Abstract
Objective:
Gastrointestinal duplications are rare congenital anomalies. Herein, we present a single institutional experience in pediatric gastrointestinal tract duplications.
Methods:
Patient records from 2014 to 2019 were retrospectively evaluated for demographic data, clinical presentation, diagnostic methods, surgical findings, and pathological reports.
Results:
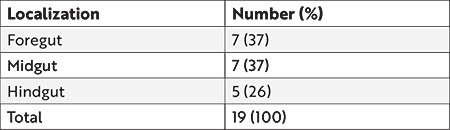
This study included 19 patients, of whom 10 were males and nine were females, with a median age of 30 (21 days-15.5 years) months. Three patients were antenatally and three were incidentally diagnosed. Abdominal pain, vomiting, constipation, and perianal accessory orifice were the most common presenting symptoms. Preoperative diagnostic workup included ultrasonography (n=13), cross-sectional imaging (n=8), and nuclear scintigraphy (n=1). A preoperative diagnosis was possible in 14 (74%) patients. The duplications originated from the foregut in seven (37%) patients, midgut in seven (37%), and hindgut in five (26%). Cystic duplications were observed in 14 (74%) patients and tubular in five (26%). The total surgical excision with (n=8) or without (n=10) associated organ resection was possible in 18 patients. Partial cyst excision with a complete mucosal removal was done in 1 patient. Heterotopic mucosa was present in six (32%) specimens. The respiratory origin with thyroid transcription factor-1 positivity was contained in two para-esophageal duplications. Among five patients with heterotopic gastric mucosa, 1 had presented with perforation and the others with hemorrhage.
Conclusions:
Duplications may involve any gastrointestinal segment. The clinical presentation is highly variable because of the wide variation in the involved segment and sizes and the possibility of bearing heterotopic mucosa. The surgery aims to totally excise the cyst or at least totally remove the inner mucosal lining.
Keywords: Gastrointestinal, duplication cyst, heterotopic mucosa, respiratory epithelium, surgery
Abstract
Amaç:
Sindirim sistemi duplikasyonları nadir görülen anomalilerdir. Çocuk sindirim sistemi duplikasyonlarına yönelik tek merkez deneyiminin sunulması amaçlanmıştır.
Yöntemler:
2014-2019 yıllarına ait hasta kayıtları demografik veri, klinik başvuru, tanı yöntemleri, cerrahi bulgular ve patoloji raporları açısından değerlendirildi.
Bulgular:
Onu erkek ve dokuzu kadın olan 19 hasta vardı. Ortanca yaş 30 (21 gün-15,5 yıl) aydı. Tanı üç hastada doğum öncesinde ve diğer üçünde tesadüfi olarak konmuştu. En sık görülen başvuru yakınmaları karın ağrısı, kusma, kabızlık ve perianal aksesuar açıklıktı. Ameliyat öncesi tanısal yöntem olarak ultrasonografi (n=13), kesitsel inceleme (n=8) ve nükleer sintigrafi (n=1) kullanılmıştı. Hastaların 14’ünde (%74) ameliyat öncesinde tanı konabilmişti. Duplikasyonların yedisi (%37) önbarsaktan, yedisi (%37) ortabarsaktan ve beşi (%26) arkabarsaktan köken alıyordu. On dördü (%74) kistik ve beşi (%26) tübüler tipteydi. Hastaların 18’inde komşu organ rezeksiyonu yapılarak (n=8) veya yapılmadan (n=10) duplikasyon kistinin tamamı çıkartılabildi. Bir hastada kısmi kist eksizyonuna ek olarak, döşeyici mukozanın tam olarak sıyrılması işlemi yapıldı. Heterotopik mukoza varlığı altı (%32) örnekte gösterildi. İki para-özofageal duplikasyon kistinde heterotopik mukozada tiroid transkripsiyon faktörü-1 pozitifliği ile birlikte solunum yolu kökeni tespit edildi. Heterotopik mide mukozası olan beş hastanın birisi perforasyon ile ve bir diğeri kanama ile başvurmuştu.
