Abstract
Introduction
High-intensity interval training (HIIT) is an efficient training method to improve vascular function, maximal oxygen consumption, and muscle mitochondrial capacity while maximizing muscular damage. Recently, functional foods have been considered a practical approach to avoiding HIIT damage and improving sports performance. Thus, the present study will evaluate the effectiveness of date seed powder as a functional food on the nutritional, oxidative stress, anti/inflammatory status, mental health, and performance of active people.
Methods
This study is a double-blind, randomized, placebo-controlled trial, which will be conducted among recreational runners at Tabriz stadiums, Iran. Thirty-six recreational runners will be randomly selected into two groups to consume 26 g/d date seed powder or placebo for 14 days. Both groups will do HIIT workouts. Body composition, food intake, total antioxidant capacity (TAC), oxidative stress index (OSI), total oxidant status (TOS), superoxide dismutase (SOD), glutathione peroxidase (GPx), malondialdehyde (MDA), 8-iso-prostaglandin F2α (8-iso-PGF2α), uric acid, protein carbonyl (PC), catalase (CAT), glutathione (GSH), nitric oxide (NO), high-sensitivity C-reactive protein (hs-CRP), tumor necrosis factor-alpha (TNF-α), interleukin-6 (IL-6), interleukin-10 (IL-10), IL-6/IL-10, creatine kinase (CK), lactate dehydrogenase (LDH), myoglobin (MYO), brain-derived neurotrophic factor (BDNF), insulin-like growth factor-1 (IGF-1), irisin, cortisol, muscle pain, aerobic and anaerobic performance will be evaluated at the beginning, end of the intervention and 24 h later.
Ethics and dissemination
This study was approved by the Medical Ethics Committee of TBZMED (No.IR.TBZMED.REC.1399.1011). This research's findings will be published in a peer-reviewed journal and presented at international conferences.
Trial registration
Iranian Registry of Clinical Trials website (www.IRCT.ir/, IRCT20150205020965N9).
Keywords: Date seed, Active people, Oxidative stress, Inflammation, Polyphenol
Highlights
-
•
Date seed powder may have the potential to improve exercise performance in healthy and active subjects performing HIIT bouts.
-
•
The date seed is known as an excellent functional food due to its being high in polyphenols, and total dietary fiber.
-
•
The consumption of date seed powder would improve oxidative stress, inflammation, mental health and performance.
-
•
The results of this trial can be used to provide evidence-based recommendations for recreational runners, and nutritionists.
Abbreviations:
- ACSM
American College of Sports Medicine
- BMI
Body mass index
- EIOS
Exercise-induced oxidative stress
- EIMD
Exercise-induced muscle damage
- HIIT
High-intensity interval training
- MDA
Malondialdehyde
- TAC
Total antioxidant capacity
- OSI
Oxidative stress index
- TOS
Total oxidant status
- SOD
Superoxide dismutase
- GPx
Glutathione peroxidase
- CAT
Catalase; 8-iso-PGF2α: 8-iso-prostaglandin F2α
- PC
Protein carbonyl
- hs-CRP
High-sensitivity C-reactive protein
- IL-6
Interleukin-6
- TNF-α:
Tumor necrosis factor-alpha
- IL-10
Interleukin-10
- MYO
Myoglobin
- CK
Creatine kinase
- LDH
Lactate dehydrogenase
- IGF-1
Insulin-like growth factor-1
- GSH
Glutathione
- NO
Nitric oxide
- HRR
Heart rate reserve
- BDNF
Brain-derived neurotrophic factor
- NF-kB
Nuclear factor kappa B
- COX
Cyclooxygenase
- Nrf2
Nuclear factor erythroid 2-related factor 2
- PAR-Q:
Physical Activity Readiness Questionnaire
- RCT
Randomized clinical trial
- ROS
Reactive oxygen species
- SPIRIT
Standard Protocol Items: Recommendations for Clinical Interventional Trials
- VAS
Visual analogue scale
1. Introduction
Regular physical activity and exercise are known as effective strategies for preventing and treating various metabolic disorders and chronic diseases, including cardiovascular disease, type 2 diabetes, cancer, metabolic syndrome, depression, and stress [1]. Long-term and intense (VO2max> 60%) exercises such as endurance exercise and high-intensity interval training (HIIT) have been proposed as an efficient method to improve metabolic adaptations [2], vascular function [3], maximum oxygen consumption [4], and muscle mitochondrial capacity [5], as well as to reduce cardiometabolic risk factors [6], hyperglycemia [5,7], and body fat [8]. However, findings indicate that high levels of reactive oxygen species (ROS) or exercise-induced oxidative stress (EIOS) and exercise-induced muscle damage (EIMD) in HIIT can significantly decrease the ability to generate action potentials and calcium uptake by the sarcoplasmic reticulum, both of which contribute to acute performance reductions during exercise (i.e., fatigue and soreness) [9,10]. Thus, it seems that improvement in the antioxidant defense system's capability may reduce skeletal muscle's sensitivity to oxidative stress and inflammation [11]. The capacity and ability of the body's antioxidant defense to neutralize ROS may be modulated by nutritional status, supplement consumption and functional foods [12,13],so it has been hypothesized that certain chemicals can decrease inflammation, oxidative stress, and subsequent skeletal muscle damage due to intense exercise and improve recovery and stimulate optimal adaption [14]. However, isolated bioactive compounds (e.g., vitamin E, vitamin C, resveratrol, and lipoic acid) have been shown to have adverse effects on symptoms and adaptive responses to training after consumption [[15], [16], [17]]. At the same time, there is no evidence that all of these are related to the consumption of antioxidant-rich foods or extracts [18,19]. Thus, natural supplements with multiple properties are probably more beneficial for sports recovery and performance [19].
