Abstract
Background
Chronic use of Angiotensin-converting enzyme (ACE) inhibitors (ACEi) and aldosterone-receptor blockers (ARB) is not associated with worse outcomes in patients with COVID-19. However, evidence on the impact of their discontinuation during hospital admission is scarce. Our aim was to determine whether withdrawal of ACEi, ARB and mineralocorticoid receptor antagonists (MRA) is associated with all-cause mortality in a real-life large cohort of patients with SARS-CoV-2 infection.
Methods
Observational cohort study from a large referral center from 1 March 2020 to 20 April 2020. Withdrawal of renin-angiotensin-aldosterone system inhibitors was defined as the absence of any received dose during hospital admission in patients receiving chronic treatment. Prescriptions during admission were confirmed by data from the central pharmacy computerized system.
Results
A total of 2042 patients (mean age 68.4±17.6, 57.1% male) with confirmed COVID-19 were included. During a median follow-up of 57 (21-55) days, 583 (28.6%) died. Prior to hospital admission 468 (22.9%), 343 (16.8%) and 83 (4.1%) patients were receiving ACEi, ARB and MRA respectively. During the study period, 216 (46.2%), 193 (56.3%) and 41 (49.4%) were withdrawn from the corresponding drug. After adjusting for age, cardiovascular risk factors, baseline comorbidities and in-hospital COVID-19 dedicated treatment, withdrawal of ACE inhibitors (hazard ration [HR] 1.48 [95% confidence interval –CI– 1.16-1.89]) and MRA (HR 2.01 [95% CI 1.30-3.10]) were shown to be independent predictors of all-cause mortality. No independent relationship between ARB withdrawal and mortality was observed.
Conclusion
ACEi and MRA withdrawal were associated with higher mortality. Strong consideration should be given to not discontinuing these medications during hospital admission.
Keywords: COVID-19, Withdrawal, Renin-angiotensin-aldosterone inhibitors, Angiotensin-converting enzyme inhibitors, Angiotensin-receptor blockers, Mineralocorticoid receptor antagonists
Resumo
Introdução
O uso crónico de inibidores da ECA (IECA) e de antagonistas dos recetores de aldosterona (ARA) não está associado a resultados piores em doentes com Covid-19. No entanto, a evidência relativa ao impacto da sua retirada durante a admissão hospitalar é escassa. O nosso objetivo foi determinar se a retirada do IECA, ARA e antagonistas dos recetores dos mineralocorticóides (ARM) está associada à mortalidade por todas as causas numa grande coorte real de doentes com infeção por SRA-CoV-2.
Métodos
Estudo coorte observacional a partir de um grande centro de referência de 1 de março de 2020 a 20 de abril de 2020. A retirada dos inibidores do sistema RAAS foi definida como a ausência de qualquer dose recebida durante a admissão hospitalar em doentes que recebem tratamento prolongado. As prescrições durante a admissão foram confirmadas por dados do sistema informático da farmácia central.
Resultados
Um total de 2042 doentes (idade média de 68,4 ±17,6, 57,1% do sexo masculino) com COVID-19 confirmado foram incluídos. Durante um acompanhamento médio de 57 (21-55) dias, 583 (28,6%) morreram.
Conclusão
A retirada do IECA e do ARM foi associada a uma mortalidade mais elevada. Deve ser dada grande atenção para não interromper estes medicamentos durante a admissão hospitalar.
Palavras-chave: COVID-19, Retirada, Inibidores de renina-angiotensina aldosterona, Inibidores de enzimas conversoras de angiotensina, Bloqueadores de receptores de angiotensina, Antagonistas de receptores de mineralocorticóides
Introduction
The angiotensin-converting enzyme 2 (ACE2) plays a key role in the renin-angiotensin-aldosterone system (RAAS) which regulates blood pressure, volume status and vascular tone.1 Concerns were raised during the early COVID-19 pandemic about the entry of SARS-CoV-2 into the host cells mediated by the ACE2 membrane-bound form.2 This led to the indiscriminate discontinuation of RAAS inhibitors in a large number of patients with cardiovascular disease. However, RAAS inhibitors are critical in the management of hypertension and other cardiovascular diseases, such as heart failure (HF). As a consequence, many cardiovascular scientific societies released statements calling for caution and recommending continuing chronic treatment with angiotensin-converting enzyme inhibitors (ACEi) and angiotensin II receptor blockers (ARB) in COVID-19 patients.3 Indeed, recent research has confirmed that the chronic use of ACEi and ARB is not associated with a higher likelihood of COVID-19 infection or poorer outcomes in this disease.4, 5, 6, 7 However, the specific impact of withdrawing ACE inhibitors, ARB or mineralocorticoid receptor antagonists (MRA) during hospital admission for COVID-19 is unknown, and published results from ongoing randomized controlled trials are pending8.
