Abstract
Background
There are significant differences in the prevalence and prognosis of atrial fibrillation (AF) between sexes. Epicardial adipose tissue (EAT) has been found as a risk factor for AF. This study aimed to evaluate whether sex-based EAT differences were correlated with AF recurrence and major adverse cardiovascular events (MACE).
Methods
In this study, postmenopausal women and age, BMI, and type of AF matched men who had received first catheter ablation were included. EAT volume was quantified based on the pre-ablation cardiac computed tomography (CT) images. Clinical, CT, and echocardiographic variables were compared by sex groups. The predictors of AF recurrence and MACE were determined through Cox proportional hazards regression.
Results
Women were found with significantly lower total EAT volumes (P < 0.001) but higher periatrial/total (P/T) EAT ratios (P = 0.009). The median follow-up duration was 444.5 days. As revealed by the result of the Kaplan-Meier survival analysis, the women were found to have a significantly higher prevalence of AF recurrence (log rank, P = 0.011) but comparable MACE (log rank, P = 0.507) than men. Multivariate analysis demonstrated that female gender (HR: 1.88 [95% CI: 1.03, 4.15], P = 0.032), persistent AF (HR: 2.46 [95% CI: 1.19, 5.05], P = 0.015), left atrial (LA) dimension (HR: 1.47 [95% CI: 1.02, 2.13], P = 0.041), and P/T EAT ratio (HR: 1.73 [95% CI: 1.12, 2.67], P = 0.013) were found as the independent predictors of AF recurrence. Sex-based subgroup multivariable analysis showed that the P/T EAT ratio was an independent predictor of AF recurrence in both men (HR: 1.13 [95% CI: 1.01, 1.46], P = 0.047) and women (HR: 1.37 [95% CI: 1.11, 1.67], P = 0.028). While age (HR: 1.81 [95% CI: 1.18, 2.77], P = 0.007), BMI (HR: 1.44 [95% CI: 1.02, 2.03], P = 0.038), and periatrial EAT volume (HR: 1.31 [95% CI: 1.01, 1.91], P = 0.046) were found to be independent of MACE.
Conclusion
Women had a higher P/T EAT ratio and AF post-ablation recurrence but similar MACE as compared with men. Female gender and P/T EAT ratio were found to be independent predictors of AF recurrence, whereas age and periatrial EAT volume were found to be independent predictors of MACE.
Keywords: atrial fibrillation, major adverse cardiovascular events, epicardial adipose tissue, recurrence, sex
Introduction
Atrial fibrillation (AF) has been found as the most prevalent cardiac arrhythmia in clinical practice, and it is correlated with an elevated risk of stroke and heart failure (1, 2). There are significant differences in the prevalence and prognosis of AF between the sexes, which are similar to other cardiovascular diseases (3). The risk of developing AF in women was 1.5–2.0 times higher than that in men (4). However, women with AF will have more severe symptoms and a poorer quality of life in contrast to men (5, 6). Several reports found that women had a higher risk of arrhythmia recurrence and periprocedural complications and hospitalization than men with AF (7–9). Although the results regarding the effect of sex differences on AF ablation outcomes have been controversial, an existing systematic review and meta-analysis proved that women were reported with lower AF-free survival than men (9, 10). However, the potential mechanisms of the observed sex difference in clinical outcomes following the AF post-ablation remain unclear.
Over the past few years, epicardial adipose tissue (EAT) has aroused increasing attention as it has been reported to play a role in the development and maintenance of AF and has a stronger relationship to AF presence and severity compared with other adiposity markers [e.g., waist circumference, waist/hip ratio, and body mass index (BMI)] (11–13). EAT, as the metabolically active visceral adipose depot closest to the myocardium, exhibits cell-to-cell contact or infiltration and shares the same blood supply as the myocardium, thus contributing to crosstalk with cardiomyocytes through direct paracrine and vasocrine pathways (11, 14). Some existing studies have suggested that when compared with total EAT, left atrial EAT is more significantly correlated with markers of atrial fibrillation (e.g., atrial structural remodeling and vulnerability), likely because the secretion of pro-inflammatory and anti-inflammatory adipokines by EAT around the left atrium could directly affect the left atrial wall and interact with cardiac autonomic nervous system (15–17). Thus, this study aimed to evaluate the sex-related difference in total EAT and periatrial EAT volume measured using cardiac computed tomography (CT) and correlate these with post-ablation AF recurrence and major adverse cardiovascular events (MACE).
Methods
Patient Population
In this study, all consecutive patients suffering from symptomatic AF who had received preprocedural cardiac CT for its ability to comprehensively evaluate pulmonary veins and left atrium, identify small variant anatomy, and rule out any suspicion of cardiac thrombus (18), and echocardiography examinations before the first-time catheter ablation between January 2018 and June 2021 at The First Affiliated Hospital of Chengdu Medical College were retrospectively recruited. The postmenopausal women and men matched for age, BMI, and type of AF were included. Clinical data were abstracted from the patient reports and the electronic medical records (e.g., demographic information, smoking, diabetes mellitus, hypertension, dyslipidemia, heart failure, coronary artery disease, history of stroke/transient ischemic attack (TIA), prior myocardial infarction, AF type (paroxysmal, persistent), and medications). The CHA2DS2VASC score was determined in accordance with the variables including congestive heart failure (1 point); hypertension (1 point); age 65–74 years (1 point); age ≥ 75 years (2 points); diabetes mellitus (1 point); prior stroke, TIA, or thromboembolism (2 points); vascular disease (1 point); and gender (female: 1 point) (19). Echocardiographic database was used to collect data on left ventricular ejection fraction (LVEF), left atrial (LA) dimension, and E/A-ratio. This study followed the principles in the Declaration of Helsinki and gained approval from the Institutional Review Board of our hospital.
Cardiac Computed Tomography Imaging
All patients were scanned with a 128-row spiral CT system (SOMATOM Definition; Siemens AS). An intravenous injection protocol with 80 ml of 370 mg I/ml iopamidol (Shanghai Bracco Sine Pharmaceutical Corporation Ltd, China) was employed at an infusion rate of 5 ml/s. Subsequently, 40 ml saline was injected at the same flow rate. The contrast-enhanced cardiac CT scans were performed based on a retrospectively gated electrocardiogram (ECG)-triggered sequential protocol. To be specific, the scanning parameters included tube voltage, 120 kV; automatic tube current modulation, 100–400 mAs; thread pitch, 0.18; field of view (FOV), 145 mm × 145 mm; and rotation time, 0.3 s. Images were reconstructed (slice thickness, 0.75 mm and slice interval, 0 mm) using retrospective ECG gating (70%−80% R–R interval). The effective radiation ranged from 5 to 5.5 mSv.
