Abstract
Rigidity (or stiffness) of materials and extracellular matrix has proven to be one of the most significant extracellular physicochemical cues that can control diverse cell behaviors, such as contractility, motility, and spreading, and the resultant pathophysiological phenomena. Many 2D materials engineered with tunable rigidity have enabled researchers to elucidate the roles of matrix biophysical cues in diverse cellular events, including migration, lineage specification, and mechanical memory. Moreover, the recent findings accumulated under 3D environments with viscoelastic and remodeling properties pointed to the importance of dynamically changing rigidity in cell fate control, tissue repair, and disease progression. Thus, here we aim to highlight the works related with material/matrix-rigidity-mediated cell and tissue behaviors, with a brief outlook into the studies on the effects of material/matrix rigidity on cell behaviors in 2D systems, further discussion of the events and considerations in tissue-mimicking 3D conditions, and then examination of the in vivo findings that concern material/matrix rigidity. The current discussion will help understand the material/matrix-rigidity-mediated biological phenomena and further leverage the concepts to find therapeutic targets and to design implantable materials for the treatment of damaged and diseased tissues.
Keywords: Matrix rigidity, Biophysical cue, Cell and tissue engineering, 3D biomaterials conditions, Therapeutic targets
Graphical abstract
Materials/matrix rigidity dictates diverse cell and tissue behaviors.
Highlights
-
•
Discuss the cutting-edge findings on the role of matrix rigidity in dictating diverse cell behaviors.
-
•
Underscore the dynamic matrix rigidity that interplays with cells, and the related pathophysiological phenomena.
-
•
Illuminate the significance of matrix rigidity in clinically-relevant settings.
Abbreviations
- ECM
extracellular matrix
- MSC
mesenchymal stem cell
- PA
polyacrylamide
- PEG
polyethylene glycol
- PDMS
polydiemethyl siloxane
- RGD
Arg-Gly-Asp peptide
- NSC
neural stem cell
- ASC
adipose stem cell
- SDF1
stromal cell-derived factor 1
- PSC
pluripotent stem cell
- LINC
linker of nucleoskeleton and cytoskeleton
- ESC
embryonic stem cell
- PDMS
polydimethyl siloxane
- YAP
yes-associated protein
- CCP
chromatin condensation parameter
- MMP
matrix metalloproteinase
- TIMP
tissue inhibitors of MMP
- IL-1β
interleukin-1β
- hiPSC
human induced pluripotent stem cell
- LSEC
liver sinusoidal endothelial cell
- HSC
hepatic stellate cell
- TGF-β
transforming growth factor-β
- α-SMA
α-smooth muscle actin
- VIC
valve interstitial cell
- AVS
aortic valve stenosis
- VEC
valvular endothelial cell
- EMT
endothelial-to-mesenchymal transition
- MLK1
megakaryoblastic leukemia factor 1
- ROCK
Rho-associated protein kinase
- CNS
central nervous system
- GFAP
glial fibrillary acidic protein
- LAP
latency-associated peptide
- ARP 2/3
actin related protein 2/3
- PI3K
phosphoinositide 3-kinase
- RTK
receptor tyrosine kinase
- Sp1
specificity protein 1
- CAF
cancer associated fibroblast
- FAK
focal adhesion kimase
- LOX
lysyl oxidase
- OPC
oligodendrocyte progenitor cell
- MRTF-A
myocardin-related transcription factor-A
- iNOS
inducible nitric oxide synthase
- TNFα
tissue necrosis factor α
- TNFR
TNFα receptor
- EV
extracellular vesicle
- CCL2
C–C motif chemokine ligand 2
- IL-2
interleukin-2
- HSPC
hematopoietic stem and progenitor cell
- PLL
poly-l-lysine
1. Introduction
Biological organisms are always under the influence of mechanical forces at tissue, cellular and subcellular levels. In particular, cells in the organism are involved in diverse and dynamic interactions with the biophysical cues of extracellular matrix (ECM). Rigidity (stiffness or elasticity), as the property of resisting degree to deformation under applied load [1], has become one of the most spotlighted extracellular cues in cell and tissue engineering field, as the cells sense the rigidity of the underlying substrate to adjust their behaviors, such as contractility, motility, and spreading [2]. This so-called ‘mechanosensing process’ entails physiological phenomena, ranging from development and differentiation to repair and regeneration of diseased and damaged tissues [3].
Indeed, tissue rigidity varies among the type of tissues and the conditions in which different sets of cells dynamically interact with their surroundings [4]. Neuronal cells lie in the soft tissue of brain and nerve, and osteocytes reside in the stiff lacunae of lamellar bone [5]. These tissue resident cells are connected to their surrounding extracellular matrices (ECMs) and affected by them. For instance, the fate of the mesenchymal stem cells (MSCs) could shift toward nerve-, muscle-, or bone-like lineage upon the substrate with an appropriate elasticity value [6]. Also, myoblasts showed more aligned morphology on a substrate with a fibrous texture [7].
Since the monumental study by Pelham and Wang in late 1990s, where the rigidity of polyacrylamide (PA) hydrogels was shown to affect cell adhesion, spreading, and migration [8], a number of studies have demonstrated the effects of matrix rigidity on various cellular behaviors [2,6], such as cell differentiation, death, and senescence, in both cytoskeletal and nuclear regimes, under various conditions (static or dynamic; 2D or 3D), linking the phenomena with diverse physiological and pathological tissue conditions (as illustrated in Fig. 1A). Of note, there have been gradual but significant shifts in research trend over the last decade; i) from cells on 2D gels to those encapsulated in 3D gels, ii) from static elasticity concept to dynamic viscoelastic properties, iii) from actin-myosin contractile machineries to nuclear components, iv) from single cell responses to collective (cooperative) cell behaviours, and v) from in vitro model studies to clinically relevant in vivo experiments (Fig. 1B), which will be emphasized in our communication in this review.
Fig. 1.
Schematic showing (A) the implication of material/matrix rigidity (how soft or stiff it is) in diverse cell behaviors, tissue responses, and pathophysiological phenomena, and (B) the recent research trend in material/matrix-rigidity-related mechanobiology studies. Rigidity (either static or dynamic) of matrix at extracellular space is sensed by cell receptors (i.e., integrins) in a way that more receptors are clustered and activated on more rigid matrix (A-i); the signal is transmitted through actomyosin contractility to nucleus (A-ii), leading to nuclear deformation and chromatin (de)condensation (A-iii) that consequently modulates transcriptional profile and various cellular and tissue responses, such as spreading, migration, proliferation, and differentiation, and influences the pathophysiological phenomena, such as tissue healing/regeneration, senescence/aging, tumor onset/metastasis, and immune/inflammation.
Thus, here in this review, we are motivated to highlight such research trends in the rigidity-mediated cell and tissue behaviors. First, we briefly look into the studies of static rigidity effects on some representative cell behaviors in 2D conditions, then examine the importance of dynamic rigidity involved with viscoelastic and remodeling matrix, which is closer to tissue-mimicking 3D conditions. We lastly view the impact of matrix rigidity with clinical relevance, mainly probing the areas that have been intensely investigated, such as tissue healing, tumor progression, and immune/inflammation. We further discuss the findings to envisage rigidity-related therapeutic targets and to design strategies for biomaterials (3D hydrogels and scaffolds) that can ultimately recapitulate the microenvironments for the treatment of damaged and diseased tissues. This review is considered to help researchers in materials/matrix-, cell-, and tissue-engineering to better understand the phenomena related with rigidity and to develop artificial ECMs for targeted tissues.
2. Overview of 2D material/matrix rigidity effects on cell behaviors
Earlier studies have investigated the rigidity effects on cells mainly under 2D conditions using engineered gels or flexible arrays of microposts [2,9,10]. Rigidity of the 2D systems was tailored to mimic the level of target tissues (as schematically shown in Fig. 2A). Cells cultivated on 2D matrix can sense the underlying substrate mechanics and adapt to it in a different manner from those cultivated on plastic culture dish, an extremely rigid material. Therefore, the cellular phenomena generally conceptualized in the plastic dish need to be redefined based on the findings found in the soft engineered culture systems.
Fig. 2.
(A) Rigidity scale of various tissues and (B) the engineered 2D systems (either synthetic gels, array of flexible microposts, or in situ stiffening/softening gels) to study the effects of rigidity on diverse cell behaviors, such as spreading, migration, differentiation, and mechanical memory.
As for the gel culture system, the composition of the substrate varies from synthetic (polyacrylamide (PAA), polyethylene glycol (PEG), polydimethyl siloxane (PDMS)) to natural polymers (alginate, gelatin, collagen, hyaluronic acid (HA)). While the synthetic gels lack adhesive ligands, and thus require chemical modification (often with Arg-Gly-Asp (RGD) peptide or fibronectin) to initiate cell adhesion, natural polymer-based gels like collagen and gelatin (methacrylated form) have the innate ability to allow cell anchorage, and thus can be used without addition of adhesive ligands. However, ligand density changes along with rigidity level, making it difficult to decouple the two parameters. For this reason, synthetic polymers have been preferred for use in 2D gel matrices. As an alternative method to gels, microposts array system was developed. It can successfully decouple substrate rigidity from molecular-scale properties, whereas simultaneous alterations in both rigidity and molecular-level material characteristics are inevitable in gel culture system [10].
Depending on target tissues and pathophysiological conditions, various types of cells were studied, e.g., fibroblasts, epithelial cells, endothelial cells, mesenchymal stem cells (MSCs), neural stem cells (NSCs), neurons, myoblasts, cardiomyocytes, chondrocytes, osteoblasts, osteoclasts, macrophages, neutrophils, cancer cells, etc. Regarding the cell behavior, some of the most extensively studied aspects are briefly discussed in this part, which includes cell spreading, migration (along the rigidity gradient), lineage specification of stem cells, and cellular mechanical memory (as depicted in Fig. 2B).
One of the most fundamental studies on matrix rigidity effects deals with cell-adhesion-mediated spreading. Cells cultured on rigid gels generally spread more than those on compliant ones, as they go through active mechano-signaling processes, such as integrin clustering, recruitment of focal adhesion molecules, and polymerization of cytoskeletal actin molecules. In doing so, cells exert higher traction forces against the matrix, conforming to the physical properties of the substrate. The cell spreading area and cytoskeletal development thus scale with the increase in matrix rigidity, as confirmed in many different types of cells, including dermal fibroblasts, MSCs, and epithelial cells [11]. Along with cell spreading, migration along the rigidity gradient has been considered an important issue in many pathophysiological conditions, such as wound healing, development, and cancer invasion [12,13]. This unique phenomenon of cellular preferential migration toward stiffer region is called ‘durotaxis’ [14], which has been demonstrated in many types of cells, such as fibroblasts [[14], [15], [16]], vascular smooth muscle cells [17], MSCs [[18], [19], [20], [21]], epithelial cells [22], adipose stem cells (ASCs) [23], skeletal muscle cells [23], and cancer cells [12]. A wealth of in vitro model study has proven that the mechanosensitive durotaxis migration involves multiple variables and occurs in various patterns, i.e., cells not only recognize the rigidity gradient but also perceive the width, curvature, and orientation of the substrate. The representative cell migration experiments regarding the effects of rigidity (and sometimes with the addition of other substrate parameters, e.g., nano/micro-topographies) are summarized in Table 1. Based on these findings, a general tendency can be outlined as follows: i) a certain threshold jump is required in rigidity gradient (e.g., 30–40 kPa) and narrow width of gradient are necessary to provoke durotaxis [15], ii) different threshold rigidity gradients should exist for each region with specific level of rigidity to induce durotaxis [20], iii) durotaxis velocity correlates with the rigidity gradient degree [19], iv) durotaxis depends on the curvature (either convex or concave) [16] of the rigidity boundary and the orientation [17], and v) the durotaxis behavior depends on the type of cells [23].
Table 1.
Summary of the representative in vitro cell migration studies related with matrix rigidity variation.
| Rigidity range | Cells | Engineered matrix | Findings/Comments | Refs |
|---|---|---|---|---|
| 140–300 kdyn/cm2 | 3T3 fibroblast | Collagen-coated polyacrylamide gel | Cells preferentially migrate toward stiffer substrate | Lo et al. [14] |
| 0–90 kPa | 3T3-Swiss albino fibroblast | Microelasticity-patterned gelatinous gel | Induction of durotaxis requires certain threshold jump in elasticity and sufficiently narrow width | Kawano et al. [15] |
| convex/concave 50–1000 R/μm | 3T3-Swiss albino fibroblast | Microelasticity-patterned gelatinous gel | Manipulation on curvature of elasticity boundary can enhance or reverse durotaxis | Ueki et al. [16] |
| 1–80 kPa | Bovine aortic vascular smooth muscle cell | Collagen-coated polyacrylamide gel | Stiffness gradient may be more influential than absolute stiffness magnitude for induction of durotaxis | Isenberg et al. [17] |
| 1 kPa, 34 kPa | Human bone marrow-derived mesenchymal stem cell | Collagen-coated polyacrylamide gel | Nonmuscle myosin-II phosphorylation and polarization are affected by durotaxis | Raab et al. [18] |
| 1 Pã12 kPa | Human mesenchymal stem cell | Fibronectin-coated polyacrylamide hydrogel | Migration during durotaxis is dependent on rigidity gradient. Actin and microtubule each seems to play distinctive role in migration. | Vincent et al. [19] |
| 2.8 Pã83 kPa | Human mesenchymal stem cell | Microelasticity-patterned gelatinous gel | Initial substrate rigidity determines the threshold rigidity gradient for induction of durotaxis | Moriyama et al. [20] |
| 8–49 kPa | Human mesenchymal stem cell, 3T3 fibroblast | Photocurable styrenated gelatin | Cell type and cell-scale heterogeneity of matrix rigidity affect durotactic activity | Ebata et al. [21] |
| 6.6 kPã55 kPa | Human mammary epithelial cell (MCF-10A) | Fibronectin-coated polydimethylsiloxane gel | Collective cell durotaxis can be explained by long-range transmission of force throughout the cells connected by cell junctions | Sunyer et al. [22] |
| 0.1–160 kPa | Human adipose-derived stem cell | Fibronectin-coated polyacrylamide hydrogel | Fabrication of system that can modulate various, yet subtle linear rigidity gradients may be anticipated for wide adoption in the field of mechanobiology | Hadden et al. [23] |
Among other studies, one recent work by Ebata et al. [21] is noteworthy as the authors carried out 2D durotaxis experiments that are more realistic and relevant to in vivo conditions of general fibrous tissues. They designed a gel system with cell-scale heterogeneity (100, 300, 600 μm) and mild rigidity gradient (10–70 kPa of fibrous tissue levels). Intriguingly, the fibroblastic cells migrated toward a rigid strip of 600 μm width (size suited for multiple cells) whereas MSCs favored a rigid strip of 100 μm width (size suitable for single cells), underscoring that depending on the type of cells the durotactic activity adopts the cell-scale heterogeneity of the matrix rigidity (Fig. 3A). Not only rigidity variation, but other physical cues, such as ligand density and topography as well as chemical gradient work together in altering the cellular migration direction and speed along the matrix [24]. The cellular migration across the rigidity-gradient is generally determined by the balance between focal adhesion strength and actomyosin contractility [12], and the matrix rigidity affects both cellular adhesion via integrin receptors and contractile force through actomyosin machinery; for this reason, the rigidity-dependent cell migration should be interpreted by taking other physico-chemical parameters that can affect both phenomena into consideration. While here we briefly outline the durotaxis behaviors of cells, readers are referred to other comprehensive reviews to gain detailed information on cellular durotaxis mainly in vitro and even some in vivo [25,26].
Fig. 3.
