Abstract
Bacillus thuringiensis δ-endotoxins insert into the brush border membranes of insect larval cells to form ion channels. A possible interaction of these toxins with a cytoplasmic component was examined by preloading vesicles from insect larval cells with protease K followed by incubation with toxin. There was no evidence for toxin antigens smaller than the intact toxin in extracts of solubilized vesicles, nor was there an effect of the inclusion of protease K on either of two functional properties, the formation of toxin aggregates or of ion pores. These toxins, physically and functionally, appear to be confined to the membrane.
During sporulation, Bacillus thuringiensis synthesizes a mixture of insecticidal protoxins which are deposited as intracellular inclusions (1). Following ingestion by insect larvae, the protoxins are solubilized and converted to active toxins which bind reversibly to receptors on the surfaces of midgut cells (12). Subsequently, the toxins insert into the membrane and form cation-selective channels (7, 13). It appears that lethality is due to the resulting osmotic lysis of the midgut cells (7).
These toxins are comprised of three domains (6, 9), with the amphipathic alpha-helical bundle of domain I being the most likely to be involved in the formation of ion channels (12). Following binding to the receptor, the entire toxin molecule, with the exception of alpha-helix 1, becomes resistant to the addition of protease K (2). An umbrella-like model has been proposed, with several toxin molecules aggregating and the most hydrophobic part of the toxin, helices α4 and α5 of domain I, making up the pore (5, 10, 14).
There is much experimental evidence to support the formation of an ion channel as the basis for toxicity (5, 7, 12–14). There are other aspects of larval behavior in response to toxin, such as avoidance and feeding paralysis, however, which may be more rapid and perhaps not readily accounted for by pore formation and the subsequent osmotic effects. An alternative or additional function of the toxin could depend upon the projection of part of the toxin into the cell cytoplasm, where there may be interactions with cytoplasmic components. Many of the amphipathic alpha-helices in domain I are long enough to project through the plane of the membrane (6, 9), so such an arrangement and its potential functional implications should be considered. In order to determine if part of the membrane-bound toxin was exposed to the cytoplasmic face, larval brush border membrane vesicles (BBMV) were prepared in the presence or absence of protease K. The effects of the incorporated protease on toxin integrity and function were then examined.
Manduca sexta eggs were purchased from Carolina Biologicals and were hatched and grown to fourth- to fifth-instar larvae on a synthetic diet (3). Midguts were isolated and frozen at −70°C. BBMV were prepared (2, 15) in the presence or absence of 200 μg of protease K (Sigma) per ml per midgut suspended in 5 ml of 0.3 M mannitol–5 mM EGTA–17 mM Tris, pH 7.5, plus 24 mM MgCl2 (2, 15). After a thorough washing with the suspension buffer, the protein contents were determined (bicinchoninic reagent kit; Pierce) and the BBMV were aliquoted and stored at −70°C.
Cry1Ab3 and Cry1Ac1 toxins were purified as previously described (2, 11) from inclusions produced by an acrystalliferous derivative of B. thuringiensis subsp. kurstaki (strain CryB) containing a clone of either the cry1Ab3 or cry1Ac1 gene. Following elution from a MonoQ cartridge (Pharmacia), the toxins were stored at −20°C in 0.03 M NaHCO3–0.3 M NaCl, pH 8.5.
Protease K activity in intact vesicles and in vesicles solubilized in 1% deoxycholate (DOC) was measured by incubation with 5 μg of bovine serum albumin (BSA) at 37°C for 30 and 60 min in a total volume of 20 μl. The DOC treatment totally solubilized BBMV protein (and did not inhibit protease K activity). Following the incubation, an equal volume of 6 M urea–1% sodium dodecyl sulfate (SDS)–0.05 M dithiothreitol–2 mM phenylmethylsulfonyl fluoride, pH 9.8 (UDS), was added, and the solutions were heated at 90°C for 3 min before analysis by SDS–10% polyacrylamide gel electrophoresis (PAGE). Gels were stained with GelCode Blue (Pierce).
BBMV solubilized in 1% DOC were used directly in SDS–10% PAGE to examine the stained protein profiles. The proteins were also transferred to a polyvinylidene difluoride membrane (Immobilon-P; Millipore) for immunoblotting with anti-aminopeptidase N antibody (11). The latter was prepared with purified aminopeptidase N from Bombyx mori larvae as the immunogen (courtesy of D. Dean, Ohio State University).
