Highlights
-
•
The FT1 shading treatment yielded the largest values for blueberry single fruit weight.
-
•
The highest total phenol, anthocyanin and vitamin C contents under the FT1 shading treatment.
-
•
470 known metabolites were obtained from blueberry fruits.
-
•
This study provides scientific basis for improving the quality of blueberry fruit.
Keywords: Blueberry, Fruit quality, Antioxidant system, Metabolite, Pathway
Abbreviations: CAT, catalase; SOD, superoxide dismutase; POD, peroxidase; FT1, black one-layer shading net; FT2, black two-layer shading net; FCK, no black shading net; MDA, malondialdehyde; TBA, thiobarbituric acid; PPO, polyphenol oxidase; LC, liquid chromatography; MS, mass spectrometry; PCA, principal component analysis; PLS-DA, partial least squares discriminant analysis; OPLS-DA, orthogonal partial least squares discriminant analysis; DAMs, differentially abundant metabolites; VIP, variable importance in projection; ROS, reactive oxygen species
Abstract
With the advancement of blueberry industrialization, cultivation measures for obtaining high-quality fruits and technologies for obtaining high levels of the main secondary metabolites have become inevitable requirements for further development of the blueberry industry. This study applied different shading treatments and found that the FT1 shading treatment yielded the largest values for the single fruit weight, solid longitudinal diameter and transverse diameter of blueberry fruit as well as the highest solidity-acid ratio and total phenol and vitamin C contents. Moreover, 470 known metabolites were obtained from blueberry fruits. Interestingly, the differentially abundant metabolites related to ABC transporters, pyrimidine metabolism, and purine metabolism pathways were commonly identified from the three comparisons, which indicated that these three metabolic pathways in blueberry fruits are vulnerable to shading treatment. This study provides a theoretical basis for the application of summer shading to improve the quality and antioxidant substances of small berries.
1. Introduction
Blueberry (Vaccinium spp.) is an evergreen or perennial deciduous shrub belonging to Vaccinium L. (Ericaceae) that is native to North America; it has oblate fruits that are dark blue at maturity. Known as “the king of berries”, these species boast true blue fruits, which are rare in the natural world (Chu et al., 2018). Blueberries are mainly distributed in tropical, subtropical and temperate regions. The main production locations worldwide include the United States, Chile, Canada, Spain, China, Japan, Morocco and over 30 other countries (Yousuf et al., 2016). Blueberry fruits are rich in many nutrients, including sugars, vitamins, amino acids, anthocyanins, catalase (CAT), superoxide dismutase (SOD), peroxidase (POD), and other enzymes (Han et al., 2021). Blueberry fruit anthocyanins can prevent and treat hypertension, clear capillaries and slow aging (Wang et al., 2019). With low fructose and fat contents and strong antioxidant capacity, blueberry fruit is listed as among the top five healthiest foods by the FAO (Kader et al., 1996) and is considered a third-generation fruit. Anthocyanin is a water-soluble pigment widely present in plants, and the colors of fruits and flowers are mainly determined by anthocyanin type and quantity (Honda & Moriya, 2018). Generally, there are few free anthocyanins in nature, and anthocyanins are often synthesized with various monosaccharide bonds. Anthocyanins are branched products of flavonoids, which are glycosylated derivatives formed by the combination of anthocyanin with various monosaccharides through glycosidic bonds. In general, the color differences caused by anthocyanins depend on the differences in the R3' and R5' substituents and are not affected by glycoside formation. There are six main anthocyanins in plants. In addition to pelargonidin, five anthocyanins have been found in blueberry fruits, including delphinidin, cyanidin, peonidin, petunidin, and malvidin (Srivastava et al., 2007, Somerset and Johannot, 2008). In general, the more hydroxyl groups at R3' and R5' there are, the bluer the color will be, while methoxidation (–OCH3) will increase the red tone (Honda & Saito, 2002). Fruit size depends not only on heredity but also on cultivation techniques and environmental conditions. Genotypes determine the type and content of anthocyanins in blueberry fruits of different cultivars. Other factors, such as cultivation area, soil quality, plant age, different treatments and harvesting time, also affect the content and nutritional composition of anthocyanins in blueberries (Han et al., 2021).
In recent years, research on blueberries has mainly focused on breeding improved varieties, increasing nutritional value and soil improvement and management, which has promoted the development of the blueberry industry. The anthocyanin content in blueberries is an important index that directly affects the nutritional value, sensory quality and medicinal value of the fruit. In production practice, blueberry fruit quality and anthocyanin contents cannot meet the actual demand. In the actual production of economic tree fruit in China, the appropriate cultivation conditions and management practices have not been fully determined, resulting in a need to improve fruit quality and yield. With the industrialization of blueberry production, cultivation measures aimed at obtaining high-quality fruits and technologies aimed at maximizing their main secondary metabolites have become inevitable requirements for the development of the blueberry industry.
Light is the energy source for all plants on earth and the main driving force for crops to carry out various life activities. Light plays a crucial role in plant growth and development, morphogenesis, material production, energy metabolism, yield formation and quality improvement (Castronuovo et al., 2019, Raza et al., 2020, Liu et al., 2021, Proietti et al., 2021). The effect of shading on crops is mainly due to the decrease in light intensity, which affects plant growth and fruit quality. Heindl and Brun (1983) found that light intensity was significantly positively correlated with the effective pod number of soybean, and shading treatment increased the pod drop rate of soybean. Raza et al. (2020) found that appropriate shading could improve photosynthetic characteristics, sugar and protein accumulation and optimize antioxidant defense in soybean. Light intensity is high in summer in China, and the terminal buds of blueberries tend to wilt. Different plants have different light requirements, and different plants have different response mechanisms under different light conditions. Thus, the mechanism by which different shading gradients affect blueberry fruit size, quality, antioxidant and secondary metabolite contents was studied to reveal the influence of summer shading on fruit quality formation and to provide a theoretical basis for improving the quality of small berries.