Methods
Study design and participants
We screened all consecutive patients with a clinical suspicion of COVID-19 disease who attended the emergency room in a large tertiary care center, from 1 March 2020 to 20 April 2020. Patients were only included in the study if they had confirmation of SARS-CoV2 infection confirmed by an RNA reverse-transcriptase–polymerase-chain-reaction (RT-PCR) assay of nasal or pharyngeal swab specimens and if they were ultimately admitted to hospital. Acknowledging that patients with COVID-19 may ultimately die after prolonged hospital admissions, we specifically excluded patients who were alive but with a follow-up time of less than 30 days at the time the database was locked. The present study was approved by the hospital Institutional Review Board. Individual written informed consent was waived based on legal standards for national healthcare alarm situations.
Data collection
Epidemiological, demographic, clinical, laboratory, treatment, and outcome data were extracted from electronic medical records from the index and subsequent hospital admissions using a standardized electronic data collection form. In addition, the central healthcare record system, which collects information and medical reports from all public hospitals and primary healthcare centers from the Madrid region was reviewed for additional information and follow-up. In order to avoid selection bias due to severe illness (i.e., withdrawal of RAASi due to hypotension secondary to septic shock), patients who had medication withdrawn were defined as those who had chronic treatment with ACEi, ARB or MRA before hospital admission and received no dose during hospitalization, irrespective of their clinical status. In-hospital prescription was confirmed using data from the central pharmacy computerized information system. All data were thoroughly reviewed by a team of 13 cardiologists. Special care was given to the identification of CV characteristics and outcomes. Any disagreements regarding data classification were reviewed by the whole team, and a decision was finally made by consensus.
Statistical analysis
Categorical variables are shown as rates and percentages, and continuous variables as mean (SD). The means for continuous variables were compared using independent group t-tests when the data were normally distributed, otherwise, the Mann-Whitney test was used. The normality of distributions was assessed using the Shapiro-Wilk test. Proportions for categorical variables were compared using the χ2 test or the Fisher exact test, as appropriate. Survival during follow-up was assessed using Kaplan Meier analysis and, where appropriate, the log-rank test. Risk adjustments of associations between ACE inhibitors, ARB or MRA withdrawal and all-cause mortality were evaluated using a multivariable Cox proportional-hazards model including age, sex, cardiovascular risk factors, baseline comorbidities and in-hospital treatments. All analyses were performed with Stata v14.2 (StataCorp, College Station, TX, USA).
Results
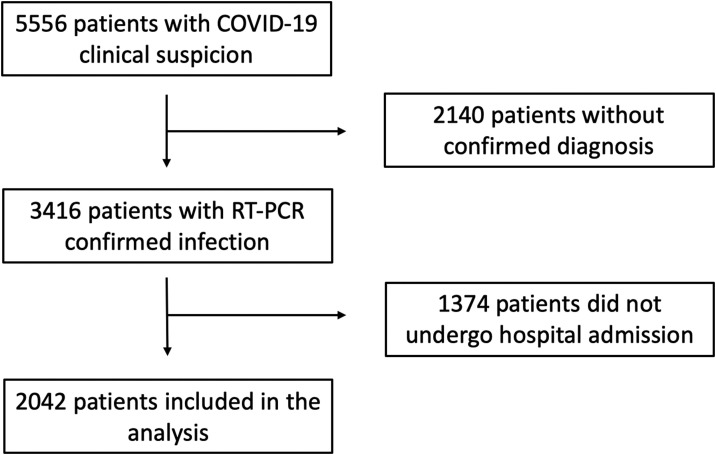
During the study period, 5556 patients with clinical suspicion of COVID-19 attended the emergency department of our tertiary care center. Of these, 3416 had confirmed SARS-CoV-2 infection and were screened for participation in the present study. Finally, 2042 patients (mean age 68.4±17.6 years, 57.1% male) were admitted to hospital and entered the present analysis (Figure 1 ). A total of 583 died during the study period, and the median length of follow-up was 57 (21-55) days. One thousand and eighty patients (53.0%) had a history of hypertension. Before hospital admission, 847 were receiving chronic treatment with RAAS inhibitors: 468 (22.9%) with ACE inhibitors, 343 (16.8%) with ARB and 83 (4.1%) with MRA.
Figure 1.
Study flow. Of the 3416 screened patients with confirmed SARS-CoV-2 infection, 2042 were admitted to hospital and included in the analysis.
Full baseline characteristics and comparisons depending on whether or not patients were receiving chronic treatment with RAAS inhibitors are shown in Supplementary Table 1. Patients in the RAASi group were older, more frequently male and had a significantly worse baseline clinical profile. Regarding laboratory data, RAASi patients showed more profound abnormalities of C-reactive protein, fibrinogen and prothrombin activity, without differences in cardiac biomarkers such as NT-proBNP and hs-troponin I. Except for steroids, both groups received similar dedicated COVID-19 treatment during hospital admission. RAASi patients presented higher mortality (36.7% vs 22.8%, p<0.001). However, no differences were observed using the log-rank test after stratifying for baseline hypertension (p=0.828). Similarly, different Cox-proportional hazards models with sequential adjustment for age, sex, baseline cardiovascular risk factors and baseline cardiovascular comorbidities showed no independent relationship between chronic treatment with RAAS inhibitors and mortality (HR 1.01 [95% CI 0.83-1.26], Supplementary Table 2).
