Abstract
Purpose: By analyzing the clinical characteristics, etiological characteristics and commonly used antibiotics of patients with ventilator-associated pneumonia (VAP) in intensive care units (ICUs) in the intensive care database. This study aims to provide guidance information for the clinical rational use of drugs for patients with VAP.
Method: Patients with VAP information were collected from the Medical Information Mart for Intensive Care IV (MIMIC-IV) database, including their sociodemographic characteristics, vital signs, laboratory measurements, complications, microbiology, and antibiotic use. After data processing, the characteristics of the medications used by patients with VAP in ICUs were described using statistical graphs and tables, and experiences were summarized and the reasons were analyzed.
Results: This study included 2,068 patients with VAP. Forty-eight patient characteristics, including demographic indicators, vital signs, biochemical indicators, scores, and comorbidities, were compared between the survival and death groups of VAP patients. Cephalosporins and vancomycin were the most commonly used. Among them, fourth-generation cephalosporin (ForGC) combined with vancomycin was used the most, by 540 patients. First-generati49n cephalosporin (FirGC) combined with vancomycin was associated with the highest survival rate (86.7%). More than 55% of patients were infected with Gram-negative bacteria. However, patients with VAP had fewer resistant strains (<25%). FirGC or ForGC combined with vancomycin had many inflammation-related features that differed significantly from those in patients who did not receive medication.
Conclusion: Understanding antibiotic use, pathogenic bacteria compositions, and the drug resistance rates of patients with VAP can help prevent the occurrence of diseases, contain infections as soon as possible, and promote the recovery of patients.
Keywords: ventilator-associated pneumonia, antibiotics, pathogenic bacteria, MIMIC-IV database, resistance rate
Introduction
Ventilator-associated pneumonia (VAP) is a common hospital-acquired infection and is the most common complication of mechanical ventilation for patients in intensive care units (ICUs) (Legras et al., 1998; Urli et al., 2002). Once VAP presents, it further aggravates the condition and seriously harms the prognosis of the patient. The VAP incidence in ICUs has been reported to range from 5.0% to as high as 67%, and the mortality rate of patients with VAP exceeds 14% (Kalil et al., 2016; Pozuelo-Carrascosa et al., 2018). The general hospitalization time of patients with VAP is also longer, as is the time required for intensive care, which also increases the economic burden on both patients and the medical system (Kollef et al., 2012; Vandana Kalwaje and Rello, 2018). In order to avoid the increased incidence of diseases and the consumption of more medical resources caused by subjective deviation in clinical diagnosis or treatment, it is very important to formulate more accurate and more detailed clinical diagnosis and treatment specifications. The particular focus of recent clinical research has been on how to apply more appropriate drug interventions to patients with VAP in a timely and accurate manner.
The most commonly used drugs for clinically treating patients diagnosed with VAP are antibiotics, especially cephalosporins and vancomycin (Tseng et al., 2012; Yue et al., 2015; Torres et al., 2017). However, medical staff often prioritize antibacterial treatment for patients based on their personal experience. Improving the rationality, safety, and effectiveness of clinical medications, and converting empirical treatment into targeted treatments as soon as possible, will help medical staff to determine more objective medical treatments for patients with VAP.
To provide a reference for guiding rational clinical drug use in patients with VAP, this study analyzed patients with VAP in ICUs from an intensive care medical database using factors such as clinical characteristics, pathogenic bacteria distribution in respiratory secretions and their drug resistance characteristics, and changes in inflammatory response indicators.
Materials and Methods
Data Source
The Medical Information Mart for Intensive Care (MIMIC) database was funded in 2003 by Beth Israel Deaconess Medical Center (BIDMC), Massachusetts Institute of Health, and the National Institutes of Health Technology, Massachusetts General Hospital, emergency room physicians, critical care physicians, computer science experts, and other professional critical care medicine database. MIMIC is the largest open source and free clinical database in the critical care and emergency department, based on BIDMC’s intensive care inpatient system. MIMIC-IV (version 1.0) is the latest version, which contains data from 2008 to 2019 (Guo et al., 2021; Lu et al., 2021; Song et al., 2021). We completed the courses required to use the database and obtained the corresponding certificate. The requirement for individual patient consent was waived because the project did not impact clinical care and all protected health information was anonymized.
Study Population and Data Extraction
We used the official MIMIC-IV tutorial to construct the study database using PostgreSQL (version 13.0, PostgreSQL Global Development Group). Structured Query Language was used to extract the data of the patients, which included sociodemographic characteristics, vital signs, laboratory measurements, complications, and microbiology and antibiotic use information (Yang et al., 2020; Wu et al., 2021). ICD-9 codes (4957 and 99,731) and ICD-10 code (J95851) were used to identify patients with VAP in the MIMIC-IV database. Data from the first hospital admission (if a patient had been admitted multiple times) was included in the study. If the data of a patient were measured multiple times, we used the first measurement. The survival rates in this article refer to in-hospital survival rates. The patient enrollment process of this study is illustrated in Figure 1.
FIGURE 1.
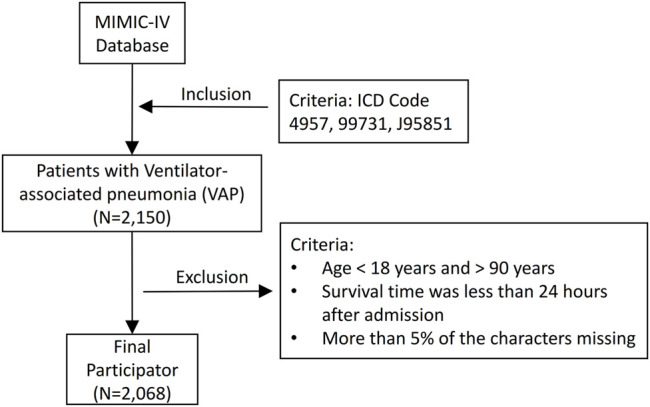
Flowchart of patients’ inclusion and exclusion.