Date seed is a waste product that is high in polyphenolic (such as hesperidin, quercetin, kaempferol), phenolic acid (allergic, epicatechin, catechol, chlorogenic), carotenoids, total dietary fiber (such as pectin, β-glucan, and arabinoxylan), fat, protein, minerals, and various other nutrients and functional elements [[20], [21], [22], [23]]. Previous human and animal studies investigating the effects of date seeds have reported its positive results on antioxidant defense systems and improvement in oxidative stress parameters [[24], [25], [26]], inflammation [27], hyperglycemia [28], hyperlipidemia [29], memory, and learning impairments [30] as a low-cost supplement. We hypothesized that consuming date seed powder can be beneficial for enhancing nutritional, oxidative stress, anti/inflammatory status, mental health, sports performance, and fatigue during a HIIT protocol in recreational runners. Up to this time, no studies have been conducted regarding the consumption of date seed powder and HIIT exercise in active people. Thus, this research will assess the impact of date seed powder on the nutritional, oxidative stress, anti/inflammatory status, mental health, and sports performance of recreational runners following a high-intensity interval training protocol.
2. Methods
2.1. Trial design and participants
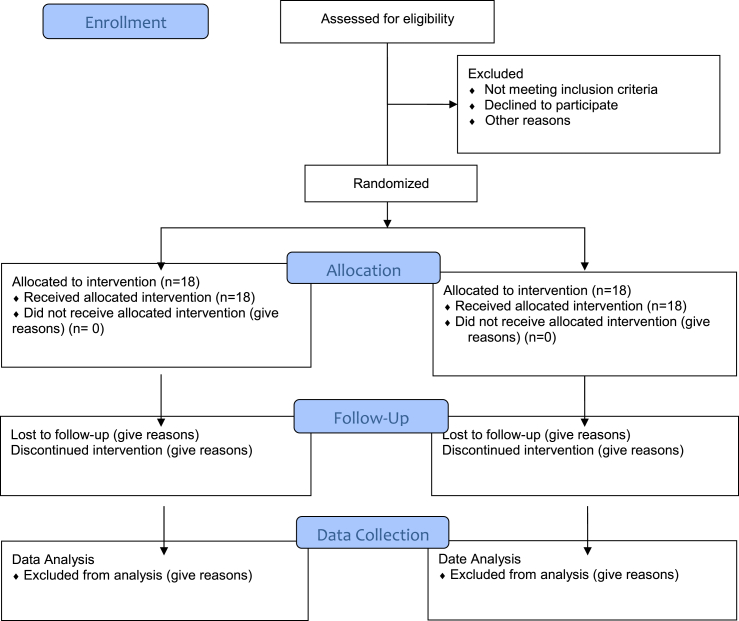
This is a double-blind, placebo-controlled, randomized clinical trial (RCT) evaluating date seed powder supplementation effects on the nutritional, oxidative, inflammatory, anti-inflammatory status, mental health, and sports performance in physically active people, which is planned to begin later this year, 2021. In this trial, 36 active people (recreational runners) will be recruited by advertisements from Tabriz Stadium. The flowchart of the trial is presented in Fig. 1. The study protocol followed the Standard Protocol Items: Recommendations for Clinical Interventional Trials (SPIRIT) guidelines (Additional File 1, SPIRIT Checklist), and Table 1 shows the diagram of the study protocol [31]. The study outcomes will be assessed at baseline (one-week pre-allocation, -t1); allocation (t0), at the beginning of the study (t1); at two weeks (t2); and 24 h after that (t3).
Fig. 1.
Consolidated Standards of Reporting Trials (CONSORT) diagram.
Table 1.
Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT) chart for study process.
| Enrolment |
Allocation |
Close-out |
|||
|---|---|---|---|---|---|
| TIMEPOINT** | -t1 | t0 | t1 | t2 | t3 |
| ENROLMENT | |||||
| Eligibility screen | X | ||||
| Informed consent | X | ||||
| General characteristics | X | ||||
| Allocation |
X |
||||
| INTERVENTIONS | |||||
| Date seed group + HIIT | |||||
| Placebo group + HIIT |
 |
||||
| ASSESSMENTS | |||||
| Dietary recall | X | X | |||
| VO2 max | X | X | X | ||
| Body composition | X | X | |||
| VAS | X | X | X | ||
| Biochemical assessments | X | X | X | ||
| Safety | X | ||||
Abbreviations: HIIT: high-intensity interval training, VAS: Visual Analogue Scale, -t1: one-week pre-allocation, t0: Allocation, t1: At the beginning of the study, t2: At two weeks, t3: 24 h after that.
The “X” is indicating what is done in the given period.
The initial participant recruitment is ongoing. The sample size was calculated based on changes in the malondialdehyde (MDA) parameter as the primary outcome of the study by Platat et al. [25]. Using Stata software (version 16), the sample size in this study was estimated to be at least 16 subjects for each group, with a power of 90% and 95% confidence levels. Based on a 25% decrease in the level of expected MDA through supplementation and a 10% dropout rate for each group, the sample size in each group increased to 18.
2.2. Inclusion criteria
The inclusion criteria are: having perfect health (confirmed by the Physical Activity Readiness Questionnaire (PAR-Q), under the supervision of a physician); age 18–35 years old; doing running workouts for at least three days a week (240 min per week) during the last two years; body mass index of 18.5–25; having a stable body mass during the previous five months (changes of less than 3 kg); abstaining from any high-intensity interval training during the last three months; and being willing to cooperate in the study.