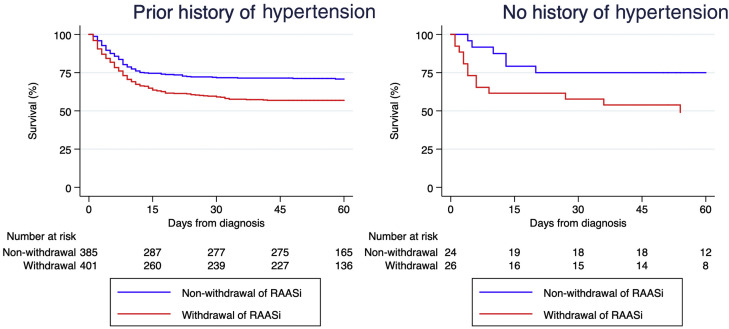
Of 847 patients on RAAS inhibitors, a total of 436 (51%) underwent RAASi withdrawal at hospital admission. There were no significant differences in baseline characteristics between those with and without RAASi withdrawal (Table 1 ). At presentation, the former had lower blood pressure and received less hydroxychloroquine and azithromycin. Patients discontinuing RAASi were more frequently admitted to an intensive care unit and had higher mortality. Log-rank test analysis with hypertension stratification showed significantly worse survival in patients who had RAAS inhibitors withdrawn (p<0.001, Figure 2 ). Multivariable analysis using a Cox-proportional hazards model adjusting for a large number of relevant covariates (Table 2 ) confirmed that RAASi withdrawal was independently associated with mortality during follow-up (HR 1.47 [95% CI 1.15-1.89]).
Table 1.
Baseline characteristics and comparisons between non-withdrawal and withdrawal groups.
| All patients on RAASi (n=847) | Non-withdrawal (n=411) | Withdrawal (n=436) | p value | |
|---|---|---|---|---|
| Age | 75.7±12.6 | 75.1±12.7 | 76.2±12.4 | 0.206 |
| Male sex | 505 (59.6%) | 239 (58.2%) | 266 (61.0%) | 0.397 |
| Hypertension | 793 (93.6%) | 386 (93.9%) | 407 (93.4%) | 0.638 |
| Diabetes | 282 (33.3%) | 130 (31.6%) | 152 (34.9%) | 0.583 |
| Dyslipidemia | 512 (60.5%) | 252 (61.3%) | 260 (59.6%) | 0.842 |
| CHD | 130 (15.4%) | 61 (14.8%) | 69 (15.8%) | 0.693 |
| HF | 97 (11.5%) | 52 (12.7%) | 45 (10.3%) | 0.287 |
| TIA/Stroke | 99 (11.7%) | 50 (12.2%) | 49 (11.2%) | 0.681 |
| CKD | 112 (13.2%) | 52 (12.7%) | 60 (13.8%) | 0.634 |
| PAD | 107 (12.6%) | 44 (10.7%) | 63 (14.5%) | 0.153 |
| SBP (mmHg) at admission | 132.8±24.0 | 136. 8±24.3 | 129.0±23.0 | <0.001 |
| SatO2 (%) at admission | 90.5±6.9 | 90.8±6.5 | 90.3±7.2 | 0.218 |
| CRP (max) | 154.4±106.1 | 152.1±103.1 | 156.7±109.0 | 0.545 |
| Fibrinogen (max) | 884.6±256.9 | 891.8±252.1 | 877.8±261.5 | 0.436 |
| Prothrombin act. (min) | 75.8±25.3 | 76.3±25.5 | 75.2±25.1 | 0.544 |
| D-Dimer (max) | 10 246±31 952 | 9034±29 680 | 11 602±34 315 | 0.316 |
| hs-TnI (max) | 711.6±8218.4 | 1095.3±11244.6 | 279.9±1176.2 | 0.3872 |
| NT-proBNP (max) | 6125±14 656 | 6008±12 850 | 6277±16 809 | 0.9065 |
| Hydroxychloroquine | 767 (90.6%) | 393 (95.6%) | 374 (85.8%) | <0.001 |
| Lopinavir/Ritonavir | 114 (13.5%) | 31 (7.5%) | 83 (19.0%) | <0.001 |
| Azithromycin | 470 (55.5%) | 249 (60.6%) | 221 (50.7%) | 0.004 |
| Corticosteroids | 190 (2.4%) | 94 (22.9%) | 96 (2.0%) | 0.766 |
| Severe bleeding | 9 (1.1%) | 4 (1.0%) | 5 (1.2%) | 1.000 |
| Thrombotic event | 33 (3.9%) | 16 (3.9%) | 17 (3.9%) | 0.996 |
| Arrhythmias | 57 (6.7%) | 23 (5.6%) | 34 (7.8%) | 0.201 |
| Critical care | 71 (8.4%) | 25 (6.1%) | 46 (10.6%) | 0.016 |
| Death | 311 (36.7%) | 118 (28.7%) | 193 (44.3%) | <0.001 |
ACEi: angiotensin converting enzyme inhibitors; ARB: angiotensin receptor blockers; CHD: coronary heart disease; CKD: chronic kidney disease; CRP: C-reactive protein; HF: heart failure; MRA: mineralocorticoid receptor antagonists; PAD: peripheral artery disease; TIA: transient ischemic attack.
