Abstract
Aim
To clarify the role of mediators of ectopic mineralization as biomarkers for arterial calcifications.
Methods
MEDLINE and Embase were searched for relevant literature, until January 4th 2022. The investigated biomarkers were: calcium, phosphate, parathyroid hormone, vitamin D, pyrophosphate, osteoprotegerin, receptor activator of nuclear factor-kappa B ligand (RANKL), fibroblast growth factor-23 (FGF-23), Klotho, osteopontin, osteocalcin, Matrix Gla protein (MGP) and its inactive forms and vitamin K. Studies solely performed in patients with kidney insufficiency or diabetes mellitus were excluded.
Results
After screening of 8985 articles, a total of 129 articles were included in this systematic review. For all biomarkers included in this review, the results were variable and more than half of the studies for each specific biomarker had a non-significant result. Also, the overall quality of the included studies was low, partly as a result of the mostly cross-sectional study designs. The largest body of evidence is available for phosphate, osteopontin and FGF-23, as a little over half of the studies showed a significant, positive association. Firm statements for these biomarkers cannot be drawn, as the number of studies was limited and hampered by residual confounding or had non-significant results. The associations of the other mediators of ectopic mineralization with arterial calcifications were not clear.
Conclusion
Associations between biomarkers of ectopic mineralization and arterial calcification are variable in the published literature. Future longitudinal studies differentiating medial and intimal calcification could add to the knowledge of biomarkers and mechanisms of arterial calcifications.
Abbreviations: MGP, matrix Gla protein; RANKL, receptor activator of nuclear factor-kappa B ligand; FGF-23, fibroblast growth factor-23; OPG, osteoprotegerin; CAC, coronary artery calcification; CAD, coronary artery disease; GACI, generalized arterial calcification of infancy; PXE, pseudoxanthoma elasticum; PTH, parathyroid hormone; uc-MGP, uncarboxylated MGP; dp-cMGP, carboxylated but dephosphorylated MGP; dp-ucMGP, uncarboxylated an dephosphorylated MGP; CVD, cardiovascular disease; 25(OH)D, 25-hydroxyvitamin D; 1,25(OH)2D, 1,25-dihydroxyvitamin D; PIVKA-2, protein induced by vitamin K absence or antagonist-2; PK, phylloquinone; MK, menaquinone
Keywords: Arterial calcification, Biomarkers, Review
Highlights
-
•
We researched the association between biomarkers and arterial calcifications.
-
•
This review focused on biomarkers of bone metabolism and Matrix Gla protein.
-
•
Associations between biomarkers and arterial calcification are variable.
-
•
Future studies should differentiate between medial and intimal calcifications.
1. Introduction
Arterial calcifications are highly prevalent, especially in older adults (van der Toorn et al., 2020). There is a large body of evidence on the strong prognostic value of arterial calcifications for major cardiovascular events and mortality. For example, coronary artery calcification (CAC) has been associated with coronary artery disease (CAD) and cerebrovascular disease (Wu et al., 2016a). Furthermore, intracranial artery calcifications have been associated with cerebrovascular disease (Wu et al., 2016a). Arterial calcifications are also strong predictors of mortality, both all-cause mortality and cardiovascular mortality (van der Toorn et al., 2020).
Arterial calcification can be present in different locations of the blood vessel wall. Calcification in the tunica intima seems to be related to lipid deposits and infiltration of inflammatory cells. It is commonly thought that these calcifications are a positive reparative mechanism that prevent plaque growth and rupture. Calcification in the tunica media seems to result from transformation of vascular smooth muscle cells into osteoblast-like cells (Lee et al., 2020). The transformation into osteoblast-like cells is an active process in which mediators of bone metabolism seem to have an important role (Rochette et al., 2019). Medial calcifications are associated with arterial stiffening and may be a cause of chronic cardiovascular disease and organ failure (Kockelkoren et al., 2017).
Important mediators of ectopic mineralization are osteoprotegerin (OPG) and receptor activators of nuclear factor kappa-B ligand (RANKL). RANKL plays an important role in bone metabolism by promoting bone resorption. Also, it seems to promote arterial calcification (Rochette et al., 2019). OPG binds RANKL, which inhibits the RANKL effects. The possible effect on arterial calcification is clear in OPG depleted mice. These mice develop spontaneous and severe arterial calcification (Rochette et al., 2019). Another mediator of ectopic mineralization is osteopontin, a protein involved in accelerating osteoclast function (Lee et al., 2020). The possible protective role of osteopontin against arterial calcification is again proposed from osteopontin depleted mice. These mice demonstrated increased arterial calcification (Lok and Lyle, 2019). Osteocalcin, a protein that can be used as a marker for bone turnover, might also have a role in arterial calcification (Millar et al., 2019).
Furthermore, arterial calcifications are more common in patients with chronic kidney disease. In this population calcium, but especially, phosphate disturbances resulting in hyperphosphatemia seem to be important for the development of arterial calcification (Bäck and Michel, 2021). The importance of phosphate for arterial calcification also becomes clear when investigating genetic disorders resulting in arterial calcification. Generalized arterial calcification of infancy (GACI) and pseudoxanthoma elasticum (PXE) for example, demonstrate severe arterial calcifications because of a genetic mutation resulting in an increase of inorganic phosphate and a decrease of inorganic pyrophosphate. The latter serves as a physiological inhibitor of calcification and growth (Rutsch et al., 2021). In preserving the balance between calcium and phosphate concentrations, parathyroid hormone (PTH) and vitamin D are important (Locatelli et al., 2002). Other proteins that have a role in the regulation of phosphate are fibroblast growth-factor 23 (FGF-23) and Klotho, a coreceptor for FGF-23. These factors decrease renal phosphate reabsorption and thereby decrease phosphate serum concentrations (Pescatore et al., 2019).
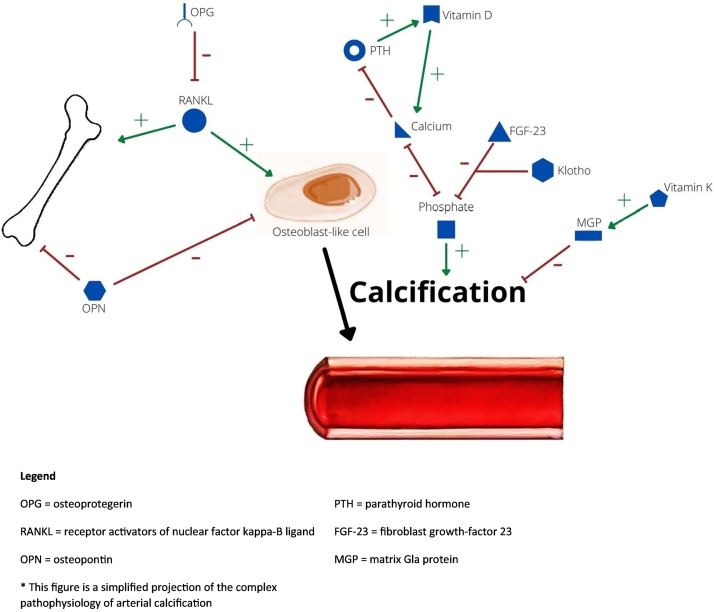
Also, matrix Gla protein (MGP) has an important role in arterial calcifications. MGP is a vitamin K-dependent protein and is considered to be a powerful inhibitor of arterial calcification. MGP in its phosphorylated and carboxylated form is the active protein. Vitamin K is necessary as an enzyme to transform MGP in this active form. In case of vitamin K deficiencies, other inactive forms of MGP can be present: uncarboxylated MGP (uc-MGP), carboxylated but dephosphorylated MGP (dp-cMGP) and uncarboxylated an dephosphorylated MGP (dp-ucMGP) (Roumeliotis et al., 2019). For an overview of these mediators of ectopic mineralization and their possible pathophysiological influence on arterial calcifications see Fig. 1.
Fig. 1.
simplified scheme of pathophysiological processes related to arterial calcifications.
As aforementioned, there are several mediators of ectopic mineralization that seem to play a role in arterial calcification. These biomarkers could potentially be used for prediction of progression of calcification, and to discover or monitor treatment options of arterial calcification. Therefore, we conducted a systematic review to summarize evidence on these possible biomarkers as etiological factors of arterial calcifications and evaluate the quality and level of evidence of the studies to elaborate on the potential therapeutic properties of these biomarkers. We focused this systematic review on patients without kidney disease or diabetes mellitus, because of the metabolic changes that influence the vascular metabolism.
2. Material and methods
The systematic review was performed according to the protocol described by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) for systematic reviews (Moher et al., 2015).
2.1. Search strategy
For this systematic review, the medical databases MEDLINE and Embase were searched until January 4th 2022. We searched on biomarkers of ectopic mineralization and the association with arterial calcifications. The search terms, generated by one of the authors (NG) in consultation with an experienced librarian, are fully described in the Supplementary appendix (Supplementary appendix 1). The search was limited to human subjects. Furthermore, the Embase search was limited to articles, reviews and articles in press. There was no limitation for language. Duplicates were removed using Rayyan software (Ouzzani et al., 2016).
2.2. Selection criteria
All original articles that investigated an association between arterial calcification and one of the following serum biomarkers of ectopic mineralization were included: calcium, phosphate, PTH, vitamin D, pyrophosphate, OPG, RANKL, FGF-23, Klotho, osteopontin, osteocalcin, MGP and its inactive forms, and vitamin K. We only included articles in adults. We excluded articles that measured arterial calcification through X-ray, because X-ray is less reliable in detecting arterial calcification than other methods like computed tomography (CT) scans (Cecelja et al., 2013). Sensitivity analysis was performed to study whether the results changed when studies with X-ray were included. There was no exclusion based on the site of the calcification, but valve calcifications were not included.
Articles in languages different from English, Dutch, German or French were excluded after screening. Articles solely targeting patients with kidney insufficiency and diabetes mellitus were excluded from the analyses because of the metabolic changes that influence the vascular metabolism in these patients.
2.3. Study selection
Study selection was performed by two of the authors independently (NG and MS) using Rayyan software (Ouzzani et al., 2016). After separately selecting all articles, the differences were discussed between those two authors. The remaining uncertainties were then discussed with a third author (HK).
2.4. Data extraction
One of the authors (NG) extracted the descriptive and outcome data from the selected articles. The following data was selected: study design, population characteristics, method of arterial calcification calculation, location of measured arterial calcification and analyzed biomarker.
2.5. Quality assessment
The quality assessment has been performed by one of the authors (NG) using the National Heart, Lung and Blood Institute (NIH) quality assessment tool (Institue TNHLaB Quality assessment tool). Observational Cohort and Cross-Sectional Studies were assessed with a tool consisting of 14 questions. The answers to the questions in the tool could be yes, moderate, no, not reported or not applicable. The exact interpretation of these questions is fully added in the Supplementary appendix (Supplementary appendix 2). After the quality assessment, all answers were added; 1 point for yes, 0.5 for moderate and 0 for no or not reported. If questions were not applicable, this question was subtracted from the total.
3. Results
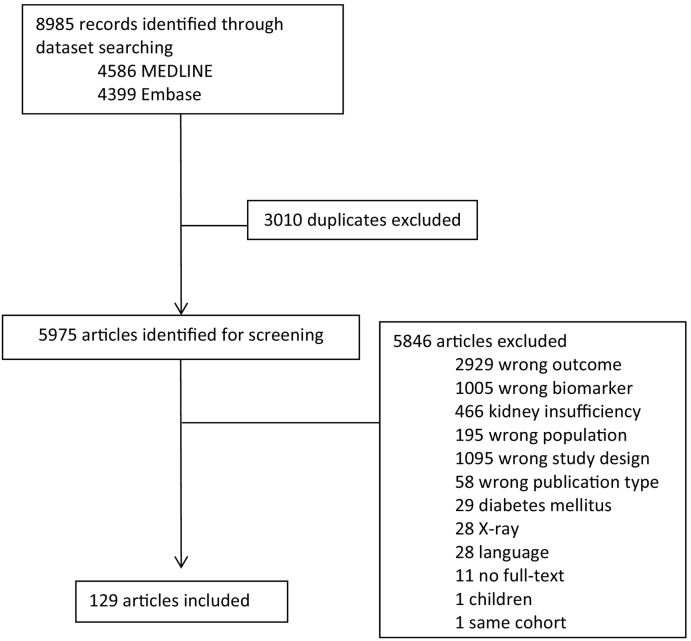
We identified 8985 articles through electronic database searching. After removing all duplicates, 5975 articles remained for screening. Of these, 5846 articles were excluded for various reasons. During the selection process there were no remaining uncertainties that had to be discussed with a third author. Of all screened articles, 28 (0,5 %) were excluded because of language. Of these articles fifteen were in Japanese, four in Russian, three in Polish and one in either Chinese, Arabic, Spanish, Italian, Croatian or Czech. Furthermore, eleven (0,2 %) had to be excluded, because there was no full text available, even after requesting the authors for a full text. Lastly, one article was excluded because it performed analyses in the same cohort as another study with the same biomarkers. Fig. 2 illustrates the complete study identification. After screening, 129 articles remained eligible for this review. The complete data extraction is added in the Supplementary appendix 3.