2.3. Exclusion criteria
The exclusion criteria are as follows: a history of non-communicable diseases such as diabetes, cancer, cardiovascular, thyroid, gastrointestinal, renal or pancreatic disease; infectious diseases; cognitive disorders; smoking; being pregnant or lactating; having anemia (hemoglobin <13 g/dl); having musculoskeletal injury; irregular physical activity; the current consumption of alcohol, antacids; anti-inflammatory; antibiotics; antihypertensive; antidiarrheal or laxative medicines; participants on special diets or dietary restrictions, as well as those who were taking antioxidant supplements, prebiotics, or probiotics before the intervention.
2.4. Randomization and allocation concealment
Eligible volunteers will be randomly assigned to either the intervention group (n = 18) or the placebo group (n = 18) for 14 days. Participants will be randomized into the two groups (1:1) using stratified randomization based on sex and VO2 max and then we will use random allocation software to perform randomized blocks of sizes 2 and 4. A third person will be assigned to divide subjects into groups to ensure concealment of research elements. The statistical consultant and principal investigator will be blind to the groups of subjects until the end of the analysis.
2.5. Intervention
After a run-in period, we will use the PAR-Q, which is a pre-study screening questionnaire to assess a person's medical history and lifestyle in several areas [32]. When the participant answers “yes” to a question on the questionnaire, he or she will be excluded from the study. The intervention group will receive 26 g/d of the date seed powder (date seed, Flavinea Co., Iran) according to a pilot study of date seed powder for two weeks in active people (data not reported). The placebo group will receive a similar volume of bran wheat powder (bran wheat, Nazhvangiah Co., Iran) as a placebo for 14 days. The powder will be divided into two packages of 13 g each to be taken before and after exercise with a cup of water. Both date seed and bran wheat powder are flavorless, odorless, and brown powders will be provided to the participants in identical opaque packages. The powders will be delivered to participants weekly for two weeks. To emphasize maintenance of physical activity, resolve issues with supplement administration, and ensure compliance, all participants will be contacted two times per week. Participants will also be given a checklist to tick off after each powder intake to check for non-compliance.
2.6. Exercise protocol
A high-intensity interval training (HIIT) exercise protocol was designed for each volunteer in both groups based on the American College of Sports Medicine (ACSM)'s physical activity recommendations [33]. The participants will do the HIIT program for two weeks (5 training sessions per week; 10 sessions during the study period). A 15-min warm-up (with various stretches, flexibility, walking, and running) will be included in each training session. The main activity of both groups consists of two sessions with 3–4 repetitions and 30 s of running with an intensity of 90–100% of the heart rate reserve (HRR) in each repetition. After each repetition and after each period, there will be 90–180 s and 2.5–4 min of active rest, respectively [34]. Each session will start with a 15-min warm-up at 50% HRR and finish with a 5-min cool-down at 45% HRR. The study team will monitor participants daily during the trial. There aren't expected to be any specific adverse effects from the test. During high-intensity exercise training, all participants will be guided to make their own cooperative decisions. Adherence to the training program will be assessed by the number of sessions attended. If less than 90% of training sessions are attended each week, the person would be excluded.
2.7. Primary and secondary outcomes
The primary outcomes used in this study will be the total antioxidant capacity (TAC), oxidative stress index (OSI), total oxidant status (TOS), superoxide dismutase (SOD), glutathione peroxidase (GPx), malondialdehyde (MDA), 8-iso-prostaglandin F2α (8-iso-PGF2α), uric acid, protein carbonyl (PC), catalase (CAT), glutathione (GSH), nitric oxide (NO), high-sensitivity C-reactive protein (hs-CRP), tumor necrosis factor-alpha (TNF-α), interleukin-6 (IL-6), interleukin-10 (IL-10), IL-6/IL-10, creatine kinase (CK), lactate dehydrogenase (LDH), myoglobin (MYO), muscle pain, aerobic and anaerobic performance. Furthermore, the secondary outcomes will include nutritional status (energy and macro and micronutrient intake), insulin-like growth factor-1 (IGF-1), brain-derived neurotrophic factor (BDNF), irisin, cortisol, and body composition.
2.8. Assessment of anthropometric measurements
Anthropometric parameters (height, weight, and BMI (body mass index)) will be evaluated at baseline and post-intervention. Height measurement will be done without shoes using a centimeter tape with a precision of 0.1 cm. Weight will be measured barefoot and in minimal clothing to the nearest 0.5 kg using a reliable scale (Seca, Hamburg, Germany). BMI will be computed as weight (kg) divided by the square of height (m). Bioelectrical impedance analysis (Tanita BC-418, Tanita Corp., Tokyo, Japan) will be used to measure the body composition of the participants before and after the training.
2.9. Physical activity, aerobic, anaerobic performance and dietary intake
Dietary intake will be assessed using a 3-day food diary (2 weekdays and one weekend day) before starting supplements and again at the end of the study during the last weeks. Food record data will be evaluated using “Nutritionist 4” software (First Databank Inc., Hearst Corp., San Bruno, CA, USA) [35]. We will determine the physical activity level of active participants using the International Physical Activity Questionnaire (IPAQ—short version) [36].The PAR-Q will be used as a pre-study screening questionnaire [32]. A visual analogue scale (VAS) will be used to determine the recreational runner's muscle pain at baseline and after the training [37]. The Cooper 12-min run test will be used to measure aerobic endurance. Participants will run continuously for 12 min on the 400-m running track [38]. We will also measure anaerobic endurance using the running-based anaerobic sprint test (RAST). The RAST test consists of six parallel 35-m sprints separated by a 10-s rest period [39]. All of the people who are going to do the exercise tests will have a 5-min warm-up session first.