Statistical Analysis
Univariate analyses were applied to all of the study variables. Shapiro-Wilk tests were used to assess the distribution of variables. Non-normally distributed continuous variables were reported as medians with interquartile ranges (IQRs), and the Kruskal–Wallis rank-sum test or the Mann-Whitney U test were used to compare these data. All classified variables were expressed as numbers and percentages, and they were compared using chi-square or Fisher’s exact tests. Values in continuous variables that exceed 1.5 times the upper and lower quartiles are judged as outliers and the outliers are deleted. The Numpy (version 1.19.5) and Pandas (version 1.1.5) packages were used for data computation. Supplementary Figure S1 shows which variables were absent. The “miceforest” (version 2.0.5) package of Python was used for the multiple interpolation of missing values (Zhao et al., 2021).
Statistical plots (include column charts, line charts, Venn diagrams, correlation charts, etc.) were used to present data distributions, and statistical tables are used to describe differences between patients. Seaborn (version 0.11.2) and Matplotlib (version 3.3.4) packages were used to graphically display data. Python software (version 3.7.0) was used for all statistical analyses. A probability value of p < 0.05 in two-sided tests was considered significant.
Results
This study included 2,068 patients with VAP (Table 1), among whom 1,608 (77.8%) survived and 460 (22.2%) died. The patients were aged 64.0 years (53.0–74.0 years) [median (IQR)], and the survival group was younger than the death group [63.0 years (51.0–73.0 years) vs. 69.0 years (59.0–79.0 years)]. The patients had a BMI of 29.0 kg/m2 (25.1–34.3 kg/m2), which was lower in the death group than in the survival group [28.5 kg/m2 (24.1–33.3 kg/m2) vs. 29.1 kg/m2 (25.4–34.6 kg/m2)]. The first admission reason of thoracic surgery was the least common, accounting for only 2.4% of cases. VAP presented in different ICUs, most commonly in medical/surgical ICUs (55.1%). Patients admitted through emergency departments accounted for 58.7% of cases. Except for diabetes, the other eight complications showed statistically significant differences between survival group and death group (Supplementary Table S1).
TABLE 1.
Basic characteristics of patients with VAP.
| Characteristics | Overall (N = 2068) | Alive (N = 1608) | Death (N = 460) | p value |
|---|---|---|---|---|
| Age, years | 64.0 (53.0, 74.0) | 63.0 (51.0, 73.0) | 69.0 (59.0, 79.0) | <0.001 |
| Sex, n | 0.490 | |||
| Female | 783 (37.9) | 602 (37.4) | 181 (39.3) | |
| Male | 1285 (62.1) | 1006 (62.6) | 279 (60.7) | |
| Race, n | <0.001 | |||
| Asian | 62 (3.0) | 38 (2.4) | 24 (5.2) | |
| White | 1216 (58.8) | 963 (59.9) | 253 (55.0) | |
| Hispanic | 65 (3.1) | 53 (3.3) | 12 (2.6) | |
| Black | 247 (11.9) | 207 (12.9) | 40 (8.7) | |
| Other | 478 (23.1) | 347 (21.6) | 131 (28.5) | |
| Frist Service, n | 0.561 | |||
| Medical | 906 (43.8) | 704 (43.8) | 202 (43.9) | |
| Surgical | 139 (6.7) | 107 (6.7) | 32 (7.0) | |
| Thoracic Surgical | 50 (2.4) | 43 (2.7) | 7 (1.5) | |
| Other | 973 (47.1) | 754 (46.9) | 219 (47.6) | |
| First Care Unit, n | <0.001 | |||
| CCU/CVICU | 416 (20.1) | 301 (18.7) | 115 (25.0) | |
| MICU/SICU | 1139 (55.1) | 880 (54.7) | 259 (56.3) | |
| NSICU | 103 (5.0) | 77 (4.8) | 26 (5.7) | |
| TSICU | 410 (19.8) | 350 (21.8) | 60 (13.0) | |
| Admission Type, n | 0.335 | |||
| Elective | 44 (2.1) | 31 (1.9) | 13 (2.8) | |
| Urgent | 505 (24.4) | 389 (24.2) | 116 (25.2) | |
| Emergency | 1214 (58.7) | 941 (58.5) | 273 (59.3) | |
| Other | 305 (14.7) | 247 (15.4) | 58 (12.6) | |
| BMI, kg/m2 | 29.0 (25.1, 34.3) | 29.1 (25.4, 34.6) | 28.5 (24.1, 33.3) | 0.004 |
| Heart Rate, bpm | 90.0 (76.0, 106.0) | 90.0 (76.0, 106.0) | 91.5 (77.0, 108.0) | 0.174 |
| Systolic Blood Pressure, mmHg | 121.0 (103.0, 139.0) | 121.0 (104.0, 139.0) | 119.0 (101.8, 138.0) | 0.086 |
| Respiratory Rate, insp/min | 20.0 (16.0, 24.0) | 20.0 (16.0, 24.0) | 20.0 (16.0, 24.0) | 0.250 |
| Temperature Fahrenheit, °F | 98.4 (97.8, 99.0) | 98.4 (97.8, 99.1) | 98.3 (97.7, 98.8) | <0.001 |
