 | |
HR - hazard ratio;
CI – confidence interval;
Ref – reference category; death (%) - unadjusted number of deaths and deaths rate; HRs were adjusted for study site, race/ethnicity, diagnosis year, and clinical stage and stratified for ER status; survival time was censored at 15 years post diagnosis;
p≤0.05
Very young age at diagnosis and parity comparison
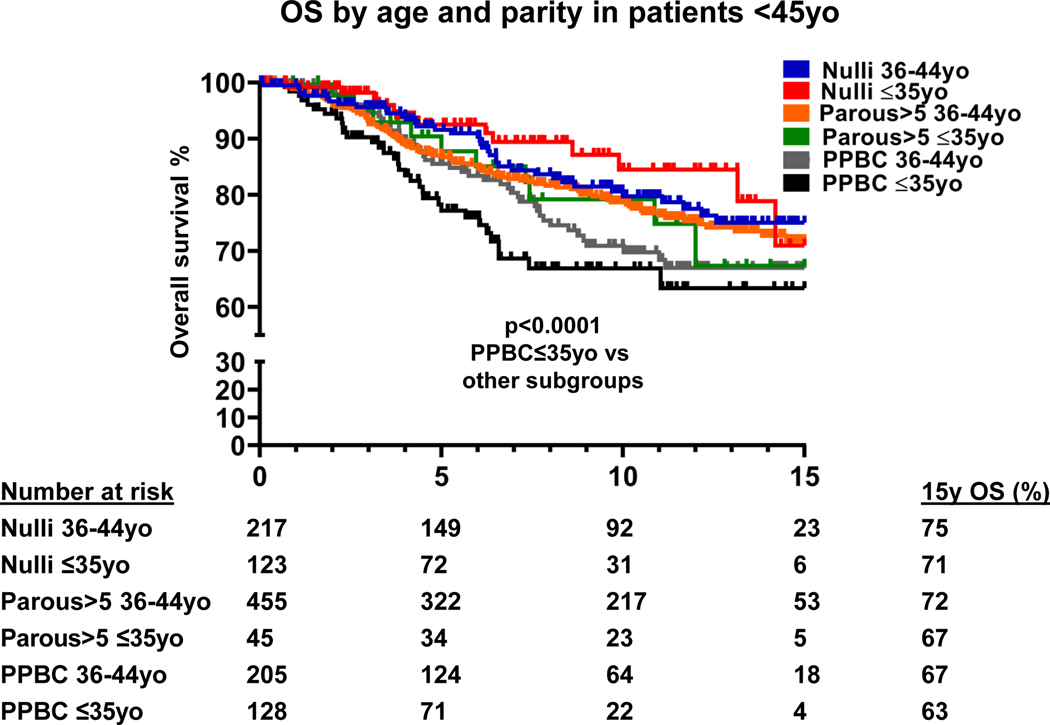
Given the known poor prognosis of a very young age at breast cancer diagnosis7, we examined the impact of parity on OS between cases diagnosed at ≤35y and 36–44y (Fig. 3, Table S1). The survival curves separated based on youngest age at diagnosis and parity. At 15 years of follow-up, PPBC cases diagnosed at ≤35y had the worst OS of only 63% (p<0.001, Fig. 3), compared with 71% and 67% for Nulliparous cases and Parous>5 cases diagnosed at ≤35y, respectively. PPBC cases diagnosed at 36–44y had a 67% OS, compared with 75% and 72% for Nulliparous cases and Parous >5 cases, respectively. In multivariable models (Table 2), PPBC status remained the main factor negatively affecting survival of very young cases, whereas Nulliparous cases had better outcomes regardless of age. Among cases diagnosed at ≤35y, OS of Nulliparous cases was 2.3 times better (HR=0.44, 95%CI 0.23–0.84) than PPBC cases diagnosed at ≤35y. Survival of PPBC cases was poor regardless of age at diagnosis (≤35y vs. 36–44y).
Fig. 3.