Sonuçlar:
Duplikasyonlar sindirim sisteminin her seviyesinde görülebilir. Tutulan segmente ek olarak, boyut ve heterotopik mukoza varlığı gibi değişkenlik gösteren nedenlerle klinik başvuru yakınmaları da çok farklı olabilir. Cerrahi girişimde kistin tam olarak çıkartılması veya en azından iç yüzeyi döşeyen mukozanın tamamen çıkartılması hedeflenir.
Keywords: Sindirim sistemi, duplikasyon kisti, heterotopik mukoza, solunum epitelyumu, cerrahi
INTRODUCTION
Gastrointestinal tract duplications are congenital anomalies, which are located in or adjacent to the wall of any part of the gastrointestinal tract1. They can be localized anywhere from the mouth to the anus, but the ileum is the most common localization2. Duplications have smooth muscle in their walls and are lined with gastrointestinal tract mucosa, which is different from the adjacent segment’s mucosa1,3. They may contain a variety of heterotopic mucosa with gastric mucosa being detected in approximately one-third of all cases2. Clinical findings are dictated by cyst localization and size, as well as the presence of heterotopic mucosa, and can be highly variable depending on these criteria. The presenting findings may be chronic, but some patients present with intestinal obstruction, perforation, or abundant bleeding. The diagnosis can also be incidental. The treatment for duplications is done aiming for total excision. With an unresectable duplication cyst, the inner mucosal lining should be completely removed because of its malignant potential3.
Gastrointestinal tract duplications are rare anomalies. Macpherson estimated that they were seen in major pediatric referral centers at a rate of up to only three cases per year1. Our study aimed to present a single institutional experience in diagnosis, treatment, pathological findings, and follow-up of pediatric patients with gastrointestinal duplications.
MATERIALS and METHODS
A retrospective search was done in operative logs for 16 years from 2014 to 2019 with an Istanbul Medeniyet University Goztepe Training and Research Hospital Clinical Research Ethics Committee approval (decision no: 2021/0019, date: 13.01.2021). The patient files and hospital records were assessed for demographic data, clinical presentation, employed diagnostic methods, surgical findings, and pathological reports. Follow-up records were given regarding the last outpatient department visit of the patient.
Statistical Analysis
Descriptive statistics were expressed as median, minimum and maximum frequency, and percentage.
RESULTS
This study included 19 patients, of whom 10 were males and nine were females. The median age was 30 months (21 days-15.5 years), wherein nine (47%) patients were aged <2 years, seven (37%) aged 2-6 years, and three (16%) aged >6 years, during the operation. The locations of the duplication cysts according to the gastrointestinal segment were as follows: intrathoracic esophageal (n=1), thoracoabdominal (n=2) (Figure 1), abdominal esophageal (n=1), gastric (n=2), gastroduodenal (n=1), jejunal (n=1), ileal (n=6) (Figures 2 and 3), colonic (n=2), and perianal (n=3) (Figure 4) (Table 1).
Figure 1.
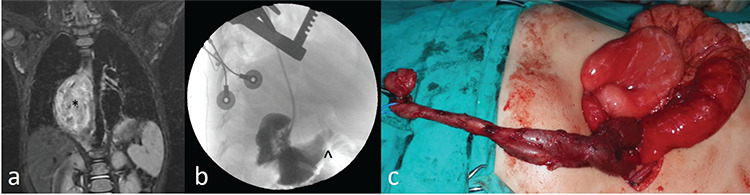
(a) Magnetic resonance imaging shows a thoracoabdominal cystic mass. (*) (b) Contrast injection into the cystic mass, which was closed at the cranial end during thoracotomy, shows the passage of contrast into the duodenum. (^) (c) Thoracoabdominal duplication cyst of the same patient during surgery.
Figure 2.