Durotactic behaviors of cells along the matrix-rigidity-gradient. (A) Cell-dependent durotaxis observed on designed striped-patterned 2D gels. (A-a) Fibroblasts and MSCs were seeded on the gels. (A-b) Soft (light blue) and rigid (dark blue) regions are illustrated below the phase-contrast images. Fibroblasts migrated toward a rigid strip of 600 μm width (size suited for multiple cells) whereas MSCs favored a rigid strip of 100 μm width (size suitable for single cells). Distribution of elastic modulus E around the elasticity boundaries measured within the white rectangular area indicated in (A-a). (A-b) Illustration showing the cell-type-dependent durotaxis; sensing rigidity gradient width differently between fibroblasts and MSCs. (A) is adapted with permission from Ebata et al. [6] in Biomater., 2020. (B) Collective durotactic behavior of cell clusters on 2D rigidity-gradient gels. (B-a) Illustration showing the collective cell behavior as a continuum mass that enables a long-range force transmission through cell-cell adhesions. (B-b) Zoomed in projection of red box in (B-a), detailing the cellular machineries involved in cell-cell and cell-ECM interactions that are cooperative in collective cell migration (cadherin, β-catenin, α-catenin, and F-actin at cell-cell interactions, integrin, focal adhesion molecules, and F-actin at cell-ECM interactions, and myosin II mediating cytoskeletal contractility). (B-c,d,e) Representative cell migration behaviors of clusters (MCF-10A cells) in monolayer, cultured (B-c) on a soft uniform gel of 6.6 kPa, (B-d) on a rigidity-gradient gel where gel rigidity increases toward the right of the panel, and (B-e) on a rigidity-gradient gel but with a depletion of α-catenin, the cell-cell adhesion signaling molecule. Numbers at the bottom or top indicate Young's modulus values measured with atomic force microscopy. Gray area indicates initial cluster position (t = 0 h), and phase-contrast image shows the cluster at 10 h. The figures except (B-b) are adapted with permission from Sunyer et al. [22] in Science, 2016. (C) Collective cell migration along in vivo self-generated stiffness gradient in an embryonic cell population (neural crest). (C-a) Model of the neural crest self-generated stiffness gradient and durotaxis. More actomyosin contractility, Rac signaling, and integrin-mediated cell-matrix interactions (vs. N-cadherin-mediated cell-cell interactions) exist in stiffer cell region. Durotaxis and chemotaxis (SDF 1 gradient) are cooperative. The neural crest is shown in red, and placodes are shown in yellow (no stiffness gradient) or a purple (stiff) to yellow (soft) gradient (stiffness gradient). Tactic index (C-b) and speed (C-c). Quantification of the rear/front polarity of actomyosin contractions along the gradient axis (C-d) and correlation of migration with this polarity strength (C-e). Adapted with permission from Shellard et al. [24] in Nature, 2021.
While most durotaxis experiments are designed on a single cell level, the collective migration of cells is the physiological phenomenon in development, wound healing, and tumor progression [24,27]. Sunyer et al. examined the collective durotaxis behavior at subcellular level, and derived an empirical model from the observation [22]. Collective migration along the rigidity gradient required not only the action of myosin motors but also the integrity of cell-cell junctions. Thus, durotaxis did not result from a local rigidity sensing but from a long-range force transmission of clustered cells (Fig. 3B). Of note, the collective cell durotaxis was far more efficient than the single cell durotaxis as the cell cluster, considered to behave as a giant ‘supracell’, increases its sensitivity to mechanical rigidity gradients [26], which highlights robust collective cellular mechanisms in vivo such as in wound closure, development, and cancer cell invasion. One recent study further highlights that an embryonic population of cells (neural crest) self-generates stiffness gradients by the cellular contractile forces, enabling collective directional cell migration for morphogenesis (Fig. 3C) [24]. In stiffer regions, cells develop higher Rac signaling and integrin-mediated cell-matrix interactions (vs. N-cadherin-mediated cell-cell interactions). The durotaxis was further shown to be cooperative with chemotaxis (made along the gradient of secreted stromal cell-derived factor 1 (SDF1) signal) for more efficient cell migration.
It is also worth noting the durotaxis behavior of MSCs as it is clearly related to the process where they emerge from the soft bone marrow and are recruited to the site of injury, which would be more rigid than physiological matrix [18]. Given the durotaxis studies undertaken so far are mostly about the rigidity-gradient and -patterning in 2D level, new experimental designs in 3D environment, that have been challenging thus far, are necessary to recapitulate in vivo conditions more accurately, i.e., to offer in vitro platforms for better interpretation of the in vivo phenomena. Although one recent work investigated the cellular durotaxis in 3D collagen gels with rigidity gradient [28], the design could not decouple the effects of ligand density, necessitating fine-tuned design of 3D platforms for further study.
The effects of rigidity-sensing ability on cellular differentiation have been intensively studied with various types of stem cells, including MSCs, NSCs, and pluripotent stem cells (PSCs). Since the pioneering report in 2006 that MSCs could differentiate into different lineages according to substrate rigidity (i.e., soft (0.1–1 kPa) [6], medium (8–17 kPa), and stiff (25–40 kPa) gels induced the differentiation of MSCs to nerve-, muscle-, and bone-like phenotype, respectively), diverse sets of experiments have consolidated the relationship between rigidity and MSC differentiation, highlighting the MSCs lineage specificity is favored on the matrix that matches the corresponding tissue stiffness. The mechanotransduction mechanisms behind the event are mainly through the actomyosin contractile force that links the extracellular gel to intracellular components, and further change in nucleus rigidity via connection between linker of nucleoskeleton and cytoskeleton (LINC) complex and activated lamin A/C. Expression of nuclear lamin A/C is of particular importance because the expression level was proportional to the rigidity of the tissue from which the cells originated, and the knockdown or overexpression of the proteins resulted in adipogenic or osteogenic shifting, respectively [5]. With regard to the cytoskeletal-to-nuclear mechanotransduction process, readers are guided to some recent review papers [29,30]. Along with MSCs, PSCs were also examined in several studies. Embryonic stem cells (ESCs) cultured on an engineered PDMS microposts-array system were able to perceive the scales of 2D rigidity and react upon it in terms of downstream differentiation process [9]. The neuronal differentiation was found to increase ten-fold on a soft substrate and the mechanotransduction mechanism was reasoned to be Hippo/YAP (Yes-associated protein) signaling and actomyosin contractility. This phenomenon was further supported with NSCs where the induction of neuronal differentiation was found to be rigidity-dependent [6,[31], [32], [33]]. It seems mechanosensitive ion channel Piezo 1 is the main sensor molecule responsible for neuronal differentiation [6]. Piezo 1 is expressed in human brain-derived neural stem/progenitor cells, and the authors revealed that neuronal differentiation was highly elevated when Piezo 1 was stimulated by alteration of rigidity.
One of the most interesting characteristics related to mechanosensing, other than the induction of differentiation, is that cells can ‘memorize’ the effect of mechanical cue on them in a duration-dependent manner. To demonstrate the mechanical memory of stem cells, human MSCs were first cultured on several different rigidity conditions of 2D gels for different periods of time (i.e., ‘mechanical dosing’), after which the cells were transferred onto softer gels. Mechanosensitive osteo-differentiation of MSCs upon the 2D gels was then interpreted by the change in nuclei/cytosol concentration of a well-known mechanosensitive transcription factor YAP. Cells that had been dosed on rigid substrates for a relatively long period retained (remembered) high level of nuclear localization of YAP even after gels had turned soft, whereas in cells dosed for a short period the nuclear localization of YAP was reversible (Fig. 4A) [34]. Following studies on mechanical memory designed phototunable hydrogels in which rigidity could be modulated in situ (i.e., on demand softening), to enable in situ observation of cells upon altered matrix rigidity. Matrix softening from a rigid gel resulted in decreased cell area, and reduced mechano-transduction signaling, such as YAP nuclear/cytoplasm ratio [35]. In particular, the authors focused on the change in nuclear mechanics, not just on the alteration in cytosol mechanosensitive components [36]. When MSCs cultured on a rigid gel for 1 day (1-day short-term mechanical dosing) were in situ switched to a softened gel for 5 days, it was found that the nuclear mechanics, such as nucleus volume and roundness, and chromatin condensation, were reversible. However, the same parameters were irreversible in the 10-day (long-term) mechanically-dosed group (Fig. 4B). Such a cellular rigidity-memorizing phenomenon was subsequently harnessed to interpret the fibrotic disease that is caused by the continuous deposition of ECM by persistently activated fibroblasts [37]. The increase in the mechanical dosing (from 1 day to 7 days of culture on stiff gel prior to in situ switching to soft gel with 2 days of culture) increased the population of persistently activated myofibroblasts (vs. transiently activated myofibroblasts) (Fig. 4C). Of note, the persistently activated myofibroblasts displayed condensed chromatin structure (i.e., less chromatin accessibility) with reduced expression of histone acetyl transferases, delineating a new biophysical mechanism that the persistent rigidity signal of ECM is directly transmitted to the nucleus, which results in the modification of histones and distinct chromatin signature. Such phenomenon implies the significance of continuous exposure of fibroblasts to rigid matrix on the possible progression of fibrotic diseases.
Fig. 4.
Cells remember the matrix rigidity at both cytoskeletal and nuclear levels. (A) MSCs mechanical memory of matrix rigidity dosed for different periods in their osteogenesis. (A-a) Test scheme showing MSCs cultured on rigid gels for different periods of time (1, 5, 10 days), followed by a transfer to soft gels and then culture for 7 days. (A-b) The longer the cells experience rigid matrix, cells differentiate more into osteoblasts (as revealed by ALP stain) even after 7 days of culture on soft matrix. (A-b) is adapted with permission from Yang et al. [34] in Nat Mater., 2014. (B) Nuclear mechanical memory by MSCs. (B-a) Test scheme showing MSCs cultured on rigid gels followed by in-situ softening (rigid-to-soft culture periods are either 1 day-to-5 days (St1-So5), or 10 days-to-10 days (St10-So10)). (B-b) Chromatin intensity distribution within nucleus along with nucleus volume and chromatin condensation parameter (CCP) measurement. St10-So10 shows irreversible nuclear mechanics whereas St1-So5 exhibits reversible change, the values being comparable to those from soft culture. (B-b) is adapted with permission from Ref. [36] by Killaars et al. in Adv. Sci., 2019. (C) Effects of rigidity dosing of fibroblasts on myofibroblasts activation and chromatin accessibility. (C-a) Test scheme showing fibroblasts cultured on rigid gels for different periods (day 1, 3, 5, and 7), prior to in situ softening and further culture for 2 days. (C-b) Longer mechanical dosing increases persistently activated myofibroblasts (vs. transiently activated fibroblasts). (C-c) Schematic showing the persistently activated fibroblasts on prolonged rigidity dosing have higher tractional force (tension), nuclear lamin AC intensity, and chromatin condensation, implying the significance of continuous exposure of fibroblasts to rigid matrix. It is an attempt to recapitulate fibrotic disease progression. (C-b,c) are adapted with permission from Walker et al. [37] in Nat Biomed Eng., 2021.
As to the matrix rigidity and the related change in nuclear mechanics, another recent study by Elosegui-Artola et al. is noteworthy [38]. It was demonstrated that the 2D matrix rigidity could regulate the nuclear transport of a key mechano-regulating transcription factor YAP, via nuclear flattening and the resultant stretching of nuclear pores induced by the rigidity-mediated cell traction forces. This study unravelled the direct physical influence of matrix rigidity on the nuclear mechanosensitive machinery mainly nuclear pore and the transcriptional regulation. In short, the matrix-rigidity signal, as a critical mechanical stimulus to cells, is transmitted to the nucleus through cytoskeletal complexes, while, in doing so, regulating the presentation of distinct epigenetic status and fate of cells. Such effects of matrix rigidity on diverse cell behaviors need to be interpreted through the nuclear mechanics and epigenetic profiles, which remains important research area for further investigation.
So far, 2D model studies conducted on stem cell behaviors with variable matrix rigidity levels are limited largely to certain types of cells (mainly MSCs, and some ESCs and NSCs), where the lineage specification highly depends on the matrix rigidity sensed by a diverse set of mechanosensitive apparatus, including integrins, actin cytoskeleton, lamin A/C, and Piezo channels. Nevertheless, the 2D studies clearly point out that rigidity is indeed ‘a significant factor’ in cell behavior, underscoring the rationale for designing tissue-rigidity-matched biomaterials at least to activate the tissue resident stem cells to differentiate properly into target cells, which would ultimately aid regeneration of damaged tissues. Furthermore, given the 2D models are limited in interpreting the matrix rigidity-driven cellular behaviors in vivo, the studies with 3D artificial matrices and scaffolds that have tissue-specific rigidity are needed to improve our understanding of the physiological phenomena related with matrix rigidity.
3. Consideration of 3D matrix and dynamic interactions
Although many studies have identified the role of rigidity in dictating diverse cell behaviors, they were observations mainly in 2D culture conditions upon engineered gels and flexible microposts. Therefore, studies conducted under physiologically-relevant 3D environments are in great demand [39]. However, one of the most significant differences between the systems is that unlike the 2D matrix conditions, where cells spread and grow actively along the 2D surface upon adherence to the substrate, the cells in 3D matrices experience unique multi-directional stress due to the 3D confining effect of matrix rigidity and thus are limited in spreading, expansion, and migration. Among the biophysical parameters that characterize the 3D matrix conditions, dynamic mechanical property (i.e., viscoelasticity) has been given the most attention as physiological ECM (in vivo tissue) is able to dissipate cell-mediated traction forces, i.e., allow relaxation of cell-generated stress over time (illustrated in Fig. 5A and B) [[40], [41], [42], [43], [44]]. Some of the representative works that report the effects of matrix dynamic mechanics (e.g., stress relaxation, plasticity) on diverse cell behaviors are summarized in Table 2.
Fig. 5.
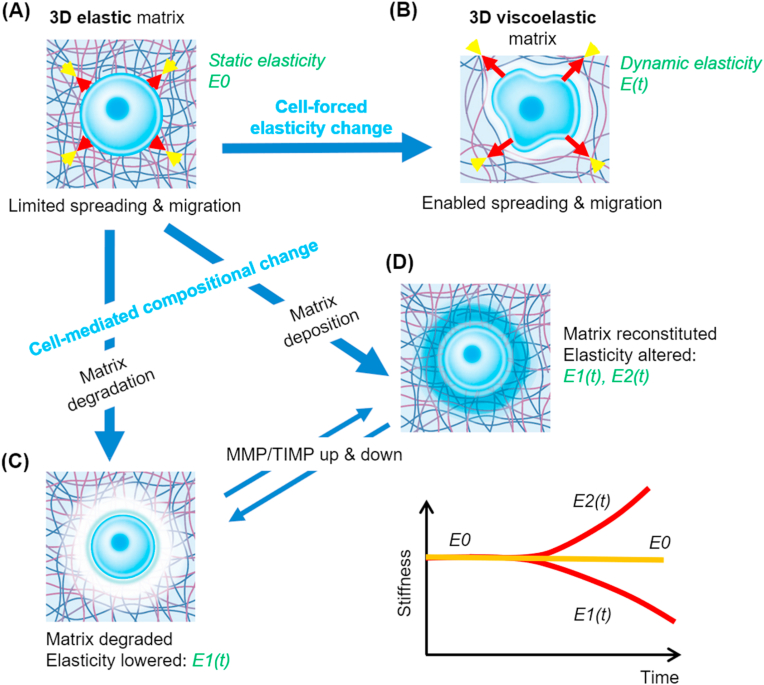
Cell behavior in 3D matrix and the dynamic cell-matrix interactions. Cells interact dynamically with the surrounding 3D matrix. When the matrix is elastic (static elasticity (E0)), cells are limited in spreading and migration (A), whereas viscoelastic matrix (dynamic elasticity E(t)) enables cells to spread and migrate (B). The matrix can also change its composition and elasticity over time due to the cell-mediated degradation (dynamic elasticity, E1(t)) (C), or new matrix deposition (dynamic elasticity, E1(t) or E2(t)) (D), wherein matrix metalloproteinases (MMPs) and tissue inhibitors of MMPs (TIMPs) become up-/down-regulated.
Table 2.