Overall survival (OS) cases <45 years (N=1,173) grouped by age at diagnosis and parity status; number of cases at risk is indicated; Nulli – Nulliparous cases; 15y OS – overall survival 15 years post diagnosis
Overall survival of patients with stage I breast cancer is influenced by parity in YWBC
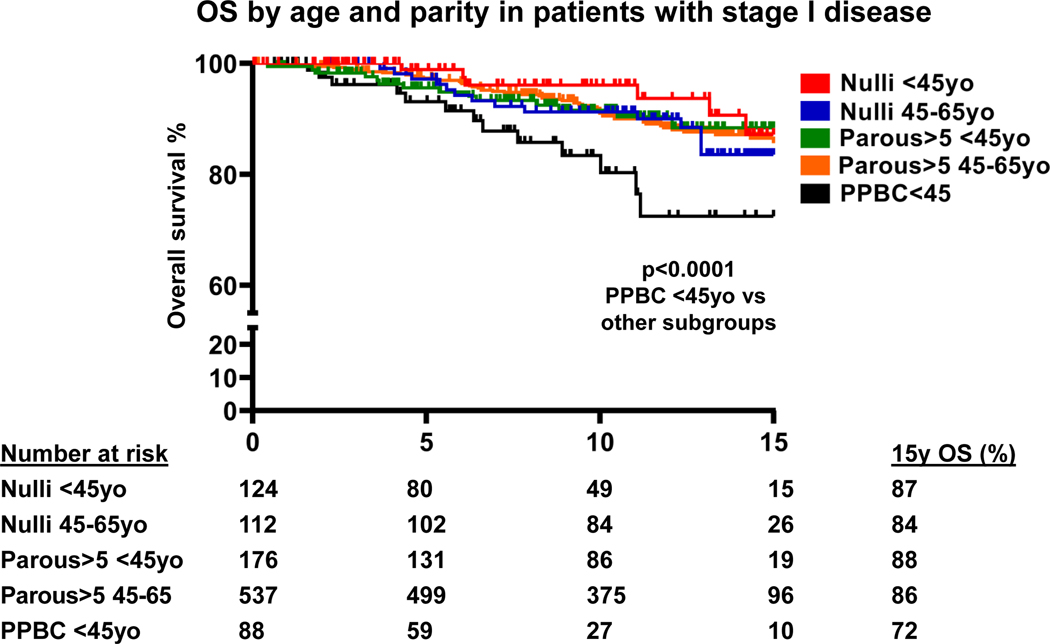
We explored the influence of parity on OS in cases diagnosed at <45y with stage I disease (Fig. 4, Table S1). We observed statistically significant differences in OS between Stage I PPBC <45y cases and all other stage I subgroups (p<0.0001, Fig. 4). PPBC <45y cases had an OS of only 72% at 15 years of follow-up in comparison with an 84–88% range for the other groups. In multivariable models (Table 2), OS of Nulliparous <45y and Parous>5 <45y cases were significantly higher (HR=0.30, 95%CI 0.11–0.79, and HR=0.46, 95%CI 0.22–0.99, respectively) compared to stage I PPBC <45y cases. Exploratory analysis showed that in patients with stage II disease, there was a trend towards better survival of Nulliparous <45y compared to PPBC <45y (p=0.08); survival of patients with stage III disease was poor regardless of parity (Table S4).
Fig. 4.
Overall survival (OS) cases with stage I disease (N=1,037) grouped by age at diagnosis and parity status; number of cases at risk is indicated; Nulli – Nulliparous cases; 15y OS – overall survival 15 years post diagnosis
Discussion:
What causes the poorer prognosis of YWBC has been under examination for many years and the current thinking is often that all young women diagnosed with breast cancer face a poorer prognosis than their older counterparts. The naturally occurring childbearing years directly overlap with early-onset breast cancer for women aged 20–45y. Here we showed that ~30% of women <45y were diagnosed within 5 years of their most recent childbirth, demonstrating that PPBC is not a rare event. We found that women diagnosed <45y and within 5 years of their most recent childbirth have the poorest OS. We also found that OS was worse for <45y women up to ten years after their last childbirth, consistent with our prior findings of a smaller but significant increased risk for metastasis persisting in women between 5 and ten years postpartum at diagnosis.3 Among these young mothers, the ‘postpartum effect’ persists when clinical and pathologic factors are adjusted for, including stage and ER status. These data confirm the importance of the time from last childbirth as a highly relevant biomarker for YWBC outcomes, as we have now shown that survival is impacted in addition to the risk for metastasis.2,3 Indeed, the postpartum cases appear to be the cases driving the poor prognosis of YWBC, as we showed, for the first time, that nulliparous women <45y and parous women <45y diagnosed more than 5 years after the most recent childbirth have similar prognosis in comparison to women diagnosed 45–65y. These data highlight that not all YWBC have poorer outcomes and research focused on postpartum breast cancer as the highest risk subset is warranted.
