Abstract
BACKGROUND & AIMS:
Postoperative Crohn’s disease (CD) surveillance relies on endoscopic monitoring. The role of cross-sectional imaging is less clear. We evaluated the concordance of cross-sectional enterography with endoscopic recurrence and the predictive ability of radiography for future CD postoperative recurrence.
METHODS:
We performed a multi-institution retrospective cohort study of postoperative adult patients with CD who underwent ileocolonoscopy and cross-sectional enterography within 90 days of each other following ileocecal resection. Imaging studies were interpreted by blinded, expert CD radiologists. Patients were categorized by presence of endoscopic postoperative recurrence (E+) (modified Rutgeerts’ score ≥i2b) or radiographic disease activity (R+) and grouped by concordance status.
RESULTS:
A total of 216 patients with CD with paired ileocolonoscopy and imaging were included. A majority (54.2%) exhibited concordance (34.7% E+/R+; 19.4% E−/R−) between studies. The plurality (41.7%; n = 90) were E−/R+ discordant. Imaging was highly sensitive (89.3%), with low specificity (31.8%), in detecting endoscopic postoperative recurrence. Intestinal wall thickening, luminal narrowing, mural hyper-enhancement, and length of disease on imaging were associated with endoscopic recurrence (all P < .01). Radiographic disease severity was associated with increasing Rutgeerts’ score (P < .001). E−/R+ patients experienced more rapid subsequent endoscopic recurrence (hazard ratio, 4.16; P = .033) and increased rates of subsequent endoscopic (43.8% vs 22.7%) and surgical recurrence (20% vs 9.5%) than E−/R− patients (median follow-up, 4.5 years).
CONCLUSIONS:
Cross-sectional imaging is highly sensitive, but poorly specific, in detecting endoscopic disease activity and postoperative recurrence. Advanced radiographic disease correlates with endoscopic severity. Patients with radiographic activity in the absence of endoscopic recurrence may be at increased risk for future recurrence, and closer monitoring should be considered.
Keywords: Enterography, Monitoring, Postoperative Crohn’s, Postoperative Recurrence
Graphical Abstract
Despite therapeutic advances, 50% of patients with Crohn’s disease (CD) require an intestinal resection within 10 years of diagnosis.1 Unfortunately, postoperative recurrence (POR) of CD is common, occurring in 35% to 85% of patients within the first postoperative year.1,2 Current postoperative monitoring strategies rely on endoscopic assessment of the neoterminal ileum (neoTI) for recurrence using the Rutgeerts’ score (RS).3 The RS correlates with clinical outcomes, including hospitalization and need for repeat surgery, and remains the gold standard for assessing postoperative disease activity.3,4
Enhanced imaging protocols for luminal assessment (eg, enterography) have improved the radiographic ability to assess CD activity. Both computed tomography enterography (CTE) and magnetic resonance enterography (MRE) have been validated in detecting CD activity in the nonoperative patient with CD and remain the radiographic test of choice to assess small bowel luminal activity.5–8 Given the transmural nature of inflammation in CD, radiography may offer additional sensitivity to detect disease activity compared with endoscopy. However, the clinical impact of discordant results between endoscopy and imaging is currently unclear. A recent retrospective study of patients with nonoperative CD undergoing clinically indicated enterography and ileocolonoscopy demonstrated that a minority had active radiographic disease, but normal endoscopic and histologic assessment.9 Most of these discordant patients experienced disease progression, suggesting that radiographic detection of disease activity may signal early detectable disease activity compared with endoscopy in a patient subset.
Prior studies have demonstrated that cross-sectional enterography has a high sensitivity and specificity to detect POR when compared with reference endoscopy.10–13 However, the clinical impact of discordant cross-sectional imaging findings compared with ileocolonoscopy have not been studied in postoperative CD. We aimed to characterize the test characteristics of cross-sectional imaging to monitor for POR compared with ileocolonoscopy and determine the long-term clinical impact of discordant imaging and ileocolonoscopy findings.
Methods
A retrospective, observational cohort study was performed on adult (>18 years) patients with CD undergoing ileocecal resection (ICR) for CD at 2 large academic institutions (Cleveland Clinic and New York University) between January 1, 2009 and January 1, 2020. Inclusion criteria included: (1) age >18 years; (2) CD diagnosis confirmed by ≥ 2 International Classification of Diseases-9 or −10 codes (K50.90) entered by a gastroenterologist or colorectal surgeon; (3) ICR indicated for CD management (Current Procedural Terminology codes: 44160, 44140, 44204, 44205) entered by a colorectal surgeon; (4) restoration of bowel continuity; (5) postoperative colonoscopy and cross-sectional imaging (CTE or MRE) at least 90 days after date of surgery or bowel continuity restoration. All inclusion criteria were confirmed via manual chart review.
Eligible patients were consecutively reviewed to determine record of ileocolonoscopy with cross-sectional imaging within 90 days of ileocolonoscopy. This time frame was chosen to minimize therapeutic changes and effects between the 2 studies. If patients had multiple matched studies, the pair closest to date of surgery was used. Patients were excluded if: (1) ICR was performed for a non-CD indication (eg, neoplasm, ischemia); (2) absence of gastroenterology follow-up (<1 outpatient clinic visit postoperatively); (3) insufficient endoscopy details to determine disease activity; (4) non-enterography cross-sectional imaging or unavailable images.
Demographic and Clinical Data
Clinical and demographic information was obtained through manual chart review by 4 independent reviewers (S.B., R.S., T.N., M.S.). Data collected included sex, age at diagnosis, age at surgery, tobacco use history, CD anatomic location and behavior according to Montreal classification, number of prior ICRs, and a history of perianal disease. Operative characteristics collected included anastomosis type, use of a diverting ileostomy, and date of ileostomy reversal. Postoperative data collected included postoperative biologic prophylaxis defined as initiation of biologic (including infliximab, adalimumab, certolizumab pegol, vedolizumab, ustekinumab) therapy within 3 months of date of surgery, exposure to postoperative biologics, and subsequent surgical recurrence defined by subsequent ileocecal resection for disease activity ≥90 days after the index resection.
Ileocolonoscopy Data
Postoperative ileocolonoscopy data was obtained via manual chart review of procedure reports. All lower endoscopic procedures performed >3 months from date of surgery were reviewed. Endoscopic severity was assessed using the RS; which grades endoscopic inflammatory activity on a scale of i0 (normal) to i4 (severe). If the RS was not prospectively available, retrospective application of the modified RS occurred utilizing colonoscopy images and report text. Retrospective RS application was done by 2 independent scorers (S.B., T.N.) that were trained by IBD gastroenterologists (B.C., J.A.) and validated (≥90% accuracy) using a sample dataset prior to data collection. Scorers were blinded to imaging and clinical data. Ileoscopy via stoma (ie, proximal to an ileocolonic anastomosis) and flexible sigmoidoscopy not reaching ileocolonic anastomosis were excluded. Endoscopic POR was defined as a modified RS of ≥ i2b.14
CTE or MRE Data
Two expert CD radiologists (M.B., B.D.) performed rereads of matched cross-sectional imaging. Radiologists were blinded to endoscopic and clinical outcomes and interpreted only their respective institution’s images. Radiologists met to discuss method of interpretation to enhance reliability. Imaging features and activity were defined by the Society of Abdominal Radiology CD consensus guidelines.8 Imaging data included: active disease sites proximal to neoTI (graded 0–5, 5–10, 10–20, and >20 cm proximal to neoTI) , intestinal wall thickening >3 mm, luminal narrowing >50% of normal luminal diameter, mural hyper-enhancement, length of disease, upstream stasis >2.5 cm, upstream dilation >3 cm, mural fat, pseudosacculations, fibrofatty proliferation, and penetrating disease. Radiographic severity was subjectively graded (none, minimal, mild, moderate, severe).