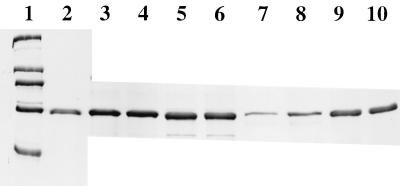
Intact vesicles and those lysed by DOC (20 μg of vesicle protein) were incubated with 5 μg of BSA for 30 or 60 min at 37°C, mixed with an equal volume of UDS, and analyzed by SDS–10% PAGE (Fig. 1). There was no protease activity in either the preparation of washed, intact BBMV or the control BBMV solubilized in DOC. Protease activity was detected only in solubilized BBMV which had been prepared in the presence of protease K. There was total digestion of the BSA (or the Cry1Ab3 or Cry1Ac1 toxins) by 0.2 μg of protease K in BBMV suspension buffer at 37°C for 30 min (A. Aronson, unpublished data).
FIG. 1.
SDS-PAGE profile of BSA incubated with intact or lysed BBMV. Lane 1, standards of 205, 116, 97, 66, and 45 kDa (from top to bottom); lane 2, 2 μg of BSA; lanes 3 and 4, BSA incubated for 60 (lane 3) and 30 (lane 4) min with solubilized BBMV; lanes 5 and 6, BSA incubated with intact BBMV for 60 (lane 5) or 30 (lane 6) min; lanes 7 and 8, BSA incubated with solubilized BBMV containing protease K for 60 (lane 7) or 30 (lane 8) min; lanes 9 and 10, BSA incubated with intact BBMV containing protease K for 60 (lane 9) or 30 (lane 10) min.
There were no major differences in the profiles of stained proteins from the two BBMV preparations resolved by SDS–10% PAGE (A. Aronson, unpublished data). In addition, a major membrane protein, aminopeptidase N, which also serves as a toxin receptor (12), was unaltered in BBMV prepared in the presence of protease K (Fig. 2).
FIG. 2.
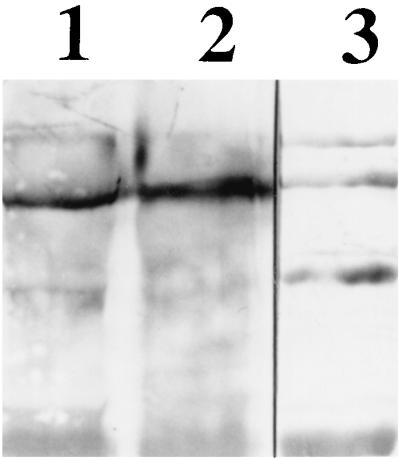
Immunoblot with aminopeptidase N antibody of solubilized M. sexta BBMV produced in the absence (lane 1) or presence (lane 2) of protease K. Lane 3, standards of 205, 116, 97, and 66 kDa (from top to bottom).
It had been previously demonstrated that following incubation of purified Cry1Ab3 or Cry1Ac1 toxins (ca. 60-kDa monomers) with BBMV, toxin antigens of ca. 130 and 200 kDa were present, indicating aggregation of the toxins either within the membrane or at the membrane surface. The former appears to be more likely on the basis of studies with fluorescently labeled peptides of the domain I alpha-helices (5). This aggregation appears to be functionally significant since toxins which were not active due to mutations of residues within helix α5 and certain other parts of domain I did not aggregate in BBMV (2). Exceptions were nonactive toxins with mutations within helix α4 which aggregated in the membrane but did not form functional ion channels (8).
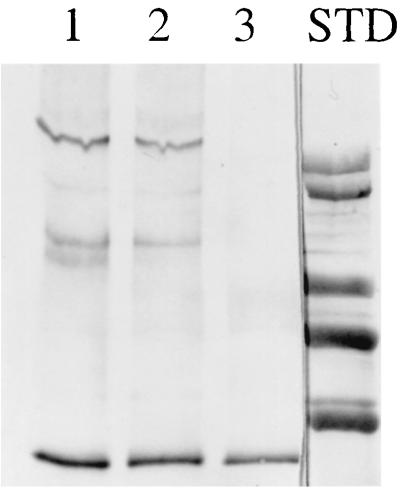
The Cry1Ac1 toxin was incubated with BBMV prepared in the presence or absence of protease K, washed, and extracted as previously described (2, 8). Cry1Ac1 antigens were resolved by SDS–6% PAGE and detected with a Cry1Ac1-specific polyclonal antibody plus an anti-mouse immunoglobulin–alkaline phosphatase conjugate following transfer to a polyvinylidene difluoride membrane (Fig. 3). Aggregates of ca. 200 and 130 kDa were present in both cases. There was no evidence for smaller Cry1Ac1 antigens in the BBMV containing protease K. Since helices α4 and α5 in domain I are the most likely ones to be directly involved in the formation of ion channels (2, 5, 10, 12–14), the loop connecting these two helices could project into the cytoplasm of larval midgut cells following toxin insertion. Cleavage by protease K within this loop would result in toxin fragments of ca. 20 and 40 kDa, but neither was detected. It should be noted, however, that the Cry1Ac1 antibody may not react with either of these fragments. It is also possible that protease K could not cleave in this region of the toxin in its membrane-bound state, although this protease is rather nonspecific and totally digests the toxin in solution.
