Abstract
Mercury-reducing biofilms from packed-bed bioreactors treating nonsterile industrial effluents were shown to consist of a monolayer of bacteria by scanning electron microscopy. Droplets of several micrometers in diameter which accumulated outside of the bacterial cells were identified as elemental mercury by electron-dispersive X-ray analysis. The monospecies biofilms of Pseudomonas putida Spi3 initially present were invaded by additional strains, which were identified to the species level by thermogradient gel electrophoresis (TGGE) and 16S rDNA sequencing. TGGE community fingerprints of the biofilms showed that they were composed of the effluent bacteria and did not contain uncultivable microorganisms. Of the 13 effluent bacterial strains, 2 were not mercury resistant, while all the others had resistance levels similar to or higher than the inoculant strain.
Mercury reduction encoded by the microbial mer operon is an efficient resistance mechanism that is widespread among gram-positive and gram-negative microorganisms (8, 11). Highly toxic water-soluble ionic mercury is taken up by the microorganisms and reduced to insoluble metallic mercury by the intracellular enzyme mercuric reductase, encoded by the merA gene. Metallic mercury subsequently diffuses out of the cells. The reduction process can be continuously performed within a submersed microbial biofilm on porous support material, resulting in accumulation of metallic mercury within the bioreactor (1, 12). Here the structure of mercury-reducing biofilms on the carrier material from packed-bed bioreactors was investigated by scanning electron microscopy and the elemental composition of droplets accumulating within the bioreactor was determined by electron-dispersive X-ray (EDX) analysis.
Industrial bioreactor operation is performed under nonsterile conditions. A monospecies biofilm initially established through inoculation may therefore be subject to colonization by ubiquitous mercury-resistant bacteria (8). The resulting multispecies biofilms may also contain organisms which are hard to cultivate separately. In this study, the invasion of monospecies mercury-reducing biofilms in model laboratory bioreactors operated with industrial waste water was analyzed (12). Genetic fingerprints of isolated effluent bacteria were determined using thermogradient gel electrophoresis (TGGE) of 16S rDNA fragments. This is a fast and sensitive screening technique which has a resolution limit intermediate between the species and genus levels for coryneform bacteria (4). It allows rapid differentiation between samples containing multiple isolates, which can subsequently be identified by 16S rDNA sequencing. However, even if applied with great care, isolation is always a subjective procedure since it relies on colony morphology to differentiate between strains. Moreover, uncultivable bacteria might be present in the biofilms. To analyze the complete community structure of the biofilms within the bioreactors independent of cultivation, 16S rDNA fragments were amplified directly from community DNA and separated by TGGE. In such a way, community fingerprints were generated, which could directly be compared to pure-culture fingerprints. This method also recognizes uncultured microorganisms within the biofilms as additional bands which do not match bands of cultivated bacteria.
Bioreactors and experimental design.
Laboratory columns were filled with glass beads (20 ml, 1 to 5 mm in diameter; Schott Glaswerke, Mainz, Germany), and the beads were covered with carrier material (20 ml) and autoclaved. The carrier material in reactor 1 was Siran beads (SiO2, 0.4 to 1.0 mm in diameter; Schott Glaswerke), the carrier material in reactors 2 and 3 was Lignocell (wood chips, 2.0 to 2.5 mm in diameter; Rettenmaier & Söhne GmbH & Co, Holzmühle, Germany), and the carrier material in reactors 4 to 6 was Arbocell (cellulose, fiber length of 700 μm, fiber diameter of 20 μm; Rettenmaier & Söhne GmbH & Co.). For inoculation, 500 ml of a pure culture of Pseudomonas putida Spi 3, a mercury-resistant isolate, was pumped through the column in upflow mode at 20 ml/h. Subsequently, sterile synthetic wastewater was pumped through the columns, followed by nonsterile chloralkali electrolysis wastewater, which had been aerated and neutralized prior to treatment. The wastewater was supplemented with nutrients (final concentration, 0.1 g of yeast extract per liter). Details of the experimental setup have been described (12).