Outcomes
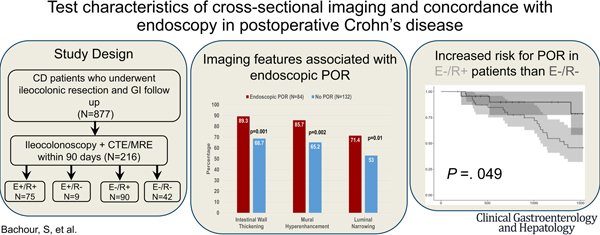
The primary outcome was test characteristics (accuracy, sensitivity, specificity, and area under the curve) of cross-sectional imaging disease activity detection compared to endoscopic recurrence. Patients were categorized by presence or absence of endoscopic POR (E+ or E−) or radiographic disease activity (R+ or R−). Patients with endoscopic and radiologic recurrence were defined as positive concordance (E+/R+), patients with no evidence of endoscopic and radiologic recurrence were defined as negative concordance (E−/R−), and patients with discordant endoscopic and radiologic activity were defined based on their CD activity findings (E+/R− or E−/R+) (Figure 1).
Figure 1.
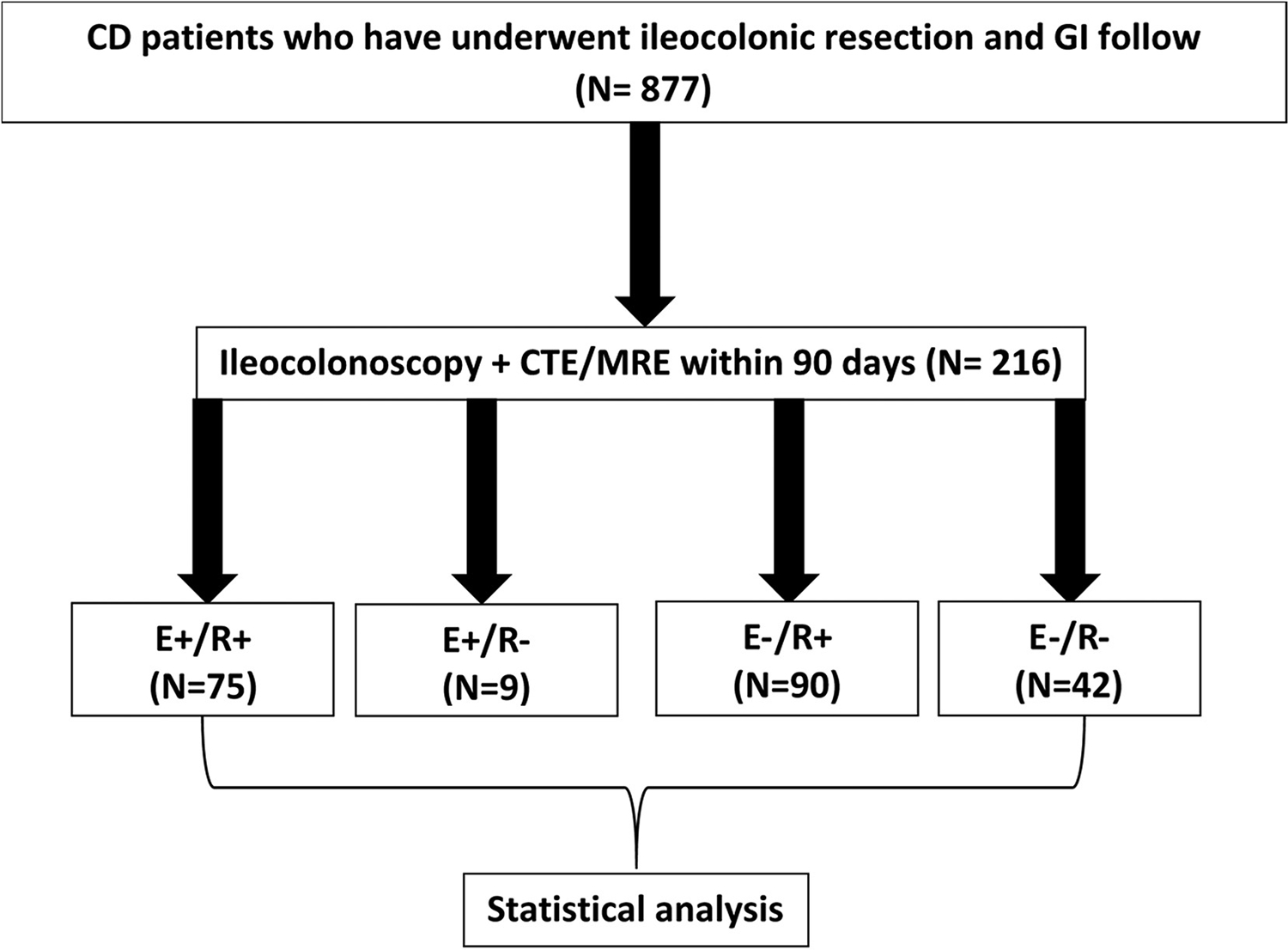
Study design flow chart. GI, Gastrointestinal.
Secondary outcomes of interest included the association of imaging features with endoscopic severity and recurrence and rates of subsequent endoscopic and surgical recurrence by concordance status.
Statistical Analysis
Data were described using medians and quartiles for non-normally distributed continuous variables, and counts and percentages for categorical variables. The χ2 and Fisher exact tests were used to compare categorical variables. The Kruskal-Wallis test was applied to compare non-normally distributed continuous variables. A receiver operating characteristic curve was conducted comparing radiology with ileocolonoscopy. Imaging test characteristics were performed utilizing endoscopic POR as gold standard. The Kaplan-Meier survival curve with 95% confidence intervals was conducted, and the log-rank test was performed to determine a significant difference in time to subsequent endoscopic or surgical POR from date of paired endoscopy. The censoring date was defined as median follow-up time (~5 years from index ileocolonoscopy). The decision was made to use median follow-up time to balance observing the disease course while minimizing confounding from time of matched studies.
To assess the impact of outcome definitions on radiography test characteristics, a sensitivity analysis defining POR as a modified RS of ≥i2a or ≥i1 was performed. All analyses were performed using R (R Foundation for Statistical Computing, Vienna, Austria) and SAS version 9.4 (SAS Institute, Cary, NC). The P-value < .05 was considered statistically significant. Bonferroni-corrected significance level was used for pairwise comparisons.
Ethical Considerations
The institutional review board approved the study at participating centers. All ethical principles laid out in the Declaration of Helsinki were followed.