2.10. Clinical assessment
A venous blood sample (10 ml) will be collected from every participant at the beginning and at the end of the last training session on the fourteenth day, as well as 24 h after the last training session. The levels of TAC, TOS, GPx, SOD, and PC will be measured using a colorimetric method using kits. Serum levels of MDA will be evaluated through a reaction with thiobarbituric acid (TBA) as a TBARS using a spectrofluorimeter [40]. Catalase activity, NO, and GSH will be assessed using the methods described by Aebi et al. [41], Griess [42], and Beutler et al. [43], respectively. Uric acid will be measured using the enzymatic method by an autoanalyzer using kits. The serum hs-CRP concentration will be determined using an immunoturbidimetric kit. TNF-α, IL-6, IL-10, MYO, BDNF, IGF-1, irisin, and 8-iso-PGF2, and cortisol concentrations will be quantified by the use of a commercial ELISA kit. The level of inflammation will be shown by the proportion of IL-6 to IL-10. CK and LDH markers will be quantified spectrophotometrically. Also, the OSI will be calculated using TAC and TOS as follows: OSI = 100 × (TOS/TAC) [44].
2.11. Data management
Data includes demographic data, PAR-Q test, physical activity, food intake, anthropometric indices, biochemical parameters, and performance markers that will be obtained in this study. Related questionnaires, including VAS, 3-day food diary, PAR-Q, and IPAQ will be used to collect data. If there are any discrepancies in answers, the respondent will be asked to answer more clearly to reduce bias. Participants who drop out of this study for any reason will be followed, and the data will be examined using the intention-to-treat (ITT) principle. All participants will be followed up for 15 days after being assigned at random.
2.12. Confidentiality
All study-related and participant data will be maintained in password-protected file cabinets with restricted access. To preserve participant confidence, a code identification number will be used to identify data collection and forms. All records, including names and other information that could be used to identify a person, will be kept separate from study data, which will be identified by a code number.
2.13. Statistical analysis
The SPSS program version 24 will be used to examine the study's findings. The Kolmogorov–Smirnov test will be used to determine the data's normality. Qualitative data will be expressed as frequency (percent), whereas quantitative data will be described as mean (standard deviation (SD)). The independent sample student's T-test and analysis of covariance (ANCOVA) will be used to compare quantitative variables between groups at baseline and after the intervention, respectively. The paired sample student's T-test will be used to identify within-group differences. A two-way repeated measure analysis of variance (ANOVA) test will analyze hematological and biochemical parameters. Statistical significance was defined as a value of p < 0.05.
3. Discussion
The interest in dietary supplements to promote sports performance has increased these days among professional and non-professional athletes. Regular consumption of nutritional supplements (including antioxidants E and C) has recently been increased in order to suppress EIOS and EIMD and improve athletic performance [15]. In contrast, there is growing evidence that vitamin C and E supplementation have no significant effect on the physiological performance of athletes [45]. Thus, natural supplements and functional foods have attracted the attention of many researchers and athletes as supplements containing multi-nutrients in patients [46,47] and healthy people [48]. Due to having different ingredients, these multi-nutrient supplements lead to several simultaneous effects, such as modulation of antioxidant capacity, inflammation, muscle fatigue, and subsequently enhancing athletes' performance [48,49]. It has been shown that date seeds as a functional food have a wide range of healthful properties, including antioxidant, anti-inflammatory, antitumor, hepatoprotective and nephroprotective, anti-hyperlipidemic, antiaging, and memory improvement [20,29,50,51]. Also, it is worth mentioning that the chemical constituents' polyphenols, flavan-3-ols, especially catechins and epicatechins, and prebiotics are the critical contributors to the mentioned conventional activities [51,52]. Limited studies have reported the modulating effects of date seed as a rich plant source of antioxidants and prebiotics on oxidative stress, inflammation, immune system, lipid profile, and sports performance [20,27,29,53]. Ali and Abdelaziz reported that aqueous suspension of date seed (1 g/d, four weeks) modulated kidney oxidative damage mediated by carbon tetrachloride (CCl4) in Wistar rats via significant decreased MDA, glutathione S-transferase (GST), and nitric oxide (NO) levels in the kidney and significantly restored SOD and GSH activities [54]. In a study by Saryono et al., date seed daily intake (0.25; 0.5; 0.75; 1 g/kg/day, seven days) in rats significantly increased SOD and GPX in the intervention groups in a dose-dependent manner [55]. In a recent study by Platat et al., administration of date seed powder (0.25 g/kg and 0.5 g/kg bodyweight for an acute dose) significantly changed the levels of GSH, protein carbonyl, and MDA 1 h after ingestion. These changes were maintained up to 8 h after ingestion [25]. In the Saryono al. study conducted on postmenopausal women, it has been indicated that date seed intake (2.5 g/day for two weeks) significantly changed the levels of MDA, SOD, GPX enzyme activities and vitamin E [56]. Likewise, in another study by Atyanti Isworo et al. (2.5 g of date seed, 14 days) showed that the expression of IL-1b, TGF-b, COX-1, and COX-2 in middle-aged women significantly decreased after consuming date seed [27]. Recently, Fahad Jubayer et al. found that taking 600 mg of date seed powder for 90 days resulted in decreased levels of total cholesterol (TC), triglycerides (TG), and low-density lipoprotein (LDL) as well as an increase in high-density lipoprotein (HDL) in the intervention group [29]. Date seed polyphenol supplementation may improve athletic performance and reduce fatigue by scavenging various forms of free radicals, inhibiting lipid peroxidation, metal iron-mediated radical formation, and preventing radical-mediated -carotene and vitamin E depletion [57,58]. Increasing the expression of the nitric oxide synthase (NOS) enzyme involved in nitric oxide (NO) production, inhibiting nuclear factor kappa B (NF-kB), modulating mitogen-activated protein kinases (MAPK), and enhancing nuclear factor erythroid 2 related factors 2 (Nrf2) may be other probable mechanisms that date seed could improve immunological, inflammatory, stress, proliferative, and apoptotic response [[59], [60], [61]].