Left Atrial Volume Assessment
Left atrial volume (LAV) was calculated on the workstation (syngo MMWP OT 46806, VE40A; Siemens Medical Systems). The endocardial contour of the LA was manually outlined on each axial image slice. Every image was carefully ruled out because of the LA appendage and pulmonary veins. All voxels at each slice were added to derive LAV.
EAT Volume Analysis
Epicardial adipose tissue volume measurement was performed with a 3D Slicer software (Boston, USA, 4.11.2 version). EAT has been defined as the adipose tissue between the pericardial visceral layer and the surface of the myocardium. The volumes of total EAT and periatrial EAT surrounding the left atrium were determined by manually tracing from the first section to the last section containing any images of the whole heart and the left atrium, respectively. The region outside the pericardium was excluded. EAT was automatically recognized using the software as tissue with HU between −195 and −15 for contrast-enhanced images as described previously (20). Subsequently, the region of interest (ROI) was examined and reviewed by the experienced operator (with 4 years of experience in cardiovascular imaging), and the voxels in the respective slice were summed to determine the total EAT volume and periatrial EAT volume (Figure 1). Moreover, the periatrial to total EAT ratios (P/T EAT ratio) of all patients were calculated. For the interobserver analysis, 50 individuals were randomly selected and then analyzed independently by two experienced radiologists (with 4 years of experience in cardiovascular imaging) blinded to clinical characteristics and CT findings. In terms of the intra-observer analysis, the results of the same 50 subjects above were measured again by one of the radiologists 2 weeks later.
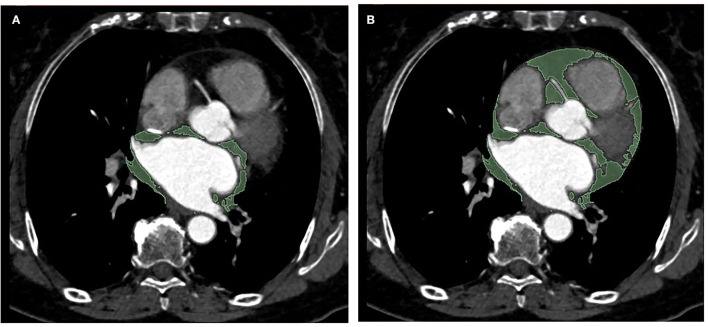
Figure 1.
Representative example of periatrial EAT (A) and total EAT (B) measurement in a single axial CT image in a patient (area in green depict traced EAT). EAT, epicardial adipose tissue.
Atrial Fibrillation Ablation
All patients included in this study received catheter ablation using radiofrequency ablation, and the indications for the procedures were based on the current guidelines (21). A femoral vein puncture was performed to place the arterial sheath. Subsequently, electrodes were delivered into the apical and coronary sinus through the arterial sheaths for intracardiac electrophysiological examination to determine the location of the trigger of AF. The atrial septal puncture was performed to push heparin. Next, pulmonary vein and LA angiography were performed. A three-dimensional LA model was built by feeding a star-shaped magnetoelectric dual-positioning calibration catheter along the arterial sheath. Afterward, a cold saline pressure ablation adjustable elbow catheter (D133604IL, QiangSheng, China) was placed for circumferential pulmonary vein isolation; on that basis, a bidirectional block was achieved in the pulmonary vein based on Carto 3D electrical labeling. The ablation target was found as the earliest point of cardiac excitation at the onset of AF, and the endpoint was the disappearance of AF after ablation and its inability to be reevoked. Finally, the electrode was withdrawn, and the arterial sheath was removed when the sinus heart rate persisted for more than 15 min.
Follow-Up Visits
All patients were followed after their first ablation procedures were completed. The follow-up examinations for AF monitoring were generally performed at 3 or earlier, 6, 9, and 12 months, then once or twice a year thereafter. The AF recurrence was determined in accordance with the clinical evaluation, 12 lead ECG, as well as 24-h Holter monitoring. One of the endpoints of recurrence was defined as AF episodes >30 s in duration documented on standard ECG recordings, event-activated ECG recordings, or 24-h Holter recordings.
Another endpoint of MACE comprised stroke/TIA, myocardial infarction, heart failure, major bleeding, cardiovascular hospitalization, and cardiovascular death during follow-up.
Statistical Analysis
Continuous variables were expressed as mean ± standard deviation (SD) if normally distributed or median and interquartile range (IQR) if not normally distributed. Categorical variables were expressed as numbers (n) and percentages (%). Continuous variables were compared through the Student's t-test or Mann–Whitney U tests as appropriate, while categorical variables were compared through chi-square tests or Fisher exact tests as appropriate. The correlation of BMI with total EAT volume, periatrial EAT volume, and P/T EAT ratio was performed using Pearson correlation analysis. Intraclass correlation coefficients (ICC) were determined to evaluate interobserver and intraobserver reproducibility. The survival curve of AF recurrence and MACE was studied using the Kaplan–Meier method, and the comparison between sex groups was evaluated based on log-rank tests. The predictors of AF recurrence and MACE were determined using the univariable and multivariable Cox proportional hazard regression analysis. Variables with P < 0.1 were included in the multivariate Cox models. Receiver-operating characteristic (ROC) curve analysis was performed to evaluate the diagnostic performance and the optimal cutoff values of the P/T EAT ratio for AF recurrence. A two-tailed P < 0.05 was considered statistically significant. Statistical analyses were conducted using SPSS (version 22, IBM, Chicago, IL, USA), and graphs were generated using the Prism software (version 8, GraphPad, San Diego, CA, USA).
Results
In this study, 71 men and 87 women were generally included. Table 1 and Supplementary Material 1 list the baseline characteristics of the study. The mean age was 69 ± 10 years, and 67 (42%) had persistent AF. There was no significant difference between men and women in age, BMI, hypertension, dyslipidemia, CAD, heart failure, prior myocardial infarction, stroke/TIA, and medications. Women had a lower proportion of smoking habits and a higher proportion of diabetics than men. Female patients had higher CHA2DS2VASC scores than male patients.
Table 1.
