Abstract
Purpose
Large studies have demonstrated improved survival outcomes with thoracic endovascular aortic repair (TEVAR) at two and five years compared to medical therapy; however, early TEVAR for acute type B aortic dissection (TBAD) remains controversial. We aimed to evaluate trends and clinical predictors of hospital readmissions in patients undergoing medical management and TEVAR for acute TBADs.
Methods
Materials and The Nationwide Readmissions Database was queried for all 30-day and 90-day index readmissions (30D-IR and 90D-IR, respectively) after a diagnosis of a TBAD from January 2012 to September 2015. Data on readmission diagnosis, patient demographics, and hospital characteristics were collected from readmitted patients and analyzed. Multivariable logistic regression models were used to identify the predictors of readmission after TEVAR or medical medical management of TBAD.
Results
We identified 53,117 patients with acute TBAD. Medical management was the initial treatment modality in 46,985 (88.4%) patients, while 6,132 (11.5%) underwent TEVAR. Factors including older patient age, lower household income, severity of comorbidities, initial hospital length of stay, and urgent procedure demonstrated an increased likelihood of experiencing 30D-IR and 90D-IR (P<0.05). The rate of unplanned readmission for patients undergoing medical management remained stable (11.3% vs. 10.0% for 30D-IR; 19.1% vs. 15.5% for 90D-IR). Reasons for unplanned readmission in the TEVAR cohort were largely related to technical complications. There was no significant difference in readmission costs between medical management and TEVAR.
Conclusion
Number of unplanned readmissions in the TEVAR arm decreased significantly over time, whereas the number of readmissions for medical management remained stable.
Keywords: Aortic dissection, Thoracic endovascular aortic repair, Readmission, Type B dissection, Nationwide readmissions database
INTRODUCTION
The short-term benefit of treatment of complicated type B aortic dissection (TBAD) with thoracic endovascular aortic repair (TEVAR) is well described, and aorta specific benefit of treating uncomplicated TBAD with TEVAR have been reported at 2 and 5 years [1]. Mounting data continues to suggest potential mortality benefit in the use of TEVAR for acute uncomplicated TBAD [2,3]. While the cost of TEVAR is initially more expensive than medical management for TBAD, access to care and the long-term cost of medication pose unique challenges to long-term medical management. Furthermore, the overall long-term compliance with surveillance and follow-up of the patient population remains poor [4]. As more TEVARs are performed for TBADs, more data are available to assess short-term complications, specifically readmission. We sought to investigate the factors that predict readmission after TEVAR and medical management of acute TBADs and how they differ between the groups.
METHODS AND MATERIALS
The data source used was the Nationwide Readmissions Database (NRD) from January 2012 to September 2015, which is available to the public and has been exempt from IRB review. The NRD is an inpatient database created by the Agency for Healthcare Research and Quality as part of the Healthcare Cost and Utilization Project (HCUP). The diagnosis and procedure codes for the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) are available in the NRD. The Clinical Classifications Software (CCS), developed as part of the HCUP, was used to collapse ICD-9-CM diagnoses into a smaller number of clinically meaningful categories. All hospitalizations for patients aged 18 years or older with a primary or secondary diagnosis of dissection of the thoracic or thoracoabdominal aorta (ICD-9-CM: 441.01, 441.02, or 441.03) were included. Patients with procedure codes for open repair were excluded (ICD-9-CM: 38.34, 38.35, 38.44, 38.45, 39.57, or 39.58). Patients were categorized as receiving TEVAR (ICD-9-CM: 39.73) or medical management. Concomitant procedures for proximal debranching (ICD-9-CM: 39.22) and iliac access (ICD-9-CM: 39.29) were also identified. Patients admitted in December were excluded to allow for at least one month of follow-up for each patient. Additionally, patients who died at the index admission were excluded.
Patients with non-urgent and urgent index admission were included in the analysis. Patient demographic and socioeconomic characteristics collected included age, sex, primary insurance, median household income by ZIP code, and patient location. Patient location was based on urban-rural and population classifications developed by the National Center for Health Statistics (central metropolitan: ≥1 million, fringe metropolitan: ≥1 million, metropolitan: 250,000-999,999, and small metro/micropolitan: <250,000). Comorbidities were summarized using a modified version of the Elixhauser score for administrative databases, which is based on the presence of 30 comorbidities [5]. The characteristics of the index admission were as follows: urgent admission, surgical treatments, the Patient Refined Diagnosis Related Group (DRG) severity of illness subclass [6], length of stay (LOS), and total charges in United States dollars. DRG severity categorizes patient complications and comorbidities into minor, moderate, major, or extreme loss of function, based on ICD-9-CM diagnosis codes. Hospital characteristics included small, medium, or large bed number and metropolitan teaching status (metro nonteaching, metro teaching, not metro). Hospital characteristics of readmission were also collected.
The primary outcome of the study was all-cause readmission within 30 or 90 days of index admission. The secondary outcomes were the rate of TEVAR over time, unplanned readmission rates, reasons for readmission based on CCS categories, hospital characteristics of readmissions, and cost of readmissions as measured by total charges. All statistical analyses were performed using weighted numbers and percentages, which allowed estimates of national statistics to be computed. Univariate comparisons between those with and without the index at 30- and 90-day all-cause readmissions were assessed using the Wald chi-squared test for categorical variables and the survey linear regression model for continuous variables. Survey logistic regression with a contrast of orthogonal polynomial coefficients was used to assess the linear trends in TEVAR and readmission rates over time. Comparisons of readmission reasons and surgical treatments between index medical management and TEVAR patients were performed using survey logistic regression. All patient and hospital covariates were entered into a multivariable survey logistic regression model to identify the factors independently associated with 30-day index readmission (30D-IR) and 90-day index readmission (90D-IR). Total charges for readmissions were stratified by hospital characteristics and compared between index medical management and TEVAR using survey linear regression. The analysis of all readmissions was repeated for the unplanned readmissions. All statistical analyses were performed using SAS 9.3 (SAS Institute, Cary, NC, USA) with two-tailed tests, and statistical significance was set at P<0.05.