In addition, it has been shown that date seed supplementation may result in decreased IGF-1 binding protein-3 levels and increased IGF-1 bioactivity that leads to muscle hypertrophy, repair of muscle damage, and eventually improved performance [[62], [63], [64]]. Also, date seed polyphenols and soluble fiber can influence the proliferation of specific bacteria such as Lactobacillus and Bifidobacterium and decrease certain pathogenic Clostridium in the gut microbiota. Previous research suggests that gut microbiota may have a positive impact on sports performance [65,66].
To our knowledge, this is the first study to examine the effects of date seed powder consumption during HIIT sessions on recreational runners' nutritional, oxidative stress, anti/inflammatory status, mental health, and sports performance. It is hypothesized that the consumption of date seed powder would decrease inflammation, oxidative stress, and fatigue and improve sports performance.
4. Trial status
The present protocol is version 1, dated October 25, 2021. The trial has not yet begun, and the process of recruiting participants is ongoing.
5. Conclusion
The consumption of date seed powder would decrease oxidative stress, inflammation, muscle pain, and improve mental health and performance. The results of this trial can be used to provide evidence-based recommendations for active people, recreational runners, and nutritionists.
Ethics and dissemination
Ethical consideration
At the baseline session, all-volunteer participants will sign an informed consent form after obtaining complete information about the study methodology. The study protocol (IR.TBZMED.REC.1399.1011) was approved by the Ethical Committee of the Tabriz University of Medical Sciences. It was then registered on the Iranian Registry of Clinical Trials website (www.IRCt.ir/) with this number (IRCT20150205020965N9).
Safety considerations
The participants and researchers will report any adverse outcomes observed during the intervention or follow-up. If necessary, the main investigator will ensure that the corrective action improves the unfavorable impact. In addition, the ethics committee will be notified of any adverse effects.
Protocol amendments
All authors will review and approve any protocol changes or adjustments. The principal investigator will examine any protocol revisions or additions, and the other study investigators will have to support them. Any changes will be reported at the end.
Dissemination
The results of this study will be written up in a peer-reviewed journal and shared both online and in print.
Contributors
All authors made substantial contributions to the conception and design. P.D, E.M, and M.K conceived and developed the idea for the study and revised the manuscript under the supervision of P.D and M.K. EM is conducting this study as part of her MS.c thesis (66809). All the authors read and approved the final manuscript.
Funding
This work is part of a government-funded project supported by the Tabriz University of Medical Sciences, Iran. Grant number: 66809.
Participants and public involvement
Participants and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Consent for publication
All the authors take public responsibility for the content of the protocol.
Availability of data and materials
All data generated or analyzed during this study will be included in published articles.
Author declaration
1)We wish to confirm that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome.
2)We confirm that the manuscript has been read and approved by all named authors and that there are no other persons who satisfied the criteria for authorship but are not listed. We further confirm that the order of authors listed in the manuscript has been approved by all of us.
3)We confirm that neither the entire paper nor any of its content has been submitted, published, or accepted by another journal. The paper will not be submitted elsewhere if accepted for publication in the Journal.
4)We confirm that we have given due consideration to the protection of intellectual property associated with this work and that there are no impediments to publication, including the timing of publication, with respect to intellectual property. In so doing we confirm that we have followed the regulations of our institutions concerning intellectual property.
5)We confirm that any aspect of the work covered in this manuscript that has involved either experimental animals or human patients has been conducted with the ethical approval of all relevant bodies and that such approvals are acknowledged within the manuscript.
6)We understand that the Corresponding Author is the sole contact for the Editorial process (including Editorial Manager and direct communications with the office). He/she is responsible for communicating with the other authors about progress, submissions of revisions and final approval of proofs.
Additional file
Additional file 1: SPIRIT 2013 Checklist: Recommended items to address in a clinical trial protocol and related documents.
Declaration of competing interest
None declared.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.conctc.2022.100951.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
Data availability
The data that has been used is confidential.