Fig. 2.
Study selection.
The results of the sensitivity analysis including studies that used X-ray as measurement for arterial calcification are presented in the Supplementary appendix 3a. The studies mostly included calcification of the abdominal aorta and the results were comparable.
3.1. Biomarkers of ectopic mineralization
3.1.1. Calcium
Thirty-four articles published results investigating a possible association between calcium and arterial calcification (Alagoz et al., 2014; Arad et al., 1998; Billington et al., 2020; Chen et al., 2006; Criqui et al., 2010; Foley et al., 2009; Grønhøj et al., 2016; Kim et al., 2013; Kwak et al., 2014; Liang et al., 2021; Nam et al., 2017; Nielsen et al., 2021; Park et al., 2011; Park et al., 2016; Shin et al., 2012; Tuersun et al., 2020; Tuttle and Short, 2009; Wei et al., 2020; Wu et al., 2016b; Zagura et al., 2011; Barbarash et al., 2016; Cancela et al., 2012; Chai et al., 2017; Davaine et al., 2016; Diederichsen et al., 2017; Donate-Correa et al., 2019; Golovkin et al., 2016; Liu et al., 2019; Masai et al., 2013; Pirro et al., 2013; Schlieper et al., 2010; Torii et al., 2016; Awan et al., 2010; Razavi et al., 2021). Of these studies, nine reported positive associations (Grønhøj et al., 2016; Kwak et al., 2014; Nielsen et al., 2021; Shin et al., 2012; Tuttle and Short, 2009; Zagura et al., 2011; Diederichsen et al., 2017; Golovkin et al., 2016; Awan et al., 2010) and 25 reported no significant association (Alagoz et al., 2014; Arad et al., 1998; Billington et al., 2020; Chen et al., 2006; Criqui et al., 2010; Foley et al., 2009; Kim et al., 2013; Liang et al., 2021; Nam et al., 2017; Park et al., 2011; Park et al., 2016; Tuersun et al., 2020; Wei et al., 2020; Wu et al., 2016b; Barbarash et al., 2016; Cancela et al., 2012; Chai et al., 2017; Davaine et al., 2016; Donate-Correa et al., 2019; Liu et al., 2019; Masai et al., 2013; Pirro et al., 2013; Schlieper et al., 2010; Torii et al., 2016; Razavi et al., 2021). Five studies were longitudinal, of which one showed a positive association (Arad et al., 1998; Foley et al., 2009; Tuttle and Short, 2009; Diederichsen et al., 2017; Liu et al., 2019). This study included 1006 people from a general population born in 1949 (60–61 years of age) or 1959 (50–51 years of age). After five years of follow-up the participants without coronary calcifications at baseline showed more CAC progression (incidence rate ratio (IRR) 201, p 0.01) (Diederichsen et al., 2017). Furthermore, in patients with coronary calcification at baseline there was no association between calcium and CAC progression (Diederichsen et al., 2017). Of the eight studies that showed a positive association between calcium and arterial calcification in a cross-sectional design, three were not adjusted for possible confounders (Nielsen et al., 2021; Golovkin et al., 2016; Awan et al., 2010). The other five were of moderate quality of evidence (Grønhøj et al., 2016; Kwak et al., 2014; Shin et al., 2012; Tuttle and Short, 2009; Zagura et al., 2011).
In summary, the majority of papers extracted in this systematic review showed no significant association between calcium and arterial calcification.
3.1.2. Phosphate
Thirty-five studies investigated a possible association between phosphate and arterial calcification (Alagoz et al., 2014; Billington et al., 2020; Chen et al., 2006; Criqui et al., 2010; Foley et al., 2009; Grønhøj et al., 2016; Kim et al., 2013; Kwak et al., 2014; Liang et al., 2021; Nam et al., 2017; Nielsen et al., 2021; Park et al., 2011; Park et al., 2016; Shin et al., 2012; Tuersun et al., 2020; Tuttle and Short, 2009; Wei et al., 2020; Wu et al., 2016b; Barbarash et al., 2016; Cancela et al., 2012; Chai et al., 2017; Diederichsen et al., 2017; Donate-Correa et al., 2019; Golovkin et al., 2016; Liu et al., 2019; Masai et al., 2013; Pirro et al., 2013; Schlieper et al., 2010; Torii et al., 2016; Awan et al., 2010; Razavi et al., 2021; Martín-Reys et al., 2016; Barbarash et al., 2019; McPherson et al., 2012; Park et al., 2020). Of these studies, fourteen reported a significant positive association (Foley et al., 2009; Kim et al., 2013; Kwak et al., 2014; Park et al., 2011; Park et al., 2016; Shin et al., 2012; Tuersun et al., 2020; Tuttle and Short, 2009; Cancela et al., 2012; Diederichsen et al., 2017; Barbarash et al., 2019; McPherson et al., 2012; Park et al., 2020), three a negative association (Chen et al., 2006; Kim et al., 2013; Razavi et al., 2021) and twenty no significant association (Alagoz et al., 2014; Billington et al., 2020; Criqui et al., 2010; Grønhøj et al., 2016; Liang et al., 2021; Nam et al., 2017; Nielsen et al., 2021; Wei et al., 2020; Wu et al., 2016b; Barbarash et al., 2016; Chai et al., 2017; Donate-Correa et al., 2019; Golovkin et al., 2016; Liu et al., 2019; Masai et al., 2013; Pirro et al., 2013; Schlieper et al., 2010; Torii et al., 2016; Awan et al., 2010; Martín-Reys et al., 2016). Six articles described a longitudinal study of which two articles were the same cohort, but with a different time to follow-up (Alagoz et al., 2014; Foley et al., 2009; Tuttle and Short, 2009; Diederichsen et al., 2017; Liu et al., 2019; McPherson et al., 2012). Of these five longitudinal cohorts, three showed a positive association between phosphate and arterial calcification at follow-up (Foley et al., 2009; Diederichsen et al., 2017; McPherson et al., 2012). In 3015 young adults (18–30 years of age) higher phosphate concentrations at baseline was associated with the presence of CAC at fifteen years of follow-up (odds ratio (OR) 1.71, p 0.03), not corrected for the presence of CAC at baseline (Foley et al., 2009). In another cohort of 1006 people born in 1949 (60–61 years of age) or 1959 (50–51 years of age) higher phosphate was associated with more CAC progression after five years when coronary calcification was present at baseline (IRR 3.60, p 0.01). There was no significant association between phosphate at baseline and CAC progression in patients without coronary calcification at baseline (Diederichsen et al., 2017). Lastly, in 721 community-dwelling adults without known cardiovascular disease (CVD) higher phosphate was associated with more progression of CAC after ten years (β 0.50, p < 0.001) (McPherson et al., 2012). Of the two longitudinal studies that did not show an association between phosphate and arterial calcification one had a small sample size of 75 participants (Alagoz et al., 2014). The other was of moderate to high quality of evidence (Liu et al., 2019). Of the nine articles with a positive association in a cross-sectional design, one study was not corrected for possible confounders (Barbarash et al., 2019). The other eight articles were of low to moderate quality of evidence (Kim et al., 2013; Kwak et al., 2014; Park et al., 2011; Park et al., 2016; Shin et al., 2012; Tuersun et al., 2020; Cancela et al., 2012; Park et al., 2020). Of the two studies that showed a negative association between phosphate and arterial calcification, one was not corrected for possible confounders (Chen et al., 2006). The other study was of moderate quality of evidence (Kim et al., 2013; Razavi et al., 2021).
There were no studies that investigated an association between pyrophosphate and arterial calcification.
In summary, even though 40 % of the longitudinal studies did not show an association, there are some indications that phosphate can predict the development of arterial calcification in the future. It is not clear if this association is only present in people with already existing arterial calcification at baseline. Most studies showed no association between phosphate and arterial calcification in a cross-sectional design.
3.1.3. Calcium‑phosphorus product
Fifteen studies reported analyses of the association of calcium‑phosphorus product and arterial calcification (Alagoz et al., 2014; Foley et al., 2009; Kim et al., 2013; Kwak et al., 2014; Nam et al., 2017; Nielsen et al., 2021; Park et al., 2011; Shin et al., 2012; Wu et al., 2016b; Diederichsen et al., 2017; Masai et al., 2013; Awan et al., 2010; Razavi et al., 2021; Leckstroem et al., 2014; Cm et al., 2009). Of these studies, five indicated a positive association (Kim et al., 2013; Kwak et al., 2014; Shin et al., 2012; Diederichsen et al., 2017; Cm et al., 2009), one a negative association (Razavi et al., 2021) and nine no significant association (Alagoz et al., 2014; Foley et al., 2009; Nam et al., 2017; Nielsen et al., 2021; Park et al., 2011; Wu et al., 2016b; Masai et al., 2013; Awan et al., 2010; Leckstroem et al., 2014). Of the three longitudinal studies, one showed a positive association (Alagoz et al., 2014; Foley et al., 2009; Diederichsen et al., 2017). This study includes 1006 participants from the general population born in 1949 (60–61 years of age) or 1959 (50–51 years of age). It showed a positive association between calcium‑phosphorus product and CAC progression after five years of follow-up for both participants without CAC at baseline (IRR 2.46, p 0.02) and participants with CAC at baseline (IRR 1.53, p 0.03) (Diederichsen et al., 2017). The two other longitudinal studies showed no significant association (Alagoz et al., 2014; Foley et al., 2009). One of these studies is of high quality (Foley et al., 2009). The other includes a small sample size of 75 participants (Alagoz et al., 2014). The cross-sectional studies were of low to moderate quality of evidence.
In summary, there were conflicting results and the majority of papers extracted in this systematic review showed no significant association.
3.1.4. Parathyroid hormone
Three of 23 studies that analyzed a possible association between PTH and arterial calcification found a positive association (Tuersun et al., 2020; Wu et al., 2016b; Billington et al., 2016) and twenty no association (Alagoz et al., 2014; Arad et al., 1998; Billington et al., 2020; Grønhøj et al., 2016; Barbarash et al., 2016; Cancela et al., 2012; Davaine et al., 2016; Diederichsen et al., 2017; Golovkin et al., 2016; Masai et al., 2013; Pirro et al., 2013; Torii et al., 2016; Razavi et al., 2021; Martín-Reys et al., 2016; Cm et al., 2009; Bellasi et al., 2013; Soares et al., 2015; Bernandes et al., 2019; Kim et al., 2012; Watson et al., 1997). In the included articles there were two studies that were longitudinal (Alagoz et al., 2014; Diederichsen et al., 2017). However, these two articles did not show an association between PTH and progression of coronary calcifications (Alagoz et al., 2014; Diederichsen et al., 2017). The three studies that did find a positive association were all cross-sectional (Tuersun et al., 2020; Wu et al., 2016b; Billington et al., 2016).
In conclusion, the majority of papers showed no significant association between PTH and arterial calcification.
