Abstract
The viable but nonculturable (VBNC) state is a survival strategy adopted by bacteria when they are exposed to hostile environmental conditions. It has been shown that VBNC forms of bacteria are no longer capable of growing on conventional bacteriological media but conserve pathogenic factors and/or genes. It is thus necessary to develop methods capable of detecting nonculturable bacteria and of establishing their viability when the microbiological quality of environments is monitored. In this study we demonstrated that a gene was expressed during the VBNC state in a low-nutrient-concentration microcosm through detection of Enterococcus faecalis pbp5 mRNA by reverse transcription-PCR over a 3-month period. The presence of mRNA correlated with metabolic activity and resuscitation capability, indicating the viability of the VBNC cells.
The viable but nonculturable (VBNC) state is a recently described survival mechanism of bacteria facing environmental stress conditions (7, 18). The VBNC state has been described for numerous gram-negative bacteria, including bacteria of medical interest such as vibrios (7, 8), Shigella dysenteriae (21), Campylobacter jejuni (23), Helicobacter pylori (6), and Escherichia coli O157:H7 (31). More recently, we have shown that a gram-positive species, Enterococcus faecalis, can also enter the VBNC state (12). When in this state, bacteria are no longer able to grow and form colonies on conventional culture media but demonstrate metabolic activity (7, 12, 21), maintain their pathogenicity (19, 21), and, in some cases, may return to active growth when optimal conditions are restored (7, 8, 12, 14, 16, 19, 28). Whether these properties have to be considered proof of VBNC bacterial viability is, at present, a controversial issue (1, 2, 4, 5, 19). For this reason, new tests and parameters should be sought in order to definitively establish the viability of the VBNC forms. Moreover, these new tests and cellular targets could also be useful for detecting VBNC bacteria in environmental samples. During evaluation of the microbiological quality of water, the need to detect bacteria in the VBNC state is of paramount importance not only for pathogenic bacteria but also for standard indicators of fecal contamination, such as E. coli and enterococci (fecal streptococci). The latter microorganisms (E. faecalis in particular) are currently regarded as the indicators of choice for medium-term fecal contamination of water for human use (both drinking and recreational use). Moreover, of all the microorganisms measured, only the presence of enterococci in water correlates with the incidence of diarrheal diseases among human users (29).
Very recently, we have described specific modifications of the cell wall of E. faecalis when it enters the VBNC state (27). E. faecalis VBNC forms have a hyper-cross-linked peptidoglycan compared with the peptidoglycan of dividing cells. Moreover, they show modifications of penicillin binding proteins (PBPs), the enzymes involved in terminal stages of peptidoglycan synthesis; PBP1 and PBP5 are the prevalent PBPs. Thus, these proteins are two potential targets which can be used to study and detect cells in the VBNC state. The gene encoding PBP5 has been cloned, sequenced, and shown to be species specific (26). For this reason, we previously used this gene as a specific probe (22) and as a target for PCR (12) and competitive PCR (13) for detection of E. faecalis cells.
Bacterial mRNAs have been proposed as markers for cell viability since they are very unstable molecules with very short half-lives inside the cell (25). Thus, it would be expected that as long as VBNC bacteria are alive, they should produce some mRNA molecules. In this study we verified that the E. faecalis pbp5 gene is expressed during the VBNC state through detection of pbp5 mRNA by reverse transcription (RT)-PCR over time and used it as a marker for cell viability.