| Arterial O2 pressure, mmHg | 114.0 (82.0, 185.0) | 113.0 (82.0, 187.0) | 115.0 (83.8, 182.2) | 0.983 |
| Arterial CO2 Pressure, mmHg | 41.0 (35.0, 48.0) | 41.0 (35.0, 48.0) | 39.0 (33.0, 46.0) | <0.001 |
| Creatinine, mg/dl | 1.0 (0.7, 1.5) | 1.0 (0.7, 1.4) | 1.2 (0.8, 1.8) | <0.001 |
| Characteristics | Overall (N = 2068) | Alive (N = 1608) | Death (N = 460) | p value |
|---|---|---|---|---|
| Urea Nitrogen, mg/dl | 21.0 (14.0, 33.0) | 20.0 (13.0, 31.0) | 26.0 (17.0, 40.0) | <0.001 |
| Glucose, mg/dl | 134.0 (109.0, 170.0) | 134.0 (108.0, 169.0) | 136.0 (111.0, 172.0) | 0.415 |
| RDW, % | 14.9 (13.7, 16.6) | 14.7 (13.6, 16.4) | 15.3 (13.9, 17.2) | <0.001 |
| RBC, m/µl | 3.4 (2.9, 4.0) | 3.4 (2.9, 4.1) | 3.3 (2.8, 3.9) | 0.003 |
| WBC, K/µl | 11.7 (8.4, 15.7) | 11.6 (8.4, 15.4) | 12.1 (8.5, 16.9) | 0.102 |
| Lymphocytes, % | 9.4 (5.9, 14.3) | 9.8 (6.1, 14.9) | 8.5 (5.0, 12.0) | <0.001 |
| Monocytes, % | 5.5 (3.7, 7.8) | 5.5 (3.7, 7.8) | 5.3 (3.2, 7.7) | 0.048 |
| Neutrophils, % | 81.0 (74.1, 86.2) | 80.3 (73.9, 86.0) | 82.8 (76.0, 87.1) | 0.001 |
| Basophils, % | 0.3 (0.2, 0.4) | 0.3 (0.2, 0.4) | 0.2 (0.2, 0.4) | 0.001 |
| Eosinophils, % | 1.0 (0.4, 2.0) | 1.0 (0.4, 2.2) | 1.0 (0.4, 2.0) | 0.041 |
| pH, units | 7.4 (7.3, 7.4) | 7.4 (7.3, 7.4) | 7.4 (7.3, 7.4) | 0.609 |
| Lactate, mmol/L | 1.6 (1.1, 2.4) | 1.5 (1.1, 2.4) | 1.7 (1.3, 2.5) | <0.001 |
| Anion Gap, mEq/L | 14.0 (12.0, 17.0) | 14.0 (12.0, 17.0) | 15.0 (13.0, 18.0) | 0.001 |
| ALT, IU/L | 27.0 (16.0, 45.0) | 27.0 (17.0, 44.0) | 28.0 (16.0, 46.0) | 0.842 |
| ALP, IU/L | 82.0 (59.0, 111.0) | 80.5 (58.8, 110.0) | 85.0 (61.0, 116.0) | 0.023 |
| AST, IU/L | 39.0 (25.0, 66.0) | 38.0 (25.0, 65.0) | 43.0 (27.0, 76.0) | 0.007 |
| LDH, IU/L | 287.5 (219.0, 406.0) | 283.0 (215.0, 400.0) | 314.0 (231.0, 453.2) | <0.001 |
| Albumin, g/dl | 2.9 (2.5, 3.3) | 2.9 (2.6, 3.4) | 2.8 (2.4, 3.3) | <0.001 |
| Hemoglobin, g/dl | 10.2 (8.6, 12.0) | 10.3 (8.7, 12.1) | 10.0 (8.4, 11.7) | 0.017 |
| Platelet Count, K/µl | 196.0 (136.8, 261.0) | 198.5 (140.0, 264.0) | 182.5 (121.8, 250.0) | 0.003 |
| INR | 1.2 (1.1, 1.4) | 1.2 (1.1, 1.4) | 1.3 (1.2, 1.5) | <0.001 |
| PT, sec | 13.7 (12.4, 15.7) | 13.6 (12.4, 15.5) | 14.3 (12.8, 16.3) | <0.001 |
| PTT, sec | 30.5 (27.1, 36.1) | 30.2 (26.9, 35.7) | 31.5 (27.5, 37.4) | <0.001 |
| SOFA Score | 1.0 (0.0, 4.0) | 1.0 (0.0, 3.0) | 2.0 (1.0, 5.0) | <0.001 |
| Respiratory Secretions, n | 0.522 | |||
| Positive | 987 (47.7) | 774 (48.1) | 213 (46.3) | |
| Negative | 1081 (52.3) | 834 (51.9) | 247 (53.7) |
Nonnormal continuous variables were presented as Median (IQR). Categorical variables were presented as number (precentage %). CCU, coronary care unit; CVICU, cardiac vascular intensive care unit; MICU, medical intensive care unit; SICU, surgical intensive care unit; NSICU, neuro surgical intensive care unit; TSICU, traume surgical intensive care unit; BMI, body mass index; RDW, red blood cell volume istribution width; RBC, red blood count; WBC, write blood count; pH, hydrogen ion concentration; ATL, alanine aminotransferase; ALP, alkaline phosphatase; AST, aspartate transaminase; LDH, lactate dehydrogenase; PT, prothrombin time; PTT, partial thromboplastin time; INR, international normalized ratio; SOFA, score, Sequential Organ Failure Assessment.
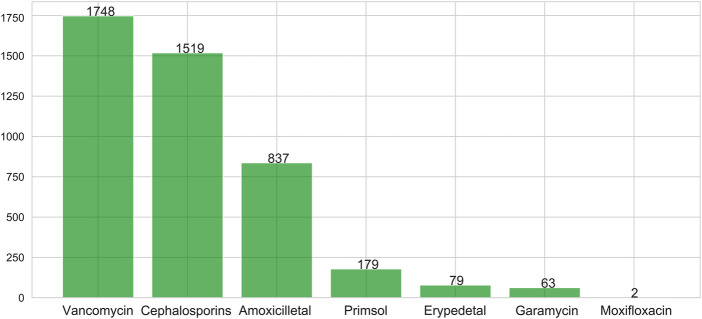
Statistics of the application of antibiotics in all patients with VAP are shown in Figure 2. The most common used was vancomycin, followed by cephalosporins. Eight patients had used the fifth generation of cephalosporin, which was too few to be included in the analyses. In the subsequent analysis, antibiotic use therefore referred to using the first through to the fourth generation of cephalosporins and vancomycin in patients with VAP.
FIGURE 2.
Antibiotic used in patients with VAP.
Figure 3 shows how antibiotics were used and the corresponding number of people. The largest number of patients used two antibiotics in combination (n = 776), while the smallest number of patients used all five antibiotics (n = 9).
FIGURE 3.