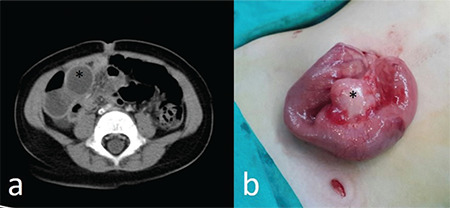
(a) Computerized tomographic appearance of an ileal duplication cyst in a 19-month-old patient who presented with abdominal pain and vomiting. (*) (b) Surgical appearance of the ileal duplication cyst of the same patient.
Figure 3.
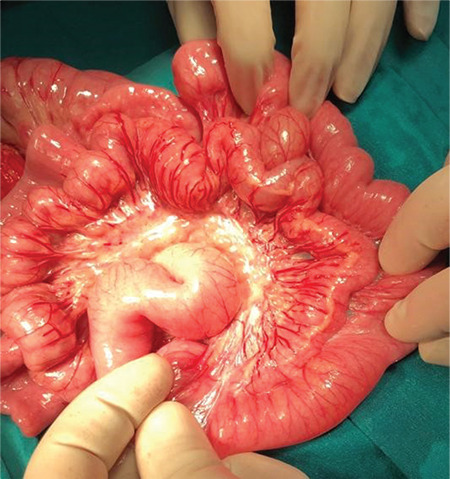
Perioperative appearance of a tubular ileal duplication cyst in a 20-month-old patient who presented with lower gastrointestinal bleeding.
Figure 4.
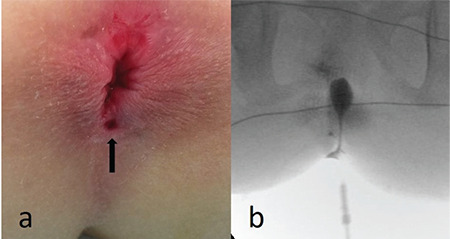
(a) Perianal orifice in a patient with anal canal duplication. (b) Contrast radiography obtained under fluoroscopic control shows the contrast-filled anal canal duplication cyst.
Table 1. Localization of the duplication cysts according to the embryological origin.
An antenatal diagnosis was evident in three (16%) patients. One of these was asymptomatic after birth; however, one of the others presented with constipation and the other with abdominal pain and vomiting. The diagnosis was incidental in three (16%) patients, of whom an intrathoracic mass was seen during echocardiography done because of an irrelevant heart murmur in one; another had an ultrasound examination after an abdominal trauma, which detected an intraabdominal cystic structure; and the last one had a splenic cyst, which was laparoscopically removed, and an esophageal cystic structure of the intraabdominal esophagus was detected and removed during the laparoscopic exploration. The presenting clinical findings in the remaining 13 patients were abdominal pain (n=3), an accessory orifice neighboring the anus (n=2), vomiting (n=2), constipation (n=2), abdominal distension and vomiting (n=1), abdominal pain and vomiting (n=1), cough (n=1), and recurring melena (n=1).
Two patients presented with acute abdominal findings and therefore operated on as emergency cases, which revealed purulent intraabdominal fluid and a perforated ileal duplication cyst in one. An ileal resection, including the cyst with ileo-ileal anastomosis, was done, and the pathological examination of the specimen showed the presence of gastric mucosa within the cyst. The other patient had a jejunal volvulus just proximal to the duplication cyst and underwent jejunal resection, including the cyst, with primary anastomosis.
A 2-month-old male baby with an ileostomy performed elsewhere presented with abdominal distension and vomiting. He had undergone surgical exploration during the neonatal period because of “intestinal obstruction”. The operating surgeon thought he had meconium ileus and performed an ileostomy as recorded in the operative report. An exploratory laparotomy revealed a perforated ileal duplication cyst with ileal atresia proximal to the cyst and distal to the ileostomy. An ileocolonic resection and anastomosis were performed. Another patient, aged 20 months, presented with profound melena and a hemoglobin value of 5.5 g/dL. She had repeated blood transfusions (12 in 1.5 years) because of life-threatening melena in several other centers. A technetium-99m scintigraphy that was performed elsewhere had revealed a negative result. After proper intravenous resuscitation, she underwent exploratory laparotomy, and a long-segment ileal tubular duplication was found, which was partially resected. However, she underwent secondary surgery 33 days later because of continuing bleeding. The remaining duplicated ileal segment was totally excised. She has been symptom-free for four years after the second operation.