2. Materials and methods
2.1. Plant material and growth conditions
Taking the southern highbush blueberry cultivar “Biloxi” as the research material, 2-year-old healthy plants with roughly the same properties were selected. The growth matrix included peat, coconut bran, pine bark and perlite at a ratio of 3:3:3:1, and the pH was approximately 5.5. From the end of July to the end of September 2020, the blueberry seedlings were shaded during the strongest light intensity in the year. A steel frame shed with a three-needle 50 % black one-layer shading net (Taizhou Jinnong Screen Factory, Zhejiang, China) (FT1), a three-needle 50 % black two-layer shading net (FT2) and no black shading net (FCK) were used as treatments. The three treatments with different light intensities were designed with different black shading nets. A digital illuminance meter (TES-1332A) was used to measure the light intensity; that is, FCK is 100 % light intensity, FT1 is 50 % light intensity relative to FCK, and FT2 is 20 % light intensity relative to FCK. Each treatment had three replicates with 3–4 plants per replicate. The cultivation and management procedures were consistent across treatments. The study was conducted from late July 2020 to the end of September 2020. The test site was the Nanjing Lishui White Horse Blueberry Research Base (119°03′ E, 31°35′ N) (Wu et al., 2022).
2.2. Measurements of the fruit appearance index, hardness, soluble solids and total acid
At the mass ripening stage of “Biloxi” blueberry fruits, the transverse diameter, longitudinal diameter and weight of 18 mature single fruits were measured randomly, and the fruit shape index was calculated. The fruit mass (g) was weighed by an electronic balance with a sensitivity of 10 mg. The transverse diameter and longitudinal diameter (mm) were measured with a digital Vernier caliper with a reading accuracy of 0.01 nm. The fruit shape index was used to calculate the ratio of the longitudinal diameter to the transverse diameter of blueberry fruit. The hardness of the fruit was measured by a fruit hardness tester (Catalog No. 9300 (KM-5); Takemura Electric Works, ltd., Kyoto, Japan). The probe diameter was 5 mm, and the pressing distance was 10 mm. The maximum breaking force was used as the hardness, with units of kg/cm2. The measurements were repeated 18 times, and the average value was taken. Soluble solids were measured by a handheld refractometer (Atago, WYT-A, Tokyo, Japan), with measurements repeated 18 times. The total acid content was calculated as citric acid according to the Determination of Total Acid in Foods (GB/T12456-2008). Moreover, the ratio of soluble solid content to total acid content was calculated as the solidity-acid ratio.
2.3. Determination of total phenol, anthocyanin, flavonoid and vitamin C contents
The total phenol content of blueberry fruit extract was determined using the Folin-Ciocalteu method (Ainsworth & Gillespie, 2007). The total anthocyanin content in the extract was measured using the pH differential method described by Elisia et al. (2007). Total flavonoids were extracted according to Siddhu and Saxena (2017) and were measured with a colorimetric assay at 510 nm. The content of vitamin C in blueberry fruits was determined by molybdenum blue colorimetry according to the GB/T6195-1986 determination of vitamin C in vegetables and fruits (2,6-dechloro-indophenol titration method).
2.4. Determination of antioxidant system activity
The malondialdehyde (MDA) content was determined by the thiobarbituric acid (TBA) method (Zeng et al., 2020). CAT and SOD activities were determined based on the ammonium molybdate method and hydroxylamine method described in Li et al. (2013). Polyphenol oxidase (PPO) and POD activities were determined according to González et al., 2000, Maehy, 1955, respectively.
2.5. Sample preparation for LC–MS
Blueberry fruits were pretreated as follows: First, 80 mg of each sample was mixed with 20 μL of internal standard and 1 mL of methanol water (Wu et al., 2022). Then, two small steel balls were added, and the mixture was placed at −20℃ for 2 min for precooling and then placed in a grinder (60 Hz, 2 min). After that, ultrasonic extraction was carried out in an ice water bath for 30 min and left at −20 °C overnight. Finally, the mixture was centrifuged for 10 min (13000 rpm, 4℃), and a syringe was used to withdraw 150 μL of supernatant. The supernatant was considered the organic phase, passed through a 0.22-μM pinhole filter, transferred to a liquid chromatography (LC) injection vial and stored at −80℃ until LC–mass spectrometry (MS) analysis.
2.6. LC–MS and data processing
The analytical instrument was an LC–MS system composed of a Nexera UPLC ultrahigh-performance liquid phase tandem QE high-resolution mass spectrometer. The chromatographic conditions were as follows: column: ACQUITY UPLC HSS T3 (100 mm × 2.1 mm, 1.8 µm); mobile phase: A-water (containing 0.1 % formic acid), B-acetonitrile (containing 0.1 % formic acid); and flow rate: 0.35 mL/min. Mass spectrometry was performed with an ESI ion source, and sample mass spectrum signal acquisition was performed in positive and negative ion scanning modes. In addition, the PMDB database was used for qualitative analysis, and specific procedures were based on studies by Li et al., 2020, Wu et al., 2022. The quantitative analysis was performed with reference to Cao et al., 2020, Zhu et al., 2021.
2.7. Data statistical analysis
Multivariate statistical analysis included principal component analysis (PCA), partial least squares discriminant analysis (PLS-DA) and orthogonal partial least squares discriminant analysis (OPLS-DA). Univariate analysis mainly focuses on univariate description and statistical inference, including interval estimation and statistical hypothesis testing. Student’s t tests and fold change analyses were used to compare metabolites between two groups. In addition, the combination of multidimensional analysis and one-dimensional analysis was used to screen the differentially abundant metabolites (DAMs) between groups. The screening criteria were as follows: a variable importance in projection (VIP) value of the first principal component of the OPLS-DA model greater than 1 and a p value from a T test < 0.05. In addition, based on the KEGG database, metabolic pathway enrichment analysis of DAMs was performed (Cao et al., 2020). For firmness, soluble solid, bioactive substance and antioxidant system activity indexes of blueberry fruits under different shade treatments, all data were analyzed using SPSS 22.0 statistical software (IBM Inc., Chicago, IL, USA). One-way analysis of variance with a post hoc Duncan’s multiple range test was performed for the different shading treatments. A p value < 0.05 was considered statistically significant.