RESULTS
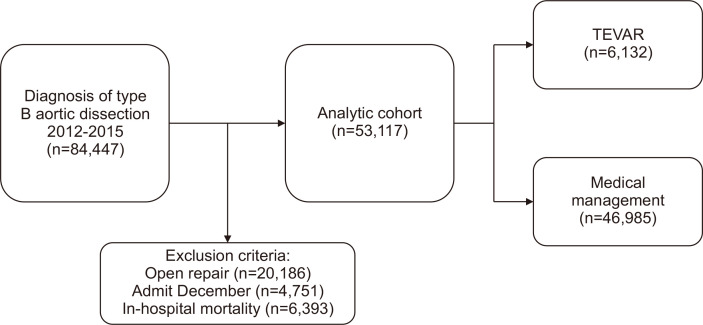
We identified 84,447 patients diagnosed with TBAD in the United States between January 2012 and September 2015. We excluded 20,186 patients who underwent open aortic repair and 6,393 patients who experienced in-hospital mortality. An additional 4,751 patients were excluded in December each year from 2012 to 2014, as patients treated in this time period could not be assessed at the 30-day mark. Thus, 53,117 patients met the inclusion criteria for the analysis. Medical management was the initial treatment modality in 46,985 (88.4%) while 6,132 underwent TEVAR (11.5%) (Fig. 1). At 30 days there were 5,770 (10.9%) unplanned readmissions, and 9,187 (17.2%) unplanned readmissions by 90 days.
Fig. 1.
Patient selection, exclusion criteria, and final cohort chosen for analysis between thoracic endovascular aortic repair (TEVAR) and medical management.
Univariate analysis of patient demographics, city demographics, hospital characteristics, and year of procedure are summarized in Table 1. Older age was significantly associated with unplanned 90D-IR. Univariate analysis also revealed that city size, lower household income, extreme DRG score, higher Elixhauser comorbidity score, earlier year, urgent procedure, concomitant iliac access, and long hospital LOS were more likely to experience unplanned 30D-IR and 90D-IR (P<0.05).
Table 1.
Baseline patient characteristics of unplanned readmissions following type B aortic dissection
| Characteristic | Overall | NR at 30 days | R at 30 days | P-value | NR at 90 days | R at 90 days | P-value |
|---|---|---|---|---|---|---|---|
| Total | 53,117 | 47,347 (89.1) | 5,770 (10.9) | - | 43,930 (82.7) | 9,187 (17.2) | - |
| Age, y | 66.6±0.2 | 66.6±0.2 | 67.1±0.3 | 0.1082 | 66.4±0.2 | 67.6±0.3 | 0.0002* |
| Age group, y | 0.3212 | 0.0039* | |||||
| <60 | 16,978 (32.0) | 15,191 (32.1) | 1,788 (31.0) | 14,256 (32.5) | 2,722 (29.6) | ||
| ≥60 | 36,139 (68.0) | 32,157 (67.9) | 3,982 (69.0) | 29,675 (67.5) | 6,465 (70.4) | ||
| Sex | 0.7313 | 0.0597 | |||||
| Male | 31,322 (59.0) | 27,941 (59.0) | 3,380 (58.6) | 26,044 (59.3) | 5,278 (57.5) | ||
| Female | 21,796 (41.0) | 19,407 (41.0) | 2,389 (41.4) | 17,887 (40.7) | 3,909 (42.5) | ||
| Location of hospital | 0.0342* | <0.0001* | |||||
| Central metro (≥1 million) | 15,717 (29.7) | 13,971 (29.5) | 1,773 (30.7) | 12,798 (29.1) | 2,956 (32.2) | ||
| Fringe metro (≥1 million) | 14,024 (26.5) | 12,448 (26.3) | 1,625 (28.2) | 11,544 (26.3) | 2,530 (27.5) | ||
| Metro (250,000-999,999) | 10,447 (19.7) | 9,440 (20.0) | 1,164 (20.2) | 8,766 (20.0) | 1,837 (20.0) | ||
| Small metro/micropolitan (<250,000) | 12,740 (24.1) | 11,488 (24.3) | 1,208 (20.9) | 10,821 (24.6) | 1,864 (20.3) | ||
| Insurance | 0.1523 | 0.9720 | |||||
| Private/medicare | 42,495 (80.1) | 37,956 (80.3) | 4,539 (78.9) | 35,151 (80.2) | 7,344 (80.1) | ||
| Medicaid/other | 10,525 (19.9) | 9,311 (19.7) | 1,214 (21.1) | 8,704 (19.8) | 1,822 (19.9) | ||
| Median household income for zip code, quartiles | 0.0048* | 0.0005* | |||||
| 1st quartile (0%-25%) | 16,223 (31.1) | 14,262 (30.6) | 1,960 (34.6) | 13,098 (30.3) | 3,125 (34.6) | ||
| 2nd quartile (26%-50%) | 12,968 (24.8) | 11,631 (25.0) | 1,337 (23.6) | 10,859 (25.1) | 2,109 (23.3) | ||
| 3rd quartile (51%-75%) | 12,009 (23.0) | 10,769 (23.1) | 1,240 (21.9) | 10,043 (23.3) | 1,966 (21.7) | ||
| 4th quartile (76%-100%) | 11,026 (21.1) | 9,897 (21.3) | 1,129 (19.9) | 9,183 (21.3) | 1,843 (20.4) | ||
| DRG severity | <0.0001* | <0.0001* | |||||
| Minor | 6,132 (11.5) | 5,461 (11.5) | 671 (11.6) | 5,065 (11.5) | 1,067 (11.6) | ||
| Moderate | 10,635 (20.0) | 9,709 (20.5) | 925 (16.0) | 9,153 (20.8) | 1,482 (16.1) | ||
| Major | 26,451 (49.8) | 23,745 (50.2) | 2,706 (46.9) | 22,088 (50.3) | 4,363 (47.5) | ||
| Extreme | 9,899 (18.6) | 8,433 (17.8) | 1,467 (25.4) | 7,625 (17.4) | 2,274 (24.7) | ||
| Elixhauser Comorbidity Score | 4.9±0.1 | 4.7±0.1 | 6.1±0.2 | <0.0001* | 4.6±0.1 | 6.0±0.2 | <0.0001* |
| Year | 0.0465* | <0.0001* | |||||
| 2012 | 12,991 (24.4) | 11,445 (24.2) | 1,546 (26.8) | 10,457 (23.8) | 2,534 (27.6) | ||
| 2013 | 13,554 (25.5) | 12,151 (25.7) | 1,404 (24.3) | 11,291 (25.7) | 2,263 (24.6) | ||
| 2014 | 14,331 (27.0) | 12,765 (27.0) | 1,566 (27.1) | 11,820 (26.9) | 2,511 (27.3) | ||
| 2015 | 12,241 (23.0) | 10,988 (23.2) | 1,254 (21.7) | 10,363 (23.6) | 1,878 (20.4) | ||
| Management | 0.0003* | 0.0723 | |||||
| Medical | 46,985 (88.5) | 42,035 (88.8) | 4,950 (85.8) | 38,951 (88.7) | 8,035 (87.5) | ||
| Thoracic endovascular aortic repair | 6,132 (11.5) | 5,313 (11.2) | 820 (14.2) | 4,980 (11.3) | 1,152 (12.5) | ||
| Urgent procedure | 46,350 (87.3) | 41,225 (87.1) | 5,125 (88.8) | 0.0319* | 38,150 (86.8) | 8,200 (89.3) | 0.0004* |
| Concomintant procedures | |||||||
| Proximal debranching | 1,206 (2.3) | 1,038 (2.2) | 168 (2.9) | 0.0894 | 955 (2.2) | 251 (2.7) | 0.0940 |
| Iliac access | 604 (1.1) | 479 (1.0) | 125 (2.2) | 0.0033* | 432 (1.0) | 172 (1.9) | 0.0012* |
Values are presented as number (%) or mean±standard error.