Figure 2.
Kaplan-Meier survival analysis stratified based on prior history of hypertension. Statistically significant differences were observed in both groups of patients: with (left panel) and without (right panel) history of hypertension.
Table 2.
Univariable and multivariable Cox proportional-hazards model regarding RAAS inhibitors withdrawal and all-cause mortality.
| Variable | Univariable |
Multivariable |
||||||
|---|---|---|---|---|---|---|---|---|
| HR | Std. Err. | p value | 95% CI | HR | Std. Err. | p value | 95% CI | |
| RAASi withdrawal | 1.71 | 0.20 | <0.001 | 1.36-2.15 | 1.60 | 0.20 | <0.001 | 1.25-2.04 |
| Age (per year) | 1.06 | 0.01 | <0.001 | 1.05-1.07 | 1.06 | 0.01 | <0.001 | 1.04-1.07 |
| Male sex | 1.20 | 0.14 | 0.118 | 0.95-1.51 | 1.33 | 0.17 | 0.029 | 1.03-1.72 |
| Hypertension | 0.97 | 0.23 | 0.891 | 0.61-1.54 | 0.90 | 0.23 | 0.681 | 0.55-1.47 |
| Diabetes Mellitus | 1.19 | 0.14 | 0.149 | 0.94-1.49 | 1.03 | 0.13 | 0.800 | 0.80-1.33 |
| Dyslipidemia | 1.34 | 0.17 | 0.020 | 1.05-1.71 | 1.21 | 0.16 | 0.157 | 0.93-1.58 |
| CHD | 1.29 | 0.19 | 0.086 | 0.96-1.73 | 0.79 | 0.16 | 0.262 | 0.53-1.19 |
| Heart Failure | 1.53 | 0.24 | 0.007 | 1.12-2.09 | 1.11 | 0.20 | 0.560 | 0.78-1.59 |
| TIA/Stroke | 1.60 | 0.25 | 0.003 | 1.17-2.18 | 1.11 | 0.18 | 0.518 | 0.80-1.54 |
| PAD | 1.63 | 0.25 | 0.001 | 1.21-2.19 | 1.42 | 0.30 | 0.099 | 0.94-2.16 |
| CKD | 1.61 | 0.24 | 0.001 | 1.20-2.15 | 0.95 | 0.15 | 0.772 | 0.70-1.31 |
| Hydroxychloroquine | 0.56 | 0.10 | 0.001 | 0.40-0.78 | 0.59 | 0.12 | 0.012 | 0.39-0.89 |
| Lopinavir/Ritonavir | 1.10 | 0.17 | 0.550 | 0.81-1.49 | 0.72 | 0.14 | 0.087 | 0.49-1.05 |
| Azithromycin | 0.62 | 0.07 | <0.001 | 0.49-0.77 | 0.84 | 0.11 | 0.175 | 0.65-1.08 |
| Corticosteroids | 1.72 | 0.21 | <0.001 | 1.36-2.18 | 1.84 | 0.23 | <0.001 | 1.43-2.36 |
ACEi: angiotensin converting enzyme inhibitors; ARB: angiotensin receptor blockers;; CHD: coronary heart disease; CKD: chronic kidney disease; CRP: C-reactive protein; MRA: mineralocorticoid receptor antagonists; PAD: peripheral artery disease; TIA: transient ischemic attack.