Baseline characteristics of the study populations.
| Male (n = 71) | Female (n = 87) | P-value | |
|---|---|---|---|
| Age, y | 67 ± 11 | 70 ± 9 | 0.064 |
| BMI, kg/m2 | 29.57 ± 2.31 | 29.40 ± 3.17 | 0.697 |
| Smoking, n (%) | 36 (41) | 3 (4) | <0.001 |
| Hypertension, n (%) | 46 (65) | 56 (64) | 0.956 |
| Diabetes, n (%) | 27 (38) | 52 (60) | 0.007 |
| Dyslipidemia, n (%) | 32 (45) | 48 (55) | 0.206 |
| CAD, n (%) | 30 (42) | 49 (56) | 0.079 |
| Heart failure, n (%) | 10 (14) | 21 (24) | 0.113 |
| Prior myocardial infarction, n (%) | 1 (1) | 4 (5) | 0.380 |
| Stroke/TIA, n (%) | 3 (4) | 6 (7) | 0.141 |
| Persistent AF, n (%) | 28 (39) | 39 (45) | 0.456 |
| CHA2DS2VASC score | 3 (1, 4) | 4 (3, 5) | <0.001 |
Values are shown as the mean±SD, median (first to third quartile) or n (%).
AF, atrial fibrillation; BMI, body mass index; CAD, coronary artery disease; TIA, transient ischemic attack.
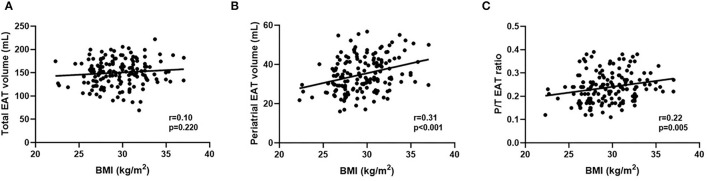
Table 2 lists the imaging variables according to sex groups. LVEF, LA dimension, E/A ratio, LAV, and periatrial EAT volume were comparable between sexes. Total EAT volume was significantly lower in female patients than in male patients (140.91 ± 20.31 vs. 161.55 ± 21.65, P < 0.001), while female patients had a higher P/T EAT ratio (0.25 ± 0.07 vs. 0.22 ± 0.06, P = 0.009) than male patients. BMI was significantly correlated with periatrial EAT volume (r = 0.31, P < 0.001) and P/T EAT ratio (r = 0.22, P = 0.005). No significant correlation was found between BMI and total EAT volume (r = 0.10, P = 0.220; Figure 2). The intraobserver and interobserver reproducibility of total EAT volume and periatrial EAT volume were found to be excellent. The ICC values in the intraobserver analysis were 0.94 (95% confidence interval [CI]: 0.90–0.97) and 0.90 (95% CI: 0.84–0.94) for total EAT volume and periatrial EAT volume, respectively. The ICC values in the interobserver analysis were 0.91 (95% CI: 0.84–0.95) and 0.89 (95% CI: 0.81–0.93) for total EAT volume and periatrial EAT volume, respectively.
Table 2.
Imaging variables of the total population and stratified according to sex.
| Male (n = 71) | Female (n = 87) | P-value | |
|---|---|---|---|
| LVEF, % | 63.14 ± 8.90 | 63.51 ± 8.62 | 0.790 |
| LA dimension, mm | 38.36 ± 6.15 | 40.25 ± 6.03 | 0.053 |
| E/A ratio | 1.51 ± 0.82 | 1.34 ± 0.63 | 0.144 |
| LAV, mL | 100.27 ± 19.94 | 102.67 ± 21.89 | 0.473 |
| Total EAT, mL | 161.55 ± 21.65 | 140.91 ± 20.31 | <0.001 |
| Periatrial EAT, mL | 35.92 ± 9.86 | 34.28 ± 8.12 | 0.252 |
| P/T ratio | 0.22 ± 0.06 | 0.25 ± 0.07 | 0.009 |
Values are mean±SD if normally distributed and median (interquartile range) if not normally distributed.
LVEF, left ventricular ejection fraction; LA, left atrial; EAT, epicardial adipose tissue. P/T EAT ratio; proportion of periatrial to total EAT.
Figure 2.
(A–C) Correlations between BMI and total EAT volume, periatrial EAT volume and P/T EAT ratio. BMI, body mass index; EAT, epicardial adipose tissue; P/T EAT ratio, proportion of periatrial to total EAT.
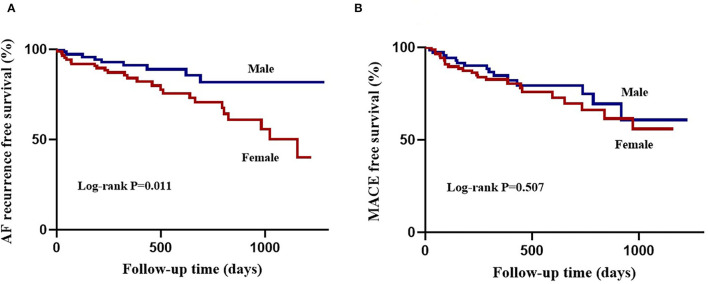
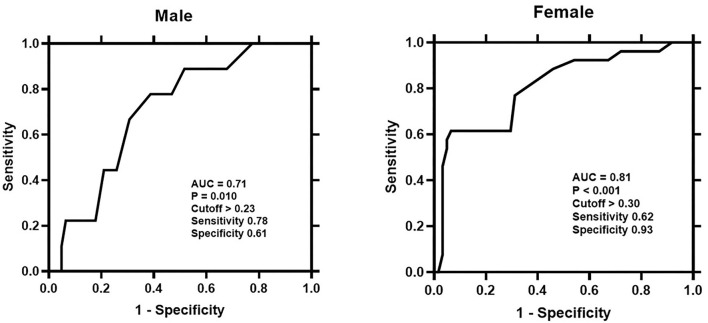
During the median follow-up period of 444.5 (IQR: 296.3, 804.2) days, female patients had lower arrhythmia-free survival than male patients (26 [30%] vs. 9 [13%], log-rank P = 0.011; Figure 3A). Table 3 lists the Cox regression analysis results. In the univariate analysis, the factors that were found to be the predictors of AF recurrence following the catheter ablation were as follows: age (HR: 1.74 [95% CI: 1.14, 2.66], P = 0.011), female gender (HR: 2.61 [95% CI: 1.22, 5.60], P = 0.014), persistent AF (HR: 2.14 [95% CI: 1.08, 4.24], P = 0.030), LA dimension (HR: 1.62 [95% CI: 1.20, 2.20], P = 0.002), periatrial EAT volume (HR: 1.72 [95% CI: 1.23, 2.40], P = 0.002), and P/T EAT ratio (HR: 1.94 [95% CI: 1.43, 2.64], P < 0.001). The result of the multivariate analysis showed that female gender (HR: 1.88 [95% CI: 1.03, 4.15], P = 0.032), persistent AF (HR: 2.46 [95% CI: 1.19, 5.05], P = 0.015), LA dimension (HR: 1.47 [95% CI: 1.02, 2.13], P = 0.041), and P/T EAT ratio (HR: 1.73 [95% CI: 1.12, 2.67], P = 0.013) were still the independent predictors of post-ablation recurrence. Subgroup multivariate Cox regression analysis of sex-based showed that P/T EAT ratio remained an independent predictor of AF recurrence in both men (HR: 1.13 [95% CI: 1.01, 1.46], P = 0.047) and women (HR: 1.37 [95% CI: 1.11, 1.67], P = 0.028) (Supplementary Material 2). ROC analysis demonstrated that the P/T EAT ratio showed good diagnostic performance of AF recurrence in both men (cutoff = 0.23, AUC = 0.71, P = 0.010)and women (cutoff = 0.30, AUC = 0.81, P < 0.001) (Figure 4).