3.1.5. Vitamin D
In total, 30 studies performed analyses on a possible association between 25-hydroxyvitamin D (25(OH)D) and arterial calcifications (Billington et al., 2020; Grønhøj et al., 2016; Tuersun et al., 2020; Zagura et al., 2011; Davaine et al., 2016; Liu et al., 2019; Pirro et al., 2013; Awan et al., 2010; Razavi et al., 2021; Bernandes et al., 2019; Kim et al., 2012; de Boer et al., 2009; Cheru et al., 2019; Doherty et al., 1997; El Mokadem et al., 2021; Eyyüpkoca et al., 2021; Ho et al., 2015; Kiani et al., 2013; Lai et al., 2013; Lee et al., 2016; Lim et al., 2012; Michos et al., 2009; Moradi and Foroutanfar, 2017; Nikolova et al., 2021; Rodrigues et al., 2021; Sajjadieh et al., 2020; Satilmis et al., 2015; Shikuma et al., 2012; Sung et al., 2016; Janus et al., 2021). Of these, ten studies found a negative association between 25(OH)D and arterial calcification (Zagura et al., 2011; Pirro et al., 2013; de Boer et al., 2009; Doherty et al., 1997; El Mokadem et al., 2021; Eyyüpkoca et al., 2021; Lai et al., 2013; Lee et al., 2016; Moradi and Foroutanfar, 2017; Nikolova et al., 2021), two a positive association (Zagura et al., 2011; Sung et al., 2016) and nineteen no significant association (Billington et al., 2020; Grønhøj et al., 2016; Tuersun et al., 2020; Davaine et al., 2016; Liu et al., 2019; Awan et al., 2010; Razavi et al., 2021; Bernandes et al., 2019; Kim et al., 2012; Cheru et al., 2019; Ho et al., 2015; Kiani et al., 2013; Lim et al., 2012; Michos et al., 2009; Rodrigues et al., 2021; Sajjadieh et al., 2020; Satilmis et al., 2015; Shikuma et al., 2012; Janus et al., 2021). There were two longitudinal studies of which one showed a significant association (Liu et al., 2019; de Boer et al., 2009). This study included 1370 community-dwelling adults (45–84 years of age) without clinical CVD. After three years of follow-up there was more CAC progression in patients without CAC at baseline with lower 25(OH)D concentration at baseline (relative risk (RR) 1.23, p 0.05) (de Boer et al., 2009). However, there was no association between 25(OH)D and CAC progression in patients with CAC at baseline (de Boer et al., 2009). The studies with a significant association in a cross-sectional design were all of low to moderate quality of evidence (Zagura et al., 2011; Pirro et al., 2013; Doherty et al., 1997; El Mokadem et al., 2021; Eyyüpkoca et al., 2021; Lai et al., 2013; Lee et al., 2016; Moradi and Foroutanfar, 2017; Nikolova et al., 2021; Sung et al., 2016). Four studies were not adjusted for possible confounders (Pirro et al., 2013; El Mokadem et al., 2021; Moradi and Foroutanfar, 2017; Nikolova et al., 2021).
Of the eight studies that analyzed a possible association between 1,25-dihydroxyvitamin D (1,25(OH)2D) and arterial calcifications (Arad et al., 1998; Barbarash et al., 2016; Diederichsen et al., 2017; Golovkin et al., 2016; Masai et al., 2013; Awan et al., 2010; Watson et al., 1997; de Boer et al., 2009), three showed a negative association (Diederichsen et al., 2017; Masai et al., 2013; Watson et al., 1997) and five showed no association (Arad et al., 1998; Barbarash et al., 2016; Golovkin et al., 2016; Awan et al., 2010; de Boer et al., 2009). There were two longitudinal study, but these papers reported no significant association (Diederichsen et al., 2017; de Boer et al., 2009). Of the three cross-sectional studies that did find a negative association, one did not adjust the results for confounders (Watson et al., 1997). One study was of high quality of evidence (Diederichsen et al., 2017). It included 1006 people from the general population born in 1949 (60–61 years of age) and 1959 (50–51 years of age). At baseline the authors reported lower 1,25(OH)2D concentrations in people with CAC (p 0.01) [410]. However, there was no association with progression of CAC after five years (Diederichsen et al., 2017). The other study has a relative small sample size of 148 participants and was of moderate quality of evidence (Masai et al., 2013).
It can be concluded that most papers on the association of vitamin D with arterial calcifications showed no significant results and results were conflicting with both positive and negative associations.
3.1.6. FGF-23
Five of ten included studies that analyzed a possible association between FGF-23 and arterial calcification showed a positive association (Masai et al., 2013; Holden et al., 2018; Kestenbaum et al., 2014; Xiao et al., 2013; Morita et al., 2015). The other five showed no significant association (Cancela et al., 2012; Razavi et al., 2021; Martín-Reys et al., 2016; Roos et al., 2008; Zamparini et al., 2018). There were no longitudinal studies included. Of the five studies that found a positive association there were two articles with large study populations (Kestenbaum et al., 2014; Xiao et al., 2013). In 6546 community-dwelling adults (45–85 years of age) without known clinical CVD, adults with a FGF-23 concentration in the highest quartile (46.4-223 pg/mL) had more frequent CAC than adults with FGF-23 in the lowest quartile (<30.5 pg/mL) (OR 1.26, 95 %-confidence interval (95 %-CI): 1.09–1.46) (Kestenbaum et al., 2014). The other large study included 2076 patients who underwent a diagnostic coronary angiogram (Xiao et al., 2013). However, these results were not adjusted for possible confounders (Xiao et al., 2013). The other three studies that found a positive association included smaller study populations, ranging from 148 to 204 participants, and lower quality of evidence (Masai et al., 2013; Holden et al., 2018; Morita et al., 2015). The papers that reported no significant associations were of moderate quality of evidence and included smaller study populations, ranging from 30 to 325 included participants (Cancela et al., 2012; Razavi et al., 2021; Martín-Reys et al., 2016; Roos et al., 2008; Zamparini et al., 2018).
One study investigated the association between intact FGF-23 and c-terminal fragments of FGF-23 and arterial calcification in patients with peripheral arterial disease and reported that both biomarkers were higher in patients with calcification (Donate-Correa et al., 2019). However, these results were not adjusted for possible confounders.
It can be concluded that results from studies on the association between FGF-23 and arterial calcifications are inconsistent with half of the studies in this systematic review showing a positive association and the other half showing no association. However, the studies that indicated no association included smaller study populations. There were no longitudinal studies analyzing an association between FGF-23 and arterial calcifications.
3.1.7. Klotho
One of two studies in this systematic review found a negative association between Klotho and arterial calcification (Morita et al., 2015; Koga et al., 2021). In 75 patients with stable angina pectoris who underwent an intravascular ultrasound (IVUS) of the coronary arteries and Klotho was higher in patients with a lower CalcIndex (OR 6.94, p 0.026) (Koga et al., 2021).
Because of the small number of studies included it is not possible to make any conclusion about a possible association between Klotho and arterial calcification.
3.1.8. Osteoprotegerin and RANKL
In total, 38 studies are published that investigated a possible association between OPG and arterial calcification (Barbarash et al., 2016; Chai et al., 2017; Davaine et al., 2016; Diederichsen et al., 2017; Golovkin et al., 2016; Liu et al., 2019; Torii et al., 2016; Bernandes et al., 2019; Abedin et al., 2007; Asanuma et al., 2007; Bakhireva et al., 2008; Berezin and Kremzer, 2013a; Bezerra et al., 2005; Dekker et al., 2021; Esteghamati et al., 2014; Frost et al., 2008; Golledge et al., 2008; Hosbond et al., 2014a; Ketlogetswe et al., 2015; Kiani et al., 2017; Lieb et al., 2010; Liu et al., 2020; Mohammadpour et al., 2012; Nugroho and Widorini, 2017; Omland et al., 2007; Park et al., 2006; Pérez de Ciriza et al., 2014; Poornima et al., 2014; Poornima et al., 2018; Quercioli et al., 2012; Vinholt et al., 2013; Hosbond et al., 2014b; Hwang et al., 2012; Kwon et al., 2016; Pesaro et al., 2018; Salari et al., 2017; Stȩpień et al., 2012; Wilund et al., 2008). Nineteen studies indicated a positive association (Barbarash et al., 2016; Chai et al., 2017; Liu et al., 2019; Torii et al., 2016; Abedin et al., 2007; Asanuma et al., 2007; Dekker et al., 2021; Esteghamati et al., 2014; Golledge et al., 2008; Ketlogetswe et al., 2015; Liu et al., 2020; Nugroho and Widorini, 2017; Omland et al., 2007; Park et al., 2006; Pérez de Ciriza et al., 2014; Poornima et al., 2014; Kwon et al., 2016; Stȩpień et al., 2012) two a negative association (Golovkin et al., 2016; Mohammadpour et al., 2012) and seventeen no association (Davaine et al., 2016; Diederichsen et al., 2017; Bernandes et al., 2019; Bakhireva et al., 2008; Bezerra et al., 2005; Frost et al., 2008; Hosbond et al., 2014a; Kiani et al., 2017; Lieb et al., 2010; Poornima et al., 2018; Quercioli et al., 2012; Vinholt et al., 2013; Hosbond et al., 2014b; Hwang et al., 2012; Pesaro et al., 2018; Salari et al., 2017; Wilund et al., 2008). There were four longitudinal studies included in this systematic review (Diederichsen et al., 2017; Liu et al., 2019; Lieb et al., 2010; Liu et al., 2020). One of these longitudinal studies indicated a positive association. However, the authors did not adjust their results for possible confounders (Liu et al., 2019). Another longitudinal study in 1106 participants ≥60 years of age without carotid plaque at baseline, higher OPG at baseline was associated with the presence of carotid calcification after five years of follow-up (hazard ratio (HR) 1.15, 95 %-CI: 1.03, 2.12) (Liu et al., 2020). In this same cohort another study was performed with coronary calcification as outcome measure after five years follow-up. There was no association between OPG and coronary calcification shown (Lieb et al., 2010). Of the cross-sectional studies included that indicated a significant association more than half were not adjusted for possible confounders and had low quality of evidence (Barbarash et al., 2016; Chai et al., 2017; Golovkin et al., 2016; Torii et al., 2016; Golledge et al., 2008; Mohammadpour et al., 2012; Nugroho and Widorini, 2017; Omland et al., 2007; Pérez de Ciriza et al., 2014; Stȩpień et al., 2012). The cross-sectional studies not indicating an association partly included small sample sizes. However, there were also a few studies including large study populations without significant associations. The study populations ranged from eleven to 152 participants.
Eleven studies were performed investigating a possible association between RANKL and arterial calcification (Davaine et al., 2016; Bernandes et al., 2019; Bakhireva et al., 2008; Bezerra et al., 2005; Ketlogetswe et al., 2015; Lieb et al., 2010; Mohammadpour et al., 2012; Poornima et al., 2014; Quercioli et al., 2012; Hwang et al., 2012; Pesaro et al., 2018). Four reported a negative association (Davaine et al., 2016; Bezerra et al., 2005; Ketlogetswe et al., 2015; Poornima et al., 2014), three a positive association (Quercioli et al., 2012; Hwang et al., 2012; Pesaro et al., 2018) and four no significant association (Bernandes et al., 2019; Bakhireva et al., 2008; Lieb et al., 2010; Mohammadpour et al., 2012). There was one longitudinal study included that did not report a significant association (Lieb et al., 2010).
Three studies investigated a possible association between OPG/RANKL ratio and arterial calcification (Davaine et al., 2016; Bernandes et al., 2019; Mohammadpour et al., 2012). One study reported a significant, positive association (Mohammadpour et al., 2012). This study did not adjust their results for any possible confounders (Mohammadpour et al., 2012).
Overall, studies on the association of OPG with arterial calcifications showed inconsistent results, with half of the studies in this systematic review showing a positive association for OPG. However, the quality of evidence is relatively low and 46 % of the studies indicated no association. The results on association between RANKL and arterial calcification are inconsistent.
3.1.9. Osteopontin
Eight of fourteen studies showed a positive association between osteopontin and arterial calcification (Golovkin et al., 2016; Berezin and Kremzer, 2013a; Aryan et al., 2009; Berezin and Kremzer, 2013b; Luna-Luna et al., 2020; Nandkeolyar et al., 2019; Peng et al., 2022; Uz et al., 2009). The other six studies did not report a significant association (Barbarash et al., 2016; Stȩpień et al., 2012; Wilund et al., 2008; Acar et al., 2012; Miyabara et al., 2011; Zwakenberg et al., 2020). One longitudinal study including 1207 patients without known CVD, but with intermediate risk for cardiovascular disease, reported more progression of CAC measured with the square root method after four years of follow-up, in people with higher osteopontin at baseline (β 1.25, p < 0.05). However, there was no significant association when the progression of CAC was measured with different methods and there was also no significant association in rapid progression (Nandkeolyar et al., 2019). The cross-sectional studies with positive results were all with small sample sizes, ranging from 39 to 126 participants, and with low to moderate quality of evidence (Golovkin et al., 2016; Berezin and Kremzer, 2013a; Aryan et al., 2009; Berezin and Kremzer, 2013b; Luna-Luna et al., 2020; Peng et al., 2022; Uz et al., 2009). In contrast, the articles reporting no significant association consisted of larger study populations, ranging from 25 to 718 participants.