To do this, an exponentially growing E. faecalis 56R culture was used to inoculate a nutrient-poor microcosm consisting of sterilized lake water at a final density of 106 cells/ml. The suspension was maintained at 4°C for about 2 weeks, as previously described (12). After this, the total cell number remained invariant while no E. faecalis colonies were detectable when 10-ml portions of the microcosm were inoculated on plates containing tryptic soy agar (Difco Laboratories, Detroit, Mich.). Active metabolism of these nonculturable cells was detected by measuring incorporation of [3H]leucine into newly synthesized proteins and was evaluated by measuring trichloroacetic acid-precipitable radioactivity, as previously described (12). Moreover, a modified direct viable count method was used to test cell viability. Because gram-positive bacteria are resistant to nalidixic acid used in the Kogure method (10), we treated the cells with benzylpenicillin at a concentration (1 μg/ml) that blocked septum formation and allowed cell elongation (11). Induction of VBNC cell resuscitation was used as an additional method to test the viability of VBNC cells. To do this, samples of E. faecalis VBNC cells were incubated under optimal growth conditions after treatment of VBNC forms with benzylpenicillin (100 μg/ml) to eliminate growing cells, as previously described (12).
In order to analyze the presence of pbp5 mRNA by RT-PCR, samples of E. faecalis VBNC cells were taken at intervals over a 5-month period and harvested by filtration with 0.22-μm-pore-size membrane filters (Millipore Corporation, Bedford, Mass.), and their RNA was extracted. Standard precautions to make sure that RNases were not present were taken: diethyl pyrocarbonate-treated water and an RNase inhibitor (Roche Diagnostic SpA, Milan, Italy) were used.
The bacterial pellets (ca. 108 cells) were suspended in 1 ml of SET buffer (20% sucrose, 50 mM EDTA, 50 mM Tris-HCl [pH 7.6]) containing 1 U of RNase inhibitor, and then 35 μl of lysozyme (5 mg/ml) was added. The suspensions were left on ice for 15 min. No differences in sensitivity to lysozyme were observed in VBNC cells compared to exponentially growing cells (there was an 18% decrease in the optical density at 640 nm per h in both cases), as opposed to the results obtained when cells were mechanically broken (27). Immediately after this incubation, 9 μl of 25% sodium dodecyl sulfate was added, and samples were incubated for 60 min at room temperature. Subsequently, 50 μl of proteinase K (20 mg/ml) was added, and the suspensions were incubated at room temperature for an additional 30 min. To remove contaminating DNA, 1 μg of RNA was treated with 30 U of RNase-free DNase (Roche Diagnostic SpA) at 37°C for 60 min. The absence of DNA was confirmed by PCR performed with PBP5-specific primers FWD (5′ CATGCGCAATTAATCGG 3′) and IS (5′ CATAGCCTGTCGCAAAAC 3′), as previously described (12). DNA-free samples were treated with phenol-chloroform, and the RNA was precipitated with 3 volumes of ethanol and 5 M ammonium acetate at −80°C. The RNA concentration was determined spectrophotometrically. Under our experimental conditions 108 cells yielded approximately 2 μg of total RNA.
RT-PCR was performed in a single-step procedure by adding ca. 200 ng of RNA (corresponding to approximately 5 × 106 to 1 × 107 cells) to a mixture consisting of EZ buffer (250 mM bicine, 575 mM potassium acetate, 40% glycerol; pH 8.2), each deoxynucleoside triphosphate at a concentration of 200 μM, 100 pmol of each of the two primers described above, 2.5 mM manganese acetate, and 5 U of rTth DNA polymerase (Roche Diagnostic SpA). Retrotranscription was performed with a thermal cycler (Perkin-Elmer, Warrington, United Kingdom) for 30 min at 60°C, and then cDNA was amplified by 40 cycles consisting of 1.5 min of denaturation at 94°C, 1.5 min of annealing at 60°C, and 2 min of extension at 72°C, followed by a final 5-min extension period at 72°C. The expected size of the amplification product was 444 bp (13). This RT-PCR protocol allowed us to detect mRNA in samples containing at least 50 VBNC cells.