Figure 3.
Kaplan-Meier curves of atrial fibrillation outcomes following ablation according to sex. (A), atrial fibrillation recurrence free survival is lower in females compared with males. (B), major adverse cardiovascular events free survival is comparable between sexes.
Table 3.
Univariable and multivariable cox regression analysis for atrial fibrillation recurrence post-ablation.
| Univariable analysis | Multivariable analysis | |||
|---|---|---|---|---|
| HR (95%CI) | P | HR (95%CI) | P | |
| Age, y | 1.74 (1.14, 2.66) | 0.011 | 1.25 (0.89, 2.25) | 0.116 |
| Female | 2.61 (1.22, 5.60) | 0.014 | 1.88 (1.03, 4.15) | 0.032 |
| BMI, kg/m2 | 1.31 (0.94, 1.84) | 0.116 | ||
| Hypertension | 1.66 (0.78, 3.56) | 0.189 | ||
| Diabetes | 1.02 (0.53, 1.99) | 0.950 | ||
| Dyslipidemia | 1.37 (0.69, 2.69) | 0.369 | ||
| CAD | 1.21 (0.62, 2.36) | 0.587 | ||
| Heart failure | 1.49 (0.64, 3.46) | 0.355 | ||
| Prior myocardial infarction | 1.39 (0.33, 5.85) | 0.655 | ||
| Stroke/TIA | 1.75 (0.42, 7.37) | 0.447 | ||
| Persistent AF | 2.14 (1.08, 4.24) | 0.030 | 2.46 (1.19, 5.05) | 0.015 |
| Anti-arrhythmic drugs | 0.98 (0.50, 1.90) | 0.942 | ||
| LVEF, % | 0.92 (0.63, 1.34) | 0.664 | ||
| LA dimension, mm | 1.62 (1.20, 2.20) | 0.002 | 1.47 (1.02, 2.13) | 0.041 |
| LAV, mL | 1.01 (0.99, 1.03) | 0.193 | ||
| Total EAT, mL | 1.39 (0.98, 1.92) | 0.052 | 1.18 (0.77, 1.81) | 0.446 |
| Periatrial EAT, mL | 1.72 (1.23, 2.40) | 0.002 | 1.18 (0.73, 1.90) | 0.502 |
| P/T EAT ratio | 1.94 (1.43, 2.64) | <0.001 | 1.73 (1.12, 2.67) | 0.013 |
BMI, body mass index; CAD, coronary artery disease; TIA, transient ischemic attack; AF, atrial fibrillation; LVEF, left ventricular ejection fraction; LA, left atrial; LAV, left atrial volume; EAT, epicardial adipose tissue. P/T EAT ratio; proportion of periatrial to total EAT. Bold values indicate P < 0.05.
Figure 4.
Receiver-operating-characteristic (ROC) curve analysis evaluating the predictive performance of P/T EAT ratio for atrial fibrillation recurrence in the male and female. The cutoff value of P/T EAT ratio for predicting AF recurrence and its sensitivity and specificity were presented. P/T EAT ratio, proportion of periatrial to total EAT.
By the time of the final follow-up, MACE had occurred in 23 (26%) female patients and 15 (21%) male patients (log-rank P = 0.507; Figure 3B), including stroke/TIA in 3 (3%) female patients, heart failure in 8 (9%) female patients and 4 (6%) male patients, cardiovascular hospitalization in 17 (20%) female patients and 13 (18%) male patients, and cardiovascular death in 1 (1%) female patient and 1 (1%) male patient. Table 4 shows the major determinants of MACE after ablation. As revealed by the univariable analysis, age (HR: 1.89 [95% CI: 1.27, 2.83], P = 0.002), BMI (HR: 1.60 [95% CI: 1.16, 2.20], P = 0.004), periatrial EAT volume (HR: 1.43 [95% CI: 1.04, 1.96], P = 0.027), and P/T EAT ratio (HR: 1.44 [95% CI: 1.08, 1.91], P = 0.012) were significantly correlated with MACE. In the multivariable analysis, age (HR: 1.81 [95% CI: 1.18, 2.77], P = 0.007), BMI (HR: 1.44 [95% CI: 1.02, 2.03], P = 0.038), and periatrial EAT volume (HR: 1.31 [95% CI: 1.01, 1.91], P = 0.046) were found to be independent of MACE post-ablation.
Table 4.
Univariable and multivariable cox regression analysis for major adverse cardiovascular events post-ablation.
| Univariable analysis | Multivariable analysis | |||
|---|---|---|---|---|
| HR (95%CI) | P | HR (95%CI) | P | |
| Age, y | 1.89 (1.27, 2.83) | 0.002 | 1.81 (1.18, 2.77) | 0.007 |
| Female | 1.25 (0.65, 2.39) | 0.508 | ||
| BMI, kg/m2 | 1.60 (1.16, 2.20) | 0.004 | 1.44 (1.02, 2.03) | 0.038 |
| Hypertension | 1.52 (0.75, 3.06) | 0.245 | ||
| Diabetes | 1.23 (0.65, 2.33) | 0.535 | ||
| Dyslipidemia | 1.40 (0.73, 2.69) | 0.310 | ||
| CAD | 1.34 (0.70, 2.55) | 0.380 | ||
| Heart failure | 1.09 (0.45, 2.65) | 0.846 | ||
| Prior myocardial infarction | 1.39 (0.33, 5.90) | 0.654 | ||
| Stroke/TIA | 1.12 (0.43, 2.89) | 0.823 | ||
| Persistent AF | 1.03 (0.52, 2.04) | 0.939 | ||
| Anti-arrhythmic drugs | 0.85 (0.45, 1.61) | 0.615 | ||
| CHA2DS2VA score | 1.39 (0.92, 2.10) | 0.114 | ||
| LVEF, % | 1.05 (0.74, 1.48) | 0.790 | ||
| LA dimension, mm | 1.23 (0.90, 1.69) | 0.196 | ||
| LAV, mL | 1.02 (0.98, 1.07) | 0.447 | ||
| Total EAT, mL | 0.87 (0.63, 1.19) | 0.383 | ||
| Periatrial EAT, mL | 1.43 (1.04, 1.96) | 0.027 | 1.31 (1.01, 1.91) | 0.046 |
| P/T EAT ratio | 1.44 (1.08, 1.91) | 0.012 | 1.01 (0.64, 1.53) | 0.971 |
AF, atrial fibrillation; BMI, body mass index; CAD, coronary artery disease; TIA, transient ischemic attack; LVEF, left ventricular ejection fraction; LA, left atrial; LAV, left atrial volume; EAT, epicardial adipose tissue. P/T EAT ratio; proportion of periatrial to total EAT. Bold values indicate P < 0.05.