Scanning electron microscopy and EDX.
Samples of different column bed levels were conventionally fixed with 2.5% glutardialdehyde growth medium for 2 h or several days at 4°C, dehydrated with an acetone series, and critical-point dried with liquid CO2 at 41°C and 85 atm. Samples were carbon coated to a thickness of 30 nm (sputter coater SCD 040; Balzers Union, Walluf, Germany) and analyzed with a field emission scanning microscope (DSM 982 Gemini; Zeiss, Oberkochen, Germany) at a working distance of 8 mm, an acceleration voltage of 20 kV, and a sampling area of 8 by 8 μm. Spectra were registered with the Link-ISIS system (Oxford Instruments, Munich, Germany), and intensities were equally scaled for direct comparison of spectral peaks.
Isolation of effluent bacteria.
Reactor effluent samples were serially diluted in phosphate-buffered saline (PBS) (2.2 g of NaH2PO4 per liter, 6.0 g of Na2HPO4 per liter, 5.8 g of NaCl per liter [pH 7.2]). Aliquots (50 μl) of the appropriate dilution were spread on agar plates containing NaCl (10 g/liter) and yeast extract (1.5 g/liter). The plates were incubated at room temperature for 2 days and then inspected carefully, and colonies showing new morphologies were picked and analyzed further.
Determination of mercury resistance level.
Isolates were grown in 5 ml of liquid growth medium [10 g of NaCl per liter, 2 g of yeast extract per liter, 4 g of sucrose per liter, 1 mg of Hg(II) per liter] for 1 day at 30°C on a rotary shaker at 180 rpm. Aliquots (100 μl) were serially diluted in PBS, and 50 μl of an appropriate dilution was spread on agar plates containing solid growth medium and various concentrations of Hg(II) [0, 1, 5, and 10 mg of Hg(II) per liter). For each resistance level, two dilutions were plated out in triplicate. The plates were incubated at room temperature for 4 days.
Extraction of DNA from pure cultures and biofilms.
Total DNA was isolated by the modified method of Wilson (13, 14) from 2 ml of an overnight culture grown in Luria-Bertani medium supplemented with 1 mg of Hg(II) per liter at 30°C on a rotary shaker. For extraction of DNA from biofilms, 2 ml of the glass beads was transferred to 6 ml of PBS and vortexed (5 min). Then 2 ml of the suspended biofilm was used for extraction of total cellular DNA as described above.
Primers and PCR amplification.
Primers for PCR were specific for conserved bacterial 16S rDNA sequences. PCR with primers R1401 and F968GC (3) amplified a bacterial 16S rDNA fragment from positions 968 to 1401 (Escherichia coli numbering). A GC-rich sequence was attached to the 5′ end of primer F968GC. PCR amplification was performed as described previously (3).
TGGE.
The TGGE system (Qiagen, Hilden, Germany) was used as described previously (3). A thermal gradient of 39 to 52°C was applied. A mixture of amplified 16S rDNA fragments of phylogenetically diverse bacteria was used as a reference pattern. The gels were silver stained (11), dried, and scanned (Umax Astra 1220U).
Determination of 16S rDNA sequences and species identification.
DNA from pure cultures of effluent bacteria was extracted as described above. Nearly complete 16S rRNA genes were amplified by PCR using a forward primer hybridizing at positions 8 to 27 and a reverse primer hybridizing at the complement of positions 1525 to 1541 (E. coli 16S rRNA gene sequence numbering). PCR was performed as described previously (13). The sequence of the amplified 16S rDNA was determined directly using an Applied Biosystems 373A DNA sequencer (Perkin-Elmer, Applied Biosystems GmbH, Weiterstadt, Germany) and the protocols recommended by the manufacturer for Taq polymerase-initiated cycle sequencing with fluorescent-dye-labeled dideoxynucleotides and standard 16S rRNA sequencing primers (6). The resulting sequences were aligned with reference 16S rRNA and 16S rRNA gene sequences from the EMBL database by using FASTA (9).