Results
Study Population
Of the 877 adult patients with CD who underwent ICR, 536 patients (61.1%) underwent ≥1 postoperative ileocolonoscopy, and 484 (55.2%) underwent ≥1 cross-sectional enterography study. Of these, 216 patients had a paired ileocolonoscopy and cross-sectional enterography within 90 days of each other. Cross-sectional enterography occurred before endoscopy in 55% (n = 119) of matched studies. The majority (56.9%) were female with median age at surgery of 31 years (interquartile range, 25–45 years) (Table 1). Nearly one-third (31.7%) of patients were actively smoking at time of surgery. Disease behavior was primarily stricturing (49.5%), and 39.1% had ≥1 prior ICR. Approximately one-third (30.1%) of patients were started on postoperative biologic prophylaxis.
Table 1.
Summaries of Disease Characteristics on Concordance
| Factor | Overall (N = 216) | E−/R− (n = 42) | E−/R+ (n = 90) | E+/R− (n = 9) | E+/R+ (n = 75) | P-value | |
|---|---|---|---|---|---|---|---|
| n | Statistics | ||||||
| Age at surgery (median [IQR]) | 216 | 31 [25,45] | 30.5 [24,40] | 31 [24,47.75] | 37 [21,48] | 31 [26.5,42.5] | .761 |
| Median age at diagnosis [IQR], y | 216 | 22 [17–29] | 21.5 [18.25–26.75] | 20 [16–27.75] | 20 [17–31] | 24 [17–31] | .729 |
| CD location | 216 | .708 | |||||
| Colon | 6 (2.8) | 0 (0.0) | 2 (2.2) | 0 (0.0) | 4 (5.3) | ||
| Ileocolon | 133 (61.6) | 27 (64.3) | 57 (63.3) | 5 (55.6) | 44 (58.7) | ||
| Terminal ileum | 77 (35.6) | 15 (35.7) | 31 (34.4) | 4 (44.4) | 27 (36.0) | ||
| CD behavior | 216 | .933 | |||||
| Inflammatory | 6 (2.8) | 2 (4.8) | 2 (2.2) | 0 (0.0) | 2 (2.7) | ||
| Penetrating | 39 (18.1) | 8 (19.0) | 14 (15.6) | 2 (22.2) | 15 (20.0) | ||
| Stricturing + penetrating | 64 (29.6) | 12 (28.6) | 30 (33.3) | 1 (11.1) | 21 (28.0) | ||
| Stricturing | 107 (49.5) | 20 (47.6) | 44 (48.9) | 6 (66.7) | 37 (49.3) | ||
| History of tobacco use | 188 | .425 | |||||
| Never | 99 (52.4) | 18 (60.0) | 46 (56.1) | 4 (57.1) | 31 (44.3) | ||
| Former | 30 (15.9) | 4 (13.3) | 9 (11.0) | 2 (28.6) | 15 (21.4) | ||
| Active | 60 (31.7) | 8 (26.7) | 27 (32.9) | 1 (14.3) | 24 (34.3) | ||
| Prior CD resection | 216 | .713 | |||||
| 0 | 131 (60.9) | 31 (75.6) | 54 (60.0) | 5 (55.6) | 41 (54.7) | ||
| 1 | 51 (23.7) | 7 (17.1) | 21 (23.3) | 3 (33.3) | 20 (26.7) | ||
| 2 | 23 (10.7) | 2 (4.9) | 10 (11.1) | 1 (11.1) | 10 (13.3) | ||
| ≥3 | 10 (4.7) | 1 (2.4) | 5 (5.6) | 0 (0.0) | 4 (5.3) | ||
| Upper GI | 216 | 40 (18.5) | 8 (19.0) | 19 (21.1) | 2 (22.2) | 11 (14.7) | .746 |
| Gender (male) | 216 | 93 (43.1) | 11 (26.2) | 44 (48.9) | 3 (33.3) | 35 (46.7) | .076 |
| Perianal involvement ever | 216 | 76 (35.3) | 13 (31.0) | 29 (32.6) | 2 (22.2) | 32 (42.7) | .378 |
| Postoperative biologic prophylaxis | 216 | 65 (30.1) | 17 (40.5) | 29 (32.2) | 3 (33.3) | 16 (21.3) | .163 |
| Imaging modality (MRE) | 216 | 82 (38.0) | 23 (54.8) | 31 (34.4) | 4 (44.4) | 24 (32.0) | .079 |
Note: Data are presented as number (%) except where indicated.
CD, Crohn’s disease; E+, endoscopic postoperative recurrence; E−, no endoscopic postoperative recurrence; GI, gastrointestinal; IQR, interquartile range; MRE, magnetic resonance enterography; R+, radiologic active disease; R−, radiologic inactive disease.
At the time of the matched studies, 84 patients (38.9%) had endoscopic POR (42.9% i2b, 26.2% i3, 31.0% i4), and 165 patients (76.4%) had active radiologic disease. A majority (54.2%) exhibited concordance between matched imaging and endoscopy, with 34.7% (n = 75) having positive concordance (E+/R+) and 19.4% (n = 42) having negative concordance (E−/R−). The plurality (41.7%; n = 90) were E−/R+ discordant, and 4.2% (n = 9) were E+/R− discordant. The median time to matched colonoscopy was 709.5 days (interquartile range, 351.5–1329.25 days) from ICR and did not differ among concordance subgroups (P = .32). CTE was the predominant imaging modality (62.0% vs 38.0% MRE). Concordance status was not associated with imaging modality (CTE: 44% E−/R+, 38.1% E+/R+, 14.2% E−/R−, 3.7% E+/R−; MRE: 37.8% E−/R+, 29.3% E+/R+, 28% E−/R−, 4.9% E+/R−; P = .08). Surgical POR occurred in 47 patients and was associated with concordance status (20% E−/R+, 28% E+/R+, 9.5% E−/R−, 44.4% E+/R−; P = .03); however, no statistical association was found on pairwise comparison.
Test Characteristics
When comparing cross-sectional enterography with ileocolonoscopy for assessment of POR, the area under the curve of the receiver operating characteristic curve was 0.70. Cross-sectional imaging was 54.2% accurate in detecting POR. Imaging was highly sensitive (89.3%), but had low specificity (31.8%) (positive predictive value, 45.5% and negative predictive value, 82.4%) (Supplemental Table 1). CTE detected radiographic activity at significantly higher rates than MRE studies (82.1% vs 67.1%; P = .02). CTE had greater sensitivity (91.1% vs 85.7%) and positive predictive value (46.3% vs 43.6%) than MRE; however, MRE had significantly greater specificity (42.6% vs 24.3%; P = .03) and slightly greater accuracy (57.3% vs 52.2%) and negative predictive value (85.2% vs 79.2%).
Radiologic Features of Endoscopic Recurrence
The most common radiographic features observed were intestinal wall thickening (76.7%), mural hyper-enhancement (73.1%), and luminal narrowing (60.2%). Imaging features associated with endoscopic POR included intestinal wall thickening (89.3%; P = .001), mural hyper-enhancement (85.7%; P = .002), luminal narrowing (71.4%; P = .011), upstream dilation (56.6%; P = .001), and length of disease >10 cm (40.4%; P < .001) (Table 2). Imaging features not associated with POR included active disease sites proximal to neoTI (P = .49), upstream stasis (P = .99), mural fat (P = .99), pseudosacculations (P = .37), and fibrofatty proliferation (P = .15). Radiographic graded severity was also associated with endoscopic POR(P<.001). Endoscopic POR was associated with surgical recurrence (29.8% vs 16.7%; P = .035); however, there was no association of surgical POR with radiologic activity (23.6% vs 18.6%; P = .31).