Discussion
This study demonstrated that there were significant sex-related differences in the distribution of EAT in patients with AF. Postmenopausal women had a higher P/T EAT ratio while men had a higher total EAT volume. Female patients had a higher incidence of post-ablation AF recurrence but a comparable prevalence of MACE compared with male patients. Female gender and P/T EAT ratio were independent of post-ablation AF recurrence. Sex-based subgroup multivariable analysis showed that the P/T EAT ratio was an independent predictor of AF recurrence in both sexes. While age and periatrial EAT volume were independent predictors of MACE.
EAT and AF
It is generally known that the risk of AF is higher in men compared with women, even after adjusting for age (22). Reasons for the differences may comprise sex-specific atrial electrophysiologic properties, atrial remodeling, and mechanisms of atrial fibrosis (23). Obesity-related AF has been gradually increased, and obesity has been considered a crucial and modifiable risk factor for AF development (11, 24, 25). However, obesity can be an attribute of general adiposity with variable degrees of cardiovascular and metabolic presentations. EAT, the more specific fat depot, has aroused rising attention for its close anatomic proximity to coronary arteries and crosstalk with cardiomyocytes. Some evidence has demonstrated that EAT can play a certain role in the pathogenesis and progression of AF, and the relationship was not dependent on total adiposity and LA enlargement (11, 14, 26). A Framingham Heart Study of 2,317 patients discovered that a higher total EAT volume was significantly associated with 40% higher hazards of AF independent of traditional risk factors (13). In addition to total EAT, existing studies further suggest that specific periatrial EAT is more significantly correlated with AF, and it may directly facilitate structural and electric remodeling (11, 27). Van Rosendael et al. suggested that for every gram increase in posterior LA adipose tissue mass, the risk of AF increased by 32% (28).
While the mechanisms correlating EAT with AF were unclear, some potential hypotheses were proposed in accordance with existing prior studies. One of the vital extrapolations may be the role played by EAT in the AF electrophysiological substrate. Direct infiltration of lipocytes within the atrial myocardium could accelerate side-to-side cells' connection loss and induce remodeling of the atrial substrate. Moreover, adipocyte infiltration interpenetrating between myocytes was implicated in decreased voltage and increased voltage heterogeneity within the posterior left atrium, thus jointly leading to subsequent conductive defects (conduction deceleration or heterogeneity) (29–31). A study using a distinctive 3D merge process and dominant frequency LA map determined that fat locations corresponded to high dominant frequency. EAT co-localizes with high dominant frequency areas, revealing that it could be most likely to harbor high-frequency regions, creating beneficial circumstances for the perpetuation of AF (32, 33). For another potential mechanism, EAT was found as the anatomical location of the cardiac intrinsic autonomic nervous system (e.g., ganglionated plexi (GP) and interconnecting nerves) located near the pulmonary veins (34, 35). The ganglia were the key determinants responsible for triggering and perpetuating AF (36). GP activation contributed to the short action potential duration and increased calcium release, thus evoking triggered firing due to a delayed post-depolarization of the atrial/pulmonary vein, as demonstrated by the high dominant frequency sites (35). Another additional mechanism was that EAT, as an endocrine organ, could produce variable pro-inflammatory cytokines, pro-fibrotic factors, reactive oxygen species, and adipocytokines through paracrine or vasocrine pathways, which exerted deleterious local effects on the atrium (e.g., functional disorganization) and further structurally remodeled and produced arrhythmogenic substrates (35). Matrix metalloproteinases, a vital modulator of extracellular matrix turnover that contributes to atrial fibrosis, were upregulated in the AF period, and their secretion increased within EAT (37, 38). An existing study reported that female patients with AF had more advanced atrial remodeling compared with male patients (39).
EAT and AF Recurrence
Some reports found sex differences in the AF recurrence (7, 8, 39). Consistent with the above results, this study demonstrated that women experienced a higher incidence of post-ablation AF recurrence. However, the mechanisms of the sex-related differences remain unclear. It has been assumed that periatrial EAT is more significantly correlated with AF recurrence after catheter ablation (20, 40, 41). EI Mahdiui et al. quantified posterior LA EAT attenuation and explored its relationship with AF recurrence following catheter ablation (41). They reported that patients suffering from higher posterior LA EAT attenuation had a significantly more frequent recurrence rate, and the fat attenuation could serve as a promising predictor of AF recurrence (41). Existing studies evaluated both periatrial and periventricular EAT and found both the fat in the two regions were correlated with AF recurrence, but the closer relationship existed in periatrial EAT (31, 40, 42). As revealed by the above results, EAT promoted AF recurrence to varying extents in different myocardial sites, and the evaluation of EAT around the left atrium might be a preferable methodological evaluation (41). Our study showed that total EAT volume was significantly higher, whereas the P/T EAT ratio was lower in male patients than in female patients. In general, body fat distribution has been different between the sexes: visceral fat obesity predominates in men, while subcutaneous fat obesity predominates in women (43). Existing studies reported that although men had significantly larger pericardial fat volumes than women, the adipose was correlated with more adverse risk factors in women (44). Kim et al. reported that women had more periatrial adiposity, which probably adversely affected LA voltage and transport function (45). The bioactivity of periatrial EAT may be affected by the degree of periatrial adiposity, and metabolically abnormal periatrial EAT further affects the atrium, leading to atrial remodeling and dysfunction (45). Besides, female patients and the P/T EAT ratio instead of periatrial or total EAT were independently correlated with AF recurrence. The above findings reveal that a relatively abundant P/T EAT ratio in women may affect AF recurrence on the basis of several potential mechanisms: Catheter ablation targeted regions for substrate modifications overlap with the majority of LA EAT and adipose tissue had a lower electrical conduction than atrial myocardium, and the increased amount of periatrial EAT may directly decrease the opportunity for successful procedure (35, 46). Furthermore, atrial fibrosis as a result of a fat-induced local inflammatory course may facilitate the development of intra-atrial reentry circuits, thus decreasing the success of catheter ablation (47, 48).