Among very young women diagnosed at ≤35y, where age already impacts prognosis7, we show for the first time that a PPBC diagnosis confers a significantly poorer OS, with a survival probability of only 63% by 15 years post diagnosis. Nulliparous cases had 2.3 times better OS compared to very young PPBC cases. These data highlight for the first time the importance of parity status among our very young cases as a driving feature for worse survival. We also noted that 15-year survival of Parous>5 ≤35y cases (67%), and PPBC 36–44y cases (67%) was worse in comparison to Parous>5 36–44y cases or nulliparous cases. These findings are consistent with our analysis of OS defining PPBC up to 10 years after the last childbirth for the <45y cases, as more women in the younger group are within ten years as opposed to beyond ten years postpartum.
Even when the ‘best case scenario’ of an early stage I diagnosis occurs in young women, which we found in only ~30% of cases, prognosis was significantly worse for PPBC <45y cases. A nulliparous young woman has a 3.3 times better chance of surviving a stage I diagnosis than her young mother counterpart.
Our study has several strengths. It is a large cohort across multiple geographic areas with Hispanic women accounting for 38% of cases. It has sufficient clinical detail to permit for important statistical adjustments [i.e., stage and ER status]. It is the first study that has compared OS of YWBC stratified by parity status and the additional factor of very young age. It is also the first study to compare these YWBC subgroups with outcomes of women diagnosed 45–65y, allowing the contribution of parity as a poor risk factor, and conversely, nulliparity or later parous status as a favorable risk factor, to be highlighted in comparison to a more common age range of diagnosis. These data demonstrate the “one risk fits all” thinking of higher risk for YWBC is not accurate. There are some limitations to the study. BMI and tumor grade were not included in multivariable model because of cases with missing values. Missing HER2 status prevented full analysis of biologic subtypes and adjustment of survival probabilities for cancer subtype beyond ER. Lastly, we were not able to adjust for treatment, and instead adjusted for year of diagnosis as a surrogate.
The poorer survival of women with PPBC may have multiple underlying mechanisms15, 27. The postpartum changes in the breast at the time of involution (weaning) are sufficient to increase tumor invasion and metastasis in murine models28, 29 and similar changes are present in the healthy breast tissue of post-lactating women30. Moreover, these changes are durable, explaining how a biologic window could alter prognosis of a subsequent breast cancer years into the future31. Recent research has suggested potential targets for PPBC interventions, including pathways related to lymphangiogenesis and immune modulation, and offering hope for reversing the poor prognosis15, 27.
In conclusion, this study highlights the poor survival for women diagnosed <45y with PPBC across a large and diverse cohort. We hope to bring enhanced recognition to the postpartum conundrum of YWBC and emphasize the opportunity to systematically collect parity data, asking the age at most recent childbirth prior to diagnosis, to better hone our knowledge of the ‘postpartum effect’. PPBC remains an under-recognized high-risk breast cancer group. In published research, YWBC are commonly grouped together regardless of parity, or early PPBC cases are grouped with breast cancer arising during pregnancy as “pregnancy associated breast cancer”, which obscures the different outcomes in these biologically distinct groups2, 19. We demonstrate that PPBC is an independent adverse prognostic factor for breast cancer survival and potentially a main factor determining poor OS in YWBC. With improved understanding of the factors driving prognosis in PPBC, we can achieve tailored management of this breast cancer group that faces the greatest need to improve survival.