Biofilm structure.
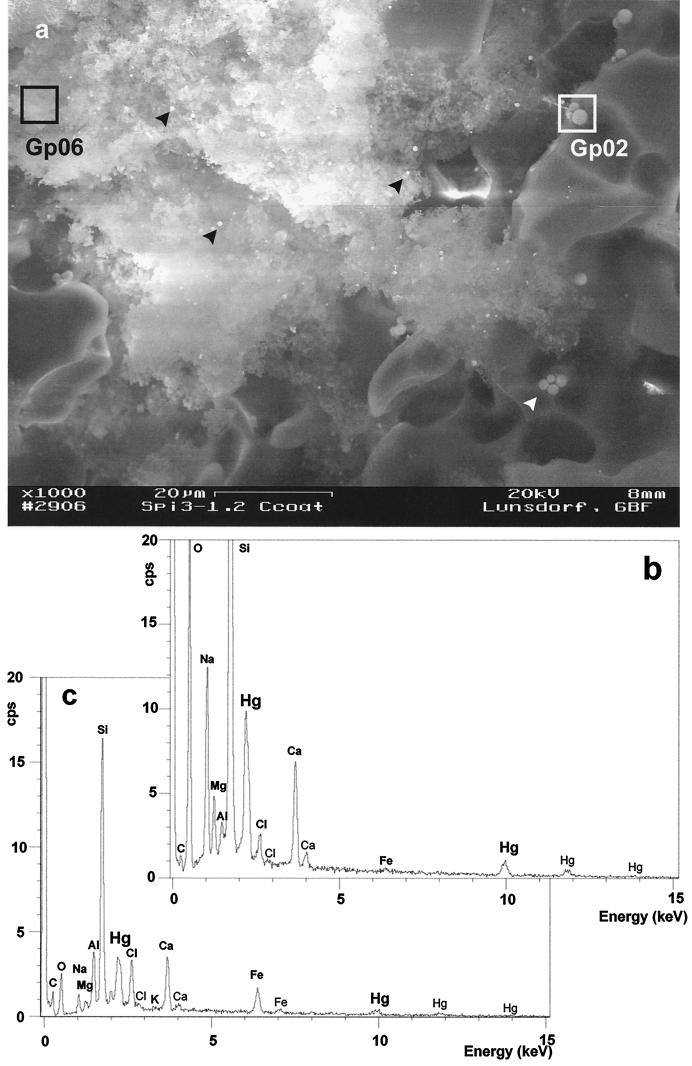
Figure 1 shows a biofilm that had developed on the carrier material after 14 days of sterile reactor operation with model wastewater. A single microbial cell layer can be seen attached to the surface of the carrier material. The bacteria stuck firmly to the substratum and were not lost during bioreactor operation. This thin microbial biofilm acted as the catalyst for mercury removal, illustrating that it is not adsorption to biomass which is the main mechanism operating here. Figure 2a shows a larger field from a lower level of the reactor. Here, biomass had massively grown and filled the substratum cavity with an amorphous extracellular polysaccharide cell matrix, dotted with irregularly dispersed mercury droplets. EDX analysis of boxed areas, 64 μm2 in size, revealed prominent Hg peaks (Mα1 = 2,195 keV; Lα1 = 9,987 keV), which correlated well with the droplet size and peak height. The number and size of mercury droplets increased during continuous operation of the reactor. Similar intensity ratios for O to Si to Ca to Na were found on the pure Siran surface (EDX data not shown) and around mercury droplets on the Siran surface (1:2.83:0.35:0.5 and 1:2.95:0.33:0.57, respectively [Fig. 2b]), contrasting with the element intensity ratios of the biofilm area (1:6.40:1.60:0.24 [Fig. 2c]). Siran not only is composed of SiO2 but also contains small amounts of Na, Ca, Mg, and Al, and thus the corresponding EDX intensities are derived from the carrier material. Additionally, the biofilm matrix contained Fe (Kα1 = 6,403 keV) from the yeast extract medium and chlorine (Kα1 = 2,622 keV) from the NaCl present in the wastewater. Thus, mercury droplets of different sizes were identified throughout the carrier material, some of them in close contact with clumps of biomass glued together by extracellular polysaccharide material.