Table 2.
Cross-sectional Imaging Features Association With Endoscopic POR
| Factor | Overall (N = 216) | No POR (n = 132) | Endoscopic POR (n = 84) | P-value | |
|---|---|---|---|---|---|
| N | Statistics | ||||
| Time from start time-point to matched endoscopy, d | 216 | 709.5 [351.5–1329.25] | 680.5 [297.95–1294.5] | 805 [429.75–1353.75] | .15a |
| Active disease sites proximal to neoTI | 216 | 40 (18.5) | 22 (16.7) | 18 (21.4) | .49b |
| Disease presence relative to neoTI, cm | 216 | .003c | |||
| No disease | 47 (21.8) | 38 (28.8) | 9 (10.7) | ||
| 0–5 | 163 (75.5) | 91 (68.9) | 72 (85.7) | ||
| 5–10 | 3 (1.4) | 0 (0.00) | 3 (3.6) | ||
| 10–20 | 1 (0.46) | 1 (0.76) | 0 (0.00) | ||
| >20 cm | 2 (0.93) | 2 (1.5) | 0 (0.00) | ||
| Intestinal wall thickening | 215 | 165 (76.7) | 90 (68.7) | 75 (89.3) | .001b |
| Luminal narrowing | 216 | 130 (60.2) | 70 (53.0) | 60 (71.4) | .011b |
| Hyper-enhancement | 216 | 158 (73.1) | 86 (65.2) | 72 (85.7) | .002b |
| Length of disease, cm | 216 | < .001b | |||
| No disease | 46 (21.3) | 37 (28.0) | 9 (10.7) | ||
| 1–10 | 114 (52.8) | 73 (55.3) | 41 (48.8) | ||
| >10 | 56 (25.9) | 22 (16.6) | 34 (40.4) | ||
| Upstream stasis >2.5 cm | 207 | 14 (6.8) | 9 (7.1) | 5 (6.2) | .99b |
| Upstream dilation >3 cm | 214 | 90 (42.1) | 43 (32.8) | 47 (56.6) | .001b |
| Mural fat | 213 | 37 (17.4) | 23 (17.6) | 14 (17.1) | .99b |
| Pseudosacculations | 214 | 13 (6.1) | 10 (7.6) | 3 (3.6) | .37b |
| Fibrofatty proliferation | 212 | 43 (20.3) | 22 (16.8) | 21 (25.9) | .15b |
| Penetrating disease | 215 | 10 (4.7) | 1 (0.76) | 9 (10.8) | .008c |
| Graded radiologic severity | 216 | < .001c | |||
| No | 51 (23.6) | 42 (31.8) | 9 (10.7) | ||
| Minimal | 48 (22.2) | 38 (28.8) | 10 (11.9) | ||
| Mild | 70 (32.4) | 35 (26.5) | 35 (41.7) | ||
| Moderate | 39 (18.1) | 14 (10.6) | 25 (29.8) | ||
| Severe | 8 (3.7) | 3 (2.3) | 5 (6.0) | ||
| Any radiologic activity | 216 | 165 (76.4) | 90 (68.2) | 75 (89.3) | .001b |
Note: Data are presented as median [IQR] or number (%).
Note: Boldface P-values indicate statistical significance.
IQR, Interquartile range; neoTI, neoterminal ileum; POR, postoperative recurrence.
P-values: Kruskal-Wallis test.
P-values: Pearson χ2 test.
P-values: Fisher exact test.
Radiologic Features of Endoscopic Rutgeerts’ Severity
Increasing RS was associated with increased prevalence of intestinal wall thickening (P = .004) and mural hyper-enhancement (P = .001) (Table 3). Radiologic disease activity was seen at higher rates with increasing endoscopic severity (P = .001) with 96.2% of i4 disease having corresponding radiographic activity. Increased prevalence of radiographic luminal narrowing (P = .025) and upstream dilation (P = .014) were associated with endoscopic severity; however, there was less discrepancy between i3 and i4 disease (Table 3).
Table 3.
Cross-sectional Imaging Features Association With Rutgeerts’ Score
| Factor | Overall (N = 216) | Rutgeerts’ score | P-value | ||||||
|---|---|---|---|---|---|---|---|---|---|
| N | Statistics | i0 (n = 55) | i1 (n = 23) | i2a (n = 54) | i2b (n = 36) | i3 (n = 22) | i4 (n = 26) | ||
| Time from start time-point to matched endoscopy, d | 216 | 709.5 [351.5–1329.25] | 590.0 [245.5– 1011.5] | 797.0 [363.5–1094.5] | 709.5 [360.25–1660.75] | 917.0 [434.25–1329.25] | 556.0 [329.0–822.0] | 971.5 [540.0–1589.0] | .052a |
| Active disease sites proximal to neoTI | 216 | 40 (18.5) | 11 (20.0) | 4 (17.4) | 7 (13.0) | 6 (16.7) | 4 (18.2) | 8 (30.8) | .57c |
| Disease presence relative to neoTI, cm | 216 | .015c | |||||||
| No disease | 47 (21.8) | 20 (36.4)g | 7 (30.4) | 11 (20.4) | 5 (13.9) | 3 (13.6) | 1 (3.8)d | ||
| 0–5 | 163 (75.5) | 34 (61.8) | 14 (60.9) | 43 (79.6) | 29 (80.6) | 19 (86.4) | 24 (92.3) | ||
| 5–10 | 3 (1.4) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 2 (5.6) | 0 (0.00) | 1 (3.8) | ||
| 10–20 | 1 (0.46) | 0 (0.00) | 1 (4.3) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | ||
| >20 | 2 (0.93) | 1 (1.8) | 1 (4.3) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | ||
| Intestinal wall thickening | 215 | 165 (76.7) | 33 (61.1) | 15 (65.2) | 42 (77.8) | 31 (86.1) | 19 (86.4) | 25 (96.2) | .004b |