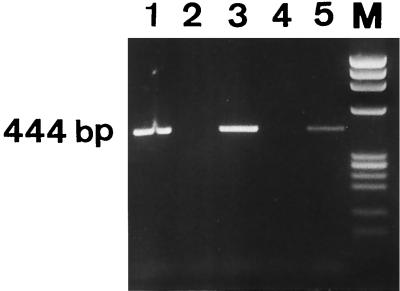
Figure 1 shows the products obtained from the RT-PCR performed with samples containing RNA extracted from exponentially growing cells, killed cells (cells boiled for 5 min and then left to stand for 5 h at room temperature, as described by Sheridan et al. [25]), and VBNC cells and separated on a 2.5% agarose gel. VBNC cells, like growing cells, contain pbp5-specific mRNA which has been retrotranscribed and amplified, as demonstrated by the presence of the corresponding 444-bp DNA fragment visible on the agarose gel. RT-PCR performed with a 1,000-fold dilution of a VBNC population containing less then 1 CFU/10 ml yielded a similar amplification product, thus eliminating the possibility of amplification of mRNA from undetected dividing cells still present in the VBNC population (data not shown). Heat-killed cells, as expected (25), yielded no amplification products. When a sample of RNA was treated with RNase, no amplification was observed, indicating that RNA was the real target of RT-PCR (data not shown).
FIG. 1.
RT-PCR detection of pbp5 mRNA from exponentially growing cells (lane 3), from growing cells killed by boiling for 5 min and then left to stand for 5 h at room temperature (lane 4), and from 3-month-old VBNC cells (lane 5). The RT-PCR positive (lane 1) and negative (lane 2) controls contained E. faecalis DNA and sterile water, respectively. Lane M contained markers.
Using RT-PCR, we then studied expression of the pbp5 gene as a marker to evaluate the viability over time of an E. faecalis population that was maintained in a laboratory microcosm and entered the VBNC state. Table 1 shows that pbp5 mRNA was detectable during the 3 months after entry of E. faecalis cells into the VBNC state. In contrast, mRNA was no longer detectable in a sample taken 5 months after the VBNC state was reached. Table 1 also shows a good correlation between detection of this specific mRNA and other cellular parameters which were used to test cell viability during the VBNC state. The positive results of the other viability tests, such as tests for resuscitation capability, protein synthesis, and elongation of nondividing cells, indicate that, all things considered, these parameters may be useful. At 5 months, the cells seemed to be dead, since not only was pbp5 mRNA undetectable but protein synthesis had also ceased and cells no longer elongated or had the ability to resuscitate.
TABLE 1.
pbp5 mRNA detection by RT-PCR in an E. faecalis population maintained for 5 months in the VBNC state compared to the results of other cell viability tests
| Cells | Cell viability test results
|
|||
|---|---|---|---|---|
| RT-PCR | Protein synthesisa | Direct viable counting (%)b | Resuscitation | |
| VBNC cells afterc: | ||||
| 0.5 month | + | + | 84 (4) | + |
| 1 month | + | + | 60 (3) | + |
| 3 months | + | + | 15 (5) | ±d |
| 5 months | − | − | <1 | − |
| Growing cells (positive control)e | + | + | 100 | + |
| Killed cells (negative control)f | − | − | <1 | − |
Protein synthesis was considered positive when the level of radioactivity incorporated (in counts per minute) was threefold higher than that of the same sample treated with formaldehyde, as described previously (12).
Percentage of cells that, in the presence of 0.05% yeast extract, showed elongation after 6 h of treatment with benzylpenicillin. The numbers in parentheses are coefficients of variation.
Time zero is considered the time of inoculation of the microcosm; 0.5 month, therefore, coincides with the loss of culturability and the beginning of the VBNC state.
Resuscitation was observed in two of the three experiments performed.
The optical density at 640 nm of the exponentially growing culture used as a positive control was 0.5.
Exponentially growing cells collected by centrifugation were boiled for 5 min and then left to stand for 5 h at room temperature and used as a negative control.