Summary of representative studies that report the 3D in vitro cell behaviors altered by matrix properties, mainly the dynamically-changing matrix mechanics.
| Matrix properties | Cells | Engineered matrix | Findings/comments | Refs |
|---|---|---|---|---|
| Rigidity (with adhesion ligand) | Mouse and human mesenchymal stem cell | Alginate hydrogel, PEG dimethacrylate gel | Differentiation of MSCs is regulated by substrate rigidity in 3D environment. However, unlike in 2D, adhesion ligand rather than cell morphology seems more critical in lineage commitment | Huebsch et al. [42] |
| Stress relaxation | 3T3 fibroblast, D1 cell | Alginate hydrogel | Stress relaxation is a key mechanical parameter that influences cell spreading, differentiation, and proliferation in 3D environment | Chaudhuri et al. [46] |
| Stress relaxation | Bovine chondrocyte | Alginate hydrogel | Fast stress relaxation induces pro-chondrogenesis pathway for chondrocytes whereas slow stress relaxation leads to cartilage degradation and cell death | Lee et al. [49] |
| Stress relaxation | Adenocarcinoma cell line (MDA-MB-231) | Alginate hydrogel | Cancer cells in the fast-relaxing deform the surrounding matrix effectively to allow mitotic elongation and cell division, whereas those in slow-relaxing gels fail to complete mitosis | Nam et al. [50] |
| Stress relaxation (with adhesion ligand) | Human induced pluripotent stem cell | Alginate hydrogel | RGD density and stress relaxation are crucial factors in stem cell morphogenesis | Indana et al. [51] |
| Fiber elasticity, anisotropy | Fibroblast | Dextran methacrylated form (DexMa) | Cells dynamically interact with flexible fibers. Cell mechano-responses to 3D fibrillar anisotropic environment are different from those to isotropic gel matrix | Baker et al. [54] |
| Degradability | Human mesenchymal stem cell | Methacrylated hyaluronic acid (MeHA) | Degradation-mediated cellular traction regulates stem cell differentiation | Khetan et al. [55] |
| Elasticity change (due to void formation) | Mouse mesenchymal stem cell | High guluronic acid (GA)-content alginate hydrogel | Manipulation of elasticity by void-forming hydrogels results in enhanced in vitro osteogenesis | Huebsch et al. [56] |
| Plasticity | Adenocarcinoma cell line (MDA-MB-231) | Interpenetrating network of alginate and basement membrane matrix | Cells physically widen pores of matrices with protrusion of invadopodia to make space for squeeze-through migration, a mechanism that is protease-independent | Wisdom et al. [43] |
| Plasticity | human mesenchymal stem cell | PEG-coupled/free alginate hydrogel | Intermediate level of plasticity induces most dynamic cell-spreading, with activation of mechanotransductory molecules | Grolman et al. [52] |
| Plasticity | Primary rat cardiac, lung fibroblast | collagen type 1 hydrogel | When matrix plasticity decreases, cytoskeletal tension and YAP nuclear translocation increase, leading to enhanced fibroblast activation and spreading | Jia et al. [53] |
| Matrix deposition | Human mesenchymal stem cell | Norbornene hyaluronic acid hydrogel (NorHA), PEG-diacrylate, agarose and alginate hydrogel, guest-host double-network hyaluronic acid hydrogel | Locally deposited nascent proteins alter cell behavior in different types of hydrogels by masking the effect of synthetic substrates and interacting with the cells themselves | Loebel et al. [44] |
In this context, many synthetic polymer gels used in 2D studies become unsuitable in the new dimension as they are not viscoelastic [45]. As chemical (photo)-crosslinked networks of these gels are not stress-relaxing, physical-crosslinking is employed in the hydrogel designs for 3D cell cultures instead; some representative examples are alginate, HA, and PEG, which are crosslinked physically or semi-chemically [42,46,47]. Even with the stress-relaxing gels, the existence of adhesive ligands is a prerequisite for the 3D cultures, and thus RGD is generally introduced to the polymer networks to provide adhesion sites for further cellular processes. One of the earliest studies undertaken to examine the cell behaviors in 3D gel matrix was carried out by Huebsch et al. [42], where methacrylated-PEG with RGD modification was utilized. Researchers observed that MSC lineage polarized along the rigidity variation (i.e., preferred osteogenesis with increasing gel rigidity), which was quite consistent with the results from 2D conditions. Interestingly, osteogenesis enhancement elicited by rigid environment decreased when the elasticity level rose above 30 kPa, suggesting there is a parabolic tendency yet unidentified in 2D gel conditions. In fact, even with the dramatic change in lineage specification, the MSCs embedded in the 3D gels were shown to adopt a more spherical shape (minimal alteration in the morphology), regardless of the rigidity of the gels, which contrasted with the well-spread morphology in stiff 2D gel conditions.
Cell-mediated alteration of gel composition via ECM deposition and enzymatic degradation should also be considered importantly. Cells interact dynamically with the surrounding matrix, altering the elasticity by degrading the components and/or recreating them over time (as illustrated in Fig. 5C and D) [48]. The altered composition drives the change in elasticity level, which continues during the cellular remodeling process. When 3D matrix is not easily degradable, cells require a viscoelastic environment to transmit mechano-signals. When cells are in a degradable 3D matrix, however, they will carve out space for themselves for mechano-permissive growth and migration.
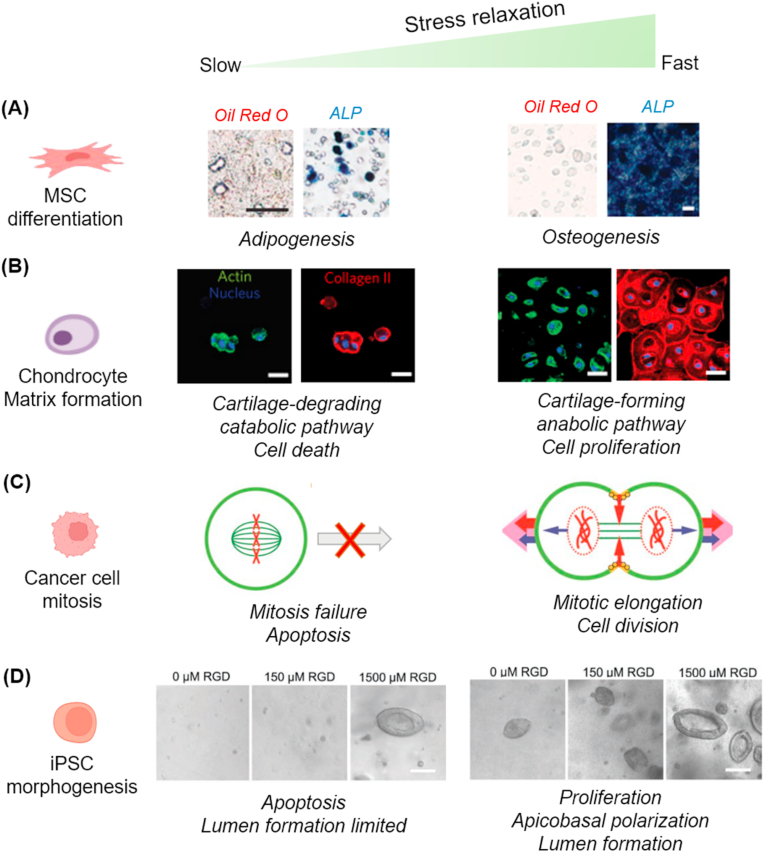
With regard to the cellular phenomena in non-degradable but viscoelastic 3D gels, Mooney and Chaudhuri et al. have intensely explored cell behaviors using engineered alginate gels, where they focused on stress-relaxation among other viscoelastic properties. The cellular behaviors in which they have taken interest include MSC lineage specification [46], chondrocyte matrix formation [49], cancer cell mitosis [50], and pluripotent stem cell morphogenesis [51]. The alginate-based gels were tuned to have varying stress relaxation rates, independent of the initial elastic modulus, rate of degradation, and cell-adhesion-ligand density. When cells are confined in 3D gel matrix, they exert strains on the matrix that result in force resisting the strain, a factor determined by the initial rigidity (elasticity) of the matrix. Within elastic or slow-relaxing gels, such forces are not relaxed without remodeling of the matrix environment. In viscoelastic fast-relaxing gels, however, the forces are relaxed, allowing chondrocytes to remodel the matrix, facilitating cellular adhesion-ligand clustering, cell shape change, proliferation, and matrix formation [46]. In an experiment with MSCs, the fast-relaxing gels permitted MSCs to exhibit higher ligand clustering, actomyosin contractility, and matrix reorganization, thereby enabling them to spread and proliferate more actively, which ultimately led to enhanced osteogenic differentiation (Fig. 6A) [46]. In another study, chondrocytes were cultured in alginate-based viscoelastic gels and the cartilage matrix formation was investigated [49]. Cells in fast-relaxing 3D gels exhibited higher proliferation and anabolic (or matrix-forming) phenotype expressions, whereas those in slow-relaxing gels induced cell death and catabolic (or matrix-degrading) phenotype expressions (Fig. 6B). Furthermore, authors found the restriction of chondrocyte volume expansion induced interleukin-1β (IL-1β) expression, which is considered as an essential molecular mechanism for upregulating cellular catabolic activity and death, signifying possible relevance to osteoarthritis. The significance of a stress relaxing viscoelastic environment has also been implicated in cell mitosis [50]. Cells dividing in confined microenvironments generate protrusive extracellular forces that push against the surrounding microenvironments to acquire space for cell division. The stress relaxing gel allows the cells to deform the surrounding matrix sufficiently for mitotic elongation and cell division to transpire. However, when cells are placed in elastic or low-stress-relaxing gel, those at metaphase fail to complete mitosis (Fig. 6C). In a recent work, human induced pluripotent stem cells (hiPSCs) were able to go through morphogenesis in 3D viscoelastic gel setting [51]. Within fast stress relaxation gels, hiPSCs displayed promoted viability, proliferation, apicobasal polarization, and ultimately lumen formation, whereas slow stress relaxation gels triggered apoptosis of the cells (Fig. 6D). The lumen formation was found to be regulated by mechanotransduction signaling via actomyosin contractility (but not Rho-associated protein kinase (ROCK) inhibition) and YAP translocation, suggesting volume regulation and associated signaling might be responsible for regulating hiPSCs lumen formation. This study highlights matrix viscoelasticity as a key regulating factor for stem cell morphogenesis and offers new insights into engineering of 3D matrix for stem cell-enabled organoids.
Fig. 6.
Significance of 3D matrix stress relaxation on diverse cell behaviors. (A) MSC lineage specification; MSCs cultured in fast-relaxing alginate-based 3D gels show enhanced osteogenesis (alkaline phosphatase stain) and reduced adipogenesis (Oil red O stain). Adapted with permission from Chaudhuri et al. [46] in Nat. Mater., 2016. (B) Chondrocyte matrix formation; chondrocytes in fast-relaxing gels exhibit greater proliferation rate, anabolic metabolism, and cartilage-forming behavior, whereas those in slow-relaxing gels express catabolic, cartilage-degrading pathway and end in cell death. Adapted with permission from Lee et al. [49] in Nat. Mater., 2017. (C) Cancer cell mitosis; cancer cells in the fast-relaxing deform the surrounding matrix effectively to allow mitotic elongation and cell division, whereas those in slow-relaxing gels fail to complete mitosis. Adapted with permission from Nam et al. [50] in Nat. Phys., 2018. (D) Induced pluripotent stem cell (iPSC) morphogenesis. Human iPSCs in fast-relaxing gels show promoted viability, proliferation, and apicobasal polarization, and ultimately lumen formation. Those in slow-relaxing gels end up in apoptosis. Other than stress relaxation, increasing RGD ligand density enhances iPSC morphogenesis. Adapted with permission from Indana et al. [51] in Adv. Mater., 2021.
Along with stress relaxation behavior, the matrix plasticity (irreversible matrix deformation in response to force) was also found to be a key dynamic matrix mechanics that determines diverse cell behaviors [43,52,53]. For instance, Wisdom et al. discovered that high plasticity of a hydrogel matrix promotes protease-independent mode of cancer cell migration in which cells can mechanically widen miniscule pores of the plastically deforming matrix by exerting force via protrusion of invadopodia, whereas low plasticity of a matrix does not permit such cell migration [43]. Grolman et al. also reported that there is a biphasic relationship between matrix plasticity and MSC spreading [52]. Using a nondegradable polymer model that specifically decouples plastic deformation from stress relaxation and modulus the authors could demonstrate the MSC spreading on different levels of plasticity. Cells were shown to spread maximally with the highest focal adhesions at an intermediate plasticity level. The integrin activation, actin polymerization, and actin-myosin contractility were all responsible for such phenomena. The work by Jia et al. further demonstrated the importance of matrix plasticity in fibroblast activation and spreading [53]. Using a set of 3D collagen nanofibrous matrix with constant modulus but tunable plasticity by adjusting crosslinking degree, the authors demonstrated that the decrease of matrix plasticity promoted fibroblast activation and revealed the activation was mediated through cytoskeletal tension and nuclear translocation of YAP, highlighting the impact of matrix plasticity in the possible development of fibrotic diseases.
The importance of dynamic matrix mechanics has also been implicated in the cell behaviors with ECM-like fibrous polymer network [54]. Due to its fibrous form, the photo-crosslinked (methacrylated) polymer is flexible and at the same time stress-relaxing. In this case, rigidity was controllable by adjusting ultraviolet wavelength exposure. Fibroblasts were more active on soft fiber (2.8 kPa) than on rigid one (55 kPa), i.e., cells were actively recruited on to the soft fibers by exertion of traction forces, resulting in increased focal adhesion and spreading, whereas increased fiber rigidity restricted cellular traction forces which led to less clustered fibers and decreased focal adhesion formation. Of note is that the behaviors observed in the engineered fiber gel matrix were similar to those in fibrous collagen matrices with variable rigidity, which are considered representative platforms for native ECM. However, these were not readily observable in 2D non-fibrous gels of the same composition with similar stiffness variation. This study implies that the fibrous nature of a matrix (considered ‘anisotropy’ of a matrix), together with rigidity, should also be considered as one of the crucial 3D matrix cues by which cells recruit, cluster, and remodel the matrix during the transduction of mechano-signals.
Like the cells in viscoelastic but non-degradable 3D matrices, those in degradable gels experience dynamic mechanical elasticity over time [55,56]. An exemplar study by Huebsch et al. pointed to the importance of 3D matrix degradation in the osteogenesis of MSCs. They used methacrylated hyaluronic acid gels that encapsulate cells and are permissive for cellular mechanosensing events, such as focal adhesion formation, actomyosin cytoskeletal development, and cell spreading. The permissive gels ultimately induced intense osteogenesis of MSCs. However, when the gels were made restrictive by secondary crosslinks, actomyosin traction force and cell spreading decreased, which subsequently led to progressive adipogenic induction. This finding runs parallel with the phenomenon observed in stress-relaxing viscoelastic gels, highlighting once again the importance of matrix dynamic mechanical properties that can respond to cellular forces by providing a space for cells to protrude, extend, divide, and conform to the microenvironment (as noted in Fig. 5).
Deposition of new matrix substance from dynamic cellular engagement also alters matrix composition and biochemical status. Importance of the cell-secreted matrix has recently been identified in a pioneering work by Loebel et al. [44]. Authors demonstrated the significance of 3D matrix deposition and remodeling by MSCs, in dictating their own fate in differentiation process toward osteogenesis or adipogenesis. The nascent proteins deposited by the stem cells can provide new matrix cues (as noted in Fig. 5D), and the remodeling of nascent proteins was found to be required for cell spreading and osteogenesis in dynamic hydrogels. This study underscores the importance of considering not only the initial material properties but also the secreted ECM components in interpreting the materials interactions with cells over time. It can also be assumed that the previously identified rigidity-dependent cell responses might be ascribed in part to indirect effects of the newly deposited proteins. As discussed, novel strategies are envisaged to harness the concept of cell-secreted ECM for future development of biomaterials and scaffolds optimized for tissue repair [57]. For instance, inductive signals or genetically engineered cells can be integrated with biomaterials, to guide and instruct endogenous (e.g., homed) or delivered cells to synthesize and organize ECM (e.g., cases in which defective tissues regenerate) or degrade and soften dense ECM (e.g., conditions that need to relieve fibrotic reaction).
4. In vivo phenomena in damaged and diseased tissues related with matrix rigidity: hinting at therapeutic targets and biomaterial designs
As discussed, the cell behaviors in 3D matrices with altered rigidity have significant implications in many pathophysiological conditions. In this Part, we examine the findings of the effects of matrix rigidity in some of the clinically-relevant in vivo conditions. The in vivo phenomena can provide clues to development of new therapeutic targets for the treatments of diseases where the alteration of tissue rigidity is quite influential (Fig. 7A). For example, cancer cell migration is boosted in a stiffened tissue matrix, a characteristic that can be alleviated by softening of the matrix (e.g., blocking of collagen crosslinking or boosting enzymatic degradation). In fact, many intracellular apparatuses that are sensitive to tissue rigidity (e.g., integrins, actin/myosin, ion channels, nuclear lamina) have been identified and proposed as possible therapeutic targets for a number of diseases [58]. To elucidate the underlying mechanisms mentioned earlier, it is often necessary to design in vitro matrix models that can recapitulate the in vivo pathological environments (Fig. 7B). However, it should be reminded that in vivo findings offer ample rationales for the design of novel implantable therapeutic biomaterials. For the injured tissues, biomaterials or those encapsulating stem cells are commonly applied. Therefore, in vivo examination of the matrix-mediated behaviors of cells (either exogenously delivered through 3D gels or endogenously existent in proximity to implants) can guide us to design biomaterials with better therapeutic efficacy (Fig. 7C). This part will discuss the in vivo phenomena related to matrix rigidity, with particular emphasis on some diseased and damaged tissues that represent the pathological (e.g., fibrosis, cancer) cellular behaviors with altered ECM rigidity or the cellular responses to implantable biomaterials under inflammatory conditions. Some representative studies on this subject are briefly summarized in Table 3.