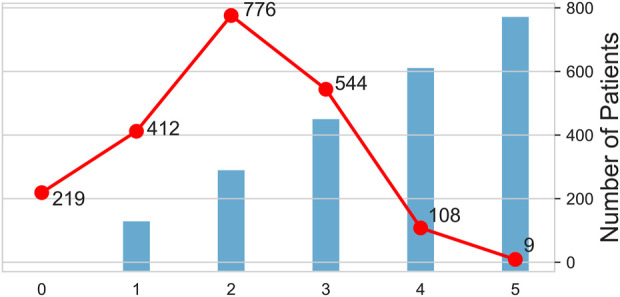
Pattern and number of antibiotics used. The columns show the number of antibiotics used, and the broken lines shows the number of people taking drugs. The horizontal axis shows the number of antibiotics used. 0 indicates that no antibacterial drugs have been used, one indicates that only one antibacterial drug has been used, and two indicates the combination of two antibacterial drugs, and so on for 3, 4, and 5.
Supplementary Figure S2 shows the use of different antibiotics and their combinations in patients with VAP. The most common combination was using vancomycin and fourth-generation cephalosporin (ForGC) (n = 540). Vancomycin alone was used by 336 patients, while relatively few patients used cephalosporin antibiotics alone.
Supplementary Figure S3 shows the correlations between different antibiotics. The medical staff in the data source center most commonly used ForGC and vancomycin in combination, followed by first-generation cephalosporin (FirGC) and vancomycin.
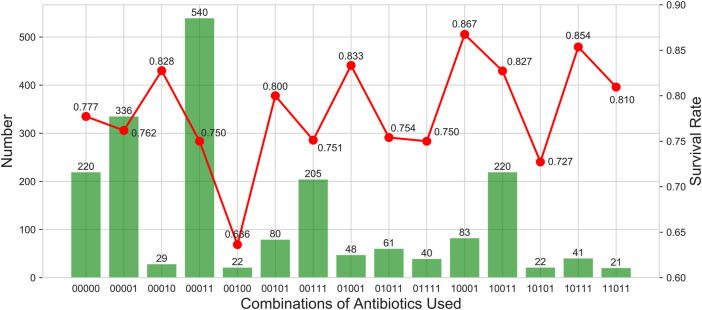
We further counted the use of antibiotics, the number of people, and survival rates (Figure 4). The results indicated that ForGC combined with vancomycin (ForGC + Van) was the most common. Patients who used FirGC combined with vancomycin (FirGC + Van) had the highest survival rate (86.7%).
FIGURE 4.
Describe the use of antibiotics, the number of people and survival rates. The columns show the number of people who took drugs. The broken line shows the survival rate. The horizontal coordinate is a 5-digit sequence from left to right representing the first to fourth generations of cephalosporins and vancomycin. 0 indicates that it is not used, and one indicates that it is used.
To explore the reasons behind medical staff using antibiotics, we compared the differences between FirGC + Van or ForGC + Van and no antibiotics (Table 2). This revealed that only four inflammation indicators of patients who used FirGC + Van differed from those in the no-antibiotics group, and that all inflammatory indicators of patients who used ForGC + Van differed from those in the no-antibiotics group.
TABLE 2.
Comparison of differences in inflammatory related characteristics. (a) FirGC + Vancomycin vs. Unused antibiotics; (b) ForGC + Vancomycin vs. Unused antibiotics.
| (a) FirGC + Vancocin vs. Unused antibiotics | |||
|---|---|---|---|
| Inflammation Characteristics | Unused (N = 220) | FirGC + Vancomycin (N = 83) | p value |
| Temperature Fahrenheit, °F | 98.3 (97.8,98.8) | 98.3 (97.8,98.8) | 0.468 |
| Basophils, % | 0.3 (0.2,0.5) | 0.3 (0.2,0.4) | 0.064 |
| Eosinophils, % | 1.6 (0.8,2.7) | 1.0 (0.3,2.0) | 0.002 |
| Lymphocytes, % | 10.4 (7.0,16.3) | 10.8 (7.4,14.2) | 0.578 |
| Neutrophils, % | 79.5 (71.9,84.8) | 81.6 (75.8,85.8) | 0.019 |
| RBC, m/ul | 3.3 (2.8,3.7) | 3.3 (2.7,3.9) | 0.646 |
| WBC, K/ul | 10.1 (7.3,13.8) | 12.3 (9.1,15.5) | 0.018 |
| RDW, % | 15.4 (14.0,17.0) | 14.4 (13.6,15.4) | <0.001 |
| (b) ForGC + Vancocin vs. Unused antibiotics | |||
| Inflammation Characteristics | Unused (N = 220) | ForGC + Vancomycin (N = 540) | p Value |
| Temperature Fahrenheit, °F | 98.3 (97.8,98.8) | 98.4 (97.7,99.1) | 0.018 |
| Basophils, % | 0.3 (0.2,0.5) | 0.3 (0.2,0.4) | 0.003 |
| Eosinophils, % | 1.6 (0.8,2.7) | 1.0 (0.4,2.0) | <0.001 |
| Lymphocytes, % | 10.4 (7.0,16.3) | 8.9 (5.0,13.3) | <0.001 |
| Neutrophils, % | 79.5 (71.9,84.8) | 81.3 (75.4,87.0) | <0.001 |
| RBC, m/ul | 3.3 (2.8,3.7) | 3.4 (2.9,4.1) | 0.002 |
| WBC, K/ul | 10.1 (7.3,13.8) | 12.3 (8.5,16.3) | <0.001 |
| RDW, % | 15.4 (14.0,17.0) | 15.0 (13.7,16.6) | 0.026 |
Table 3 lists the distributions of pathogenic bacteria in respiratory tract specimens of patients with VAP treated using FirGC + Van. For FirGC + Van treatment, 428 pathogens were detected among 83 patients with VAP. These comprised 254 strains (59.35%) of Gram-negative bacteria, 155 strains (36.21%) of Gram-positive bacteria, and 19 strains (4.44%) of fungi. Table 4 lists the resistance characteristics of these pathogens. The resistance rate to cephalosporin or vancomycin in respiratory tract specimens of patients with VAP treated using FirGC + Van was 0%.