In summary, there was insufficient evidence that there is an association between osteopontin and arterial calcification.
3.1.10. Osteocalcin
In total, eighteen studies were performed investigating an association between osteocalcin and arterial calcification (Barbarash et al., 2016; Golovkin et al., 2016; Liu et al., 2019; Pirro et al., 2013; Torii et al., 2016; Awan et al., 2010; Bernandes et al., 2019; Kim et al., 2012; Watson et al., 1997; Salari et al., 2017; Wilund et al., 2008; Miyabara et al., 2011; Zwakenberg et al., 2020; Choi et al., 2015; Kim et al., 2016; Millar et al., 2020; Cahalane et al., 2021; Zhelyazkova-Savova et al., 2021). Two studies reported a positive association (Liu et al., 2019; Salari et al., 2017), five a negative association (Golovkin et al., 2016; Awan et al., 2010; Kim et al., 2012; Wilund et al., 2008; Cahalane et al., 2021) and eleven no association (Barbarash et al., 2016; Pirro et al., 2013; Torii et al., 2016; Bernandes et al., 2019; Watson et al., 1997; Miyabara et al., 2011; Zwakenberg et al., 2020; Choi et al., 2015; Kim et al., 2016; Millar et al., 2020; Zhelyazkova-Savova et al., 2021). One longitudinal study reported higher osteocalcin in people with a calcified coronary or carotid plaque after five years of follow-up in 1571 participants, ≥60 years of age, without plaque at baseline. However, this study did not adjust their results for possible confounders (Liu et al., 2019). The studies with a cross-sectional design showed conflicting results (Barbarash et al., 2016; Golovkin et al., 2016; Pirro et al., 2013; Torii et al., 2016; Awan et al., 2010; Bernandes et al., 2019; Kim et al., 2012; Watson et al., 1997; Salari et al., 2017; Wilund et al., 2008; Miyabara et al., 2011; Zwakenberg et al., 2020; Choi et al., 2015; Kim et al., 2016; Millar et al., 2020; Cahalane et al., 2021; Zhelyazkova-Savova et al., 2021).
Furthermore, three studies investigated uncarboxylated osteocalcin in which one indicated a positive association with arterial calcification (Okura et al., 2010) and the other two showed no association (Choi et al., 2015; Kim et al., 2016). The study with a positive result has a cross-sectional design and consists of a small sample size of 92 patients with essential hypertension (Okura et al., 2010).
Lastly, two cross-sectional studies investigated an association between uncarboxylated/carboxylated osteocalcin ratio and arterial calcification (Choi et al., 2015; Rennenberg et al., 2010). One of these studies showed a positive association with coronary calcification in men, but not in women (Choi et al., 2015).
It can be concluded that studies on the association of osteocalcin with arterial calcifications showed inconsistent results.
3.1.11. MGP
Eight studies reported on an association between MGP and arterial calcification (Janus et al., 2021; Pesaro et al., 2018; Wilund et al., 2008; Acar et al., 2012; Jin et al., 2021; Jono et al., 2004; O'Donnell et al., 2006; Silaghi et al., 2013). Five reported a positive association (Janus et al., 2021; Pesaro et al., 2018; Jin et al., 2021; O'Donnell et al., 2006; Silaghi et al., 2013), one a negative association (Jono et al., 2004) and two a non-significant association (Wilund et al., 2008; Acar et al., 2012). There were no longitudinal studies included. The cross-sectional studies were of low quality of evidence except for two studies. In 267 women who participated in a randomized controlled trial, higher MGP was reported with more severe CAC (p 0.04) (O'Donnell et al., 2006). However, there was no significant association in men who participated in the same randomized controlled trial or in the general population (O'Donnell et al., 2006). In 130 patients without known CAD who were referred for a coronary CT higher MGP was reported in patients with CAC (OR 3.12, p 0.02) (Pesaro et al., 2018).
Furthermore, six studies investigated dp-ucMGP with arterial calcifications [127,133,135-137,144,167,168,170,172,175]. One of these studies found a positive association. However, this cross-sectional study did not adjust their results for possible confounders (Dofferhoff et al., 2021).
Nine studies assessed a possible association between uc-MGP and arterial calcification (Schlieper et al., 2010; Torii et al., 2016; Rennenberg et al., 2010; Zhelyazkova-Savova et al., 2021; Dalmeijer et al., 2013a; Dalmeijer et al., 2013b; Malhotra et al., 2022; Shea et al., 2011; Zwakenberg et al., 2018) of which two reported a significant negative association (Schlieper et al., 2010; Torii et al., 2016) and seven no significant association (Rennenberg et al., 2010; Zhelyazkova-Savova et al., 2021; Dalmeijer et al., 2013a; Dalmeijer et al., 2013b; Malhotra et al., 2022; Shea et al., 2011; Zwakenberg et al., 2018). Both studies with a significant association did not adjust for possible confounders (Schlieper et al., 2010; Torii et al., 2016).
Two studies found no association between dp-MGP and arterial calcifications (Dalmeijer et al., 2013a; Dalmeijer et al., 2013b).
In summary, although there was some evidence that MGP has a positive association with arterial calcification, the studies were of low quality of evidence.
3.1.12. Vitamin K
For the biomarkers vitamin K1, protein induced by vitamin K absence or antagonist-2 (PIVKA-2), phylloquinone (PK), menaquinone (MK)-4 and MK-7 three studies analyzed an association with arterial calcification and no significant associations were reported (Torii et al., 2016; Zwakenberg et al., 2020; Shea et al., 2013).
3.2. Quality assessment
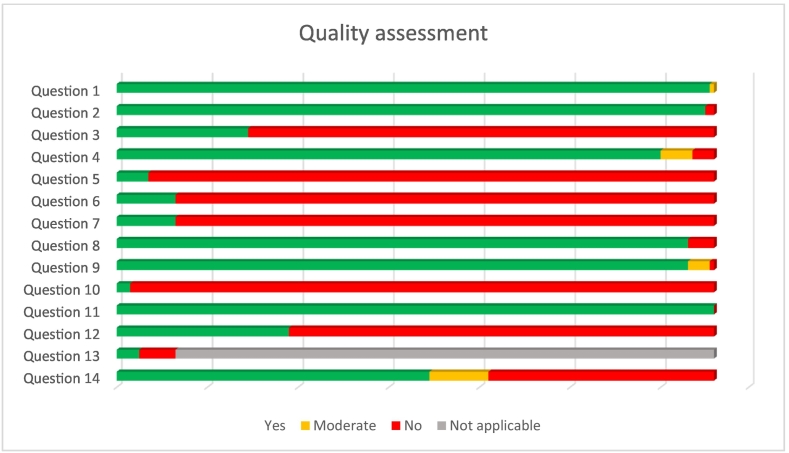
The quality assessment showed that the average quality of the studies was low to modest. Most studies had a cross-sectional design which makes one of the questions (question 13: “Was loss to follow-up after baseline 20% or less?”) not applicable. The mean score of the other thirteen questions for all the studies was 7.2. Of the longitudinal studies, 62 % had >20 % loss to follow-up after baseline. Furthermore, studies had particularly a poor result for the questions about sample size justification and the absence of repeated measures. For an overview on the quality assessment see Fig. 3. For a detailed quality assessment of all individual studies see Supplementary appendix 4.
Fig. 3.
Quality assessment.
4. Discussion
This systematic review shows that the associations of biomarkers of ectopic mineralization with arterial calcifications are not clear. For all biomarkers included in this review, the results are highly variable. For most biomarkers more than half of the studies showed a non-significant result.
There are several possible explanations for our findings. First, it may well be that the pathophysiology for intimal arterial calcification and medial arterial calcification differs. As stated before, it could be that tunica media calcifications originate from transformation of vascular smooth muscle cells into osteoblast-like cells (Lee et al., 2020). This transformation presumably results because of alterations in the investigated biomarkers of this systematic review. On the other hand, calcifications in the tunica intima seem to be more related to lipid deposits and infiltration of inflammatory cells (Lee et al., 2020). Biomarkers more related to these processes were not part of this systematic review. In most studies the differences between these dominant types of calcifications are not taken into account. Second, many studies have a cross-sectional design which has limitations for etiologic associations. Although we might conclude that the serum markers seem to have no relevant diagnostic value for arterial calcification. Third, the measurement of serum markers and/or arterial calcification varies, as well as the investigated study populations. Fourth, there were quality issues in several studies and overall quality was rated as modest. The largest body of evidence was available for phosphate, FGF-23 and osteopontin, where a little over half of the studies showed a significant, positive association. Still, firm conclusions for these biomarkers cannot be drawn, as the number of studies was limited and hampered by residual confounding or had non-significant results. This implicates that the complex mechanisms of arterial calcifications and its association with the biomarkers are not yet clearly understood based on these serum marker studies. Our etiologic findings should be weighed with evidence from other sources such as genetic insights and from clinical trials (van Varik et al., 2012; Vossen et al., 2020). Studies considering genetic diseases that cause arterial calcification show possible etiological factors associated with the development of arterial calcification, such as increased inorganic phosphate in PXE, Primary Familial Brain Calcification (PFBC) and GACI. However, our study does not support that association in other populations. Furthermore, studies performing clinical trials with interventions mediating the investigated biomarkers have not proven decrease of arterial calcification with these interventions (van Varik et al., 2012).
There were several strengths of this study. The systematic review consists of a wide number of possible biomarkers of ectopic mineralization. Furthermore, the search, data extraction and quality assessment has been thorough and the appropriate, widely acknowledged protocols and assessment tools have been used.
There were a few limitations of this systematic review. It is possible that publication bias resulted in less publication of non-significant results. This would make the level of evidence for the associations even less strong. Also, the investigated biomarkers were all measured in serum, whereas the calcifying process is in the blood vessel wall. This could indicate that measuring serum biomarkers might not be the designated method. However, serum measures are one of the less invasive methods with only few disadvantages. Furthermore, it was preferred to use severity of calcification as the outcome measure. However, some studies only reported the absence or presence of arterial calcification. When taking only studies that noted severity of calcification into account, the overall conclusions did not change. Lastly, there are several more biomarkers analyzed as possible biomarkers for ectopic mineralization in literature that we did not include in our systematic review. For example, biomarkers of inflammation, including Fetuin A, were not included.
The implications of the results of this systematic review are that with the current available evidence it is not clear what the etiological role is of the investigated biomarkers in arterial calcification. Therefore, at this time there is no implication that therapeutic options influencing these biomarkers will influence arterial calcifications. Also, at this point the biomarkers cannot be used to identify patients who could benefit from in-depth imaging of the site and severity of calcification. Future longitudinal studies of high quality are recommended in well-defined populations at risk for arterial calcifications, with differentiation of medial and intimal calcification in the arterial wall to further improve the knowledge of biomarkers and mechanisms of arterial calcifications.
5. Conclusion
Associations of biomarkers of ectopic mineralization with arterial calcifications are variable in the published literature. This hampers etiological insight into arterial calcifications and decisions for future intervention studies. Future longitudinal studies differentiating medial and intimal calcification could add to the knowledge of biomarkers and mechanisms of arterial calcifications and our review should be weighted with other sources of evidence.
The following are the supplementary data related to this article.
Full search terms.
Quality assessment using the National Heart, Lung and Blood Institute (NIH) quality assessment tool: Observational Cohort and Cross-Sectional Studies.
Data extraction.
Full quality assessment.
Supplementary appendix 5 Data extraction and quality assessment in case of calcification measurement with X-ray.
Supplementary appendix 6 References of supplementary appendix 5.