On the basis of the tenet that viability is equivalent to culturability, a nonculturable microorganism has to be automatically considered nonviable, i.e., dead. However, many observations have shown that microorganisms may remain viable and maintain active metabolism after ceasing to divide and losing culturability (7, 18, 30). To date, several parameters have been used and proposed as alternatives to culturability for defining a viable cell; these include the ability to take up amino acids or sugars (15, 21), protein synthesis (12), detection of intact DNA (24), and respiration (12, 21). Moreover, in some cases it has been possible to resuscitate nonculturable cells, even if the experiments have been severely criticized, since some authors believe that VBNC samples may still contain undetectable dividing cells (8, 30). It is clear that lack of resuscitation of nonculturable cells in the laboratory is not proof of cell death because some VBNC cells can be resuscitated only during infection of humans or animals (6, 7, 19, 28).
Because mRNA is a short-lived molecule due to the presence of nucleases that digest it very rapidly (25), the presence of mRNA can be regarded as a valid and convincing criterion for assessing cell viability (3, 9, 20, 25). In this study we demonstrated that nonculturable E. faecalis cells are capable of expressing the pbp5 gene for at least 3 months, indicating that they remain viable in a low-nutrient-concentration microcosm for this period of time. To the best of our knowledge, this is the first time that mRNA has been detected in unculturable cells considered to be in the VBNC state. Detection of pbp5 mRNA over a 3-month period confirms our findings indicating that specific alterations of the E. faecalis cell wall and peptidoglycan occur in conjunction with entry of this bacterial species into the VBNC state (27). The absence of pbp5 mRNA in cells maintained in the VBNC state for more than 3 months, together with the negative results for the other viability parameters at that time, may indicate that these cells have probably reached a state leading to death and from which it is no longer possible to return to division. A “window” between loss of culturability and death can thus be identified for each bacterial species. This window can be years for some bacteria, such as Vibrio cholerae (17), or a shorter time, e.g., 3 months in E. faecalis. Considering the term viability from this point of view, we can define a dead cell not as a cell that is unable to multiply but as a cell that has lost the ability to express genes and/or to return to the culturable state. Consequently, new tests which are also capable of detecting unculturable but potentially viable microorganisms have to be included when the presence of bacteria in clinical, environmental, and food samples is monitored. We regard mRNA detection by RT-PCR as the most appropriate method for evaluating the impact of the presence of VBNC bacteria when the microbiological quality of water has to be defined. This consideration stems not only from the high specificity and sensitivity of this technique but also from the fact that it allows us to establish cell viability. Though the other parameters tested here (i.e., protein synthesis capability, elongation of nondividing cells, and resuscitation of VBNC cells) may appear to be equally valid, they cannot be easily standardized for practical use and thus are less reliable than RT-PCR. VBNC-specific mRNAs need to be identified in several bacterial species in order to set up protocols for enumeration of the VBNC forms of at least the main bacterial species of medical interest.
Acknowledgments
Rita R. Colwell is gratefully acknowledged for helpful comments and suggestions.
This study was supported by grants 99.00320.PF49 and 99.03586.PF49 (Target Project on “Biotechnology”) from the Consiglio Nazionale delle Richerche (CNR) and by 1998 cofinancing from Ministero dell'Università e della Ricerca Scientifica e Tecnologica (MURST), Rome, Italy.