TABLE 3.
Types and composition ratio of main pathogenic bacteria in respiratory tract specimens of patients with VAP treated with first-generation of cephalosporin combined with vancomycin.
| Pathogenic bacteria | Number of strains (n) | Constituent ratio (%) |
|---|---|---|
| Fungus | 19 | 4.44 |
| Yeast | 18 | 4.21 |
| Mold | 1 | 0.23 |
| Gram Positive Bacteria | 155 | 36.21 |
| Staph Aureus Coag + | 147 | 34.35 |
| Streptococcus pneumoniae | 6 | 1.40 |
| Other | 2 | 0.47 |
| Gram Negative Bacteria | 254 | 59.35 |
| Enterobacteriaceae | 138 | 32.24 |
| Pseudomonas aeruginosa | 49 | 11.45 |
| Serratia marcescens | 42 | 9.81 |
| Klebsiella pneumoniae | 12 | 2.80 |
| Other | 13 | 3.05 |
| Total | 428 | 100.00 |
TABLE 4.
Resistance of main Gram bacteria to different antibacterial drugs in respiratory tract specimens of patients with VAP treated with first-generation cephalosporin combined with vancomycin.
| Antimicrobials | Gram positive bacteria | Gram Negative bacteria | ||||
|---|---|---|---|---|---|---|
| Staph aureus coag + (N = 147) | Streptococcus pneumoniae (N = 6) | Pseudomonas aeruginosa (N = 49) | ||||
| Number of resistant strains (n) | Resistant ratio (%) | Number of resistant strains (n) | Resistant ratio (%) | Number of resistant strains (n) | Resistant ratio (%) | |
| Clidamycin | 4 | 2.72 | — | — | ||
| Erythromycin | 7 | 4.76 | 1 | 16.67 | — | |
| Levofloxacin | 4 | 2.72 | — | — | ||
| Oxacillin | 5 | 3.40 | — | — | ||
| Penicillin | 3 | 2.04 | — | — | ||
| Trimethoprim/Sulfa | — | 1 | 16.67 | — | ||
| Meropenem | — | — | 3 | 6.12 | ||
Supplementary Table S2 presents the distribution of pathogenic bacteria in the respiratory tract specimens of patients with VAP treated using ForGC + Van. For ForGC + Van treatment, 2,320 pathogens were detected in 540 patients with VAP, comprising 1,505 strains (64.87%) of Gram-negative bacteria, 700 strains (30.17%) of Gram-positive bacteria, and 115 strains (4.96%) of fungi. Supplementary Table S3 presents the drug resistance characteristics of Gram-positive bacteria in the respiratory tract specimens of patients with VAP treated using ForGC + Van. The results indicated that among 700 strains of Gram-positive bacteria, 163 (23.28%) were resistant, comprising 155 resistant strains of Staphylococcus aureus coagulase+, and eight resistant strains of Streptococcus pneumoniae. Supplementary Table S4 presents the drug resistance characteristics of Gram-negative bacteria in the respiratory tract specimens of patients with VAP treated using. ForGC + Van. The results indicated that among 1,505 strains of Gram-negative bacteria, 147 were resistant, which included 46 strains resistant to cephalosporin or vancomycin, although the resistance was low (<2.4%).
Discussion
This is the first study based on the clinical characteristics and medication characteristics of patients with VAP in the latest and largest open database in the field of severe and emergency medicine, aiming to have a deeper understanding of antibiotic use and bacterial infection in patients with VAP, and provide more medical evidence for drug intervention. This was achieved by understanding the distributions and drug resistance rates of pathogenic bacteria in patients with VAP, and further analyzing factors related to inflammatory characteristics. Formulating a more-scientific treatment plan would be helpful in reducing the occurrence of drug-resistant pathogenic bacteria. This has important clinical significance for diagnostic and prognostic evaluations of VAP.
According to the significant difference between the survival group and the death group, such as sex, heart rate, systolic blood pressure, respiratory rate, arterial O2 pressure, glucose, pH, ALT, positive/negative microorganisms in respiratory secretions showed no difference between the groups (p > 0.05). Sharpe et al. (2014) reported higher mortality in women with VAP, but our results and other studies suggest that gender is an irrelevant factor (de Miguel-Díez et al., 2017) for VAP. Other features with no difference between groups, such as heart rate, systolic blood pressure and respiratory rate, were the patients’ vital signs; arterial O2 pressure, glucose, pH, ALT, positive/negative microorganisms in respiratory secretions were the general blood gas, biochemical and microbiological status of patients. These characteristics may differ significantly before and after patients progress to VAP. This suggests that we need to further analyze the changes of these indicators when studying whether they are directly related to in-hospital death of VAP patients, and conclusion drawn from only one test may not be reliable. However, the characteristics that were significantly different between groups (p < 0.05), such as RDW, lymphocytes, monocytes, neutrophils, basophils, eosinophils and other inflammatory indicators; complications such as hypertension, liver disease, renal disease and cancer; biochemical indicators such as albumin and platelet count; and scores such as SOFA score reflected the types and severity of diseases in patients at admission, and their values showed that the diseases in the death group were more complex and severe than those in the survival group, which was consistent with clinical experience. BMI is a special indicator. According to our results, the BMI of the death group was lower than that of the survival group, which is consistent with the “obesity paradox” (Park et al., 2020), indicating that patients with higher BMI at admission may be more able to support the body’s consumption of nutrients during the disease, and thus have a higher probability of survival.
The analysis results indicated that the most commonly used medications for patients with VAP were vancomycin and cephalosporins, which was consistent with the findings of [Agrafiotis et al., 2011; Bassetti et al., 2012; Zhang et al. (2019)]. This indicates that the clinical drug intervention measures (including diagnosis and treatment plans) for patients with VAP are currently relatively consistent between China and other countries. The results of the present study indicated that the composition of the primary pathogens in patients with VAP was consistent with that reported by [Giard et al., 2008; Shorr et al., 2009; Djordjevic et al. (2017); Kradin and Digumarthy, 2017]. This indicated that several pathogens (e.g., yeast, Staphylococcus aureus coagulase+, and Pseudomonas aeruginosa) invading the respiratory tract of patients were the main cause of lung damage, and so should receive attention from clinical medical staff. Patients in ICUs also often use ventilators more frequently than patients in conventional wards due to the severity of their medical conditions, which may also increase the occurrence of pathogenic bacteria, thereby aggravating lung damage, leading to VAP.