References
- 1.Eime R.M., et al. A systematic review of the psychological and social benefits of participation in sport for children and adolescents: informing development of a conceptual model of health through sport. Int. J. Behav. Nutr. Phys. Activ. 2013;10(1):1–21. doi: 10.1186/1479-5868-10-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gibala M.J., McGee S.L. Metabolic adaptations to short-term high-intensity interval training: a little pain for a lot of gain? Exerc. Sport Sci. Rev. 2008;36(2):58–63. doi: 10.1097/JES.0b013e318168ec1f. [DOI] [PubMed] [Google Scholar]
- 3.Ramos J.S., et al. The impact of high-intensity interval training versus moderate-intensity continuous training on vascular function: a systematic review and meta-analysis. Sports Med. 2015;45(5):679–692. doi: 10.1007/s40279-015-0321-z. [DOI] [PubMed] [Google Scholar]
- 4.Milanović Z., Sporiš G., Weston M. Effectiveness of high-intensity interval training (HIT) and continuous endurance training for VO 2max improvements: a systematic review and meta-analysis of controlled trials. Sports Med. 2015;45(10):1469–1481. doi: 10.1007/s40279-015-0365-0. [DOI] [PubMed] [Google Scholar]
- 5.Little J.P., et al. Low-volume high-intensity interval training reduces hyperglycemia and increases muscle mitochondrial capacity in patients with type 2 diabetes. J. Appl. Physiol. 2011;111(6):1554–1560. doi: 10.1152/japplphysiol.00921.2011. [DOI] [PubMed] [Google Scholar]
- 6.Kessler H.S., Sisson S.B., Short K.R. The potential for high-intensity interval training to reduce cardiometabolic disease risk. Sports Med. 2012;42(6):489–509. doi: 10.2165/11630910-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 7.Khalafi M., et al. The impact of high-intensity interval training on postprandial glucose and insulin: a systematic review and meta-analysis. Diabetes Res. Clin. Pract. 2022 doi: 10.1016/j.diabres.2022.109815. [DOI] [PubMed] [Google Scholar]
- 8.Khalafi M., Symonds M.E. The impact of high intensity interval training on liver fat content in overweight or obese adults: a meta-analysis. Physiol. Behav. 2021;236 doi: 10.1016/j.physbeh.2021.113416. [DOI] [PubMed] [Google Scholar]
- 9.Vollaard N.B., Shearman J.P., Cooper C.E. Exercise-induced oxidative stress. Sports Med. 2005;35(12):1045–1062. doi: 10.2165/00007256-200535120-00004. [DOI] [PubMed] [Google Scholar]
- 10.Howatson G., Van Someren K.A. The prevention and treatment of exercise-induced muscle damage. Sports Med. 2008;38(6):483–503. doi: 10.2165/00007256-200838060-00004. [DOI] [PubMed] [Google Scholar]
- 11.Chow C.K. Nutritional influence on cellular antioxidant defense systems. Am. J. Clin. Nutr. 1979;32(5):81–1066. doi: 10.1093/ajcn/32.5.1066. [DOI] [PubMed] [Google Scholar]
- 12.Saleh-Ghadimi S., et al. Co-supplementation of camelina oil and a prebiotic is more effective for in improving cardiometabolic risk factors and mental health in patients with NAFLD: a randomized clinical trial. Food Funct. 2021;12(18):8594–8604. doi: 10.1039/d1fo00448d. [DOI] [PubMed] [Google Scholar]
- 13.Farhangi M.A., Dehghan P., Namazi N. Prebiotic supplementation modulates advanced glycation end-products (AGEs), soluble receptor for AGEs (sRAGE), and cardiometabolic risk factors through improving metabolic endotoxemia: a randomized-controlled clinical trial. Eur. J. Nutr. 2020;59(7):3009–3021. doi: 10.1007/s00394-019-02140-z. [DOI] [PubMed] [Google Scholar]
- 14.Bongiovanni T., et al. Nutritional interventions for reducing the signs and symptoms of exercise-induced muscle damage and accelerate recovery in athletes: current knowledge, practical application and future perspectives. Eur. J. Appl. Physiol. 2020:1–32. doi: 10.1007/s00421-020-04432-3. [DOI] [PubMed] [Google Scholar]
- 15.Lieberman H.R., et al. Patterns of dietary supplement use among college students. Clin. Nutr. 2015;34(5):976–985. doi: 10.1016/j.clnu.2014.10.010. [DOI] [PubMed] [Google Scholar]
- 16.Poljsak B., Milisav I. The neglected significance of “antioxidative stress”. Oxid. Med. Cell. Longev. 2012;2012:480–895. doi: 10.1155/2012/480895. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Poljsak B. Strategies for reducing or preventing the generation of oxidative stress. Oxid. Med. Cell. Longev. 2011;2011:194–586. doi: 10.1155/2011/194586. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prior R.L. Fruits and vegetables in the prevention of cellular oxidative damage. Am. J. Clin. Nutr. 2003;78(3):570S–578S. doi: 10.1093/ajcn/78.3.570S. [DOI] [PubMed] [Google Scholar]
- 19.Bowtell J., Kelly V. Fruit-derived polyphenol supplementation for athlete recovery and performance. Sports Med. 2019;49(1):3–23. doi: 10.1007/s40279-018-0998-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dardjito E., et al. IOP Conference Series: Earth and Environmental Science. IOP Publishing; 2019. Date seeds (Phoenix dactylifera L.) consumption as anti-inflammatory and immunostimulant: a systematic review. [Google Scholar]
- 21.Hamada J., Hashim I., Sharif F. Preliminary analysis and potential uses of date pits in foods. Food Chem. 2002;76(2):135–137. [Google Scholar]