Any form of the preoperative radiological investigation was not done in four patients and radiological assessments could not be identified in two patients. An ultrasound was performed in all of the remaining 13 patients and additionally, a computerized tomography (CT) in five, magnetic resonance imaging (MRI) in three, and/or nuclear scintigraphy in one. Ultrasound examination revealed a cystic structure with a probable duplication cyst diagnosis in all but four patients. It was non-diagnostic in a thoracal duplication cyst, a thoracoabdominal duplication cyst, an ileal cyst with perforation, and an ileal tubular duplication with gastrointestinal bleeding. CT and MRI were diagnostic in one with thoracal and the other with thoracoabdominal duplications. All three patients with anal canal duplication cysts underwent fluoroscopic contrast radiography (Figure 4b). Overall, a preoperative presumptive diagnosis of a duplication cyst could be made in 14 (74%) patients.
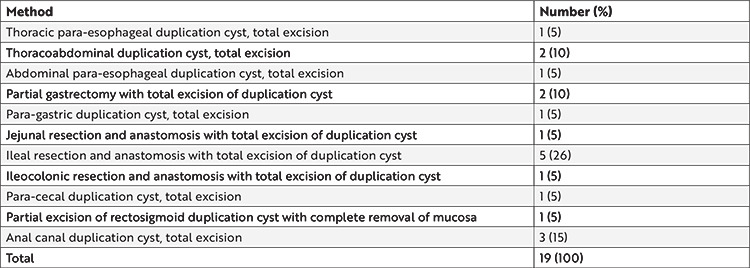
Among the 19 duplication cysts, 14 (74%) were cystic and five (26%) were tubular. The duplication cyst was removed via total cyst removal only (n=10) or relevant organ resection and anastomosis (n=9) in all but one patient (Table 2). The cyst and the colon shared a common wall in a patient with a rectosigmoid tubular duplication cyst; therefore, a partial cystectomy, accompanied by complete removal of the mucosa, was performed.
Table 2. Surgical treatment methods employed in 19 patients.
Pathological examination revealed the presence of heterotopic mucosa in six (32%) specimens, which included gastric in four, gastric and respiratory in one, and respiratory only in one. One patient had ileal triplication. Among the patients with heterotopic gastric mucosa, one presented with ileal perforation and the other with gastrointestinal bleeding (Figure 3). Coexistent heterotopic gastric and respiratory mucosa was detected in an intrathoracic esophageal duplication cyst, which was lined by typical gastric columnar epithelium, as well as ciliated columnar respiratory epithelium, that was positive with thyroid transcription factor (TTF)-1 (Figure 5). The other specimen with isolated heterotopic respiratory mucosa was from a patient with an intraabdominal esophageal duplication cyst. The cyst was lined by ciliated columnar epithelium that resembles the respiratory epithelium with TTF-1 positivity, and the two-layered muscularis propria was positive with smooth muscle antigen (SMA).
Figure 5.
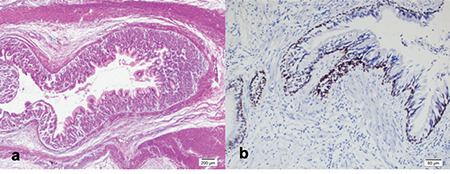
(a) Gastric mucosa, submucosa, and muscular layer. (H&Ex4) (b) Nuclear positive reactivity in the respiratory epithelium with TTF-1 immunohistochemistry (TTF-1×20).