Fig. 7.
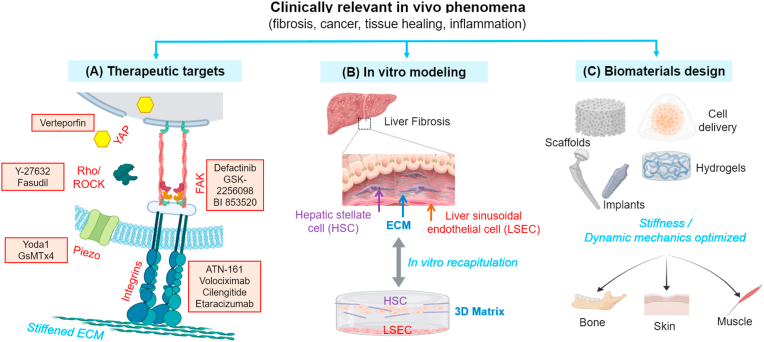
Schematic showing the research streams based on in vivo findings related with matrix rigidity. In vivo evidence can (A) hint at exploring new therapeutic targets for disease treatments (exemplar chemicals that can perturb some key mechanosensitive machineries are written in red; Verteporfin for YAP, Y-27632/Fasudil for Rho/ROCK, Yoda1/GsMTx4 for Piezo channel, ATN-161/Volociximab/Cilengitide/Etaracizumab for integrins, and Defactinib/GSK-2256098/BI 853520 for focal adhesion kinase FAK), (B) allow design of in vitro matrix models that can recapitulate and interpret the in vivo phenomena (liver fibrosis and the in vitro model to recapitulate HSC-LSEC interactions in 3D matrix is displayed as an example), and (C) guide how to approach the development of implantable biomaterials for therapeutic efficacy (noted are the example biomaterials that can be directly implanted or after cell encapsulation, for the regeneration damaged target tissues, such as bone, skin and muscle).
Table 3.
Summary of representative studies that report the effects of matrix rigidity on in vivo phenomena mainly related with fibrosis, cancer, aging, and inflammation.
| In vivo phenomena | Cells | Engineered matrix | Findings/comments | Refs |
|---|---|---|---|---|
| Fibrosis (liver, cardiac, lung, vessel, brain) | Rat hepatic stellate cell | Collagen-/fibronectin-/PLL-coated polyacrylamide gel | Hepatic stellate cells differentiate into myofibroblasts on stiff substrates, and the degree of differentiation increases along with stiffness | Olsen et al. [64] |
| Rat hepatocyte, rat hepatic stellate cell, rat liver sinusoidal endothelial cell | Collagen-coated polyacrylamide gel | High level of stiffness induces intracellular tension through cytoskeleton, inducing nuclear deformation of liver cells. Disruption of cytoskeleton connection attenuates the effect | Guixé-Muntet et al. [65] | |
| Human liver sinusoidal endothelial cell (hLSEC), human hepatic stellate cell (hHSC), mouse cells (mLSEC and mHSC) for in vivo | Polyethylene glycol gel, collagen type 1 hydrogel | Study with in vitro liver model reveals that angiogenesis is important in early-stage model, while late-stage model is more concerned with collagen deposition. Findings offer idea for stage-specific therapeutic targets | Liu et al. [66] | |
| Rat cardiac fibroblast | Collagen type 1 hydrogel | Reduced serum amount and increased matrix stiffness promote the myofibroblast phenotype in the myocardium | Galie et al. [67] | |
| Porcine valve interstitial cell (VIC) | Collagen-coated polyacrylamide gel | Matrix stiffness modulates myofibroblast differentiation of VIC by rendering the cells to be more responsive to TGF-β stimulation | Chen et al. [68] | |
| Porcine valvular endothelial cell (VEC) | Collagen-coated polydimethyl siloxane gel | TGF-β selectively activates endothelial-to-mesenchymal transition of VECs on stiffer substrates | Zhong et al. [69] | |
| Mouse lung fibroblast | Collagen-coated polyacrylamide gel | MKL-1 activation as a result of actin cytoskeletal remodeling leads to the promotion of myofibroblast differentiation | Huang et al. [70] | |
| Human lung fibroblast | Gelatin methacryloylated hydrogel (GelMA) | Both isotypes of ROCK are involved in myofibroblast differentiation | Htwe et al. [71] | |
| Rat brain tissue | N/A | CNS tissue softens when injured, unlike other mammalian tissues | Moeendarbary et al. [74] | |
| Bovine aortic endothelial cell, human umbilical vein endothelial cell | Collagen-coated polyacrylamide gel | Stiffening of intima ECM alters cell contractility and leads to endothelial leukocyte extravasation, which is a critical step in atherosclerosis | Huynh et al. [99] | |
| Tumor (onset, progression, metastasis) | Glioblastoma, adenocarcinoma, fibrosarcoma cell line (87-MG, T98G, MDA-MB-231, and HT1080) | Fibronectin-coated polyacrylamide hydrogel | Cancer cells go through durotaxis, the degree of which depends highly on the local stiffness gradient | Duchez et al. [87] |
| Human mammary epithelial cell (MCF-10A) | Interpenetrating network of alginate and basement membrane matrix | High stiffness of ECM induces malignant phenotype for mammary epithelial cells, which can be counteracted by altering ECM composition | Chaudhuri et al. [89] | |
| MCF10A, HEK293 | Collagen-coated polyacrylamide gel, hyaluronan and gelatin-based 3D hydrogel | Increase in ECM rigidity can transform normal cells into tumor precursors, a process bolstered by RTK-Ras oncogenes | Panciera et al. [91] | |
| Epithelial cell and cancer cell (MCF10A, hTERT-HME1, MCF7, MDA-MB-231) | Interpenetrating network of alginate and basement membrane matrix, polyacrylamide hydrogel | Increased stiffness leads to wrinkled nuclei and more accessible chromatin sites for Sp1-mediated tumorigenicity | Stowers et al. [92] | |
| Human gastric cell line (MKN74, KATO3) | Interpenetrating network of alginate and collagen type 1 | Physical properties of microenvironment epigenetically reprogram gastric tumor cells | Jang et al. [93] | |
| Human umbilical vein endothelial cells, human hepatic sinusoid endothelial cells | Fibronectin-coated polyacrylamide gel | Reduction of rigidity at metastatic site improves colorectal cancer response to anti-tumor drug | Shen et al. [96] | |
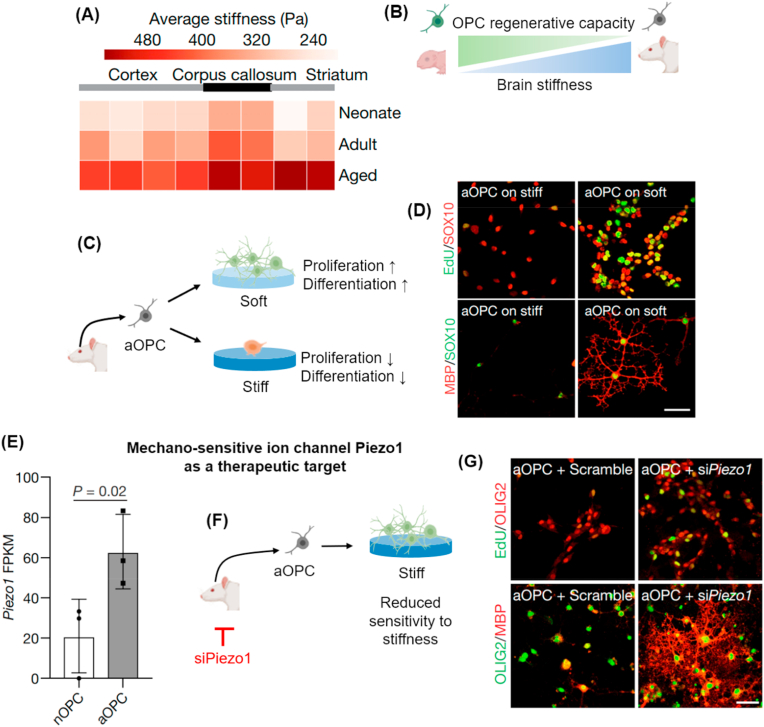
| Aging (brain) | Oligodendrocyte progenitor cell (OPC) | Laminin-coated polyacrylamide gel | Less stiff scaffold, mimicking the microenvironment of young brain, rejuvenates OPC | Segel et al. [101] |
| Inflammation | Mouse fibroblast | Polydimethyl siloxane disc | Soft silicone coating around the stiff implant reduces inflammatory reaction and ECM deposition in vivo | Noskovicova et al. [78] |
| Monocyte (primary, THP-1, U937) | Fibrin hydrogel, collagen gel, PEG diacrylate hydrogel, fibrinogen-coated polyacrylamide gel | Pro-inflammatory reaction of MSC is modulated by adjustment of substrate rigidity | Meli et al. [112] | |
| Human mesenchymal stem cell | Alginate-based hydrogel | MSC primed by soft substrates reacts more profoundly to TNFα stimulation | Wong et al. [116] |
4.1. Fibrosis and scar tissue formation
During the regeneration process at the site of injury, irregular collections of fibrous connective tissue called scars may form [59]. Typically, scar tissue consists of poorly reconstructed dense collagen bundles with fewer elastin fibers compared to normal tissue, which usually results in stiffening of the ECM [60]. A prolonged and continuous activation of myofibroblast-like cells develops excessive amount of collagen, which elevates the stiffness of ECM. This stiffening is often aggravated by the dysregulated secretion of MMPS (which degrade collagen matrices) and TIMPs [61].
Scars can cause serious impairment of surrounding tissue functions, as observed in dysfunctional liver, cardiac, lung, and brain. For instance, liver cirrhosis is a form of scar mainly followed by long term liver damages, i.e. hepatitis B, C, or chronic alcohol consumption, and impedes liver circulation by shunting the portal/arterial blood supply and closing down endothelial fenestration, leading to liver hypertension and other serious systemic dysfunctions [62,63]. Intriguingly, stiffness level of the liver differs among patients as it correlates with the stage of the fibrosis patients are at. Olsen et al. reported the effects of tissue stiffness on the functions of hepatic stellate cells, which are crucial for the onset of cirrhosis [64]. Generally, the hepatic stellate cells undergo myofibroblastic differentiation during liver fibrosis, and the authors observed that the cell activation requires a stiff environment. Other cells in liver (hepatocytes or sinusoidal endothelial cells) can also sense and respond to the matrix stiffness [65]. A recent study by Guixé-Muntet et al. showed that hepatocytes, hepatic stellate cells, and sinusoidal endothelial cells from rat liver with cirrhosis presented significant amelioration of the cirrhosis phenotypes when cultured on soft matrix (0.5 kPa), when those cultured on rigid matrix did not (30 kPa). In this case, protein called nesprin 1 mediated the transmission of rigidity recognition to nucleus, and disruption of the protein led to attenuation in expression of downstream pathway, suggesting the molecules involved in nuclear mechanics could be proper therapeutic targets in case of cirrhosis.
Another recent study further pointed to the importance of matrix-rigidity-targeting therapeutic approach in liver fibrosis particularly at early-stage [66]. The clinical evaluation of liver fibrosis reveals sinusoidal angiogenesis is dominant at early-stage whereas insoluble scar formation is prevalent at late-stage (Fig. 8A), signifying the importance of reciprocal mechanical signaling between liver sinusoidal endothelial cells (LSECs) and hepatic stellate cells (HSCs) in the early liver fibrosis. To recapitulate the biological mechanisms underlying the LSECs-HSCs crosstalk, the authors devised an in vitro model of liver fibrosis which consists of LSECs placed on different rigidity substrates (soft: 0.14–0.61 kPa; stiff: higher than 1.2 kPa) at the bottom surface and HSCs encapsulated in 3D fibrotic microniche (FμN), where LSECs and HSCs mechanically interact (Fig. 8B). Of note, the LSECs on the soft substrate showed evidence of promoting angiogenesis, which further activated HSCs through FμN-mediated mechano-signalings. The collagenous fiber formation in FμN by HSCs was consequently higher in early-stage LSECs than in late-stage LSECs, implying the stimulation of HSCs on secretion of ECM via soft matrix-induced (early-stage) signals (Fig. 8C). Therefore, a proper therapeutic approach at the early-stage of fibrosis was proposed to suppress the angiogenic process, which was indeed proven by the treatment with anti-angiogenic drugs (Fig. 8D and E). This study signifies the necessity of time-matched selective therapies considering matrix-stiffening mechanism, i.e., anti-angiogenic therapy at the early-stage of liver fibrosis is favored whereas inhibition of collagen condensation would be more effective at the late-stage.
Fig. 8.
Therapeutic approach to target matrix stiffening in liver fibrosis. (A) Clinical evaluation of liver fibrosis reveals sinusoidal angiogenesis is dominant at early-stage whereas insoluble scar formation is prevalent at late-stage. (B)In vitro model to recapitulate liver fibrosis wherein liver sinusoidal endothelial cells (LSECs) cultured upon varying rigidities (soft: 0.14–0.61 kPa; stiff: higher than 1.2 kPa) mechanically interact with hepatic stellate cells (HSCs) encapsulated in fibrotic microniche (FμN). LSECs upon soft substrate have promoted angiogenesis, which activates HSCs through FμN-mediated mechano-signalings. (C) Collagenous fiber formation in FμN by HSCs was higher in early-stage LSECs than in late-stage LSECs, implying the stimulation of HSCs secretion of ECM via soft matrix-induced (early-stage) signals. (D) Schematic showing the phenomena of matrix-rigidity-mediated interactions between LSECs and HSCs, i.e., soft matrix promotes angiogenesis of LSECs, which further activates HSCs to secrete ECM and fibrosis. Thus, inhibition of pro-angiogenic process of LSECs blocks HSCs activation and fibrosis progression. (E) Inhibition with anti-angiogenic drugs (Sorafenib, Captopril, anti-vascular endothelial growth factor 2) is effective in preventing fibrosis (as assessed by Sirius red stain of collagen matrix) at early-stage. (C,E) are adapted with permission from Liu et al. [66] in Nat. Mater., 2017.
As noted in liver fibrosis, the myofibroblastic transition of cells initiates the fibrosis and scar formation in other tissues as well. The cardiac tissue is another popular example where the transition transpires depending on the matrix stiffness. Myofibroblastic cells express significant levels of collagen, transforming growth factor-β (TGF-β), and α-smooth muscle actin (α-SMA) [67,68]. Not only the isolated cardiac fibroblasts shaped into myofibroblastic phenotype when cultured on a stiff matrix [67], but the cardiac valve interstitial cells (VIC) also went through myofibroblastic transition under stiffening conditions, although the transition of the latter is more closely related to the Wnt-β-catenin signaling [68]. Chen et al. revealed that a stiffened matrix, which is relevant to calcified aortic valve disease (e.g., aortic valve stenosis (AVS)), induced valve interstitial cells to undergo myofibroblastic differentiation via TGF-β-Wnt crosstalk, implying that stiffer fibrosa is prone to disease. In addition to VICs, valvular endothelial cells (VEC), which also contribute to the myofibroblastic population of AVS by endothelial-to-mesenchymal transition (EMT), were revealed to be affected by matrix stiffness via β-catenin signaling [69]. Among the substrates engineered with tissue-relevant stiffness levels (5–50 kPa), the soft substrate (5 kPa, representing physiological tissue) displayed a low degree of EMT of valvular endothelial cells, whereas the stiff one (50 kPa, representing pathologic tissue) activated the cells to express myofibroblastic marker α-SMA. Collectively, scars in cardiac tissue are related with not only parenchyma (i.e., valve interstitial cells), but also stroma (i.e., valvular endothelial cells), in the process of multiplication of myofibroblastic population and consequent disease aggravation.
In the case of lung fibrosis, the stiffened matrix is also known to activate the fibroblasts to shift toward myofibroblasts with highly expressed α-SMA through megakaryoblastic leukemia factor 1 (MLK1) nuclear translocation [70]. Actin polymerization derived from the stiffened matrix resulted in nuclear translocation of MLK1 and increased α-SMA expression. The MLK1-deficient mouse lung fibroblasts failed to respond to the substrate stiffness, while the MLK-1 transfection rescued the α-SMA expression. Another report unravelled that both ROCK isoforms (both 1 and 2) are heavily involved in lung fibrosis [71]. The α-SMA expression on a stiff matrix is attenuated by ROCK inhibitor (Y-27632), while the knockdown of ROCK isoform could not decrease the α-SMA expression. As expected, the absence of ROCK 2 isoform resulted in aggressive fiber assembly, implying that the control of α-SMA expression is more related to ROCK 1, although each isoform can complement the function of the other.