3. Results
3.1. Fruit appearance index and hardness
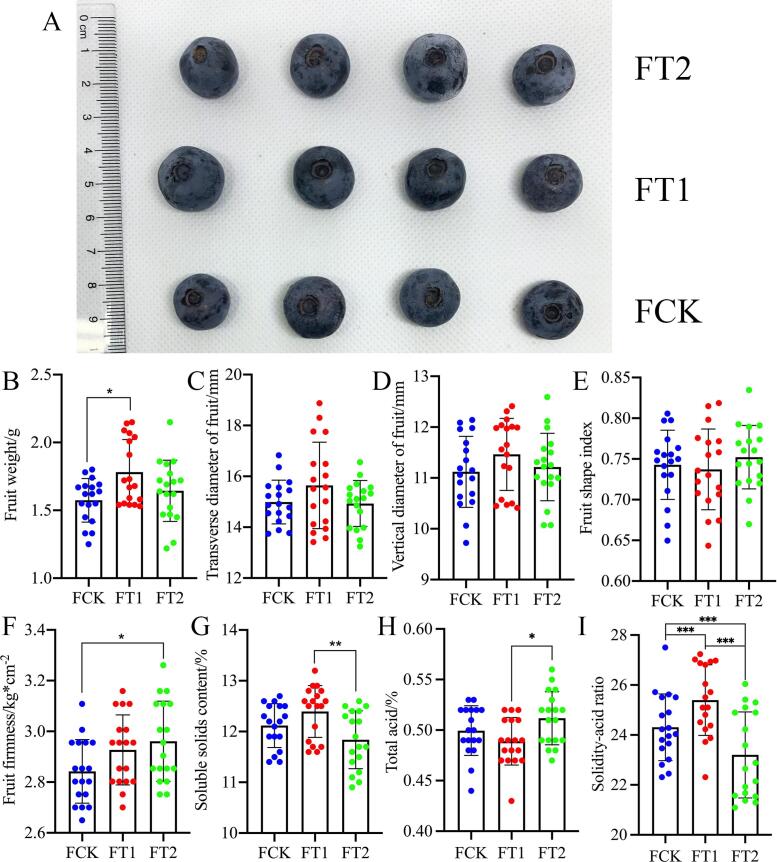
Of the different shading treatments, the FT1 group exhibited the largest values for the appearance, single fruit weight, solid longitudinal diameter and transverse diameter of blueberry fruit (Fig. 1A-D); in particular, the single fruit weight of the FT1 group was significantly higher than that of the FCK group. The average shape index (the ratio of the longitudinal diameter to the transverse diameter) of the fruit showed the following trend: FT1 < FCK < FT2 (Fig. 1E). The average fruit hardness of blueberries under the different shading treatments showed the following trend, FT2 > FT1 > FCK, and the hardness of the FT2 group was significantly higher than that of the FCK group (Fig. 1F). Herein, under the different shading treatments, the soluble solid content of the FT1 group was the highest and was significantly higher than that of the FT2 group (Fig. 1G), which indicated that the blueberry fruit quality was better with the FT1 treatment than with the FT2 treatment. However, no significant differences were observed between FCK and FT1 fruits. Because approximately 80 % of fruit soluble solids are soluble sugars, the content of soluble solids is often approximately expressed as the content of soluble sugars. Sugar content, sweetness and total acid content are important indexes to evaluate fruit quality and flavor. There was a significant difference in the total acid content of blueberry fruit between the FT1 and FT2 treatment groups, but there was no significant difference between the FCK group and the FT1 or FT2 group (Fig. 1H). The solidity-acid ratio is one of the important indexes of fruit quality, and there were significant differences in the solidity-acid ratio of blueberry fruit under different shading treatments. The solidity-acid ratio of the FT1 group was the highest, while that of the FCK group was the second highest, and that of the FT2 group was the lowest (Fig. 1I).
Fig. 1.
Appearance, firmness and soluble solids of blueberry fruits under different shade treatments. All data are expressed as the mean ± standard deviation. Through Duncan’s multiple range test in SPSS 22.0, * and ** represent a significant correlation at the 0.05 and 0.01 levels, respectively.
3.2. Fruit bioactive substances and antioxidant system indexes
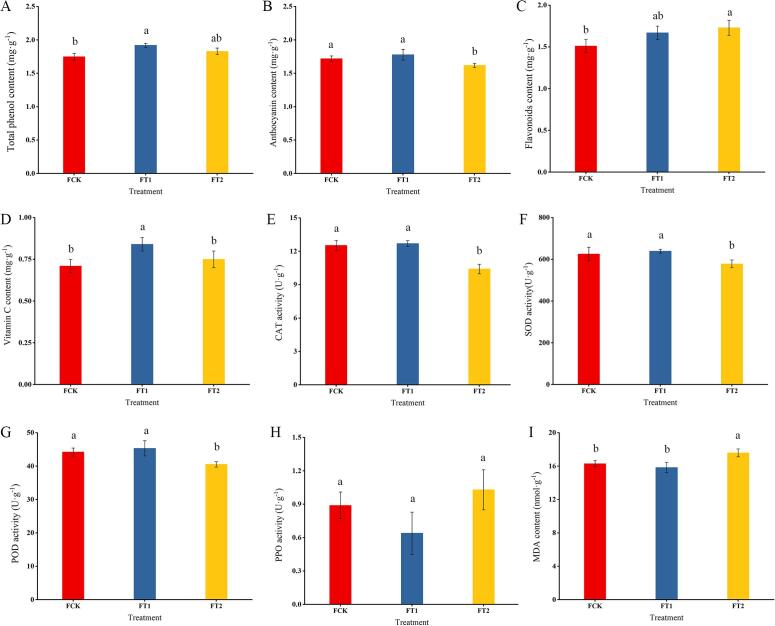
Four bioactive substances of blueberry fruits, including the total phenol, anthocyanin, flavonoid and vitamin C contents, exhibited significant differences among the different treatment groups (Fig. 2A-D). The total phenol content in blueberry fruits of the FT1 group was significantly higher than that of the FCK group under the different shade treatments (Fig. 2A). The anthocyanin content of the FCK and FT1 groups was higher than that of the FT2 group (Fig. 2B). Interestingly, the flavonoid content in the FCK group was significantly lower than that in the FT2 group (Fig. 2B). Moreover, the vitamin C content of the FT1 group was significantly higher than those of the FCK and FT2 groups, and no difference in these contents was found between the FCK and FT2 groups (Fig. 2D). In the antioxidant enzyme system, SOD, CAT and POD are three important active oxygen scavenging enzymes in plants. These three enzymes in blueberry fruits were not significant different between the FCK and FT1 groups, but they were significantly higher than those in the FT2 group (Fig. 2E, F, G). The PPO enzyme activity of blueberry fruits were not significantly different among the different treatments (Fig. 2H). The MDA content represents the degree of oxidation of plant cells. Herein, the MDA content was not significantly different between the FCK and FT1 groups, but these values were significantly lower than that in the FT2 group (Fig. 2I).
Fig. 2.