Vancomycin is a glycopeptide antibiotic widely used to treat infections caused by pathogenic bacteria such as Staphylococcus aureus and Streptococcus pneumoniae. It is more effective than cephalosporin antibiotics, exhibits no cross-resistance with other antibiotics, and very few strain are resistant to it (van der Veen et al., 2021). The present study also found that no strain was resistant to vancomycin. In addition, according to the results of this paper, most patients were co-infected with gram positive bacteria and gram negative bacteria, so the combined use of vancomycin and cephalosporin was consistent with the medication habits of clinicians.
The pathogens targeted by each generation of cephalosporins are different (Pomorska-Mól et al., 2015; Belley et al., 2021; Pilmis et al., 2021; Roach et al., 2021). FirGC was developed during 1962–1970. The antibacterial spectrum of FirGC has good effects on anti-Gram-positive, poor antistreptococcal effects, and a weak ability for blood–brain barrier penetration, meaning it is not suitable for central infections. Second-generation cephalosporins were mostly developed during 1970–1976, and their antibacterial effect on Gram-negative bacteria was superior to that of FirGC. The antibacterial spectrum of third-generation cephalosporins expanded on that of the second generation, but their effect on Gram-positive bacteria is weaker than that of FirGC. The recently developed ForGC has a broader antibacterial spectrum, which not only has a good antibacterial effect on Gram-negative bacteria, but also can resist Staphylococcus aureus.
Considering the research results of this study and the characteristics of different cephalosporins, we speculated that the patients treated with FirGC + Van would have the highest survival rate, which was due to the mild condition of these patients and the clearer bacterial flora of the infection, so it would have the highest cure rate. Patients were most commonly treated using ForGC + Van. This might be due to these two drugs having the broadest antibacterial spectrum, therefore making them suitable for a wider range of patients. It is the first choice for clinical antibiotic treatment for patients with VAP infected with different bacteria or with more complex flora. However, the survival rate of patients treated using ForGC + Van was low. This reminds us that in order to maximize the survival of patients with VAP, we need to be aware of the occurrence of drug abuse and accurately administer drugs at the appropriate time.
We therefore suggest that early treatment of patients with VAP should employ more adaptable broad-spectrum antibiotic therapies based on the clinical symptoms of the patient. Later, as the understanding of pathogen drug resistance increases, more sensitive antibiotics should be used. In clinical practice, doctors should designate more personalized and precise drug intervention programs for patients, and make timely adjustments to the dosage, treatment course, and antibiotic administration so as to improve the recovery of patients with VAP.
Inflammation-related indicators of patients can assist in diagnosing VAP occurrence and the rational use of drugs (Stoeppel et al., 2014; Nobahar et al., 2016; Moradi Moghaddam et al., 2019). However, the results of this study did not show that the median and IQR values of inflammatory indicators in the medication group were significantly higher than the median and IQR values of inflammatory indicators in the untreated group. This may be attributed to the high sensitivity but poor specificity of the inflammatory response index. No significant increase was observed in the median and IQR values. This may be because the data used were from the first admission of patients with VAP, when the inflammatory responses of the patients were still in their initial stages. Although there were significant differences between groups, the value of inflammatory indicators did not change significantly.
The results of this study on the drug resistance of pathogens indicated that the included patients with VAP had poor pathogen resistance, meaning that antibiotic treatment was suitable and would have a better curative effect, which affects the mortality of patients with VAP. This further indicates that the medication experience of the medical institution or center of the study data can be used as a reference by other medical institutions.
This study inevitably had some limitations. First, the MIMIC-IV database includes mostly white patients. Therefore, due to genetic differences, the results described in this study may need further confirmation in different populations. Second, we did not attempt to analyze the factors that may lead to VAP occurrence and the factors that may affect the prognosis of patients with VAP. These work will be performed as one of the main focuses of our next study.
Conclusion
This study has described the basic clinical characteristics, pathogen compositions, drug resistances, and antibiotic use of patients with VAP in ICUs from the MIMIC-IV database. The findings provide a reference path and theoretical basis for formulating more reasonable drug intervention measures for patients with VAP.
Acknowledgments
We appreciate the researchers at the MIT Laboratory for Computational Physiology for publicly sharing of the MIMIC-IV clinical database.
Data Availability Statement
Publicly available datasets were analyzed in this study. This data can be found here: https://mimic.mit.edu/.
Author Contributions
RY, TH, and LS conceived of and designed the work. TH, AF, and LL acquired and check the data. RY and TH performed statistical and computational analyses. LS, WH, SL, and LL provided professional clinical analyses. RY, LS, and TH drafted the work. LH and NH assisted with manuscript revision and data confirmation. All authors participated in interpretation of the data and substantively revised the work.
Funding
This work was supported by the Guangdong Provincial Key Laboratory of Traditional Chinese Medicine Informatization (2021B1212040007) and the Scientific research project of Guangdong Bureau of Traditional Chinese Medicine (No.20221106).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2022.869499/full#supplementary-material
Abbreviations
VAP, ventilator-associated pneumonia; ICUs, Intensive Care Units; MIMIC, Medical Information Mart for Intensive Care; ForGC, forth-generation of cephalosporin; FirGC, first-generation of cephalosporin; ForGC + Van, ForGC combined with vancomycin; FirGC + Van, FirGC combined with vancomycin.
