Abstract
Introduction
The SARS-CoV-2 pandemic has impacted the care of cancer patients. This study sought to assess the pandemic’s impact on the clinical presentations and outcomes of newly referred patients with lung cancer from the Greater Paris area.
Methods
We retrospectively retrieved the electronic health records and administrative data of 11.4 million patients pertaining to Greater Paris University Hospital (AP-HP). We compared indicators for the 2018–2019 period to those of 2020 in regard to newly referred lung cancer cases. We assessed the initial tumour stage, the delay between the first multidisciplinary tumour board (MTB) and anticancer treatment initiation, and 6-month overall survival (OS) rates depending on the anticancer treatment, including surgery, palliative systemic treatment, and best supportive care (BSC).
Result
Among 6240 patients with lung cancer, 2179 (35%) underwent tumour resection, 2069 (33%) systemic anticancer therapy, 775 (12%) BSC, whereas 1217 (20%) did not receive any treatment. During the first lockdown, the rate of new diagnoses decreased by 32% compared with that recorded in 2018–2019. Initial tumour stage, repartition of patients among treatment categories, and MTB-related delays remained unchanged. The 6-month OS rates of patients diagnosed in 2018–2019 who underwent tumour resection were 98% versus 97% (HR = 1.2; 95% CI: 0.7–2.0) for those diagnosed in 2020; the respective rates for patients who underwent systemic anticancer therapy were 78% versus 79% (HR = 1.0; 95% CI: 0.8–1.2); these rates were 20% versus 13% (HR = 1.3; 95% CI: 1.1–1.6) for those who received BSC. COVID-19 was associated with poorer OS rates (HR = 2.1; 95% CI: 1.6–3.0) for patients who received systemic anticancer therapy.
Conclusions
The SARS-CoV-2 pandemic has not exerted any deleterious impact on 6-month OS of new lung cancer patients that underwent active anticancer therapy in Greater Paris University hospitals.
Keywords: COVID-19, Delivery of health care, Early cancer detection, Lung neoplasms, Quality of health care, Routinely collected health data
Abbreviations: AP-HP, Assistance Publique - Hôpitaux de Paris; CDW, Clinical Data Warehouse; ICD, International Classification of Diseases; MTB, Multidisciplinary Tumor Board; OS, Overall Survival
1. Introduction
The SARS-CoV-2 pandemic has impacted the care trajectories of patients suffering from lung cancer, given that screening and lung nodule investigations were postponed [1]. Due to potential delays in both diagnosis and treatment, modellers anticipated increasing lung cancer-related mortality rates in the forthcoming years [2]. However, evidence of the pandemic’s impact on care trajectories and outcomes of patients with lung cancer remains limited. Most published studies are small-sized, focused on the first 2020 semester only, and based on declarative surveys [3].
This study sought to assess the impact of the SARS-CoV-2 pandemic on newly referred lung cancer cases from the Greater Paris area in regard to their tumour stage at diagnosis, anticancer upfront treatments, and 6-month overall survival (OS) during and after the SARS-CoV-2 epidemic outbreak in early 2020.
2. Material and methods
We performed a retrospective cohort study using the Clinical Data Warehouse (CDW) of the Greater Paris University Hospitals (Assistance Publique - Hôpitaux de Paris, AP-HP), which contains routinely collected medical and administrative data pertaining to 11.4 million patients [4]. We selected patients with lung cancer who were newly referred to one of the 27 AP-HP teaching hospitals with clinical data available. Patients were included provided that C33, C34, D021 or D022 ICD 10 codes were recorded between 1st January 2018 and 31st December 2020, that no recurrences were noted in the previous two years, and that these patients were not affected by another cancer.
We classified cancer treatments at AP-HP as follows: tumour resection, including peri-operative treatment (codes GFFA001-002, GFFA004, GFFA006-013, GFFA015-016, GFFA018-019, GFFA021-031, GFFA033-034 and GFFC002 of the French Common Classification of Medical Procedures, 11th edition); systemic anticancer therapy, including chemotherapy ICD-10 code Z511, irrespective of radiotherapy; either best supportive care (ICD-10 code Z515) or no cancer-related treatment.
Overall survival (OS) was defined as the time period between the date of the first occurrence of an ICD-10 lung cancer code and the patient’s death. The patient data were censored at the date the patient was last known to be alive. Patients’ mortality follow-up ended on 31st June 2021, on account of the availability of out-of-hospital mortality data. The cohort definition permitted a minimum follow-up period of 6 months for all patients.