Bioactive substances and antioxidant system activity indexes of blueberry fruits under different shade treatments. All the data are shown as the mean ± standard deviation (error bar) of three biological replicates. Means with different letters are significantly different at p < 0.05, as determined by one-way ANOVA with Duncan’s multiple range tests.
3.3. Multivariate statistical analysis
Through qualitative and quantitative analyses of blueberry fruit metabolites, 470 known metabolites were obtained, including 194 metabolites in negative ion mode and 276 metabolites in positive ion mode. Unsupervised PCA of metabolites can reflect the variability between and within sample groups, represent the overall distribution trend among samples, and determine the possible discrete points. The main parameter of the PCA model is R2X. The square of the percentage of original data information retained in the x-axis direction (R2X) was more than 0.76 cμm among the different comparison groups (Table 1). The scores of the sum of the first principal component and the second principal component of blueberry samples between each comparison group were greater than 60 %. PLS and OPLS analyses indicated that R2X greater than 0.88, R2Y greater than 0.99, and Q2Y = 0.94 between different comparison groups. The parameter indexes of these models were more than 0.8, which is in line with the expectations of the experimental data model, indicating that the PLS and OPLS models can effectively explain the differences in metabolites in blueberry fruits between different comparison groups.
Table 1.
Model parameters for the comparative analysis of different shade treatment groups.
| Group | Type | PRE | ORT | N | R2X(cμm) | R2Y(cμm) | Q2(cμm) | R2 | Q2 |
|---|---|---|---|---|---|---|---|---|---|
| FT1/FCK | PCA | 3 | 0 | 6 | 0.766 | ||||
| FT1/FCK | PLS | 3 | 0 | 6 | 0.888 | 0.999 | 0.98 | ||
| FT1/FCK | OPLS | 1 | 2 | 6 | 0.888 | 0.999 | 0.963 | 0.972 | 0.158 |
| FT2/FCK | PCA | 3 | 0 | 6 | 0.797 | ||||
| FT2/FCK | PLS | 3 | 0 | 6 | 0.905 | 0.998 | 0.957 | ||
| FT2/FCK | OPLS | 1 | 2 | 6 | 0.905 | 0.998 | 0.948 | 0.961 | 0.186 |
| FT2/FT1 | PCA | 3 | 0 | 6 | 0.778 | ||||
| FT2/FT1 | PLS | 3 | 0 | 6 | 0.887 | 0.999 | 0.986 | ||
| FT2/FT1 | OPLS | 1 | 2 | 6 | 0.887 | 0.999 | 0.978 | 0.936 | 0.265 |
Note: Type: Multivariate statistical analysis model; PRE: the number of principal components during modeling; ORT: the number of orthogonal components during modeling; N: the number of samples during modeling; R2X (cμm): the cumulative interpretation rate of the model in the x-axis direction during multivariate statistical analysis modeling; cμm represents the cumulative results of several principal components. R2Y(cμm) represents the cumulative interpretation rate of the model in the y-axis direction. Q2(cμm) represents the cumulative prediction rate of the model. R2, Q2: The parameters of the response ranking test were used to measure whether the model was overfitted.
3.4. DAM analysis of different comparison groups
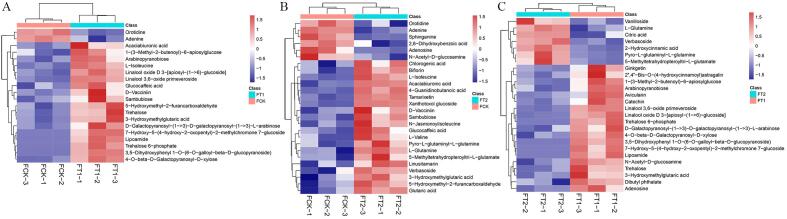
To more intuitively show the relationship between samples and the abundance differences of metabolites between different samples, we performed hierarchical clustering on the abundance of all DAMs (Fig. 3). The color from blue to red indicates the abundance abundance of metabolites from low to high; that is, the redder color indicates that the abundance of DAMs is higher. Among them, adenine was present in all the groups (Fig. 3). Between the FT1 and FCK groups, 20 DAMs were mainly distributed in seven classes. d-vacciniin, orotidine, l-isoleucine, adenine and lipoamide belonged to the five classes, benzene and substituted derivatives, pyrimidine nucleosides, carboxylic acids and derivatives, imidazopyrimidines, and dithiolanes, respectively; 3-hydroxymethylglutaric acid and 1-(3-methyl-2-butenoyl)-6-apiosylglucose belonged to the fatty acyls class. The thirteen other DAMs belonged to the organooxygen compound class (Fig. 3A). Twenty-six DAMs were mainly distributed in 11 classes; specifically, three DAMs (tamarixetin, xanthotoxol glucoside and linusitamarin) between the FT2 and FCK groups belonged to the super class of phenylpropanoids and polyketides (Fig. 3B). In addition, tamarixetin belonged to the subclass of O-methylated flavonoids within the flavonoid class and was upregulated 1.0-fold in the FT2 group compared with the FCK group (Fig. 3B). Interestingly, the FT2/FT1 comparison revealed 26 DAMs, which were classified into 9 classes (Fig. 3C). Among them, avicularin (flavonoid glycoside subclass), ginkgetin (biflavonoid and polyflavonoid subclass), catechin (flavan subclass) and 2′',4′'-bis-O-(4-hydroxycinnamoyl) astragalin (flavonoid glycoside subclass) belonged to the flavonoid class and were downregulated 0.4-fold, 0.7-fold, 1.8-fold and 1.1-fold in the FT2 group, respectively (Fig. 3C).
Fig. 3.
Heatmap of differentially abundant metabolites of blueberry fruit in different comparison groups. The abscissa represents the sample name, and the ordinate represents the differentially abundant metabolites.