Glial scar, which commonly occurs after brain and spinal cord injury, can act as a physical barrier to the surrounding tissues interrupting axonal growth [72,73]. In contrast to other scar tissues, the scar in central nervous system (CNS) is actually softer than the surrounding tissue [74]. In the injured spinal cord, the early (1–3 weeks) post-injury lesions softened due to the decreased intermediate filaments, e.g., glial fibrillary acidic protein (GFAP), vimentin, and other ECM proteins like laminin and collagen. Glial scar is actually rich in proteoglycans, (i.e., chondroitin sulphate proteoglycan) [75] which are highly hydrated bodies. That may be the possible reason behind the decrease in stiffness for glial scars. This atypical phenomenon in which CNS scar becomes softened, contrary to the scars of other tissues, implies that the therapeutic approach to trauma in CNS might be different from that for other tissues, especially in the context of scaffold mechanics and mechano-tissue-engineering.
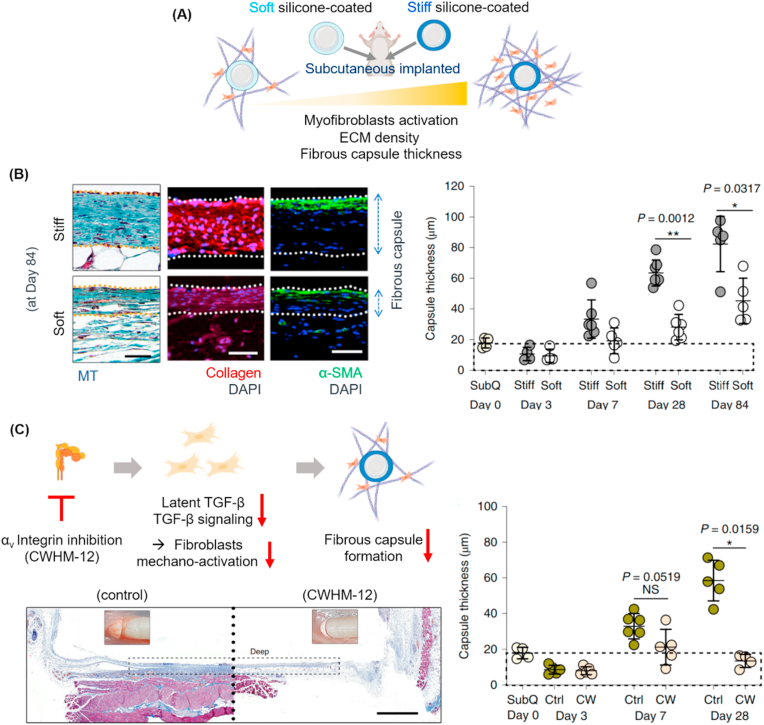
The fibrous tissue formation is also a well-recognized event around engineered medical devices and implants [[76], [77], [78]], which causes problematic issues such as chronic tissue inflammation and implant failure. A recent study by Hinz group has demonstrated the in vivo phenomena of activated fibrotic capsule formation around rigid silicone implants [78]. The authors designed soft- or stiff-coating silicone (elastic modulus of ∼2 kPa or ∼2 MPa) around a conventionally stiff silicone (∼2 MPa) to compare the in vivo fibrotic encapsulation around the surface when subcutaneously implanted in mice (as illustrated in Fig. 9A). They observed that the soft-silicone-coating substantially reduced the collagen ECM deposition, α-SMA expression in fibroblasts, and fibrous capsule thickness (Fig. 9B), without significantly affecting the number and polarization state of macrophages. Underlying the events were myofibroblasts that played a key role in the matrix-rigidity-induced fibrosis activation. The myofibroblasts were highly stimulated by the process of recruited integrins and TGF-β signaling, which was mediated by latency-associated peptide (LAP) that helps latent TGF-β bind to recruited integrin (mainly αvβ1) and TGF-β downstream signaling. The blocking of integrin subset αv by chemical inhibitor (CWHM-12) was further proven to be effective in suppressing the activation of latent TGF-β and TGF-β downstream signaling in myofibroblasts, suggesting a novel therapeutic approach of mitigating fibrosis and foreign body response to medical implants (Fig. 9C).
Fig. 9.
Implant-rigidity-dependent tissue fibrosis and therapeutic intervention. (A) In vivo experimental design involving two implants with soft (pale blue) or stiff (dark blue) coating of silicone (elastic modulus of ∼2 kPa or ∼2 MPa, respectively) on a conventionally stiff silicone body (∼2 MPa) which were subcutaneously implanted in mice. Matrix rigidity activates myofibroblasts, resulting in increased ECM density and fibrous capsule formation. (B) Soft-silicone-coating substantially reduced the collagen ECM deposition, α-SMA expression in fibroblasts, and fibrous capsule thickness. (C) Strategy to attenuating fibrosis around implant via integrin inhibition is schematically displayed; inhibition of integrin (mainly αv subset with inhibitor CWHM-12) recruitment decreases latent TGF-β binding and related downstream signaling, thus attenuating myofibroblasts mechano-activation and fibrous capsule formation. In vivo tissue sample treated with or without CWHM-12 is analyzed to compare fibrous capsule thickness. (B,C) are adapted with permission from Noskovicova et al. [78] in Nat. Biomed. Eng., 2021.
As witnessed, fibrosis and scars occur commonly in many tissue conditions, wherein matrix remodeling of cells is dysregulated, and the severity of phenomena is largely dependent on the matrix or biomaterials rigidity change and the mechanosensitive responses of tissue resident cells [62,64,72,73,[79], [80], [81]], implying the matrix stiffening can be both the cause and effect of fibrosis progress. Therefore, strategies to modulate matrix rigidity and the related mechanotransductory pathways could be new therapeutic intervention sites of fibrotic scar lesions which, if left untreated, become irreversible and severe [58,82]. Along with the inhibition of matrix-mechanosensing integrin subset to alleviate fibrosis around implants, as described above, down-regulation of YAP mechanosensor has also been suggested as a target candidate to prevent liver fibrosis in animal models [58], underpinning that the therapeutic intervention of mechanosensitive transcription factor is an effective strategy for fibrosis treatment.
4.2. Tumor initiation, progression, and metastasis
It is widely known that tumors have higher stiffness than the surrounding normal tissues [83]. Breast cancer development is closely related with the matrix stiffening accompanied by collagen deposition, cross-linking, and re-orientation, which is similar to the physiological growth and morphogenesis of mammary epithelial cells under regulation by the matrix rigidity [84]. Indeed, it is well known that mammographically dense breast tissue is closely related to breast carcinoma [85,86]. Furthermore, a recent report revealed that human cancer cells in glioblastoma, metastatic breast cancer, and fibrosarcoma tend to migrate toward more rigid matrix, and this essentially involves the actin related protein 2/3 (Arp2/3) complex, a well-known actin nucleating protein complex and one of the crucial factors for lamellipodia protrusion [87]. It is also well established that cancer cells alter their fundamental physiology to acquire malignant phenotypes, such as invasion of basement membrane, uncontrolled proliferation, and loss of apicobasal polarity [[88], [89], [90]]. Here we highlight some recent in vivo findings on these events of tumor initiation, growth, invasion, and distant metastasis, all of which are influenced by the altered matrix rigidity of tumor microenvironment (as schemed in Fig. 10A).
Fig. 10.
Significance of matrix rigidity in tumor progression and metastasis, related with nuclear epigenetic change. (A) Schematic showing that matrix rigidity differs depending on cancer status, from onset to distant metastasis (indicated as E0, E1, E2, E3, E4). (B) Matrix stiffening induces activation of tumorigenesis related with nuclear chromatin state; (B-a) matrix stiffening changes chromatin state and lowers activation barrier to metastatic transition of cells, and (B-b) representative images reveal that breast cancer cells embedded in stiffer matrix exhibit wrinkled nuclei and thick heterochromatin arrangement. Adapted with permission from Stowers et al. [92] in Nat. Biomed. Eng., 2019. (C) Matrix rigidity implicated in gastric cancer progression via YAP signaling and epigenetic modification; (C-a) tissue rigidity (herein annotated as G′) of tumor is recorded to be significantly higher than that of normal tissues in patients with gastric cancer, (C-b) activated expression of YAP in tumor tissue, and (C-c) down-regulation of DNA methylation of YAP in tumor tissue. (C-d) Proposed mechanism of positive feedback between YAP activation and epigenetic alteration (GRHL2, TET2, and KMT2A are methylation inhibitors) in response to matrix rigidity. (C-e) In vitro design of collagen-alginate gel system with different rigidities to interpret the in vivo phenomena, and (C-f) representative results with YAP expression and methylation index reduced on soft gel, by stiff-to-soft substrate transition or chemical YAP inhibition. Adapted with permission from Jang et al. [93] in Nat. Biomed. Eng., 2020.
Tumor malignancy of epithelium tissue has proven to be significantly enhanced by the stiffened matrix [89]. Stiffened ECM, unlike the normal ECM where the integrin α6β4 clustering is feasible for hemidesmosome formation, gathers less integrins, leading to malignancy via a molecular axis of phosphoinositide 3-kinase (PI3K)/Rac. This was abrogated by increasing laminin density and restoring integrin clustering, underscoring the importance of matrix rigidity cue sensed by integrins and the intracellular mechanotransduction signaling process. A very recent study by Panciera et al. unravelled that matrix rigidity is a crucial environmental driver of the tumorigenesis of cells [91]. They found that the programming of normal cells into tumor-initiating cells requires a process of increased force transmission between oncogene-expressing cells and their surrounding ECM. Using a cellular model involving receptor tyrosine kinase (RTK)–Ras oncogenes, they observed the oncogenic cells that had gone through a subtle increase in matrix rigidity (from 0.1 kPa to 0.5 kPa) were converted to pancreatic tumor-initiating cells. In contrast, the blunting of the mechanical interplay between oncogene-expressing cells and their substrate by lowering either ECM or intracellular mechanics was proven to prevent the tumor initiation in vivo. Furthermore, they demonstrated that these events are mediated by the mechanotransduction through YAP and transcriptional co-activator with PDZ-binding motif (TAZ). The study highlights that the cellular mechano-signaling related with matrix rigidity is pivotal to converting normal cells into tumor-initiating cells, and the regulation of such a mechanism is a possible therapeutic target for tumor prevention, primarily at the early stage of tumorigenesis.
A notable mechanistic view underlying the tumorigenic events was recently found to relate to chromatin accessibility, as reported by Stowers et al. (Fig. 10B) [92]. They demonstrated that the matrix rigidity-induced tumorigenic phenotype in mammary epithelium was due to the changes in chromatin accessibility and epigenetic change. Breast epithelial cells experiencing stiffer matrix showed wrinkled nuclei and increased chromatin accessibility, and the subsequent up-regulated binding of transcription factor specificity protein 1 (Sp1), which ultimately led to increased cellular tumorigenicity. This study emphasizes the importance of matrix rigidity in cancer cell epigenomic changes and reveals that chromatin state is a critical barometer of mechanotransduction in tumorigenesis. In a similar context, the gastric cancer progression associated with increased matrix rigidity was recently identified to be transduced by YAP signaling and epigenetic regulation (Fig. 10C) [93]. This study revealed that the matrix rigidity reversibly regulated DNA methylation and the promoter region of mechanosensitive YAP protein, and thus, the softening of matrix could reverse the YAP activity and the epigenetic program, suggesting that epigenetic regulation of cells (e.g., DNA methylation) via matrix rigidity-induced nuclear mechanics may be a therapeutic strategy to inhibiting cancer progression.
Matrix rigidity further influences the migration and invasion of cancer cells, highlighting its possible role in activation of cancer cell metastasis. As the cancer associated fibroblasts (CAFs) are highly sensitive to matrix mechanical cues like rigidity, many studies have focused on how to suppress matrix-stiffening. These efforts include the inhibition of production of matrix proteins, intracellular mechano-signalings (Rho, YAP/TAZ, and focal adhesion kinase (FAK)), and collagen crosslinking molecule lysyl oxidase (LOX) [58]. Metastasized tumor as well as primary tumor site is also considered to be stiffened by CAFs, a status primed for the colonization of metastatic cancer cells. Therefore, effort to suppress the matrix rigidity-induced metastasis has recently been actively taken. Softening of matrix by the treatment of collagen crosslinking inhibitor β-aminopropionitrile was successful in reducing metastasis in various mouse cancer models [94,95], implying the matrix rigidity of metastatic site is an effective target to deter cancer colonization. One recent study by Shen et al. demonstrated the reduction of rigidity in metastasized cancer on liver improved the response to anti-tumor drug bevacizumab in metastatic colorectal cancer [96]. They found that tissue rigidity is higher in secondary liver metastasized tumor than in primary colorectal tumor. This is associated with the matrix-stiffening effect of activated metastasis-associated fibroblasts, which enhance angiogenesis and anti-angiogenic drug resistance. When drugs targeting the renin-angiotensin system were treated, fibroblast contraction and ECM deposition were significantly inhibited, thereby reducing the stiffening of liver metastatic sites while amplifying the anti-angiogenic effects.
4.3. Aging-related dysfunctions
Aging is an inevitable process in which the biological system goes through gradual functional decline over time [97,98]. The hallmarks of aging are described as the typical changes at molecular and cellular levels (i.e., telomerase shortening, stem cell exhaustion, cellular senescence) [98]. At tissue level, it is well known that many tissues (muscle, vessel, brain, etc.) become stiffer with age [[99], [100], [101]]. Elastin fragmentation, collagen deposition, and matrix protein crosslinking collectively contribute to stiffening of the aging ECM. For instance, old murine tibialis anterior muscle (28–30-month-old) showed two-fold increase in elastic modulus of fiber bundle and three-fold increase in hydroxyproline level, which is indicative of extensive cross-links between collagen proteins [100]. Vessels of elder patients do demonstrate distinct increase in rigidity due to the change in the ECM properties, and the stiffened vessels raise incidence of the cardiovascular disorders and mortality of patients with stroke, coronary heart diseases, and atherosclerosis [99].
A study by Huynh et al. revealed a close relationship between tissue rigidity and age-related vessel disease of intima [99]. They found that matrix stiffening of intima increased permeability and aggravated intercellular junctions followed by leukocyte extravasation, which is an indispensable step in plaque formation of atherosclerosis. The treatment of ROCK inhibitor Y-27632 and ROCK siRNA could attenuate the increased permeability, revealing that the Rho pathway is involved in the mechanotransduction of the stiffened matrix in vessels. The study implies the regulation of mechanosensitive Rho pathway can be potentially exploited for therapeutic purposes in cases of cardiovascular diseases related to matrix stiffening. A recent study by Segel et al. highlighted that the age-related ECM stiffening in brain is closely related with the impaired brain functions (as explained in Fig. 11) [101]. They revealed that aged brain ECM was stiffened compared to new (neonatal) ECM, and that it could impair the function of the oligodendrocyte progenitor cells (OPCs), which generate central nerve myelination cells (oligodendrocytes). Interestingly, when the OPCs from aged brain were seeded on the decellularized ECM from neonatal brain, they recovered proliferation capability. This phenomenon was explained via the mechanotransduction pathways involving actomyosin contractility, lamin A/C density, and Piezo 1 ion channel expression, all of which were upregulated in aged OPCs. In particular, the aged OPCs in Piezo1-knockdown animal, which are less susceptible to matrix rigidity, exhibited enhanced remyelination in vivo, suggesting that Piezo1-mediated mechanotransduction signal might be an effective target for restoration of aging-related functional impairment in OPCs. While this study is specific to brain, the concept may also be applicable to other aged tissues and related diseases.
Fig. 11.
Aging-related brain matrix stiffening and its impact on regenerative capacity. (A) Average stiffness of brain tissues, measured by AFM indentation method, increases with aging (neonate < adult < aged). (B) Schematic to describe that oligodendrocyte progenitor cell (OPC) proliferation capacity decreases with increasing brain stiffness. (C,D) OPCs cultured on young brain-relevant soft matrix show more proliferation and differentiation capacity compared to those on aged brain-relevant rigid matrix. (E) Expression of mechano-sensitive ion channel Piezo 1 is higher in neonate OPCs than in aged OPCs. (F,G) Silencing Piezo 1 (siPiezo 1) allowed adult OPCs (aOPC) to be less sensitive to matrix rigidity, enabling them to restore proliferation and differentiation capacity. (A,D,E,G) are adapted with permission from Segel et al. [101] in Nature, 2019.