EAT and MACE
Some recent studies have demonstrated EAT as a risk factor for future adverse cardiovascular events in CAD, diabetes, and even asymptomatic populations (14, 49, 50). Eisenberg et al. assessed the prognostic value of EAT volume and attenuation with the use of deep learning-based algorithms quantification from non-contrast cardiac computed tomography in 2,068 asymptomatic individuals with 14 ± 3 years of follow-up (49). They reported that EAT volume and attenuation could be employed to predict MACE independent of traditional risk factors and coronary calcified score (49). Christensen et al. revealed that a high amount of EAT were correlated with incident cardiovascular disease and mortality in patients with type 1 diabetes (T2DM) after they were adjusted for cardiovascular risk factors (50). However, no research has been conducted on the effect of periatrial EAT on MACE in patients with AF following the catheter ablation. Accordingly, the present research extended previous findings to AF patients and explored the correlation between periatrial EAT volume and the incidence of MACE, determining whether there existed sex-related differences. This study revealed that women were not an independent risk factor for MACE in AF patients with post-ablation. The results of a large prospective study suggested that although female patients had a higher incidence of thromboembolism than male patients, they did not emerge as an independent risk factor for stroke and systemic embolism (51). Furthermore, this study also found that periatrial EAT volume was independently correlated with MACE, suggesting that therapeutic targeting of periatrial EAT for MACE prevention in patients with AF post-ablation is promising. A recent review also supports the concept that EAT is a central pathogenetic mechanism and therapeutic target for AF (52).
Study Limitations
This study had several limitations. First, there was a limited sample size with a single center design and retrospective data evaluation, which probably limited evaluation of causality. Accordingly, the above findings should be confirmed in a larger and prospective cohort. Second, the follow-up period was relatively short to determine clinical events, which may affect our results. Third, the results of this study might not be extrapolated to other ethnical populations, since the demographics in this study were dominated by Chinese.
Conclusion
Through the comparison of the sex-based differences in the EAT distribution and AF outcomes post-ablation, it was reported that postmenopausal women had a higher P/T EAT ratio and a higher incidence of AF recurrence than men, but both sexes had similar rates of MACE. In addition, female gender and P/T EAT ratio were found to be independent predictors of AF recurrence, and P/T EAT ratio is still independently associated with AF recurrence in both sexes. While age and periatrial EAT volume were independently correlated with MACE, which suggested that the difference in the distribution of EAT might have an effect on MACE, and the regional distribution of EAT depending on sex may have an impact on AF recurrence.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author Contributions
JZ designed study, wrote the study, reviewed the study, gave input to improve the study, and read and approved the final manuscript. KZ collected data, measured imaging parameters, performed research, and wrote the study. BZ reviewed and analyzed data and performed the data interpretation. ZX measured imaging parameters. WL reviewed data. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2022.905351/full#supplementary-material
References
- 1.Go AS, Hylek EM, Phillips KA, Chang Y, Henault LE, Selby JV, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. (2001) 285:2370–5. 10.1001/jama.285.18.2370 [DOI] [PubMed] [Google Scholar]
- 2.Calkins H, Hindricks G, Cappato R, Kim YH, Saad EB, Aguinaga L, et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Heart Rhythm. (2017) 14:e275–444. 10.1016/j.hrthm.2017.05.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Linde C, Bongiorni MG, Birgersdotter-Green U, Curtis AB, Deisenhofer I, Furokawa T, et al. Sex differences in cardiac arrhythmia: a consensus document of the European Heart Rhythm Association, endorsed by the Heart Rhythm Society and Asia Pacific Heart Rhythm Society. Europace. (2018) 20:1565. 10.1093/europace/euy067 [DOI] [PubMed] [Google Scholar]
- 4.Ko D, Rahman F, Schnabel RB, Yin X, Benjamin EJ, Christophersen IE. Atrial fibrillation in women: epidemiology, pathophysiology, presentation, and prognosis. Nat Rev Cardiol. (2016) 13:321–32. 10.1038/nrcardio.2016.45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xiong Q, Proietti M, Senoo K, Lip GY. Asymptomatic versus symptomatic atrial fibrillation: a systematic review of age/gender differences and cardiovascular outcomes. Int J Cardiol. (2015) 191:172–7. 10.1016/j.ijcard.2015.05.011 [DOI] [PubMed] [Google Scholar]
- 6.Scheuermeyer FX, Mackay M, Christenson J, Grafstein E, Pourvali R, Heslop C, et al. There are sex differences in the demographics and risk profiles of Emergency Department (ED) patients with atrial fibrillation and flutter, but no apparent differences in ED management or outcomes. Acad Emerg Med. (2015) 22:1067–75. 10.1111/acem.12750 [DOI] [PubMed] [Google Scholar]
- 7.Cheng X, Hu Q, Gao L, Liu J, Qin S, Zhang D. Sex-Related differences in catheter ablation of atrial fibrillation: a systematic review and meta-analysis. Europace. (2019) 21:1509–18. 10.1093/europace/euz179 [DOI] [PubMed] [Google Scholar]
- 8.Kuck KH, Brugada J, Fürnkranz A, Chun KRJ, Metzner A, Ouyang F, et al. Impact of female sex on clinical outcomes in the fire and ice trial of catheter ablation for atrial fibrillation. Circ Arrhythm Electrophysiol. (2018) 11:e006204. 10.1161/CIRCEP.118.006204 [DOI] [PubMed] [Google Scholar]