CRediT authorship contribution statement
All authors were involved in conception and design of the study. NG and MS contributed to the data acquisition. NG contributed to the writing of the manuscript. All authors critically reviewed and approved the final manuscript.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Abedin M., Omland T., Ueland T., Khera A., Aukrust P., Murphy S.A., et al. Relation of osteoprotegerin to coronary calcium and aortic plaque (from the Dallas heart Study) Am. J. Cardiol. 2007;99(4):513–518. doi: 10.1016/j.amjcard.2006.08.064. [DOI] [PubMed] [Google Scholar]
- Acar A., Cevik M.U., Arikanoglu A., Evliyaoglu O., Basarili M.K., Uzar E., et al. Serum levels of calcification inhibitors in patients with intracerebral hemorrhage. Int. J. Neurosci. 2012;122(5):227–232. doi: 10.3109/00207454.2011.642039. [DOI] [PubMed] [Google Scholar]
- Alagoz S., Cebi D., Akman C., Altiparmak M.R., Serdengecti K., Seyahi N. Progression of coronary artery calcification in living kidney donors: a follow-up study. Nephron Clin. Pract. 2014;126(3):144–150. doi: 10.1159/000362169. [DOI] [PubMed] [Google Scholar]
- Arad Y., Spadaro L.A., Roth M., Scordo J., Goodman K., Sherman S., et al. Serum concentration of calcium, 1,25 vitamin D and parathyroid hormone are not correlated with coronary calcifications. An electron beam computed tomography study. Coron. Artery Dis. 1998;9:513–518. doi: 10.1097/00019501-199809080-00007. [DOI] [PubMed] [Google Scholar]
- Aryan M., Kepez A., Atalar E., Hazirolan T., Haznedaroglu I., Akata D., et al. Association of plasma osteopontin levels with coronary calcification evaluated by tomographic coronary calcium scoring. J. Bone Miner. Metab. 2009;27(5):591–597. doi: 10.1007/x00774-009-0078-2. [DOI] [PubMed] [Google Scholar]
- Asanuma Y., Chung C.P., Oeser A., Solus J.F., Avalos I., Gebretsadik T., et al. Serum osteoprotegerin is increased and independently associated with coronary-artery atherosclerosis in patients with rheumatoid arthritis. Atherosclerosis. 2007;195(2):e135–e141. doi: 10.1016/j.atherosclerosis.2007.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awan Z., Alwaili K., Alshahrani A., Langsetmo L., Goltzman D., Genest J. Calcium homeostasis and skeletal integrity in individuals with familial hypercholesterolemia and aortic calcification. Clin. Chem. 2010;56(10):1599–1607. doi: 10.1373/clinchem.2010.147066. [DOI] [PubMed] [Google Scholar]
- Bäck M., Michel J.-B. From organic and inorganic phosphate to valvular and vascular calcifications. Cardiovasc. Res. 2021 doi: 10.1093/cvr/cvab038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakhireva L.N., Laughlin G.A., Bettencourt R., Barrett-Connor E. Does osteoprotegerin or receptor activator of nuclear factor-κB ligand mediate the association between bone and coronary artery calcification? J. Clin. Endocrinol. Metab. 2008;93(5):2009–2012. doi: 10.1210/jc.2007-2624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbarash O., Zykov M., Kashtalap V., Hryachokova O., Kokov A., Gruzdeva O., et al. Increased serum parathyroid hormone, osteocalcin and alkaline phosphatase are associated with a long-term adverse cardiovascular outcome after coronary artery bypass graft surgery. Diagnostics (Basel) 2019;9(4):143. doi: 10.3390/diagnostics9040143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbarash O.L., Lebedeva N.B., Kokov A.N., Novitskaya A.A., Hryachkova O.N., Voronkina A.V., et al. Decreased cathepsin K plasma level may reflect an association of osteopenia/osteoporosis with coronary atherosclerosis and coronary artery calcification in male patients with stable angina. Heart Lung Circ. 2016;25(7):691–697. doi: 10.1016/j.hlc.2016.02.002. [DOI] [PubMed] [Google Scholar]
- Bellasi A., Zona S., Orland G., Carli F., Ligabue G., Rochira V., et al. Inverse correlation between vascular calcification and bone mineral density in human immunodeficiency virus-infected patients. Calcif. Tissue Int. 2013;93(5):413–418. doi: 10.1007/s00223-013-9767-x. [DOI] [PubMed] [Google Scholar]
- Berezin A.E., Kremzer A.A. Serum uric acid as a marker of coronary calcification in patients with asymptomatic coronary artery disease with preserved left ventricular pump function. Cardiol. Res. Pract. 2013;2013 doi: 10.1155/2013/129369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berezin A.E., Kremzer A.A. Circulating osteopontin as a marker of early coronary vascular calcification in type two diabetes mellitus patients with known asymptomatic coronary artery disease. Atherosclerosis. 2013;229(2):475–481. doi: 10.1016/j.atherosclerosis.2013.06.003. [DOI] [PubMed] [Google Scholar]
- Bernandes M., Madureira A., Oliveira A., Martins M.J., Lucas R., Costa L., et al. Coronary artery calcium score in female rheumatoid arthritis patients: associations with apolipoproteins and disease biomarkers. Int. J. Rheum. Dis. 2019;22(10):1841–1856. doi: 10.1111/1756-185X.13685. [DOI] [PubMed] [Google Scholar]
- Bezerra M.C., Calomeni G.D., Caparbo V.F., Gebrim E.S., Rocha M.S., Pereira R.M.R. Low bone density and low serum levels of soluble RANK ligand are associated with severe arterial calcification in patients with takayasu arteritis. Rheumatology (Oxford) 2005;44(12):1503–1506. doi: 10.1093/rheumatology/kei045. [DOI] [PubMed] [Google Scholar]
- Billington E.O., Gamble G.D., Reid I.R. Parathyroid hormone reflects adiposity and cardiometabolic indices but not bone density in normal men. Bonekey Rep. 2016;5:852. doi: 10.1038/bonekey.2016.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billington E.O., Burt L.A., Plett R., Rose M.S., Boyd S.K., Hanley D.A. Effect of high-dose vitamin D supplementation on peripheral arterial calcification: secondary analysis of a randomized controlled trial. Osteoporos. Int. 2020;31(11):2141–2150. doi: 10.1007/s00198-020-05500-2. [DOI] [PubMed] [Google Scholar]
- de Boer I.H., Kestenbaum B., Shoben A.B., Michos E.D., Sarnak M.J., Siscovick D.S. 25-hydroxyvitamin D levels inversely associate with risk for developing coronary artery calcification. J. Am. Soc. Nephrol. 2009;20(8):1805–1812. doi: 10.1681/ASN.2008111157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahalane R.M., Barrett H.E., Ross A.M., Mulvihill J.J.E., Purtill H., Selvarajah L., et al. On the association between circulating biomarkers and atherosclerotic calcification in a cohort of arterial disease participants. Nutr. Metab. Cardiovasc. Dis. 2021;31(5):1533–1541. doi: 10.1016/j.numecd.2021.02.005. [DOI] [PubMed] [Google Scholar]
- Cancela A.L., Santos R.D., Sm Titan, Goldenstein P.T., Rochitte C.E., Lemos P.A., et al. Phosphorus is associated with coronary artery disease in patients with preserved renal function. PLoS One. 2012;7(5) doi: 10.1371/journal.pone.0036883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cecelja M., Frost M.L., Spector T.D., Chowienczyk P. Abdominal aortic calcification detection using dual-energy X-ray absorptiometry: validation study in healthy women compared with computed tomography. Calcif. Tissue Int. 2013;92:495–500. doi: 10.1007/s00223-013-9704-z. [DOI] [PubMed] [Google Scholar]
- Chai M., Zhang H.-T., Zhou Y.-J., Ji Q.-W., Yang Q., Liu Y.-Y., et al. Elevated IL-37 levels in the plasma of patients with severe coronary artery calcification. J. Geriatr. Cardiol. 2017;14(5):285–291. doi: 10.11909/j.issn.1671-5411.2017.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X.-Y., Lam W.W.M., Ng H.K., Fan Y.-H., Wong K.S. The frequency and determinants of calcification in intracranial arteries in chinese patients who underwent computed tomography examinations. Cerebrovasc. Dis. 2006;21(1–2):91–97. doi: 10.1159/000090206. [DOI] [PubMed] [Google Scholar]
- Cheru L.T., Saylor C.F., Fitch K.V., Looby S.E., Lu M., Hoffmann U., et al. Low vitamin D is associated with coronary atherosclerosis in women with HIV. Antivir. Ther. 2019;24(7):505–512. doi: 10.3851/IMP3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi B.-H., Joo N.-S., Kim M.J., Kim K.-M., Park K.-C., Kim Y.-S. Coronary artery calcification is associated with high serum concentration of uncarboxylated osteocalcin in asymptomatic korean men. Clin. Endocrinol. 2015;83(3):320–326. doi: 10.1111/cen.12792. [DOI] [PubMed] [Google Scholar]
- Cm McEniery, McDonnell B.J., So A., Aitken S., Bolton C.E., Munnery M., et al. Aortic calcification is associated with aortic stiffness and isolated systolic hypertension in healthy individuals. Hypertension. 2009;53(3):524–531. doi: 10.1161/HYPERTENSIONAHA.108.126615. [DOI] [PubMed] [Google Scholar]
- Criqui M.H., Kamineni A., Allison M.A., Ix J.H., Carr J.J., Cushman M., et al. Risk factor differences for aortic versus coronary calcified atherosclerosis: the multi-ethnic study of atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2010;30(11):2289–2296. doi: 10.1161/ATVBAHA.110.208181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalmeijer G.W., van der Schouw Y.T., Vermeer C., Magdeleyns E.J., Schurgers L.J., Beulens J.W.J. Circulating matrix gla protein is associated with coronary artery calcification and vitamin K status in healthy women. J. Nutr. Biochem. 2013;24(4):624–628. doi: 10.1016/j.jnutbio.2012.02.012. [DOI] [PubMed] [Google Scholar]
- Dalmeijer G.W., van der Schouw Y.T., Magdeleyns E.J., Vermeer C., Elias S.G., Velthuis B.K., et al. Circulating species of matrix gla protein and the risk of vascular calcification in healthy women. Int. J. Cardiol. 2013;168(6):e168–e170. doi: 10.1016/j.ijcard.2013.08.062. [DOI] [PubMed] [Google Scholar]
- Davaine J.-M., Quillard T., Chatelais M., Guilbaud F., Brion R., Guyomarch B., et al. Bone like arterial calcification in femoral atherosclerotic lesions: prevalence and role of osteoprotegerin and pericytes. Eur. J. Vasc. Endovasc. Surg. 2016;51(2):259–267. doi: 10.1016/j.ejvs.2015.10.004. [DOI] [PubMed] [Google Scholar]
- Dekker M., Waissi F., Silvis M.J.M., Bennekom J.V., Schoneveld A.H., de Winter R.J., et al. High levels of osteoprotegerin are associated with coronary artery calcification in patients suspected of a chronic coronary syndrome. Sci. Rep. 2021;11(1):18946. doi: 10.1038/s41598-021-98177-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diederichsen S.Z., Grønhøj M.H., Mickley H., Gerke O., Steffensen F.H., Lambrechtsen J., et al. CT-detected growth of coronary artery calcification in asymptomatic middle-aged subjects and association with 15 biomarkers. JACC Cardiovasc. Imaging. 2017;10(8):858–866. doi: 10.1016/j.jcmg.2017.05.010. [DOI] [PubMed] [Google Scholar]
- Dofferhoff A.S.M., Piscaer I., Schurgers L.J., Visser M.P.J., van den Ouweland J.M.W., de Jong P.A., et al. Reduced vitamin K status as a potentially modifiable risk factor of severe COVID-19. Clin. Infect. Dis. 2021;73(11):e4039–e4046. doi: 10.1093/cid/ciaa1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty T.M., Tang W., Dascalos S., Watson K.E., Demer L.L., Shavelle R.M., et al. Ethnic origin and serum levels of 1alpha,25-dihydroxyvitamin D3 are independent predictors of coronary calcium mass measured by electron-beam computed tomography. Circulation. 1997;96(5):1477–1481. doi: 10.1161/01.cir.96.5.1477. [DOI] [PubMed] [Google Scholar]