Supplementary Material
Highlights:
Postpartum breast cancer (PPBC) is diagnosed within 5 years since childbirth
In multivariate analysis, PPBC an independent driver of poor prognosis
Young women with PPBC have the worst overall survival (OS) at 15 years of follow up
Negative effect of PPBC on OS is the greatest in patients diagnosed at ≤35 years
Even in stage I disease, negative effect of PPBC on OS is significant
Funding:
AACR-BCRF 09-06-26BORG grant to VB, Glass and Connor Family Foundations awards to VB; CDMRP DOD BC060531 and R01CA169175 award to VB and PS; NIH/NCATS CCTSI KL2TR001080 and NIH 1K08CA241071 awards to ES; NIH/NCATS CTSI UL1 TR001082 for RedCap; University of Colorado Cancer Center support grant P3CA046934. The Breast Cancer Health Disparities Study funded by NIH CA14002. The 4-Corners Breast Cancer Study supported by NIH CA078682, CA078762, CA078552, and CA078802; NIH contract #N01-PC-67000 with Utah Cancer Registry with support from Utah Department of Health; CDC and National Program of Cancer Registries funds to New Mexico, Arizona and Colorado Cancer Registries, with state support. The San Francisco Bay Area Breast Cancer Study supported by NIH CA63446 and CA77305, DOD DAMD17-96-1-6071 award, and 7PB-0068 from the California Breast Cancer Research Program.
Footnotes
Declaration of Interest Statement:
All authors declare no conflict of interest.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- 1.National Cancer Institute. Surveillance Epidemiology and End Results. SEER Stat Fact Sheets; 2009 - 2015: Breast. [Google Scholar]
- 2.Callihan EB, Gao D, Jindal S, et al. Postpartum diagnosis demonstrates a high risk for metastasis and merits an expanded definition of pregnancy-associated breast cancer. Breast Cancer Res Treat. 2013;138: 549–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goddard ET, Bassale S, Schedin T, et al. Association Between Postpartum Breast Cancer Diagnosis and Metastasis and the Clinical Features Underlying Risk. JAMA Netw Open. 2019;2: e186997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schedin P. Pregnancy-associated breast cancer and metastasis. Nat Rev Cancer. 2006;6: 281–291. [DOI] [PubMed] [Google Scholar]
- 5.Lambe M, Hsieh C, Trichopoulos D, Ekbom A, Pavia M, Adami HO. Transient increase in the risk of breast cancer after giving birth. N Engl J Med. 1994;331: 5–9. [DOI] [PubMed] [Google Scholar]
- 6.Slepicka PF, Cyrill SL, Dos Santos CO. Pregnancy and Breast Cancer: Pathways to Understand Risk and Prevention. Trends Mol Med. 2019;25: 866–881. [DOI] [PubMed] [Google Scholar]
- 7.Anders CK, Hsu DS, Broadwater G, et al. Young age at diagnosis correlates with worse prognosis and defines a subset of breast cancers with shared patterns of gene expression. J Clin Oncol. 2008;26: 3324–3330. [DOI] [PubMed] [Google Scholar]
- 8.Azim HA Jr., Michiels S, Bedard PL, et al. Elucidating prognosis and biology of breast cancer arising in young women using gene expression profiling. Clin Cancer Res. 2012;18: 1341–1351. [DOI] [PubMed] [Google Scholar]
- 9.Nixon AJ, Neuberg D, Hayes DF, et al. Relationship of patient age to pathologic features of the tumor and prognosis for patients with stage I or II breast cancer. J Clin Oncol. 1994;12: 888–894. [DOI] [PubMed] [Google Scholar]
- 10.Gnerlich JL, Deshpande AD, Jeffe DB, Sweet A, White N, Margenthaler JA Elevated breast cancer mortality in women younger than age 40 years compared with older women is attributed to poorer survival in early-stage disease. J Am Coll Surg. 2009;208: 341–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anders CK, Johnson R, Litton J, Phillips M, Bleyer A. Breast cancer before age 40 years. Semin Oncol. 2009;36: 237–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnson RH, Chien FL, Bleyer A. Incidence of Breast Cancer With Distant Involvement Among Women in the United States, 1976 to 2009Incidence Trends of Breast Cancer. JAMA. 2013;309: 800–805. [DOI] [PubMed] [Google Scholar]
- 13.Fredholm H, Magnusson K, Lindstrom LS, et al. Long-term outcome in young women with breast cancer: a population-based study. Breast Cancer Res Treat. 2016;160: 131–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Partridge AH, Hughes ME, Warner ET, et al. Subtype-Dependent Relationship Between Young Age at Diagnosis and Breast Cancer Survival. J Clin Oncol. 2016;34: 3308–3314. [DOI] [PubMed] [Google Scholar]