FIG. 1.
Scanning electron micrograph survey of a P. putida Spi3 biofilm on ceramic carrier material (Siran beads) from a packed-bed mercury-reducing bioreactor after 14 days of operation with sterile synthetic wastewater. A morphologically homogenous bacterial population is growing on the carrier as a monolayer. Little EPS can be observed (arrowheads).
FIG. 2.
EDX analysis of a P. putida Spi3 biofilm from a packed-bed mercury-reducing bioreactor after 14 days of operation with sterile synthetic wastewater. (a) Scanning electron micrograph of a biofilm aggregate, which fills a cavity of the carrier material (Siran beads) and contains several brightly shining droplets of mercury (black arrowheads). These droplets are larger when in direct contact with the carrier surface (white arrowhead, white-framed box). Boxed areas, i.e., Gp06 and Gp02, represent the detection area of the EDX analysis. (b) EDX spectrum of area Gp02 on Hg droplets in direct contact with the Siran surface. (c) EDX spectrum of area Gp06 as a characteristic in situ biomass sample.
TGGE fingerprints and identification of effluent bacteria.
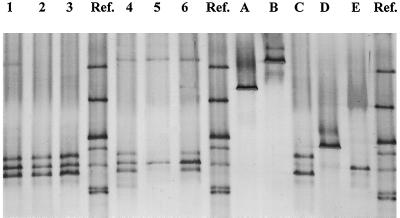
From the effluents of six laboratory bioreactors operated continuously with chloralkali electrolysis wastewater, 13 bacterial strains were isolated on the basis of differences in colony morphology. These strains could be grouped into four clearly distinguishable TGGE fingerprint types. The TGGE fingerprints of the inoculant strain, P. putida Spi3, and representatives of the four effluent TGGE fingerprint types are shown in Fig. 3. Most isolates generated single bands, indicating that their 16S rDNA amplicons showed no sequence heterogeneity, similar to results of Felske et al. (4) for coryneform bacteria. They could be distinguished from each other on the basis of their position relative to each other and to the standard. Two rDNA bands were observed for TGGE fingerprint type C.
FIG. 3.
TGGE fingerprints of biofilm samples from six mercury-reducing bioreactors treating nonsterile chemical wastewater (lanes 1 to 6), effluent bacterial cultures (lanes A to D), and the inoculant strain, P. putida Spi3 (lane E). Ref, reference standard.
Representatives of these fingerprint types were subsequently identified by 16S rDNA sequencing and sequence comparison with the EMBL sequence database. Thus, fingerprint type A corresponded to Stenotrophomonas maltophilia (100% sequence identity), represented by isolates 1 and 12; fingerprint type B was Bacillus cereus (99.6% sequence identity), represented by isolates 3, 6, 7, 8, and 11; fingerprint type C was Paenibacillus amylolyticus (97.7% sequence identity), represented by isolates 4, 5, 9, 10, and 13; and fingerprint type D was Citrobacter freundii (99.6% sequence identity), represented by isolate 2. Identifications were confirmed by sequencing several isolates from the same fingerprint type, which always yielded the same results. The identity of C. freundii was confirmed by sequencing through the Deutsche Sammlung von Mikroorganismen und Zellkulturen (DSMZ) and by phenotypic tests. Fingerprint type C (Paenibacillus amylolyticus) showed two TGGE bands, confirming the results of Nübel et al. (7), who previously found different sequences for 16SrDNA amplicons from P. polymyxa.