NR, not readmitted; R, readmitted; DRG, Patient Refined Diagnosis Related Group.
*Statistically significant P<0.05.
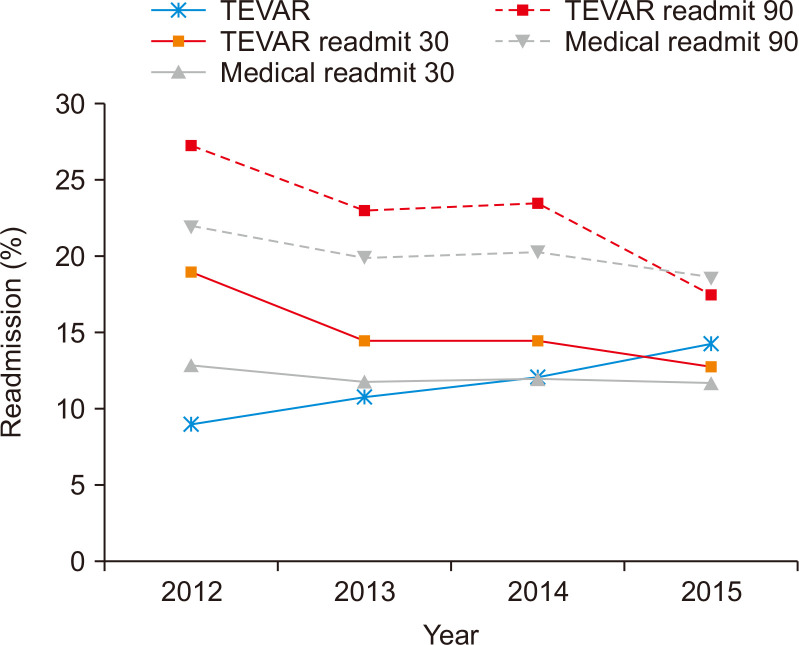
Over the study period, the 30D-IR and 90D-IR for patients undergoing TEVAR decreased significantly (17.7% in 2012 vs. 11.6% in 2015 for 30D-IR; 23.6% in 2012 vs. 14.6% in 2015 for 90D-IR) as the number of TEVARs performed increased slightly (9.0% in 2012 vs. 14.3% in 2015). During this time, the rate of unplanned readmissions for patients undergoing medical management remained stable (11.3% in 2012 vs. 10.0% in 2015 for 30D-IR; 19.1% in 2012 vs. 15.5% in 2015 for 90D-IR) (Fig. 2).
Fig. 2.
Readmissions trends following management of type B aortic dissections. As the number of thoracic endovascular aortic repair (TEVAR) procedures performed increased, the percentage of readmissions significantly decreased over time. The percentage of medical management readmissions remained stable throughout the study.
The primary reasons for unplanned readmissions were analyzed for both the medical and TEVAR cohorts at 30D-IR and 90D-IR, and are shown in Table 2. Overall, the most common reasons for unplanned readmission were vascular complications (aortic, peripheral, and visceral artery aneurysms, 24%), congestive heart failure (CHF, 6%), and septicemia (6%). Patients who underwent TEVAR were significantly more likely to be readmitted for procedural complications related to TEVAR than for other reasons; TEVAR-related complications included aortic, peripheral, and visceral artery aneurysms at both 30D-IR and 90D-IR. Patients who underwent medical management were significantly more likely to be readmitted for medical conditions including CHF, septicemia, renal failure, and respiratory failure.
Table 2.