- 22.Maqsood S., et al. Bioactive compounds from date fruit and seed as potential nutraceutical and functional food ingredients. Food Chem. 2020;308 doi: 10.1016/j.foodchem.2019.125522. [DOI] [PubMed] [Google Scholar]
- 23.Al-Farsi M., et al. Compositional and functional characteristics of dates, syrups, and their by-products. Food Chem. 2007;104(3):943–947. [Google Scholar]
- 24.Djaoudene O., et al. Phoenix dactylifera L. seeds: a by-product as a source of bioactive compounds with antioxidant and enzyme inhibitory properties. Food Funct. 2019;10(8):4953–4965. doi: 10.1039/c9fo01125k. [DOI] [PubMed] [Google Scholar]
- 25.Platat C., et al. Urine metabolites and antioxidant effect after oral intake of date (phoenix dactylifera L.) seeds-based products (powder, bread and extract) by human. Nutrients. 2019;11(10):2489. doi: 10.3390/nu11102489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alem C., et al. Phytochemical compositions and antioxidant capacity of three date (Phoenix dactylifera L.) seeds varieties grown in the South East Morocco. J. Saudi Soc. Agric. Sci. 2017;16(4):350–357. [Google Scholar]
- 27.Isworo A. Anti-inflammatory activity of date palm seed by downregulating interleukin-1β, TGF-β, cyclooxygenase-1 and-2: a study among middle age women. Saudi Pharmaceut. J. 2020;28(8):1014–1018. doi: 10.1016/j.jsps.2020.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ahmed F., Ahmed A.M., Darwish H.H. Hypoglycemic effect of an extract from date seeds on diabetic rats. Saudi Med. J. 2010;31:747–751. [PubMed] [Google Scholar]
- 29.Jubayer F., Kayshar S., Rahaman M. Effects of Ajwa date seed powder on serum lipids in humans: a randomized, double-blind, placebo-controlled clinical trial. J. Herb. Med. 2020;24 [Google Scholar]
- 30.Dehghanian F., et al. Date seed extract ameliorates β-amyloid-induced impairments in hippocampus of male rats. Biomed. Pharmacother. 2017;89:221–226. doi: 10.1016/j.biopha.2017.02.037. [DOI] [PubMed] [Google Scholar]
- 31.Chan A.-W., et al. SPIRIT 2013 statement: defining standard protocol items for clinical trials. Ann. Intern. Med. 2013;158(3):200–207. doi: 10.7326/0003-4819-158-3-201302050-00583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shephard R.J. PAR-Q, Canadian Home Fitness Test and exercise screening alternatives. Sports Med. 1988;5(3):185–195. doi: 10.2165/00007256-198805030-00005. [DOI] [PubMed] [Google Scholar]
- 33.Roy B.A. High-intensity interval training: efficient, effective, and a fun way to exercise: brought to you by the American college of sports medicine. ACSM's Health & Fit. J. 2013;17(3):3. www.acsm.org [Google Scholar]
- 34.Bogdanis G., et al. Short-term high-intensity interval exercise training attenuates oxidative stress responses and improves antioxidant status in healthy humans. Food Chem. Toxicol. 2016;61:171–177. doi: 10.1016/j.fct.2013.05.046. [DOI] [PubMed] [Google Scholar]
- 35.Yang Y.J., et al. Relative validities of 3-day food records and the food frequency questionnaire. Nutr. Res. Pract. 2010;4(2):142–148. doi: 10.4162/nrp.2010.4.2.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ács P., et al. Criterion validity and reliability of the International Physical Activity Questionnaire–Hungarian short form against the RM42 accelerometer. BMC Publ. Health. 2021;21(1):1–10. doi: 10.1186/s12889-021-10372-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ueda T., Nabetani T., Teramoto K. Differential perceived exertion measured using a new visual analogue scale during pedaling and running. J. Physiol. Anthropol. 2006;25(2):171–177. doi: 10.2114/jpa2.25.171. [DOI] [PubMed] [Google Scholar]
- 38.Bandyopadhyay A. Validity of Cooper's 12-min run test for estimation of maximum oxygen uptake in female university students. Indian J. Physiol. Pharmacol. 2014;58(2):184–186. [PubMed] [Google Scholar]
- 39.Adamczyk J. The estimation of the RAST test usefulness in monitoring the anaerobic capacity of sprinters in athletics. Pol. J. Sport Tourism. 2011;18(3):214–218. [Google Scholar]
- 40.Jentzsch A.M., et al. Improved analysis of malondialdehyde in human body fluids. Free Radic. Biol. Med. 1996;20(2):251–256. doi: 10.1016/0891-5849(95)02043-8. [DOI] [PubMed] [Google Scholar]
- 41.Maqbool A., et al. Management of tannery wastewater for improving growth attributes and reducing chromium uptake in spinach through citric acid application. Environ. Sci. Pollut. Control Ser. 2018;25(11):10848–10856. doi: 10.1007/s11356-018-1352-4. [DOI] [PubMed] [Google Scholar]
- 42.Ricart-Jane D., Llobera M., Lopez-Tejero M. Anticoagulants and other preanalytical factors interfere in plasma nitrate/nitrite quantification by the Griess method. Nitric Oxide. 2002;6(2):178–185. doi: 10.1006/niox.2001.0392. [DOI] [PubMed] [Google Scholar]
- 43.Beutler E., Gelbart T. Plasma glutathione in health and in patients with malignant disease. J. Lab. Clin. Med. 1985;105(5):581–584. [PubMed] [Google Scholar]