| Luminal narrowing | 216 | 130 (60.2) | 23 (41.8) | 14 (60.9) | 33 (61.1) | 24 (66.7) | 17 (77.3) | 19 (73.1) | .025b |
| Hyper-enhancement | 216 | 158 (73.1) | 32 (58.2) | 12 (52.2) | 42 (77.8) | 30 (83.3) | 18 (81.8) | 24 (92.3) | .001b |
| Length of disease, cm | 216 | < .001c | |||||||
| No disease | 46 (21.3) | 19 (34.5)f,g | 7 (30.4) | 11 (20.4)g | 5 (13.9) | 3 (13.6)d | 1 (3.8)d,e | ||
| 1–10 | 114 (52.8) | 30 (54.5) | 9 (39.1) | 34 (63.0) | 23 (63.9) | 9 (40.9) | 9 (34.6) | ||
| >10 | 56 (25.9) | 6 (10.9) | 4 (30.4) | 9 (16.7) | 8 (22.3) | 10 (45.4) | 16 (61.5) | ||
| Upstream stasis >2.5 cm | 207 | 14 (6.8) | 4 (7.8) | 0 (0.00) | 5 (9.4) | 2 (5.7) | 2 (10.0) | 1 (3.8) | .70c |
| Upstream dilation >3 cm | 214 | 90 (42.1) | 15 (27.8) | 10 (43.5) | 18 (33.3) | 18 (51.4) | 14 (63.6) | 15 (57.7) | .014b |
| Mural fat | 213 | 37 (17.4) | 4 (7.4) | 6 (26.1) | 13 (24.1) | 8 (22.9) | 3 (13.6) | 3 (12.0) | .15c |
| Pseudosacculations | 214 | 13 (6.1) | 2 (3.6) | 2 (8.7) | 6 (11.3) | 2 (5.7) | 0 (0.00) | 1 (3.8) | .41c |
| Fibrofatty proliferation | 212 | 43 (20.3) | 9 (16.4) | 4 (17.4) | 9 (17.0) | 6 (18.2) | 8 (36.4) | 7 (26.9) | .38b |
| Penetrating disease | 215 | 10 (4.7) | 1 (1.8) | 0 (0.0) | 0 (0.0) | 4 (11.4) | 0 (0.0) | 5 (19.2) | .001c |
| Graded radiologic severity | 215 | < .001c | |||||||
| No | 51 (23.6) | 21 (38.2)f,g | 10 (43.5)g | 11 (20.4)g | 5 (13.9) | 3 (13.6) | 1 (3.8)d | ||
| Minimal | 48 (22.2) | 16 (29.1) | 4 (17.4) | 18 (33.3) | 7 (19.4) | 1 (4.5) | 2 (7.7) | ||
| Mild | 70 (32.4) | 12 (21.8) | 6 (26.1) | 17 (31.5) | 13 (36.1) | 14 (63.6) | 8 (30.8) | ||
| Moderate | 39 (18.1) | 5 (9.1) | 3 (13.0) | 6 (11.1) | 10 (27.8) | 3 (13.6) | 12 (46.2) | ||
| Severe | 8 (3.7) | 1 (1.8) | 0 (0.0) | 2 (3.7) | 1 (2.8) | 1 (4.5) | 3 (11.5) | ||
| Any radiologic activity | 216 | 165 (76.4) | 34 (61.8) | 13 (56.5) | 43 (79.6) | 31 (86.1) | 19 (86.4) | 25 (96.2) | .001b |
Note: Data are presented as median [IQR] or number (%).
Note: Boldface P-values indicate statistical significance.
IQR, Interquartile range; neoTI, neoterminal ileum.
P-values: Kruskal-Wallis test.
P-values: Pearson χ2 test.
P-values: Fisher exact test.
Significantly different from i0.
Significantly different from i2a.
Significantly different from i3.
Significantly different from i4.
Radiologic Activity in the Absence of Endoscopic Recurrence
Of the 90 E−/R+ individuals, the majority had endoscopic activity, with 47.8% (n = 43) having i2a disease, 14.4% (n = 13) i1 disease, and 37.8% (n = 34) having i0 disease. Three E−/R+ patients had isolated radiographic activity ≥10 centimeters proximal to the neoTI. Pairwise comparison of E−/R+ and E+/R+ groups demonstrated that radiographic length of disease ≥10 cm (24.5% E−/R+; 45.3% E+/R+; P = .007) and upstream dilation (40.4% E−/R+; 63.5% E+/R+; P = .004) were associated with concordance (Supplementary Table 2). E−/R+ patients had minimal radiographic severity at significantly higher rates than E+/R+ patients (42.2% E−/R+; 13.3% E+/R+; P < .001).
The majority (n = 48; 53.3%) of E−/R+ patients had at least 1 subsequent colonoscopy after matched study (Supplementary Table 4). Of these, 21 (43.8%; i0 = 8, i1 = 3, i2a = 10) experienced subsequent endoscopic POR. No radiographic features at time of paired imaging were predictive of future endoscopic POR (Supplementary Table 3). In E−/R− patients, 22 (52.4%) had at least 1 subsequent colonoscopy with 5 patients (22.7%; i0 = 1, i1 = 1, i2a = 3) having subsequent endoscopic POR (median follow-up time of 4.3 years). On survival analysis and Cox proportional hazards analysis, E−/R+ patients had a faster time interval from matched endoscopy to subsequent endoscopic POR (hazard ratio, 4.16; 95% confidence interval, 1.1–13.4; P = .033) (Figure 2). Median survival time to subsequent endoscopic POR in E−/R+ patients was 3.5 years. E−/R+ patients had double the rate of surgical POR than E−/R− individuals (median follow-up time of 4.5 years), but was not statistically significant (20% vs 9.5%; P = .35) (Supplementary Figure 1).
Figure 2.
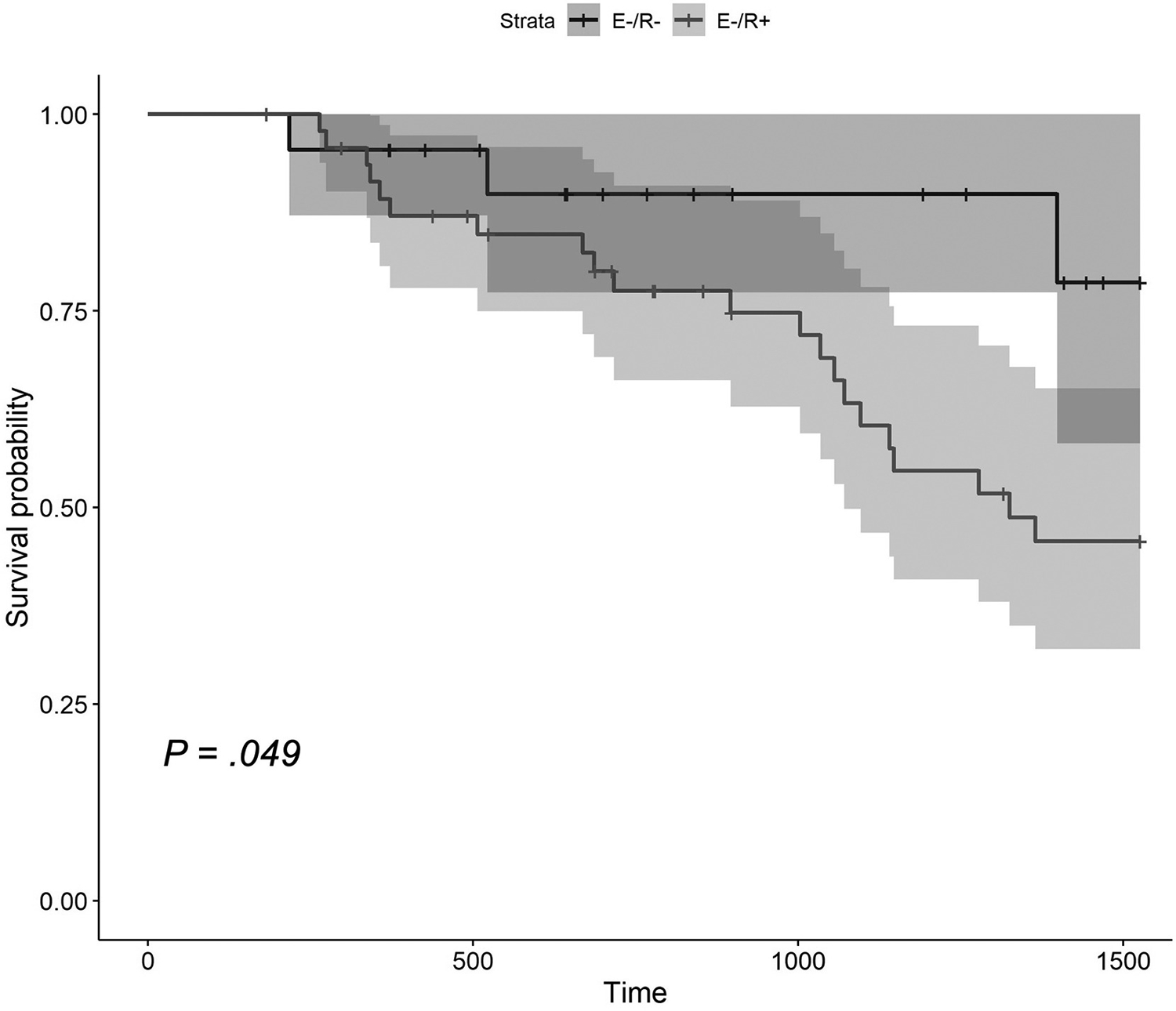
Kaplan-Meier survival analysis of time (days) to subsequent endoscopic POR after matched endoscopy between E−/R−and E−/R+ patients.