3.5. Correlation analysis of DAMs
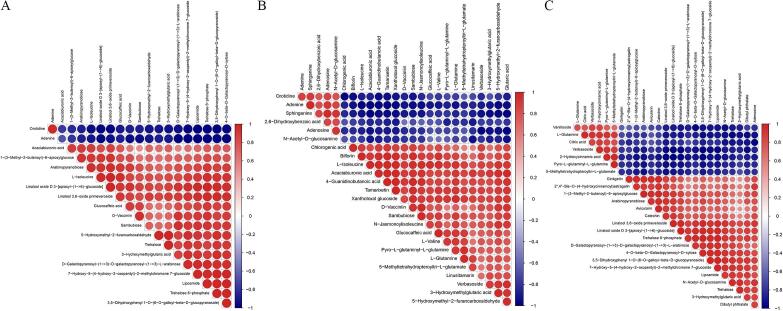
Correlation analysis can help to measure the correlations between significant DAMs and further understand the relationship between metabolites during biological changes. Correlation analysis was performed using the Pearson correlation coefficient to measure the degree of linear correlation between two metabolites. The results showed that except for a positive correlation between orotidine and adenine, orotidine was negatively correlated with all other DAMs between the FT1 and FCK groups (Fig. 4A). Adenine was negatively correlated with all DAMs in the FT1/FCK group (Fig. 4A). There was a positive correlation between the other DAMs (Fig. 4A). Between the FT2 and FCK groups or the FT1 and FCK groups, the correlation analysis between different metabolites showed that the number of positive correlations between the two DAMs was greater than that of negative correlations (Fig. 4B and C). Moreover, the positive and negative correlations between common DAMs had synergistic abundance patterns in different comparison groups.
Fig. 4.
Correlation analysis of differentially abundant metabolites (DAMs). Red indicates a positive correlation, and blue represents a negative correlation. (A) Between the FT1 and FCK groups; (B) Between the FT2 and FCK groups; and (C) Between the FT2 and FT1 groups.
3.6. KEGG enrichment analysis
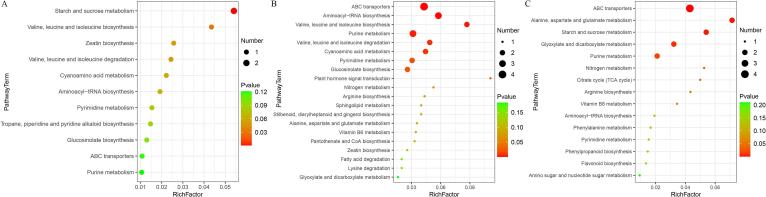
Pathway enrichment analysis of DAMs is helpful for understanding the changes in metabolic pathways in different blueberry fruit samples. ABC transporters, pyrimidine metabolism, and purine metabolism pathways were common in the three comparison groups (Fig. 5). Between the FCK and FT1 groups, starch and sucrose metabolism was the most enriched pathway, followed by the valine, leucine and isoleucine biosynthesis pathways (Fig. 5A). In the comparison of FT2 and FCK, there were more enriched pathways; specifically, ABC transport was the most enriched pathway, followed by aminoacyl-tRNA biosynthesis and valine, leucine and isoleucine biosynthesis pathways (Fig. 5B). ABC transport was also the most enriched pathway between the FT2 and FT1 groups, followed by the alanine, aspartate and glutamate metabolism pathways (Fig. 5C). Interestingly, through the observation of each comparison group, it was found that there were few enriched pathways and fewer DAMs between the FT1 and FCK groups.
Fig. 5.
Bubble diagram of differentially abundant metabolites (DAMs) in the top 20 metabolic pathways. The ordinate shows the names of the metabolic pathways. The abscissa is the enrichment factor (rich factor, rich factor = number of significant DAMs/number of total metabolites in the pathway). A larger rich factor indicates a greater enrichment degree. The color ranging from green to red indicates a decrease in the p value. A larger point indicates that more DAMs were enriched in the pathway.
4. Discussion
Light is an important energy source that green plants use to carry out life activities. More than 90 % of the biological yield of plants comes from photosynthesis. Light intensity affects the photosynthesis of plants. Within a certain light range, the photosynthesis of plants increases with increasing light intensity. When the light intensity reaches the light saturation point of plants, light inhibition will occur, which will affect photosynthesis (Ishida et al., 1999). If plants are in a weak light environment for a long time, plant growth and development become difficult, and crop yields can be reduced. Due to the high light intensity in summer, blueberries are prone to leaf wilting during the normal growth process. Changes in light intensity through shading nets can be used as a physical tool to improve the chemical quality and antioxidant activity of plants. Shading nets have been incorporated into the current protective cultivation practices for Melissa officinalis, Mentha piperita, and Ocimum basilicum to produce higher levels of essential oils (Ilić et al., 2022). Herein, fruit appearance and metabolites were investigated in blueberries grown under different shade levels to observe acclimation under shade conditions and to determine the optimal light conditions for agricultural purposes in summer.
Many scientific research studies and agricultural production practices show that light conditions will affect the quality of fruits (Beraud and Ulloa, 2015, Proietti et al., 2021). With the improvement of people’s living standards and the progress of cultivation methods, improving the quality and economic benefits of blueberries has become particularly important. Blueberries are composed of sugar, acid and vitamin C, and many factors affect their quality, such as temperature, light and humidity. Fruit development depends not only on heredity, but also on cultivation techniques and external conditions. Shade netting can significantly reduce light intensity and canopy temperature and significantly increase relative humidity (Mditshwa et al., 2019). Growers often use shading nets to protect fruit trees from various abiotic and biotic stresses (Mditshwa et al., 2019). When the biological yield is certain, the main determinants of the economic yield of fruit trees are fruit quantity and single fruit quality (Scalzo et al., 2013). Shading nets have the potential to positively affect the yield and quality of blueberry fruits by reducing light and temperature stress (Lobos et al., 2013). A statistical analysis of fruit-related characteristics revealed that the single fruit weight of the FT1 group was significantly higher than that of the FCK group. The results of this study showed that when the breeding goal of blueberry was a large fruit, a layer of shading net could be used to shade the blueberry plant in summer to improve the single fruit quality. The shading treatment had little effect on the fruit appearance quality (Fig. 1) but a significant effect on the contents of internal primary and secondary metabolites. Sugars and acids are not only important nutritional components of fruits but also important flavor indicators, and their contents and compositions are important factors determining the sour and sweet flavors of fruit (Duran-Soria et al., 2020). Stone et al. (2022) found that light conditions had a certain effect on fruit yield and quality. This study found that the blueberry fruits belonging to the FT1 group exhibited the highest soluble solid contents, followed by those in the FCK group. However, there was no significant difference in the SSC content between FT1 and FCK fruits (Fig. 1G). The solidity-acid ratio has a great influence on fruit flavor; the greater the solidity-acid ratio is, the sweeter the fruit tases. In this study, the FT1 group had the highest solidity-acid ratio, and the FT2 group had the lowest solidity-acid ratio (Fig. 1I), which indicated that blueberry fruits taste better under one layer of shade, making this a suitable treatment for fresh berries. Excessive shading reduces the assimilation capacity of source organs and intensifies the competition for preferential assimilation products between sink organs, which will lead to a decrease in the soluble solid content of fruits at harvest. Vitamin C, also known as ascorbic acid, cannot be synthesized in the human body and must be obtained from food. Vitamin C affects the formation of collagen, participates in a variety of redox reactions in the human body and promotes the metabolism of amino acids (Perla et al., 2016). The content of vitamin C in fruits belonging to the FT1 group was significantly higher than that of control fruits without shading and those under two-layer shading treatment, which indicated that the one-layer shading treatment is a better method for significantly improving the content of vitamin C in blueberry fruit.