References
- Agrafiotis M., Siempos I. I., Ntaidou T. K., Falagas M. E. (2011). Attributable Mortality of Ventilator-Associated Pneumonia: a Meta-Analysis. Int. J. Tuberc. Lung Dis. 15 (9), 1154–1163. i-v. 10.5588/ijtld.10.0498 [DOI] [PubMed] [Google Scholar]
- Bassetti M., Taramasso L., Giacobbe D. R., Pelosi P. (2012). Management of Ventilator-Associated Pneumonia: Epidemiology, Diagnosis and Antimicrobial Therapy. Expert Rev. Anti Infect. Ther. 10 (5), 585–596. 10.1586/eri.12.36 [DOI] [PubMed] [Google Scholar]
- Belley A., Morrissey I., Hawser S., Kothari N., Knechtle P. (2021). Third-generation Cephalosporin Resistance in Clinical Isolates of Enterobacterales Collected between 2016-2018 from USA and Europe: Genotypic Analysis of β-lactamases and Comparative In Vitro Activity of Cefepime/enmetazobactam. J. Glob. Antimicrob. Resist 25, 93–101. 10.1016/j.jgar.2021.02.031 [DOI] [PubMed] [Google Scholar]
- de Miguel-Díez J., López-de-Andrés A., Hernández-Barrera V., Jiménez-Trujillo I., Méndez-Bailón M., Miguel-Yanes J. M., et al. (2017). Decreasing Incidence and Mortality Among Hospitalized Patients Suffering a Ventilator-Associated Pneumonia: Analysis of the Spanish National Hospital Discharge Database from 2010 to 2014. Med. Baltim. 96 (30), e7625. 10.1097/MD.0000000000007625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djordjevic Z. M., Folic M. M., Jankovic S. M. (2017). Distribution and Antibiotic Susceptibility of Pathogens Isolated from Adults with Hospital-Acquired and Ventilator-Associated Pneumonia in Intensive Care Unit. J. Infect. Public Health 10 (6), 740–744. 10.1016/j.jiph.2016.11.016 [DOI] [PubMed] [Google Scholar]
- Giard M., Lepape A., Allaouchiche B., Guerin C., Lehot J. J., Robert M. O., et al. (2008). Early- and Late-Onset Ventilator-Associated Pneumonia Acquired in the Intensive Care Unit: Comparison of Risk Factors. J. Crit. Care 23 (1), 27–33. 10.1016/j.jcrc.2007.08.005 [DOI] [PubMed] [Google Scholar]
- Guo Q., Li H., Ouyang H., Sun R., Wang J., Wu M., et al. (2021). Heart Rate Fluctuation and Mortality in Critically Ill Myocardial Infarction Patients: A Retrospective Cohort Study. Front. Cardiovasc Med. 8, 577742. 10.3389/fcvm.2021.577742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalil A. C., Metersky M. L., Klompas M., Muscedere J., Sweeney D. A., Palmer L. B., et al. (2016). Executive Summary: Management of Adults With Hospital-Acquired and Ventilator-Associated Pneumonia: 2016 Clinical Practice Guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin. Infect. Dis. 63 (5), 575–582. 10.1093/cid/ciw504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollef M. H., Hamilton C. W., Ernst F. R. (2012). Economic Impact of Ventilator-Associated Pneumonia in a Large Matched Cohort. Infect. Control Hosp. Epidemiol. 33 (3), 250–256. 10.1086/664049 [DOI] [PubMed] [Google Scholar]
- Kradin R. L., Digumarthy S. (2017). The Pathology of Pulmonary Bacterial Infection. Semin. Diagn Pathol. 34 (6), 498–509. 10.1053/j.semdp.2017.06.001 [DOI] [PubMed] [Google Scholar]
- Legras A., Malvy D., Quinioux A. I., Villers D., Bouachour G., Robert R., et al. (1998). Nosocomial Infections: Prospective Survey of Incidence in Five French Intensive Care Units. Intensive Care Med. 24 (10), 1040–1046. 10.1007/s001340050713 [DOI] [PubMed] [Google Scholar]
- Lu X., Wang X., Gao Y., Yu S., Zhao L., Zhang Z., et al. (2021). Efficacy and Safety of Corticosteroids for Septic Shock in Immunocompromised Patients: A Cohort Study from MIMIC. Am. J. Emerg. Med. 42, 121–126. 10.1016/j.ajem.2020.02.002 [DOI] [PubMed] [Google Scholar]
- Moradi Moghaddam O., Niakan Lahiji M., Talebi-Taher M., Mahmoodiyeh B. (2019). Effect of Inhaled Colistin on the Treatment of Ventilator-Associated Pneumonia Due to Multi-Drug Resistant Acinetobacter. Tanaffos 18 (1), 66–73. [PMC free article] [PubMed] [Google Scholar]
- Nobahar M., Razavi M. R., Malek F., Ghorbani R. (2016). Effects of Hydrogen Peroxide Mouthwash on Preventing Ventilator-Associated Pneumonia in Patients Admitted to the Intensive Care Unit. Braz J. Infect. Dis. 20 (5), 444–450. 10.1016/j.bjid.2016.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S. J., Ha K. H., Kim D. J. (2020). Body Mass Index and Cardiovascular Outcomes in Patients with Acute Coronary Syndrome by Diabetes Status: the Obesity Paradox in a Korean National Cohort Study. Cardiovasc Diabetol. 19 (1), 191. 10.1186/s12933-020-01170-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilmis B., Jiang O., Mizrahi A., Nguyen Van J. C., Lourtet-Hascoët J., Voisin O., et al. (2021). No Significant Difference between Ceftriaxone and Cefotaxime in the Emergence of Antibiotic Resistance in the Gut Microbiota of Hospitalized Patients: A Pilot Study. Int. J. Infect. Dis. 104, 617–623. 10.1016/j.ijid.2021.01.025 [DOI] [PubMed] [Google Scholar]