- 9.Cheung JW, Cheng EP, Wu X, Yeo I, Christos PJ, Kamel H, et al. Sex-Based differences in outcomes, 30-day readmissions, and costs following catheter ablation of atrial fibrillation: the United States Nationwide Readmissions Database 2010-14. Eur Heart J. (2019) 40:3035–43. 10.1093/eurheartj/ehz151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Calkins H, Hindricks G, Cappato R, Kim YH, Saad EB, Aguinaga L, et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Europace. (2018) 20:e1–160. 10.1093/europace/eux274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Couselo-Seijas M, Rodríguez-Mañero M, González-Juanatey JR, Eiras S. Updates on epicardial adipose tissue mechanisms on atrial fibrillation. Obes Rev. (2021) 22:e13277. 10.1111/obr.13277 [DOI] [PubMed] [Google Scholar]
- 12.Wong CX, Sun MT, Odutayo A, Emdin CA, Mahajan R, Lau DH, et al. Associations of epicardial, abdominal, and overall adiposity with atrial fibrillation.]Circ Arrhythm Electrophysiol. (2016) 9:e004378. 10.1161/CIRCEP.116.004378 [DOI] [PubMed] [Google Scholar]
- 13.Thanassoulis G, Massaro JM, O'Donnell CJ, Hoffmann U, Levy D, Ellinor PT, et al. Pericardial fat is associated with prevalent atrial fibrillation: the framingham heart study. Circ Arrhythm Electrophysiol. (2010) 3:345–50. 10.1161/CIRCEP.109.912055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ansaldo AM, Montecucco F, Sahebkar A, Dallegri F, Carbone F. Epicardial adipose tissue and cardiovascular diseases. Int J Cardiol. (2019) 278:254–60. 10.1016/j.ijcard.2018.09.089 [DOI] [PubMed] [Google Scholar]
- 15.Schauerte P, Scherlag BJ, Pitha J, Scherlag MA, Reynolds D, Lazzara R, et al. Catheter ablation of cardiac autonomic nerves for prevention of vagal atrial fibrillation. Circulation. (2000) 102:2774–80. 10.1161/01.CIR.102.22.2774 [DOI] [PubMed] [Google Scholar]
- 16.Rudski LG, Lai WW, Afilalo J, Hua L, Handschumacher MD, Chandrasekaran K, et al. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr. (2010) 23:685–713. 10.1016/j.echo.2010.05.010 [DOI] [PubMed] [Google Scholar]
- 17.Sacks HS, Fain JN. Human epicardial adipose tissue: a review. Am Heart J. (2007) 153:907–17. 10.1016/j.ahj.2007.03.019 [DOI] [PubMed] [Google Scholar]
- 18.Markman TM, Khoshknab M, Nazarian S. Catheter ablation of atrial fibrillation: cardiac imaging guidance as an adjunct to the electrophysiological guided approach. Europace. (2021) 23:520–8. 10.1093/europace/euaa249 [DOI] [PubMed] [Google Scholar]
- 19.Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest. (2010) 137:263–72. 10.1378/chest.09-1584 [DOI] [PubMed] [Google Scholar]
- 20.Sanghai SR, Sardana M, Hansra B, Lessard DM, Dahlberg ST, Aurigemma GP, et al. Indexed left atrial adipose tissue area is associated with severity of atrial fibrillation and atrial fibrillation recurrence among patients undergoing catheter ablation. Front Cardiovasc Med. (2018) 5:76. 10.3389/fcvm.2018.00076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sepehri Shamloo A, Dagres N, Hindricks G. [2020 ESC guidelines on atrial fibrillation: Summary of the most relevant recommendations and innovations]. Herz. (2021) 46:28–37. 10.1007/s00059-020-05005-y [DOI] [PubMed] [Google Scholar]
- 22.Godeau D, Petit A, Richard I, Roquelaure Y, Descatha A. Return-to-work, disabilities and occupational health in the age of COVID-19. Scand J Work Environ Health. (2021) 47:408–9. 10.5271/sjweh.3960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Poorthuis MHF, Sherliker P, de Borst GJ, Carter JL, Lam KBH, Jones NR, et al. Joint associations between body mass index and waist circumference with atrial fibrillation in men and women. J Am Heart Assoc. (2021) 10:e019025. 10.1161/JAHA.120.019025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang TJ, Parise H, Levy D, D'Agostino RB, Sr., Wolf PA, Vasan RS, et al. Obesity and the risk of new-onset atrial fibrillation. JAMA. (2004) 292:2471–7. 10.1001/jama.292.20.2471 [DOI] [PubMed] [Google Scholar]
- 25.Pathak RK, Middeldorp ME, Lau DH, Mehta AB, Mahajan R, Twomey D, et al. Aggressive risk factor reduction study for atrial fibrillation and implications for the outcome of ablation: the ARREST-AF cohort study. J Am Coll Cardiol. (2014) 64:2222–31. 10.1016/j.jacc.2014.09.028 [DOI] [PubMed] [Google Scholar]
- 26.Lau DH, Schotten U, Mahajan R, Antic NA, Hatem SN, Pathak RK, et al. Novel mechanisms in the pathogenesis of atrial fibrillation: practical applications. Eur Heart J. (2016) 37:1573–81. 10.1093/eurheartj/ehv375 [DOI] [PubMed] [Google Scholar]
- 27.Hasebe H, Yoshida K, Nogami A, Ieda M. Difference in epicardial adipose tissue distribution between paroxysmal atrial fibrillation and coronary artery disease. Heart Vessels. (2020) 35:1070–8. 10.1007/s00380-020-01575-3 [DOI] [PubMed] [Google Scholar]
- 28.van Rosendael AR, Dimitriu-Leen AC, van Rosendael PJ, Leung M, Smit JM, Saraste A, et al. Association between posterior left atrial adipose tissue mass and atrial fibrillation. Circ Arrhythm Electrophysiol. (2017) 10:e004614. 10.1161/CIRCEP.116.004614 [DOI] [PubMed] [Google Scholar]
- 29.Hatem SN, Redheuil A, Gandjbakhch E. Cardiac adipose tissue and atrial fibrillation: the perils of adiposity. Cardiovasc Res. (2016) 109:502–9. 10.1093/cvr/cvw001 [DOI] [PubMed] [Google Scholar]
- 30.Mahajan R, Lau DH, Brooks AG, Shipp NJ, Manavis J, Wood JP, et al. Electrophysiological, electroanatomical, and structural remodeling of the atria as consequences of sustained obesity. J Am Coll Cardiol. (2015) 66:1–11. 10.1016/j.jacc.2015.04.058 [DOI] [PubMed] [Google Scholar]
- 31.Wong CX, Abed HS, Molaee P, Nelson AJ, Brooks AG, Sharma G, et al. Pericardial fat is associated with atrial fibrillation severity and ablation outcome. J Am Coll Cardiol. (2011) 57:1745–51. 10.1016/j.jacc.2010.11.045 [DOI] [PubMed] [Google Scholar]
- 32.Takahashi Y, Sanders P, Jaïs P, Hocini M, Dubois R, Rotter M, et al. Organization of frequency spectra of atrial fibrillation: relevance to radiofrequency catheter ablation. J Cardiovasc Electrophysiol. (2006) 17:382–8. 10.1111/j.1540-8167.2005.00414.x [DOI] [PubMed] [Google Scholar]