Primary reasons for 30- and 90-day unplanned index readmission following type B dissection
| Variable | All | Medical | TEVAR | P-value |
|---|---|---|---|---|
| 30-day | ||||
| Total patients | 5,770 | 4,950 | 820 | - |
| Total readmissions | 6,457 | 5,535 | 932 | - |
| Aortic, peripheral, and visceral artery aneurysms | 1,395 (24.2) | 1,011 (20.4) | 384 (46.8) | <0.0001* |
| Congestive heart failure; nonhypertensive | 340 (5.9) | 319 (6.4) | 20 (2.4) | 0.0001* |
| Septicemia (except in labor) | 340 (5.9) | 316 (6.4) | 25 (3.0) | 0.0041* |
| Complication of device; implant or graft | 201 (3.5) | 152 (3.1) | 49 (6.0) | 0.2559 |
| Hypertension with complications and secondary HTN | 160 (2.8) | 147 (3.0) | 12 (1.5) | 0.0416* |
| Cardiac dysrhythmias | 163 (2.8) | 155 (3.1) | 7 (0.9) | 0.0003* |
| Nonspecific chest pain | 105 (1.8) | 94 (1.9) | 12 (1.5) | 0.4108 |
| Pneumonia (except that caused by TB or STD) | 143 (2.5) | 131 (2.6) | 12 (1.5) | 0.0917 |
| Acute and unspecified renal failure | 132 (2.3) | 128 (2.6) | 4 (0.5) | <0.0001* |
| Respiratory failure, insufficiency, arrest (adult) | 113 (2.0) | 105 (2.1) | 8 (1.0) | 0.0483* |
| Complications of surgical procedures or medical care | 163 (2.8) | 132 (2.7) | 31 (3.8) | 0.2768 |
| Acute myocardial infarction | 94 (1.6) | 89 (1.8) | 5 (0.6) | 0.0215* |
| Acute cerebrovascular disease | 107 (1.9) | 101 (2.0) | 6 (0.7) | 0.0193* |
| COPD and bronchiectasis | 77 (1.3) | 77 (1.6) | 0 (0.0) | - |
| UTIs | 80 (1.4) | 64 (1.3) | 16 (2.0) | 0.3531 |
| Gastrointestinal hemorrhage | 102 (1.8) | 88 (1.8) | 14 (1.7) | 0.9371 |
| Fluid and electrolyte disorders | 90 (1.6) | 76 (1.5) | 14 (1.7) | 0.7939 |
| Peripheral and visceral atherosclerosis | 51 (0.9) | 47 (0.9) | 4 (0.5) | 0.2840 |
| Intestinal obstruction without hernia | 50 (0.9) | 46 (0.9) | 4 (0.5) | 0.3660 |
| Deficiency and other anemia | 55 (1.0) | 46 (0.9) | 9 (1.1) | 0.7848 |
| 90-day | ||||
| Total patients | 9,187 | 8,035 | 1,152 | - |
| Total readmissions | 12,295 | 10,723 | 1,572 | - |
| Aortic, peripheral, and visceral artery aneurysms | 2,178 (23.7) | 1,614 (20.1) | 563 (48.9) | <0.0001* |
| Congestive heart failure; nonhypertensive | 543 (5.9) | 509 (6.3) | 34 (3.0) | 0.0002* |
| Septicemia (except in labor) | 519 (5.6) | 483 (6.0) | 36 (3.1) | 0.0024* |
| Complication of device; implant or graft | 336 (3.7) | 274 (3.4) | 63 (5.5) | 0.2488 |
| Hypertension with complications and secondary HTN | 294 (3.2) | 271 (3.4) | 23 (2.0) | 0.1014 |
| Cardiac dysrhythmias | 272 (3.0) | 266 (3.3) | 6 (0.5) | <0.0001* |
| Nonspecific chest pain | 225 (2.5) | 210 (2.6) | 16 (1.4) | 0.0174* |
| Pneumonia (except that caused by TB or STD) | 233 (2.5) | 214 (2.7) | 19 (1.6) | 0.1135 |
| Acute and unspecified renal failure | 210 (2.3) | 199 (2.5) | 11 (1.0) | 0.0069* |
| Respiratory failure, insufficiency, arrest (adult) | 203 (2.2) | 193 (2.4) | 10 (0.9) | 0.0015* |
| Complications of surgical procedures or medical care | 187 (2.0) | 153 (1.9) | 34 (3.0) | 0.1788 |
| Acute myocardial infarction | 172 (1.9) | 164 (2.0) | 8 (0.7) | 0.0042* |
| Acute cerebrovascular disease | 179 (1.9) | 160 (2.0) | 18 (1.6) | 0.5294 |
| COPD and bronchiectasis | 171 (1.9) | 165 (2.1) | 6 (0.5) | 0.0004* |
| UTIs | 125 (1.4) | 111 (1.4) | 15 (1.3) | 0.8588 |
| Gastrointestinal hemorrhage | 119 (1.3) | 108 (1.3) | 11 (1.0) | 0.3594 |
| Fluid and electrolyte disorders | 113 (1.2) | 104 (1.3) | 9 (0.8) | 0.2183 |
| Peripheral and visceral atherosclerosis | 91 (1.0) | 86 (1.1) | 4 (0.3) | 0.0299* |
| Intestinal obstruction without hernia | 90 (1.0) | 84 (1.0) | 6 (0.5) | 0.2105 |
| Deficiency and other anemia | 87 (0.9) | 78 (1.0) | 9 (0.8) | 0.7000 |
Values are presented as number (%).
TEVAR, thoracic endovascular aortic repair; HTN, hypertension; TB, tuberculosis; STD, sexually transmitted disease; COPD, chronic obstructive pulmonary disease; UTI, urinary tract infection.
*Statistically significant P<0.05.
A multivariate analysis of predictors for index unplanned readmission was completed, which found that older age, hospital locations in metro or small metro/micropolitan areas, lower household income, increased comorbidity score, earlier year of diagnosis, TEVAR procedure, need for urgent procedures, and concomitant procedures such as iliac devices were statistically significant predictors of both 30D-IR and 90D-IR (Table 3). Medicaid insurance was found to be a predictor of 30D-IR but not 90D-IR. These factors remained statistically significant in the sub-analysis of urgent procedures, whereas the sub-analysis of non-urgent procedures showed no difference in the rate of readmission by comorbidity, year of diagnosis, TEVAR, or concomitant procedures (Table 3).
Table 3.