Relevant studies have shown that plant polyphenols are important secondary metabolites. The term “polyphenols” is the general name for polyhydroxyphenols, and these compounds have a variety of medicinal and healthcare benefits (Naczk & Shahidi, 2004). Polyphenols are also important bioactive substances in blueberry fruits. In this study, the total phenol content of the FT1 group was significantly higher than that of the FCK group, which suggested that the FT1 fruits have strong antioxidant capacity. In addition, flavonoids and anthocyanins are key metabolites in blueberry fruit (Wu et al., 2021). These types of polyphenols exert antioxidation and anticancer effects and reduce blood sugars, blood lipids and eye fatigue (Li et al., 2017). Blueberry fruits belonging to the FT1 and FCK groups had higher contents of anthocyanins than those in the FT2 group. These results preliminarily show that blueberry fruits treated with one layer of shading are rich in nutrients and have higher contents of different types of antioxidant substances. Reactive oxygen species (ROS) are inevitable products of plant photosynthesis, respiration, nitrogen fixation and other metabolic processes. Under normal circumstances, the production and clearance of ROS in plants are in dynamic equilibrium. ROS clearance is mainly coordinated by antioxidant enzymes such as SOD and POD and other antioxidant substances. As important components of the antioxidant protective enzyme system, POD, SOD, and CAT play key roles in maintaining biofilm structure and function (Wang et al., 2017). The activities of POD, SOD and CAT in blueberry fruit in the FT1 and FCK groups were significantly higher than those in the FT2 group, indicating that the antioxidant capacity of blueberry fruit in the FT1 and FCK groups was strong and the ability to eliminate intracellular oxygen free radicals was also strong. MDA is the final product of membrane lipid peroxidation in plants under stress and can be used as the basis for judging the degree of membrane lipid peroxidation in plants (Yang et al., 2022). The MDA content of blueberry fruit in the FT2 group was higher than that of fruits in the FCK and FT1 groups, indicating that the degree of stress injury in the FT2 shading group was greater than that in the other groups. To summarize, fruits treated with one layer of shading presented higher levels of bioactive substances and nutrients and a higher antioxidant degree, and these findings provide a reference for improving fruit quality.
Different environments lead to the development of different plant traits, which can also be transformed into variations in metabolomes (Pegiou et al., 2021). For example, Lee et al. (2013) analyzed the effect of shade treatment on the nutritional and sensory qualities of green tea using metabolomics technology. A metabolic pathway related to the low light effect was proposed and which can preliminarily clarify the relationship between low light and tea quality. Wu et al. (2021) studied five representative Rubus fruit DAMs and indicated that cyanidin-3-sambubioside may be predominantly responsible for color differences. Several studies have shown that the shading of tea plants to decrease the light intensity improves the taste of green tea (Lee et al., 2013). In this study, tamarixetin was upregulated 1.0-fold in the FT2 group compared with the FCK group, which suggested that too much shade in the external environment may lead to the accumulation of tamarixetin, a natural flavonoid derivative of quercetin. Four DAMs belonging to the flavonoid class were downregulated in the FT2 group compared with the FT1 group, which indicated that a layer of shade is more conducive to the formation of anti-inflammatory and antioxidant substances. In addition, this study showed that ABC transporters, pyrimidine metabolism, and purine metabolism pathways were common in the three comparison groups, indicating that these three metabolic pathways of blueberry fruits are vulnerable to shading treatment, which led to the differences in DAMs in these pathways, thus suggesting that they may have an important impact on fruit quality. Several studies have shown that shading of blueberry plants usually leads to delayed fruit development (Godoy et al., 2018) and may be considered a promising tool to delay harvest for commercial purposes (Beraud & Ulloa, 2015). Blueberries, as shrubby plants, can readily be shaded for agricultural purposes, and Kim et al. (2011) showed that to achieve the best photosynthesis and blueberry growth, we must avoid shading levels exceeding 60 % of the full light level. This study showed that the FT1 treatment, 50 % shading, had a good effect on blueberry fruit quality and related antioxidants and confirmed that appropriate shading was conducive to blueberry fruit production. In short, it is necessary to properly shade blueberries in summer.
5. Conclusion
In short, we found that the FT1 shading treatment yielded the largest values for the single fruit weight, solid longitudinal diameter, transverse diameter, soluble solids content and solidity-acid ratio of blueberry fruit as well as the highest total phenol, anthocyanin and vitamin C contents. The solidity-acid ratio, anthocyanin, CAT, SOD and POD activities were significantly lower in blueberry fruits under the FT2 shading treatment. Moreover, 470 known metabolites were obtained in blueberry fruits, and the enrichment degree and types of DAMs were different among the different comparison groups. These results provide a theoretical basis for improving the quality and antioxidant substances of blueberry.
Funding
This research was supported by the Science and Technology Planning Project of Jiangsu Province (BE2019399), the Jiangsu Agriculture Science and Technology Innovation Fund (JASTIF)(CX(21)3172), and the Earmarked Fund for Jiangsu Agricultural Industry Technology System (JATS[2021]511).
Role of the funding source.
The funding bodies had no role in the design of the study, the collection, analysis, and interpretation of the data, or in the writing of the manuscript.
CRediT authorship contribution statement
Yaqiong Wu: Writing – original draft, Conceptualization, Data curation, Funding acquisition, Investigation, Methodology. Hao Yang: Formal analysis. Haiyan Yang: Formal analysis. Chunhong Zhang: Investigation, Methodology. Lianfei Lyu: Resources. Weilin Li: Writing – review & editing. Wenlong Wu: Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Contributor Information
Yaqiong Wu, Email: ya_qiong@126.com.