Isolates having the same TGGE fingerprint showed the same 16S rDNA sequence. Thus, multiple isolates of Paenibacillus amylolyticus, S. maltophilia, and B. cereus, which had been picked because of differences in colony morphology and which also differed with respect to mercury resistance (see below), were not distinguishable on the basis of their TGGE fingerprints. To differentiate on the strain level, genomic fingerprint techniques with a higher resolution would be needed.
Biofilm community composition.
Community fingerprints from the bioreactors are shown in Fig. 3, lane 1 to 6. The inoculant strain, P. putida Spi3, could clearly be detected in all bioreactors, and up to four additional bands were present. Community fingerprints from bioreactors 1, 2, 3, 4, and 6 showed a double band which was identical to fingerprint type C, identified as Paenibacillus amylolyticus. This organism was absent from the TGGE fingerprint of bioreactor 5, although it was isolated from its effluent. Bioreactors 1, 4, 5, and 6 showed a TGGE band corresponding to fingerprint type B, identified as B. cereus. TGGE fingerprint type A, identified as S. maltophilia, was detected only once, in bioreactor 6. TGGE bands of fingerprint type D, identified as C. freundii, were not seen in any of the community fingerprints.
The community profiles from the six different bioreactors consisted of two to five bands. These bands could clearly be matched to the TGGE bands of isolated, pure strains of effluent bacteria. Thus, it was possible to determine the structure of the biofilm communities in the bioreactors. They were composed of the inoculant strain, P. putida Spi3, and one to three additional species of bacteria. There were no bands in the community fingerprints which could not be found in effluent bacteria; thus, there was no indication of unculturable bacteria in the bioreactor. Conversely, one of the effluent bacteria, C. freundii, was not detected in the community fingerprint. The reason might be that it colonized a different part of the bioreactor, not the glass beads in the bottom part from which biofilm samples were taken. Alternatively, its abundance in the biofilms might have been below the detection limit of TGGE, which is estimated to be around 1% in complex communities.
Mercury resistance of invading strains.
The mercury resistance levels of bacteria strongly depend on the number of cells used in the assay (2), with much higher resistance levels found at high cell densities, and on the buffering and complexing capacity of the test medium. Moreover, much higher resistance is generally observed on plates than in liquid cultures (5), because on plates the growth of the test strains can locally reduce mercury concentrations. The ability of a single cell to form a colony on an agar plate of a given mercury concentration was used here to determine in a rapid and reproducible way the resistance levels of our strains. The medium was unbuffered to obtain data which would be relevant to chemical wastewater conditions.
Table 1 shows the MICs for previously constructed mercury-reducing genetically engineered strains, the inoculant strain P. putida Spi3, and the 13 reactor effluent isolates. Three strains were not able to grow on mercury-containing plates, namely, P. putida KT2442::mer73::gfp11 and B. cereus Tin3 and Tin6. All other strains, including the inoculant strain P. putida Spi3, grew at 1 mg of Hg(II) per liter. Four strains were able to grow on plates with 5 mg of Hg(II) per liter, namely P. putida KT2442::mer73 and Paenibacillus amylolyticus Tin9, Tin10, and Tin13. Thus, some of the invading strains showed stronger mercury resistance than did the inoculant strain P. putida Spi3, most had a similar resistance level, and two were not mercury resistant. Strains belonging to the same species showed differences in resistance levels; e.g., B. cereus Tin3 and Tin6 did not grow on mercury-containing plates, while B. cereus Tin7, Tin8, and Tin11 grew at 1 mg of Hg(II) per liter. Similarly, Paenibacillus amylolyticus Tin9, Tin10, and Tin13 grew at 5 mg of Hg(II) per liter while Paenibacillus amylolyticus Tin4 and Tin5 only grew at 1 mg of Hg(II) per liter.