Together, the findings above demonstrate that the matrix rigidity is altered in aged tissues, shedding light on the importance of matrix rigidity in aging-related dysfunction of tissues, and imply that rigidity-sensing mechano-active cellular machineries may be targeted for therapeutic restoration of aging-related diseases. Because aging is accompanied by profound changes in mitochondrial metabolism, stem cell exhaustion, and epigenetics [98], it would be wise to keep all those aspects in mind to interpret the aging phenomena related with matrix rigidity. Possibly, the matrix-rigidity-dependent mechanotransduction signals will become an important source for aging studies in near future.
4.4. Inflammation and related phenomena
Inflammation is a protective mechanism for the host to protect itself and fight against foreign bodies. A variety of cells participate in the response, including neutrophils and macrophages [102]. Recent findings have identified that inflammatory cells, not surprisingly, are able to sense the mechanical cue (primarily rigidity) through certain receptors [[103], [104], [105], [106], [107]]. Compared to the studies of rigidity effects on other cell types (e.g., fibroblasts and cancer cells), those on inflammatory cells are still in its infancy. However, growing evidence supports the significance of matrix rigidity on inflammatory responses. For instance, some of the in vitro studies have recently found that inflammatory actions of macrophages and neutrophils are heavily influenced by the matrix rigidity. Mouse primary macrophages cultured on stiff hydrogels secrete more pro-inflammatory mediators than those cultured on soft hydrogels [103], and human monocyte cell line (THP-1) expresses higher M1 macrophage markers on rigid plastic dishes than on soft agarose gels [108]. Considering that macrophages are activated in the ECM remodeling process, especially in lesions that involve stiffening of tissues (e.g., atherosclerosis and tumors) [[108], [109], [110]], such stimulation of macrophages upon rigid matrices is conceivable. Moreover, a recent study by Jain et al. hints at matrix-related mechanosensing mechanisms that may be the plausible driving force behind the initiation of macrophage activation [111]. The lipopolysaccharide (LPS)-induced macrophages (RAW 264.7), when cultured under confined environments (e.g., within micropores), had their spreading ability impaired, with reduced actin polymerization and nuclear translocation of mechanosensitive transcription factor myocardin-related transcription factor-A (MRTF-A), which ultimately downsized macrophage activation. These in vitro studies have opened the discussion that matrix rigidity is indeed a sensitive mechanical cue recognized by neutrophils and macrophages, either aggravating or alleviating the inflammatory responses.
One notable in vivo study was recently reported by Meli et al. in such aspect. To investigate how matrix rigidity affects in vivo wound healing, authors prepared photo-crosslinkable synthetic PEG diacrylate hydrogels with different rigidities (1 kPa, 140 kPa), and implanted them subcutaneously inside the surgically prepared dorsal pockets of mice. Harvested samples revealed increased cell recruitment (at days 3 and 14) and collagen deposition (at day 14) around more rigid hydrogels. Moreover, pan-macrophages (positive for F4/80) were identified to be more populated around stiffer implants at day 3, and the activation of M1 macrophages (positive for inducible nitric oxide synthase (iNOS)) was even higher, implying the rigidity-dependent activation of pro-inflammatory reactions. The results suggest that rigidity of biomaterials considerably affects inflammatory responses of the host tissues through recruitment of macrophages at different levels, which would ultimately influence the wound healing process (Fig. 12A) [112].
Fig. 12.
Impact of matrix rigidity on tissue inflammatory responses. (A) Inflammatory responses around subcutaneously implanted PEG diacrylate gels with different rigidities (soft: 1 kPa and stiff: 140 kPa); (A-a) confocal images of immunohistochemistry, and (A-b) quantification for iNOS+, F4/80+, and YAP + cells. Adapted with permission from ref by Meli et al. [112] in Sci. Adv., 2020. (B) Rigidity-mediated MSC responses modulating immune cell recruitment in acute inflammatory lung injury; (B-a) simple schematic explaining the matrix rigidity gradient inside bone marrow (becomes more rigid as draws near to bone), wherein MSCs deliver mechanical signal to immune/inflammatory cells. (B-b) Higher secretion of monocyte recruitment cytokines (C–C motif chemokine ligand 2 (CCL2) and interleukin-2 (IL-2)) from MSCs upon rigid alginate-based gel matrix, in a TNFα dose-dependent manner. (B-c) Higher TNFα receptor clustering in rigid matrix. (B-d) In vivo model describing lung rigidity-primed MSC transplantation for treatment of lung inflammation; NSG mice(immunodeficient mice) irradiated in sublethal dose were injected intravenously with human cord blood hematopoietic stem and progenitor cells (HSPCs). After 2 weeks, MSCs primed in soft or rigid gels for 1 day followed by ± TNFα treatment for 3 days were delivered to the tibia by an intrabone (i.b.) route, while on the other tibia they were delivered in PBS. (B-e) Representative fluorescence images at day 0 and 4 after MSC delivery. (B-f) TNFα-treated MSCs in soft matrix increase migration of human peripheral blood CD14+ monocytes via secretion of CCL2. (B-b,c,d,e,f) are adapted with permission from Wong et al. [116] in Sci. Adv., 2020.
Not only neutrophils and macrophages, but MSCs in bone marrow are also crucial in inflammatory responses. MSCs interact with the inflammatory cells through secretion of chemokines and cytokines that recruit neutrophils and polarize macrophages [113,114]. Upon injury, MSCs travel across the bone marrow toward stromal wound site, and along the journey they experience varying rigidities (e.g., a few to tens of kPa across bone marrow [115], and even higher in stromal connective and osteoid tissues), thereby adopting the mechanical inputs for their signaling with inflammatory cells and immune modulation. In this regard, the rigidity-mediated crosstalk of MSCs with inflammatory cells is considered critical to interpret the inflammatory and healing events in injured tissues. The work by Wong et al. highlights the significance of matrix rigidity in priming MSCs to modulate immune cell functions under inflammation (Fig. 12B) [116]. They demonstrated that soft alginate-based gels could enhance tissue necrosis factor α (TNFα)-mediated induction of chemokines and cytokines in MSCs that are involved in recruitment of monocytes and their differentiation. Soft matrices were shown to increase the clustering of TNF receptors (TNFRs) and binding of TNFα to the cell surface. Mechanistically, the reduced actin polymerization and increased lipid rafts in soft matrices were proven to be the key machineries that aid TNFR clustering and TNFα signaling. They further proved that MSCs primed with TNFα in soft matrices could enhance in vivo production of human monocytes in marrow of xenografted mice and also trafficking of monocytes. While most works have focused on the effects of biochemical cues that can modulate inflammatory cells, this study underscored the importance of biomechanical rigidity cue in regulating the sensitivity of bone marrow MSCs to inflammatory cytokine TNFα and their subsequent ability to modulate monocytes. It provides subtle hints at how MSCs should be engineered and delivered to control inflammatory responses in vivo. This issue of rigidity effects on MSCs and their role in inflammation was also dealt with in a slightly different angle, i.e., secretion of extracellular vesicles (EVs). On soft matrices, the MSCs secreted more EVs, which are mediators involved in cell-cell communications [117]. When cultured on alginate-based soft gels, MSCs secreted approximately 10-fold more EVs per cell than those seeded on ultra-stiff plastic dishes, without compromising the quality of cargo content in EVs. Mechanistically, MSCs on soft matrix generated less-developed actin cytoskeletons, mainly related with Arp2/3 complex (not myosin II). As a result, the trafficking of intracellular multivesicular bodies, such as their transport in the cytosol and the fusion with plasma membrane, could be enhanced on soft matrix, leading to increased secretion of EVs. They further validated the efficacy of rigidity-modulated EVs in significant amelioration of acute inflammation in lung at 24 h post-injury. This study offers a new strategy to improve the immunomodulatory stem cell functions, mainly the EVs secretion, through adjustment of matrix rigidity and the responsive cellular mechanotransduction behavior.
Although the field of mechano-immunology has begun to take its initial step, new discoveries are being made with regard to the mechanical sensing ability of various immune/inflammatory cells (neutrophils, macrophages, lymphocytes) and the implication of matrix rigidity in diverse biological processes, such as extravasation of blood vessels, recognition of foreign bodies, and interactions with antigen presenting cells and their activation; therefore, the field would be able to offer novel therapeutic strategies for treating various kinds of immune/inflammatory dysfunctions and diseases.
5. Concluding remarks
Rigidity varies widely across types of tissues; from a few kPa in soft tissues to hundreds of kPa-to-GPa in hard tissues. Also, rigidity of tissues changes with disease progression and aging. Therefore, rigidity is considered a hallmark of ECM with which cells interact to further decide their responses in diverse patterns, including spreading, migration, proliferation, differentiation, and apoptosis. Importantly, such rigidity-dependent cellular behaviors are closely linked to the phenomena observed in many physiological and pathological conditions. Therefore, investigations in this area can aid in better understanding of the biological events in tissues, and offer new therapeutic strategies for the treatment of diseases.
Experiments over the last two decades performed primarily with engineered hydrogels that have varying rigidities have accumulated evidence regarding the decisive roles of matrix rigidity in altering diverse cellular behaviors and tissue functions. As witnessed, earlier findings in 2D matrices have been re-evaluated in 3D hydrogel conditions. Of note is the time-dependent rigidity change (i.e., viscoelasticity, plasticity), which has become a crucial factor for matrix models used to study cellular interactions. Also the dynamic change in matrix rigidity due to compositional remodeling mediated by cellular degradation and deposition process has just drawn attention, awaiting more extensive studies. Further investigations into the areas of dynamic cell-matrix interactions mainly in 3D models and in vivo conditions will indeed lead to technological advances in the design and processing of biomaterials and cell delivery scaffolds.
By far, numerous cellular machineries, such as integrins, focal adhesion complex, actomyosin unit, LINC molecules, nuclear pore, and ion channels have been revealed to be heavily involved in the rigidity-sensing mechanisms. This implies that those mechano-signaling molecules can be considered as appropriate therapeutic targets for the restoration of diseased or damaged tissues, which would get worse over time due to dysregulated cellular mechanics if left untreated. Recent studies have highlighted the physical transmission of extracellular cues to nuclear components across the cytoskeletal system. For instance, flattened cells on a rigid matrix exert higher actomyosin traction forces, which leads to widened aperture of nuclear pores, ultimately increasing the translocation of transcriptional factors in and out of the nuclei [38]; the change in matrix rigidity directly forces the chromosomes to be more open for transcription by altering the structure through physically connected integrin-actin-LINC molecules, by which transcriptional process is accelerated [36]; and overcoming of mechanical memory in cells placed in a persistently-stiffened matrix condition is closely related with the epigenetic signatures, i.e., histone modification (methylation and acetylation) of chromatins [118]. These compelling evidences hint at potential research opportunities in the field of rigidity-mediated nuclear mechanics, i.e., identification of the roles of nuclear mechanosensing molecules, linking them with the chromatin remodeling and modifications, and investigation of their engagement in transcriptional and post-transcriptional processes. Advances in knowledge across this area will deepen our views on interpretating the complex and dynamic biological phenomena related to matrix rigidity and approaching unidentified therapeutic targets around/in nucleus.
Some of the notable in vivo findings in matrix-rigidity-related tissue dysfunctions highlight the impact of the mechanical cue in dictating the fate of surrounding cells and tissue functions. For example, aged brain cells (myelination progenitor cells) lose their innate function primarily due to the stiffened matrix [101]; increased rigidity in early fibrotic liver weakens sinusoidal endothelial activity and their mechanical interplay with hepatic stellate cells, leading to chronic fibrosis [119]; and stiffened tumor environments drive oncogenic cells to reprogram into cancer cells [91]. These pioneering studies strongly underpin the importance of matrix rigidity in many pathological settings. As the matrix rigidity change is a common in vivo feature in which cells reform their microenvironment to adopt to altered conditions, more in vivo phenomena will continue to be unveiled, particularly those related to immune responses and aging as these are considered general causes of many chronic diseases and tissue dysfunctions.
Although our views here are mainly focused on the rigidity of matrix, rigidity is not the only parameter that the native tissue matrix provides; rather, the type and density of ligands, the nano/microstructure and orientation of matrix, and the matrix-bound soluble molecules (e.g., growth factors) are essential parts of the matrix properties. These matrix parameters are not decoupled in tissues but intimately cooperate with each other to coordinate cell and tissue function. To name a few, nanostructured surface can complicate or dampen the effect of rigidity of an underlying matrix; also, exogenously administered growth factors and signaling molecules can override the rigidity effect of wounded tissue in the stimulated healing process. As such, future studies may need to understand comprehensively the interactive role of those parameters in cell and tissue functions. Certainly, in situations where stiffening is prolonged or becomes a dominant microenvironmental cue (e.g., chronic wounds, fibrosis, aged tissue), extra effort would be needed to investigate the cause-and-effect of matrix rigidity and develop the ideal way to reverse the dysregulated role.
Declaration of interests
The authors declared no conflict of interests.
Ethics approval and consent to participate
All the authors contributed significantly to writing this review article, and agree to the content and being listed as authors on the manuscript.
Author contributions
All authors made substantial contributions to the content, writing, and editing of the manuscript.
Acknowledgements
This work was supported by the grants (2021R1A5A2022318, 2019R1C1C1002490, 2018R1A2B3003446, 2018K1A4A3A01064257, 2019R1A6A1A11034536), National Research Foundation, Republic of Korea.