- Pomorska-Mól M., Czyżewska-Dors E., Kwit K., Wierzchosławski K., Pejsak Z. (2015). Ceftiofur Hydrochloride Affects the Humoral and Cellular Immune Response in Pigs after Vaccination against Swine Influenza and Pseudorabies. BMC Vet. Res. 11, 268. 10.1186/s12917-015-0586-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozuelo-Carrascosa D. P., Torres-Costoso A., Alvarez-Bueno C., Cavero-Redondo I., López Muñoz P., Martínez-Vizcaíno V. (2018). Multimodality Respiratory Physiotherapy Reduces Mortality but May Not Prevent Ventilator-Associated Pneumonia or Reduce Length of Stay in the Intensive Care Unit: a Systematic Review. J. Physiother. 64 (4), 222–228. 10.1016/j.jphys.2018.08.005 [DOI] [PubMed] [Google Scholar]
- Roach E. J., Uehara T., Daigle D. M., Six D. A., Khursigara C. M. (2021). The Next-Generation β-Lactamase Inhibitor Taniborbactam Restores the Morphological Effects of Cefepime in KPC-Producing Escherichia coli . Microbiol. Spectr. 9 (2), e0091821. 10.1128/Spectrum.00918-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpe J. P., Magnotti L. J., Weinberg J. A., Brocker J. A., Schroeppel T. J., Zarzaur B. L., et al. (2014). Gender Disparity in Ventilator-Associated Pneumonia Following Trauma: Identifying Risk Factors for Mortality. J. Trauma Acute Care Surg. 77 (1), 161–165. 10.1097/TA.0000000000000251 [DOI] [PubMed] [Google Scholar]
- Shorr A. F., Zilberberg M. D., Kollef M. (2009). Cost-effectiveness Analysis of a Silver-Coated Endotracheal Tube to Reduce the Incidence of Ventilator-Associated Pneumonia. Infect. Control Hosp. Epidemiol. 30 (8), 759–763. 10.1086/599005 [DOI] [PubMed] [Google Scholar]
- Song K., Guo C., Yang K., Li C., Ding N. (2021). Clinical Characteristics of Aortic Aneurysm in MIMIC-III. Heart Surg. Forum 24 (2), E351–e358. 10.1532/hsf.3571 [DOI] [PubMed] [Google Scholar]
- Stoeppel C. M., Eriksson E. A., Diaz-Flores R., Coffie P., Koshy J., Kacir C., et al. (2014). Guiding the Management of Intubated Patients with Pneumonia and Ventilator-Associated Events Using Serial Catheter-Directed Bronchoalveolar Lavage. J. Trauma Acute Care Surg. 76 (5), 1264–1269. 10.1097/TA.0000000000000219 [DOI] [PubMed] [Google Scholar]
- Torres A., Niederman M. S., Chastre J., Ewig S., Fernandez-Vandellos P., Hanberger H., et al. (2017). International ERS/ESICM/ESCMID/ALAT guidelines for the management of hospital-acquired pneumonia and ventilator-associated pneumonia: Guidelines for the management of hospital-acquired pneumonia (HAP)/ventilator-associated pneumonia (VAP) of the European Respiratory Society (ERS), European Society of Intensive Care Medicine (ESICM), European Society of Clinical Microbiology and Infectious Diseases (ESCMID) and Asociación Latinoamericana del Tórax (ALAT). Eur. Respir. J. 50 (3). 1700582. 10.1183/13993003.00582-2017 [DOI] [PubMed] [Google Scholar]
- Tseng C. C., Huang K. T., Chen Y. C., Wang C. C., Liu S. F., Tu M. L., et al. (2012). Factors Predicting Ventilator Dependence in Patients with Ventilator-Associated Pneumonia. ScientificWorldJournal 2012, 547241. 10.1100/2012/547241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urli T., Perone G., Acquarolo A., Zappa S., Antonini B., Ciani A. (2002). Surveillance of Infections Acquired in Intensive Care: Usefulness in Clinical Practice. J. Hosp. Infect. 52 (2), 130–135. 10.1053/jhin.2002.1271 [DOI] [PubMed] [Google Scholar]
- van der Veen A., Somers A., Vanhaesebrouck S., Ter Heine R., Brüggemann R., Allegaert K., et al. (2021). Variation in Vancomycin Dosing and Therapeutic Drug Monitoring Practices in Neonatal Intensive Care Units. Int. J. Clin. Pharm. 44 (2), 564–56910. 10.1007/s11096-021-01345-9 [DOI] [PubMed] [Google Scholar]
- Vandana Kalwaje E., Rello J. (2018). Management of Ventilator-Associated Pneumonia: Need for a Personalized Approach. Expert Rev. Anti Infect. Ther. 16 (8), 641–653. 10.1080/14787210.2018.1500899 [DOI] [PubMed] [Google Scholar]
- Wu W. T., Li Y. J., Feng A. Z., Li L., Huang T., Xu A. D., et al. (2021). Data Mining in Clinical Big Data: the Frequently Used Databases, Steps, and Methodological Models. Mil. Med. Res. 8 (1), 44–12. 10.1186/s40779-021-00338-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J., Li Y., Liu Q., Li L., Feng A., Wang T., et al. (2020). Brief Introduction of Medical Database and Data Mining Technology in Big Data Era. J. Evid. Based Med. 13 (1), 57–69. 10.1111/jebm.12373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue M., Liu F., Zhao L., Zhang F., Wang C. (2015). A Multicenter Clinical Study of Bundle Treatment for Moderate or Severe Acute Respiratory Distress Syndrome. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue 27 (7), 601–605. 10.3760/cma.j.issn.2095-4352.2015.07.012 [DOI] [PubMed] [Google Scholar]
- Zhang Y., Wang Y., Van Driel M. L., McGuire T. M., Zhang T., Dong Y., et al. (2019). Network Meta-Analysis and Pharmacoeconomic Evaluation of Antibiotics for the Treatment of Patients Infected with Complicated Skin and Soft Structure Infection and Hospital-Acquired or Ventilator-Associated Penumonia. Antimicrob. Resist Infect. Control 8, 72. 10.1186/s13756-019-0518-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X., Shen W., Wang G. (2021). Early Prediction of Sepsis Based on Machine Learning Algorithm. Comput. Intell. Neurosci. 2021, 6522633. 10.1155/2021/6522633 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Publicly available datasets were analyzed in this study. This data can be found here: https://mimic.mit.edu/.