In E−/R+ patients with at least 1 subsequent colonoscopy, 15 (31.3%) were started on a biologic after date of paired imaging. Three of the 15 patients (20%) experienced subsequent endoscopic POR compared with 18 of the 33 patients (54.5%) who were not initiated on biologic therapy (P = .05), of which 9 were subsequently started on biologic therapy.
Sensitivity Analysis
When defining POR as a RS of ≥ i2a, we observed a 13% increase in accuracy of imaging to detect endoscopic POR (67.6%), an 8% increase in specificity (39.7%), and a 6% decrease in sensitivity (83.3%) (Supplementary Table 5). There were no changes to imaging features associated with endoscopic POR or with concordance status. There was no difference in rates of future endoscopic POR between E−/R+(46.2%) and E−/R+ (55.6%) patients, in addition to no difference in time to future endoscopic POR (P = .79) (Supplementary Figure 2).
A second sensitivity analysis was performed to evaluate cross-sectional enterography’s ability to detect endoscopic inflammation (RS ≥i1). We observed an increase in accuracy (69.0%) and specificity (38.2%); however, we observed a 10% decrease in sensitivity (79.5%) when compared with POR ≥i2b (Supplementary Table 6).
Discussion
In this multi-institutional, retrospective study of adult postoperative patients with CD, we found that cross-sectional enterography was highly sensitive, but with limited specificity, in detecting postoperative recurrence of CD. The majority of paired studies resulted in concordant findings; however, a considerable portion of patients displayed active radiographic activity without evidence of endoscopic POR. These discordant, radiographically active individuals experienced faster time to endoscopic POR and approximately twice the rates of subsequent endoscopic and surgical POR compared with negative concordant patients. To our knowledge, this is the first study assessing the clinical impact of paired endoscopic and radiographic assessments in postoperative patients with CD. This data suggests that cross-sectional imaging can play an adjunctive role in postoperative disease activity assessment and may detect individuals at risk of impending endoscopic recurrence.
Our study demonstrated that cross-sectional enterography is highly sensitive in detecting endoscopic POR, similar to previous literature in the nonoperative and postoperative CD populations.5–7 In contrast, our study showed limited specificity in detecting endoscopic POR. The high sensitivity with lower specificity suggests that imaging performs strongly in detecting intestinal inflammation; however, it is not specific when comparing any radiologic activity with endoscopic POR or inflammation (RS ≥i1), as demonstrated in our sensitivity analyses. This outcome may be due to the transmural nature of CD. As ileocolonoscopies only visualize intestinal mucosa, cross-sectional enterography offers a distinct advantage in evaluating intramural and mesenteric disease. Our study population demonstrated a statistical association between length of disease, upstream dilation, and radiologic disease severity between E−/R+ and E+/R+ patient populations. Additionally, when defining POR as an RS of ≥i2a, we observed an increase in accuracy and specifity. These findings suggest that as features of radiologic disease severity progresses, CD activity begins to manifest as endoscopically visible mucosal pathology at higher rates. Thus, enterography may be useful to detect active disease in individuals who have contraindications or increased risk for endoscopic assessments, incomplete ileocolonoscopies, or potentially as an adjunctive noninvasive modality in the absence of prior endoscopic activity, particularly in patients with isolated anastomotic inflammation.
Although ileocolonoscopy is established as the standard for postoperative surveillance of CD, it has its limitations. Ileocolonoscopy cannot visualize the small bowel beyond the terminal ileum (TI), which could contribute to decreased radiologic accuracy and specificity. However, in our study cohort, there was no difference in prevalence of active disease sites proximal to the neoTI between E+/R+ and E−/R+ patients, suggesting that the visualization of the proximal small bowel did not impact concordance status. Additionally, only 3 (3.3%) E−/R+ patients had isolated proximal small bowel radiographic activity—further suggesting that activity proximal to the neoTI did not play a significant role in discordance.
Similar to previous literature, we found that intestinal wall thickening, luminal narrowing, mural hyper-enhancement, and increased length disease on cross-sectional enterography were all associated with endoscopic POR.5,7,8,15 Recently, the MONITOR index has been developed and validated in predicting endoscopic POR.16 The imaging features incorporated in the MONITOR index, including wall thickening, length of disease, and hyperenhancement, were found to be significantly associated with endoscopic POR in our study cohort among patients who underwent MRE. Additionally, we observed similar test characteristics and presence of other imaging features, such as upstream dilation and luminal narrowing, as described in the MONITOR index development and validation cohorts. However, in our cohort, 67.4% of patients without evidence of endoscopic POR had active radiologic disease. This phenomenon has previously been described in the nonoperative patient population with CD and can be a result of either intramural and mesenteric ileal disease or endoscopic skipping of the distal TI entirely.8,9,17,18 The prevalence of endoscopic skipping of the TI in nonoperative CD has varied significantly from 8% to 56%.9,17,18 In smaller cohort studies, patients exhibiting endoscopic skipping of the TI have had more aggressive CD phenotypes; however, in our cohort, clinical and demographic history was not associated with concordance.17 Furthermore, we had low rates of radiographic skipping of the distal neoTI, suggesting this TI-skipping phenomenon is not as common in postoperative CD recurrence. Thus, certain radiographic features may differentiate those with endoscopic recurrence and clinical documentation should describe these features when present.