TTF-1: Thyroid transcription factor-1
Vesical globe (n=1) and subcutaneous emphysema (n=1) were observed in two patients in the early postoperative period, which were both conservatively managed. The median follow-up period was 9.5 months (1.5 months-2.8 years) from the time of the surgery to the last recorded outpatient department visit. Four major complications were encountered after hospital discharge. Recurrent bleeding after the first surgery in one patient was managed by a second surgery as described above. One patient with ileal duplication, who had undergone ileal resection and anastomosis, presented with abdominal pain and fever 3 weeks postoperatively. An ultrasound examination detected a-6×2×2-cm fluid collection with internal echoes in the right lower quadrant. He was conservatively managed with antibiotics. Another patient presented with ileal adhesion three weeks postoperatively and was conservatively managed. A patient developed an incisional hernia, which was primarily repaired 1 year postoperatively.
DISCUSSION
Gastrointestinal duplications are rare malformations with an estimated incidence of 1 in 4,500 live births4. They may be located throughout the gastrointestinal tract from the mouth to the anus but they are more commonly encountered in the small intestine. One report with 73 patients indicated that the duplication location by frequency includes the ileum (n=23, 31.5%), ileocecal valve (n=22, 30.2%), duodenum (n=7, 9.6%), stomach (n=6, 8.2%), jejunum (n=6, 8.2%), colon (n=5, 6.8%), and rectum (n=4, 5.5%)5. Duplications can also be embryologically categorized into foregut, midgut, and hindgut2. Accordingly, another large series with 77 duplications revealed the location by frequency as foregut (n=39, 51%), midgut (n=30, 39%), hindgut (n=7, 9%), and spine (n=1, 1%)3. Our series was generally consistent with literature data in terms of the segmentary lesion location with the ileum being the most commonly involved gastrointestinal segment (32%), equally affecting the foregut and the midgut (37%, each). Anal canal duplications are considered the rarest form of all subtypes with only a limited number of case reports6, but interestingly, 3 (16%) of our patients had anal canal duplications. The presenting complaint was an accessory orifice posterior to the anus in two and constipation in the remaining with the duplication diagnosed by perineal inspection. Simple stripping of the duplicated mucosa is proposed by some authors7; however, we opted for duplicated anal removal through the perineal approach.
A male predominance has been previously reported in digestive system duplications4,5; however, the sex distribution was equal in our patients. As in the presented series, most duplications are more cystic than tubular and do not communicate with the intestinal lumen. They vary a lot in size and may contain heterotopic mucosa. All these parameters, namely gastrointestinal tract localization, size, cystic or tubular nature, and presence of heterotopic mucosa, resulted in a wide spectrum of presenting signs and symptoms2. Abdominal pain is reported as the most common presenting symptom in symptomatic patients as in our series, yet a wide range of symptomatology, such as vomiting, palpable abdominal mass, respiratory distress, melena, constipation, acute abdominal findings, including intestinal obstruction, etc., were observed. The presented series is a typical example of this wide range of presenting symptomatology.
Antenatal diagnosis is possible and reports about antenatally diagnosed cases have been increasing8; however, only three (16%) patients were antenatally diagnosed in our series. Among these, two were already symptomatic at presentation. Nevertheless, most reported patients present before two years of age, whether antenatally diagnosed or not2,3,8. Approximately, half of our patients (n=9, 47%) were operated on before the age of two years. Among these, three had an antenatal diagnosis. However, duplications can be encountered at any age.
Gastrointestinal duplications may remain silent and incidentally discovered2. In our series, among the three incidentally diagnosed patients, two were detected during investigations for other reasons and one was detected during an irrelevant surgery.