- 15.Lefrere H, Lenaerts L, Borges VF, Schedin P, Neven P, Amant F. Postpartum breast cancer: mechanisms underlying its worse prognosis, treatment implications, and fertility preservation. Int J Gynecol Cancer. 2021;31: 412–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sun X, Nichols HB, Tse C-K, et al. Association of Parity and Time since Last Birth with Breast Cancer Prognosis by Intrinsic Subtype. Cancer Epidemiology Biomarkers & Prevention. 2016;25: 60–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Amant F, von Minckwitz G, Han SN, et al. Prognosis of women with primary breast cancer diagnosed during pregnancy: results from an international collaborative study. J Clin Oncol. 2013;31: 2532–2539. [DOI] [PubMed] [Google Scholar]
- 18.Puchar A, Despierres M, Boudy AS, et al. Prognosis of triple-negative breast cancer associated with pregnancy: A propensity score-matched analysis from the French CALG (Cancer Associe a la Grossesse) network. Breast. 2022;61: 168–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Azim HA Jr., Santoro L, Russell-Edu W, Pentheroudakis G, Pavlidis N, Peccatori FA Prognosis of pregnancy-associated breast cancer: a meta-analysis of 30 studies. Cancer Treat Rev. 2012;38: 834–842. [DOI] [PubMed] [Google Scholar]
- 20.Amant F, Lefrere H, Borges VF, et al. The definition of pregnancy-associated breast cancer is outdated and should no longer be used. Lancet Oncol. 2021;22: 753–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lefrere H, Floris G, Schmidt MK, et al. Breast cancer diagnosed in the post-weaning period is indicative for a poor outcome. Eur J Cancer. 2021;155: 13–24. [DOI] [PubMed] [Google Scholar]
- 22.Slattery ML, John EM, Torres-Mejia G, et al. Genetic variation in genes involved in hormones, inflammation and energetic factors and breast cancer risk in an admixed population. Carcinogenesis. 2012;33: 1512–1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hines LM, Sedjo RL, Byers T, et al. The Interaction between Genetic Ancestry and Breast Cancer Risk Factors among Hispanic Women: The Breast Cancer Health Disparities Study. Cancer Epidemiol Biomarkers Prev. 2017;26: 692–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Slattery ML, Sweeney C, Edwards S, et al. Body size, weight change, fat distribution and breast cancer risk in Hispanic and non-Hispanic white women. Breast Cancer Res Treat. 2007;102: 85–101. [DOI] [PubMed] [Google Scholar]
- 25.John EM, Phipps AI, Davis A, Koo J. Migration history, acculturation, and breast cancer risk in Hispanic women. Cancer Epidemiol Biomarkers Prev. 2005;14: 2905–2913. [DOI] [PubMed] [Google Scholar]
- 26.Goddard ET, Hill RC, Nemkov T, et al. The Rodent Liver Undergoes Weaning-Induced Involution and Supports Breast Cancer Metastasis. Cancer Discov. 2017;7: 177–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Borges VF, Lyons TR, Germain D, Schedin P. Postpartum Involution and Cancer: An Opportunity for Targeted Breast Cancer Prevention and Treatments? Cancer Res. 2020;80: 1790–1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lyons TR, O’Brien J, Borges VF, et al. Postpartum mammary gland involution drives progression of ductal carcinoma in situ through collagen and COX-2. Nat Med. 2011;17: 1109–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martinson HA, Jindal S, Durand-Rougely C, Borges VF, Schedin P. Wound healing-like immune program facilitates postpartum mammary gland involution and tumor progression. Int J Cancer. 2015;136: 1803–1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jindal S, Gao D, Bell P, et al. Postpartum breast involution reveals regression of secretory lobules mediated by tissueremodeling. Breast Cancer Res. 2014;16: R31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lyons TR, Borges VF, Betts CB, et al. Cyclooxygenase-2-dependent lymphangiogenesis promotes nodal metastasis of postpartum breast cancer. J Clin Invest. 2014;124: 3901–3912. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.