Hao Yang, Email: yanghao_19940720@163.com.
Haiyan Yang, Email: haiyanyang_025@126.com.
Chunhong Zhang, Email: chzhang0714@163.com.
Lianfei Lyu, Email: njbglq@163.com.
Weilin Li, Email: wlli@njfu.edu.cn.
Wenlong Wu, Email: 1964wwl@163.com.
References
- Ainsworth E.A., Gillespie K.M. Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin-Ciocalteu reagent. Nature Protocol. 2007;2:875–877. doi: 10.1038/nprot.2007.102. [DOI] [PubMed] [Google Scholar]
- Beraud M.M.R., Ulloa D.M.M. Shading nets effect on the production and quality of blueberry fruit (Vaccinium corymbosum L.) cv. Brigitta. Scientia Agropecuaria. 2015;6:41–50. doi: 10.17268/sci.agropecu.2015.01.04. [DOI] [Google Scholar]
- Cao M., Liu Y., Jiang W., Meng X., Zhang W., Chen W.…Xing S. UPLC/MS-based untargeted metabolomics reveals the changes of metabolites profile of Salvia miltiorrhiza bunge during sweating processing. Scientific Reports. 2020;10:19524. doi: 10.1038/s41598-020-76650-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castronuovo D., Russo D., Libonati R., Faraone I., Candido V., Picuno P.…Milella L. Influence of shading treatment on yield, morphological traits and phenolic profile of sweet basil (Ocimum basilicum L.) Scientia Horticulturae. 2019;254:91–98. doi: 10.1016/j.scienta.2019.04.077. [DOI] [Google Scholar]
- Chu W., Gao H., Chen H., Fang X., Zheng Y. Effects of cuticular wax on the postharvest quality of blueberry fruit. Food Chemistry. 2018;239:68–74. doi: 10.1016/j.foodchem.2017.06.024. [DOI] [PubMed] [Google Scholar]
- Duran-Soria S., Pott D.M., Osorio S., Vallarino J.G. Sugar signaling during fruit ripening. Frontiers in Plant Science. 2020;11 doi: 10.3389/fpls.2020.564917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elisia L., Hu C., Popovich D.G., Kitts D.D. Antioxidant assessment of anthocyanin-enriched blackberry extract. Food Chemistry. 2007;101:1052–1058. doi: 10.1016/j.foodchem.2006.02.060. [DOI] [Google Scholar]
- Godoy C.A., Monterubbianesi G., Sanchez E., Tognetti J.A. Cluster illumination differentially affects growth of fruits along their ontogeny in highbush blueberry (Vaccinium corymbosum L.) Scientia Horticulturae. 2018;230:1–10. doi: 10.1016/j.scienta.2017.11.008. [DOI] [Google Scholar]
- González E.M., de Ancos B., Cano M.P. Partial characterization of peroxidase and polyphenol oxidase activities in blackberry fruits. Journal of Agricultural and Food Chemistry. 2000;48:5459–5464. doi: 10.1021/jf000169w. [DOI] [PubMed] [Google Scholar]
- Han T., Wu W., Li W. Transcriptome analysis revealed the mechanism by which exogenous ABA increases anthocyanins in blueberry fruit during veraison. Frontiers in Plant Science. 2021;12 doi: 10.3389/fpls.2021.758215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heindl J.C., Brun W.A. Light and shade effects on abscission and 14C-photoassimilate partitioning among reproductive structures in soybean. Plant Physiology. 1983;73:434–439. doi: 10.1104/pp.73.2.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda C., Moriya S. Anthocyanin biosynthesis in apple fruit. The Horticulture Journal. 2018;87:305–314. doi: 10.2503/hortj.okd-r01. [DOI] [Google Scholar]
- Honda T., Saito N. Recent progress in the chemistry of polyacylated anthocyanins as flower color pigments. Heterocycles. 2002;56:1–2. doi: 10.3987/REV-01-SR(K)2. [DOI] [Google Scholar]
- Ilić Z.S., Milenković L., Tmušić N., Stanojević L., Stanojević J., Cvetković D. Essential oils content, composition and antioxidant activity of lemon balm, mint and sweet basil from Serbia. LWT. 2022;153 doi: 10.1016/j.lwt.2021.112210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida A., Toma T., Marjenah M. Limitation of leaf carbon gain by stomatal and photochemical processes in the top canopy of Macaranga conifera, a tropical pioneer tree. Tree Physiology. 1999;19:467–473. doi: 10.1093/treephys/19.7.467. [DOI] [PubMed] [Google Scholar]
- Kader F., Rovel B., Girardin M., Metche M. Fractionation and identification of the phenolic compounds of highbush blueberries (Vaccinium corymbosum, L.) Food Chemistry. 1996;55:35–40. doi: 10.1016/0308-8146(95)00068-2. [DOI] [Google Scholar]
- Kim S.J., Yu D.J., Kim T.C., Lee H.J. Growth and photosynthetic characteristics of blueberry (Vaccinium corymbosum cv. bluecrop) under various shade levels. Scientia Horticulturae. 2011;129:486–492. doi: 10.1016/j.scienta.2011.04.022. [DOI] [Google Scholar]
- Lee L.S., Choi J.H., Son N., Kim S.H., Park J.D., Jang D.J.…Kim H.J. Metabolomic analysis of the effect of shade treatment on the nutritional and sensory qualities of green tea. Journal of Agricultural and Food Chemistry. 2013;61:332–338. doi: 10.1021/jf304161y. [DOI] [PubMed] [Google Scholar]
- Li D.N., Li B., Ma Y., Sun Y.Y., Lin Y., Meng X.J. Polyphenols, anthocyanins, and flavonoids contents and the antioxidant capacity of various cultivars of highbush and half-high blueberries. Journal of Food Composition and Analysis. 2017;62:84–93. doi: 10.1016/j.jfca.2017.03.006. [DOI] [Google Scholar]
- Li H.X., Xiao Y., Cao L.L., Yan X., Li C., Shi H.Y.…Ye Y.H. Cerebroside C increases tolerance to chilling injury and alters lipid composition in wheat roots. PLoS One. 2013;8:e73380. doi: 10.1371/journal.pone.0073380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N., Zhou J., He C., Fu Y., Wang C., Liu L.…Hu D. GC-MS-based metabolomics reveal that light intensity during indoor overwintering affects the metabolism of Scylla paramamosain. Aquaculture Research. 2020;52:1013–1025. doi: 10.1111/are.14956. [DOI] [Google Scholar]