FIG. 3.
Immunoblot of extracts of BBMV which had been preincubated with Cry1Ac1 toxin. Lane 1, BBMV (30 μg of protein) incubated with 100 ng of toxin; lane 2, BBMV (30 μg of protein) formed in the presence of protease K incubated with 100 ng of toxin; lane 3, 50 ng of Cry1Ac1 toxin. STD, standards of 66, 97, 116, and 205 kDa (with an extra band of >205 kDa), from bottom to top.
In order to confirm these results, the aggregation experiments were repeated with the Cry1Ab3 toxin and a monoclonal antibody. This toxin aggregates in solution much less than the Cry1Ac1 toxin (2), and there was an equivalent increase of a ca. 200-kDa antigen in extracts from BBMV which had been prepared in the presence or absence of protease K. In addition, there were no antigens smaller than the 60-kDa toxin in extracts from either the control or protease K-containing BBMV (A. Aronson, unpublished data).
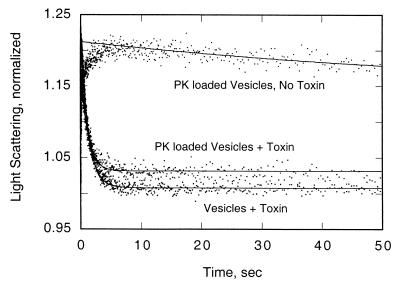
The formation of pores in BBMV in the absence or presence or protease K was examined by light scattering following the addition of 150 mM KCl (4, 8). Suspensions of BBMV (0.2 mg/ml) were equilibrated with 10 mM 2(cyclohexylamino)ethanesulfonic acid (CHES)-KOH (pH 9.0)–1% (wt/vol) BSA with or without Cry1Ab3 toxin (36 pmol/mg of BBMV) at 25°C. These suspensions were rapidly equilibrated with an equal volume of CHES-KOH–0.1% BSA containing 150 mM KCl, pH 9.0. The decrease in light scattering at 450 nm was recorded over 50 s in a SpectraKinetic stopped-flow spectrophotometer (Applied Physics). On the basis of the light-scattering measurements, functional potassium ion pores were formed by Cry1Ab3 in both BBMV preparations (Fig. 4).
FIG. 4.
Normalized light scattering of vesicles formed in the absence or presence of protease K (PK). The averages of three measurements taken over 50 s are plotted (standard deviations were 2 to 4%).
Formation of vesicles in the presence of protease K resulted in the entrapment of this enzyme, either within the vesicles or in some other nonaccessible location (bound to a membrane protein, for example). If it is assumed that at least some of the enzyme is within the vesicles and is functional there, then any part of the toxin molecule projecting onto the cytoplasmic side of the membrane should be digested by this very active, nonspecific protease. Within the limits of the assays used, it appears that toxin molecules inserted into vesicles containing protease K remained intact, aggregated, and formed functional pores for potassium ions. It is therefore likely that toxin function is confined to the larval membrane.
Acknowledgments
Stanislav Zakharov was most helpful with the light-scattering measurements. The technical assistance of Lan Wu is appreciated.