Cross-sectional imaging in nonoperative patients with CD has shown to be highly accurate in detecting and monitoring CD activity.7,16,19 In the current study, patients with radiographic activity in the absence of endoscopic recurrence that underwent subsequent ileocolonoscopies demonstrated nearly double the rate of endoscopic and surgical recurrence compared to those with no radiographic or endoscopic disease. These differences further highlight the potential risk of radiographic activity in this postoperative CD population. Although we saw a near doubling in rates of endoscopic and surgical recurrence, these differences were not statistically significant over the entire follow-up period, possibly due to a smaller sample size. However, patients with radiographic activity in the absence of endoscopic recurrence had faster time to subsequent endoscopic POR. This may be due to transmural and mesenteric disease activity that is observed on radiology, but absent on endoscopy. Furthermore, initiation of biologic therapy was associated with lower likelihood of progress, suggesting a protective effect. Our findings were similar in comparison to a previous study in the nonoperative patient population with CD that showed that 67% of patients with imaging findings of inflammation with negative ileocolonoscopy and ileal biopsy had subsequent confirmation of disease progression.9 To our knowledge, this is the first study comparing the relative risk of patients with discordant radiographic-endoscopic findings to patients in endoscopic and radiologic remission. Thus, radiographic activity in the absence of criteria for endoscopic recurrence may identify an at-risk population that may benefit from therapeutic intervention and close monitoring.
Several limitations to this study exist. Limited sample size of subgroups may have resulted in an inability to detect differences in prevalence or time to subsequent endoscopic or surgical recurrence. However, this study serves as one of the largest retrospective cohort studies evaluating the prevalence of radiologic postoperative recurrence of CD in patients with negative surveillance ileocolonoscopies. Additionally, by not limiting the study to only patients with discordant findings, we were able to contribute to the sparse literature of radiologic features associated with endoscopic POR and severity. We did not limit to index postoperative ileocolonoscopy or imaging study when capturing pairs, thus the test characteristics of cross-sectional imaging to detect early postoperative findings is unknown. All studies were clinically indicated, and thus individuals undergoing concurrent endoscopy and radiography may differ from those receiving only 1 surveillance modality, and timing of subsequent studies may be influenced. Additionally, we did not collect biopsy and histologic activity data, which may be better in detecting activity than endoscopic data.20 Another limitation was the application of retrospective RS, which can be limited by quality and quantity of images captured. To mitigate this limitation, scorers underwent training and high accuracy validation prior to data collection. There is discrepancy in the literature in using i2a vs i2b to define endoscopic POR.14,21 To alleviate any outcome bias, we performed sensitivity analyses using i1 and i2a disease as our definition of POR, which showed robustness of our outcomes. In our study, we only included cross-sectional enterography due to enhanced CD detection with these modalities, but this limits generalizability to other imaging protocols. Additionally, the 2 radiologists did not use a published imaging-based assessment of CD activity, as these largely exist for MRE and the present study is primarily composed of CTE studies.16 Another limitation of the study is that expert radiology review was utilized; limiting the findings’ generalizability. Lastly, this study was conducted at 2 large academic institutions, which may limit the study’s generalizability and was retrospective in nature, subject to the typical limitations of this study design.
Conclusions
Cross-sectional enterography is highly sensitive in detecting endoscopic POR. Radiologic features of increased severity of disease correlate well with increased endoscopic severity. Patients with radiologic active disease, in the absence of endoscopic ileal disease, may be at increased risk for future endoscopic recurrence and may benefit from therapeutic intervention and close monitoring though confirmatory studies are needed. Cross-sectional imaging plays a key adjunctive role in monitoring and surveillance of this at-risk postoperative population.
Supplementary Material
Supplementary Table 1. Test Characteristics of Cross-sectional Imaging in Detecting Endoscopic POR
Supplementary Table 2. Cross-sectional Imaging Features at Time of Matched Endoscopy Imaging By Concordance Group
Supplemental Table 3. Summaries of Radiologic Features at Time of Paired Imaging on Future Endoscopic Recurrence Among Patients With E−/R+
Supplemental Table 4. Subsequent Endoscopic and Surgical POR Following Matched Studies in E−/R+ and E−/R− Patients
Supplemental Table 5. Sensitivity Analysis of Test Characteristics Between Radiology Activity and Endoscopic POR Defined as Rutgeerts’ Score ≥i2a
Supplemental Table 6. Sensitivity Analysis Between Radiology Activity and Endoscopic Inflammation Defined as Rutgeerts’ Score ≥i1
Supplementary Figure 2. Kaplan-Meier survival analysis of time to endoscopic POR or surgical recurrence between E−/R+ and E−/R4 patients with endoscopic POR defined as RS ≥i2a.
Supplementary Figure 1. Kaplan-Meier survival analysis of time to surgical recurrence between E−/R+ and E−/R− patients with endoscopic POR defined as RS ≥i2b.
What You Need to Know.
Background
The use of cross-sectional enterography in postoperative Crohn’s disease surveillance is unclear. We evaluated the concordance of radiologic activity with endoscopic recurrence and the predictive ability of imaging for future Crohn’s disease postoperative recurrence.
Findings
Cross-sectional enterography was accurate and highly sensitive in detecting endoscopic postoperative recurrence. Patients with radiologic activity in the absence of endoscopic recurrence were at increased risk for future endoscopic postoperative recurrence.
Implications for patient care
Clinicians may consider utilizing cross-sectional enterography following negative surveillance ileocolonoscopy in select patient populations.
Funding
This work was funded in part by a Cleveland Clinic Lerner Research Institute Research Program Committee grant.
Abbreviations used in this paper:
- CD
Crohn’s disease
- CTE
computed tomography enterography
- E+
endoscopic postoperative recurrence
- E−
no endoscopic postoperative recurrence
- ICR
ileocecal resection
- MRE
magnetic resonance enterography
- neoTI
neoterminal ileum
- POR
postoperative recurrence
- R+
radiologic active disease
- R−
radiologic inactive disease
- RS
Rutgeerts’ score
- TI
terminal ileum
Footnotes
Supplementary Material
Note: To access the supplementary material accompanying this article, visit the online version of Clinical Gastroenterology and Hepatology at www.cghjournal.org, and at http://doi.org/10.1016/j.cgh.2021.12.033.
CRediT Authorship Contributions
Salam P. Bachour (Conceptualization: Equal; Data curation: Lead; Formal analysis: Equal; Methodology: Supporting; Writing – original draft: Lead; Writing – review & editing: Equal)
Ravi S. Shah (Data curation: Equal; Writing – original draft: Supporting; Writing – review & editing: Supporting)
Ruishen Lyu (Formal analysis: Lead; Visualization: Lead)
Takahiro Nakamura (Data curation: Supporting; Writing – review & editing: Supporting)
Michael Shen (Data curation: Supporting; Writing – review & editing: Supporting)
Terry Li (Data curation: Supporting; Writing – review & editing: Supporting)
Bari Dane (Data curation: Equal; Methodology: Equal; Writing – review & editing: Equal)
Edward L. Barnes (Writing – review & editing: Equal)
Florian Rieder (Methodology: Supporting; Writing – review & editing: Equal)
Benjamin Cohen (Methodology: Supporting; Writing – review & editing: Equal)
Taha Qazi (Methodology: Supporting; Writing – review & editing: Equal)
Bret Lashner (Methodology: Supporting; Writing – review & editing: Equal)
Jean Paul Achkar (Writing – review & editing: Equal)
Jessica Philpott (Writing – review & editing: Equal)
Stefan D. Holubar (Writing – review & editing: Equal)
Amy L. Lightner (Methodology: Supporting; Writing – review & editing: Equal)
Miguel Regueiro (Conceptualization: Supporting; Methodology: Supporting; Writing – review & editing: Equal)
Jordan Axelrad (Conceptualization: Equal; Investigation: Lead; Methodology: Equal; Writing – review & editing: Equal)
Mark E. Baker (Conceptualization: Lead; Data curation: Equal; Methodology: Lead; Writing – review & editing: Equal)
Benjamin Click (Conceptualization: Lead; Investigation: Lead; Methodology: Lead; Project administration: Lead; Supervision: Lead; Writing – original draft: Equal; Writing – review & editing: Equal)
Conflicts of interest
These authors disclose the following conflicts directly relevant to the data presented: Mark Baker receives support from Siemens Healthineers investigating the effect of lower exposure CTE in detecting and characterizing Crohn’s disease. The remaining authors disclose no conflicts.