The presence of heterotopic mucosa is a well-known but relatively uncommon occurrence in gastrointestinal duplications2,9,10. Heterotopic gastric mucosa is seen in approximately one-third of all duplications and may cause complications, such as ulceration, hemorrhage, and perforation, as well as probable malignant degeneration3,11,12,13. Heterotopic gastric mucosa was present in specimens of 5 (26%) of our patients and two of these presented with complications, such as gastrointestinal bleeding and perforation. Both two occurrences of heterotopic respiratory mucosa were detected in duplications adjacent to the esophagus. Respiratory differentiation in a duplication cyst is extremely rare14. Both specimens were positive for the homeobox transcription factor, TTF-1. Its expression is specifically limited to the respiratory tract in the developing foregut14. The first one contained additional gastric mucosa and the second one was positive for SMA, which is typical for the gastrointestinal tract. This latter patient was reported elsewhere15. Consequently, both of these cysts were reported as “foregut cysts” from embryological and pathological standpoints.
As a non-invasive and inexpensive tool, ultrasound is usually the first line of radiological assessment. The presence of a cystic structure related to the gut with a double-wall or muscular rim sign, such as inner hyperechoic mucosa with an outer hypoechoic smooth muscle layer, is considered diagnostic for intraabdominal duplication cysts9. However, ultrasound is usually not helpful in detecting thoracal lesions as in the presented series herein. A preoperative ultrasound examination was obtained in 76% of 17 patients with recorded radiological assessment data. Ultrasound alone was diagnostic in 69%. Generally, CT has the disadvantage of radioactivity, and MRI needs sedation in children. However, they play important roles in patients in whom a proper diagnosis cannot be established to delineate the anatomy of the cyst concerning adjacent structures before a planned surgery9. Thus, in addition to ultrasound, 5 of our patients underwent a CT examination and 4 had MRI. Both techniques were found to be useful in diagnosing thoracal lesions in particular. In total, a preoperative presumptive diagnosis for a duplication cyst could be made in 74% of all our patients.
Gastrointestinal duplications are generally benign lesions with only a few case reports with malignant degeneration11. Nevertheless, their total excision should be attempted in all cases because they may reach enormous sizes with associated symptomatology and have the potential of causing complications, such as bleeding and perforation. Total cyst excision is the main goal of surgery and attached luminal organ resection can be done if safe and necessary. A total cyst excision was accomplished in 95% of our patients with (42%) or without (53%) associated luminal organ resection. Resecting the adjacent luminal organ is usually needed because of a common blood supply with the duplication cyst5. If the cyst cannot be completely resected for any reason, a partial resection with complete inner mucosal layer removal should be done3,8,10. Thus, we could not completely remove the cyst in 1 (5%) patient in our series because it shared a common wall with the rectum but complete removal of the inner mucosal layer could be performed.
CONCLUSIONS
Children with digestive system duplications present a variety of symptoms and signs. Physical examination findings combined with radiological assessments are helpful in a majority of patients. Approximately, one-third of all duplications harbor heterotopic mucosa, which may result in a complicated presentation. Complete cyst removal can be achieved in most patients. The inner mucosal layer should be removed if a total cyst excision is impossible to prevent the patient from the possible future development of complications including malignant degeneration.
Footnotes
Ethics
Ethics Committee Approval: A retrospective search was done in operative logs for 16 years from 2014 to 2019 with an Istanbul Medeniyet University Goztepe Training and Research Hospital Clinical Research Ethics Committee approval (decision no: 2021/0019, date: 13.01.2021).
Informed Consent: Retrospective study.
Peer-review: Externally and internally peer-reviewed.
Author Contributions
Surgical and Medical Practices: M.C.O., F.E., N.G., A.P., S.K.O., S.O., C.U.D., Concept: M.C.O., N.G., S.K.O., C.U.D., Design: M.C.O., C.U.D., Data Collection and/or Processing: M.C.O., F.E., N.G., A.P., S.K.O., S.O., C.U.D., Analysis and/or Interpretation: M.C.O., S.O., C.U.D., Literature Search: M.C.O., F.E., A.P., S.O., C.U.D., Writing: M.C.O., C.U.D.
Conflict of Interest: The authors have no conflict of interest to declare.
Financial Disclosure: The authors declared that this study has received no financial support.