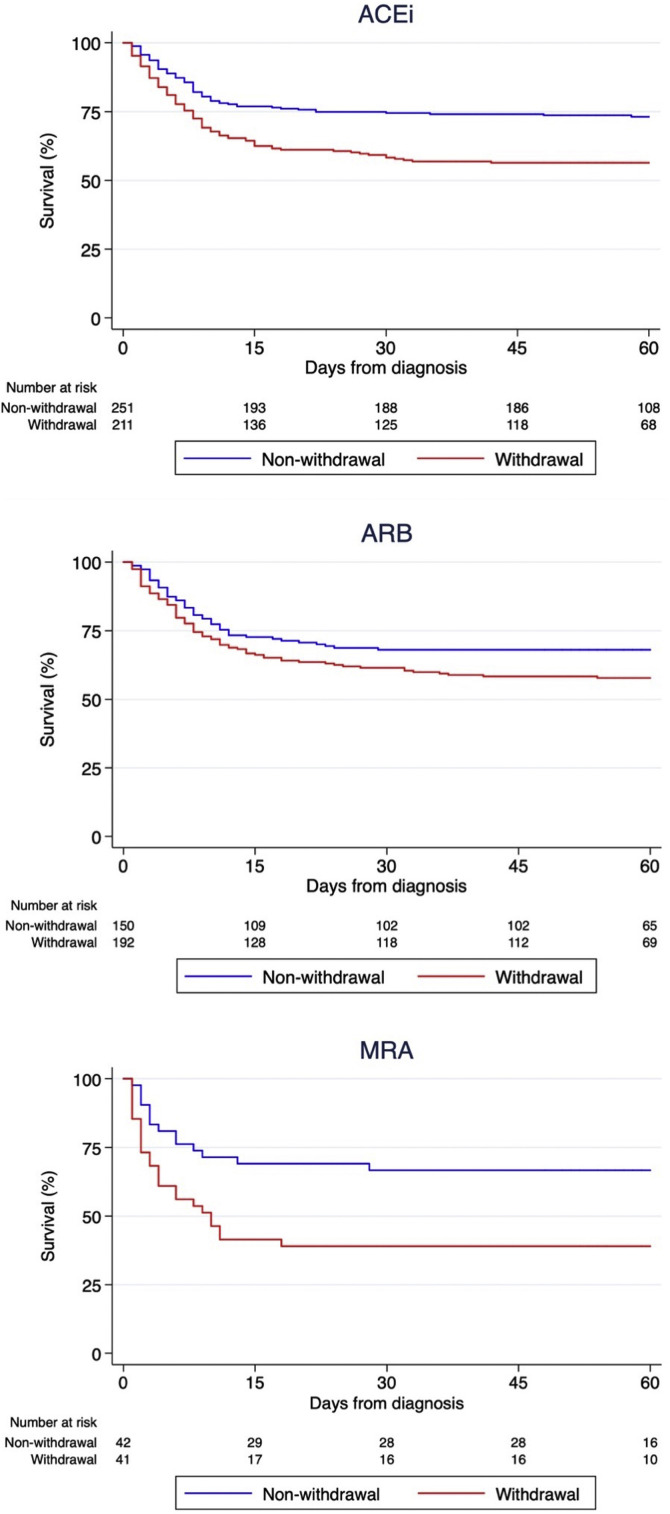
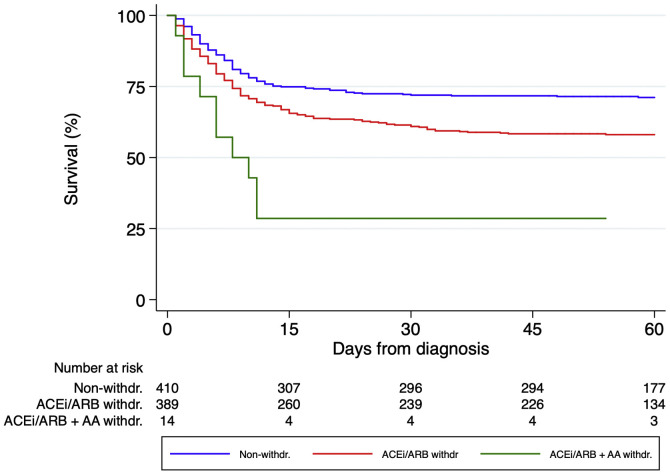
Regarding the impact of each different RAASi therapeutic group, 216 (46.2%), 193 (56.3%) and 41 (49.4%) patients discontinued ACEi, ARB and MRA respectively during hospital stay. Survival analysis using the log-rank test stratified by hypertension (Figure 3 ) showed that ACEi withdrawal (p<0.001) and MRA withdrawal (p=0.007) were associated with higher mortality. Among patients discontinuing ARB, there was a trend for lower mortality (p=0.056), but the association did not reach statistical significance. A multivariable analysis adjusted for multiple confounders confirmed that ACEi withdrawal (HR 1.62 [95% CI 1.23-2.14]) and MRA withdrawal (HR 2.10 [95% CI 1.32-3.34]) were independent predictors of mortality while ARB withdrawal (1.25 [95% CI 0.93-1.68]) did not reach statistical significance (Table 3 ). Patients discontinuing ACEi or ARB plus MRA had the worst prognosis (p<0.001 by the log-rank test, Figure 4 ).
Figure 3.
Kaplan-Meier survival analysis among strata of patients with different groups of chronic renin-angiotensin-aldosterone system inhibitors: Angiotensin converting enzyme inhibitors (upper panel), angiotensin receptor blockers (middle panel) and mineralocorticoid receptor antagonists (lower panel).
Table 3.