Multivariable analysis of predictors for unplanned readmissions following type B dissection
| Variable | Total | Urgent | Elective | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 30 Days | 90 Days | 30 Days | 90 Days | 30 Days | 90 Days | ||||||||||||
| OR (95% CI) | P-value | OR (95% CI) | P-value | OR (95% CI) | P-value | OR (95% CI) | P-value | OR (95% CI) | P-value | OR (95% CI) | P-value | ||||||
| Age, per year increase | 1.05 (1.01-1.08) | 0.0147* | 1.06 (1.03-1.10) | 0.0003* | 1.04 (1.01-1.08) | 0.0221* | 1.05 (1.02-1.09) | 0.0017* | 1.08 (0.96-1.21) | 0.1941 | 1.16 (1.05-1.29) | 0.0054* | |||||
| Sex | |||||||||||||||||
| Male | 0.99 (0.89-1.10) | 0.8064 | 0.95 (0.87-1.03) | 0.1821 | 1.01 (0.90-1.12) | 0.8878 | 0.96 (0.88-1.05) | 0.3573 | 0.82 (0.61-1.10) | 0.1803 | 0.82 (0.64-1.05) | 0.1164 | |||||
| Female | Reference | - | Reference | - | Reference | - | Reference | - | Reference | - | Reference | - | |||||
| Location of hospital | |||||||||||||||||
| Central metro (≥1 million) | Reference | - | Reference | - | Reference | - | Reference | - | Reference | - | Reference | - | |||||
| Fringe metro (≥1 million) | 1.02 (0.90-1.15) | 0.7990 | 1.01 (0.91-1.13) | 0.8402 | 1.02 (0.89-1.17) | 0.7847 | 1.01 (0.91-1.14) | 0.8088 | 0.93 (0.63-1.36) | 0.6901 | 0.94 (0.68-1.29) | 0.6905 | |||||
| Metro (250,000-999,999) | 0.96 (0.84-1.09) | 0.4994 | 0.86 (0.77-0.96) | 0.0068* | 1.00 (0.87-1.17) | 0.9871 | 0.88 (0.78-0.99) | 0.0361* | 0.64 (0.42-0.97) | 0.0350* | 0.68 (0.47-0.96) | 0.0299* | |||||
| Small metro/micropolitan (<250,000) | 0.81 (0.69-0.95) | 0.0076* | 0.71 (0.63-0.81) | <0.0001* | 0.81 (0.69-0.95) | 0.0101* | 0.70 (0.61-0.81) | <0.0001* | 0.76 (0.47-1.23) | 0.2589 | 0.74 (0.52-1.05) | 0.0931 | |||||
| Insurance | |||||||||||||||||
| Private/medicare | Reference | - | Reference | - | Reference | - | Reference | - | Reference | - | Reference | - | |||||
| Medicaid/other | 1.16 (1.01-1.33) | 0.0325* | 1.09 (0.97-1.22) | 0.1438 | 1.13 (0.99-1.30) | 0.0674 | 1.06 (0.95-1.18) | 0.3321 | 1.48 (0.96-2.26) | 0.0730 | 1.45 (1.01-2.08) | 0.0453* | |||||
| Median household income for zip code, quartiles | |||||||||||||||||
| 1st quartile (0%-25%) | Reference | - | Reference | - | Reference | - | Reference | - | Reference | - | Reference | - | |||||
| 2nd quartile (26%-50%) | 0.84 (0.74-0.95) | 0.0045* | 0.81 (0.72-0.90) | <0.0001* | 0.86 (0.75-0.99) | 0.0291* | 0.83 (0.74-0.93) | 0.0016* | 0.67 (0.45-1.01) | 0.0550 | 0.65 (0.47-0.88) | 0.0061* | |||||
| 3rd quartile (51%-75%) | 0.81 (0.71-0.93) | 0.0018* | 0.78 (0.70-0.87) | <0.0001* | 0.80 (0.70-0.93) | 0.0023* | 0.78 (0.69-0.88) | <0.0001* | 0.86 (0.57-1.28) | 0.4575 | 0.77 (0.55-1.09) | 0.1412 | |||||
| 4th quartile (76%-100%) | 0.79 (0.69-0.91) | 0.0010* | 0.75 (0.67-0.84) | <0.0001* | 0.79 (0.69-0.92) | 0.0017* | 0.76 (0.68-0.86) | <0.0001* | 0.74 (0.49-1.13) | 0.1649 | 0.66 (0.45-0.94) | 0.0220* | |||||
| DRG severity | |||||||||||||||||
| Minor | Reference | - | Reference | - | Reference | - | Reference | - | Reference | - | Reference | - | |||||
| Moderate | 0.75 (0.63-0.89) | 0.0013* | 0.75 (0.64-0.86) | <0.0001* | 0.82 (0.68-0.98) | 0.0279* | 0.79 (0.67-0.92) | 0.0021* | 0.46 (0.26-0.81) | 0.0076* | 0.52 (0.33-0.82) | 0.0050* | |||||
| Major | 0.83 (0.70-0.98) | 0.0301* | 0.85 (0.74-0.98) | 0.0257* | 0.86 (0.72-1.02) | 0.0856 | 0.87 (0.75-1.01) | 0.0578 | 0.69 (0.41-1.09) | 0.1074 | 0.75 (0.49-1.15) | 0.1842 | |||||
| Extreme | 1.13 (0.92-1.38) | 0.2375 | 1.16 (0.98-1.36) | 0.0772 | 1.18 (0.97-1.44) | 0.0988 | 1.18 (1.00-1.39) | 0.0502 | 0.79 (0.42-1.49) | 0.4671 | 1.00 (0.58-1.73) | 0.9974 | |||||
| Elixhauser Comorbidity Score, per unit increase | 1.02 (1.01-1.03) | <0.0001* | 1.02 (1.02-1.03) | <0.0001* | 1.02 (1.01-1.03) | <0.0001* | 1.02 (1.02-1.03) | <0.0001* | 1.02 (0.99-1.06) | 0.1628 | 1.01 (0.98-1.05) | 0.3727 | |||||
| Year | |||||||||||||||||
| 2012 | Reference | - | Reference | - | Reference | - | Reference | - | Reference | - | Reference | - | |||||
| 2013 | 0.91 (0.79-1.04) | 0.1527 | 0.89 (0.79-0.99) | 0.0464* | 0.89 (0.76-1.03) | 0.1080 | 0.88 (0.77-0.99) | 0.0430* | 1.13 (0.75-1.71) | 0.5678 | 1.05 (0.71-1.54) | 0.8149 | |||||
| 2014 | 0.96 (0.84-1.10) | 0.5355 | 0.94 (0.83-1.06) | 0.2867 | 0.94 (0.81-1.09) | 0.3878 | 0.92 (0.81-1.06) | 0.2403 | 1.15 (0.74-1.71) | 0.5419 | 1.06 (0.68-1.64) | 0.8090 | |||||