TABLE 1.
Mercury resistance levels of genetically engineered mercury-reducing strains and natural isolates
| Strain | Source or reference | MIC (mg/liter)a |
|---|---|---|
| P. putida KT2442::mer-73 | 5 | 10 |
| P. putida KT2442::mer-73::gfp-11 | 5 | <1 |
| P. putida F1::mer | 5 | 5 |
| P. putida Spi 3 | 12 | 5 |
| S. maltophilia Tin 1 | This work | 5 |
| S. maltophilia Tin 12 | This work | 5 |
| B. cereus Tin 3 | This work | <1 |
| B. cereus Tin 6 | This work | <1 |
| B. cereus Tin 7 | This work | 5 |
| B. cereus Tin 8 | This work | 5 |
| B. cereus Tin 11 | This work | 5 |
| Paenibacillus amylolyticus Tin 4 | This work | 5 |
| Paenibacillus amylolyticus Tin 5 | This work | 5 |
| Paenibacillus amylolyticus Tin 9 | This work | 10 |
| Paenibacillus amylolyticus Tin 10 | This work | 10 |
| Paenibacillus amylolyticus Tin 13 | This work | 10 |
| C. freundii Tin 2 | This work | 5 |
MICs were determined by plating serial dilutions of the test strains on media containing 0, 1, 5, and 10 mg of Hg(II) per liter.
None of our strains showed growth at 10 mg of Hg(II) per liter. The recombinant strain, P. putida KT2442::mer-73, could not grow above 5 mg of Hg(II) per liter, although growth at 80 mg/liter has been reported previously under different test conditions (5). Three other strains were able to grow at 5 mg of Hg2+ per liter, namely, Paenibacillus amylolyticus Tin9, Tin10, and Tin13. Thus, the resistance levels determined under the conditions described above were an order of magnitude lower than those reported in the literature.
The invading bacteria colonized the biofilms during the operation of the bioreactors, which had been autoclaved prior to inoculation with P. putida Spi3. No mercury-resistant strains could be enriched from the original wastewater, which had a pH of 2.5. However, for the remediation experiments, the wastewater was neutralized and stayed in contact with air for several weeks. Thus, the main colonization route can be assumed to be the air.
The colonization of the biofilms by additional species of bacteria did not impair the activity of the bioreactors (see reference 12 for data on bioreactor removal efficiency). The mercury present in the continuous wastewater inflow maintained the necessary selective pressure to exclude non-mercury-resistant invaders from colonizing the biofilms. Since mercury reduction is a one-step enzymatic process, the sequential attack of different groups of bacteria to complete the degradation reaction, which is well known for the degradation of recalcitrant xenobiotics (e.g., for polychlorinated biphenyl degradation), can be excluded. However, niche differentiation might operate in the bioreactors on the microscale for parameters like mercury concentration, oxygen concentration, flow velocity, and nutrient concentration and thus allow the development of the observed mercury-resistant multispecies biofilm communities.
Acknowledgments
We thank Ina Grammel for excellent technical assistance. Thanks to Sven Panke and Rajan Hollmann for help with maintenance of the bioreactors.
This work was supported by the European Community LIFE (LIFE97-ENV/D/000463) and BIOTECHNOLOGY (BIO4-CT98-0168) programs and by a grant from the government of Lower Saxony (Forschungsschwerpunkt Meeresbiotechnologie).