- Liu S., Baret F., Abichou M., Manceau L., Andrieu B., Weiss M., Martre P. Importance of the description of light interception in crop growth models. Plant Physiology. 2021;186:977–997. doi: 10.1093/plphys/kiab113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobos G.A., Retamales J.B., Hancock J.F., Flore J.A., Romero-Bravo S., del Pozo A. Productivity and fruit quality of Vaccinium corymbosum cv. Elliott under photo-selective shading nets. Scientia Horticulturae. 2013;153:143–149. doi: 10.1016/j.scienta.2013.02.012. [DOI] [Google Scholar]
- Maehy A.C. Plant peroxidase. Methods in Enzymology. 1955;801–813 doi: 10.1016/S0076-6879(55)02307-0. [DOI] [Google Scholar]
- Mditshwa A., Magwaza L.S., Tesfay S.Z. Shade netting on subtropical fruit: Effect on environmental conditions, tree physiology and fruit quality. Scientia Horticulturae. 2019;256 [Google Scholar]
- Naczk M., Shahidi F. Extraction and analysis of phenolics in food. Journal of Chromatography A. 2004;1054:95–111. doi: 10.1016/j.chroma.2004.08.059. [DOI] [PubMed] [Google Scholar]
- Pegiou E., Zhu Q., Pegios P., De Vos R.C.H., Mumm R., Hall R.D. Metabolomics reveals heterogeneity in the chemical composition of green and white spears of Asparagus (A. officinalis) Metabolites. 2021;11:708. doi: 10.3390/metabo11100708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perla V., Nimmakayala P., Nadimi M., Alaparthi S., Hankins G.R., Ebert A.W., Reddy U.K. Vitamin C and reducing sugars in the world collection of Capsicum baccatum L. genotypes. Food Chemistry. 2016;202:189–198. doi: 10.1016/j.foodchem.2016.01.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proietti S., Moscatello S., Riccio F., Downey P., Battistelli A. Continuous lighting promotes plant growth, light conversion efficiency, and nutritional quality of Eruca vesicaria (L.) Cav. in controlled environment with minor effects due to light quality. Frontiers. Plant Science. 2021;12 doi: 10.3389/fpls.2021.730119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raza M.A., Feng L.Y., Iqbal N., Khan I., Meraj T.A., Xi Z.J.…Yang W. Effects of contrasting shade treatments on the carbon production and antioxidant activities of soybean plants. Functional Plant Biology. 2020;47:342–354. doi: 10.1071/fp19213. [DOI] [PubMed] [Google Scholar]
- Scalzo J., Stevenson D., Hedderley D. Blueberry estimated harvest from seven new cultivars: Fruit and anthocyanins. Food Chemistry. 2013;139:44–50. doi: 10.1016/j.foodchem.2013.01.091. [DOI] [PubMed] [Google Scholar]
- Siddhu N., Saxena J. Quantification of total phenolic and total flavonoid content of extracts of tagetes erecta flowers. Asian Journal of Pharmaceutical and Clinical Research. 2017;10:328. doi: 10.22159/ajpcr.2017.v10i6.14598. [DOI] [Google Scholar]
- Somerset S.M., Johannot L. Dietary flavonoid sources in Australian adults. Nutrition and Cancer. 2008;60:442–449. doi: 10.1080/01635580802143836. [DOI] [PubMed] [Google Scholar]
- Srivastava A., Akoh C.C., Fischer J., Krewer G. Effect of anthocyanin fractions from selected cultivars of Georgia-grown blueberries on apoptosis and phase II enzymes. Journal of Agricultural and Food Chemistry. 2007;55:3180–3185. doi: 10.1021/jf062915o. [DOI] [PubMed] [Google Scholar]
- Stone C.H., Close D.C., Bound S., Hunt I. Training systems for sweet cherry: Light relations, fruit yield and quality. Agronomy. 2022;12:643. doi: 10.3390/agronomy12030643. [DOI] [Google Scholar]
- Wang H., Wu Y., Yu R., Wu C., Fan G., Li T. Effects of postharvest application of methyl jasmonate on physicochemical characteristics and antioxidant system of the blueberry fruit. Scientia Horticulturae. 2019;258 doi: 10.1016/j.scienta.2019.108785. [DOI] [Google Scholar]
- Wang Y., Stevanato P., Yu L., Zhao H., Sun X., Sun F.…Geng G. The physiological and metabolic changes in sugar beet seedlings under different levels of salt stress. Journal of Plant Research. 2017;130:1079–1093. doi: 10.1007/s10265-017-0964-y. [DOI] [PubMed] [Google Scholar]
- Wu Y., Yang H., Huang Z., Zhang C., Lyu L., Li W., Wu W. Metabolite profiling and classification of highbush blueberry leaves under different shade treatments. Metabolites. 2022;12:79. doi: 10.3390/metabo12010079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y., Zhang C., Huang Z., Lyu L., Li J., Li W., Wu W. The color difference of rubus fruits is closely related to the composition of flavonoids including anthocyanins. LWT. 2021;149 doi: 10.1016/j.lwt.2021.111825. [DOI] [Google Scholar]
- Yang H., Wu Y., Zhang C., Wu W., Lyu L., Li W. Growth and physiological characteristics of four blueberry cultivars under different high soil pH treatments. Environmental and Experimental Botany. 2022;197 doi: 10.1016/j.envexpbot.2022.104842. [DOI] [Google Scholar]
- Zeng P., Huang F., Guo Z., Xiao X., Peng C. Physiological responses of Morus alba L. in heavy metal(loid)–contaminated soil and its associated improvement of the microbial diversity. Environmental Science and Pollution Research. 2020;27:4294–4308. doi: 10.1007/s11356-019-07124-4. [DOI] [PubMed] [Google Scholar]
- Zhu Y., Zhang S., Sun J., Wang T., Liu Q., Wu G.…Wang W. Cigarette smoke promotes oral leukoplakia via regulating glutamine metabolism and M2 polarization of macrophage. International Journal of Oral Science. 2021;13:25. doi: 10.1038/s41368-021-00128-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yousuf B., Gul K., Wani A.A., Singh P. Health benefits of anthocyanins and their encapsulation for potential use in food systems: a review. Critical Reviews in Food Science and Nutrition. 2016;56:2223–2230. doi: 10.1080/10408398.2013.805316. [DOI] [PubMed] [Google Scholar]