Univariable and multivariable Cox proportional-hazards model regarding each group of renin-angiotensin-aldosterone system inhibitor withdrawal and all-cause mortality.
| Variable | HR | Std. Err. | p value | 95% CI | HR | Std. Err. | p value | 95% CI |
|---|---|---|---|---|---|---|---|---|
| ACEi withdrawal | 1.40 | 0.17 | 0.010 | 1.10-1.79 | 1.62 | 0.23 | <0.001 | 1.23-2.14 |
| ARB withdrawal | 1.25 | 0.16 | 0.080 | 0.97-1.60 | 1.25 | 0.19 | 0.140 | 0.93-1.68 |
| MRA withdrawal | 2.43 | 0.50 | <0.001 | 1.62-3.63 | 2.10 | 0.50 | <0.001 | 1.32-3.34 |
| Age (per year) | 1.06 | 0.01 | <0.001 | 1.05-1.07 | 1.06 | 0.01 | <0.001 | 1.04-1.07 |
| Male sex | 1.20 | 0.14 | 0.120 | 0.95-1.51 | 1.34 | 0.18 | 0.030 | 1.03-1.73 |
| Hypertension | 0.97 | 0.23 | 0.890 | 0.61-1.54 | 0.95 | 0.24 | 0.850 | 0.58-1.56 |
| Diabetes Mellitus | 1.19 | 0.14 | 0.149 | 0.94-1.49 | 1.07 | 0.14 | 0.580 | 0.83-1.38 |
| Dyslipidemia | 1.34 | 0.17 | 0.020 | 1.05-1.71 | 1.19 | 0.16 | 0.200 | 0.91-1.56 |
| CHD | 1.29 | 0.19 | 0.0860 | 0.96-1.73 | 0.78 | 0.16 | 0.220 | 0.52-1.16 |
| HF | 1.53 | 0.24 | 0.007 | 1.12-2.09 | 0.95 | 0.18 | 0.770 | 0.65-1.38 |
| TIA/Stroke | 1.60 | 0.25 | 0.003 | 1.17-2.18 | 1.14 | 0.19 | 0.424 | 0.82-1.58 |
| PAD | 1.63 | 0.25 | 0.001 | 1.21-2.19 | 1.43 | 0.31 | 0.092 | 0.94-2.18 |
| CKD | 1.61 | 0.24 | 0.001 | 1.20-2.15 | 0.94 | 0.15 | 0.680 | 0.68-1.29 |
| Hydroxychloroquine | 0.56 | 0.10 | 0.001 | 0.40-0.78 | 0.55 | 0.12 | 0.006 | 0.36-0.84 |
| Lopinavir/Ritonavir | 1.10 | 0.17 | 0.550 | 0.81-1.49 | 0.74 | 0.14 | 0.112 | 0.50-1.07 |
| Azithromycin | 0.62 | 0.07 | <0.001 | 0.49-0.77 | 0.84 | 0.11 | 0.177 | 0.65-1.08 |
| Corticosteroids | 1.72 | 0.21 | <0.001 | 1.36-2.18 | 1.85 | 0.24 | <0.001 | 1.44-2.38 |
ACEi: angiotensin converting enzyme inhibitors; ARB: angiotensin receptor blockers; CHD: coronary heart disease; CKD: chronic kidney disease; CRP: C-reactive protein; HF: heart failure; MRA: mineralocorticoid receptor antagonists; PAD: peripheral artery disease; RAAS: renin-angiotensin-aldosterone system; TIA: transient ischemic attack.
Figure 4.
Kaplan-Meier survival analysis among all patients with RAASi treatment. Patients discontinuing both an angiotensin converting enzyme inhibitors/angiotensin receptor blockers plus a mineralocorticoid receptor antagonists showed the worst prognosis.
Alternatively, in order to test the hypothesis that discontinuation of oral medications may be a surrogate for the severity of COVID-19, we studied the association of withdrawing beta-blockers and statins (two drugs commonly included in the chronic treatment of patients being admitted for COVID-19) and mortality. Among the study participants, 337 patients were receiving beta-blockers and 827 were receiving statins before hospital admission. Respectively, 78 (23.2%) and 456 (55.1%) discontinued that medication after being diagnosed with SARS-CoV-2 infection. Neither of these situations were associated with higher mortality during follow-up (p=0.821 and p=0.247 according to the log-rank test).
Discussion
Concerns regarding a possible association between ACEi/ARB treatment and poor outcomes in SARS-CoV-2 infection9 were raised early in the COVID-19 pandemic. They were based on the potential interaction between SARS-CoV spike protein and its host receptor ACE2.2 However, recent research suggests that their use is not linked to more severe disease4, 5, 6, 7 and there is a clear potential for harm related to RAAS inhibitor withdrawal in patients with established indications.10 A multicenter retrospective study11 suggested that in-patient use of ACEi/ARB may actually result in better outcomes. However, only 188 patients with ACEi/ARB were included in the analysis and prior use of these medications before hospital admission was not reported.