References
- 1.Macpherson RI. Gastrointestinal tract duplications: clinical, pathologic, etiologic, and radiologic considerations. Radiographics. 1993;13:1063–80. doi: 10.1148/radiographics.13.5.8210590. [DOI] [PubMed] [Google Scholar]
- 2.Stern LE, Warner BW. Gastrointestinal duplications. Semin Pediatr Surg. 2000;9:135–40. doi: 10.1053/spsu.2000.7565. [DOI] [PubMed] [Google Scholar]
- 3.Stringer MD, Spitz L, Abel R, et al. Management of alimentary tract duplication in children. Br J Surg. 1995;82:74–8. doi: 10.1002/bjs.1800820126. [DOI] [PubMed] [Google Scholar]
- 4.Rattan KN, Bansal S, Dhamija A. Gastrointestinal Duplication Presenting as Neonatal Intestinal Obstruction: An Experience of 15 Years at Tertiary Care Centre. J Neonatal Surg. 2017;6:5. doi: 10.21699/jns.v5i4.432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Puligandla PS, Nguyen LT, St-Vil D, et al. Gastrointestinal duplications. J Pediatr Surg. 2003;38:740–4. doi: 10.1016/jpsu.2003.50197. [DOI] [PubMed] [Google Scholar]
- 6.Özbey H. Anal Canal Duplication in a 12-Year-old Girl. J Pediatr Gastroenterol Nutr. 2021;72:e21. doi: 10.1097/MPG.0000000000002850. [DOI] [PubMed] [Google Scholar]
- 7.Tiryaki T, Senel E, Atayurt H. Anal canal duplication in children: a new technique. Pediatr Surg Int. 2006;22:560–1. doi: 10.1007/s00383-006-1654-3. [DOI] [PubMed] [Google Scholar]
- 8.Erginel B, Soysal FG, Ozbey H, et al. Enteric Duplication Cysts in Children: A Single-Institution Series with Forty Patients in Twenty-Six Years. World J Surg. 2017;41:620–4. doi: 10.1007/s00268-016-3742-4. [DOI] [PubMed] [Google Scholar]
- 9.Sangüesa Nebot C, Llorens Salvador R, Carazo Palacios E, Picó Aliaga S, Ibañez Pradas V. Enteric duplications cysts in children: varied presentations, varied imaging findings. Insights Imaging. 2018;9:1097–106. doi: 10.1007/s13244-018-0660-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xang L, Lan J, Chen B, Li P, Guo C. Clinical characteristics of gastrointestinal tract duplications in children: A single-institution series review. Medicine (Baltimore) 2019;98:e17682. doi: 10.1097/MD.0000000000017682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Viswanath UVA, Medappil N, Rajan A, et al. Adenocarcinoma of Jejunal Duplication Cyst-Case Report and Review of Literature. Indian J Surg Oncol. 2021;12(Suppl 2):327–31. doi: 10.1007/s13193-021-01349-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leng S, Ghionzoli M, Caporalini C, Buccoliero AM. Long-term intestinal bleeding in a child: a rare case of heterotopic gastric mucosa in the jejunum. BMJ Case Rep. 2016;2016:bcr2016216949. doi: 10.1136/bcr-2016-216949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brown RL, Azizkhan RG. Gastrointestinal bleeding in infants and children: Meckel’s diverticulum and intestinal duplication. Semin Pediatr Surg. 1999;8:202–9. doi: 10.1016/s1055-8586(99)70027-2. [DOI] [PubMed] [Google Scholar]
- 14.Laurent S, Vivario M, Lardinois F. Gastric Duplication Cyst with Respiratory Epithelium: a Rare Entity. Acta Chir Belg. 2015;115:379–81. doi: 10.1080/00015458.2015.11681134. [DOI] [PubMed] [Google Scholar]
- 15.Olgun ZC, Ozkanli S, Seneldir H, Aksu B, Mutus M, Durakbasa CU. Report of a “foregut cyst” with oesophageal and bronchogenic features. Virchows Archiv. 2021;479(Suppl1):S290. [Google Scholar]