| 2015 | 0.81 (0.71-0.93) | 0.0021* | 0.73 (0.65-0.81) | <0.0001* | 0.80 (0.70-0.92) | 0.0022* | 0.72 (0.63-0.82) | <0.0001* | 0.99 (0.64-1.54) | 0.9641 | 0.84 (0.57-1.22) | 0.3493 | |||||
| Management | |||||||||||||||||
| Medical | Reference | - | Reference | - | Reference | - | Reference | - | Reference | - | Reference | - | |||||
| TEVAR | 1.43 (1.24-1.66) | <0.0001* | 1.26 (1.11-1.44) | 0.0005* | 1.48 (1.25-1.76) | <0.0001* | 1.31 (1.13-1.52) | 0.0003* | 1.22 (0.90-1.67) | 0.2041 | 1.10 (0.82-1.47) | 0.5291 | |||||
| Urgent procedure | 1.21 (1.02-1.42) | 0.0290* | 1.23 (1.07-1.42) | 0.0042* | - | - | - | - | - | - | - | - | |||||
| Concomintant procedures | |||||||||||||||||
| Proximal debranching | 1.24 (0.91-1.70) | 0.1764 | 1.33 (1.03-1.74) | 0.0308* | 1.11 (0.76-1.62) | 0.5867 | 1.26 (0.92-1.72) | 0.1570 | 1.45 (0.90-1.67) | 0.1391 | 1.49 (0.95-2.33) | 0.0828 | |||||
| Iliac access | 2.05 (1.40-3.02) | 0.0003* | 1.93 (1.40-2.67) | <0.0001* | 2.41 (1.58-3.68) | <0.0001* | 2.24 (1.57-3.21) | <0.0001* | 0.99 (0.32-3.13) | 0.9937 | 1.01 (0.42-2.43) | 0.9878 | |||||
| Hospital bed size | |||||||||||||||||
| Small | Reference | - | Reference | - | Reference | - | Reference | - | Reference | - | Reference | - | |||||
| Medium | 1.04 (0.85-1.26) | 0.7249 | 0.97 (0.82-1.14) | 0.7014 | 0.99 (0.81-1.21) | 0.9120 | 0.95 (0.79-1.13) | 0.5556 | 1.65 (0.94-2.91) | 0.0837 | 1.19 (0.74-1.90) | 0.4708 | |||||
| Large | 0.99 (0.83-1.18) | 0.8888 | 0.94 (0.81-1.09) | 0.3834 | 0.94 (0.79-1.13) | 0.4989 | 0.92 (0.79-1.08) | 0.3170 | 1.54 (0.99-2.39) | 0.0545 | 1.05 (0.71-1.53) | 0.8207 | |||||
| Hospital teaching status | |||||||||||||||||
| Metro nonteaching | Reference | - | Reference | - | Reference | - | Reference | - | Reference | - | Reference | - | |||||
| Metro teaching | 0.95 (0.85-1.06) | 0.3250 | 0.94 (0.86-1.02) | 0.1499 | 0.92 (0.82-1.03) | 0.1639 | 0.91 (0.83-1.00) | 0.0513 | 1.23 (0.86-1.76) | 0.2488 | 1.29 (0.96-1.73) | 0.0950 | |||||
| Not metro | 1.35 (1.03-1.78) | 0.0313* | 1.36 (1.08-1.73) | 0.0106* | 1.35 (1.01-1.80) | 0.0436* | 1.35 (1.04-1.73) | 0.0219* | 1.53 (0.71-3.30) | 0.2772 | 1.72 (0.94-3.13) | 0.0766 | |||||
DRG, Patient Refined Diagnosis Related Group; TEVAR, thoracic endovascular aortic repair; OR, odds ratio; CI, confidence interval.
*Statistically significant P<0.05.
When looking at the hospital characteristics of unplanned IRs, TEVAR patients were more likely to be admitted to a larger teaching hospital than those who received medical management (Table 4). Furthermore, no significant difference in the cost of readmission was found between the medical and TEVAR readmission groups for 30D-IR or 90D-IR.
Table 4.
Hospital characteristics of unplanned readmissions following type B aortic dissection
| Hospital characteristic | 30-Day | 90-Day | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Total | Medical | TEVAR | P-value | Total | Medical | TEVAR | P-value | ||
| Total patients | 5,770 | 4,950 | 820 | - | 9,187 | 8,035 | 1,152 | - | |
| Total readmissions | 6,457 | 5,535 | 932 | - | 12,295 | 10,723 | 1,572 | - | |
| Hospital bed size | <0.0001* | 0.0003* | |||||||
| Small | 515 (8.9) | 473 (9.6) | 42 (5.1) | 875 (9.5) | 783 (9.8) | 91 (7.9) | |||
| Medium | 1,344 (23.3) | 1,198 (24.2) | 146 (17.9) | 2,123 (23.1) | 1,925 (24.0) | 198 (17.2) | |||
| Large | 3,910 (67.8) | 3,279 (66.2) | 631 (77.0) | 6,189 (67.4) | 5,326 (66.3) | 863 (74.9) | |||
| Same bed size as index | 4,900 (84.9) | 4,206 (85.0) | 694 (84.6) | 0.8764 | 7,662 (83.4) | 6,722 (83.7) | 941 (81.6) | 0.3293 | |
| Hospital teaching status | 0.0484* | 0.0451* | |||||||
| Metro nonteaching | 1,572 (27.3) | 1,372 (27.7) | 200 (24.4) | 2,615 (28.5) | 2,334 (29.1) | 281 (24.4) | |||
| Metro teaching | 3,863 (67.0) | 3,273 (66.1) | 590 (72.0) | 6,005 (65.4) | 5,192 (64.6) | 814 (70.6) | |||
| Not metro | 334 (5.8) | 305 (6.2) | 30 (3.6) | 566 (6.2) | 509 (6.3) | 58 (5.0) | |||
| Same teaching status as index | 4,904 (85.0) | 4,200 (84.9) | 703 (85.8) | 0.6394 | 7,749 (84.4) | 6,802 (84.7) | 947 (82.2) | 0.2032 | |
| Same hospital as index admission | 4,104 (71.1) | 3,510 (70.9) | 593 (72.4) | 0.5695 | 6,316 (68.7) | 5,547 (69.0) | 769 (66.7) | 0.3051 | |
| Total charges, in thousands of dollars | 71.8±2.6 | 72.6±2.8 | 66.9±5.0 | 0.3110 | 68.0±2.5 | 66.3±2.0 | 79.4±11.3 | 0.2356 | |
Values are presented as number (%) or mean±standard error.
TEVAR, thoracic endovascular aortic repair.
*Statistically significant P<0.05.