Our group recently demonstrated that withdrawal of guideline-directed medical therapy in patients with history of chronic HF was associated with higher COVID-19-related mortality.12, 13 However, data on the whole population of COVID-19 patients are scarce. We report here data from a large cohort of real-life consecutive patients with a significant length of follow-up, for whom in-hospital prescriptions were carefully reviewed and confirmed. Overall, these data convincingly illustrate that, after adjustment for a large number of relevant potential confounders, withdrawal of ACEi during hospital admission for COVID-19 was associated with higher mortality. Moreover, the association with higher mortality was not confirmed for the withdrawal of other therapeutic groups, such as beta-blockers or statins. This disputes the hypothesis that withdrawal of any oral medications may act as a surrogate for the severity of COVID-19. It is in agreement with previous data from patients with viral pneumonia of other etiologies.14 The different effects of ACEi and ARB in COVID-19 patients have not been reported before. However, there is conflicting evidence regarding their effects on angiotensin II, the substrate of ACE2, and uniform responses should not be expected for both groups of drugs.10 Therefore, ACEi and ARB may result in differential effects on bradykinin levels via their interaction with the prekallikrein/kallikrein system.15 Bradykinin interacts with nitric oxide and prostaglandins, therefore influencing platelet activation and release of von Willebrand factor,16 which may play a role in COVID-19 coagulopathy.17 However, there is the alternative possibility that our study did not have enough statistic power to detect harm from ARB withdrawal.
In addition, we report for the first time that the withdrawal of mineralocorticoid receptor antagonists is independently associated with mortality in COVID-19 patients after adjusting for multiple relevant confounders, and in our series, patients who stopped both types of medications (an ACEi/ARB plus a MRA) had a significantly worse prognosis than those who only discontinued ACEi/ARB.
Even though the underlying link between ACEi withdrawal and higher mortality is unknown, there is the possibility that it may be related to a stronger inflammatory state in the patients discontinuing these drugs.18 In addition, elevated levels of plasma angiotensin II have been reported in COVID-19 patients, which correlates with viral load and lung injury.19 ACE2 is the most potent enzyme that converts the vasoconstrictor angiotensin II to angiotensin (1–7). Angiotensin (1–7) has organ-protective properties that oppose and counterbalance those of angiotensin II.20 Mineralocorticoid receptor antagonists increase ACE2 activity in patients with HF,21 and this effect theoretically may act against angiotensin II accumulation and local RAAS activation.22 However, a recent study also including non-COVID-19 HF patients showed that neither ACEi, nor ARB were associated with higher plasma ACE2 concentrations.23 Effects reported from MRA were small and inconsistent. Thereby, further studies are needed to understand the physiopathological pathways underlying RAAS inhibitors and COVID-19.
Limitations
This was an observational study and therefore may be affected by the inherent bias of this type of design (i.e., the impossibility of establishing definitive causal associations). Therefore, our results should be considered hypothesis-generating and confirmed by ongoing randomized controlled trials.8 Even though we report data from a large cohort of consecutive patients, the study may lack statistical power to detect a potential harm from ARB withdrawal at admission. Besides, we did not include in the present analysis patients managed in an outpatient setting and, therefore, this population deserves further study.
Conclusions
In our large cohort of hospitalized patients with COVID-19, withdrawal of ACE inhibitors and mineralocorticoid receptor antagonists is independently associated with higher mortality. Strong consideration should be given to not discontinuing this medication during hospital admission. These decisions should be individualized based on the whole clinical picture.
Funding
This work has received no funding.
Conflict of interest
J.L.M. reports grants and personal fees from Bayer, grants and personal fees from Correvio, grants from Daiichi-Sankyo, personal fees from Sanofi, outside the submitted work; E.L.S reports grants from Zoll Medical Corporation, Boehringer Ingelheim and Servier; personal fees from Daiichi Sankyo, Rovi, Servier, BARD and Astra Zeneca, all outside the submitted work. All other authors had no conflict of interest.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.repc.2021.06.021.