REFERENCES
- 1.Brunke M, Deckwer W-D, Frischmuth A, Horn J M, Lünsdorf H, Rhode M, Röhricht M, Timmis K N, Weppen P. Microbial retention of mercury from waste streams in a laboratory columns containing merA gene bacteria. FEMS Microbiol Rev. 1993;11:145–152. doi: 10.1111/j.1574-6976.1993.tb00278.x. [DOI] [PubMed] [Google Scholar]
- 2.Chang J-S, Hong J. Estimation of kinetics of mercury detoxification from low-inoculum batch cultures of Pseudomonas aeruginosa PU21 (Rip64) J Biotechnol. 1995;42:85–90. doi: 10.1016/0168-1656(95)00032-l. [DOI] [PubMed] [Google Scholar]
- 3.Eichner C A, Erb R W, Timmis K N, Wagner-Döbler I. Thermal gradient gel electrophoresis analysis of bioprotection from pollutant shocks in the activated sludge microbial community. Appl Environ Microbiol. 1999;65:102–109. doi: 10.1128/aem.65.1.102-109.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Felske A, Vancanneyt M, Kersters K, Akkermans A D L. Application of temperature-gradient gel electrophoresis in taxonomy of coryneform bacteria. Int J Syst Bacteriol. 1999;49:113–121. doi: 10.1099/00207713-49-1-113. [DOI] [PubMed] [Google Scholar]
- 5.Horn J M, Brunke M, Deckwer W-D, Timmis K N. Pseudomonas putida strains which constitutively overexpress mercury resistance for biodetoxification of organomercurial pollutants. Appl Environ Microbiol. 1994;60:357–362. doi: 10.1128/aem.60.1.357-362.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lane D J. 16S/23S rRNA sequencing. In: Stackebrandt E, Goodfellow M, editors. nucleic acid techniques in bacterial systematics. Chichester, England: John Wiley & Sons Ltd.; 1991. pp. 115–175. [Google Scholar]
- 7.Nübel U, Engelen B, Felske A, Snaidr J, Wieshuber A, Amann R L, Ludwig W, Backhaus H. Sequence heterogeneities of genes encoding 16S rRNAs in Paenibaxillus polymxa detected by temperature gradient gel electrophoresis. J Bacteriol. 1996;178:5636–5643. doi: 10.1128/jb.178.19.5636-5643.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Osborn M, Bruce K D, Strike P, Ritchie D A. Distribution, diversity and evolution of the bacterial mercury resistance (mer) operon. FEMS Microbiol Rev. 1997;19:239–262. doi: 10.1111/j.1574-6976.1997.tb00300.x. [DOI] [PubMed] [Google Scholar]
- 9.Pearson W R, Lipman D J. Improved tools for biological sequence comparison. Proc Natl Acad Sci USA. 1988;85:2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Riesner D, Steger G, Zimmat R, Owens R A, Wagenhöfer M, Hillen W, Vollbach S, Henco K. Temperature-gradient gel electrophoresis of nucleic acids: analysis of conformational transitions, sequence variations, and protein-nucleic acid interactions. Electrophoresis. 1989;10:377–389. doi: 10.1002/elps.1150100516. [DOI] [PubMed] [Google Scholar]
- 11.Summers A O. Organization, expression and evolution of genes for mercury resistance. Annu Rev Microbiol. 1986;40:607–634. doi: 10.1146/annurev.mi.40.100186.003135. [DOI] [PubMed] [Google Scholar]
- 12.von Canstein H, Li Y, Timmis K N, Deckwer W-D, Wagner-Döbler I. Removal of mercury from chloralkali electrolysis waste water by a mercury-resistant Pseudomonas putida strain. Appl Environ Microbiol. 1999;65:5279–5284. doi: 10.1128/aem.65.12.5279-5284.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wagner-Döbler I, Bennasar A, Vancanneyt M, Strompl C, Brummer I, Eichner C, Grammel I, Moore E R B. Microcosm enrichment of biphenyl-degrading microbial communities from soils and sediments. Appl Environ Microbiol. 1998;64:3014–3022. doi: 10.1128/aem.64.8.3014-3022.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wilson K. Preparation of genomic DNA from bacteria. In: Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Current protocols in molecular biology. New York, N.Y: John Wiley & Sons, Inc.; 1987. pp. 2.4.1–2.4.2. [Google Scholar]