- 33.Atienza F, Calvo D, Almendral J, Zlochiver S, Grzeda KR, Martínez-Alzamora N, et al. Mechanisms of fractionated electrograms formation in the posterior left atrium during paroxysmal atrial fibrillation in humans. J Am Coll Cardiol. (2011) 57:1081–92. 10.1016/j.jacc.2010.09.066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Iacobellis G. Local and systemic effects of the multifaceted epicardial adipose tissue depot. Nat Rev Endocrinol. (2015) 11:363–71. 10.1038/nrendo.2015.58 [DOI] [PubMed] [Google Scholar]
- 35.Gaborit B, Sengenes C, Ancel P, Jacquier A, Dutour A. Role of epicardial adipose tissue in health and disease: a matter of fat? Compr Physiol. (2017) 7:1051–82. 10.1002/cphy.c160034 [DOI] [PubMed] [Google Scholar]
- 36.Coumel P. Paroxysmal atrial fibrillation: a disorder of autonomic tone? Eur Heart J. (1994) 15 (Suppl. A):9–16. 10.1093/eurheartj/15.suppl_A.9 [DOI] [PubMed] [Google Scholar]
- 37.Boixel C, Fontaine V, Rücker-Martin C, Milliez P, Louedec L, Michel JB, et al. Fibrosis of the left atria during progression of heart failure is associated with increased matrix metalloproteinases in the rat. J Am Coll Cardiol. (2003) 42:336–44. 10.1016/S0735-1097(03)00578-3 [DOI] [PubMed] [Google Scholar]
- 38.Venteclef N, Guglielmi V, Balse E, Gaborit B, Cotillard A, Atassi F, et al. Human epicardial adipose tissue induces fibrosis of the atrial myocardium through the secretion of adipo-fibrokines. Eur Heart J. (2015) 36:795–805a. 10.1093/eurheartj/eht099 [DOI] [PubMed] [Google Scholar]
- 39.Wong GR, Nalliah CJ, Lee G, Voskoboinik A, Chieng D, Prabhu S, et al. Sex-related differences in atrial remodeling in patients with atrial fibrillation: relationship to ablation outcomes. Circ Arrhythm Electrophysiol. (2022) 15:e009925. 10.1161/CIRCEP.121.009925 [DOI] [PubMed] [Google Scholar]
- 40.Kocyigit D, Gurses KM, Yalcin MU, Turk G, Evranos B, Yorgun H, et al. Periatrial epicardial adipose tissue thickness is an independent predictor of atrial fibrillation recurrence after cryoballoon-based pulmonary vein isolation. J Cardiovasc Comput Tomogr. (2015) 9:295–302. 10.1016/j.jcct.2015.03.011 [DOI] [PubMed] [Google Scholar]
- 41.El Mahdiui M, Simon J, Smit JM, Kuneman JH, van Rosendael AR, Steyerberg EW, et al. Posterior left atrial adipose tissue attenuation assessed by computed tomography and recurrence of atrial fibrillation after catheter ablation. Circ Arrhythm Electrophysiol. (2021) 14:e009135. 10.1161/CIRCEP.120.009135 [DOI] [PubMed] [Google Scholar]
- 42.Batal O, Schoenhagen P, Shao M, Ayyad AE, Van Wagoner DR, Halliburton SS, et al. Left atrial epicardial adiposity and atrial fibrillation. Circ Arrhythm Electrophysiol. (2010) 3:230–6. 10.1161/CIRCEP.110.957241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Camhi SM, Bray GA, Bouchard C, Greenway FL, Johnson WD, Newton RL, et al. The relationship of waist circumference and BMI to visceral, subcutaneous, and total body fat: sex and race differences. Obesity (Silver Spring). (2011) 19:402–8. 10.1038/oby.2010.248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rosito GA, Massaro JM, Hoffmann U, Ruberg FL, Mahabadi AA, Vasan RS, et al. Pericardial fat, visceral abdominal fat, cardiovascular disease risk factors, and vascular calcification in a community-based sample: the Framingham heart study. Circulation. (2008) 117:605–13. 10.1161/CIRCULATIONAHA.107.743062 [DOI] [PubMed] [Google Scholar]
- 45.Kim JS, Shin SY, Kang JH, Yong HS, Na JO, Choi CU, et al. Influence of sex on the association between epicardial adipose tissue and left atrial transport function in patients with atrial fibrillation: a multislice computed tomography study. J Am Heart Assoc. (2017) 6:e006077. 10.1161/JAHA.117.006077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Suárez AG, Hornero F, Berjano EJ. Mathematical modeling of epicardial RF ablation of atrial tissue with overlying epicardial fat. Open Biomed Eng J. (2010) 4:47–55. 10.2174/1874120701004020047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Abe I, Teshima Y, Kondo H, Kaku H, Kira S, Ikebe Y, et al. Association of fibrotic remodeling and cytokines/chemokines content in epicardial adipose tissue with atrial myocardial fibrosis in patients with atrial fibrillation. Heart Rhythm. (2018) 15:1717–27. 10.1016/j.hrthm.2018.06.025 [DOI] [PubMed] [Google Scholar]
- 48.Verma A, Wazni OM, Marrouche NF, Martin DO, Kilicaslan F, Minor S, et al. Pre-existent left atrial scarring in patients undergoing pulmonary vein antrum isolation: an independent predictor of procedural failure. J Am Coll Cardiol. (2005) 45:285–92. 10.1016/j.jacc.2004.10.035 [DOI] [PubMed] [Google Scholar]
- 49.Eisenberg E, McElhinney PA, Commandeur F, Chen X, Cadet S, Goeller M, et al. Deep learning-based quantification of epicardial adipose tissue volume and attenuation predicts major adverse cardiovascular events in asymptomatic subjects. Circ Cardiovasc Imaging. (2020) 13:e009829. 10.1161/CIRCIMAGING.119.009829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Christensen RH, von Scholten BJ, Hansen CS, Jensen MT, Vilsbøll T, Rossing P, et al. Epicardial adipose tissue predicts incident cardiovascular disease and mortality in patients with type 2 diabetes. Cardiovasc Diabetol. (2019) 18:114. 10.1186/s12933-019-0917-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lan DH, Jiang C, Du X, He L, Guo XY, Zuo S, et al. Female sex as a risk factor for ischemic stroke and systemic embolism in chinese patients with atrial fibrillation: a report from the China-AF study. J Am Heart Assoc. (2018):7:e009391 10.1161/JAHA.118.009391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Packer M. Drugs that ameliorate epicardial adipose tissue inflammation may have discordant effects in heart failure with a preserved ejection fraction as compared with a reduced ejection fraction. J Card Fail. (2019) 25: 986–1003 10.1016/j.cardfail.2019.09.002 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.