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
References
- 1.Baumgart E. Stiffness--an unknown world of mechanical science? Injury. 2000;31(Suppl 2):B14–B23. [PubMed] [Google Scholar]
- 2.Discher D.E., Janmey P., Wang Y.L. Tissue cells feel and respond to the stiffness of their substrate. Science. 2005;310(5751):1139–1143. doi: 10.1126/science.1116995. [DOI] [PubMed] [Google Scholar]
- 3.Shaw T.J., Martin P. Wound repair at a glance. J. Cell Sci. 2009;122(Pt 18):3209–3213. doi: 10.1242/jcs.031187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Irianto J., Pfeifer C.R., Xia Y., Discher D.E. SnapShot: mechanosensing matrix. Cell. 2016;165(7):1820–1820 e1. doi: 10.1016/j.cell.2016.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Swift J., Ivanovska I.L., Buxboim A., Harada T., Dingal P.C., Pinter J., Pajerowski J.D., Spinler K.R., Shin J.W., Tewari M., Rehfeldt F., Speicher D.W., Discher D.E. Nuclear lamin-A scales with tissue stiffness and enhances matrix-directed differentiation. Science. 2013;341(6149) doi: 10.1126/science.1240104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Engler A.J., Sen S., Sweeney H.L., Discher D.E. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126(4):677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 7.Wang L., Wu Y., Guo B., Ma P.X. Nanofiber Yarn/hydrogel core-shell scaffolds mimicking native skeletal muscle tissue for guiding 3D myoblast alignment, elongation, and differentiation. ACS Nano. 2015;9(9):9167–9179. doi: 10.1021/acsnano.5b03644. [DOI] [PubMed] [Google Scholar]
- 8.Pelham R.J., Jr., Wang Y. Cell locomotion and focal adhesions are regulated by substrate flexibility. Proc. Natl. Acad. Sci. U. S. A. 1997;94(25):13661–13665. doi: 10.1073/pnas.94.25.13661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sun Y., Yong K.M., Villa-Diaz L.G., Zhang X., Chen W., Philson R., Weng S., Xu H., Krebsbach P.H., Fu J. Hippo/YAP-mediated rigidity-dependent motor neuron differentiation of human pluripotent stem cells. Nat. Mater. 2014;13(6):599–604. doi: 10.1038/nmat3945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fu J., Wang Y.K., Yang M.T., Desai R.A., Yu X., Liu Z., Chen C.S. Mechanical regulation of cell function with geometrically modulated elastomeric substrates. Nat. Methods. 2010;7(9):733–736. doi: 10.1038/nmeth.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Georges P.C., Janmey P.A. Cell type-specific response to growth on soft materials. J. Appl. Physiol. 1985;98(4):1547–1553. doi: 10.1152/japplphysiol.01121.2004. 2005. [DOI] [PubMed] [Google Scholar]
- 12.Yeoman B., Shatkin G., Beri P., Banisadr A., Katira P., Engler A.J. Adhesion strength and contractility enable metastatic cells to become adurotactic. Cell Rep. 2021;34(10) doi: 10.1016/j.celrep.2021.108816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yamada K.M., Sixt M. Mechanisms of 3D cell migration. Nat. Rev. Mol. Cell Biol. 2019;20(12):738–752. doi: 10.1038/s41580-019-0172-9. [DOI] [PubMed] [Google Scholar]
- 14.Lo C.M., Wang H.B., Dembo M., Wang Y.L. Cell movement is guided by the rigidity of the substrate. Biophys. J. 2000;79(1):144–152. doi: 10.1016/S0006-3495(00)76279-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kawano T., Kidoaki S. Elasticity boundary conditions required for cell mechanotaxis on microelastically-patterned gels. Biomaterials. 2011;32(11):2725–2733. doi: 10.1016/j.biomaterials.2011.01.009. [DOI] [PubMed] [Google Scholar]
- 16.Ueki A., Kidoaki S. Manipulation of cell mechanotaxis by designing curvature of the elasticity boundary on hydrogel matrix. Biomaterials. 2015;41:45–52. doi: 10.1016/j.biomaterials.2014.11.030. [DOI] [PubMed] [Google Scholar]
- 17.Isenberg B.C., Dimilla P.A., Walker M., Kim S., Wong J.Y. Vascular smooth muscle cell durotaxis depends on substrate stiffness gradient strength. Biophys. J. 2009;97(5):1313–1322. doi: 10.1016/j.bpj.2009.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Raab M., Swift J., Dingal P.C., Shah P., Shin J.W., Discher D.E. Crawling from soft to stiff matrix polarizes the cytoskeleton and phosphoregulates myosin-II heavy chain. J. Cell Biol. 2012;199(4):669–683. doi: 10.1083/jcb.201205056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vincent L.G., Choi Y.S., Alonso-Latorre B., del Alamo J.C., Engler A.J. Mesenchymal stem cell durotaxis depends on substrate stiffness gradient strength. Biotechnol. J. 2013;8(4):472–484. doi: 10.1002/biot.201200205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moriyama K., Kidoaki S. Cellular durotaxis revisited: initial-position-dependent determination of the threshold stiffness gradient to induce durotaxis. Langmuir. 2019;35(23):7478–7486. doi: 10.1021/acs.langmuir.8b02529. [DOI] [PubMed] [Google Scholar]
- 21.Ebata H., Moriyama K., Kuboki T., Kidoaki S. General cellular durotaxis induced with cell-scale heterogeneity of matrix-elasticity. Biomaterials. 2020;230 doi: 10.1016/j.biomaterials.2019.119647. [DOI] [PubMed] [Google Scholar]
- 22.Sunyer R., Conte V., Escribano J., Elosegui-Artola A., Labernadie A., Valon L., Navajas D., Garcia-Aznar J.M., Munoz J.J., Roca-Cusachs P., Trepat X. Collective cell durotaxis emerges from long-range intercellular force transmission. Science. 2016;353(6304):1157–1161. doi: 10.1126/science.aaf7119. [DOI] [PubMed] [Google Scholar]
- 23.Hadden W.J., Young J.L., Holle A.W., McFetridge M.L., Kim D.Y., Wijesinghe P., Taylor-Weiner H., Wen J.H., Lee A.R., Bieback K., Vo B.N., Sampson D.D., Kennedy B.F., Spatz J.P., Engler A.J., Choi Y.S. Stem cell migration and mechanotransduction on linear stiffness gradient hydrogels. Proc. Natl. Acad. Sci. U. S. A. 2017;114(22):5647–5652. doi: 10.1073/pnas.1618239114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shellard A., Mayor R. Collective durotaxis along a self-generated stiffness gradient in vivo. Nature. 2021;600(7890):690–694. doi: 10.1038/s41586-021-04210-x. [DOI] [PubMed] [Google Scholar]
- 25.Shellard A., Mayor R. Durotaxis: the hard path from in vitro to in vivo. Dev. Cell. 2021;56(2):227–239. doi: 10.1016/j.devcel.2020.11.019. [DOI] [PubMed] [Google Scholar]
- 26.Sunyer R., Trepat X., Durotaxis Curr. Biol. 2020;30(9):R383–R387. doi: 10.1016/j.cub.2020.03.051. [DOI] [PubMed] [Google Scholar]
- 27.Theveneau E., Mayor R. Collective cell migration of epithelial and mesenchymal cells. Cell. Mol. Life Sci. 2013;70(19):3481–3492. doi: 10.1007/s00018-012-1251-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hadjipanayi E., Mudera V., Brown R.A. Guiding cell migration in 3D: a collagen matrix with graded directional stiffness. Cell Motil Cytoskeleton. 2009;66(3):121–128. doi: 10.1002/cm.20331. [DOI] [PubMed] [Google Scholar]
- 29.Uhler C., Shivashankar G.V. Regulation of genome organization and gene expression by nuclear mechanotransduction. Nat. Rev. Mol. Cell Biol. 2017;18(12):717–727. doi: 10.1038/nrm.2017.101. [DOI] [PubMed] [Google Scholar]
- 30.Lee J.H., Kim D.H., Lee H.H., Kim H.W. Role of nuclear mechanosensitivity in determining cellular responses to forces and biomaterials. Biomaterials. 2019;197:60–71. doi: 10.1016/j.biomaterials.2019.01.010. [DOI] [PubMed] [Google Scholar]
- 31.Keung A.J., de Juan-Pardo E.M., Schaffer D.V., Kumar S. Rho GTPases mediate the mechanosensitive lineage commitment of neural stem cells. Stem Cell. 2011;29(11):1886–1897. doi: 10.1002/stem.746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leipzig N.D., Shoichet M.S. The effect of substrate stiffness on adult neural stem cell behavior. Biomaterials. 2009;30(36):6867–6878. doi: 10.1016/j.biomaterials.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 33.Saha K., Keung A.J., Irwin E.F., Li Y., Little L., Schaffer D.V., Healy K.E. Substrate modulus directs neural stem cell behavior. Biophys. J. 2008;95(9):4426–4438. doi: 10.1529/biophysj.108.132217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang C., Tibbitt M.W., Basta L., Anseth K.S. Mechanical memory and dosing influence stem cell fate. Nat. Mater. 2014;13(6):645–652. doi: 10.1038/nmat3889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rosales A.M., Vega S.L., DelRio F.W., Burdick J.A., Anseth K.S. Hydrogels with reversible mechanics to probe dynamic cell microenvironments. Angew. Chem., Int. Ed. 2017;56(40):12132–12136. doi: 10.1002/anie.201705684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Killaars A.R., Grim J.C., Walker C.J., Hushka E.A., Brown T.E., Anseth K.S. Extended exposure to stiff microenvironments leads to persistent chromatin remodeling in human mesenchymal stem cells. Adv. Sci. 2019;6(3) doi: 10.1002/advs.201801483. doi.org/ARTN 180148310.1002/advs.201801483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Walker C.J., Crocini C., Ramirez D., Killaars A.R., Grim J.C., Aguado B.A., Clark K., Allen M.A., Dowell R.D., Leinwand L.A., Anseth K.S. Nuclear mechanosensing drives chromatin remodelling in persistently activated fibroblasts. Nat Biomed Eng. 2021;5(12):1485–1499. doi: 10.1038/s41551-021-00709-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Elosegui-Artola A., Andreu I., Beedle A.E.M., Lezamiz A., Uroz M., Kosmalska A.J., Oria R., Kechagia J.Z., Rico-Lastres P., Le Roux A.L., Shanahan C.M., Trepat X., Navajas D., Garcia-Manyes S., Roca-Cusachs P. Force triggers YAP nuclear entry by regulating transport across nuclear pores. Cell. 2017;171(6):1397–1410. doi: 10.1016/j.cell.2017.10.008. e14. [DOI] [PubMed] [Google Scholar]
- 39.Cukierman E., Pankov R., Stevens D.R., Yamada K.M. Taking cell-matrix adhesions to the third dimension. Science. 2001;294(5547):1708–1712. doi: 10.1126/science.1064829. [DOI] [PubMed] [Google Scholar]
- 40.Levental I., Georges P.C., Janmey P.A. Soft biological materials and their impact on cell function. Soft Matter. 2007;3(3):299–306. doi: 10.1039/b610522j. [DOI] [PubMed] [Google Scholar]
- 41.Pioletti D.P., Rakotomanana L.R. Non-linear viscoelastic laws for soft biological tissues. Eur. J. Mech. Solid. 2000;19(5):749–759. doi.org/10.1016/S0997-7538(00)00202-3. [Google Scholar]
- 42.Huebsch N., Arany P.R., Mao A.S., Shvartsman D., Ali O.A., Bencherif S.A., Rivera-Feliciano J., Mooney D.J. Harnessing traction-mediated manipulation of the cell/matrix interface to control stem-cell fate. Nat. Mater. 2010;9(6):518–526. doi: 10.1038/nmat2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wisdom K.M., Adebowale K., Chang J., Lee J.Y., Nam S., Desai R., Rossen N.S., Rafat M., West R.B., Hodgson L., Chaudhuri O. Matrix mechanical plasticity regulates cancer cell migration through confining microenvironments. Nat. Commun. 2018;9(1):4144. doi: 10.1038/s41467-018-06641-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Loebel C., Mauck R.L., Burdick J.A. Local nascent protein deposition and remodelling guide mesenchymal stromal cell mechanosensing and fate in three-dimensional hydrogels. Nat. Mater. 2019;18(8):883–891. doi: 10.1038/s41563-019-0307-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cameron A.R., Frith J.E., Cooper-White J.J. The influence of substrate creep on mesenchymal stem cell behaviour and phenotype. Biomaterials. 2011;32(26):5979–5993. doi: 10.1016/j.biomaterials.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 46.Chaudhuri O., Gu L., Klumpers D., Darnell M., Bencherif S.A., Weaver J.C., Huebsch N., Lee H.P., Lippens E., Duda G.N., Mooney D.J. Hydrogels with tunable stress relaxation regulate stem cell fate and activity. Nat. Mater. 2016;15(3):326–334. doi: 10.1038/nmat4489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Benoit D.S., Schwartz M.P., Durney A.R., Anseth K.S. Small functional groups for controlled differentiation of hydrogel-encapsulated human mesenchymal stem cells. Nat. Mater. 2008;7(10):816–823. doi: 10.1038/nmat2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bonnans C., Chou J., Werb Z. Remodelling the extracellular matrix in development and disease. Nat. Rev. Mol. Cell Biol. 2014;15(12):786–801. doi: 10.1038/nrm3904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee H.P., Gu L., Mooney D.J., Levenston M.E., Chaudhuri O. Mechanical confinement regulates cartilage matrix formation by chondrocytes. Nat. Mater. 2017;16(12):1243–1251. doi: 10.1038/nmat4993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nam S., Chaudhuri O. Mitotic cells generate protrusive extracellular forces to divide in three-dimensional microenvironments. Nat. Phys. 2018;14(6):621–628. doi: 10.1038/s41567-018-0092-1. [DOI] [Google Scholar]
- 51.Indana D., Agarwal P., Bhutani N., Chaudhuri O. Viscoelasticity and adhesion signaling in biomaterials control human pluripotent stem cell morphogenesis in 3D culture. Adv. Mater. 2021;33(43) doi: 10.1002/adma.202101966. [DOI] [PubMed] [Google Scholar]
- 52.Grolman J.M., Weinand P., Mooney D.J. Extracellular matrix plasticity as a driver of cell spreading. Proc. Natl. Acad. Sci. U. S. A. 2020;117(42):25999–26007. doi: 10.1073/pnas.2008801117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jia Y., Wang Y., Niu L., Zhang H., Tian J., Gao D., Zhang X., Lu T.J., Qian J., Huang G., Xu F. The plasticity of nanofibrous matrix regulates fibroblast activation in fibrosis. Adv. Health. Mater. 2021;10(8) doi: 10.1002/adhm.202001856. [DOI] [PubMed] [Google Scholar]
- 54.Baker B.M., Trappmann B., Wang W.Y., Sakar M.S., Kim I.L., Shenoy V.B., Burdick J.A., Chen C.S. Cell-mediated fibre recruitment drives extracellular matrix mechanosensing in engineered fibrillar microenvironments. Nat. Mater. 2015;14(12):1262–1268. doi: 10.1038/nmat4444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Khetan S., Guvendiren M., Legant W.R., Cohen D.M., Chen C.S., Burdick J.A. Degradation-mediated cellular traction directs stem cell fate in covalently crosslinked three-dimensional hydrogels. Nat. Mater. 2013;12(5):458–465. doi: 10.1038/nmat3586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Huebsch N., Lippens E., Lee K., Mehta M., Koshy S.T., Darnell M.C., Desai R.M., Madl C.M., Xu M., Zhao X., Chaudhuri O., Verbeke C., Kim W.S., Alim K., Mammoto A., Ingber D.E., Duda G.N., Mooney D.J. Matrix elasticity of void-forming hydrogels controls transplanted-stem-cell-mediated bone formation. Nat. Mater. 2015;14(12):1269–1277. doi: 10.1038/nmat4407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Blache U., Stevens M.M., Gentleman E. Harnessing the secreted extracellular matrix to engineer tissues. Nat Biomed Eng. 2020;4(4):357–363. doi: 10.1038/s41551-019-0500-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lampi M.C., Reinhart-King C.A. Targeting extracellular matrix stiffness to attenuate disease: from molecular mechanisms to clinical trials. Sci. Transl. Med. 2018;10(422) doi: 10.1126/scitranslmed.aao0475. [DOI] [PubMed] [Google Scholar]
- 59.Martin P. Wound healing--aiming for perfect skin regeneration. Science. 1997;276(5309):75–81. doi: 10.1126/science.276.5309.75. [DOI] [PubMed] [Google Scholar]
- 60.Kordestani S.S. In: Atlas of Wound Healing. Kordestani S.S., editor. Elsevier; 2019. Chapter 5 - wound care management; pp. 31–47. [Google Scholar]
- 61.Ulrich D., Ulrich F., Unglaub F., Piatkowski A., Pallua N. Matrix metalloproteinases and tissue inhibitors of metalloproteinases in patients with different types of scars and keloids. J. Plast. Reconstr. Aesthetic Surg. 2010;63(6):1015–1021. doi: 10.1016/j.bjps.2009.04.021. [DOI] [PubMed] [Google Scholar]
- 62.Tsochatzis E.A., Bosch J., Burroughs A.K. Liver cirrhosis. Lancet. 2014;383(9930):1749–1761. doi: 10.1016/S0140-6736(14)60121-5. [DOI] [PubMed] [Google Scholar]
- 63.Foucher J., Chanteloup E., Vergniol J., Castera L., Le Bail B., Adhoute X., Bertet J., Couzigou P., de Ledinghen V. Diagnosis of cirrhosis by transient elastography (FibroScan): a prospective study. Gut. 2006;55(3):403–408. doi: 10.1136/gut.2005.069153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Olsen A.L., Bloomer S.A., Chan E.P., Gaca M.D., Georges P.C., Sackey B., Uemura M., Janmey P.A., Wells R.G. Hepatic stellate cells require a stiff environment for myofibroblastic differentiation. Am. J. Physiol. Gastrointest. Liver Physiol. 2011;301(1):G110–G118. doi: 10.1152/ajpgi.00412.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Guixe-Muntet S., Ortega-Ribera M., Wang C., Selicean S., Andreu I., Kechagia J.Z., Fondevila C., Roca-Cusachs P., Dufour J.F., Bosch J., Berzigotti A., Gracia-Sancho J. Nuclear deformation mediates liver cell mechanosensing in cirrhosis. JHEP Rep. 2020;2(5) doi: 10.1016/j.jhepr.2020.100145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liu L., You Z., Yu H., Zhou L., Zhao H., Yan X., Li D., Wang B., Zhu L., Xu Y., Xia T., Shi Y., Huang C., Hou W., Du Y. Mechanotransduction-modulated fibrotic microniches reveal the contribution of angiogenesis in liver fibrosis. Nat. Mater. 2017;16(12):1252–1261. doi: 10.1038/nmat5024. [DOI] [PubMed] [Google Scholar]