- Donate-Correa J., Martín-Núñez E., Hernández-Carballo C., Ferri C., Tagua V.G., Delgado-Molinos A., et al. Fibroblast growth factor 23 expression in human calcified vascular tissues. Aging. 2019;11(18):7899–7913. doi: 10.18632/aging.102297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Mokadem M., Boshra H., El Hady Y.A., El Hameed A.S.A. Relationship of serum vitamin D deficiency with coronary artery disease severity using multislice CT coronary angiography. Clin. Investig. Arterioscler. 2021;33(6):282–288. doi: 10.1016/j.arteri.2021.02.008. [DOI] [PubMed] [Google Scholar]
- Esteghamati A., Sheikhbahaei S., Hafezi-Nejad N., Mousavizadeh M., Noshad S., Larimi N.G., et al. Serum osteoprotegerin in relation to metabolic status, severity, and estimated risk of subsequent coronary heart disease. Arch. Iran. Med. 2014;17(9):596–601. [PubMed] [Google Scholar]
- Eyyüpkoca F., Yüksel Y., Altintaş M.S., Yildirim O., Koçak A. Patients with vitamin D deficiency are at higher risk of developing calcified and mixed plaques. EJCM. 2021;9(3):158–168. doi: 10.32596/ejcm.galenos.2021-03-024. [DOI] [Google Scholar]
- Foley R.N., Collins A.J., Herzog C.A., Ishani A., Kalra P.A. Serum phosphorus levels associate with coronary atherosclerosis in young adults. J Am Soc Nephrol. 2009;20(2):397–404. doi: 10.1681/ASN.2008020141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost M.L., Grella R., Millasseau S.C., Jiang B.-Y., Hampson G., Fogelman I. Relationship of calcification of atherosclerotic plaque and arterial stiffness to bone mineral density and osteoprotegerin in postmenopausal women referred for osteoporosis screening. Calcif. Tissue Int. 2008;83(2):112–120. doi: 10.1007/s00223-008-9153-2. [DOI] [PubMed] [Google Scholar]
- Golledge J., Jayalath R., Oliver L., Parr A., Schurgers L., Clancy P. Relationship between CT anthropometric measurements, adipokines and abdominal aortic calcification. Atherosclerosis. 2008;197(1):428–434. doi: 10.1016/j.atherosclerosis.2007.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golovkin A.S., Kokov A.N., Masenko V., Khryachkova O.N., Malyuta E., Barbarash O. Markers of calcium and phosphate metabolism and osteopenic syndrome in patients with coronary artery disease. Panminerva Med. 2016;58(4):253–262. [PubMed] [Google Scholar]
- Grønhøj M.H., Gerke O., Mickley H., Steffensen F.H., Lambrechtsen J., Sand N.P.R., et al. Associations between calcium-phosphate metabolism and coronary artery calcification; a cross sectional study of a middle-aged general population. Atherosclerosis. 2016;251:101–108. doi: 10.1016/j.atherosclerosis.2016.06.001. [DOI] [PubMed] [Google Scholar]
- Ho J.S., Cannaday J.J., Barlow C.E., Reinhardt D.B., Wade W.A., Ellis J.R. Low 25-OH vitamin D levels are not associated with coronary artery calcium or obstructive stenosis. Coron. Artery Dis. 2015;26(6):521–525. doi: 10.1097/MCA.0000000000000261. [DOI] [PubMed] [Google Scholar]
- Holden R.M., Hétu M.-F., Ty Li, Ward E., Couture L.E., Herr J.E., et al. The heart and kidney: abnormal phosphate homeostasis is associated with atherosclerosis. J. Endocr. Soc. 2018;3(1):159–170. doi: 10.1210/js.2018-00311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosbond S.E., Diederichsen A.C.P., Saaby L., Rasmussen L.M., Lambrechtsen J., Munkholm H., et al. Can osteoprotegerin be used to identify the presence and severity of coronary artery disease in different clinical settings? Atherosclerosis. 2014;236(2):230–236. doi: 10.1016/j.atherosclerosis.2014.07.013. [DOI] [PubMed] [Google Scholar]
- Hosbond S.E., Diederichsen A.C.P., Pedersen L., Rasmussen L.M., Munkholm H., Gerke O., et al. Lipocalin-type prostaglandin D syntahse is not a biomarker of athersclerotic manifestations. Scand. J. Clin. Lab. Invest. 2014;74(3):219–227. doi: 10.3109/00365513.2013.877595. [DOI] [PubMed] [Google Scholar]
- Hwang J.J., Wei J., Abbara S., Grinspoon S.K., Lo J. Receptor activator of nuclear factor-κB ligand (RANKL) and its relationship to coronary atherosclerosis in HIV patients. J. Acquir. Immune Defic. Syndr. 2012;61(3):359–363. doi: 10.1097/QAI.0b013e31826a6c16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Institue TNHLaB Quality assessment tool https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools
- Janus S.E., Durieux J.C., Hajjari J., Carneiro H., McComsey G.A. Inflammation mediated vitamin K and vitamin D effects on vascular calcifications in people with HIV on active antiretroviral therapy. AIDS. 2021 doi: 10.1097/QAD.0000000000003149. [DOI] [PubMed] [Google Scholar]
- Jin H., Ji J.-J., Zhu Y., Wang X.-D., Li Y.-P., Shi Q.-Y., et al. Brain-derived neurotrophic factor, a new predictor of coronary artery calcification. Clin. Appl. Thromb. Hemost. 2021;27 doi: 10.1177/1076029621989813. 1076029621989813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jono S., Ikari Y., Vermeer C., Dissel P., Hasegawa K., Shioi A., et al. Matrix gla protein is associated with coronary artery calcification as assessed by electron-beam computed tomography. Thromb. Haemost. 2004;91(4):790–794. doi: 10.1160/TH03-08-0572. [DOI] [PubMed] [Google Scholar]
- Kestenbaum B., Sachs M.C., Hoofnagle A.N., Siscovick D.S., Ix J.H., Robinson-Cohen C., et al. Fibroblast growth factor-23 and cardiovascular disease in the general population: the multi-ethnic study of atherosclerosis. Circ. Heart Fail. 2014;7(3):409–417. doi: 10.1161/CIRCHEARTFAILURE.113.000952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ketlogetswe K.S., McKibben R., Jacobson L.P., Li X., Dobs A.S., Budoff M., et al. Osteoprotegerin (OPG), but not receptor activator for nuclear factor kappa B ligand (RANKL), is associated with subclinical coronary atherosclerosis in HIV-infected men. J. Acquir. Immune Defic. Syndr. 2015;70(4):362–369. doi: 10.1097/QAI.0000000000000725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiani A.N., Fang H., Magder L.S., Petri M. Vitamin D deficiency does not predict progression of coronary artery calcium, carotid intima-media thickness or high-sensitivity C-reactive protein in systemic lupus erythematosus. Rheumatology (Oxford) 2013;52(11):2071–2076. doi: 10.1093/rheumatology/ket271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiani A.N., Aukrust P., Ueland T., Hollan I., Barr E., Magder I.S., et al. Serum osteoprotegerin (OPG) in subclinical atherosclerosis in systemic lupus erythematosus. Lupus. 2017;26(8):865–870. doi: 10.1177/0961203316682101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K.J., Kim K.M., Park K.H., Choi H.S., Rhee Y., Lee Y.H., et al. Aortic calcification and bone metabolism: the relationship between aortic calcification, BMD, vertebral fracture, 25-hydroxyvitamin D, and osteocalcin. Calcif. Tissue Int. 2012;91(6):370–378. doi: 10.1007/s00223-012-9642-1. [DOI] [PubMed] [Google Scholar]
- Kim K.M., Lim S., Moon J.H., Jin H., Jung K.Y., Shin C.S., et al. Lower uncarboxylated osteocalcin and higher sclerostin levels are significantly associated with coronary artery disease. Bone. 2016;83:178–183. doi: 10.1016/j.bone.2015.11.008. [DOI] [PubMed] [Google Scholar]
- Kim W.S., Lee D.-H., Youn H.-J. Calcium-phosphorus product concentration is a risk factor of coronary artery disease in metabolic syndrome. Atherosclerosis. 2013;229(1):253–257. doi: 10.1016/j.atherosclerosis.2013.04.028. [DOI] [PubMed] [Google Scholar]
- Kockelkoren R., Vos A., van Hecke W., et al. Computed tomographic distinction of intimal and medial calcification in the intracranial internal carotid artery. PLoS One. 2017;12(1) doi: 10.1371/journal.pone.0168360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koga S., Ikeda S., Akashi R., Yonekura T., Kawano H., Maemura K. Serum soluble klotho is inversely related to coronary artery calcification assessed by intravascular ultrasound in patients with stable coronary artery disease. J. Cardiol. 2021;77(6):583–589. doi: 10.1016/j.jjcc.2020.11.014. [DOI] [PubMed] [Google Scholar]
- Kwak S.M., Kim J.S., Choi Y., Chang Y., Kwon M.-J., Jung J.-G., et al. Dietary intake of calcium and phosphorus and serum concentration in relation to the risk of coronary artery calcification in asymptomatic adults. Arterioscler. Thromb. Vasc. Biol. 2014;34(8):1763–1769. doi: 10.1161/ATVBAHA.114.303440. [DOI] [PubMed] [Google Scholar]
- Kwon A., Choi Y.-S., Choi Y.-W., Chung W.-B., Park C.-S., Chung W.-S., et al. Serum osteoprotegerin is associated with calcified carotid plaque. Medicine (Baltimore) 2016;95(15) doi: 10.1097/MD.0000000000003381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai S., Fishman E.K., Gerstenblith G., Brinker J., Tai H., Chen S., et al. Vitamin D deficiency is associated with coronary artery calcification in cardiovascularly asymptomatic african americans with HIV infection. Vasc. Health Risk Manag. 2013;9:493–500. doi: 10.2147/VHRM.S48388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leckstroem D.C.T., Bhuvanakrishna T., McGrath A., Goldsmith D.J.A. Prevalence and predictors of abdominal aorta calcification in healthy living kidney donors. Int. Urol. Nephrol. 2014;46(1):63–70. doi: 10.1007/s11255-013-0485-0. [DOI] [PubMed] [Google Scholar]
- Lee S., Ahuja V., Masaki K., Evans R.W., Barinas-Mitchell E.J.M., Ueshima H., et al. A significant positive association of vitamin D deficiency with coronary artery calcification among middle-aged men: for the ERA JUMP study. J. Am. Coll. Nutr. 2016;35(7):614–620. doi: 10.1080/07315724.2015.1118651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S.J., Lee I.-K., Jeon J.-H. Vascular calcification – new insights into its mechanism. Int. J. Mol. Sci. 2020;21(8):2685. doi: 10.3390/ijms21082685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang J., Huang J., He W., Shi G., Chen J., Huang H. β-hydroxybutyric inhibits vascular calcification via autophagy enhancement in models induced by high phosphate. Front. Cardiovasc. Med. 2021;8 doi: 10.3389/fcvm.2021.685748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieb W., Gona P., Larson M.G., Massaro J.M., Lipinska I., Keaney J.F., et al. Biomarkers of the osteoprotegerin pathway: clinical correlates, subclinical disease, incident CVD and mortality. Arterioscler. Thromb. Vasc. Biol. 2010;30(9):1849–1854. doi: 10.1161/ATVBAHA.109.199661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim S., Shin H., Kim M.J., Ahn H.Y., Kang S.M., Yoon J.W., et al. Vitamin D inadequacy is associated with significant coronary artery stenosis in a community-based elderly cohort: the korean longitudinal study on health and ageing. J. Clin. Endocrinol. Metab. 2012;97(1):169–178. doi: 10.1210/jc.2011-1580. [DOI] [PubMed] [Google Scholar]
- Liu D., Chen L., Dong S., Peng Z., Yang H., Chen Y., et al. Bone mass density and bone metabolism marker are associated with progression of carotid and cardiac calcified plaque in chinese elderly population. Osteoporos. Int. 2019;30(9):1807–1815. doi: 10.1007/s00198-019-05031-5. [DOI] [PubMed] [Google Scholar]
- Liu D., Chen L., Dong S., Yang H., Li L., Liu J., et al. Low bone mass is associated with carotid calcification plaque in chinese postmenopausal women: the Chongqing osteoporosis study. Climacteric. 2020;23(3):237–244. doi: 10.1080/13697137.2019.1671818. [DOI] [PubMed] [Google Scholar]