- 44.Yirün M.C., et al. Evaluation of oxidative stress in bipolar disorder in terms of total oxidant status, total antioxidant status, and oxidative stress index. Arch. Neuropsychiatry. 2016;53(3):194. doi: 10.5152/npa.2015.10123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dutra M.T., et al. The effects of strength training combined with vitamin C and E supplementation on skeletal muscle mass and strength: a systematic review and meta-analysis. J. Sports Med. 2020;2020:350–5209. doi: 10.1155/2020/3505209. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Farhangi M.A., et al. The effects of Nigella sativa on thyroid function, serum vascular endothelial growth factor (VEGF)–1, Nesfatin-1 and anthropometric features in patients with Hashimoto's thyroiditis: a randomized controlled trial. BMC Compl. Alternative Med. 2016;16(1):1–9. doi: 10.1186/s12906-016-1432-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mirtaheri E., et al. Effects of alpha-lipoic acid supplementation on inflammatory biomarkers and matrix metalloproteinase-3 in rheumatoid arthritis patients. J. Am. Coll. Nutr. 2015;34(4):310–317. doi: 10.1080/07315724.2014.910740. [DOI] [PubMed] [Google Scholar]
- 48.Skaug A., Sveen O., Raastad T. An antioxidant and multivitamin supplement reduced improvements in VO₂max. J. Sports Med. Phys. Fit. 2014;54(1):63–69. [PubMed] [Google Scholar]
- 49.Sanguigni V., et al. Natural antioxidant ice cream acutely reduces oxidative stress and improves vascular function and physical performance in healthy individuals. Nutrition. 2017;33:225–233. doi: 10.1016/j.nut.2016.07.008. [DOI] [PubMed] [Google Scholar]
- 50.Hilary S., et al. In-vitro investigation of polyphenol-rich date (phoenix dactylifera L.) seed extract bioactivity. Front. Nutr. 2021:571. doi: 10.3389/fnut.2021.667514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hilary S., et al. Polyphenol characterisation of Phoenix dactylifera L.(date) seeds using HPLC-mass spectrometry and its bioaccessibility using simulated in-vitro digestion/Caco-2 culture model. Food Chem. 2020;311 doi: 10.1016/j.foodchem.2019.125969. [DOI] [PubMed] [Google Scholar]
- 52.Habib H.M., et al. Polyphenolic compounds in date fruit seed (Phoenix dactylifera): characterisation and quantification by using UPLC‐DAD‐ESI‐MS. J. Sci. Food Agric. 2014;94(6):1084–1089. doi: 10.1002/jsfa.6387. [DOI] [PubMed] [Google Scholar]
- 53.Hasan M., Mohieldein A. In vivo evaluation of anti diabetic, hypolipidemic, antioxidative activities of Saudi date seed extract on streptozotocin induced diabetic rats. J. Clin. Diagn. Res. 2016;10(3):FF06–12. doi: 10.7860/JCDR/2016/16879.7419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ali S.E.-m., Abdelaziz D.H.A. The protective effect of date seeds on nephrotoxicity induced by carbon tetrachloride in rats. Int. J. Pharmaceut. Sci. Rev. Res. 2014;26(2):62–68. [Google Scholar]
- 55.Rahmawati E., Hapsari E., Hidayat A. Antioxidant enzyme status on rat after date seeds (Phoenix dactylifera) steeping treatment. Int. J. Res. Med. Sci. 2016;4:1893–1896. [Google Scholar]
- 56.Saryono S., et al. Effect of antioxidant status and oxidative stress products in pre-menopausal women after treatment with date seed powder (Phoenix dactylifera L.): a study on women in Indonesia. Pakistan J. Nutr. 2017;16(6):477–481. [Google Scholar]
- 57.Urquiaga I., Leighton F. Plant polyphenol antioxidants and oxidative stress. Biol. Res. 2000;33(2):55–64. doi: 10.4067/s0716-97602000000200004. [DOI] [PubMed] [Google Scholar]
- 58.Scalbert A., Johnson I.T., Saltmarsh M. Polyphenols: antioxidants and beyond. Am. J. Clin. Nutr. 2005;81(1):215S–217S. doi: 10.1093/ajcn/81.1.215S. [DOI] [PubMed] [Google Scholar]
- 59.Pandey K.B., Rizvi S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxid. Med. Cell. Longev. 2009;2(5):270–278. doi: 10.4161/oxim.2.5.9498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Han X., Shen T., Lou H. Dietary polyphenols and their biological significance. Int. J. Mol. Sci. 2007;8(9):950–988. [Google Scholar]
- 61.Li A.-N., et al. Resources and biological activities of natural polyphenols. Nutrients. 2014;6(12):6020–6047. doi: 10.3390/nu6126020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.McMahon C.D., et al. Lifelong exercise and locally produced insulin-like growth factor-1 (IGF-1) have a modest influence on reducing age-related muscle wasting in mice. Scand. J. Med. Sci. Sports. 2014;24(6):e423–435. doi: 10.1111/sms.12200. [DOI] [PubMed] [Google Scholar]
- 63.Frystyk J. Exercise and the growth hormone-insulin-like growth factor axis. Med. Sci. Sports Exerc. 2010;42(1):58–66. doi: 10.1249/MSS.0b013e3181b07d2d. [DOI] [PubMed] [Google Scholar]
- 64.Ma Y., Guo Z., Wang X. Tribulus terrestris extracts alleviate muscle damage and promote anaerobic performance of trained male boxers and its mechanisms: roles of androgen, IGF-1, and IGF binding protein-3. J. Sport Health Sci. 2017;6(4):474–481. doi: 10.1016/j.jshs.2015.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ozdal T., et al. The reciprocal interactions between polyphenols and gut microbiota and effects on bioaccessibility. Nutrients. 2016;8(2):78. doi: 10.3390/nu8020078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Clauss M., et al. Interplay between exercise and gut microbiome in the context of human health and performance. Front. Nutr. 2021;8 doi: 10.3389/fnut.2021.637010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study will be included in published articles.
The data that has been used is confidential.