Appendix A. Supplementary material
References
- 1.Crowley S.D., Gurley S.B., Oliverio M.I., et al. Distinct roles for the kidney and systemic tissues in blood pressure regulation by the renin-angiotensin system. J Clin Invest. 2005;115:1092–1099. doi: 10.1172/JCI200523378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wan Y., Shang J., Graham R., et al. Receptor recognition by the novel coronavirus from Wuhan: an analysis based on decade-long structural studies of SARS coronavirus. J Virol. 2020;94:1–9. doi: 10.1128/JVI.00127-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Simone G. 2020. Position statement of the ESC Council on hypertension on ACE-inhibitors and angiotensin receptor blockers.https://www.escardio.org/Councils/Council-on-Hypertension-(CHT)/News/position-statement-of-the-esc-council-on-hypertension-on-ace-inhibitors-and-ang [accessed 16.3.20] [Google Scholar]
- 4.Mancia G., Rea F., Ludergnani M., et al. Renin–angiotensin–aldosterone system blockers and the risk of Covid-19. N Engl J Med. 2020:1–10. doi: 10.1056/nejmoa2006923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reynolds H.R., Adhikari S., Pulgarin C., et al. Renin–angiotensin–aldosterone system inhibitors and risk of Covid-19. N Engl J Med. 2020:1–8. doi: 10.1056/nejmoa2008975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Abajo F.J., Rodríguez-Martín S., Lerma V., et al. Use of renin–angiotensin–aldosterone system inhibitors and risk of COVID-19 requiring admission to hospital: a case-population study. Lancet. 2020;6736:1–10. doi: 10.1016/S0140-6736(20)31030-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bean DM, Kraljevic Z, Searle T, et al. ACE-inhibitors and angiotensin-2 receptor blockers are not associated with severe SARS-Covid19 infection in a multi-site UK acute Hospital Trust. Eur J Heart Fail. Accepted Author Manuscript. 10.1002/ejhf.1924 [DOI] [PMC free article] [PubMed]
- 8.Lopes R.D., Macedo A.V.S., Barros e Silva P.G.M., et al. Continuing versus suspending angiotensin-converting enzyme inhibitors and angiotensin receptor blockers: impact on adverse outcomes in hospitalized patients with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)—the BRACE CORONA trial: BRACE CORONA Trial: rationale and design. Am Heart J. 2020;226:49–59. doi: 10.1016/j.ahj.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fang L., Karakiulakis G., Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir Med. 2020;8:e21. doi: 10.1016/S2213-2600(20)30116-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vaduganathan M., Vardeny O., Michel T. Renin–angiotensin–aldosterone system inhibitors in patients with Covid-19. N Engl J Med. 2020:1653–1659. doi: 10.1056/NEJMsr2005760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang P., Zhu L., Cai J., et al. Association of inpatient use of angiotensin converting enzyme inhibitors and angiotensin II receptor blockers with mortality among patients with hypertension hospitalized with COVID-19. Circ Res. 2020 doi: 10.1161/circresaha.120.317134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rey J.R., Caro-Codón J., Rosillo S.O., et al. Heart failure in Covid-19 patients: prevalence, incidence and prognostic implications. Eur J Heart Fail. 2020 doi: 10.1002/ejhf.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang N., Cao J., Lal S. COVID-19: getting to the heart of the matter. Eur J Heart Fail. 2020:6–8. doi: 10.1002/ejhf.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Henry C., Zaizafoun M., Stock E., et al. Impact of angiotensin-converting enzyme inhibitors and statins on viral pneumonia. Baylor Univ Med Cent Proc. 2018;31:419–423. doi: 10.1080/08998280.2018.1499293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Su J.B. Different cross-talk sites between the renin-angiotensin and the kallikrein-kinin systems. J Renin-Angiotensin-Aldosterone Syst. 2014;15:319–328. doi: 10.1177/1470320312474854. [DOI] [PubMed] [Google Scholar]
- 16.Conway E.M. Reincarnation of ancient links between coagulation and complement. J Thromb Haemost. 2015;13:S121–S132. doi: 10.1111/jth.12950. [DOI] [PubMed] [Google Scholar]
- 17.Rodriguez-Iturbe B., Pons H., Johnson R.J. Role of the immune system in hypertension. Physiol Rev. 2017;97:1127–1164. doi: 10.1152/physrev.00031.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Becker R.C. COVID-19 update: Covid-19-associated coagulopathy. J Thromb Thrombolysis. 2020;50:54–67. doi: 10.1007/s11239-020-02134-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu Y., Yang Y., Zhang C., et al. Clinical and biochemical indexes from 2019-nCoV infected patients linked. Sci China Life Sci. 2020;63:364–374. doi: 10.1007/s11427-020-1643-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Danser A.H.J., Epstein M., Batlle D. Renin-angiotensin system blockers and the COVID-19 pandemic: at present there is no evidence to abandon renin-angiotensin system blockers. Hypertension. 2020:1382–1385. doi: 10.1161/HYPERTENSIONAHA.120.15082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Keidar S., Gamliel-Lazarovich A., Kaplan M., et al. Mineralocorticoid receptor blocker increases angiotensin-converting enzyme 2 activity in congestive heart failure patients. Circ Res. 2005;97:946–953. doi: 10.1161/01.RES.0000187500.24964.7A. [DOI] [PubMed] [Google Scholar]
- 22.Verdecchia P., Cavallini C., Spanevello A., et al. The pivotal link between ACE2 deficiency and SARS-CoV-2 infection. Eur J Intern Med. 2020;76:14–20. doi: 10.1016/j.ejim.2020.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sama I.E., Ravera A., Santema B.T., et al. Circulating plasma concentrations of angiotensin-converting enzyme 2 in men and women with heart failure and effects of renin-angiotensin-aldosterone inhibitors. Eur Heart J. 2020;41:1810–1817. doi: 10.1093/eurheartj/ehaa373. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.