- Locatelli F., Cannata-Andia J.B., Drüeke T.B., Hörl W.H., Fouque D., Heimburger O., et al. Management of disturbances of calcium and phosphate metabolism in chronic renal insufficiency, with emphasis on the control of hyperphosphataemia. Nephrol. Dial. Transplant. 2002;17(5):723–731. doi: 10.1093/ndt/17.5.723. [DOI] [PubMed] [Google Scholar]
- Lok Z.S.Y., Lyle A.N. Osteopontin in vascular disease: friend or foe? Arterioscler. Thromb. Vasc. Biol. 2019;39(4):613–622. doi: 10.1161/ATVBAHA.118.311577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luna-Luna M., Criales-Vera S., Medina-Leyte D., Díaz-Zamudio M., Flores-Zapata A., Cruz-Robles D., et al. Bone morphogenetic protein-2 and osteopontin gene expression in epicardial adipose tissue from patients with the presence of calcified atherosclerotic plaques. Diabetes Metab. Syndr. Obes. 2020;13:1943–1951. doi: 10.2147/DMSO.S253632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhotra R., Nicholson C.J., Wang D., Bhambhani V., Paniagua S., Slocum C., et al. Matrix gla protein levels are associated with arterial stiffness and incident heart failure with preserved ejection fraction. Arterioscler. Thromb. Vasc. Biol. 2022;42(2):e61–e73. doi: 10.1161/ATVBAHA.121.316664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martín-Reys R., Franco-Peláez J.A., Lorenzo O., González-Casaus M.L., Pello A.M., Aceña A., et al. Plasma levels of monocyte chemoattractant protein-1, n-terminal fragment of brain natriuretic peptide and calcidiol are independently associated with the complexity of coronary artery disease. PLoS One. 2016;11(5) doi: 10.1371/journal.pone.0152816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masai H., Joki N., Sugi K., Moroi M. A preliminary study of the potential role of FGF-23 in coronary calcification in patients with suspected coronary artery disease. Atherosclerosis. 2013;226(1):228–233. doi: 10.1016/j.atherosclerosis.2012.10.045. [DOI] [PubMed] [Google Scholar]
- McPherson S., Barbosa-Leiker C., Short R., Tuttle K.R. Classification of chronic kidney disease biomarkers to predict coronary artery calcium. Kidney Blood Press. Res. 2012;36(1):26–35. doi: 10.1159/000339024. [DOI] [PubMed] [Google Scholar]
- Michos E.D., Streeten E.A., Ryan K.A., Rampersaud E., Peyser P.A., Bielak L.F., et al. Serum 25-hydroxyvitamin D levels are not associated with subclinical vascular disease or C-reactive protein in the old order amish. Calcif. Tissue Int. 2009;84(3):195–202. doi: 10.1007/s00223-008-9209-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millar S.A., Anderson S.I., O’Sullivan S.E. Osteokines and the vasculature: a review of the in vitro effects of osteocalcin, fibroblast growth factor-23 and lipocalin-2. PeerJ. 2019;7 doi: 10.7717/peerj.7139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millar S.A., John S.G., McIntyre C.W., Ralevic V., Anderson S.I., O’Sullivan S.E. An investigation into the role of osteocalcin in human arterial smooth muscle cell calcification. Front. Endocrinol. 2020;11:369. doi: 10.3389/fendo.2020.00369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyabara Y., Camp J., Holmes D., Lahr B., Bailey K., Miller V.M., et al. Coronary arterial calcification and thoracic spine mineral density in early menopause. Climacteric. 2011;14(4):438–444. doi: 10.3109/13697137.2010.537409. [DOI] [PubMed] [Google Scholar]
- Mohammadpour A.H., Shamsara J., Nazemi S., Ghadirzadeh S., Shahsavand S., Ramezani M. Evaluation of RANKL/OPG serum concentration ratio as a new biomarker for coronary artery calcification: a pilot study. Thrombosis. 2012;2012 doi: 10.1155/2012/306263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher D., Shamseer L., Clarke M., Ghersi D., Liberati A., Petticrew M., et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015;4(1):1. doi: 10.1186/2046-4053-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moradi M., Foroutanfar A. Evaluation of vitamin D levels in relation to coronary CT angiographic findings in an iranian population. Vasc. Health Risk Manag. 2017;13:361–367. doi: 10.2147/VHRM.S142721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita H., Takeda Y., Fujita S.-I., Okamoto Y., Sakane K., Teramoto K., et al. Gender specific association between serum fibroblast growth factor 23/α-klotho and coronary artery and aortic valve calcification. J. Atheroscler. Thromb. 2015;22(1):1338–1346. doi: 10.5551/jat.30635. [DOI] [PubMed] [Google Scholar]
- Nam S.-H., Kang S.-G., Song S.-W. The neutrophil-lymphocyte ratio is associated with coronary artery calcification in asymptomatic korean males: a cross-sectional study. Biomed. Res. Int. 2017;2017:1989417. doi: 10.1155/2017/1989417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nandkeolyar S., Naqvi A., Fan W., Sharma A., Rana J.S., Rozanski A., et al. Utility of novel serum biomarkers to predicts subclinical atherosclerosis: a sub-analysis of the EISNER study. Atherosclerosis. 2019;282:80–84. doi: 10.1016/j.atherosclerosis.2019.01.012. [DOI] [PubMed] [Google Scholar]
- Nielsen C.V., Underbjerg L., Grove-Laugesen D., Sikjaer T., Rejnmark L. Lower leg arterial calcifications assessed by high-resolution peripheral quantitative computed tomography in hypoparathyroid and pseudohypoparathyroid patients. Calcif. Tissue Int. 2021;108(6):775–784. doi: 10.1007/s00223-021-00814-7. [DOI] [PubMed] [Google Scholar]
- Nikolova M., Nazifova-Tasinova N., Vankova D., Gerova D., Yotov Y., Atanasov A., et al. Vitamin D status in patients with atrial fibrillation and heart failure – is there a link? Clin. Lab. 2021;67(6) doi: 10.7754/Clin.Lab.2020.200902. [DOI] [PubMed] [Google Scholar]
- Nugroho J., Widorini W. Correlation between osteoprotegerin serum level and coronary calcification using coronary artery calcium score in patients with moderate-severe cardiovascular risk factor. Int. J. Angiol. 2017;26(4):234–237. doi: 10.1055/s-0037-1607050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donnell C.J., Shea M.K., Price P.A., Gagnon D.R., Wilson P.W.F., Larson M.G., et al. Matrix gla protein is associated with risk factors for atherosclerosis but not with coronary artery calcification. Arterioscler. Thromb. Vasc. Biol. 2006;26(12):2769–2774. doi: 10.1161/01.ATV.0000245793.83158.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okura T., Kurata M., Enomoto D., Jotoku M., Nagao T., Desilva V.R., et al. Undercarboxylated osteocalcin is a biomarker of carotid calcification in patients with essential hypertension. Kidney Blood Press. Res. 2010;33(1):66–71. doi: 10.1159/000289575. [DOI] [PubMed] [Google Scholar]
- Omland T., Drazner M.H., Ueland T., Abedin M., Murphy S.A., Aukrust P., et al. Plasma osteoprotegerin levels in the general population: relation to indices of left ventricular structure and function. Hypertension. 2007;49(6):1392–1398. doi: 10.1161/HYPERTENSIONAHA.107.087742. [DOI] [PubMed] [Google Scholar]
- Ouzzani M., Hammady H., Fedorowicz Z., Elmagarmid A. Rayyan – a web and mobile app for systematic reviews. Syst. Rev. 2016;5:210. doi: 10.1186/s13643-016-0384-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park C.-S., Chung W.-S., Youn H.-J., Shin Y.-J., Kim J.-H., Oh Y.-S., et al. Association between the serum osteoprotegerin level and target lesion calcium in coronary artery disease. Korean Circ. J. 2006;36:337–342. [Google Scholar]
- Park K.S., Chang J.W., Kim T.Y., Kim H.W., Lee E.K., Kim H.-S., et al. Lower concentrations of serum phosphorus with the normal range could be associated with less calcification of the coronary artery in koreans with normal renal function. Am. J. Clin. Nutr. 2011;94(6):1465–1470. doi: 10.3945/ajcn.110.001974. [DOI] [PubMed] [Google Scholar]
- Park K.S., Park J., Choi S.H., Ann S.H., Singh G.B., Shin E.-S., et al. Serum phosphorus concentration and coronary artery calcification in subjects with renal dysfunction. PLoS One. 2016;11(3) doi: 10.1371/journal.pone.0151007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park K.S., Lee Y., Park G.-M., Park J., Kim Y.-G., Yang D.H. Association between serum phosphorus and subclinical coronary atherosclerosis in asymptomatic korean individuals without kidney dysfunction. Am. J. Clin. Nutr. 2020;112(1):66–73. doi: 10.1093/ajcn/nqaa091. [DOI] [PubMed] [Google Scholar]
- Peng J., Liu M.-M., Liu H.-H., Xu R.-X., Zhu C.-G., Guo Y.-L., et al. Lipoprotein (a)-mediated vascular calcification: population-based and in vitro studies. Metabolism. 2022;127 doi: 10.1016/j.metabol.2021.154960. [DOI] [PubMed] [Google Scholar]
- Pérez de Ciriza C., Moreno M., Restituto P., Bastarrika G., Simón I., Colina I., et al. Circulating osteoprotegerin is increased in the metabolic syndrome and associates with subclinical atherosclerosis and coronary arterial calcification. Clin. Biochem. 2014;47(18):272–278. doi: 10.1016/j.clinbiochem.2014.09.004. [DOI] [PubMed] [Google Scholar]
- Pesaro A.E., Katz M., Liberman M., Pereira C., Mangueira C.L.P., de Carvalho A.E.Z., et al. Circulating osteogenic proteins are associated with coronary artery calcification and increase after myocardial infarction. PLoS One. 2018;13(8) doi: 10.1371/journal.pone.0202738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pescatore L.A., Gamarra L.F., Liberman M. Multifaceted mechanisms of vascular calcification in aging. Arterioscler. Thromb. Vasc. Biol. 2019;39(7):1307–1316. doi: 10.1161/ATVBAHA.118.311576. [DOI] [PubMed] [Google Scholar]
- Pirro M., Manfredelli M.R., Schillaci G., Helou R.S., Bagaglia F., Melis F., et al. Association between circulating osteoblast progenitor cells and aortic calcifications in women with postmenopausal osteoporosis. Nutr. Metab. Cardiovasc. Dis. 2013;23(5):466–473. doi: 10.1016/j.numecd.2011.08.006. [DOI] [PubMed] [Google Scholar]
- Poornima I.G., Mackey R.H., Buhari A.M., Cauley J.A., Matthews K.A., Kuller L.H. Relationship between circulating serum osteoprotegerin and total receptor activator of nuclear κ-B ligand levels, triglycerides, and coronary calcification in postmenopausal women. Menopause. 2014;21(7):702–710. doi: 10.1097/GME.0000000000000127. [DOI] [PubMed] [Google Scholar]
- Poornima I.G., Shields K., Kuller L.H., Manzi S.M., Ramsey-Goldman R., Richardson C. Associations of osteoprotegerin with coronary artery calcification among women with systemic lupus erythematosus and healthy controls. Lupus. 2018 doi: 10.1177/0961203317751060. 961203317751060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quercioli A., Mach F., Bertolotto M., Lenglet S., Vuilleumier N., Galan K., et al. Receptor activator of NF-κB ligang (RANKL) increases the release of neutrophil products associated with coronary vulnerability. Thromb. Haemost. 2012;107(1):127–139. doi: 10.1160/TH11-05-0324. [DOI] [PubMed] [Google Scholar]