References
- 1.Frolkis AD, Dykeman J, Negrón ME, et al. Risk of surgery for inflammatory bowel diseases has decreased over time: a systematic review and meta-analysis of population-based studies. Gastroenterology 2013;145:996–1006. [DOI] [PubMed] [Google Scholar]
- 2.Cunningham MF, Docherty NG, Coffey JC, et al. Postsurgical recurrence of ileal Crohn’s disease: an update on risk factors and intervention points to a central role for impaired host-microflora homeostasis. World J Surg 2010;34:1615–1626. [DOI] [PubMed] [Google Scholar]
- 3.Rutgeerts P, Geboes K, Vantrappen G, et al. Predictability of the postoperative course of Crohn’s disease. Gastroenterology 1990;99:956–963. [DOI] [PubMed] [Google Scholar]
- 4.Regueiro M, Velayos F, Greer JB, et al. American Gastroenterological Association Institute Technical Review on the management of Crohn’s disease after surgical resection. Gastroenterology 2017;152:277–295.e3. [DOI] [PubMed] [Google Scholar]
- 5.Qiu Y, Mao R, Chen B-L, et al. Systematic review with metaanalysis: magnetic resonance enterography vs. computed tomography enterography for evaluating disease activity in small bowel Crohn’s disease. Aliment Pharmacol Ther 2014;40:134–146. [DOI] [PubMed] [Google Scholar]
- 6.Kim SH. Computed tomography enterography and magnetic resonance enterography in the diagnosis of Crohn’s disease. Intest Res 2015;13:27–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deepak P, Fletcher JG, Fidler JL, et al. Computed tomography and magnetic resonance enterography in Crohn’s disease: assessment of radiologic criteria and endpoints for clinical practice and trials. Inflamm Bowel Dis 2016;22:2280–2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bruining DH, Zimmermann EM, Loftus EV, et al. Society of Abdominal Radiology Crohn’s Disease-Focused Panel. Consensus recommendations for evaluation, interpretation, and utilization of computed tomography and magnetic resonance enterography in patients with small bowel Crohn’s disease. Radiology 2018;286:776–799. [DOI] [PubMed] [Google Scholar]
- 9.Nehra AK, Sheedy SP, Wells ML, et al. Imaging findings of ileal inflammation at computed tomography and magnetic resonance enterography: what do they mean when ileoscopy and biopsy are negative? J Crohns Colitis 2020;14:455–464. [DOI] [PubMed] [Google Scholar]
- 10.Mao R, Gao X, Zhu Z, et al. CT enterography in evaluating postoperative recurrence of Crohn’s disease after ileocolic resectioncomplementary role to endoscopy. Inflamm Bowel Dis 2013;19:977–982. [DOI] [PubMed] [Google Scholar]
- 11.Choi IY, Park SH, Park SH, et al. CT enterography for surveillance of anastomotic recurrence within 12 months of bowel resection in patients with Crohn’s disease: an observational study using an 8-year registry. Korean J Radiol 2017; 18:906–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Soyer P, Boudiaf M, Sirol M, et al. Suspected anastomotic recurrence of Crohn disease after ileocolic resection: evaluation with CT enteroclysis. Radiology 2010;254:755–764. [DOI] [PubMed] [Google Scholar]
- 13.Paparo F, Revelli M, Puppo C, et al. Crohn’s disease recurrence in patients with ileocolic anastomosis: value of computed tomography enterography with water enema. Eur J Radiol 2013; 82:e434–e440. [DOI] [PubMed] [Google Scholar]
- 14.Ollech JE, Aharoni-Golan M, Weisshof R, et al. Differential risk of disease progression between isolated anastomotic ulcers and mild ileal recurrence after ileocolonic resection in patients with Crohn’s disease. Gastrointest Endosc 2019; 90:269–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bodily KD, Fletcher JG, Solem CA, et al. Crohn disease: mural attenuation and thickness at contrast-enhanced CT enterography—correlation with endoscopic and histologic findings of inflammation. Radiology 2006;238:505–516. [DOI] [PubMed] [Google Scholar]
- 16.Schaefer M, Laurent V, Grandmougin A, et al. A magnetic resonance imaging index to predict Crohn’s disease postoperative recurrence: the MONITOR index. Clin Gastroenterol Hepatol 2021. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- 17.Samuel S, Bruining DH, Loftus EV, et al. Endoscopic skipping of the distal terminal ileum in Crohn’s disease can lead to negative results from ileocolonoscopy. Clin Gastroenterol Hepatol 2012; 10:1253–1259. [DOI] [PubMed] [Google Scholar]
- 18.Siddiki HA, Fidler JL, Fletcher JG, et al. Prospective comparison of state-of-the-art MR enterography and CT enterography in small-bowel Crohn’s disease. Am J Roentgenol 2009;193:113–121. [DOI] [PubMed] [Google Scholar]
- 19.Hara AK, Alam S, Heigh RI, et al. Using CT enterography to monitor Crohn’s disease activity: a preliminary study. Am J Roentgenol 2008;190:1512–1516. [DOI] [PubMed] [Google Scholar]
- 20.Christensen B, Erlich J, Gibson PR, et al. Histologic healing is more strongly associated with clinical outcomes in ileal Crohn’s disease than endoscopic healing. Clin Gastroenterol Hepatol 2020;18:2518–2525.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hirten RP, Ungaro RC, Castaneda D, et al. Anastomotic ulcers after ileocolic resection for Crohn’s disease are common and predict recurrence. Inflamm Bowel Dis 2020;26:1050–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1. Test Characteristics of Cross-sectional Imaging in Detecting Endoscopic POR
Supplementary Table 2. Cross-sectional Imaging Features at Time of Matched Endoscopy Imaging By Concordance Group
Supplemental Table 3. Summaries of Radiologic Features at Time of Paired Imaging on Future Endoscopic Recurrence Among Patients With E−/R+
Supplemental Table 4. Subsequent Endoscopic and Surgical POR Following Matched Studies in E−/R+ and E−/R− Patients
Supplemental Table 5. Sensitivity Analysis of Test Characteristics Between Radiology Activity and Endoscopic POR Defined as Rutgeerts’ Score ≥i2a
Supplemental Table 6. Sensitivity Analysis Between Radiology Activity and Endoscopic Inflammation Defined as Rutgeerts’ Score ≥i1
Supplementary Figure 2. Kaplan-Meier survival analysis of time to endoscopic POR or surgical recurrence between E−/R+ and E−/R4 patients with endoscopic POR defined as RS ≥i2a.
Supplementary Figure 1. Kaplan-Meier survival analysis of time to surgical recurrence between E−/R+ and E−/R− patients with endoscopic POR defined as RS ≥i2b.