DISCUSSION
TBADs have historically been treated with medical management rather than operative interventions. DeBakey et al. [7] and Daily et al. [8] initially recognized this treatment paradigm in the late 1960s when they discovered that the anatomic distribution of dissections directly affected patient outcomes. Mortality was found to be significantly improved when patients with aortic dissection in the ascending aorta underwent surgery compared to medical treatment, but this benefit was not seen in dissection of the descending aorta. The Stanford classification, developed from this finding, has been used to drive treatment for the last 50 years. Therefore, operative intervention has been reserved for type B dissections that are considered complicated, including those with end-organ malperfusion, aortic rupture, hypotension or shock, neurologic sequelae, recurrent or refractory pain, hypertension refractory to medical therapy, early aortic dilation, or propagation of the dissection. It has been well described that patients with complicated type B dissection have worse in-hospital survival than those with uncomplicated type B dissection [9].
Despite technical innovation in TEVAR and a growing body of evidence, medical treatment focused on blood pressure and heart rate control to minimize stress on the aortic wall remains the gold standard of treatment for acute uncomplicated type B dissection. However, chronic medical treatment is not without challenges. Multiple antihypertensive medications are often required to reach blood pressure and heart rate goals in outpatients, and this treatment fails in younger and obese patients [10]. Furthermore, lack of access to care makes sustainable medical management challenging, especially for lower-income families. As such, our dataset demonstrates that lower household income led to a higher rate of unplanned readmissions for both medical and TEVAR-managed TBADs, highlighting the continued disparity seen in the outcomes of patients with complex diseases.
Literature comparing medical management and TEVAR for uncomplicated type B dissection is mixed and evolving. Early studies favored the medical management of TEVAR with regard to cost and outcomes [11]. Initial studies demonstrated technical success using TEVAR for TBAD, but most studies were designed to compare open vs. endovascular approaches for complicated type B dissections and are less likely to be applicable now [12]. Development of the International Registry of Acute Aortic Dissection (IRAD) has allowed for a large analysis of aortic dissection management. Using this registry, Booher et al. [2] identified that performing TEVAR for acute uncomplicated TBADs improved survival 30 days after the onset of dissection symptoms. Similar studies using state registries have demonstrated that although initially more expensive, TEVAR repair for acute uncomplicated TBAD results in improved survival at one and five years [3].
Larger studies using the IRAD have favored decreased 5-year mortality in patients treated with TEVAR when compared to those treated with medical management [13]. Durham et al. [14] reported their experience in treating acute uncomplicated TBADs with TEVAR vs. medical management: over 50% of the medical group ultimately failed medical therapy, leading to 38% mortality and 29% aortic repair. Furthermore, patients who underwent TEVAR demonstrated increased survival starting at the 2-year mark compared with the medical group. The data were further supported by the Investigation of STEnt Grafts in Aortic Dissection trial (INSTEAD-XL) [15]. This study is one of the few randomized controlled trials studying patients with uncomplicated TBAD in the subacute and chronic phases, treated with medical therapy alone, or with medical therapy and TEVAR. Again, following patients for up to five years revealed that patients undergoing TEVAR experienced lower aorta-specific mortality (7% vs. 19%) and trended toward lower all-cause mortality (11% vs. 19%) compared to the medical treatment group [1].
The timing and execution of TEVAR for TBAD are dynamic. Although long-term mortality benefits have been established, immediate complications and costs are less well understood. We have demonstrated that TEVAR and medical management for acute TBAD reach similar unplanned readmissions over time. Importantly, the readmission rates for patients who underwent TEVAR were significantly lower. We also found that unplanned readmission rates in the TEVAR group were driven by the following procedural variables: urgent vs. elective surgery, adjunct procedures, and hospital characteristics. We believe this suggests that hospital and surgeon experience with TEVAR for acute TBADs affects unplanned readmissions. Furthermore, this suggests that performing TEVAR for acute TBADs as first-line treatment in aortic centers of excellence should be studied.
Recently, a group in Boston completed a similar analysis of readmissions in patients with acute TBADs. Carroll et al. [16] reported high rates of unplanned readmissions within 90 days, which were the highest among patients who underwent TEVAR and the lowest among those who underwent medical management alone. In their analysis, patients who underwent medical management were more likely to receive aortic intervention on readmission. Mortality and costs were similar between the two groups. Mody et al. [17] also evaluated readmission in the Medicare population in 2011 and found lower readmission rates in the medical management patients (19.8% medical vs. 25.5% open vs. 22.3% TEVAR).
However, our conclusions differ from these findings. Our data accumulation included a more recent timeframe. As noted above, we demonstrate that the performance of TEVAR has increased, whereas unplanned readmissions from TEVAR have decreased. We postulate that our results may differ because our patient population included more patients who underwent TEVAR more recently. Furthermore, our data demonstrated that patients who underwent TEVAR were readmitted for complications, notably in the iliac or peripheral systems. We also postulate that readmissions may have decreased with time in our dataset due to device improvements such as lower profiles and better conformability, translating into a reduction in procedural complications. In addition, our data did not include patients who underwent open repair for TBAD. We believe that by excluding open repair, we can more clearly compare medical management with TEVAR, and thus evaluate the two most common treatment modalities for acute TBADs.
1) Study limitations
We acknowledge that when using an ICD coding system, coding only exists for thoracic aortic dissection and does not distinguish between type A and type B dissection. To limit the inclusion of type A dissections, we excluded patients who underwent open repair, as this is the gold standard for type A dissections. However, we were unable to confirm that our patient population did not exclusively represent those with dissections involving the descending aorta. In addition, the complex survey design of the NRD must be acknowledged. Our results are based on nationally representative estimates from a smaller (20%) population sample. In addition, the limitation of using a coding database, such as the NRD, includes the inability to abstractly or directly correlate patient-level information from the encounter. As such, we cannot comment directly on the specifics of the aortic changes that resulted in readmission and the reason for TEVAR or other procedures, nor qualitatively discern the specific development of all the diagnoses coded with that specific readmission encounter. We can only indirectly surmise that an aorta-related issue arose to a high degree given that the collective inclusion of a vascular procedure, a code for graft complication, or aneurysmal dilation were coded for that patient encounter.
CONCLUSION
Recent data suggest a role for TEVAR in acute, uncomplicated TBAD. We found that readmission from TEVAR was related to the technical aspects of the procedure. As operative experience increased, the number of unplanned readmissions after TEVAR statistically decreased, whereas unplanned readmissions for medical management remained unchanged. Thus, we conclude that there continues to be support for TEVAR as the initial treatment for acute uncomplicated TBAD in experienced aortic centers.
Footnotes
FUNDING
None.
CONFLICTS OF INTEREST
The authors have nothing to disclose.