- Razavi A.C., Cardoso R., Dzaye O., Budoff M., Thanassoulis G., Post W.S., et al. Risk markers for limited coronary artery calcium in persons with significant aortic valve calcium (from the multi-ethnic study of atherosclerosis) Am. J. Cardiol. 2021;156:58–64. doi: 10.1016/j.amjcard.2021.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rennenberg R.J.M.W., de Leeuw P.W., Kessels A.G.H., Schurgers L.J., Vermeer C., van Engelshoven J.M.A., et al. Calcium scores and matrix gla protein levels: association with vitamin K status. Eur. J. Clin. Investig. 2010;40(4):344–349. doi: 10.1111/j.1365-2362.2010.02275.x. [DOI] [PubMed] [Google Scholar]
- Rochette L., Meloux A., Rigal E., Zeller M., Malka G., Cottin Y., et al. The role of osteoprotegerin in vascular calcification and bone metabolism: the basis for developing new treatments. Calcif. Tissue Int. 2019;105(3):239–251. doi: 10.1007/s00223-019-00573-6. [DOI] [PubMed] [Google Scholar]
- Rodrigues I.G., Pinho C.P.S., Filho D.S., Leão A.P.D., Oliveira M.C.M., Barbosa G.P., et al. The impact of visceral fat and levels of vitamin D on coronary artery calcification. Rev. Assoc. Med. Bras. 2021;67(1):88–93. doi: 10.1590/1806-9282.67.01.20200388. [DOI] [PubMed] [Google Scholar]
- Roos M., Lutz J., Salmhofer H., Luppa P., Knauss A., Braun S., et al. Relation between plasma fibroblast growth factor-23, serum fetuin-a levels and coronary artery calcification evaluated by multislice computed tomography in patients with normal kidney function. Clin. Endocrinol. 2008;68(4):660–665. doi: 10.1111/j.1365-2265.2007.03074.x. [DOI] [PubMed] [Google Scholar]
- Roumeliotis S., Dounousi E., Eleftheriadis T., Liakopoulos V. Association of the inactive circulating matrix gla protein with vitamin K intake, calcification, mortality and cardiovascular disease: a review. Int. J. Mol. Sci. 2019;20(3):628. doi: 10.3390/ijms20030628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutsch F., Buers I., Nitschke Y. Hereditary disorders of cardiovascular calcification. Arterioscler. Thromb. Vasc. Biol. 2021;41(1):35–47. doi: 10.1161/ATVBAHA.120.315577. [DOI] [PubMed] [Google Scholar]
- Sajjadieh H., Sajjadieh A., Koopaei Z.K., Oveisgharan S. Correlation between vitamin D level and coronary artery calcification. J. Res. Med. Sci. 2020;25:51. doi: 10.4103/jrms.JRMS_1080_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salari P., Keshtkar A., Shirani S., Mounesan L. Coronary artery calcium score and bone metabolism: a pilot study in postmenopausal women. J. Bone Metab. 2017;24(1):15–21. doi: 10.11005/jbm.2017.24.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satilmis S., Celik O., Biyik I., Ozturk D., Celik K., Akin F., et al. Association between serum vitamin D levels and subclinical coronary atherosclerosis and plaque burden/composition in young adult population. Bosn. J. Basic Med. Sci. 2015;15(1):67–72. doi: 10.17305/bjbms.2015.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlieper G., Grotemeyer D., Aretz A., Schurgers L.J., Krüger T., Rehbein H., et al. Analysis of calcifications in patients with coral reef aorta. Ann. Vasc. Surg. 2010;24(3):408–414. doi: 10.1016/j.avsg.2009.11.006. [DOI] [PubMed] [Google Scholar]
- Shea M.K., O’Donnell C.J., Vermeer C., Magdeleyns E.J.P., Crosier M.D., Gundberg C.M., et al. Circulating uncarboxylated matrix gla protein is associated with vitamin K nutritional status, but nog coronary artery calcium, in older adults. J. Nutr. 2011;141(8):1529–1534. doi: 10.3945/jn.111.139634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shea M.K., Booth S.L., Miller M.E., Burke G.L., Chen H., Cushman M., et al. Association between circulating vitamin K1 and coronary calcium progression in community-dwelling adults: the multi-ethnic study of atherosclerosis. Am. J. Clin. Nutr. 2013;98(1):197–208. doi: 10.3945/ajcn.112.056101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shikuma C.M., Seto T., Liang C.-Y., Bennett K., DeGruttola V., Gerschenson M., et al. Vitamin D levels and markers of arterial dysfunction in HIV. AIDS Res. Hum. Retrovir. 2012;28(8):793–797. doi: 10.1089/AID.2011.0086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin S., Kim K.-J., Chang H.-J., Cho I., Kim Y.J., Choi B.-W., et al. Impact of serum calcium and phosphate on coronary atherosclerosis detected by cardiac computed tomography. Eur. Heart J. 2012;33(22):2873–2881. doi: 10.1093/eurheartj/ehs152. [DOI] [PubMed] [Google Scholar]
- Silaghi C.N., Fodor D., Crăciun A.M. Circulating matrix gla protein: a potential tool to identify minor carotid stenosis with calcification in a risk population. Clin. Chem. Lab. Med. 2013;51(5):1115–1123. doi: 10.1515/cclm-2012-0329. [DOI] [PubMed] [Google Scholar]
- Soares A.A.S., Freitas W.M., Japiassú A.V.T., Quaglia L.A., Santos S.N., Pereira A.C., et al. Enhance parathyroid hormone levels are associated with left ventricle hypertrophy in very elderly men and women. J. Am. Soc. Hypertens. 2015;9(9):697–704. doi: 10.1016/j.jash.2015.06.020. [DOI] [PubMed] [Google Scholar]
- Stȩpień E., Fedak D., Klimeczek P., Wilkosz T., Banyś R.P., Starzyk K., et al. Osteoprotegerin, but not osteopontin, as a potential predictor of vascular calcification in normotensive subjects. Hypertens. Res. 2012;35(5):531–538. doi: 10.1038/hr.2011.231. [DOI] [PubMed] [Google Scholar]
- Sung K.-C., Chang Y., Ryu S., Chung H.-K. High levels of serum vitamin D are associated with a decreased risk of metabolic diseases in both men and women, but an increased risk for coronary artery calcification in korean men. Cardiovasc. Diabetol. 2016;15(1):112. doi: 10.1186/s12933-016-0432-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Toorn J.E., Rueda-Ochoa O.L., van der Schaft N., Vernoij M.W., Arfan Ikram M., Bos D., Kavousi M. Arterial calcification at multiple sites: sex-specific cardiovascular risk profiles and mortality risk – the Rotterdam study. BMC Med. 2020;18(1):263. doi: 10.1186/s12916-020-01722-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torii S., Ikari Y., Tanabe K., Kakuta T., Hatori M., Shioi A., et al. Plasma phylloquinone, menaquinone-4 and menaquinone-7 and coronary artery calcification. J. Nutr. Sci. 2016;5 doi: 10.1017/jns.2016.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuersun T., Luo Q., Zhang Z., Wang G., Zhang D., Wang M., et al. Abdominal aortic calcification is more severe in unilateral primary aldosteronism patients and is associated with elevated aldosterone and parathyroid hormone levels. Hypertens. Res. 2020;43(12):1413–1420. doi: 10.1038/s41440-020-0529-7. [DOI] [PubMed] [Google Scholar]
- Tuttle K., Short R.A. Longitudinal relationships among coronary artery calcification, serum phosphorus, and kidney function. Clin. J. Am. Soc. Nephrol. 2009;4(12):1968–1973. doi: 10.2215/CJN.01250209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uz O., Kadeşoğlu E., Yiğiner O., Baş S., Ipçioğlu O.M., Ozmen N., et al. The relationship between coronary calcification and the metabolic markers of osteopontin, fetuin-a, and visfatin. Turk Kardiyol. Dern. Ars. 2009;37(6):397–402. [PubMed] [Google Scholar]
- van Varik B.J., Rennenberg R.J.M.W., Reutelingsperger C.P., Kroon A.A., de Leeuw P.W., Schurgers L.J. Mechanisms of arterial remodeling: lessons from genetic diseases. Front. Genet. 2012;3:290. doi: 10.3389/fgene.2012.00290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinholt P.J., Overgaard M., Diederichsen A.C.P., Mickley H., Poulsen T.S., Sand N.P.R., et al. An ELISA for the quantitation of von willebrand factor: osteoprotegerin complexes in plasma. Thromb. Res. 2013;131(5):396–400. doi: 10.1016/j.thromres.2013.03.001. [DOI] [PubMed] [Google Scholar]
- Vossen L.M., Kroon A.A., Schurgers L.J., de Leeuw P.W. Pharmacological and nutritional modulation of vascular calcification. Nutrients. 2020;12(1):100. doi: 10.3390/nu12010100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson K.E., Abrolat M.L., Malone L.L., Hoeg J.M., Doherty T., Detrano R., et al. Active serum vitamin D levels are inversely correlated with coronary calcification. Circulation. 1997;96(6):1755–1760. doi: 10.1161/01.cir96.6.1755. [DOI] [PubMed] [Google Scholar]
- Wei N., Lu L., Zhang H., Gao M., Ghosh S., Liu Z., et al. Warfarin accelerates aortic calcification by upregulating senescence-associated secretory phenotype maker expression. Oxidative Med. Cell. Longev. 2020;2020 doi: 10.1155/2020/2043762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilund K.R., Tomayko E.J., Evans E.M., Kim K., Ishaque M.R., Fernhall B. Physical activity, coronary artery calcium, and bone mineral density in elderly men and women: a preliminary investigation. Metabolism. 2008;57(4):584–591. doi: 10.1016/j.metabol.2007.11.024. [DOI] [PubMed] [Google Scholar]
- Wu G.-Y., X B.-D., Wu T., Wang X.-Y., Wang T.-X., Zhang Z. Correlation between serum parathyroid hormone levels and coronary artery calcification in patients without renal failure. Biomed. Rep. 2016;5(5):601–606. doi: 10.3892/br.2016.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X.H., Chen X.-Y., Wang L.J., Wong K.S. Intracranial artery calcification and its clinical significance. J. Clin. Neurol. 2016;12(3):253–261. doi: 10.3988/jcn.2016.2.3.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Y., Peng C., Huang W., Zhang J., Xia M., Zhang Y., et al. Circulating fibroblast growth factor 23 is associated with angiographic severity and extent of coronary artery disease. PLoS One. 2013;8(8) doi: 10.1371/journal.pone.0072545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagura M., Serg M., Kampus P., Zilmer M., Eha J., Unt E., et al. Aortic stiffness and vitamin D are independent markers of aortic calcification in patients with peripheral arterial disease and in healthy subjects. Eur. J. Vasc. Endovasc. Surg. 2011;42(5):689–695. doi: 10.1016/j.ejvs.2011.07.027. [DOI] [PubMed] [Google Scholar]
- Zamparini J.M., Immelman A.R., Raal F.J. Fibroblast growth factor-23 in patients with homozygous familial hypercholesterolemia. J. Clin. Lipidol. 2018;12(3):767–772. doi: 10.1016/j.jacl.2018.02.009. [DOI] [PubMed] [Google Scholar]
- Zhelyazkova-Savova M.D., Yotov Y.T., Nikolova M.N., Nazifova-Tasinova N.F., Vankova D.G., Atanasov A.A., et al. Statins, vascular calcification, and vitamin K-dependent proteins: is there a relation? Kaohsiung J. Med. Sci. 2021;37(7):624–631. doi: 10.1002/kjm2.12373. [DOI] [PubMed] [Google Scholar]
- Zwakenberg S.R., van der Schouw Y.T., Vermeer C., Pasterkamp G., den Ruijter H.M., Beulens J.W.J. Matrix gla protein, plaque stability, and cardiovascular events in patients with severe atherosclerotic disease. Cardiology. 2018;141(1):32–36. doi: 10.1159/000493006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwakenberg S.R., de Jong P.A., Hendriks E.J., Westerink J., Spiering W., de Borst G.J., et al. Intimal and medial calcification in relation to cardiovascular risk factors. PLoS One. 2020;15(7) doi: 10.1371/journal.pone.0235228. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Full search terms.
Quality assessment using the National Heart, Lung and Blood Institute (NIH) quality assessment tool: Observational Cohort and Cross-Sectional Studies.
Data extraction.
Full quality assessment.
Supplementary appendix 5 Data extraction and quality assessment in case of calcification measurement with X-ray.
Supplementary appendix 6 References of supplementary appendix 5.