- 67.Galie P.A., Westfall M.V., Stegemann J.P. Reduced serum content and increased matrix stiffness promote the cardiac myofibroblast transition in 3D collagen matrices. Cardiovasc. Pathol. 2011;20(6):325–333. doi: 10.1016/j.carpath.2010.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chen J.H., Chen W.L., Sider K.L., Yip C.Y., Simmons C.A. beta-catenin mediates mechanically regulated, transforming growth factor-beta1-induced myofibroblast differentiation of aortic valve interstitial cells. Arterioscler. Thromb. Vasc. Biol. 2011;31(3):590–597. doi: 10.1161/ATVBAHA.110.220061. [DOI] [PubMed] [Google Scholar]
- 69.Zhong A., Mirzaei Z., Simmons C.A. The roles of matrix stiffness and ss-catenin signaling in endothelial-to-mesenchymal transition of aortic valve endothelial cells. Cardiovasc. Eng. Technol. 2018;9(2):158–167. doi: 10.1007/s13239-018-0363-0. [DOI] [PubMed] [Google Scholar]
- 70.Huang X., Yang N., Fiore V.F., Barker T.H., Sun Y., Morris S.W., Ding Q., Thannickal V.J., Zhou Y. Matrix stiffness-induced myofibroblast differentiation is mediated by intrinsic mechanotransduction. Am. J. Respir. Cell Mol. Biol. 2012;47(3):340–348. doi: 10.1165/rcmb.2012-0050OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Htwe S.S., Cha B.H., Yue K., Khademhosseini A., Knox A.J., Ghaemmaghami A.M. Role of rho-associated coiled-coil forming kinase isoforms in regulation of stiffness-induced myofibroblast differentiation in lung fibrosis. Am. J. Respir. Cell Mol. Biol. 2017;56(6):772–783. doi: 10.1165/rcmb.2016-0306OC. [DOI] [PubMed] [Google Scholar]
- 72.Horner P.J., Gage F.H. Regenerating the damaged central nervous system. Nature. 2000;407(6807):963–970. doi: 10.1038/35039559. [DOI] [PubMed] [Google Scholar]
- 73.Camand E., Morel M.P., Faissner A., Sotelo C., Dusart I. Long-term changes in the molecular composition of the glial scar and progressive increase of serotoninergic fibre sprouting after hemisection of the mouse spinal cord. Eur. J. Neurosci. 2004;20(5):1161–1176. doi: 10.1111/j.1460-9568.2004.03558.x. [DOI] [PubMed] [Google Scholar]
- 74.Moeendarbary E., Weber I.P., Sheridan G.K., Koser D.E., Soleman S., Haenzi B., Bradbury E.J., Fawcett J., Franze K. The soft mechanical signature of glial scars in the central nervous system. Nat. Commun. 2017;8 doi: 10.1038/ncomms14787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Adams K.L., Gallo V. The diversity and disparity of the glial scar. Nat. Neurosci. 2018;21(1):9–15. doi: 10.1038/s41593-017-0033-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Klopfleisch R., Jung F. The pathology of the foreign body reaction against biomaterials. J. Biomed. Mater. Res. 2017;105(3):927–940. doi: 10.1002/jbm.a.35958. [DOI] [PubMed] [Google Scholar]
- 77.Franz S., Rammelt S., Scharnweber D., Simon J.C. Immune responses to implants - a review of the implications for the design of immunomodulatory biomaterials. Biomaterials. 2011;32(28):6692–6709. doi: 10.1016/j.biomaterials.2011.05.078. [DOI] [PubMed] [Google Scholar]
- 78.Noskovicova N., Schuster R., van Putten S., Ezzo M., Koehler A., Boo S., Coelho N.M., Griggs D., Ruminski P., McCulloch C.A., Hinz B. Suppression of the fibrotic encapsulation of silicone implants by inhibiting the mechanical activation of pro-fibrotic TGF-beta. Nat Biomed Eng. 2021;5(12):1437–1456. doi: 10.1038/s41551-021-00722-z. [DOI] [PubMed] [Google Scholar]
- 79.McHedlidze T., Waldner M., Zopf S., Walker J., Rankin A.L., Schuchmann M., Voehringer D., McKenzie A.N., Neurath M.F., Pflanz S., Wirtz S. Interleukin-33-dependent innate lymphoid cells mediate hepatic fibrosis. Immunity. 2013;39(2):357–371. doi: 10.1016/j.immuni.2013.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Suki B., Bates J.H. Extracellular matrix mechanics in lung parenchymal diseases. Respir. Physiol. Neurobiol. 2008;163(1–3):33–43. doi: 10.1016/j.resp.2008.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ohayon J., Gharib A.M., Garcia A., Heroux J., Yazdani S.K., Malvè M., Tracqui P., Martinez M.-A., Doblare M., Finet G., Pettigrew R.I. Is arterial wall-strain stiffening an additional process responsible for atherosclerosis in coronary bifurcations?: an in vivo study based on dynamic CT and MRI. Am. J. Physiol. Heart Circ. Physiol. 2011;301(3):H1097–H1106. doi: 10.1152/ajpheart.01120.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Alsamman S., Christenson S.A., Yu A., Ayad N.M.E., Mooring M.S., Segal J.M., Hu J.K., Schaub J.R., Ho S.S., Rao V., Marlow M.M., Turner S.M., Sedki M., Pantano L., Ghoshal S., Ferreira D.D.S., Ma H.Y., Duwaerts C.C., Espanol-Suner R., Wei L., Newcomb B., Mileva I., Canals D., Hannun Y.A., Chung R.T., Mattis A.N., Fuchs B.C., Tager A.M., Yimlamai D., Weaver V.M., Mullen A.C., Sheppard D., Chen J.Y. Targeting acid ceramidase inhibits YAP/TAZ signaling to reduce fibrosis in mice. Sci. Transl. Med. 2020;12(557) doi: 10.1126/scitranslmed.aay8798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Butcher D.T., Alliston T., Weaver V.M. A tense situation: forcing tumour progression. Nat. Rev. Cancer. 2009;9(2):108–122. doi: 10.1038/nrc2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kass L., Erler J.T., Dembo M., Weaver V.M. Mammary epithelial cell: influence of extracellular matrix composition and organization during development and tumorigenesis. Int. J. Biochem. Cell Biol. 2007;39(11):1987–1994. doi: 10.1016/j.biocel.2007.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Boyd N.F., Lockwood G.A., Martin L.J., Knight J.A., Byng J.W., Yaffe M.J., Tritchler D.L. Mammographic densities and breast cancer risk. Breast Dis. 1998;10(3–4):113–126. doi: 10.3233/bd-1998-103-412. [DOI] [PubMed] [Google Scholar]
- 86.Provenzano P.P., Inman D.R., Eliceiri K.W., Knittel J.G., Yan L., Rueden C.T., White J.G., Keely P.J. Collagen density promotes mammary tumor initiation and progression. BMC Med. 2008;6:11. doi: 10.1186/1741-7015-6-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.DuChez B.J., Doyle A.D., Dimitriadis E.K., Yamada K.M. Durotaxis by human cancer cells. Biophys. J. 2019;116(4):670–683. doi: 10.1016/j.bpj.2019.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gkretsi V., Stylianopoulos T. Cell adhesion and matrix stiffness: coordinating cancer cell invasion and metastasis. Front. Oncol. 2018;8:145. doi: 10.3389/fonc.2018.00145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chaudhuri O., Koshy S.T., Branco da Cunha C., Shin J.W., Verbeke C.S., Allison K.H., Mooney D.J. Extracellular matrix stiffness and composition jointly regulate the induction of malignant phenotypes in mammary epithelium. Nat. Mater. 2014;13(10):970–978. doi: 10.1038/nmat4009. [DOI] [PubMed] [Google Scholar]
- 90.Lee G., Han S.B., Lee J.H., Kim H.W., Kim D.H. Cancer mechanobiology: microenvironmental sensing and metastasis. ACS Biomater. Sci. Eng. 2019;5(8):3735–3752. doi: 10.1021/acsbiomaterials.8b01230. [DOI] [PubMed] [Google Scholar]
- 91.Panciera T., Citron A., Di Biagio D., Battilana G., Gandin A., Giulitti S., Forcato M., Bicciato S., Panzetta V., Fusco S., Azzolin L., Totaro A., Dei Tos A.P., Fassan M., Vindigni V., Bassetto F., Rosato A., Brusatin G., Cordenonsi M., Piccolo S. Reprogramming normal cells into tumour precursors requires ECM stiffness and oncogene-mediated changes of cell mechanical properties. Nat. Mater. 2020;19(7):797–806. doi: 10.1038/s41563-020-0615-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Stowers R.S., Shcherbina A., Israeli J., Gruber J.J., Chang J., Nam S., Rabiee A., Teruel M.N., Snyder M.P., Kundaje A., Chaudhuri O. Matrix stiffness induces a tumorigenic phenotype in mammary epithelium through changes in chromatin accessibility. Nat Biomed Eng. 2019;3(12):1009–1019. doi: 10.1038/s41551-019-0420-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Jang M., An J., Oh S.W., Lim J.Y., Kim J., Choi J.K., Cheong J.H., Kim P. Matrix stiffness epigenetically regulates the oncogenic activation of the Yes-associated protein in gastric cancer. Nat Biomed Eng. 2021;5(1):114–123. doi: 10.1038/s41551-020-00657-x. [DOI] [PubMed] [Google Scholar]
- 94.Rachman-Tzemah C., Zaffryar-Eilot S., Grossman M., Ribero D., Timaner M., Maki J.M., Myllyharju J., Bertolini F., Hershkovitz D., Sagi I., Hasson P., Shaked Y. Blocking surgically induced lysyl oxidase activity reduces the risk of lung metastases. Cell Rep. 2017;19(4):774–784. doi: 10.1016/j.celrep.2017.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Pickup M.W., Laklai H., Acerbi I., Owens P., Gorska A.E., Chytil A., Aakre M., Weaver V.M., Moses H.L. Stromally derived lysyl oxidase promotes metastasis of transforming growth factor-beta-deficient mouse mammary carcinomas. Cancer Res. 2013;73(17):5336–5346. doi: 10.1158/0008-5472.CAN-13-0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Shen Y., Wang X., Lu J., Salfenmoser M., Wirsik N.M., Schleussner N., Imle A., Freire Valls A., Radhakrishnan P., Liang J., Wang G., Muley T., Schneider M., Ruiz de Almodovar C., Diz-Munoz A., Schmidt T. Reduction of liver metastasis stiffness improves response to bevacizumab in metastatic colorectal cancer. Cancer Cell. 2020;37(6):800–817. doi: 10.1016/j.ccell.2020.05.005. e7. [DOI] [PubMed] [Google Scholar]
- 97.Gopinath S.D., Rando T.A. Stem cell review series: aging of the skeletal muscle stem cell niche. Aging Cell. 2008;7(4):590–598. doi: 10.1111/j.1474-9726.2008.00399.x. [DOI] [PubMed] [Google Scholar]
- 98.Lopez-Otin C., Blasco M.A., Partridge L., Serrano M., Kroemer G. The hallmarks of aging. Cell. 2013;153(6):1194–1217. doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Huynh J., Nishimura N., Rana K., Peloquin J.M., Califano J.P., Montague C.R., King M.R., Schaffer C.B., Reinhart-King C.A. Age-related intimal stiffening enhances endothelial permeability and leukocyte transmigration. Sci. Transl. Med. 2011;3(112):112ra122. doi: 10.1126/scitranslmed.3002761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wood L.K., Kayupov E., Gumucio J.P., Mendias C.L., Claflin D.R., Brooks S.V. Intrinsic stiffness of extracellular matrix increases with age in skeletal muscles of mice. J. Appl. Physiol. 1985;117(4):363–369. doi: 10.1152/japplphysiol.00256.2014. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Segel M., Neumann B., Hill M.F.E., Weber I.P., Viscomi C., Zhao C., Young A., Agley C.C., Thompson A.J., Gonzalez G.A., Sharma A., Holmqvist S., Rowitch D.H., Franze K., Franklin R.J.M., Chalut K.J. Niche stiffness underlies the ageing of central nervous system progenitor cells. Nature. 2019;573(7772):130–134. doi: 10.1038/s41586-019-1484-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Waisman A., Liblau R.S., Becher B. Innate and adaptive immune responses in the CNS. Lancet Neurol. 2015;14(9):945–955. doi: 10.1016/S1474-4422(15)00141-6. doi.org/10.1016/S1474-4422(15)00141-6. [DOI] [PubMed] [Google Scholar]
- 103.Previtera M.L., Sengupta A. Substrate stiffness regulates proinflammatory mediator production through TLR4 activity in macrophages. PLoS One. 2015;10(12) doi: 10.1371/journal.pone.0145813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.O'Connor R.S., Hao X., Shen K., Bashour K., Akimova T., Hancock W.W., Kam L.C., Milone M.C. Substrate rigidity regulates human T cell activation and proliferation. J. Immunol. 2012;189(3):1330–1339. doi: 10.4049/jimmunol.1102757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Saitakis M., Dogniaux S., Goudot C., Bufi N., Asnacios S., Maurin M., Randriamampita C., Asnacios A., Hivroz C. Different TCR-induced T lymphocyte responses are potentiated by stiffness with variable sensitivity. Elife. 2017;6 doi: 10.7554/eLife.23190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zeng Y., Yi J., Wan Z., Liu K., Song P., Chau A., Wang F., Chang Z., Han W., Zheng W., Chen Y.H., Xiong C., Liu W. Substrate stiffness regulates B-cell activation, proliferation, class switch, and T-cell-independent antibody responses in vivo. Eur. J. Immunol. 2015;45(6):1621–1634. doi: 10.1002/eji.201444777. [DOI] [PubMed] [Google Scholar]
- 107.Zhu C., Chen W., Lou J., Rittase W., Li K. Mechanosensing through immunoreceptors. Nat. Immunol. 2019;20(10):1269–1278. doi: 10.1038/s41590-019-0491-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Okamoto T., Takagi Y., Kawamoto E., Park E.J., Usuda H., Wada K., Shimaoka M. Reduced substrate stiffness promotes M2-like macrophage activation and enhances peroxisome proliferator-activated receptor gamma expression. Exp. Cell Res. 2018;367(2):264–273. doi: 10.1016/j.yexcr.2018.04.005. [DOI] [PubMed] [Google Scholar]
- 109.Adlerz K.M., Aranda-Espinoza H., Hayenga H.N. Substrate elasticity regulates the behavior of human monocyte-derived macrophages. Eur. Biophys. J. 2016;45(4):301–309. doi: 10.1007/s00249-015-1096-8. [DOI] [PubMed] [Google Scholar]
- 110.Sridharan R., Cavanagh B., Cameron A.R., Kelly D.J., O'Brien F.J. Material stiffness influences the polarization state, function and migration mode of macrophages. Acta Biomater. 2019;89:47–59. doi: 10.1016/j.actbio.2019.02.048. [DOI] [PubMed] [Google Scholar]
- 111.Jain N., Vogel V. Spatial confinement downsizes the inflammatory response of macrophages. Nat. Mater. 2018;17(12):1134–1144. doi: 10.1038/s41563-018-0190-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Meli V.S., Atcha H., Veerasubramanian P.K., Nagalla R.R., Luu T.U., Chen E.Y., Guerrero-Juarez C.F., Yamaga K., Pandori W., Hsieh J.Y., Downing T.L., Fruman D.A., Lodoen M.B., Plikus M.V., Wang W., Liu W.F. YAP-mediated mechanotransduction tunes the macrophage inflammatory response. Sci. Adv. 2020;6(49) doi: 10.1126/sciadv.abb8471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wang Y., Chen X., Cao W., Shi Y. Plasticity of mesenchymal stem cells in immunomodulation: pathological and therapeutic implications. Nat. Immunol. 2014;15(11):1009–1016. doi: 10.1038/ni.3002. [DOI] [PubMed] [Google Scholar]
- 114.Le Blanc K., Mougiakakos D. Multipotent mesenchymal stromal cells and the innate immune system. Nat. Rev. Immunol. 2012;12(5):383–396. doi: 10.1038/nri3209. [DOI] [PubMed] [Google Scholar]
- 115.Park D., Spencer J.A., Koh B.I., Kobayashi T., Fujisaki J., Clemens T.L., Lin C.P., Kronenberg H.M., Scadden D.T. Endogenous bone marrow MSCs are dynamic, fate-restricted participants in bone maintenance and regeneration. Cell Stem Cell. 2012;10(3):259–272. doi: 10.1016/j.stem.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wong S.W., Lenzini S., Cooper M.H., Mooney D.J., Shin J.W. Soft extracellular matrix enhances inflammatory activation of mesenchymal stromal cells to induce monocyte production and trafficking. Sci. Adv. 2020;6(15) doi: 10.1126/sciadv.aaw0158. eaaw0158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lenzini S., Debnath K., Joshi J.C., Wong S.W., Srivastava K., Geng X., Cho I.S., Song A., Bargi R., Lee J.C., Mo G.C.H., Mehta D., Shin J.W. Cell-matrix interactions regulate functional extracellular vesicle secretion from mesenchymal stromal cells. ACS Nano. 2021 doi: 10.1021/acsnano.1c03231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wang P., Dreger M., Madrazo E., Williams C.J., Samaniego R., Hodson N.W., Monroy F., Baena E., Sanchez-Mateos P., Hurlstone A., Redondo-Munoz J. WDR5 modulates cell motility and morphology and controls nuclear changes induced by a 3D environment. Proc. Natl. Acad. Sci. U. S. A. 2018;115(34):8581–8586. doi: 10.1073/pnas.1719405115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Higashi T., Friedman S.L., Hoshida Y. Hepatic stellate cells as key target in liver fibrosis. Adv. Drug Deliv. Rev. 2017;121:27–42. doi: 10.1016/j.addr.2017.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]