REFERENCES
- 1.Aronson A I. The two faces of Bacillus thuringiensis: insecticidal proteins and post-exponential survival. Mol Microbiol. 1993;7:489–496. doi: 10.1111/j.1365-2958.1993.tb01139.x. [DOI] [PubMed] [Google Scholar]
- 2.Aronson A I, Geng C, Wu L. Aggregation of Bacillus thuringiensis Cry1A toxins upon binding to target insect larval midgut vesicles. Appl Environ Microbiol. 1999;65:2503–2507. doi: 10.1128/aem.65.6.2503-2507.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arvidson H, Dunn P E, Strnad S, Aronson A I. Specificity of Bacillus thuringiensis for lepidopteran larvae: factors involved in vivo and in the structure of a purified protoxin. Mol Microbiol. 1989;3:1533–1543. doi: 10.1111/j.1365-2958.1989.tb00139.x. [DOI] [PubMed] [Google Scholar]
- 4.Carroll J, Wolfersberger M G, Ellar D J. The Bacillus thuringiensis Cry1Ac toxin-induced permeability change in Manduca sexta midgut brush border membrane vesicles proceeds by more than one mechanism. J Cell Sci. 1997;110:3099–3104. doi: 10.1242/jcs.110.24.3099. [DOI] [PubMed] [Google Scholar]
- 5.Gazit E, Rocca P L, Sansom M S P, Shai Y. The structure and organization within the membrane of the helices composing the pore-forming domain of Bacillus thuringiensis δ-endotoxin are consistent with an “umbrella-like” structure of the pore. Proc Natl Acad Sci USA. 1998;95:12289–12294. doi: 10.1073/pnas.95.21.12289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grochulski P, Masson L, Borisova S, Pusztai-Carey M, Schwartz J-L, Brousseau R, Cygler M. Bacillus thuringiensis CryIAa insecticidal toxin: crystal structure and channel formation. J Mol Biol. 1995;254:1–18. doi: 10.1006/jmbi.1995.0630. [DOI] [PubMed] [Google Scholar]
- 7.Knowles B H. Mechanism of action of Bacillus thuringiensis insecticidal δ-endotoxins. Adv Insect Physiol. 1994;24:275–308. [Google Scholar]
- 8.Kumar A S M, Aronson A I. Analysis of mutations in the pore-forming region essential for insecticidal activity of a Bacillus thuringiensis δ-endotoxin. J Bacteriol. 1999;181:6103–6107. doi: 10.1128/jb.181.19.6103-6107.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li J, Carroll J, Ellar D J. Crystal structure of an insecticidal protein. The δ-endotoxin from Bacillus thuringiensis subsp. tenebrionis at 2.5 Å resolution. Nature. 1991;353:815–821. doi: 10.1038/353815a0. [DOI] [PubMed] [Google Scholar]
- 10.Masson L, Tabashnik B E, Liu Y-B, Brousseau R, Schwartz J-L. Helix 4 of the Bacillus thuringiensis Cry1Aa toxin lines the lumen of the ion channel. J Biol Chem. 1999;274:31996–32000. doi: 10.1074/jbc.274.45.31996. [DOI] [PubMed] [Google Scholar]
- 11.Mohammed S I, Johnson D E, Aronson A I. Altered binding of the Cry1Ac toxin to larval membranes but not to the toxin-binding protein in Plodia interpunctella selected for resistance to different Bacillus thuringiensis isolates. Appl Environ Microbiol. 1996;62:4168–4173. doi: 10.1128/aem.62.11.4168-4173.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schnepf E, Crickmore N, Van Rie J, Lereclus D, Baum J, Feitelson J, Zeigler D R, Dean D H. Bacillus thuringiensis and its pesticidal crystal proteins. Microbiol Mol Biol Rev. 1998;62:775–806. doi: 10.1128/mmbr.62.3.775-806.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schwartz J-L, Juteau M, Grochulski P, Cygler M, Prefontaine C, Brosseau R, Masson L. Restriction of intramolecular movements within the CryIA(a) toxin molecule of Bacillus thuringiensis through disulphide bond engineering. FEBS Lett. 1997;410:397–402. doi: 10.1016/s0014-5793(97)00626-1. [DOI] [PubMed] [Google Scholar]
- 14.Schwartz J-L, Lu Y-J, Sohnlein P, Brousseau R, Laprade R, Masson L, Adang M J. Ion channels formed in planar lipid bilayers by Bacillus thuringiensis toxins in the presence of Manduca sexta midgut receptors. FEMS Microbiol Lett. 1997;412:270–276. doi: 10.1016/s0014-5793(97)00801-6. [DOI] [PubMed] [Google Scholar]
- 15.Wolfersberger M G, Luthy P, Mauer A, Parenti P, Sacchi V F, Giordano B, Hanozet G M. Preparation and partial characterization of amino acid transporting brushborder membrane vesicles from the larval midgut of the cabbage butterfly (Pieris brassicae) Comp Biochem Physiol A. 1987;86:301–312. [Google Scholar]