AUTHOR CONTRIBUTIONS
Conception and design: CJL. Analysis and interpretation: KMK, CJL, AJW, SS, OM, BL. Data collection: KMK, CJL. Writing the article: AJW, SS, CJL, KMK, SG. Critical revision of the article: CJL, OM, BL, LP, AD. Final approval of the article: all authors. Statistical analysis: KMK. Obtained funding: not applicable. Overall responsibility: CJL.
REFERENCES
- 1.Nienaber CA, Kische S, Rousseau H, Eggebrecht H, Rehders TC, Kundt G, et al. Endovascular repair of type B aortic dissection: long-term results of the randomized investigation of stent grafts in aortic dissection trial. Circ Cardiovasc Interv. 2013;6:407–416. doi: 10.1161/CIRCINTERVENTIONS.113.000463. [DOI] [PubMed] [Google Scholar]
- 2.Booher AM, Isselbacher EM, Nienaber CA, Trimarchi S, Evangelista A, Montgomery DG, et al. The IRAD classification system for characterizing survival after aortic dissection. Am J Med. 2013;126:730.e19–24. doi: 10.1016/j.amjmed.2013.01.020. [DOI] [PubMed] [Google Scholar]
- 3.Iannuzzi JC, Stapleton SM, Bababekov YJ, Chang D, Lancaster RT, Conrad MF, et al. Favorable impact of thoracic endovascular aortic repair on survival of patients with acute uncomplicated type B aortic dissection. J Vasc Surg. 2018;68:1649–1655. doi: 10.1016/j.jvs.2018.04.034. [DOI] [PubMed] [Google Scholar]
- 4.Kret MR, Azarbal AF, Mitchell EL, Liem TK, Landry GJ, Moneta GL. Compliance with long-term surveillance recommendations following endovascular aneurysm repair or type B aortic dissection. J Vasc Surg. 2013;58:25–31. doi: 10.1016/j.jvs.2012.12.046. [DOI] [PubMed] [Google Scholar]
- 5.van Walraven C, Austin PC, Jennings A, Quan H, Forster AJ. A modification of the Elixhauser comorbidity measures into a point system for hospital death using administrative data. Med Care. 2009;47:626–633. doi: 10.1097/MLR.0b013e31819432e5. [DOI] [PubMed] [Google Scholar]
- 6.Averill R, Goldfield N, Steinbeck B, Grant T, Muldoon J, Brough A, et al. Development of the all patient refined DRGs (APR-DRGs) 3M; St. Paul (MN): pp. 3M HIS Research Report 8–97. [Google Scholar]
- 7.DeBakey ME, Henly WS, Cooley DA, Morris GC, Jr, Crawford ES, Beall AC., Jr Surgical management of dissecting aneurysms of the aorta. J Thorac Cardiovasc Surg. 1965;49:130–149. doi: 10.1016/S0022-5223(19)33323-9. [DOI] [PubMed] [Google Scholar]
- 8.Daily PO, Trueblood HW, Stinson EB, Wuerflein RD, Shumway NE. Management of acute aortic dissections. Ann Thorac Surg. 1970;10:237–247. doi: 10.1016/S0003-4975(10)65594-4. [DOI] [PubMed] [Google Scholar]
- 9.Nauta FJ, Trimarchi S, Kamman AV, Moll FL, van Herwaarden JA, Patel HJ, et al. Update in the management of type B aortic dissection. Vasc Med. 2016;21:251–263. doi: 10.1177/1358863X16642318. [DOI] [PubMed] [Google Scholar]
- 10.Eggebrecht H, Schmermund A, von Birgelen C, Naber CK, Bartel T, Wenzel RR, et al. Resistant hypertension in patients with chronic aortic dissection. J Hum Hypertens. 2005;19:227–231. doi: 10.1038/sj.jhh.1001800. [DOI] [PubMed] [Google Scholar]
- 11.Estrera AL, Miller CC, Goodrick J, Porat EE, Achouh PE, Dhareshwar J, et al. Update on outcomes of acute type B aortic dissection. Ann Thorac Surg. 2007;83:S842–S845. discussion S846–S850. doi: 10.1016/j.athoracsur.2006.10.081. [DOI] [PubMed] [Google Scholar]
- 12.Nienaber CA, Fattori R, Lund G, Dieckmann C, Wolf W, von Kodolitsch Y, et al. Nonsurgical reconstruction of thoracic aortic dissection by stent-graft placement. N Engl J Med. 1999;340:1539–1545. doi: 10.1056/NEJM199905203402003. [DOI] [PubMed] [Google Scholar]
- 13.Fattori R, Montgomery D, Lovato L, Kische S, Di Eusanio M, Ince H, et al. Survival after endovascular therapy in patients with type B aortic dissection: a report from the International Registry of Acute Aortic Dissection (IRAD) JACC Cardiovasc Interv. 2013;6:876–882. doi: 10.1016/j.jcin.2013.05.003. [DOI] [PubMed] [Google Scholar]
- 14.Durham CA, Cambria RP, Wang LJ, Ergul EA, Aranson NJ, Patel VI, et al. The natural history of medically managed acute type B aortic dissection. J Vasc Surg. 2015;61:1192–1198. doi: 10.1016/j.jvs.2014.12.038. [DOI] [PubMed] [Google Scholar]
- 15.Kwolek CJ, Watkins MT. The INvestigation of STEnt grafts in aortic dissection (INSTEAD) trial: the need for ongoing analysis. Circulation. 2009;120:2513–2514. doi: 10.1161/CIRCULATIONAHA.109.911883. [DOI] [PubMed] [Google Scholar]
- 16.Carroll BJ, Schermerhorn M, Kennedy KF, Swerdlow N, Soriano KM, Yeh RW, et al. Readmissions after acute type B aortic dissection. J Vasc Surg. 2020;72:73–83.e2. doi: 10.1016/j.jvs.2019.08.280. [DOI] [PubMed] [Google Scholar]
- 17.Mody PS, Wang Y, Geirsson A, Kim N, Desai MM, Gupta A, et al. Trends in aortic dissection hospitalizations, interventions, and outcomes among medicare beneficiaries in the United States, 2000-2011. Circ Cardiovasc Qual Outcomes. 2014;7:920–928. doi: 10.1161/CIRCOUTCOMES.114.001140. [DOI] [PMC free article] [PubMed] [Google Scholar]