Rule-based natural language processing algorithms were used to identify the initial tumour stage using: 1/baseline computer tomography (CT) text reports (90 days before up to 30 days after diagnosis date); 2/first postoperative pathology report for resected tumours [5]. We classified the pTNM tumour stage (8th WHO TNM classification) according to the relapse risk into low- and high-risk defined as pT1-2aN0 and pT2b-T4 N0, xN1, pT1-2bN2, respectively. Based on identified multidisciplinary tumour board (MTBs) dates, we calculated the time from the first MTB to the first anticancer treatment administration.
A positive recent polymerase chain reaction (PCR) serologic test for SARS-CoV-2 or one of the U071x ICD-10 codes from the cancer diagnosis date to the one-year follow-up period defined SARS-CoV-2 infection. We compared the indicator values from the 2018–19 period with those of 2020, with a focus made on the French national lockdowns (17th March to 11th May 2020; 30th October to 15th December 2020) periods. For comparing the 2018–2019 data with those of 2020, the Kaplan–Meier methodology was applied to estimate the cumulative probabilities of death from all causes, using a Cox proportional hazard model to estimate the hazard ratio (HR) with its 95% confidence intervals (CI) and p-values. Categorical variables were compared using chi-squared testing.
This study was approved by the institutional AP-HP review board (IRB 00011591) (CSE 20–55_COVONCO-AP), and was in line with the French data privacy regulator (CNIL 1980120), as well.
Final data extraction was performed on 1st February 2022.
3. Results
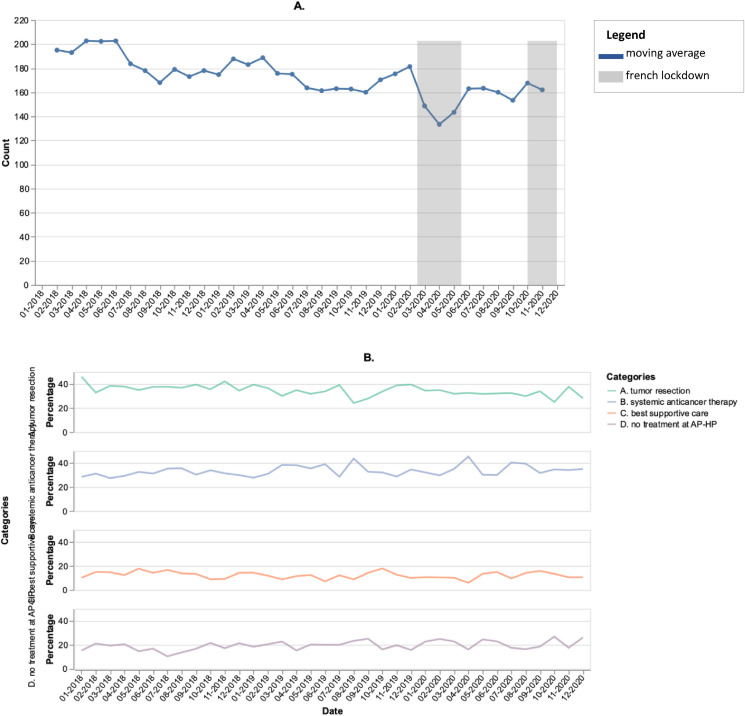
Between January 2018 and December 2020, overall, 6240 patients with lung cancer were newly referred to an AP-HP hospital. In 2018, 2019 and 2020, the median age reached was 67 (IQR, 60–74), 68 (IQR, 60–75) and 68 (60–74) years, respectively. The rate of female patients was 37%, 40% and 38%, respectively. Among them, 2179 (35%) underwent tumour resection, 2069 (33%) received systemic anticancer therapy, 775 (12%) were given BSC, while 1217 (20%), mostly managed at other centres than AP-PH hospitals, received either targeted therapies or no anticancer treatment at all at AP-HP. Main patient characteristics did not change over time (median age: 68 years; 38% of women). During the first national lockdown (March–May 2020), the number of new lung cancer diagnosis cases decreased by 32% compared with the average of the same months in 2018–2019 (587 versus 401), with no catch-up observed afterwards (Fig. 1 A) [4].
Fig. 1.