REFERENCES
- 1.Barer M R, Gribbon L T, Harwood C R, Nwoguh C E. The viable but non-culturable hypothesis and medical bacteriology. Rev Med Microbiol. 1998;4:183–191. [Google Scholar]
- 2.Barer M R, Harwood C R. Bacterial viability and culturability. Adv Microb Physiol. 1999;41:93–137. doi: 10.1016/s0065-2911(08)60166-6. [DOI] [PubMed] [Google Scholar]
- 3.Bej A K, Ng W Y, Morgan S, Jones D, Mahbubani M H. Detection of viable Vibrio cholerae by reverse-transcriptase polymerase chain reaction (RT-PCR) Mol Biotechnol. 1996;5:1–10. doi: 10.1007/BF02762407. [DOI] [PubMed] [Google Scholar]
- 4.Bloomfield S F, Stewart G S, Dodd C E R, Booth I R, Power E G M. The viable but nonculturable phenomenon explained? Microbiology. 1998;144:1–3. doi: 10.1099/00221287-144-1-1. [DOI] [PubMed] [Google Scholar]
- 5.Bogosian G. Viable but nonculturable, or dead? ASM News. 1998;64:547. [Google Scholar]
- 6.Cellini L, Allocati N, Angelucci D, Iezzi T, Di Campli E, Marzio L, Dainelli B. Coccoid Helicobacter pylori not culturable in vitro reverts in mice. Microbiol Immunol. 1994;38:843–850. doi: 10.1111/j.1348-0421.1994.tb02136.x. [DOI] [PubMed] [Google Scholar]
- 7.Colwell R R, Huq H. Vibrios in the environment: viable but nonculturable Vibrio cholerae. In: Kaye T, editor. Vibrio cholerae and cholera: molecular global perspectives. Washington, D.C.: American Society for Microbiology; 1994. pp. 117–133. [Google Scholar]
- 8.Jians X, Chai T. Survival of Vibrio parahaemolyticus at low temperatures under starvation conditions and subsequent resuscitation of viable nonculturable cells. Appl Environ Microbiol. 1996;62:1300–1305. doi: 10.1128/aem.62.4.1300-1305.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Klein P G, Juneja V K. Sensitive detection of viable Listeria monocytogenes by reverse transcription-PCR. Appl Environ Microbiol. 1997;63:4441–4448. doi: 10.1128/aem.63.11.4441-4448.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kogure K, Simidu U, Taga N. A tentative direct microscopic method for counting living bacteria. Can J Microbiol. 1979;25:415–420. doi: 10.1139/m79-063. [DOI] [PubMed] [Google Scholar]
- 11.Lleò M M, Canepari P, Fontana R, Satta G. Inhibition of bacterial cell surface extension by various means causes blocking of macromolecular synthesis. Res Microbiol. 1997;148:11–20. doi: 10.1016/S0923-2508(97)81895-5. [DOI] [PubMed] [Google Scholar]
- 12.Lleò M M, Tafi M C, Canepari P. Nonculturable Enterococcus faecalis cells are metabolically active and capable of resuming active growth. Syst Appl Microbiol. 1998;21:333–339. doi: 10.1016/s0723-2020(98)80041-6. [DOI] [PubMed] [Google Scholar]
- 13.Lleò M M, Tafi M C, Signoretto C, Dal Cero C, Canepari P. Competitive polymerase chain reaction for quantification of noncultivable Enterococcus faecalis cells in lake water. FEMS Microbiol Ecol. 1999;30:345–353. doi: 10.1111/j.1574-6941.1999.tb00662.x. [DOI] [PubMed] [Google Scholar]
- 14.Mizunoe Y, Wai S N, Takade A, Yoshida S. Restoration of culturability of starvation-stressed and low-temperature-stressed Escherichia coli O157:H7 cells by using H2O2-degrading compounds. Arch Microbiol. 1999;172:63–67. doi: 10.1007/s002030050741. [DOI] [PubMed] [Google Scholar]
- 15.Morgan J A W, Cranwell P A, Pickup R W. Survival of Aeromonas salmonicida in lake water. Appl Environ Microbiol. 1991;57:1777–1782. doi: 10.1128/aem.57.6.1777-1782.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mukamolova G V, Kormer S S, Kell D B, Kaprelyants A S. Stimulation of the multiplication of Micrococcus luteus by a autocrine growth factor. Arch Microbiol. 1999;172:9–14. doi: 10.1007/s002030050733. [DOI] [PubMed] [Google Scholar]
- 17.Munro P M, Colwell R R. Fate of Vibrio cholerae O1 in seawater microcosms. Water Res. 1996;30:47–50. [Google Scholar]