Three-point moving average of the monthly number of lung cancer cases newly referred to AP-HP teaching hospitals (A), and distribution of related upfront therapeutic strategies between 2018 and 2020 (B).
Among the 2213 pathology reports available following tumour resection, overall, 1893 exhibited pTNM assessments. The distribution of pTNM risk groups did not change over time, from being 62% versus 64% for the low-risk and 38% versus 36% for the high-risk categories in 2018–2019 versus 2020, respectively (p = 0.40). Among the 2602 patients with baseline CT-scan reports available in the AP-HP CDW, the metastatic cancer rates did not change over time, being 29% versus 28% in 2018–2019 and 2020, respectively (p = 0.78). The rates of non-metastatic lung cancer patients undergoing tumour resection, non-surgical multimodal treatment, and BSC before versus after the outbreak reached 42% versus 42%, 49% versus 50%, and 9% versus 8%, respectively.
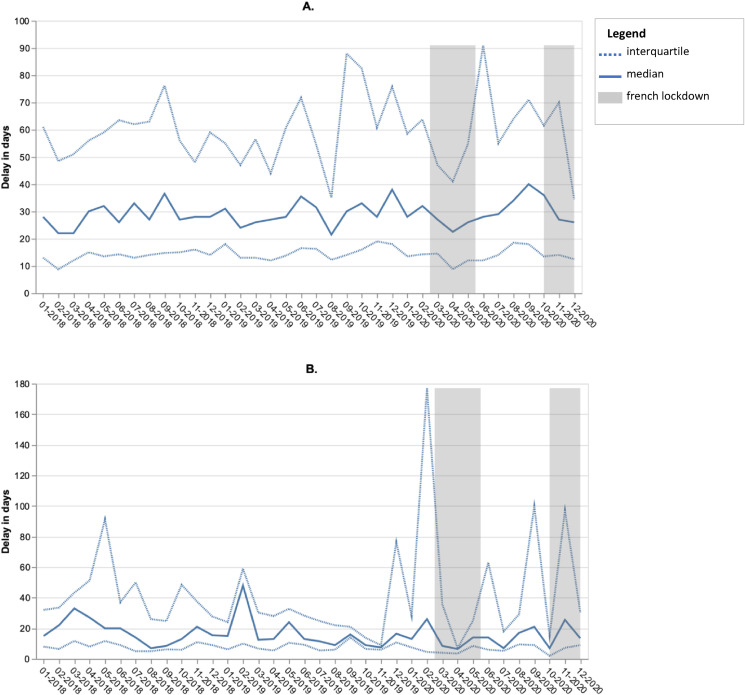
Among the 3436 cases with a MTB report available in the AP-HP CDW, the median delay between the first MTB and first anticancer therapeutic procedure remained unchanged over time, including the lockdown-related time periods (Fig. 2 A and B).
Fig. 2.
Evolution of the median delay between the 1st multidisciplinary tumour board (MTB) and the 1st therapeutic procedure for patients newly referred to the AP-HP hospitals between 2018 and 2020 for lung cancer: patients for whom anticancer treatment was initiated before (A) (n = 2,507, 77%) or after (B) MTB (n = 736, 23%).
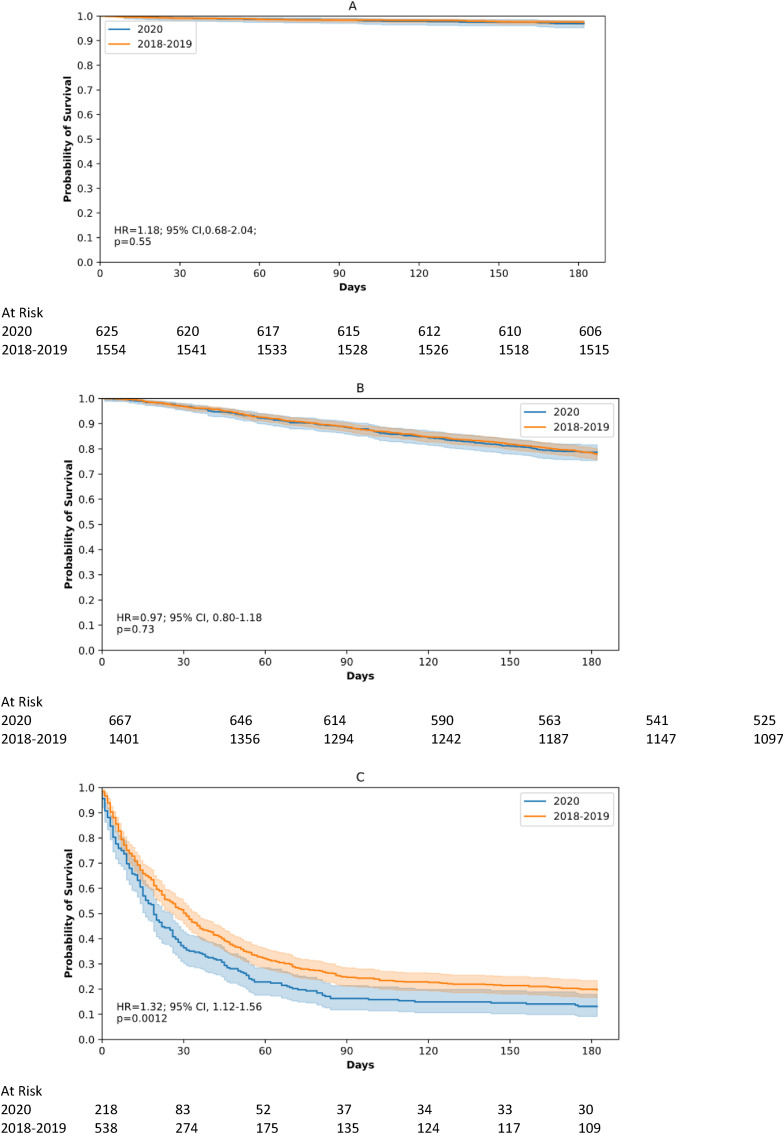
The distribution of patients among each treatment category did not vary either over time (Fig. 1B). The 12-month OS rates among those who underwent tumour resection and systemic anticancer therapy were comparable in 2018–2019 versus 2020 (98% versus 97% [HR = 1.2; 95% CI: 0.7–2.0]) and (78% versus 79% [HR = 1.0; 95% CI: 0.8–1.2]), whereas OS rates decreased in 2020 compared with 2018–2019 among patients receiving BSC (20% versus 13% [HR = 1.3; 95% CI: 1.1–1.6]) (Fig. 3 ).
Fig. 3.
Six-month overall survival rates of newly referred lung cancer patients undergoing upfront tumour resection (A), anticancer systemic therapy (B) or best supportive care (C) in 2018–2019 and in 2020 at AP-HP University hospitals and hazard ratio (HR) estimated by a Cox proportional hazard model.
The SARS-CoV-2 infection rates among patients referred in 2020 were 23%, 16%, and 15% for the three abovementioned treatment categories. Among the patients diagnosed with COVID-19, the median time duration between the date of cancer diagnosis and the date of Sars-Cov2 infection reached 68 days (IQR 14; 167). In this subpopulation, 8%, 18% and 75% were infected before, during, and after the hospital visit for the first administration of their anticancer treatment, respectively. Among patients referred in 2020 and undergoing tumour resection, OS rates were not significantly decreased in the SARS-CoV-2 group versus the no COVID-19 group (HR = 1.9; 95% CI: 0.7–5.2). Among patients referred in 2020 who received systemic anticancer therapy, OS was shorter in the SARS-CoV-2 group compared with the no COVID-19 group (HR = 2.1, 95% CI: 1.5–3.0). Due to the limited number of patients in the BSC group, such an analysis could not be performed for this group.
4. Discussion
We observed a decrease in the number of patients with lung cancer newly referred to any AP-HP hospital during the first national COVID-19 lockdown, without any catch-up afterwards. This suggests that some patients might have been managed in other general hospitals, and this, in an effort to avoid overloaded University hospitals during the COVID-19-related hospitalisation peaks. The transient interruption in lung cancer diagnostic procedures during the first wave likely accounts for the lower number of newly diagnosed patients [1]. Of note, such a decrease was no longer observed during the second lockdown (Fig. 1A). This suggests that both patients and healthcare providers adapted differently to that situation.
We did not identify any impairment in either initial clinical presentation or intent of anticancer therapeutic strategies concerning patients diagnosed during and after the SARS-CoV-2 outbreak, encompassing the surgical option, as well. Tumour stage shift related to the SARS-CoV-2 pandemic in lung cancer is under debate in the literature [6]. Some incidental new diagnoses might have been made at an early tumour stage during chest CT scans related to COVID diagnostic procedures. Again, this suggests that patients presented no higher tumour burden due to either delayed diagnosis, reported surgical decision, or both. Local guidelines emphasised the need to maintain optimal access to tumour resection for patients diagnosed with lung or head and neck cancer at APHP during the pandemic.