- 18.Oliver J D. Formation of viable but nonculturable cells. In: Kjelleberg S, editor. Starvation in bacteria. New York, N.Y: Plenum Press; 1993. pp. 239–272. [Google Scholar]
- 19.Oliver J D, Bockian R. In vivo resuscitation and virulence towards mice of viable but nonculturable cells of Vibrio vulnificus. Appl Environ Microbiol. 1995;61:2620–2623. doi: 10.1128/aem.61.7.2620-2623.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Patel B K R, Vanjerjee D K, Butcher P D. Determination of Mycobacterium leprae viability by polymerase chain reaction amplification of 71-kDa heat shock protein RNA. J Infect Dis. 1993;168:799–800. doi: 10.1093/infdis/168.3.799. [DOI] [PubMed] [Google Scholar]
- 21.Rahman I, Shahamat M, Kirchman P A, Rissek-Cohen E, Colwell R R. Methionine uptake and cytopathogenicity of viable but nonculturable Shigella dysenteriae type 1. Appl Environ Microbiol. 1994;60:3573–3578. doi: 10.1128/aem.60.10.3573-3578.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Robbi C, Signoretto C, Boaretti M, Canepari P. The gene encoding for penicillin binding protein 5 of Enterococcus faecalis is useful for development of a species-specific DNA probe. Microb Drug Resist. 1996;2:215–218. doi: 10.1089/mdr.1996.2.215. [DOI] [PubMed] [Google Scholar]
- 23.Rollins D M, Colwell R R. Viable but nonculturable stage of Campylobacter jejuni and role in survival in the natural aquatic environment. Appl Environ Microbiol. 1986;52:531–538. doi: 10.1128/aem.52.3.531-538.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roth B L, Poot M, Yue S T, Millard P J. Bacterial viability and antibiotic susceptibility testing with SYTOX green nucleic acid stain. Appl Environ Microbiol. 1997;63:2421–2431. doi: 10.1128/aem.63.6.2421-2431.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sheridan G E C, Masters C I, Shallcross J A, Mackey B M. Detection of mRNA by reverse transcription-PCR as an indicator of viability in Escherichia coli cells. Appl Environ Microbiol. 1998;64:1313–1318. doi: 10.1128/aem.64.4.1313-1318.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Signoretto C, Boaretti M, Canepari P. Cloning, sequencing and expression in Escherichia coli of the low-affinity penicillin-binding protein of Enterococcus faecalis. FEMS Microbiol Lett. 1994;123:99–106. doi: 10.1111/j.1574-6968.1994.tb07207.x. [DOI] [PubMed] [Google Scholar]
- 27.Signoretto C, Lleò M M, Tafi M C, Canepari P. Cell wall chemical composition of Enterococcus faecalis in the viable but nonculturable state. Appl Environ Microbiol. 2000;66:1953–1959. doi: 10.1128/aem.66.5.1953-1959.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Steinert M, Emody L, Amann R, Hacker J. Resuscitation of viable but nonculturable Legionella pneumophila Philadelphia JR 32 by Acanthamoeba castellanii. Appl Environ Microbiol. 1997;63:2047–2053. doi: 10.1128/aem.63.5.2047-2053.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Toranzos G A, McFetters G A. Detection of indicator microorganisms in environmental freshwaters and drinking waters. In: Kurst C J, Knudsen G R, McInnerney M J, Stetzenbach L D, Walter M V, editors. Manual of environmental microbiology. Washington, D.C.: American Society for Microbiology; 1997. pp. 184–194. [Google Scholar]
- 30.Votyakova T V, Kaprelyants A S, Kell D B. Influence of viable cells on the resuscitation of dormant cells in Micrococcus luteus cultures held in an extended stationary phase: the population effect. Appl Environ Microbiol. 1994;60:3284–3291. doi: 10.1128/aem.60.9.3284-3291.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang G, Doyle M P. Survival of enterohemorrhagic Escherichia coli O157:H7 in water. J Food Prot. 1998;6:662–667. doi: 10.4315/0362-028x-61.6.662. [DOI] [PubMed] [Google Scholar]