Like others, we found that the difference in the mortality rate of patients resected in 2018–19 versus those of 2020 was not statistically significant [[6], [7], [8]]. In the resected patient subgroup, the HR value was consistent with that observed in the other patient subgroups, with differences being not statistically significant, possibly due to a lack of power. Nevertheless, this association turned out to be statistically significant in a subgroup of resected patients with ongoing COVID-19, as previously reported in France [9].
Similarly, the survival of patients receiving systemic anticancer therapy was not likely to be significantly worse. However, in this category, patients referred in 2020 and presenting with a SARS-CoV-2 infection displayed a poorer survival than those without SARS-CoV-2 infection. This observation is perfectly in line with the 30–43% COVID-19-related mortality rate in patients with lung cancer, as reported in previous studies [10,11].
The mortality rate was significantly higher among patients receiving BSC in 2020 versus those receiving it in 2018–2019. Advanced lung cancers have been recognised to be poor prognosticators in the event of SARS-CoV-2 infection [12,13]. Indeed, some authors reported that outbreak-related changes in care pathways impacted a greater extent of palliative therapies versus curative ones, meaning surgery-based treatments [14,15].
Considering this study’s limitations, we had no access to the death causes of COVID-19 patients. A lead-time bias could account for the poorer survival in patients diagnosed after the SARS-CoV-2 outbreak [16].
Our results suggest that, although fewer patients were referred to the Greater Paris University Hospitals during the first lockdown, those who were treated did not actually experience a care trajectory and a clinical outcome that differed from that they would have undergone prior to the COVID-19 period. The additional mortality may have been more related to SARS-CoV-2 infection itself than to any treatment delays [17]. However, longer follow-up periods are warranted to assess the pandemic’s long-term impact on staging and anticancer therapeutic strategies of new lung cancer cases.
Author contributions
Emmanuelle Kempf: Conceptualisation, Methodology, Formal analysis, Data Curation, Writing - Original Draft, Funding acquisition, Project administration, Investigation. Guillaume Lamé: Conceptualisation, Methodology, Formal analysis, Writing - Original Draft. Sonia Priou: Conceptualisation, Methodology, Formal analysis, Data Curation, Writing - Original Draft. Gilles Chatellier: Conceptualisation, Methodology, Formal analysis, Writing - Original Draft, Supervision, Project administration. Romain Bey: Conceptualisation, Methodology, Resources, Writing - review & editing. Xavier Tannier: Conceptualisation, Methodology, Writing - review & editing. Christel Daniel: Conceptualisation, Methodology, Formal analysis, Resources, Writing - review & editing, Supervision, Project administration. Christophe Tournigand: Conceptualisation, Methodology, Formal analysis, Writing - review & editing, Supervision. Marie Wislez: Conceptualisation, Methodology, Resources, Writing - review & editing Jacques Cadranel: Conceptualisation, Methodology, Resources, Writing - review & editing. Laurent Zelek: Conceptualisation, Methodology, Resources, Writing - review & editing. Gérard Zalcman: Conceptualisation, Methodology, Resources, Writing - review & editing.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We would like to thank Mrs. Patricia Serre, Mrs. Cécile Poret, Mr. Ariel Cohen, Mr. Thomas Petit-Jean, Mr. Alexandre Mouchet, and Mr. Stéphane Bréant for their assistance in data access, quality assessment, and analysis.
This research was supported by the teams of the Clinical Data Warehouse of Greater Paris University Hospitals (AP-HP).
This research was supported by a grant from ARC Foundation for cancer research (grant reference COVID202001343).
References
- 1.Mazzone P.J., Gould M.K., Arenberg D.A., et al. Management of lung nodules and lung cancer screening during the COVID-19 pandemic: CHEST expert panel report. Chest. 2020;158(1):406–415. doi: 10.1016/j.chest.2020.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maringe C., Spicer J., Morris M., et al. The impact of the COVID-19 pandemic on cancer deaths due to delays in diagnosis in England, UK: a national, population-based, modelling study. Lancet Oncol. 2020;21(8):1023–1034. doi: 10.1016/S1470-2045(20)30388-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Round T., L'Esperance V., Bayly J., et al. COVID-19 and the multidisciplinary care of patients with lung cancer: an evidence-based review and commentary. Br J Cancer. 2021;125(5):629–640. doi: 10.1038/s41416-021-01361-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kempf E., Lamé G., Layese R., et al. New cancer cases at the time of SARS-Cov2 pandemic and related public health policies: a persistent and concerning decrease long after the end of the national lockdown. Eur J Cancer. 2021;150:260–267. doi: 10.1016/j.ejca.2021.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kempf E., Priou S., Lamé G., et al. Impact of two waves of Sars-Cov2 outbreak on the number, clinical presentation, care trajectories and survival of patients newly referred for a colorectal cancer: a French multicentric cohort study from a large group of University hospitals. Int J Cancer. 2022;150(10):1609–1618. doi: 10.1002/ijc.33928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang S.H., Zervos M., Kent A., et al. Safety of patients and providers in lung cancer surgery during the COVID-19 pandemic. Eur J Cardio-thoracic Surg. 2020;58(6):1222–1227. doi: 10.1093/ejcts/ezaa332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leclère J.B., Fournel L., Etienne H., et al. Maintaining surgical treatment of non-small cell lung cancer during the COVID-19 pandemic in Paris. Ann Thorac Surg. 2021;111(5):1682–1688. doi: 10.1016/j.athoracsur.2020.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Challine A., Dousset B., de'Angelis N., et al. Impact of coronavirus disease 2019 (COVID-19) lockdown on in-hospital mortality and surgical activity in elective digestive resections: a nationwide cohort analysis. Surgery. 2021;170(6):1644–1649. doi: 10.1016/J.SURG.2020.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pages P.B., Cottenet J., Bonniaud P., et al. Impact of the SARS-CoV-2 epidemic on lung cancer surgery in France: a nationwide study. Cancers (Basel) 2021;13(24) doi: 10.3390/CANCERS13246277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lièvre A., Turpin A., Ray-Coquard I., et al. Risk factors for Coronavirus Disease 2019 (COVID-19) severity and mortality among solid cancer patients and impact of the disease on anticancer treatment: a French nationwide cohort study (GCO-002 CACOVID-19) Eur J Cancer. 2020;141:62–81. doi: 10.1016/J.EJCA.2020.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Benderra M.A., Aparicio A., Leblanc J., et al. Clinical characteristics, care trajectories and mortality rate of SARS-CoV-2 infected cancer patients: a multicenter cohort study. Cancers. 2021;13(19):4749. doi: 10.3390/CANCERS13194749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garassino M.C., Whisenant J.G., Huang L.C., et al. COVID-19 in patients with thoracic malignancies (TERAVOLT): first results of an international, registry-based, cohort study. Lancet Oncol. 2020;21(7):914–922. doi: 10.1016/S1470-2045(20)30314-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grivas P., Khaki A.R., Wise-Draper T.M., et al. Association of clinical factors and recent anticancer therapy with COVID-19 severity among patients with cancer: a report from the COVID-19 and Cancer Consortium. Ann Oncol. 2021;32(6):787–800. doi: 10.1016/j.annonc.2021.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Elkrief A., Kazandjian S., Bouganim N. Changes in lung cancer treatment as a result of the coronavirus disease 2019 pandemic. JAMA Oncol. 2020;6(11):1805–1806. doi: 10.1001/JAMAONCOL.2020.4408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Glasbey J., Ademuyiwa A., Adisa A., et al. Effect of COVID-19 pandemic lockdowns on planned cancer surgery for 15 tumour types in 61 countries: an international, prospective, cohort study. Lancet Oncol. 2021;0(0):1–11. doi: 10.1016/S1470-2045(21)00493-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang S.C., Wang J Der, Wang S.Y. Considering lead-time bias in evaluating the effectiveness of lung cancer screening with real-world data. Sci Rep. 2021;11(1) doi: 10.1038/S41598-021-91852-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sud A., Torr B., Jones M.E., et al. Effect of delays in the 2-week-wait cancer referral pathway during the COVID-19 pandemic on cancer survival in the UK: a modelling study. Artic Lancet Oncol. 2020;21:1035–1079. doi: 10.1016/S1470-2045(20)30392-2. [DOI] [PMC free article] [PubMed] [Google Scholar]